Modulating Linker Composition of Haptens Resulted in Improved Immunoassay for Histamine
Abstract
1. Introduction
2. Experimental
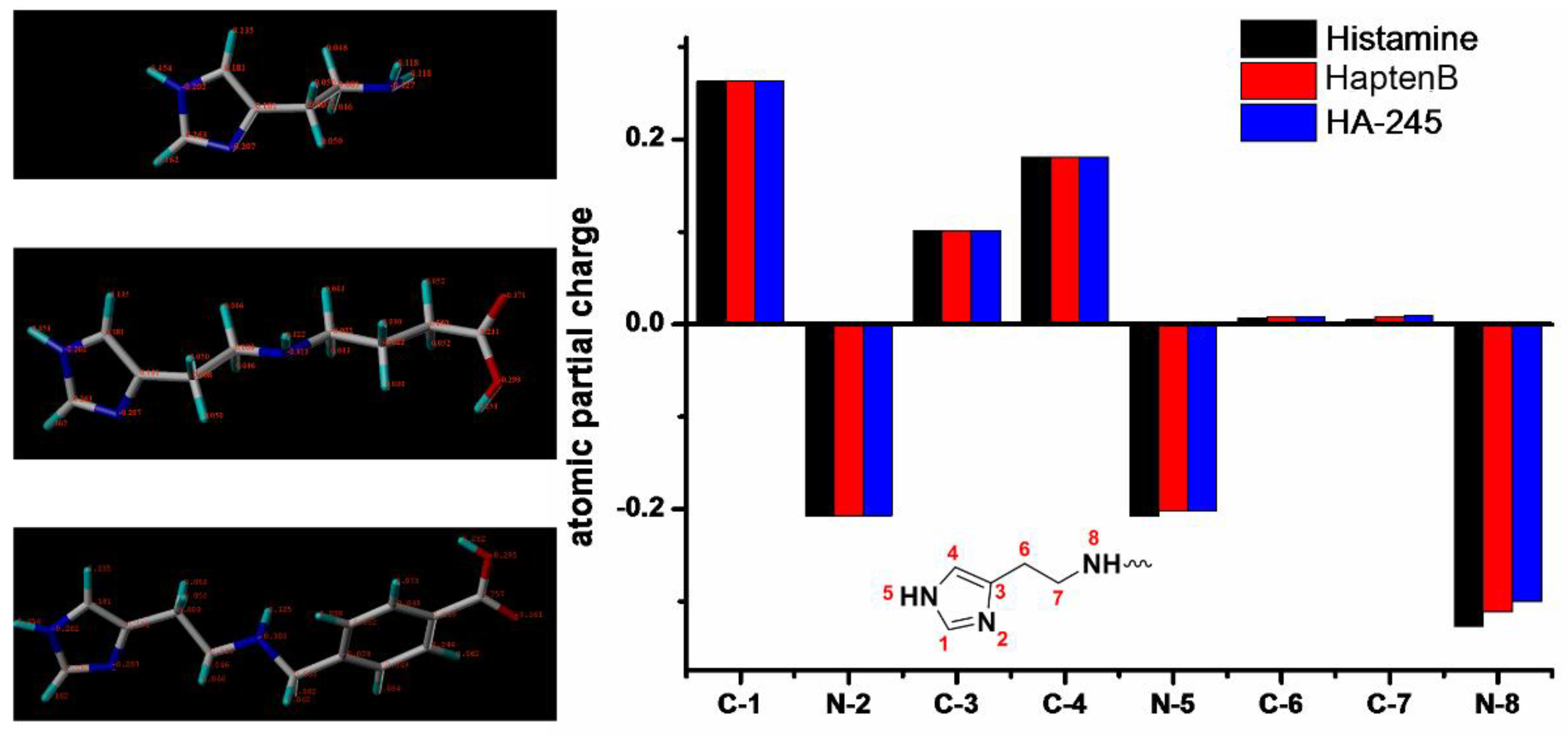
2.1. Molecular Modeling
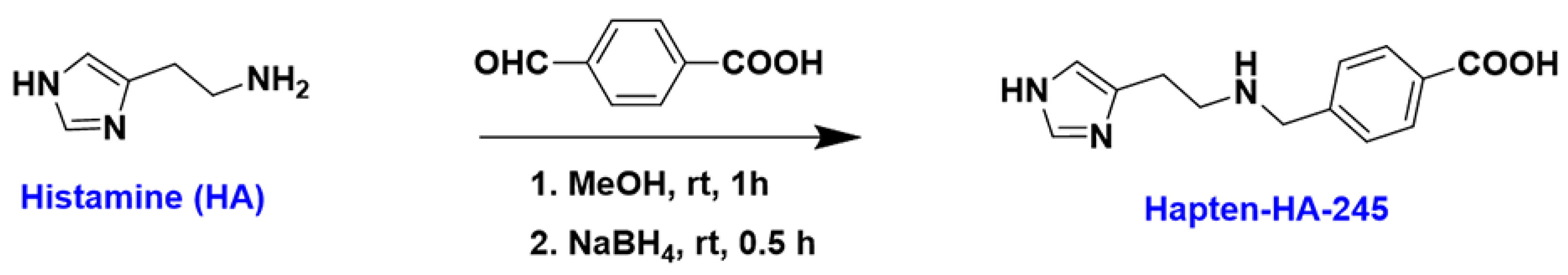
2.2. Hapten Synthesis
2.3. Conjugation of Haptens
2.4. Antibody Production
2.5. ELISA Protocol
2.6. Cross-Reactivity Test
3. Results
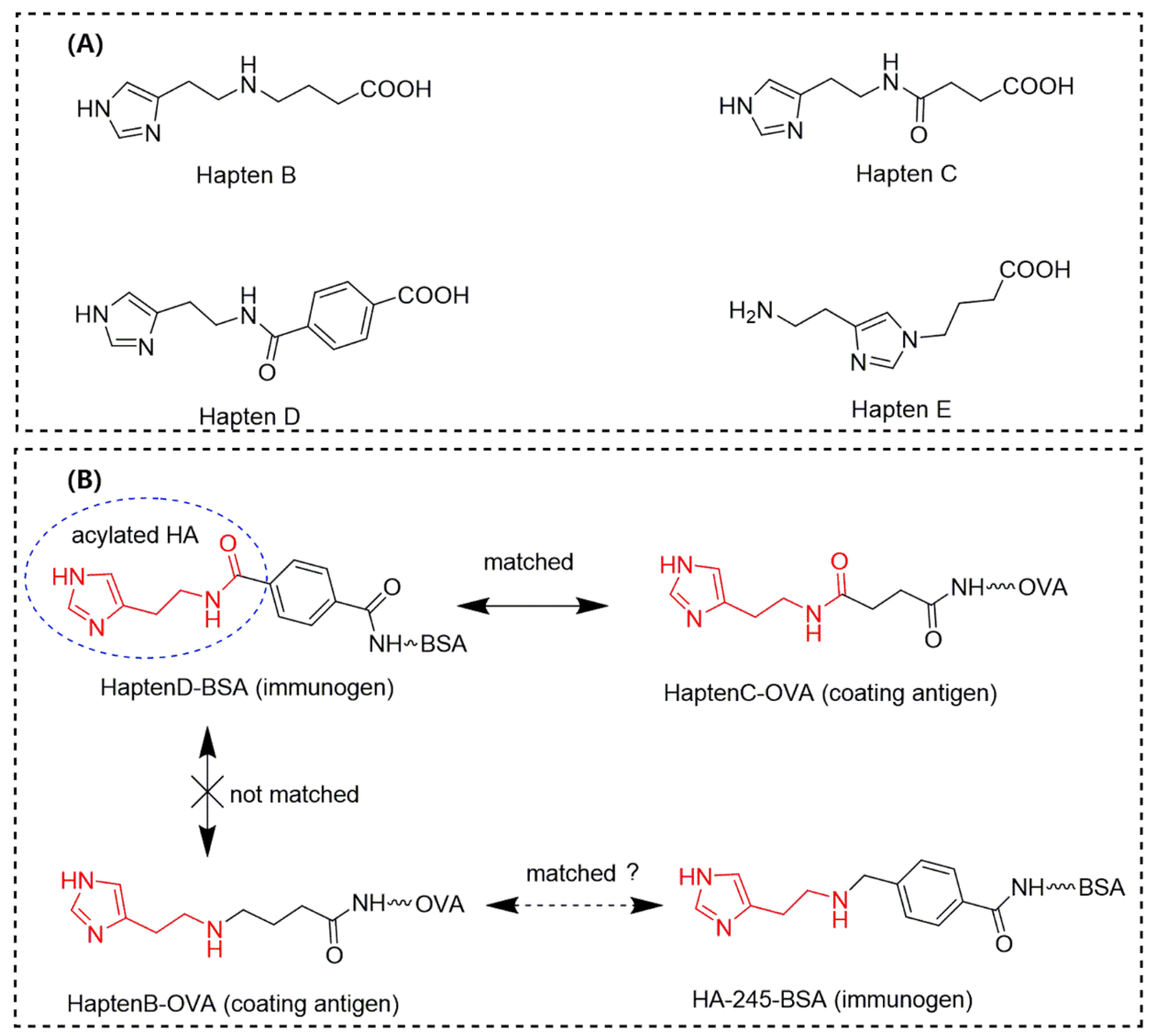
3.1. Characterization of Hapten and Artificial Antigens
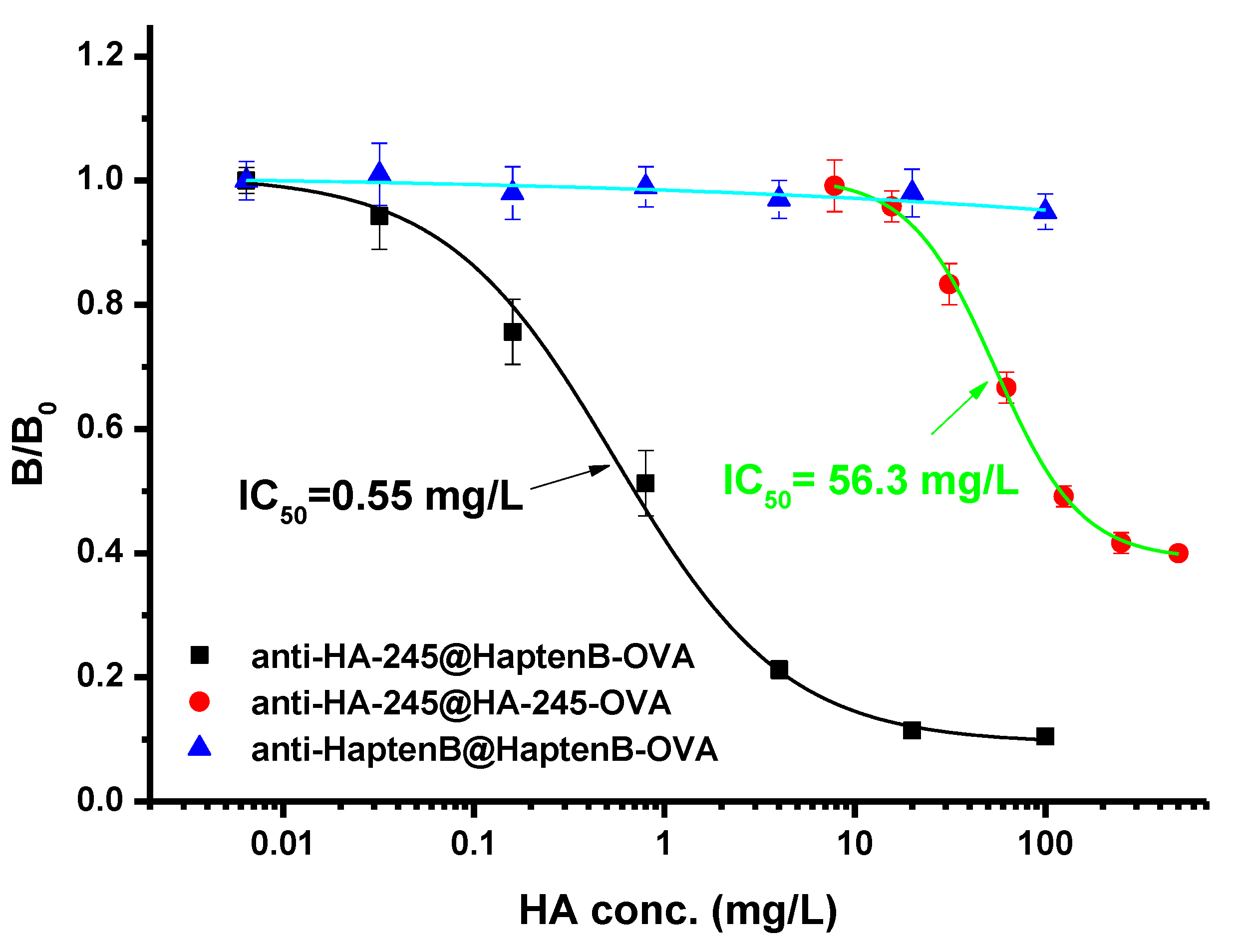
3.2. Production of Monoclonal Antibody
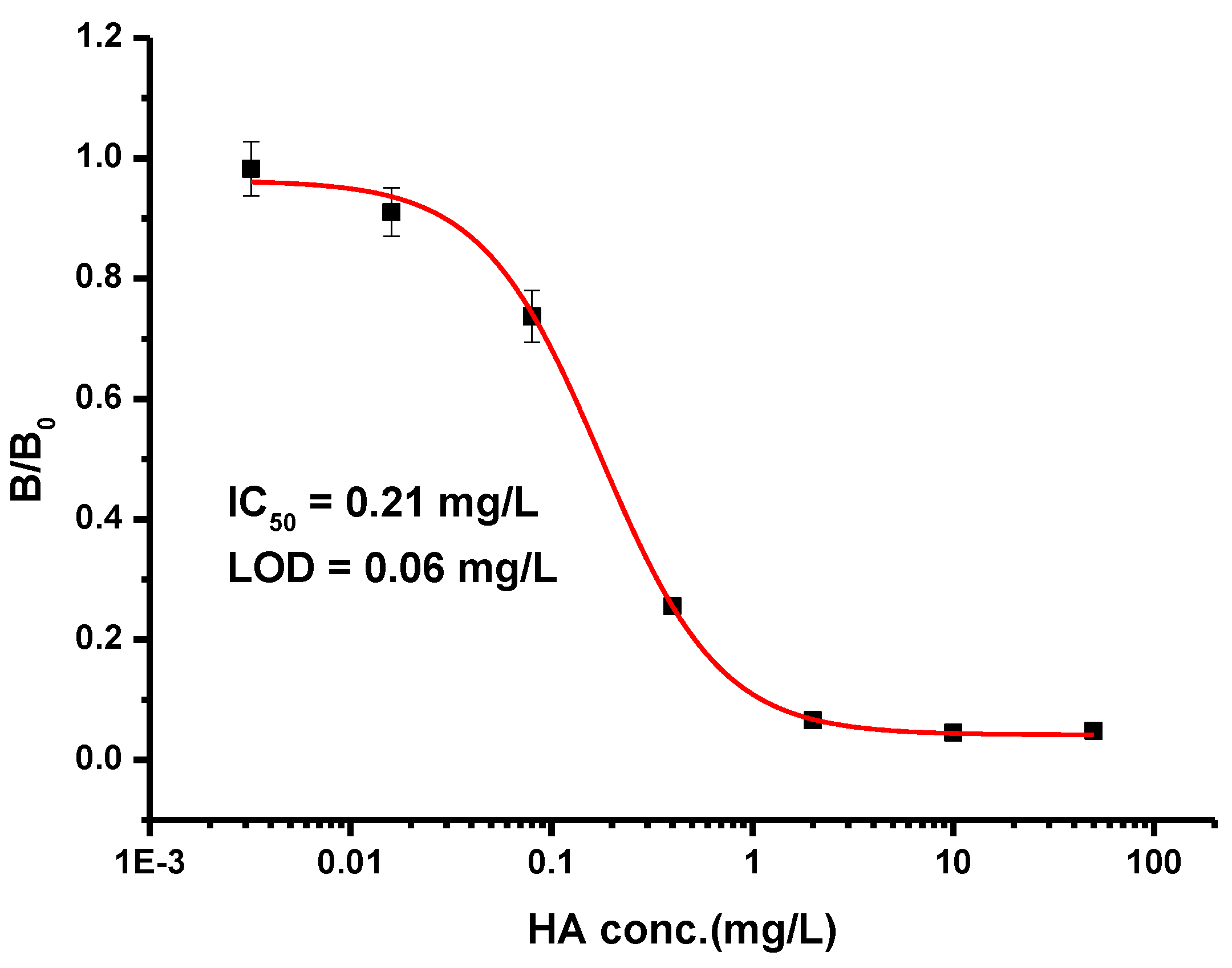
3.3. Development of ic-ELISA for Histamine
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ordonez, J.L.; Troncoso, A.M.; Garcia-Parrilla, M.C.; Callejon, R.M. Recent trends in the determination of biogenic amines in fermented beverages—A review. Anal. Chim. Acta 2016, 939, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Veseli, A.; Vasjari, M.; Arbneshi, T.; Hajrizi, A.; Svorc, L.; Samphao, A.; Kalcher, K. Electrochemical determination of histamine in fish sauce using heterogeneous carbon electrodes modified with rhenium(IV) oxide. Sens. Actuators B. 2016, 228, 774–781. [Google Scholar] [CrossRef]
- Mattsson, L.; Jungmann, C.; Lieberzeit, P.A.; Preininger, C. Modified carbon black as label in a colorimetric on-chip immunoassay for histamine. Sens. Actuators B. 2017, 246, 1092–1099. [Google Scholar] [CrossRef]
- Morel, A.M.; Delaage, M.A. Immunoanalysis of histamine through a novel chemical derivatization. J. Allergy Clin. Immunol. 1988, 82, 646–654. [Google Scholar] [CrossRef]
- Guesdon, J.L.; Chevrier, D.; Fadel, R.; Avrameas, S. Immunoenzyme assay for histamine. Allergie et Immunologie. 1988, 20, 336–338, 340–342. [Google Scholar] [PubMed]
- Serrar, D.; Brebant, R.; Bruneau, S.; Denoyel, G.A. The development of a monoclonal antibody-based ELISA for the determination of histamine in food: Application to fishery products and comparison with the HPLC assay. Food Chem. 1995, 54, 85–91. [Google Scholar] [CrossRef]
- Claeys-Bruno, M.; Vandenabeele-Trambouze, O.; Sergent, M.; Geffard, M.; Bodet, D.; Dobrijevic, M.; Commeyras, A.; Phan Tan Luu, R. Methodological approaches for histamine quantification using derivatization by chloroethylnitrosourea and ELISA measurement. Part II: Optimisation of the derivatization step. Chemom. Intell. Lab. Syst. 2006, 80, 186–197. [Google Scholar] [CrossRef]
- Luo, L.; Xu, Z.-L.; Yang, J.-Y.; Xiao, Z.-L.; Li, Y.-J.; Beier, R.C.; Sun, Y.-M.; Lei, H.-T.; Wang, H.; Shen, Y.-D. Synthesis of Novel Haptens and Development of an Enzyme-Linked Immunosorbent Assay for Quantification of Histamine in Foods. J. Agric. Food Chem. 2014, 62, 12299–12308. [Google Scholar] [CrossRef] [PubMed]
- Hammar, E.; Berglund, A.; Hedin, A.; Norrman, A.; Rustas, K.; Ytterstrom, U.; Akerblom, E. An immunoassay for histamine based on monoclonal antibodies. J. Immunol. Methods. 1990, 128, 51–58. [Google Scholar] [CrossRef]
- Schneider, E.; Usleber, E.; Martlbauer, E. Production and Characterization of Antibodies Against Histamine. In Immunoassays for Residue Analysis: Food Safety; Beier, R.C., Stanker, L.H., Eds.; ACS Symposium Series 621; American Chemical Society: Washington, DC, USA, 1996; pp. 413–420. [Google Scholar]
- Kane, M.M.; Banks, J.N. Raising Antibodies. In Immunoassays: A Practical Approach; Gosling, J.P., Ed.; Oxford University Press: Oxford, UK, 2000; pp. 37–50. [Google Scholar]
- Chen, J.H.; Wang, L.T.; Lu, L.L.; Shen, X.; Huane, X.A.; Liu, Y.J.; Sun, X.L.; Wang, Z.H.; Eremin, S.A.; Sun, Y.M.; et al. Four Specific Hapten Conformations Dominating Antibody Specificity: Quantitative Structure-Activity Relationship Analysis for Quinolone Immunoassay. Anal. Chem. 2017, 89, 6740–6748. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, A.F.S.A. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 1966, 14, 328–336. [Google Scholar] [CrossRef]
- Taylor, S.L. Histamine food poisoning: toxicology and clinical aspects. Crit. Rev. Toxicol. 1986, 17, 91–128. [Google Scholar] [CrossRef] [PubMed]
- Miki, M.; Ishikawa, T.; Okayama, H. An outbreak of histamine poisoning after ingestion of the ground saury paste in eight patients taking isoniazid in tuberculous ward. Intern Med. 2005, 44, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Toro-Funes, N.; Bosch-Fuste, J.; Latorre-Moratalla, M.L.; Veciana-Nogues, M.T.; Vidal-Carou, M.C. Biologically active amines in fermented and non-fermented commercial soybean products from the Spanish market. Food Chem. 2015, 173, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Matsumoto, K. Daily yogurt consumption in infancy is associated with reduced skin hypersensitivity to histamine. Allergy 2018, 73, 683. [Google Scholar]
- Shukla, S.; Khan, I.; Bajpai, V.K.; Lee, H.; Kim, T.; Upadhyay, A.; Huh, Y.S.; Han, Y.-K.; Tripathi, K.M. Sustainable Graphene Aerogel as an Ecofriendly Cell Growth Promoter and Highly Efficient Adsorbent for Histamine from Red Wine. ACS ACS Appl. Mater. Interfaces. 2019, 11, 18165–18177. [Google Scholar] [CrossRef] [PubMed]
- Mercader, J.V.; Agulló, C.; Abad-Somovilla, A.; Abad-Fuentes, A. Synthesis of site-heterologous haptens for high-affinity anti-pyraclostrobin antibody generation. Org. Biomol. Chem. 2011, 9, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, W.; Shen, X.; Li, X.; Huang, X.-A.; Xu, Z.; Sun, Y.; Chan, S.-W.; Zeng, L.; Eremin, S.A.; et al. Four Hapten Spacer Sites Modulating Class Specificity: Nondirectional Multianalyte Immunoassay for 31 beta-Agonists and Analogues. Anal. Chem. 2018, 90, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Tsuchikama, K.; Janda, K.D. Modulating Cocaine Vaccine Potency through Hapten Fluorination. J. Am. Chem. Soc. 2013, 135, 2971–2974. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Powers, K.; Hu, Y.; Raleigh, M.; Pentel, P.; Zhang, C.M. Engineering of a hybrid nanoparticle-based nicotine nanovaccine as a next-generation immunotherapeutic strategy against nicotine addiction: A focus on hapten density. Biomaterials 2017, 123, 107–117. [Google Scholar] [CrossRef] [PubMed]
| Coating Antigen | ||||
|---|---|---|---|---|
| Antibody | Hapten B-OVA | HA-245-OVA | ||
| Titerb (×103) | Inhibitionc (%) | Titer (×103) | Inhibition (%) | |
| anti-Hapten B#1 | 4 | — | <1 | — |
| anti-Hapten B#2 | 2 | — | <1 | — |
| anti-Hapten B#3 | 4 | — | <1 | — |
| anti-HA-245#1a | 16 | 89.8 | 128 | 26.6 |
| anti-HA-245#2 | 16 | 95.1 | 128 | 37.5 |
| anti-HA-245#3 | 32 | 92.3 | 256 | 31.8 |
| Compound | Structure | IC50 (mg/L) | CR (%) |
|---|---|---|---|
| HA | 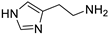 | 0.21 | 100 |
| Hapten-HA-245 | 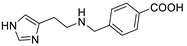 | 0.0012 | 39,583.3 |
| L-histidine | 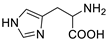 | >5000 | <0.1 |
| 1-Methyl-histamine | 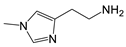 | >5000 | <0.1 |
| Tryptophan | 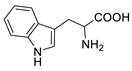 | >5000 | <0.1 |
| Tryptamine | 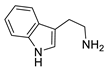 | >5000 | <0.1 |
| Tyramine | 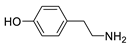 | >5000 | <0.1 |
| Phenethylamine | 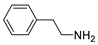 | >5000 | <0.1 |
| 4-(aminomethyl)-benzoic acid | 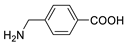 | >5000 | <0.1 |
| Benzoic acid | 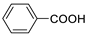 | >5000 | <0.1 |
| Sample | Spiked (mg /kg or mg/L) | Intra-Assay a | Inter-Assay b | ||||
|---|---|---|---|---|---|---|---|
| Measured (mg /kg or mg/L) | Recovery (%) | CV (%) | Measured (mg /kg or mg/L) | Recovery (%) | CV (%) | ||
| Saury | 0 | 2.31 | — | 13.0 | 2.40 | — | 12.5 |
| 2.0 | 4.05 | 87.5 | 15.0 | 4.32 | 96.0 | 14.0 | |
| 5.0 | 6.72 | 88.4 | 10.4 | 7.27 | 97.4 | 11.1 | |
| 10.0 | 11.25 | 89.5 | 11.6 | 10.81 | 84.1 | 13.0 | |
| Red Wine | 0 | 0.91 | — | 11.1 | 1.13 | — | 10.7 |
| 2.0 | 2.80 | 95.0 | 10.7 | 2.95 | 92.5 | 10.3 | |
| 5.0 | 6.44 | 106.8 | 7.8 | 6.36 | 105.2 | 7.9 | |
| 10.0 | 10.58 | 96.8 | 10.5 | 11.22 | 101.2 | 11.6 | |
| Soy Sauce | 0 | 1.22 | — | 12.6 | 1.30 | — | 11.8 |
| 2.0 | 3.08 | 93.0 | 10.5 | 3.28 | 99.0 | 10.5 | |
| 5.0 | 6.35 | 102.6 | 13.2 | 6.24 | 98.8 | 12.1 | |
| 10.0 | 10.1 | 88.8 | 12.1 | 9.82 | 85.2 | 13.2 | |
| Yoghurt | 0 | ND c | — | — | ND c | — | — |
| 2.0 | 1.92 | 96.0 | 10.2 | 2.16 | 108.5 | 12.6 | |
| 5.0 | 5.24 | 104.8 | 11.4 | 4.96 | 99.2 | 10.8 | |
| 10.0 | 9.76 | 97.6 | 13.6 | 10.24 | 102.4 | 11.8 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Wei, X.-Q.; Jia, B.-Z.; Yang, J.-Y.; Shen, Y.-D.; Hammock, B.; Dong, J.-X.; Wang, H.; Lei, H.-T.; Xu, Z.-L. Modulating Linker Composition of Haptens Resulted in Improved Immunoassay for Histamine. Biomolecules 2019, 9, 597. https://doi.org/10.3390/biom9100597
Luo L, Wei X-Q, Jia B-Z, Yang J-Y, Shen Y-D, Hammock B, Dong J-X, Wang H, Lei H-T, Xu Z-L. Modulating Linker Composition of Haptens Resulted in Improved Immunoassay for Histamine. Biomolecules. 2019; 9(10):597. https://doi.org/10.3390/biom9100597
Chicago/Turabian StyleLuo, Lin, Xiao-Qun Wei, Bao-Zhu Jia, Jin-Yi Yang, Yu-Dong Shen, Bruce Hammock, Jie-Xian Dong, Hong Wang, Hong-Tao Lei, and Zhen-Lin Xu. 2019. "Modulating Linker Composition of Haptens Resulted in Improved Immunoassay for Histamine" Biomolecules 9, no. 10: 597. https://doi.org/10.3390/biom9100597
APA StyleLuo, L., Wei, X.-Q., Jia, B.-Z., Yang, J.-Y., Shen, Y.-D., Hammock, B., Dong, J.-X., Wang, H., Lei, H.-T., & Xu, Z.-L. (2019). Modulating Linker Composition of Haptens Resulted in Improved Immunoassay for Histamine. Biomolecules, 9(10), 597. https://doi.org/10.3390/biom9100597








