Viral Infections and Interferons in the Development of Obesity
Abstract
1. Viral Infectobesity: The Association of Chronic Viral Infections with Obesity
2. IFNs Emerging as a Key Factor to Mediate Viral Persistence and Relevant Infectobesity
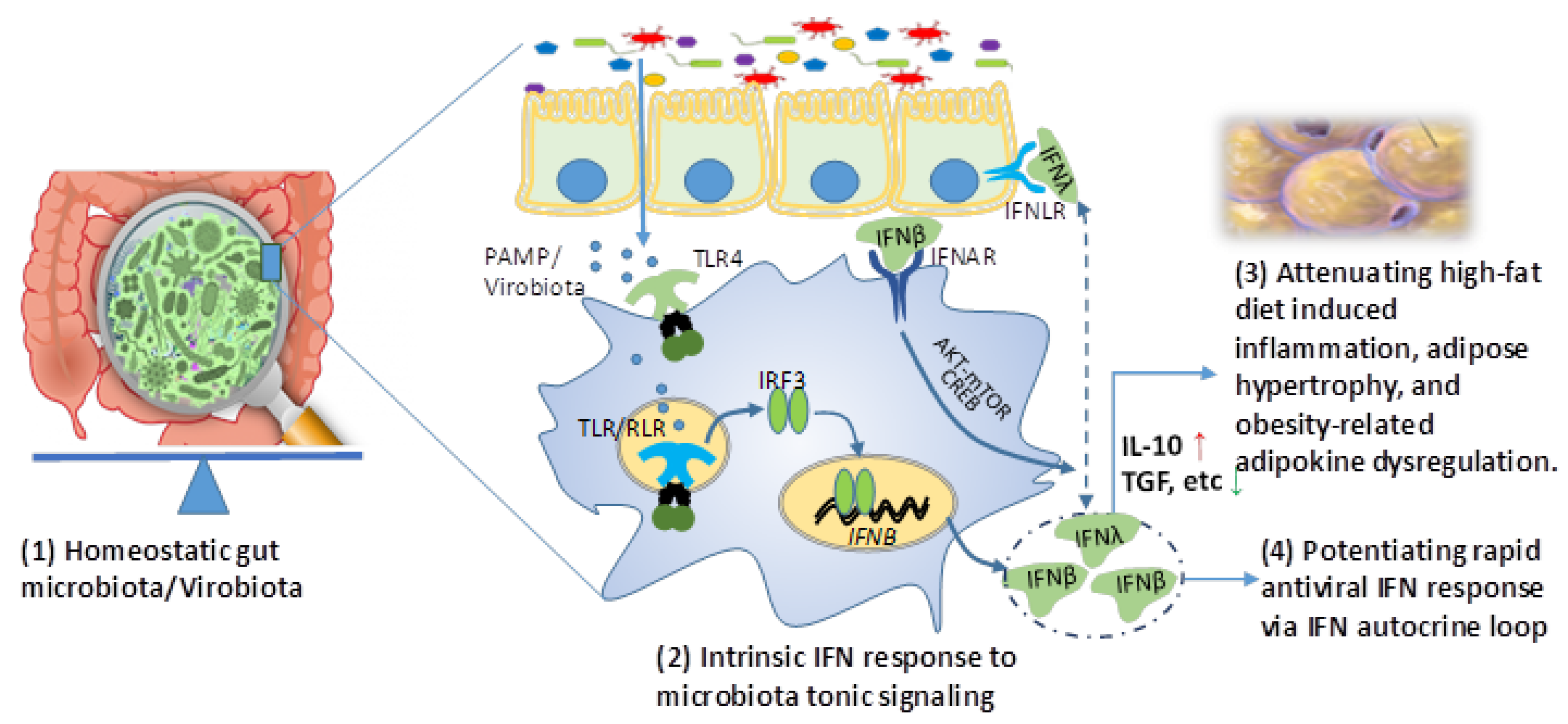
3. Gut Microbiota and Intrinsic Interferon Response
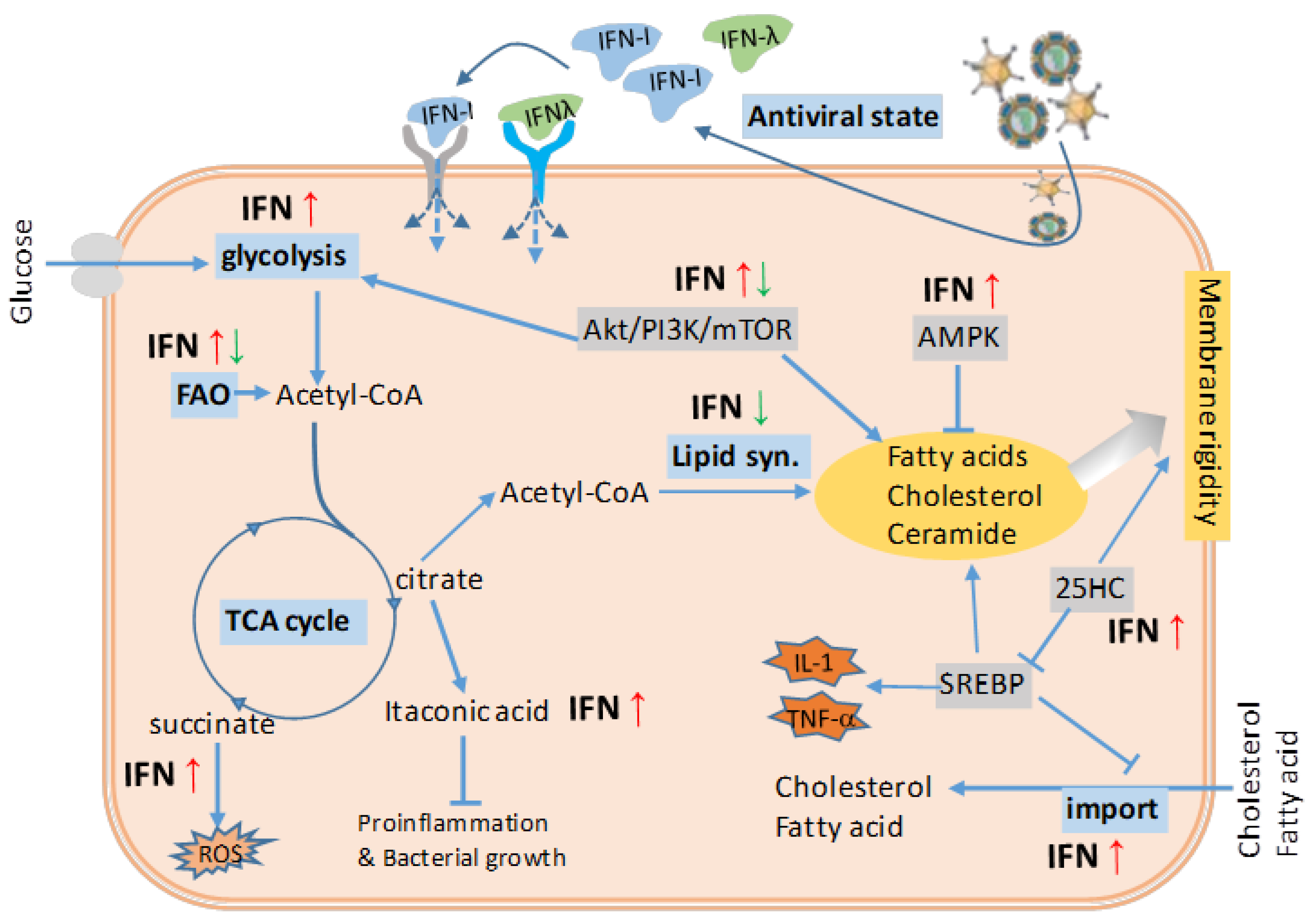
4. Regulation of Energy and Lipid Metabolism by Interferons
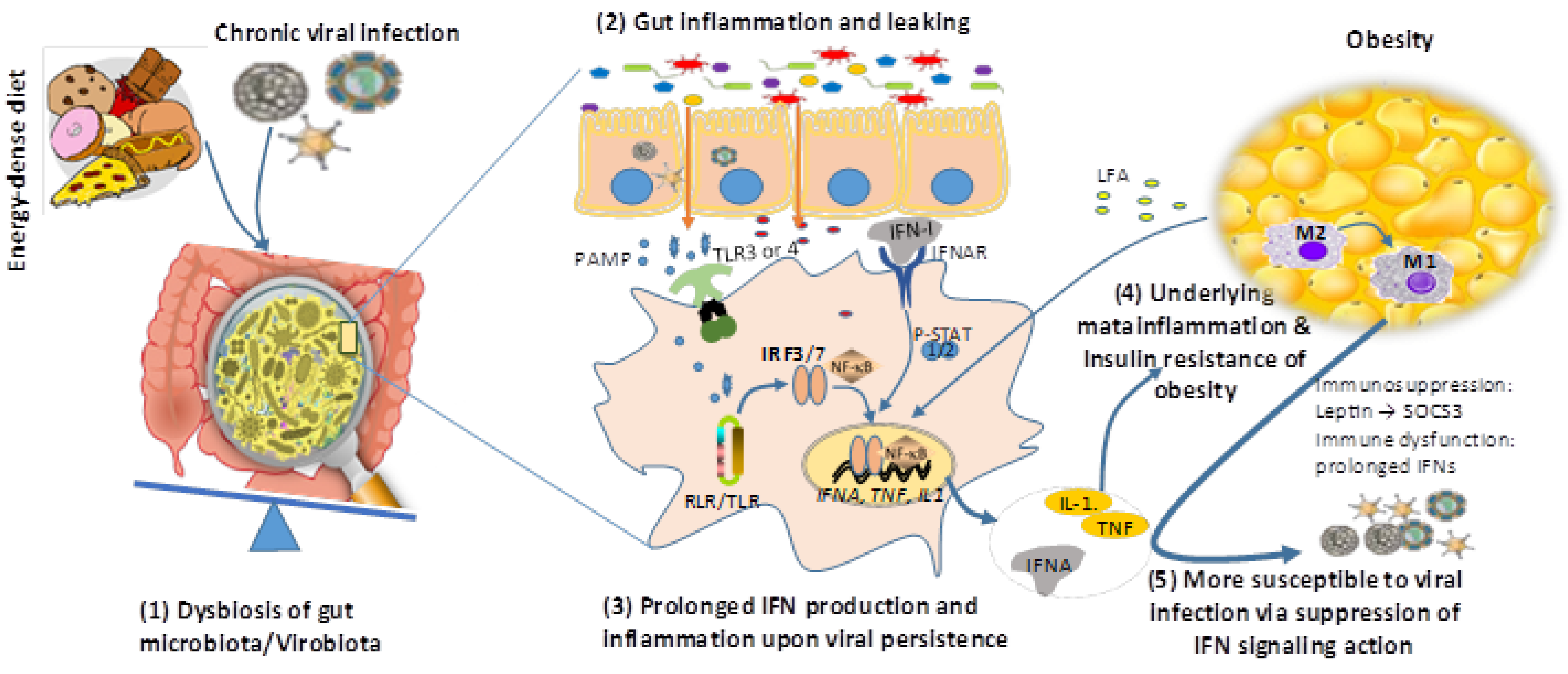
5. Interferon Responses Underlying the Reciprocal Causality of Obesity and Persistent Viral Infections
6. Prospects of Interferon-Based Anti-Obesity Therapies
7. Conclusive Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Adult Obesity Facts. Available online: https://www.cdc.gov/obesity/data/adult.html (accessed on 15 July 2019).
- Obesity. Available online: https://www.who.int/topics/obesity/en/ (accessed on 15 July 2019).
- Ludwig, D.S.; Friedman, M.I. Increasing adiposity: Consequence or cause of overeating? JAMA 2014, 311, 2167–2168. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Honce, R.; Schultz-Cherry, S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef] [PubMed]
- Preveden, T.; Scarpellini, E.; Milić, N.; Luzza, F.; Abenavoli, L. Gut microbiota changes and chronic hepatitis C virus infection. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M. Inflammasomes in the gastrointestinal tract: Infection, cancer and gut microbiota homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Debédat, J.; Clément, K.; Aron-Wisnewsky, J. Gut Microbiota dysbiosis in human obesity: Impact of bariatric surgery. Curr. Obes. Rep. 2019, 8, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Toor, D.; Wsson, M.K.; Kumar, P.; Karthikeyan, G.; Kaushik, N.K.; Goel, C.; Singh, S.; Kumar, A.; Prakash, H. Dysbiosis disrupts gut immune homeostasis and promotes gastric diseases. Int. J. Mol. Sci. 2019, 20, E2432. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Naito, Y.; Takagi, T. Intestinal microbiome as a novel therapeutic target for local and systemic inflammation. Pharm. Ther. 2019, 199, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Snell, L.M.; McGaha, T.L.; Brooks, D.G. Type I interferon in chronic virus infection and cancer. Trends Immunol. 2017, 38, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Dagenais-Lussier, X.; Loucif, H.; Murira, A.; Laulhé, X.; Stäger, S.; Lamarre, A.; van Grevenynghe, J. Sustained IFN-I expression during established persistent viral infection: A “bad seed” for protective immunity. Viruses 2017, 10, E12. [Google Scholar] [CrossRef] [PubMed]
- Sohrab, S.S.; Kamal, M.A.; Atkinson, R.L.; Alawi, M.M.; Azhar, E.I. Viral infection and obesity: Current status and future prospective. Curr. Drug Metab. 2017, 18, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Hainer, V.; Zamrazilová, H.; Kunešová, M.; Bendlová, B.; Aldhoon-Hainerová, I. Obesity and infection: Reciprocal causality. Physiol. Res. 2015, 64 (Suppl. 2), S105–S119. [Google Scholar]
- Voss, J.D.; Dhurandhar, N.V. Viral infections and obesity. Curr. Obes. Rep. 2017, 6, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Pasarica, M.; Dhurandhar, N.V. Infectobesity: Obesity of infectious origin. Adv. Food Nutr. Res. 2007, 52, 61–102. [Google Scholar] [PubMed]
- Voss, J.D.; Leon, J.C.; Dhurandhar, N.V.; Robb, F.T. Pawnobiome: Manipulation of the hologenome within one host generation and beyond. Front. Microbiol. 2015, 6, 697. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, D.; Sanin, D.E.; Everts, B.; Chen, Q.; Qiu, J.; Buck, M.D.; Patterson, A.; Smith, A.M.; Chang, C.H.; Liu, Z.; et al. Type 1 Interferons induce changes in core metabolism that are critical for immune function. Immunity 2016, 44, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Nice, T.J.; Osborne, L.C.; Tomov, V.T.; Artis, D.; Wherry, E.J.; Virgin, H.W. Type I interferon receptor deficiency in dendritic cells facilitates systemic murine norovirus persistence despite enhanced adaptive immunity. PLoS Pathog. 2016, 12, e1005684. [Google Scholar] [CrossRef] [PubMed]
- Tan, V.P.K.; Ngim, C.F.; Lee, E.Z.; Ramadas, A.; Pong, L.Y.; Ng, J.I.; Hassan, S.S.; Ng, X.Y.; Dhanoa, A. The association between obesity and dengue virus (DENV) infection in hospitalised patients. PLoS ONE 2018, 13, e0200698. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.; Velummailum, R.; Ryoo, S.G.; Senthinathan, A.; Yaghoubi, S.; Vasileva, D.; Ostermeier, E.; Plishka, M.; Soosaipillai, M.; Arora, P. Prevalence of chronic comorbidities in dengue fever and West Nile virus: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0200200. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.E. The fat of the matter: Obesity and visceral adiposity in treated HIV infection. Curr. HIV/AIDS Rep. 2017, 14, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.E.; Stanley, T.L.; Apovian, C.M.; Bhasin, S.; Brown, T.T.; Capeau, J.; Currier, J.S.; Dube, M.P.; Falutz, J.; Grinspoon, S.K.; et al. Practical review of recognition and management of obesity and lipohypertrophy in human immunodeficiency virus infection. Clin. Infect. Dis. 2017, 64, 1422–1429. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Voss, J.D.; Atkinson, R.L.; Dhurandhar, N.V. Role of adenoviruses in obesity. Rev. Med. Virol. 2015, 25, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Floyd, Z.E.; Stephens, J.M. Controlling a master switch of adipocyte development and insulin sensitivity: Covalent modifications of PPARγ. Biochim. Biophys. Acta 2012, 1822, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, O.; Dhurandhar, E.J.; Krishnapuram, R.; Kirk-Ballard, H.; Gupta, A.K.; Hegde, V.; Floyd, E.; Gimble, J.M.; Dhurandhar, N.V. PPARgamma-independent increase in glucose uptake and adiponectin abundance in fat cells. Endocrinology 2011, 152, 3648–3660. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, E.J.; Dubuisson, O.; Mashtalir, N.; Krishnapuram, R.; Hegde, V.; Dhurandhar, N.V. E4orf1: A novel ligand that improves glucose disposal in cell culture. PLoS ONE 2011, 6, e23394. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, N.V. Insulin sparing action of adenovirus 36 and its E4orf1 protein. J. Diabetes Complicat. 2013, 27, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Vangipuram, S.D.; Yu, M.; Tian, J.; Stanhope, K.L.; Pasarica, M.; Havel, P.J.; Heydari, A.R.; Dhurandhar, N.V. Adipogenic human adenovirus-36 reduces leptin expression and secretion and increases glucose uptake by fat cells. Int. J. Obes. 2007, 31, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1251214. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Shanahan, F.; Stanton, C.; Hill, C.; Coffey, A.; Ross, R.P. Movers and shakers: Influence of bacteriophages in shaping the mammalian gut microbiota. Gut Microbes. 2013, 4, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Bahra, S.M.; Weidemann, B.J.; Castro, A.N.; Walsh, J.W.; deLeon, O.; Burnett, C.M.; Pearson, N.A.; Murry, D.J.; Grobe, J.L.; Kirby, J.R. Risperidone-induced weight gain is mediated through shifts in the gut microbiome and suppression of energy expenditure. EBioMedicine 2015, 2, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Gogokhia, L.; Buhrke, K.; Bell, R.; Hoffman, B.; Brown, D.G.; Hanke-Gogokhia, C.; Ajami, N.J.; Wong, M.C.; Ghazaryan, A.; Valentine, J.F.; et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 2019, 25, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ke, Q.; Jin, Z.; Wang, H.; Kocher, O.; Morgan, J.P.; Zhang, J.; Crumpacker, C.S. Cytomegalovirus infection causes an increase of arterial blood pressure. PLoS Pathog. 2009, 5, e1000427. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Batty, G.D.; Kivimäki, M. Obesity, metabolic health, and history of cytomegalovirus infection in the general population. J. Clin. Endocrinol. Metab. 2016, 101, 1680–1685. [Google Scholar] [CrossRef] [PubMed]
- Liberski, P.P.; Gajos, A.; Sikorska, B.; Lindenbaum, S. Kuru, the first human prion disease. Viruses 2019, 11, E232. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Carp, R.I.; Callahan, S.M.; Wisniewski, H.M. Scrapie induced obesity in mice. J. Infect. Dis. 1987, 156, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Carp, R.I.; Callahan, S.M.; Sersen, E.A.; Moretz, R.C. Preclinical changes in weight of scrapie-infected mice as a function of scrapie agent-mouse strain combination. Intervirology 1984, 21, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Carp, R.I.; Callahan, S.M.; Wisniewski, H.M. Adrenal involvement in scrapie-induced obesity. Proc. Soc. Exp. Biol. Med. 1988, 189, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Strom, A.; Yutzy, B.; Kruip, C.; Ooms, M.; Schloot, N.C.; Roden, M.; Scott, F.W.; Loewer, J.; Holznagel, E. Foodborne transmission of bovine spongiform encephalopathy to non-human primates results in preclinical rapid-onset obesity. PLoS ONE 2014, 9, e104343. [Google Scholar] [CrossRef] [PubMed]
- de Brito, G.; Lupinacci, F.C.; Beraldo, F.H.; Santos, T.G.; Roffé, M.; Lopes, M.H.; de Lima, V.C.; Martins, V.R.; Hajj, G.N. Loss of prion protein is associated with the development of insulin resistance and obesity. Biochem. J. 2017, 474, 2981–2991. [Google Scholar] [CrossRef] [PubMed]
- Kralj, D.; VirovićJukić, L.; Stojsavljević, S.; Duvnjak, M.; Smolić, M.; Čurčić, I.B. Hepatitis C virus, insulin resistance, and steatosis. J. Clin. Transl. Hepatol. 2016, 4, 66–75. [Google Scholar] [PubMed]
- Lazo, M.; Nwankwo, C.; Daya, N.R.; Thomas, D.L.; Mehta, S.H.; Juraschek, S.; Willis, K.; Selvin, E. Confluence of epidemics of hepatitis C, diabetes, obesity, and chronic kidney disease in the United States population. Clin. Gastroenterol. Hepatol. 2017, 15, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.H.; Kee, K.M.; Chen, C.H.; Hu, T.H.; Lu, S.N.; Wang, J.H.; Hung, C.H. Sustained virological response and metabolic risk factors are associated with mortality in patients with chronic hepatitis C. PLoS ONE 2019, 14, e0208858. [Google Scholar] [CrossRef] [PubMed]
- Shields, L.E.; Jennings, J.; Liu, Q.; Lee, J.; Ma, W.; Blecha, F.; Miller, L.C.; Sang, Y. Cross-species genome-wide analysis reveals molecular and functional diversity of the unconventional interferon-ω subtype. Front. Immunol. 2019, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Shields, L.E.; Sang, E.R.; Si, H.; Pigg, A.; Blecha, F. Ileal transcriptome analysis in obese rats induced by high-fat diets and an adenoviral infection. Int. J. Obes. 2019, 43, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Terán-Cabanillas, E.; Hernández, J. Role of leptin and SOCS3 in inhibiting the type I interferon response during obesity. Inflammation 2017, 40, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Nice, T.J.; Robinson, B.A.; Van Winkle, J.A. The role of interferon in persistent viral infection: Insights from murine norovirus. Trends Microbiol. 2018, 26, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Suprunenko, T.; Hofer, M.J. Complexities of type I interferon biology: Lessons from LCMV. Viruses 2019, 11, E172. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, S.; Wang, J. MicroRNA-373 facilitates HSV-1 replication through suppression of type I IFN response by targeting IRF1. Biomed. Pharmacother. 2018, 97, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Sun, H.; Fan, H.; Wang, C.; Li, Y.; Liu, M.; Tang, H. MiR-23a facilitates the replication of HSV-1 through the suppression of interferon regulatory factor 1. PLoS ONE 2014, 9, e114021. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; Marsden, M.; Stacey, M.A.; Abdul-Karim, J.; GimenoBrias, S.; Costa Bento, D.; Scurr, M.J.; Ghazal, P.; Weaver, C.T.; Carlesso, G.; et al. Cytomegalovirus-specific IL-10-producing CD4+ T cells are governed by type-I IFN-induced IL-27 and promote virus persistence. PLoS Pathog. 2016, 12, e1006050. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E.E.; Geballe, A.P. Multifaceted evasion of the interferon response by cytomegalovirus. J. Interferon Cytokine Res. 2009, 29, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Biolatti, M.; Dell’Oste, V.; Pautasso, S.; Gugliesi, F.; von Einem, J.; Krapp, C.; Jakobsen, M.R.; Borgogna, C.; Gariglio, M.; De Andrea, M.; et al. Human cytomegalovirus tegument protein pp65 (pUL83) dampens type I interferon production by inactivating the DNA sensor cGAS without affecting STING. J. Virol. 2018, 92, e01774-17. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, D.; Homma, T.; Nakagaki, T.; Fuse, T.; Sano, K.; Satoh, K.; Mori, T.; Atarashi, R.; Nishida, N. Type I interferon protects neurons from prions in in vivo models. Brain 2019, 142, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Malachin, G.; Reiten, M.R.; Salvesen, Ø.; Aanes, H.; Kamstra, J.H.; Skovgaard, K.; Heegaard, P.M.H.; Ersdal, C.; Espenes, A.; Tranulis, M.A.; et al. Loss of prion protein induces a primed state of type I interferon-responsive genes. PLoS ONE 2017, 12, e0179881. [Google Scholar] [CrossRef] [PubMed]
- Sandler, N.G.; Bosinger, S.E.; Estes, J.D.; Zhu, R.T.; Tharp, G.K.; Boritz, E.; Levin, D.; Wijeyesinghe, S.; Makamdop, K.N.; del Prete, G.Q.; et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 2014, 511, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Scagnolari, C.; Monteleone, K.; Cacciotti, G.; Antonelli, G. Role of interferons in chronic hepatitis C infection. Curr. Drug Targets 2017, 18, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Wu, C.; Zhang, Y.J. Interplay between Janus kinase/signal transducer and activator of transcription signaling activated by type I interferons and viral antagonism. Front. Immunol. 2017, 8, 1758. [Google Scholar] [CrossRef] [PubMed]
- García-Sastre, A. Ten strategies of interferon evasion by viruses. Cell Host Microbe 2017, 22, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, R.; Syrjänen, J. Obesity and the risk and outcome of infection. Int. J. Obes. 2013, 37, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Backhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Meijnikman, A.S.; Gerdes, V.E.; Nieuwdorp, M.; Herrema, H. Evaluating causality of gut microbiota in obesity and diabetes in humans. Endocr. Rev. 2017, 39, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ma, L.; Ma, Y.; Zhang, F.; Zhao, C.; Nie, Y. Insights into the role of gut microbiota in obesity: Pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell 2018, 9, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Clément, K. Gut microbiota and obesity: Concepts relevant to clinical care. Eur. J. Intern. Med. 2018, 48, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Cadwell, K. The virome in host health and disease. Immunity 2015, 42, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, L.A.; Jones, B.V. The human gut virome: A multifaceted majority. Front. Microbiol. 2015, 6, 918. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Jain, S.; Nagpal, R.; Marotta, F. Increased fecal viral content associated with obesity in mice. World J. Diabetes 2016, 7, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Segal, J.P.; Carding, S.R.; Hart, A.L.; Hold, G.L. The gut virome: The ‘missing link’ between gut bacteria and host immunity? Ther. Adv. Gastroenterol. 2019, 12, 1756284819836620. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Abt, M.C.; Osborne, L.C.; Monticelli, L.A.; Doering, T.A.; Alenghat, T.; Sonnenberg, G.F.; Paley, M.A.; Antenus, M.; Williams, K.L.; Erikson, J.; et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012, 37, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Ganal, S.C.; Sanos, S.L.; Kallfass, C.; Oberle, K.; Johner, C.; Kirschning, C.; Lienenklaus, S.; Weiss, S.; Staeheli, P.; Aichele, P.; et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity 2012, 37, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Kosaka, A.; Yan, H.; Guo, Z.; Uchiyama, R.; Fukui, R.; Kaneko, D.; Kumagai, Y.; You, D.J.; Carreras, J.; et al. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-β. Immunity 2013, 38, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Miller, L.C.; Blecha, F.; Sang, Y. Reduction of infection by inhibiting mTOR pathway is associated with reversed repression of type I interferon by porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2017, 98, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- González-Navajas, J.M.; Lee, J.; David, M.; Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012, 12, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Brown, J.; Garcia, C.A.; Tang, Y.; Benakanakere, M.R.; Greenway, T.; Alard, P.; Kinane, D.F.; Martin, M. The role of glycogen synthase kinase 3 in regulating IFN-β-mediated IL-10 production. J. Immunol. 2011, 186, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Alsaggar, M.; Mills, M.; Liu, D. Interferon beta overexpression attenuates adipose tissue inflammation and high-fat diet-induced obesity and maintains glucose homeostasis. Gene Ther. 2017, 24, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, S.D.; Weichhart, T. Effects of interferons and viruses on metabolism. Front. Immunol. 2016, 7, 630. [Google Scholar] [CrossRef] [PubMed]
- Raniga, K.; Liang, C. Interferons: Reprogramming the metabolic network against viral infection. Viruses 2018, 10, E36. [Google Scholar] [CrossRef] [PubMed]
- York, A.G.; Williams, K.J.; Argus, J.P.; Zhou, Q.D.; Brar, G.; Vergnes, L.; Gray, E.E.; Zhen, A.; Wu, N.C.; Yamada, D.H.; et al. Limiting cholesterol biosynthetic flux spontaneously engages type I IFN signaling. Cell 2015, 163, 1716–1729. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, M.; Mercer, J. Lipid interactions during virus entry and infection. Cell Microbiol. 2014, 16, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Chukkapalli, V.; Heaton, N.S.; Randall, G. Lipids at the interface of virus-host interactions. Curr. Opin. Microbiol. 2012, 15, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Ramos, J.F.; Reyes-Ruiz, J.M.; Del Ángel, R.M. The Role of Host Cholesterol During Flavivirus Infection. Front. Cell. Infect. Microbiol. 2018, 8, 388. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Aliyari, R.; Chikere, K.; Li, G.; Marsden, M.D.; Smith, J.K.; Pernet, O.; Guo, H.; Nusbaum, R.; Zack, J.A.; et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 2013, 38, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Soudani, N.; Hage-Sleiman, R.; Karam, W.; Dbaibo, G.; Zaraket, H. Ceramide Suppresses Influenza A Virus Replication In Vitro. J. Virol. 2019, 93, e00053-19. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.D.; Platanias, L.C.; Fish, E.N. Beta interferon regulation of glucose metabolism is PI3K/Akt dependent and important for antiviral activity against coxsackievirus B3. J. Virol. 2014, 88, 3485–3495. [Google Scholar] [CrossRef] [PubMed]
- Pantel, A.; Teixeira, A.; Haddad, E.; Wood, E.G.; Steinman, R.M.; Longhi, M.P. Direct type I IFN but not MDA5/TLR3 activation of dendritic cells is required for maturation and metabolic shift to glycolysis after poly IC stimulation. PLoS Biol. 2014, 12, e1001759. [Google Scholar] [CrossRef] [PubMed]
- Grunert, T.; Leitner, N.R.; Marchetti-Deschmann, M.; Miller, I.; Wallner, B.; Radwan, M.; Vogl, C.; Kolbe, T.; Kratky, D.; Gemeiner, M.; et al. A comparative proteome analysis links tyrosine kinase 2 (Tyk2) to the regulation of cellular glucose and lipid metabolism in response to poly(I:C). J. Proteom. 2011, 74, 2866–2880. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hedl, M.; Yan, J.; Abraham, C. IRF5 and IRF5 disease-risk variants increase glycolysis and human M1 macrophage polarization by regulating proximal signaling and Akt2 activation. Cell Rep. 2016, 16, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A.; Huq, A.; Najarro, P. Inhibition of mitochondrial function by interferon. J. Biol. Chem. 1996, 271, 13184–13190. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Sassano, A.; Majchrzak-Kita, B.; Baker, D.P.; Su, B.; Fish, E.N.; Platanias, L.C. Regulatory effects of mTORC2 complexes in type I IFN signaling and in the generation of IFN responses. Proc. Natl. Acad. Sci. USA 2012, 109, 7723–7728. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yu, Y.; Zhong, Y.; Giannopoulou, E.G.; Hu, X.; Liu, H.; Cross, J.R.; Rätsch, G.; Rice, C.M.; Ivashkiv, L.B. Interferon-gamma regulates cellular metabolism and mRNA translation to potentiate macrophage activation. Nat. Immunol. 2015, 16, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, R.; Stambas, J.; Selemidis, S. Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends Pharm. Sci. 2012, 33, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Ma, F.; Ma, X.; Jia, W.; Pan, E.; Cheng, G.; Chen, L.; Sun, C. Regulating innate and adaptive immunity for controlling SIV infection by 25-hydroxycholesterol. Front. Immunol. 2018, 9, 2686. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.Y.; Yang, Y.; Lim, J.S.; Lee, M.S.; Zhang, D.E.; Kim, K.I. The mitochondrial pathway and reactive oxygen species are critical contributors to interferon-α/β-mediated apoptosis in Ubp43-deficient hematopoietic cells. Biochem. Biophys. Res. Commun. 2012, 423, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Suzuki, O.; Haruyama, T.; Akaike, T. Interferon-gamma induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. J. Cell. Biochem. 2003, 89, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Bougnoux, P.; Salem, N.; Lyons, C.; Hoffman, T. Alteration in the membrane fatty acid composition of human lymphocytes and cultured transformed cells induced by interferon. Mol. Immunol. 1985, 22, 1107–1113. [Google Scholar] [CrossRef]
- Pfeffer, L.M.; Landsberger, F.R.; Tamm, I. Beta-interferon-induced time-dependent changes in the plasma membrane lipid bilayer of cultured cells. J. Interferon Res. 1981, 1, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Furlong, S.T.; Mednis, A.; Remold, H.G. Interferon-gamma stimulates lipid metabolism in human monocytes. Cell. Immunol. 1992, 143, 108–117. [Google Scholar] [CrossRef]
- Chatterjee, S.; Cheung, H.C.; Hunter, E. Interferon inhibits Sendai virus-induced cell fusion: An effect on cell membrane fluidity. Proc. Natl. Acad. Sci. USA 1982, 79, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Kroczynska, B.; Rafidi, R.L.; Majchrzak-Kita, B.; Kosciuczuk, E.M.; Blyth, G.T.; Jemielity, J.; Warminska, Z.; Saleiro, D.; Mehrotra, S.; Arslan, A.D.; et al. Interferon gamma (IFNgamma) signaling via mechanistic target of rapamycin complex 2 (mTORC2) and regulatory effects in the generation of type II interferon biological responses. J. Biol. Chem. 2016, 291, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, B.; Tippetts, T.S.; Monibas, R.M.; Liu, J.; Li, Y.; Wang, L.; Wilkerson, J.L.; Sweeney, C.R.; Pereira, R.F.; Sumida, D.H.; et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 2019, 365, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Ottenlinger, F.M.; Mayer, C.A.; Ferreirós, N.; Schreiber, Y.; Schwiebs, A.; Schmidt, K.G.; Ackermann, H.; Pfeilschifter, J.M.; Radeke, H.H. Interferon-beta increases plasma ceramides of specific chain length in multiple sclerosis patients, unlike fingolimod or natalizumab. Front. Pharmacol. 2016, 7, 412. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, D.; Hogan, A.E. Dysregulation of natural killer cells in obesity. Cancers 2019, 11, E573. [Google Scholar]
- Ghazarian, M.; Revelo, X.S.; Nøhr, M.K.; Luck, H.; Zeng, K.; Lei, H.; Tsai, S.; Schroer, S.A.; Park, Y.J.; Chng, M.H.Y.; et al. Type I interferon responses drive intrahepatic T cells to promote metabolic syndrome. Sci. Immunol. 2017, 2, eaai7616. [Google Scholar] [CrossRef] [PubMed]
- Easterbrook, J.D.; Dunfee, R.L.; Schwartzman, L.M.; Jagger, B.W.; Sandouk, A.; Kash, J.C.; Memoli, M.J.; Taubenberger, J.K. Obese mice have increased morbidity and mortality compared to non-obese mice during infection with the 2009 pandemic H1N1 influenza virus. Influenza Other Respir. Viruses 2011, 5, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.J.; Sauer, M.; He, L.; Tycksen, E.; Kalugotla, G.; Razani, B.; Schilling, J.D. PPARγ deficiency suppresses the release of IL-1β and IL-1α in macrophages via a type 1 IFN-dependent mechanism. J. Immunol. 2018, 201, 2054–2069. [Google Scholar] [CrossRef] [PubMed]
- McGillicuddy, F.C.; Chiquoine, E.H.; Hinkle, C.C.; Kim, R.J.; Shah, R.; Roche, H.M.; Smyth, E.M.; Reilly, M.P. Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J. Biol. Chem. 2009, 284, 31936–31944. [Google Scholar] [CrossRef] [PubMed]
- Wieser, V.; Adolph, T.E.; Grander, C.; Grabherr, F.; Enrich, B.; Moser, P.; Moschen, A.R.; Kaser, S.; Tilg, H. Adipose type I interferon signalling protects against metabolic dysfunction. Gut 2018, 67, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Kanameni, S.; Chang, C.A.; Nair, V.; Safe, S.; Bazer, F.W.; Zhou, B. Interferon tau alleviates obesity-induced adipose tissue inflammation and insulin resistance by regulating macrophage polarization. PLoS ONE 2014, 9, e98835. [Google Scholar] [CrossRef] [PubMed]
- Interferons for Hepatitis C: Understanding the Long-Term Side Effects. Available online: https://www.healthline.com/health/hepatitis-c/interferons-long-term-effects (accessed on 10 October 2019).
| Adipogenic Viruses * |  Adenoviridae [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] |  Gut Phages [31,32,33,34] |  Herpesviridae [35,36] |  Slow Virus (Prion) [37,38,39,40,41,42] |  Other Viruses [21,22,23,43,44,45] |
|---|---|---|---|---|---|
| Natural hosts or Livestock reservoirs | Avian: SMAM1 Human: Ad-5/9/31/36/37 | Gut phages likely in all animals | Human: HSV-1, CMV, HHV8 | Sheep: Scrapie Cattle: BSE/CJD Human: Kuru | Avian: RAV7 Sheep/Horse: BDV Human: DENV, HIV, HCV |
| Lipogenic in vitro | Ad-5/9/31/36/37 Cause adipocyte differen-tiation and lipogenesis | Unknown | Increase lipogenesis in cells | Unknown | HCV enhances lipid synthesis, and CDV enlarges adipocytes |
| Adipogenic in animals | Ad-36, Ad-37: chickens Ad-36, Ad-5: mice Ad-36: rats, marmosets | Gut phages following risperidone treatment: mice | Unknown | BSE: primates Scrapie, CJD variants: mice | BDV, CDV: mice RAV7: chickens |
| Obesity-association in humans | Ad-5: childhood obesity, Ad-36: childhood, adult obesity and BMI SMAM-1: BMI | Adipogenic gut microbe transfer associated with heavier human donor | CMV: metabolic Syndrome HSV-1: adult obesity | Kuru obesity and/or bulimia during early disease in humans | HCV genotype 3: insulin resistance in humans DENV: children obesity HIV: obesity in patients on ART |
| Obesity during persistent viral infections? [13,14,15] | YES | Unknown, but the effect of gut phages on microbiota is persistent | YES | YES | YES, especially in human cases |
| Suppression of acute IFN antiviral response, and [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63] | Yes, through E1A gene | Unknown | Yes, HSV-1 through miRNA; and CMV has multiple IFN-antagonistic mechanisms | Infecting prions suppress interferon expression | Multiple IFN-antagonistic mechanism in DENV, HIV, and HCV |
| Cause prolonged IFN production upon chronic infection [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63] | Likely yes in adipogenic Ad-36 infection, and persistent enteric infection in children | Expansion of gut phages induces intestinal inflammation via TLR9-mediated IFN reaction | CMV exploits prolonged IFN production to induce IL-27 production and chronic infection | Typically chronic, as reflected by the term of “slow virus” | Prolonged IFNs promote HCV and retroviral chronicity. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Jennings, J.; Gong, Y.; Sang, Y. Viral Infections and Interferons in the Development of Obesity. Biomolecules 2019, 9, 726. https://doi.org/10.3390/biom9110726
Tian Y, Jennings J, Gong Y, Sang Y. Viral Infections and Interferons in the Development of Obesity. Biomolecules. 2019; 9(11):726. https://doi.org/10.3390/biom9110726
Chicago/Turabian StyleTian, Yun, Jordan Jennings, Yuanying Gong, and Yongming Sang. 2019. "Viral Infections and Interferons in the Development of Obesity" Biomolecules 9, no. 11: 726. https://doi.org/10.3390/biom9110726
APA StyleTian, Y., Jennings, J., Gong, Y., & Sang, Y. (2019). Viral Infections and Interferons in the Development of Obesity. Biomolecules, 9(11), 726. https://doi.org/10.3390/biom9110726






