Evaluation of Novel Chalcone-Thiosemicarbazones Derivatives as Potential Anti-Leishmania amazonensis Agents and Its HSA Binding Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Organic Synthesis
2.1.1. Chemicals and Instruments
2.1.2. (2E)-3-(4-X-phenyl)-1-phenylprop-2-en-1-one (3a–g)
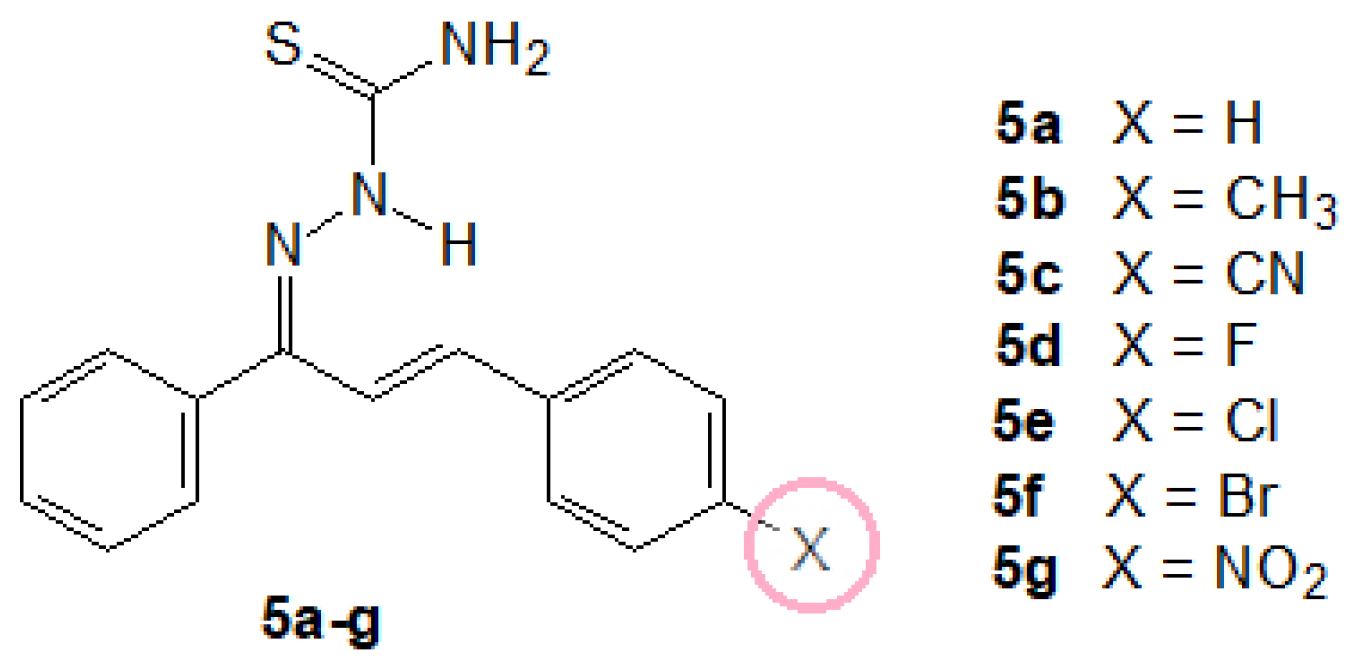
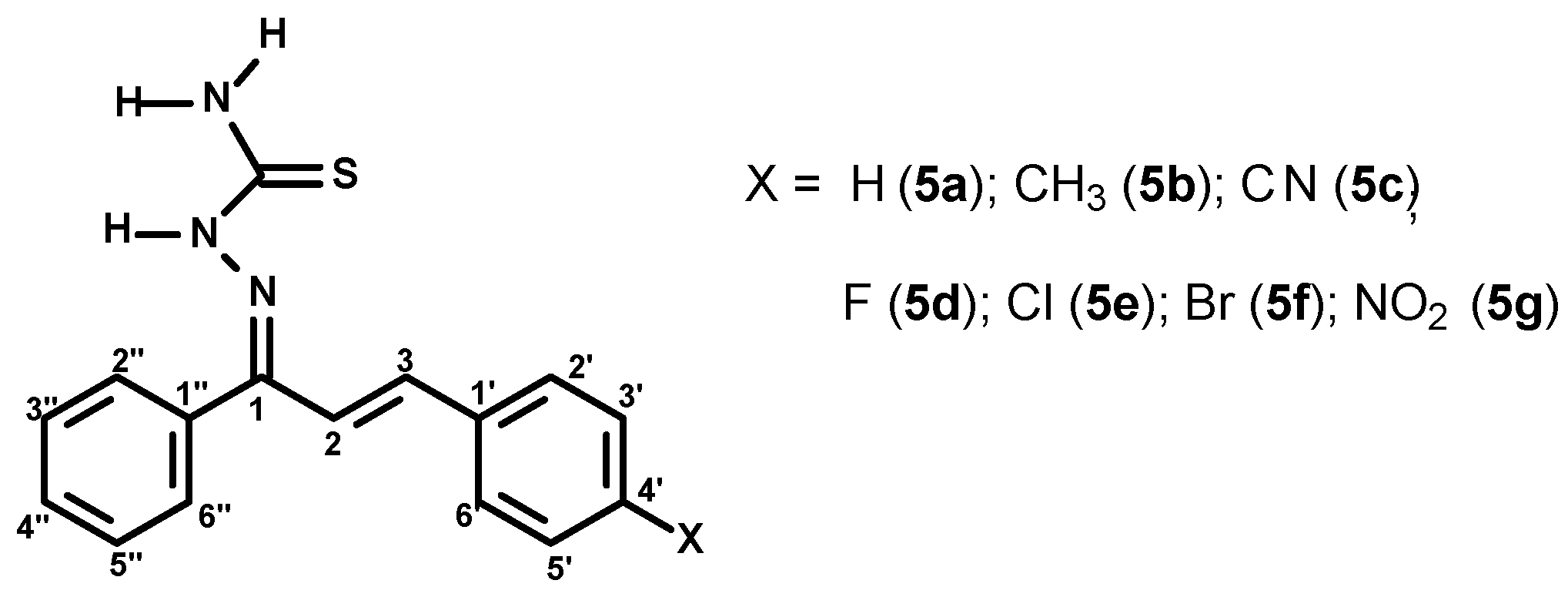
2.1.3. General Procedure for the Preparation of the (1E,2E)-3-(4-X-phenyl)-1-phenylprop-2-en-1-one Thiosemicarbazones (5a–g)
(1E,2E)-3-(phenyl)-1-phenylprop-2-en-1-one thiosemicarbazone (5a)
(1E,2E)-3-(4′-methylphenyl)-1-phenylprop-2-en-1-one thiosemicarbazone (5b)
(1E,2E)-3-(4′-cyanophenyl)-1-phenylprop-2-en-1-one thiosemicarbazone (5c)
(1E,2E)-3-(4′-fluorphenyl)-1-phenylprop-2-en-1-one thiosemicarbazone (5d)
(1E,2E)-3-(4′-chlorophenyl)-1-phenylprop-2-en-1-one thiosemicarbazone (5e)
(1E,2E)-3-(4′-bromophenyl)-1-phenylprop-2-en-1-one thiosemicarbazone (5f)
(1E,2E)-3-(4′-nitrophenyl)-1-phenylprop-2-en-1-one thiosemicarbazone (5g)
2.2. Biologic Assays
2.2.1. Chemicals
2.2.2. Parasite Cultures
2.2.3. Promastigote Assays
2.2.4. Macrophage Cytotoxicity
2.2.5. Intracellular Amastigotes Assays
2.2.6. Axenic Amastigotes Assays
2.2.7. Statistical Analysis
2.3. HSA Binding Studies
2.3.1. Spectroscopic Analysis
2.3.2. Molecular Docking Analysis for the Interaction HSA:5e
3. Results and Discussion
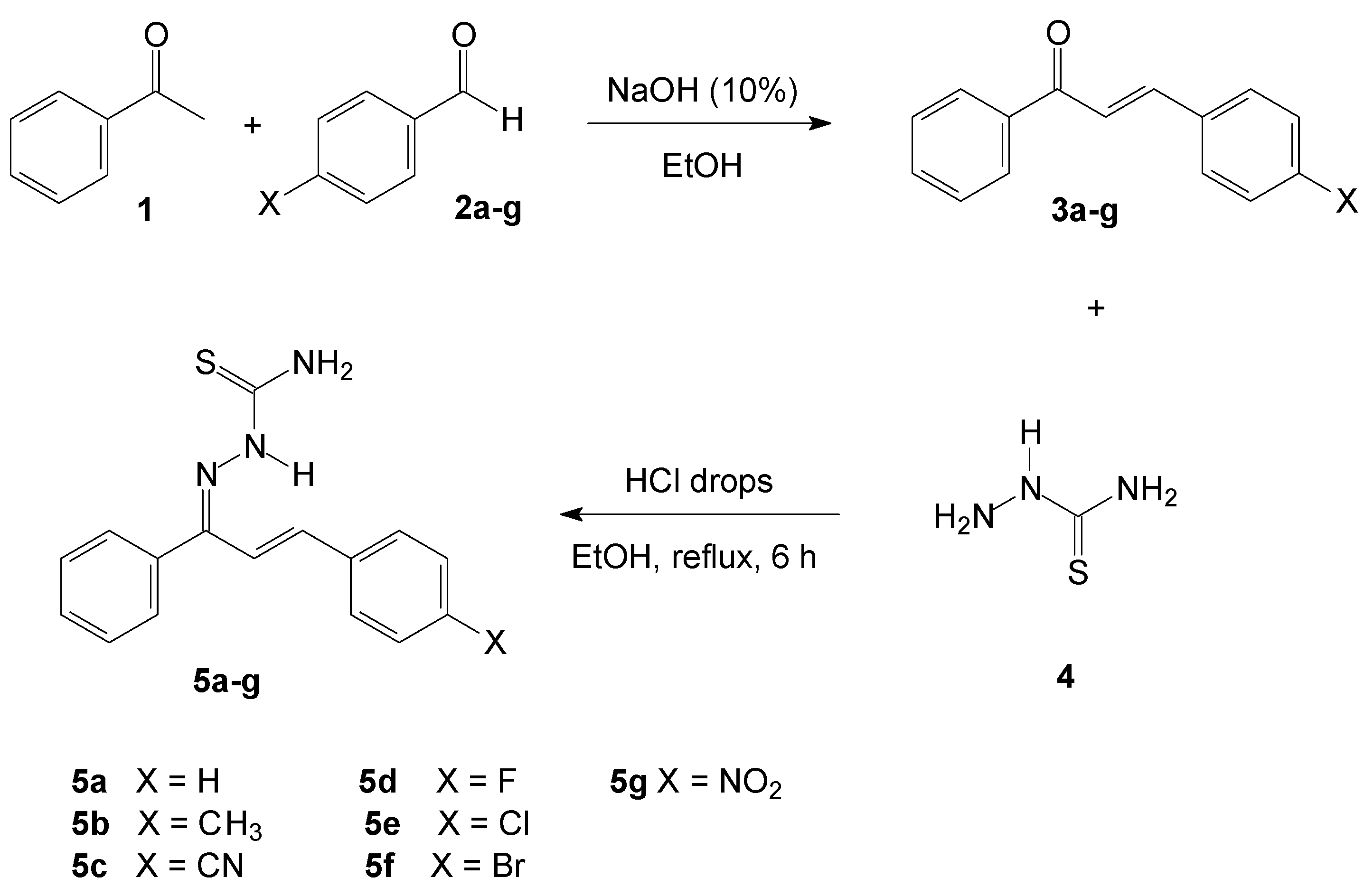
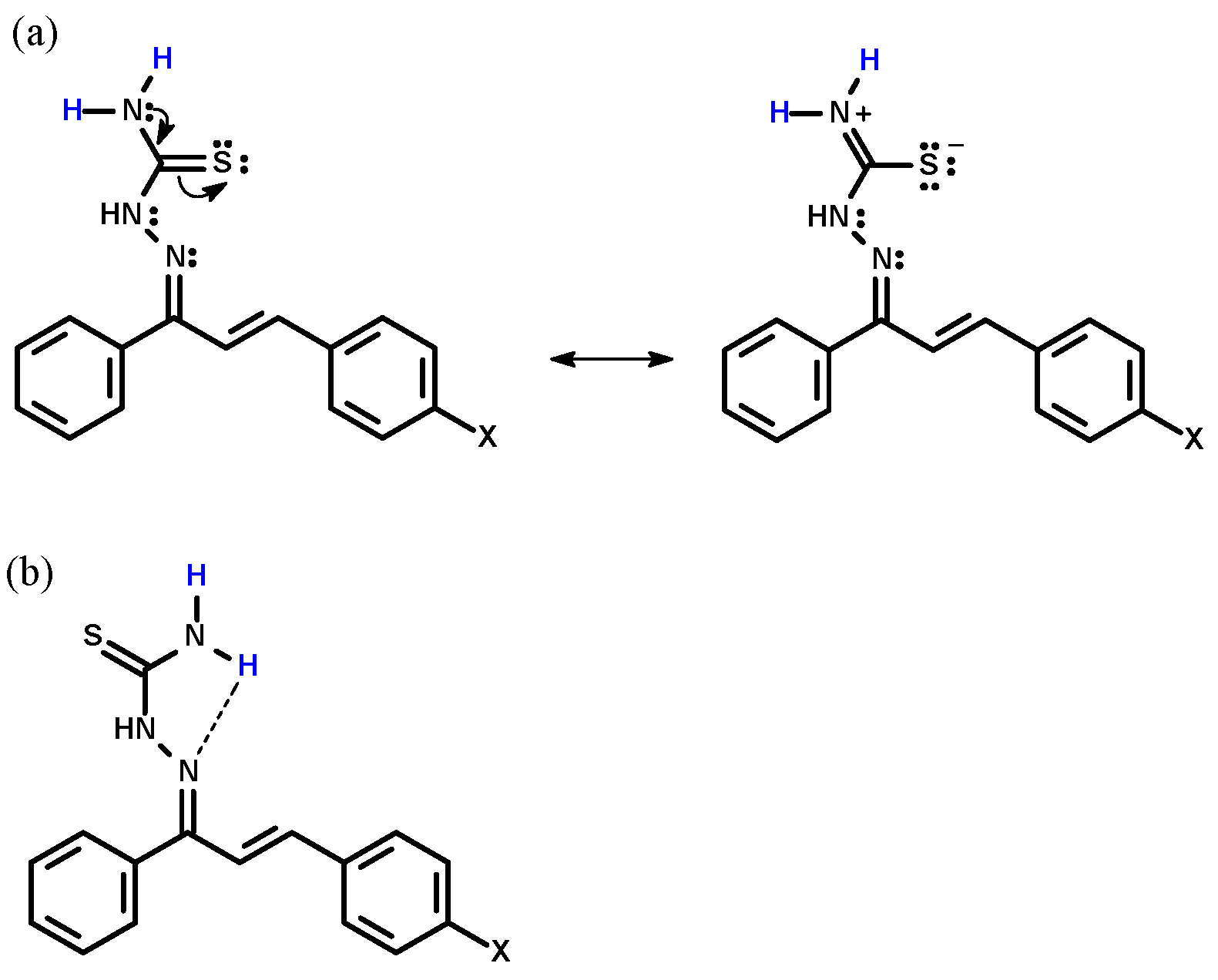
3.1. Synthesis of Chalcone-Thiosemicarbazones
3.2. Anti-Leishmanial Effects
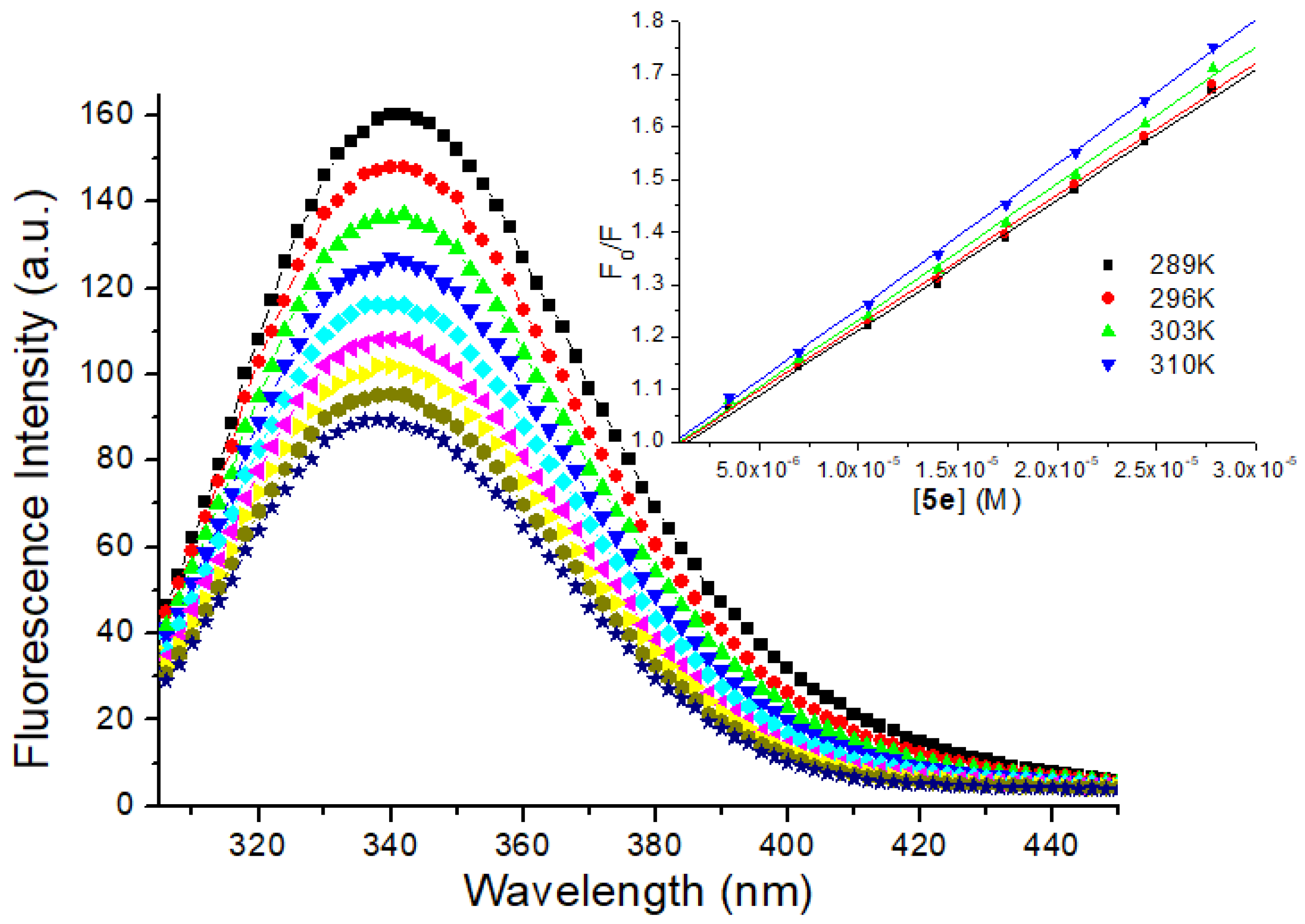
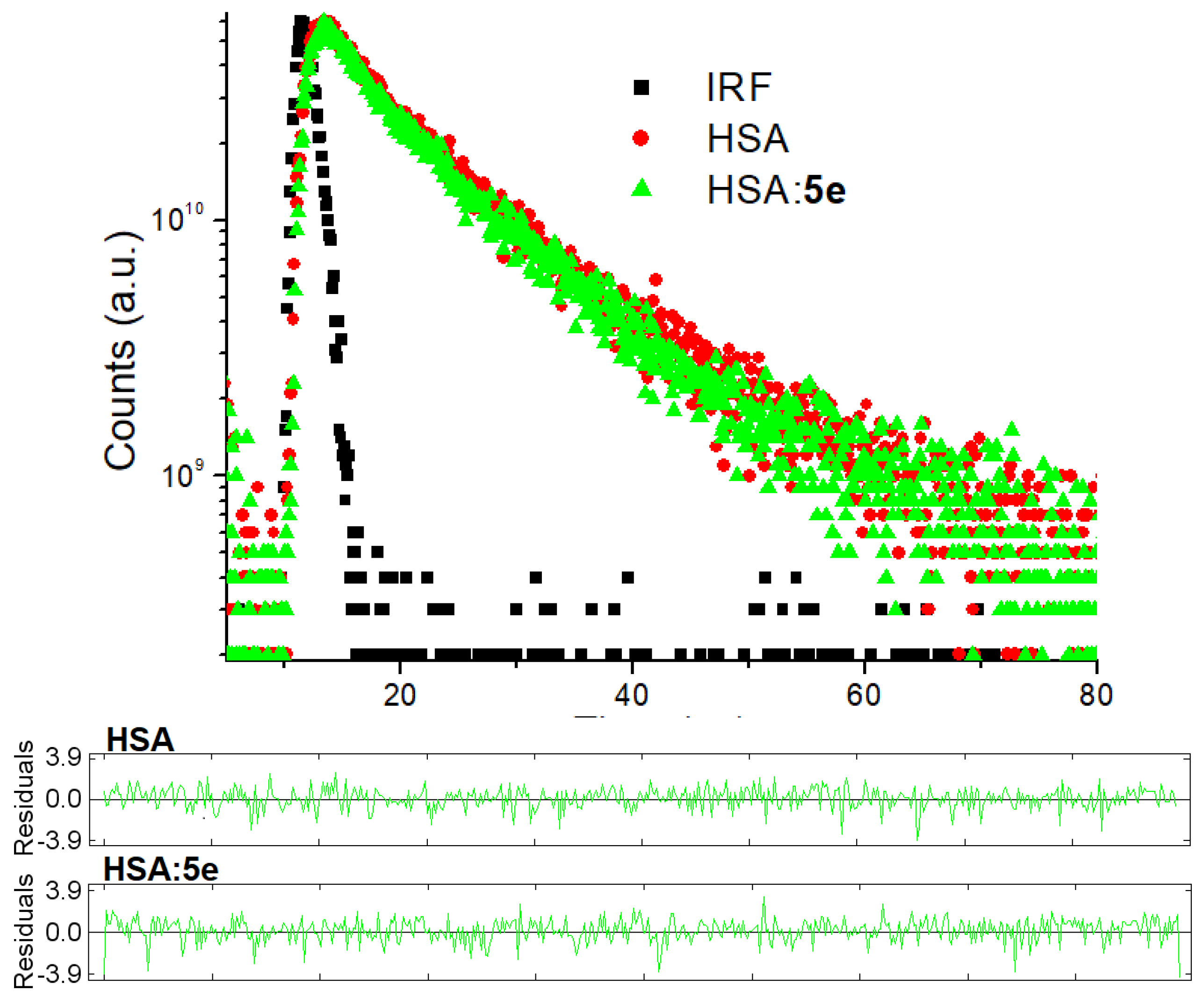
3.3. Evaluation on the Interaction between HSA and 5e
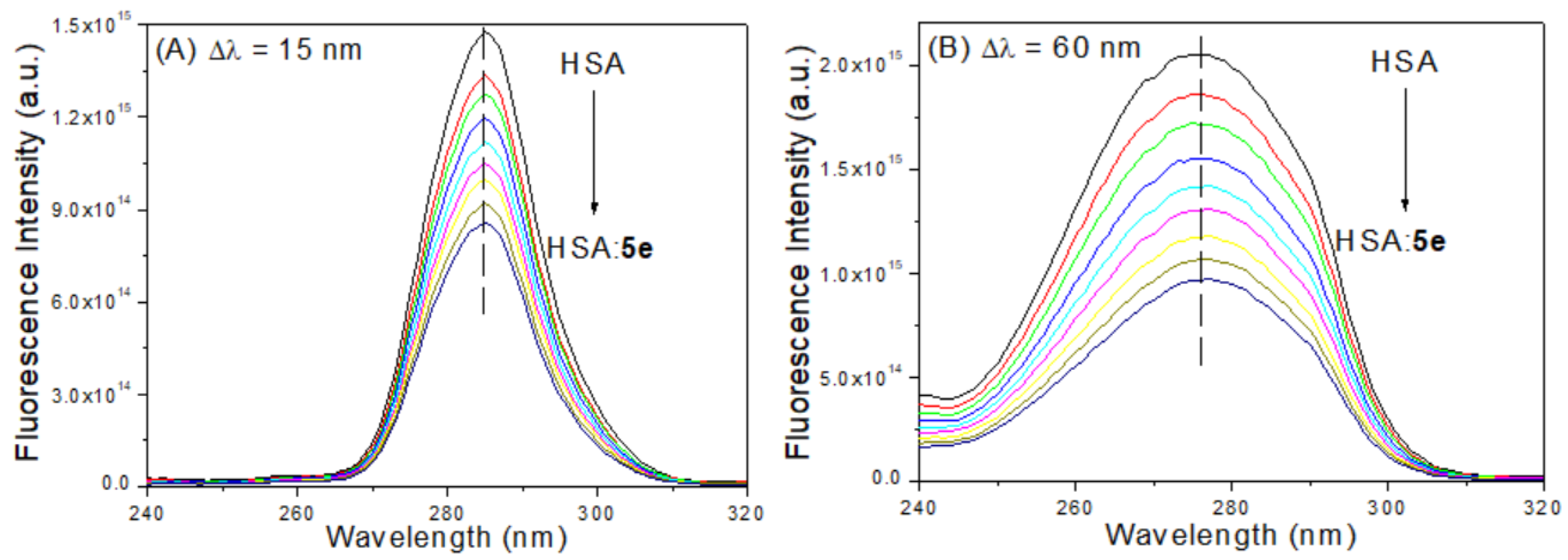
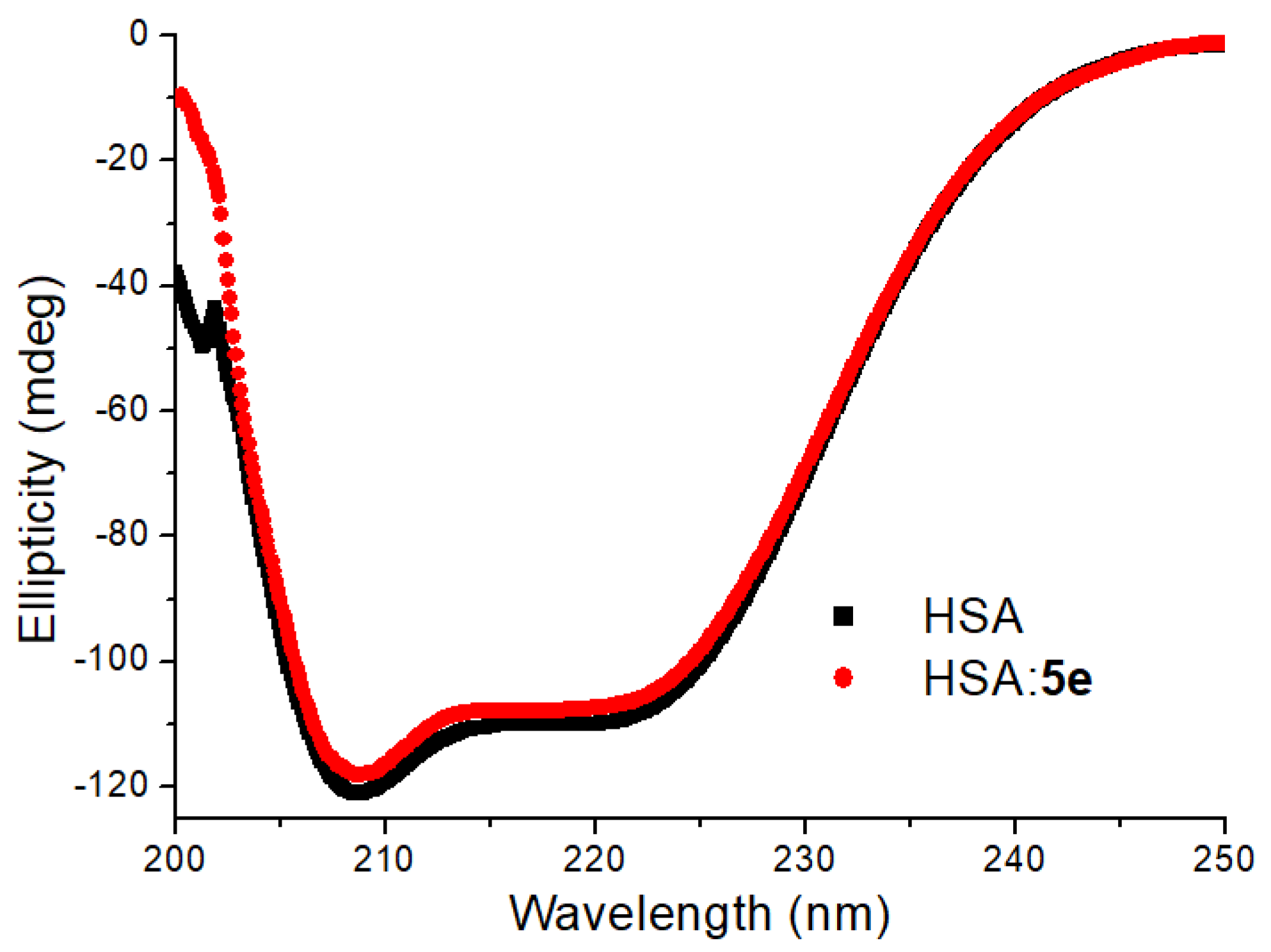
3.4. Evaluation on the Microenvironment and Structure of HSA upon 5e Binding
3.5. Molecular Docking Analysis for HSA:5e
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO—World Health Organization. Available online: www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 13 August 2019).
- Monzote, L. Current treatment of leishmaniasis: A review. Open Antimicrob. Agents J. 2009, 1, 9–19. [Google Scholar]
- Bilbe, G. Overcoming neglect of kinetoplastid diseases. Science 2015, 348, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Lindoso, J.A.L.; Costa, J.L.M.; Queiroz, I.T.; Goto, H. Review of current treatments for leishmaniasis. Res. Rep. Trop. Med. 2012, 3, 69–77. [Google Scholar] [PubMed]
- Rajasekaran, R.; Cham, Y.P. Potential therapeutic targets and the role of technology in developing novel anti-leishmanial drugs. Drug Discov. Today 2015, 20, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.F.; Castro-Pinto, D.; Echevarria, A.; Reis, C.M.; Del Cistia, C.N.; Sant’Anna, C.M.R.; Teixeira, F.; Castro, H.; Canto-Cavalheiro, M.; Leon, L.L.; et al. Investigation of thypanothione reductase inhibitory activity by 1,3,4-thiadiazolium-2-aminide derivatives and molecular docking studies. Bioorg. Med. Chem. 2012, 20, 1760. [Google Scholar] [CrossRef]
- Seifert, K. Structures, targets and recent approaches in anti-leishmanial drug discovery and development. Open Med. Chem. J. 2011, 5, 31. [Google Scholar] [CrossRef]
- Insuasty, B.; Ramírez, J.; Becerra, D.; Echeverry, C.; Quiroga, J.; Abonia, R.; Robledo, S.M.; Velez, I.D.; Upegui, Y.; Muñoz, J.A.; et al. An efficient synthesis of new caffeine-based chalcones, pyrazolines and pyrazolo [3,4-b] [1,4] diazepines as potential antimalarial, antitrypanosomal and antileishmanial agents. Eur. J. Med. Chem. 2015, 93, 401–413. [Google Scholar] [CrossRef]
- Torres-Santos, E.C.; Moreira, D.L.; Kaplan, M.A.; Meirelles, M.N.; Rossi-Bergmann, B. Selective effect of 2′,6′-dihydroxy-4′-methoxychalcone isolated from Piper aduncum on Leishmania amazonensis. Antimicrob. Agents Chemother. 1999, 43, 1234–1241. [Google Scholar] [CrossRef]
- Coskun, D.; Erkisa, M.; Ulukaya, E.; Coskun, M.F.; Ari, F. Novel 1-(7-ethoxy-1-benzofuran-2-yl) substituted chalcone derivatives: Synthesis, characterization and anticancer activity. Eur. J. Med. Chem. 2017, 136, 212–222. [Google Scholar] [CrossRef]
- Sonmez, F.; Sevmezler, S.; Atahan, A.; Ceylan, M.; Demir, D.; Gencer, N.; Arslan, O.; Kucukislamonglu, M. Evaluation of new chalcone derivatives as polyphenol oxidase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 7479–7482. [Google Scholar] [CrossRef]
- De Melos, J.L.R.; Torres-Santos, E.C.; Faiões, V.S.; Del Cistia, C.N.; Sant’Anna, C.M.R.; Rodrigues-Santos, C.E.; Echevarria, A. Novel 3,4-methylenedioxyde-6-X-benzaldehyde-thiosemicarbazones: Synthesis and anti-leishmanial effects against Leishmania amazonensis. Eur. J. Med. Chem. 2015, 103, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rodrigues, A.; Salazer-Schettino, P.M.; Bautista, J.L.; Hernandez-Luis, F.; Torrens, H.; Guevara-Gomez, Y.; Pina-Canseco, S.; Torres, M.B.; Cabrera-Bravo, M.; Martinez, C.M.; et al. In vitro antiparasitic activity of new thiosemicarbazones in strain Trypanosoma cruzi. Eur. J. Med. Chem. 2014, 87, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.O.A.; Echevarria, A.; Bellieny, M.S.S.; Pinho, R.T.; de Leo, R.M.M.; Seguins, W.S.; Machado, G.M.; Canto-Cavalheiro, M.M.; Leon, L.L. Evaluation of thiosemicarbazones and semicarbazones as potential agents anti-Trypanosoma cruzi. Exp. Parasitol. 2011, 129, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Pavan, F.R.; Maia, P.I.S.; Leite, S.R.A.; Daflon, V.M.; Batista, A.A.; Sato, D.N.; Franzblau, S.G.; Leite, C.Q.F. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti-Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Paiva, R.O.; Kneipp, L.F.; Goulart, C.M.; Albuquerque, M.A.; Echevarria, A. Antifungal activities of thiosemicarbazones and semicarbazones against mycotoxigenic fungi. Cienc. Agrotec. 2014, 38, 531–537. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W.; Shan, Y.; Liu, F.; Xu, X.; Yang, Y.; Zhang, Q.; Kuang, H.; Wang, Z.; Wang, S. Design, synthesis and anticancer activity of novel nopinone-based thiosemicarbazones derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 2360–2363. [Google Scholar] [CrossRef]
- Dos Santos, T.A.R.; da Silva, A.C.; Silva, E.B.; Gomes, P.A.T.M.; Espíndola, J.W.P.; Cardoso, M.V.O.; Moreira, D.R.M.; Leite, A.C.L.; Pereira, V.R.A. Antitumor and immunomodulatory activities of thiosemicarbazones and 1,3-thiazoles in Jurkat and HT-29 cells. Biomed. Pharmacother. 2016, 82, 555–560. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, Y.; Zhu, D.; Yang, X.; Zhu, H. Synthesis, molecular modeling and biological evaluation of chalcone-thiosemicarbazide derivatives as novel anticancer agents. Eur. J. Med. Chem. 2011, 46, 4702–4708. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.; Wu, F.; Zhao, L. Microwave-assisted synthesis and tyrosinase inhibitory activity of chalcone derivatives. Chem. Biol. Drug Des. 2013, 82, 39–47. [Google Scholar] [CrossRef]
- Da Silva, J.G.; Despaigne, A.A.R.; Ouro, S.R.W.; Bandeira, C.C.; Souza-Fagundes, E.M.; Beraldo, H. Cytotoxic activity, albumin and DNA binding of new copper (II) complexes with chalcone-derived thiosemicarbazones. Eur. J. Med. Chem. 2013, 65, 415–426. [Google Scholar] [CrossRef]
- Da Silva, J.G.; Perdigão, C.C.H.; Speziali, N.L.; Beraldo, H. Chalcone-derived thiosemicarbazones and their zinc (II) and gallium (III) complexes: Spectral studies and antimicrobial activity. J. Coord. Chem. 2013, 66, 385–401. [Google Scholar] [CrossRef]
- Franklim, T.N.; Freire-de-Lima, L.; Chaves, O.A.; LaRocque-de-Freitas, I.F.; da Silva-Trindade, J.D.; Netto-Ferreira, J.C.; Freire-de-Lima, C.G.; Decoté-Ricardo, D.; Previato, J.O.; Mendonça-Previato, L.; et al. Design, synthesis, trypanocidal activity, and studies on human albumin interaction of novel S-alkyl-1,2,4-triazoles. J. Braz. Chem. Soc. 2019, 30, 1378–1394. [Google Scholar] [CrossRef]
- Naveenraj, S.; Anandan, S. Binding of serum albumins with bioactive substances—Nanoparticles to drugs. J. Photochem. Photobiol. C 2013, 14, 53–71. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Chaves, O.A.; Amorim, A.P.O.; Castro, L.H.E.; Sant’Anna, C.M.R.; Oliveira, M.C.C.; Cesarin-Sobrinho, D.; Netto-Ferreira, J.C.; Ferreira, A.B.B. Fluorescence and Docking Studies of the Interaction between Human Serum Albumin and Pheophytin. Molecules 2015, 20, 19526–19539. [Google Scholar] [CrossRef]
- Suwito, H.; Mustofa, J.; Pudjiastuti, P.; Fanani, M.Z.; Kimata-Ariga, Y.; Katahira, R.; Kawakami, T.; Fujiwara, T.; Hase, T.; Sirat, H.M.; et al. Design and synthesis of chalcone derivatives as inhibitors of Plasmodium falciparum: Pursuing new antimalarial agents. Molecules 2014, 19, 21473–21488. [Google Scholar] [CrossRef]
- Singh, A.K.; Saxena, G.; Prasad, R.; Kumar, A. Synthesis, characterization and calculated non-linear optical properties of two new chalcones. J. Mol. Struct. 2012, 1017, 26–31. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Jonnalagadda, S.S.; Hussein, S.; Tewari, S.; Quail, J.W.; Reid, R.S.; Delbaere, L.T.J.; Prasad, L. Evaluation of some thiosemicarbazones and arylidene ketones and analogues for anticonvulsant activities. Eur. J. Med. Chem. 1990, 25, 581–588. [Google Scholar] [CrossRef]
- Wang, S.X.; Liu, C.; Duan, J. Synthesis of chalcone thiosemicarbazones under ultrasound irradiation. Asian J. Chem. 2011, 23, 4451–4453. [Google Scholar]
- Temporal, R.M.; Finkelstein, L.C.; Echevarria, A.; Souza, M.A.S.; Sertã, M.; Silva-Gonçalves, A.J.; Pirmez, C.; Leon, L.L. Effects of amidine derivatives on parasite-macrophage interaction and evaluation of toxicity. Arzneimittelforschung 2002, 6, 489–493. [Google Scholar] [CrossRef]
- Castro-Pinto, D.B.; Echevarria, A.; Genestra, M.; Cysne-Finkelstein, L.; Leon, L.L. Trypanotione reductase activity is prominent in metacyclic promastigotes and axenic amastigotes of Leishmania amazonensis. Evaluation of its potentiality as a therapeutic target. J. Enzyme Inhib. Med. Chem. 2004, 19, 57–63. [Google Scholar] [CrossRef]
- Chaves, O.A.; Jesus, C.S.H.; Cruz, P.F.; Sant’Anna, C.M.R.; Brito, R.M.M.; Serpa, C. Evaluation by fluorescence, STD-NMR, docking and semi-empirical calculations of the o-NBA photo-acid interaction with BSA. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 169, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, N.; Ajmal, M.R.; Rabbani, G.; Ahmad, E.; Khan, R.H. A Comprehensive insight into binding of hippuric acid to human serum albumin: A study to uncover its impaired elimination through hemodialysis. PLoS ONE 2013, 8, e71422. [Google Scholar] [CrossRef] [PubMed]
- Wardell, M.; Wang, Z.; Ho, J.X.; Robert, J.; Ruker, F.; Ruble, J.; Carter, D.C. The atomic structure of human methemalbumin at 1.9 A. Biochem. Biophys. Res. Commun. 2002, 291, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Chaves, O.A.; Santos, M.R.L.; Oliveira, M.C.C.; Sant’Anna, C.M.R.; Ferreira, R.C.; Echevarria, A.; Netto-Ferreira, J.C. Synthesis, tyrosinase inhibition and transportation behavior of novel β-enamino thiosemicarbazide derivatives by human serum albumin. J. Mol. Liq. 2018, 254, 280–290. [Google Scholar] [CrossRef]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef]
- Wang, Z.M.; Ho, J.X.; Ruble, J.R.; Rose, J.; Ruker, F.; Ellenburg, M.; Murphy, R.; Click, J.; Soistman, E.; Wilkerson, L.; et al. Structural studies of several clinically important oncology drugs in complex with human serum albumin. Biochim. Biophys. Acta 2013, 1830, 5356–5374. [Google Scholar] [CrossRef]
- GOLD—Protein Ligand Docking Software. Available online: https://www.ccdc.cam.ac.uk/solutions/csd-discovery/components/gold/ (accessed on 13 August 2019).
- Oliveira, R.B.; Souza-Fagundes, E.M.; Soares, R.P.P.; Andrade, A.A.; Krettli, A.U.; Zani, C.L. Synthesis and antimalarial activity of semicarbazone and thiosemicarbazone derivatives. Eur. J. Med. Chem. 2008, 43, 1983–1988. [Google Scholar] [CrossRef]
- Aksöz, B.E.; Ertanm, R. Spectral properties of chalcones II. FABAD J. Pharm. Sci. 2012, 37, 205–216. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Don, R.; Ioset, J.R. Screening strategies to identify new chemical diversity for drug development to treat kinetoplastid infections. Parasitology 2014, 141, 140–146. [Google Scholar] [CrossRef]
- Ballell, L.; Strange, M.; Cammack, N.; Fairlamb, A.H.; Borysiewicz, L. Open Lab as a source of hits and leads against tuberculosis, malaria and kinetoplastid diseases. Nat. Rev. Drug Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, N. Physical Organic Chemistry, 1st ed.; Longman Scientific & Technical, Wiley: New York, NY, USA, 1987. [Google Scholar]
- Hoekman, D. Exploring QSAR Fundamentals and Applications in Chemistry and Biology. J. Am. Chem. Soc. 1996, 118, 10678. [Google Scholar] [CrossRef]
- Chaves, O.A.; Tavares, M.T.; Cunha, M.R.; Parise-Filho, R.; Sant’Anna, C.M.R.; Netto-Ferreira, J.C. Multi-spectroscopic and theoretical analysis on the interaction between human serum albumin and a capsaicin derivative—RPF101. Biomolecules 2018, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, Y.; Lv, C.; Wang, L.; Ou, J.; Wang, M.; Liu, S. Impact of halogen substituents on interactions between 2-phenyl-2,3-dihydroquinazolin-4(1H)-one derivatives and human serum albumin. Molecules 2012, 17, 2000–2014. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Sun, H.; Liu, Y.; Li, M.; Han, S.; Yang, X.; Liu, R. Toxic effects of chrysoidine on human serum albumin: Isothermal titration calorimetry and spectroscopic investigations. Luminescence 2016, 31, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Chaves, O.A.; Sasidharan, R.; de Oliveira, C.H.C.S.; Manju, S.L.; Joy, M.; Mathew, B.; Netto-Ferreira, J.C. In vitro study of the interaction between HSA and 4-bromoindolylchalcone, a potent human MAO-B inhibitor: Spectroscopic and molecular modeling studies. ChemistrySelect 2019, 4, 1007–1014. [Google Scholar] [CrossRef]
- Paul, S.; Sepay, N.; Sarkar, S.; Roy, P.; Dasgupt, S.; Sardar, P.S.; Majhi, A. Interaction of serum albumins with fluorescentligand 4-azido coumarin: spectroscopic analysisand molecular docking studies. New J. Chem. 2017, 41, 15392–15404. [Google Scholar] [CrossRef]
- Ruiz, J.; Vicente, C.; de Haro, C.; Bautista, D. Novel bis-C, N-cyclometalated iridium (III) thiosemicarbazide antitumor complexes: Interactions with human serum albumin and DNA, and inhibition of cathepsin B. Inorg. Chem. 2013, 52, 974–982. [Google Scholar] [CrossRef]
- Chaves, O.A.; de Castro, I.S.; Goulart, C.M.; Bellieny, M.S.S.; Netto-Ferreira, J.C.; Echevarria-Lima, J.; Echevarria, A. In vitro and in vivo cytotoxic activity and human serum albumin interaction for a methoxy-styryl-thiosemicarbazone. Investig. New Drugs 2019, 37, 994–1005. [Google Scholar] [CrossRef]
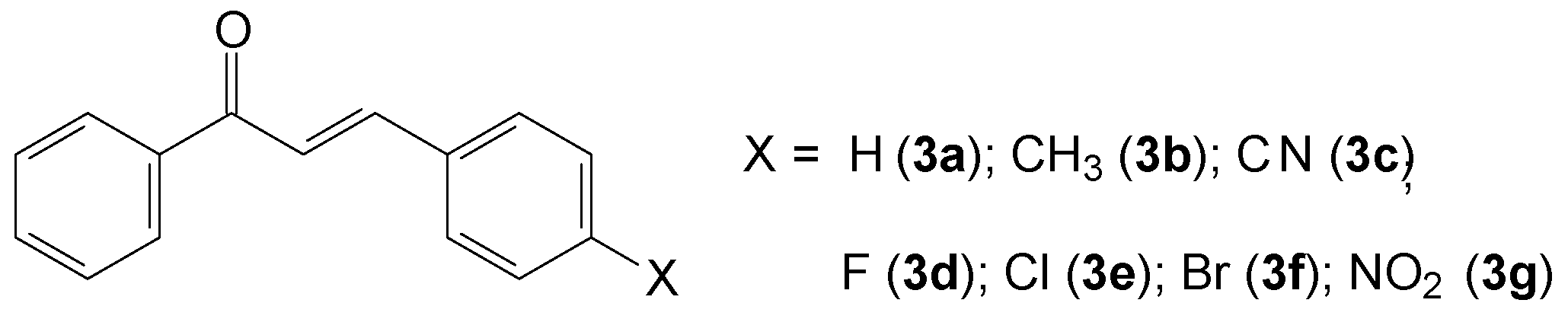

| Compound | Promastigotes IC50 a (µM) | Axenic Amastigotes IC50 (µM) | Intracellular Amastigotes IC50 (µM) | Macrophages LD50 b (µM) | Selectivity Index (SI) |
|---|---|---|---|---|---|
| 5a | 14.68 ± 0.31 | 6.88 ± 1.09 | 3.40 ± 0.28 | 40.98 ± 1.12 | 15.05 |
| 5b | 14.80 ± 1.92 | 7.08 ± 1.03 | 5.95 ± 0.61 | 56.35 ± 0.78 | 9.47 |
| 5c | n.a. c | n.d. d | n.d. | n.d. | − |
| 5d | 13.09 ± 1.85 | 5.57 ± 1.15 | > 3.13 | 35.47 ± 1.38 | − |
| 5e | 5.22 ± 0.75 | 3.19 ± 1.20 | 4.47 ± 0.42 | 32.48 ± 1.81 | 7.27 |
| 5f | 12.52 ± 0.99 | 4.24 ± 1.07 | 3.88 ± 0.24 | 44.24 ± 4.21 | 11.40 |
| 5g | n.a. | n.d. | n.d. | n.d. | − |
| Pentamidine | 4.90 ± 0.60 | 12.29 ± 1.17 | 11.12 ± 1.98 | 25.85 ± 4.06 | 2.32 |
| Compound | 4-X | σp a | Es b | MR b | π b |
|---|---|---|---|---|---|
| 5a | H | 0.00 | 0.00 | 0.00 | 0.00 |
| 5b | CH3 | −0.14 | −0.25 | 0.57 | 0.56 |
| 5c | CN | 0.71 | −0.51 | 0.63 | −0.57 |
| 5d | F | 0.15 | −0.46 | 0.10 | 0.14 |
| 5e | Cl | 0.34 | −0.97 | 0.60 | 0.71 |
| 5f | Br | 0.26 | −1.16 | 0.89 | 0.86 |
| 5g | NO2 | 0.81 | −1.01 | 0.74 | −0.28 |
| T (K) | KSV (×104) (M−1) a | kq (×1012) (M−1s−1) a | Ka (×104) (M−1) b | nb | ΔH° (kJmol−1) c | ΔS° (×10−2) (kJmol−1K−1) c | ΔG° (kJmol−1) c |
|---|---|---|---|---|---|---|---|
| 289 | 2.48 ± 0.52 | 4.15 | 5.16 ± 0.04 | 1.05 ± 0.01 | −5.79 ± 0.27 | 7.02 ± 0.09 | −26.1 |
| 296 | 2.49 ± 0.50 | 4.16 | 4.85 ± 0.06 | 1.07 ± 0.01 | −26.6 | ||
| 303 | 2.59 ± 0.51 | 4.34 | 4.57 ± 0.06 | 1.06 ± 0.01 | −27.1 | ||
| 310 | 2.75 ± 0.33 | 4.60 | 4.39 ± 0.04 | 1.05 ± 0.01 | −27.6 |
| Sites | Amino Acid Residues | Interaction | Distance (Å) |
|---|---|---|---|
| Phe-210 | Van der Waals | 2.60 | |
| Trp-214 | Van der Waals | 3.00 | |
| Ser-201 | Hydrogen bonding | 2.80 | |
| I | His-241 | Van der Waals | 3.60 |
| Val-343 | Van der Waals | 3.50 | |
| Leu-346 | Van der Waals | 2.40 | |
| Leu-480 | Van der Waals | 2.70 | |
| Val-481 | Van der Waals | 2.70 | |
| Leu-386 | Van der Waals | 3.20 | |
| Lys-413 | Van der Waals | 3.10 | |
| Val-414 | Van der Waals | 2.90 | |
| II | Val-425 | Van der Waals | 2.20 |
| Leu-429 | Van der Waals | 2.90 | |
| Leu-452 | Van der Waals | 3.60 | |
| Ser-488 | Hydrogen bonding | 1.80 | |
| Arg-113 | Hydrogen bonding | 3.30 | |
| Leu-114 | Van der Waals | 2.40 | |
| Arg-116 | Van der Waals | 2.40 | |
| III | Tyr-137 | Van der Waals | 2.40 |
| Ile-141 | Van der Waals | 2.70 | |
| Phe-156 | Van der Waals | 3.70 | |
| Arg-185 | Hydrogen bonding | 3.80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, E.P.; Goulart, C.M.; Chaves, O.A.; Faiões, V.d.S.; Canto-Carvalho, M.M.; Machado, G.C.; Torres-Santos, E.C.; Echevarria, A. Evaluation of Novel Chalcone-Thiosemicarbazones Derivatives as Potential Anti-Leishmania amazonensis Agents and Its HSA Binding Studies. Biomolecules 2019, 9, 643. https://doi.org/10.3390/biom9110643
Mendes EP, Goulart CM, Chaves OA, Faiões VdS, Canto-Carvalho MM, Machado GC, Torres-Santos EC, Echevarria A. Evaluation of Novel Chalcone-Thiosemicarbazones Derivatives as Potential Anti-Leishmania amazonensis Agents and Its HSA Binding Studies. Biomolecules. 2019; 9(11):643. https://doi.org/10.3390/biom9110643
Chicago/Turabian StyleMendes, Edinéia Pastro, Carla Marins Goulart, Otávio Augusto Chaves, Viviane dos S. Faiões, Marilene M. Canto-Carvalho, Gerzia C. Machado, Eduardo Caio Torres-Santos, and Aurea Echevarria. 2019. "Evaluation of Novel Chalcone-Thiosemicarbazones Derivatives as Potential Anti-Leishmania amazonensis Agents and Its HSA Binding Studies" Biomolecules 9, no. 11: 643. https://doi.org/10.3390/biom9110643
APA StyleMendes, E. P., Goulart, C. M., Chaves, O. A., Faiões, V. d. S., Canto-Carvalho, M. M., Machado, G. C., Torres-Santos, E. C., & Echevarria, A. (2019). Evaluation of Novel Chalcone-Thiosemicarbazones Derivatives as Potential Anti-Leishmania amazonensis Agents and Its HSA Binding Studies. Biomolecules, 9(11), 643. https://doi.org/10.3390/biom9110643






