Antioxidant Enzyme-Mimetic Activity and Neuroprotective Effects of Cerium Oxide Nanoparticles Stabilized with Various Ratios of Citric Acid and EDTA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cerium Oxide Nanoparticles
2.3. Enzyme-Mimetic Activity of Cerium Oxide Nanoparticles
2.4. Mouse Hippocampal Brain Slice Model of Ischemia
2.5. Angiotensin-II Mouse Hippocampal Brain Slice Model of Ischemia
2.5.1. Measurement of ROS Production in Ang-II/Ischemia-Treated Brain Slices
2.5.2. Assessment of Cell Death in Ang-II/Ischemia-Treated Brain Slices
2.6. Fabrication of Cyt C Microbiosensor and Electrochemical Measurements
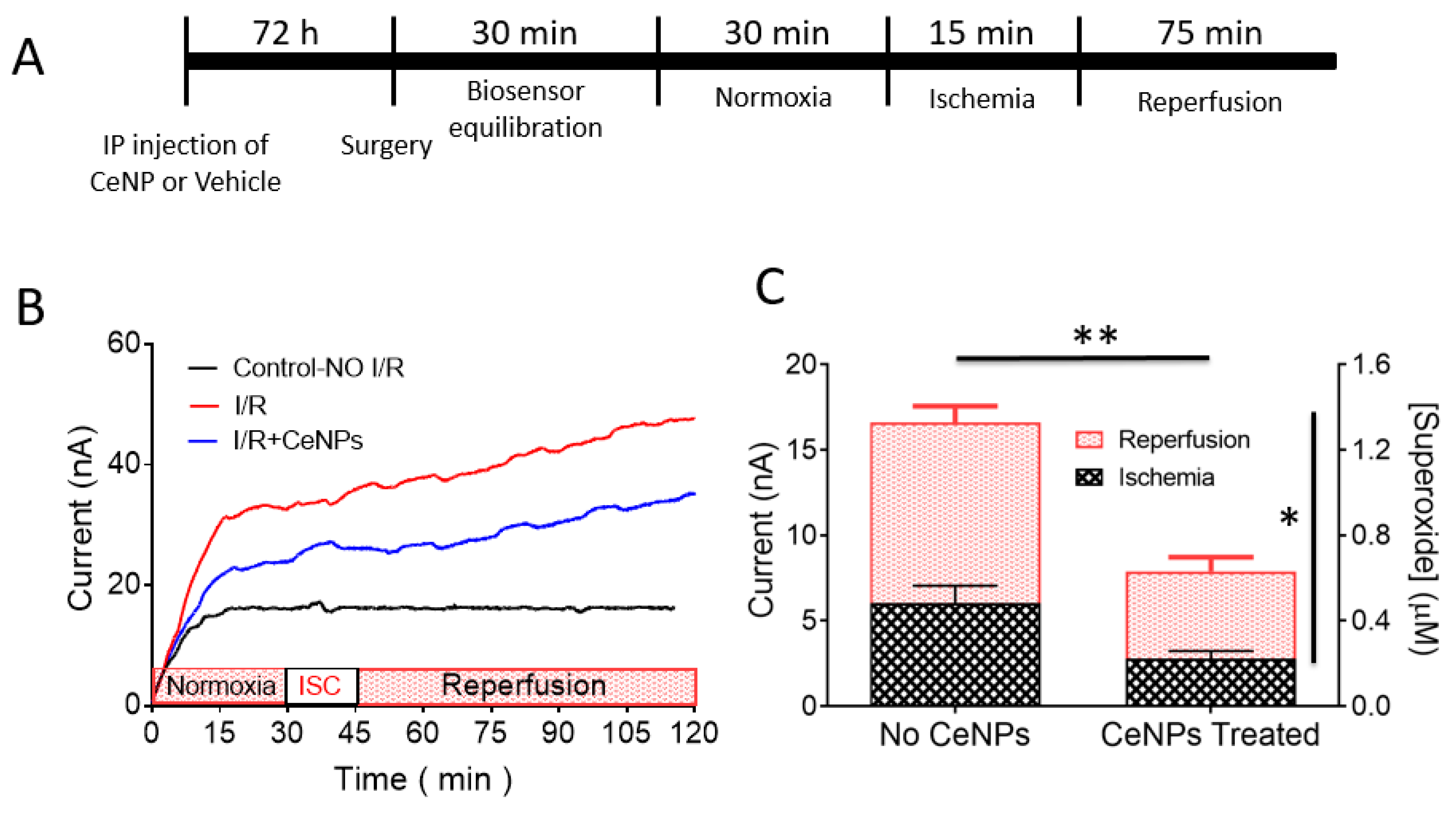
2.7. In Vivo Rat Model of Forebrain Ischemia-Reperfusion
2.8. Nanoceria Content in Rat Brains
2.9. Statistical Analysis
3. Results
3.1. Characteristics of the CeNPs Tested
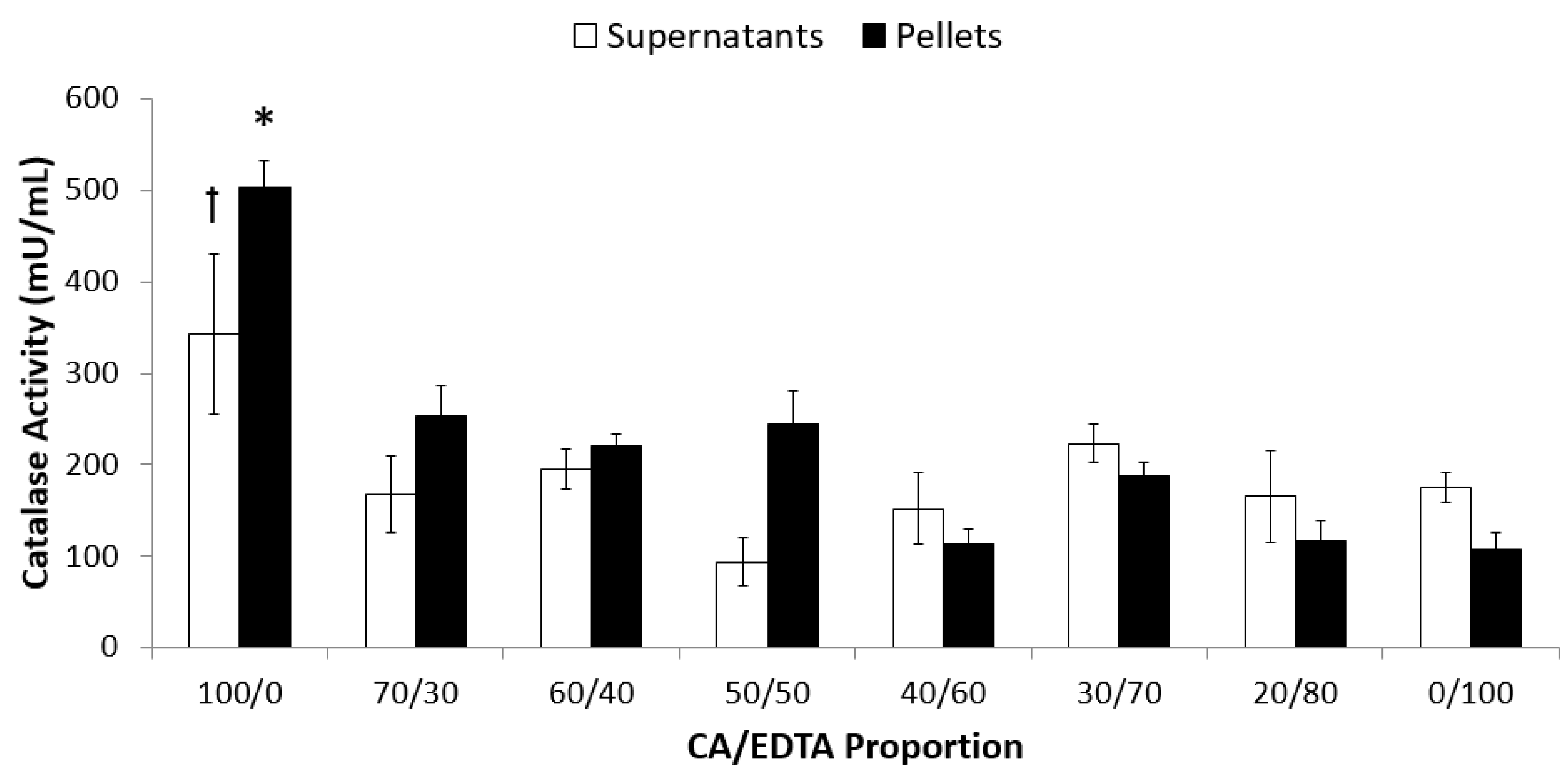
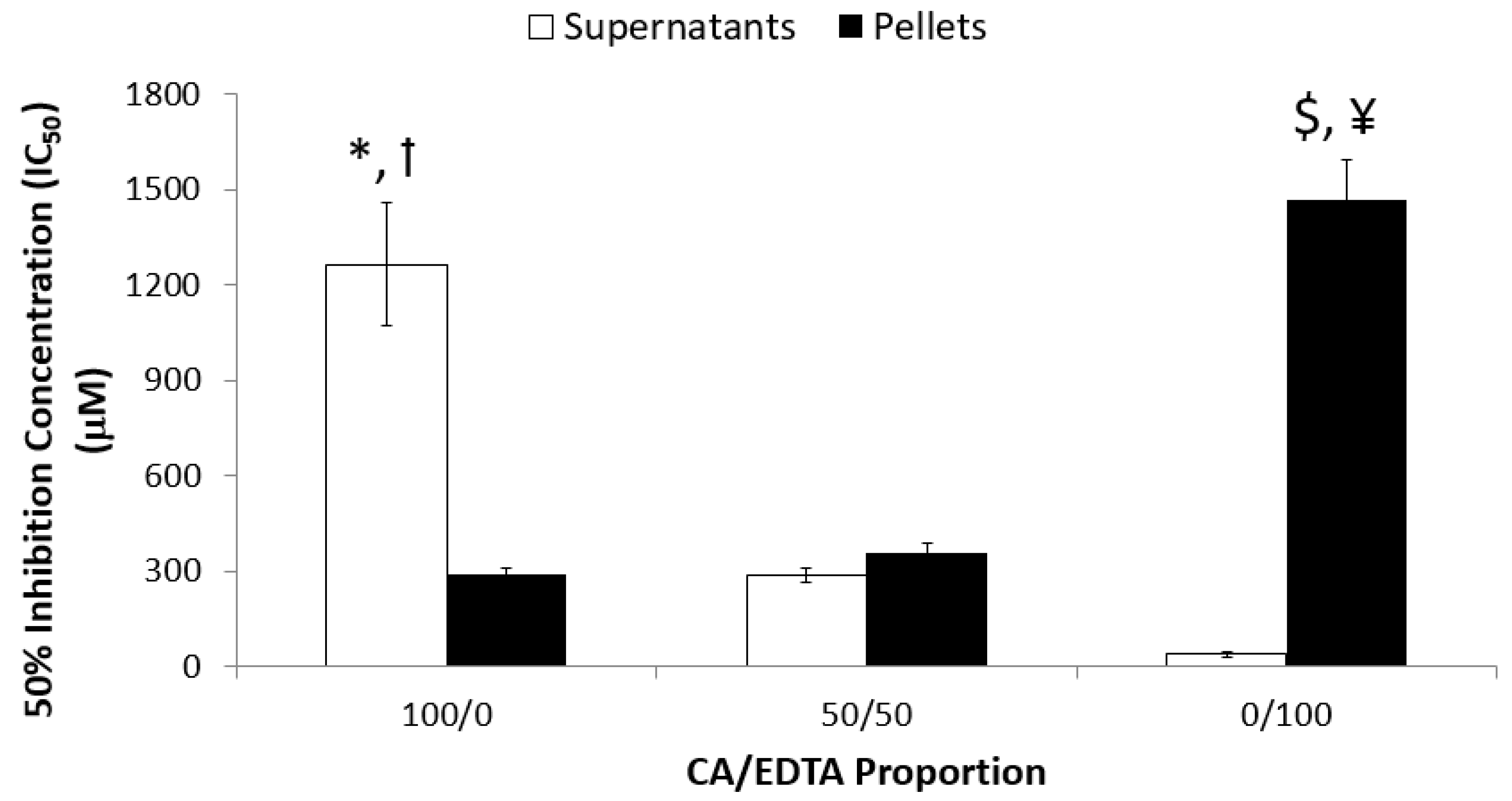
3.2. Enzyme-Mimetic Activity of CeNPs
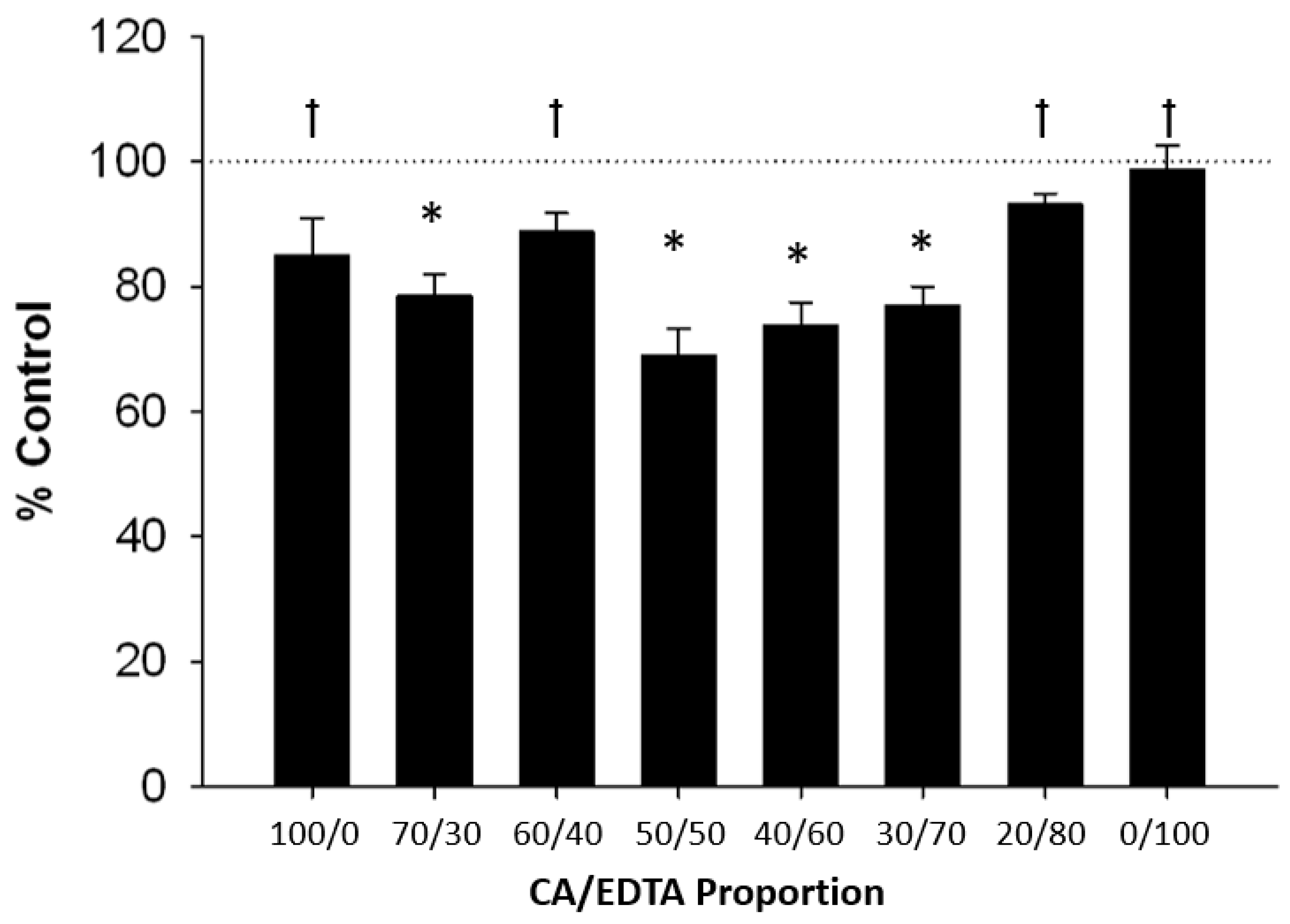
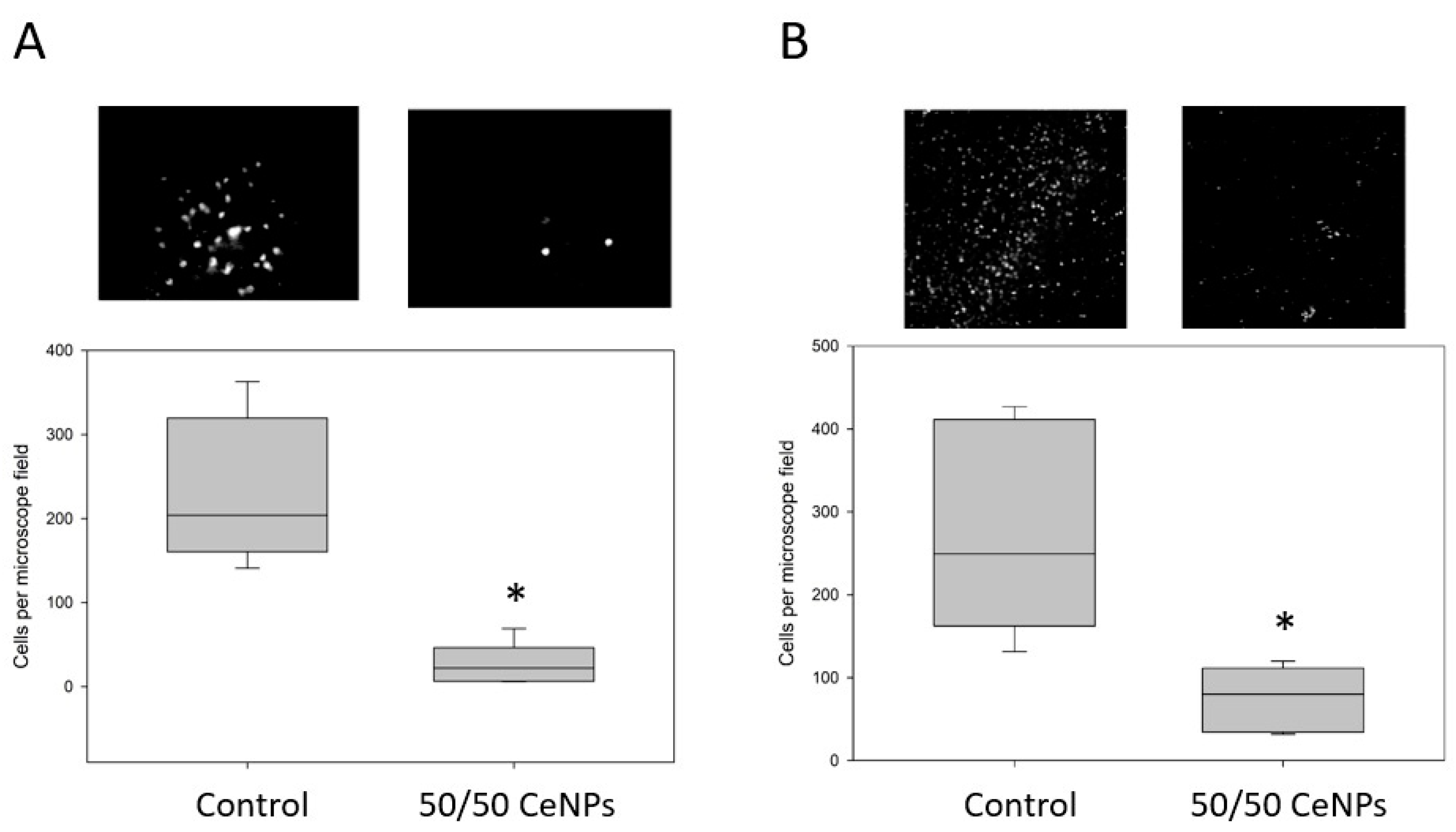
3.3. Neuroprotective Effects of CeNPs in a Hippocampal Brain Slice Model of Ischemia
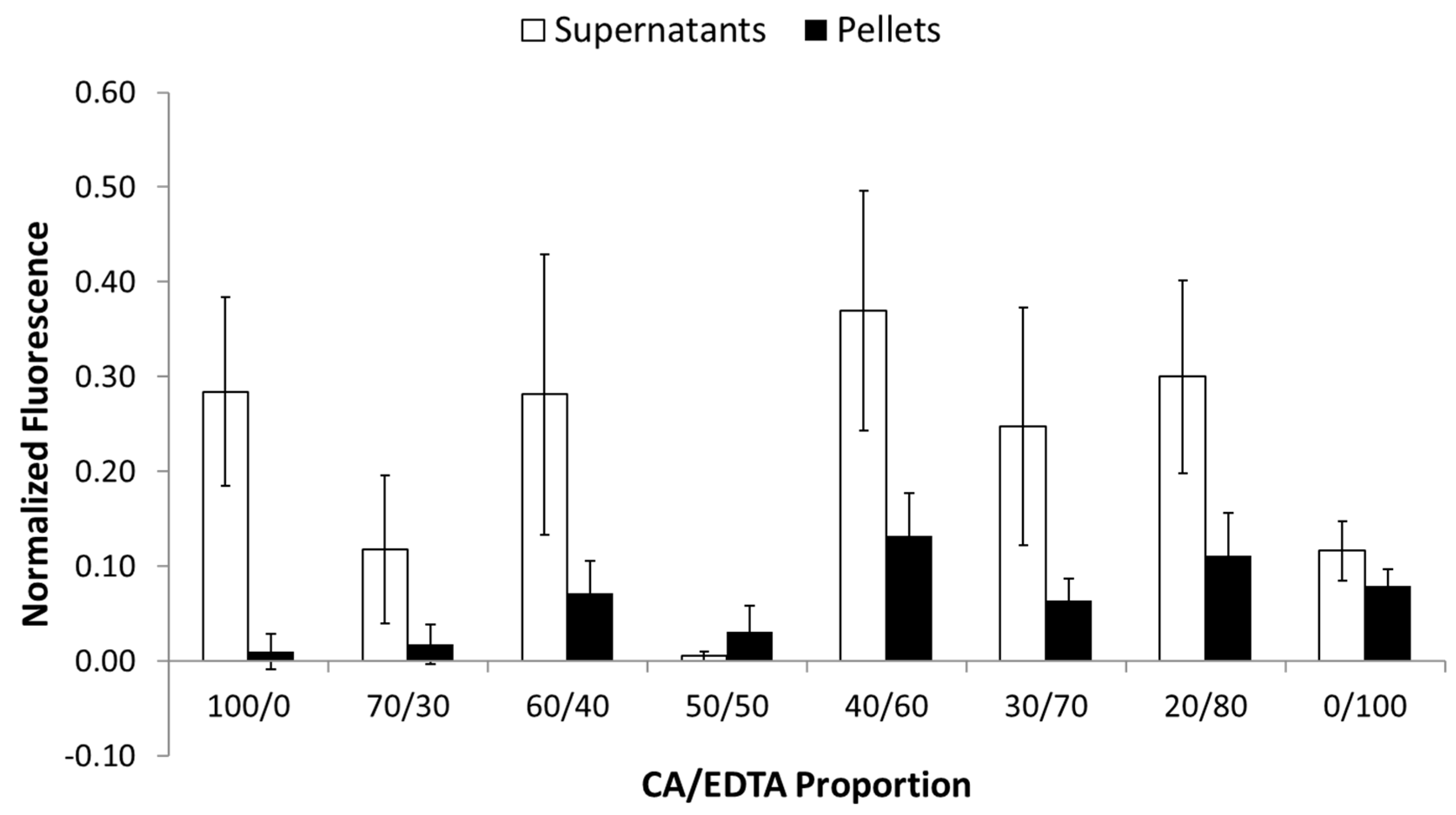
3.4. ROS Accumulation and Cell Death in Ang-II/Ischemia-Treated Brain Slices
3.5. Superoxide Monitoring in Situ in Real-Time during Forebrain Ischemia-Reperfusion
3.6. Nanoceria Content in Rat Brain
4. Discussion
4.1. Particle Biophysical Properties and Antioxidant Function
4.2. Biological Activity of CeNPs in Reduced Models
4.3. Biological Activity of CeNPs in Intact Animals
4.4. Delivery of CeNPs to the Brain
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Massaad, C.A.; Klann, E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid. Redox Signal. 2011, 14, 2013–2054. [Google Scholar] [CrossRef]
- Sena, L.; Chandel, N. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and physiological role of reactive oxygen species–the good, the bad and the ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, G.; da Cruz, I.B.M.; González-Gallego, J. Chapter Four-Manganese superoxide dismutase and oxidative stress modulation. Adv. Clin. Chem. 2015, 68, 87–130. [Google Scholar] [PubMed]
- Sepasi Tehrani, H.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef]
- Estevez, A.Y.; Erlichman, J.S. Cerium Oxide Nanoparticles for the Treatment of Neurological Oxidative Stress Diseases. In Oxidative Stress: Diagnostics, Prevention and Therapy; Andreescu, E.S., Hempel, M., Eds.; American Chemical Society: Washington, DC, USA, 2011; Volume 1083, pp. 255–288. [Google Scholar]
- Salim, S. Oxidative Stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017, 360, 201. [Google Scholar] [CrossRef]
- Umeno, A.; Biju, V.; Yoshida, Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef]
- Dolgacheva, L.P.; Berezhnov, A.V.; Fedotova, E.I.; Zinchenko, V.P.; Abramov, A.Y. Role of DJ-1 in the mechanism of pathogenesis of Parkinson’s disease. J. Bioenergy Biomembr. 2019, 51, 175–188. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. Impaired redox signaling in Huntington’s disease: Therapeutic implications. Front. Mol. Neurosci. 2019, 12, 68. [Google Scholar] [CrossRef]
- Baillet, A.; Chanteperdrix, V.; Trocmé, C.; Casez, P.; Garrel, C.; Besson, G. The role of oxidative stress in Amyotrophic Lateral Sclerosis and Parkinson’s disease. Neurochem. Res. 2010, 35, 1530–1537. [Google Scholar] [CrossRef]
- Takei, K.; Watanabe, K.; Yuki, S.; Akimoto, M.; Sakata, T.; Palumbo, J. Edaravone and its clinical development for amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, H.; Kimura, A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (Phase II study). Amyotroph Lateral Scler. 2006, 7, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.M.; Hubble, J.; Apple, S.; Takei, K.; Tsuda, K.; Liu, S.; Zhang, J.; Agnese, W. Post-hoc analyses of the edaravone clinical trials Study 16 and Study 19: A step toward more efficient clinical trial designs in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2019, 20, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Estevez, A.Y.; Erlichman, J.S. The potential of cerium oxide nanoparticles (nanoceria) for neurodegenerative disease therapy. Nanomedicine 2014, 9, 1437–1440. [Google Scholar] [CrossRef] [Green Version]
- Naz, S.; Beach, J.; Heckert, B.; Tummala, T.; Pashchenko, O.; Banerjee, T.; Santra, S. Cerium oxide nanoparticles: A ‘radical’ approach to neurodegenerative disease treatment. Nanomedicine 2017, 12, 545–553. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. Wires Nanomed. Nanobiotechnol. 2017, 9, e1444. [Google Scholar] [CrossRef]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 10, 1056–1058. [Google Scholar] [CrossRef]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef] [Green Version]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.; Seal, S.; Self, W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [Green Version]
- Baldim, V.; Bedioui, F.; Mignet, N.; Margaill, I.; Berret, J.-F. The enzyme-like catalytic activity of cerium oxide nanoparticles and its dependency on Ce3+ surface area concentration. Nanoscale 2018, 10, 6971–6980. [Google Scholar] [CrossRef]
- Das, M.; Patil, S.; Bhargava, N.; Kang, J.F.; Riedel, L.M.; Seal, S.; Hickman, J.J. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials 2007, 28, 1918–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, K.; Cormack, A.; Kulkarni, A.; Mayton, M.; Sayle, D.; Klaessig, F.; Stadler, B. Exploring the properties and applications of nanoceria: Is there still plenty of room at the bottom? Environ. Sci. Nano 2014, 1, 390–405. [Google Scholar] [CrossRef]
- Aneggi, E.; Boaro, M.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Insights into the redox properties of ceria-based oxides and their implications in catalysis. J. Alloy. Compd. 2006, 408–412, 1096–1102. [Google Scholar] [CrossRef]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Heckman, K.L.; DeCoteau, W.; Estevez, A.; Reed, K.J.; Costanzo, W.; Sanford, D.; Leiter, J.C.; Clauss, J.; Knapp, K.; Gomez, C.; et al. Custom cerium oxide nanoparticles protect against a free radical mediated autoimmune degenerative disease in the bain. ACS Nano 2013, 7, 10582–10596. [Google Scholar] [CrossRef] [PubMed]
- Eitan, E.; Hutchison, E.R.; Greig, N.H.; Tweedie, D.; Celik, H.; Ghosh, S.; Fishbein, K.W.; Spencer, R.G.; Sasaki, C.Y.; Ghosh, P.; et al. Combination therapy with lenalidomide and nanoceria ameliorates CNS autoimmunity. Exp. Neurol. 2015, 273, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, D.; Dargusch, R.; Raitano, J.; Chan, S.W. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem. Biophys. Res. Commun. 2006, 342, 86–91. [Google Scholar] [CrossRef]
- Chen, J.; Patil, S.; Seal, S.; McGinnis, J.F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006, 1, 142–150. [Google Scholar] [CrossRef]
- Bailey, Z.S.; Nilson, E.; Bates, J.A.; Oyalowo, A.; Hockey, K.S.; Sajja, V.S.S.S.; Thorpe, C.; Rogers, H.; Dunn, B.; Frey, A.S.; et al. Cerium oxide nanoparticles improve outcome after in vitro and in vivo mild traumatic brain injury. J. Neurotrauma 2016. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Maklad, H.M.; Samy, D.M.; Abdelmonsif, D.A.; El Sabaa, B.M.; Elnozahy, F.Y. Cerium oxide nanoparticles could ameliorate behavioral and neurochemical impairments in 6-hydroxydopamine induced Parkinson’s disease in rats. Neurochem. Int. 2017, 108, 361–371. [Google Scholar] [CrossRef]
- Kim, C.K.; Kim, T.; Choi, I.Y.; Soh, M.; Kim, D.; Kim, Y.J.; Jang, H.; Yang, H.S.; Kim, J.Y.; Park, H.K.; et al. Ceria nanoparticles that can protect against ischemic stroke. Angew. Chem. Int. Ed. Engl. 2012, 51, 11039–11043. [Google Scholar] [CrossRef] [PubMed]
- Estevez, A.Y.; Pritchard, S.; Harper, K.; Aston, J.W.; Lynch, A.; Lucky, J.J.; Ludington, J.S.; Chatani, P.; Mosenthal, W.P.; Leiter, J.C.; et al. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic. Biol. Med. 2011, 51, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- DeCoteau, W.; Heckman, K.L.; Estevez, A.Y.; Reed, K.J.; Costanzo, W.; Sandford, D.; Studlack, P.; Clauss, J.; Nichols, E.; Lipps, J.; et al. Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomedicine 2016, 12, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Yokel, R.A.; Hussain, S.; Garantziotis, S.; Demokritou, P.; Castranova, V.; Cassee, F.R. The Yin: An adverse health perspective of nanoceria: Uptake, distribution, accumulation, and mechanisms of its toxicity. Environ. Sci. Nano 2014, 1, 406–428. [Google Scholar] [CrossRef]
- Kumari, M.; Singh, S.P.; Chinde, S.; Rahman, M.F.; Mahboob, M.; Grover, P. Toxicity study of cerium oxide nanoparticles in human neuroblastoma cells. Int. J. Toxicol. 2014, 33, 86–97. [Google Scholar] [CrossRef]
- Gagnon, J.; Fromm, K.M. Toxicity and protective effects of cerium oxide nanoparticles (nanoceria) depending on their preparation method, particle size, cell type, and exposure route. Eur. J. Inorg. Chem. 2015, 2015, 4510–4517. [Google Scholar] [CrossRef]
- Hardas, S.S.; Sultana, R.; Warrier, G.; Dan, M.; Wu, P.; Grulke, E.A.; Tseng, M.T.; Unrine, J.M.; Graham, U.M.; Yokel, R.A.; et al. Rat hippocampal responses up to 90 days after a single nanoceria dose extends a hierarchical oxidative stress model for nanoparticle toxicity. Nanotoxicology 2013, 8, 155–166. [Google Scholar] [CrossRef]
- Grulke, E.; Reed, K.; Beck, M.; Huang, X.; Cormack, A.; Seal, S. Nanoceria: Factors affecting its pro- and anti-oxidant properties. Environ. Sci. Nano 2014, 1, 429–444. [Google Scholar] [CrossRef]
- Ferraro, D.; Tredici, I.G.; Ghigna, P.; Castillio-Michel, H.; Falqui, A.; Di Benedetto, C.; Alberti, G.; Ricci, V.; Anselmi-Tamburini, U.; Sommi, P. Dependence of the Ce(iii)/Ce(iv) ratio on intracellular localization in ceria nanoparticles internalized by human cells. Nanoscale 2017, 9, 1527–1538. [Google Scholar] [CrossRef] [Green Version]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C.W. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef]
- Lee, S.S.; Song, W.; Cho, M.; Puppala, H.L.; Nguyen, P.; Zhu, H.; Segatori, L.; Colvin, V.L. Antioxidant properties of cerium oxide nanocrystals as a function of nanocrystal diameter and surface coating. ACS Nano 2013, 7, 9693–9703. [Google Scholar] [CrossRef] [PubMed]
- Ould-Moussa, N.; Safi, M.; Guedeau-Boudeville, M.; Montero, D.; Conjeaud, H.; Berret, J. In vitro toxicity of nanoceria: Effect of coating and stability in biofluids. Nanotoxicology 2014, 8, 799–811. [Google Scholar] [PubMed]
- Popov, A.L.; Popova, N.R.; Selezneva, I.I.; Akkizov, A.Y.; Ivanov, V.K. Cerium oxide nanoparticles stimulate proliferation of primary mouse embryonic fibroblasts in vitro. Mater. Sci. Eng. C 2016, 68, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Hardas, S.S.; Sultana, R.; Warrier, G.; Dan, M.; Florence, R.L.; Wu, P.; Grulke, E.A.; Tseng, M.T.; Unrine, J.M.; Graham, U.M.; et al. Rat brain pro-oxidant effects of peripherally administered 5 nm ceria 30 days after exposure. Neurotoxicology 2012, 33, 1147–1155. [Google Scholar] [CrossRef]
- Yokel, R.A.; Au, T.C.; MacPhail, R.; Hardas, S.S.; Butterfield, D.A.; Sultana, R.; Goodman, M.; Tseng, M.T.; Dan, M.; Haghnazar, H.; et al. Distribution, elimination, and biopersistence to 90 days of a systemically introduced 30 nm ceria-engineered nanomaterial in rats. Toxicol. Sci. 2012, 127, 256–268. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem. Int. Ed. Engl. 2009, 48, 2308–2312. [Google Scholar] [CrossRef]
- Lee, C.W.; Chen, Y.C.; Ostafin, A. The accuracy of Amplex Red assay for hydrogen peroxide in the presence of nanoparticles. J. Biomed. Nanotechnol. 2009, 5, 477–485. [Google Scholar] [CrossRef]
- Didion, S.P.; Ryan, M.J.; Baumbach, G.L.; Sigmund, C.D.; Faraci, F.M. Superoxide contributes to vascular dysfunction in mice that express human renin and angiotensinogen. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H156–H1576. [Google Scholar] [CrossRef]
- Didion, S.P.; Ryan, M.J.; Didion, L.A.; Fegan, P.E.; Sigmund, C.D.; Faraci, F.M. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ. Res. 2002, 91, 938–944. [Google Scholar] [CrossRef]
- Franco, M.D.C.P.; Akamine, E.H.; Di Marco, G.S.; Casarini, D.E.; Fortes, Z.B.; Tostes, R.C.A.; Carvalho, M.H.C.; Nigro, D. NADPH oxidase and enhanced superoxide generation in intrauterine undernourished rats: Involvement of the renin–angiotensin system. Cardiovasc. Res. 2003, 59, 767–775. [Google Scholar] [CrossRef]
- Haugen, E.N.; Croatt, A.J.; Nath, K.A. Angiotensin II induces renal oxidant stress in vivo and heme oxygenase-1 in vivo and in vitro. Kidney Int. 2000, 58, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, R.A.; Yi, C.; Miller, J.D.; Mitchell, I.J.; Penninger, J.M.; Faraci, F.M.; Heistad, D.D. Impact of ACE2 deficiency and oxidative stress on cerebrovascular function with aging. Stroke 2012, 43, 3358–3363. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Coleman, C.G.; Chan, J.; Faraco, G.; Marques-Lopes, J.; Milner, T.A.; Guruju, M.R.; Anrather, J.; Davisson, R.L.; Iadecola, C.; et al. Angiotensin II slow-pressor hypertension enhances NMDA currents and NOX2-dependent superoxide production in hypothalamic paraventricular neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R109–R1106. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Real, A.; Rey, P.; Soto-Otero, R.; Mendez-Alvarez, E.; Labandeira-Garcia, J. Angiotensin-converting enzyme inhibition reduces oxidative stress and protects dopaminergic neurons in a 6-hydroxydopamine rat model of Parkinsonism. J. Neurosci. Res. 2005, 81, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Schmeißer, A.; Garlichs, C.D.; Plötze, K.; Damme, U.; Mügge, A.; Daniel, W.G. Angiotensin II-induced superoxide anion generation in human vascular endothelial cells: Role of membrane-bound NADH-/NADPH-oxidases. Cardiovasc. Res. 1999, 44, 215–222. [Google Scholar] [CrossRef]
- Chen, S.; Li, G.; Zhang, W.; Wang, J.; Sigmund, C.D.; Olson, J.E.; Chen, Y. Ischemia-induced brain damage is enhanced in human renin and angiotensinogen double-transgenic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R152–R1531. [Google Scholar] [CrossRef]
- Zimnol, A.; Amann, K.; Mandel, P.; Hartmann, C.; Schupp, N. Angiotensin II type 1a receptor-deficient mice develop angiotensin II-induced oxidative stress and DNA damage without blood pressure increase. Am. J. Physiol. Ren. Physiol. 2017, 313, F1264–F1273. [Google Scholar] [CrossRef]
- Song, C.Y.; Khan, N.S.; Liao, F.; Wang, B.; Shin, J.S.; Bonventre, J.V.; Malik, K.U. Brain cytosolic phospholipase A2α mediates angiotensin II-induced hypertension and reactive oxygen species production in male mice. AJH 2018, 31, 622–629. [Google Scholar] [CrossRef]
- Cao, X.; Peterson, J.R.; Gang, W.; Josef, A.; Young, C.N.; Guruju, M.R.; Burmeister, M.A.; Costantino, I.; Davisson, R.L. Angiotensin II–dependent hypertension requires cyclooxygenase 1–derived prostaglandin E2 and EP1 receptor signaling in the subfornical organ of the brain. Hypertension 2012, 59, 869–876. [Google Scholar] [CrossRef]
- Case, A.J.; Tian, J.; Zimmerman, M.C. Increased mitochondrial superoxide in the brain, but not periphery, sensitizes mice to angiotensin II-mediated hypertension. Redox Biol. 2017, 11, 82–90. [Google Scholar] [CrossRef]
- Nazarewicz, R.R.; Bikineyeva, A.; Dikalov, S.I. Rapid and specific measurements of superoxide using fluorescence spectroscopy. J. Biomol. Screen. 2013, 18, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Janiszewski, M. Direct detection of reactive oxygen species ex vivo. Kidney Int. 2005, 67, 1662–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.J.; Xu, K.H.; Tang, B.; Yin, L.L.; Yang, G.W.; An, L.G. Selective detection of superoxide anion radicals generated from macrophages by using a novel fluorescent probe. FEBS J. 2007, 274, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Dillon, C.; Weinlich, R.; Rodriguez, D.; Cripps, J.; Quarato, G.; Gurung, P.; Verbist, K.; Brewer, T.; Llambi, F.; Gong, Y.; et al. RIPK1 blocks early postnatal lethality mediated by Caspase-8 and RIPK3. Cell 2014, 157, 1189–1202. [Google Scholar] [CrossRef]
- Wrobel, K.; Claudio, E.; Segade, F.; Ramos, S.; Lazo, P.S. Measurement of cytotoxicity by propidium iodide staining of target cell DNA: Application to the quantification of murine TNF-α. J. Immunol. Methods 1996, 189, 243–249. [Google Scholar] [CrossRef]
- Ganesana, M.; Erlichman, J.S.; Andreescu, S. Real-time monitoring of superoxide accumulation and antioxidant activity in a brain slice model using an electrochemical cytochrome c biosensor. Free Radic. Biol. Med. 2012, 53, 2240–2249. [Google Scholar] [CrossRef] [Green Version]
- Cormack, A.N.; Lamphier, S.; Bu, W.; Gubb, T.; Reed, K. Simulations of ceria nanoparticles. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20150218. [Google Scholar] [CrossRef]
- Heckert, E.G.; Seal, S.; Self, W.T. Fenton-like reaction catalyzed by the rare earth inner transition metal cerium. Environ. Sci. Technol. 2008, 42, 5014–5019. [Google Scholar] [CrossRef]
- Cresi, J.S.P.; Spadaro, M.C.; D’Addato, S.; Valeri, S.; Amidani, L.; Boscherini, F.; Bertoni, G.; Deiana, D.; Luches, P. Contraction, cation oxidation state and size effects in cerium oxide nanoparticles. Nanotechnology 2017, 28, 495702. [Google Scholar] [CrossRef]
- Trovarelli, A. Structural properties and nonstoichiometric behavior of CeO2. In Catalysis by Ceria and Related Materials; Trovarelli, A., Ed.; Imperial College Press: London, UK, 2002; Volume 2, pp. 15–50. [Google Scholar]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Molinari, M.; Symington, A.R.; Sayle, D.C.; Sakthivel, T.S.; Seal, S.; Parker, S.C. Computer-aided design of nanoceria structures as enzyme mimetic agents: The role of bodily electrolytes on maximizing their activity. ACS Appl. Bio Mater. 2019, 2, 1098–1106. [Google Scholar] [CrossRef]
- Hailstone, R.K.; DiFrancesco, A.G.; Leong, J.G.; Allston, T.D.; Reed, K.J. A Study of lattice expansion in CeO2 nanoparticles by transmission electron microscopy. J. Phys. Chem. C 2009, 113, 15155–15159. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Andreescu, S.; Ornatska, M.; Erlichman, J.; Estevez, A.; Leiter, J.C. Biomedical Applications of Metal Oxide Nanoparticles. In Fine Particles in Medicine and Pharmacy; Matijević, E., Ed.; Springer: Medford, MA, USA, 2012; pp. 57–100. [Google Scholar]
- Al Ghouleh, I.; Khoo, N.K.H.; Knaus, U.G.; Griendling, K.K.; Touyz, R.M.; Thannickal, V.J.; Barchowsky, A.; Nauseef, W.M.; Kelley, E.E.; Bauer, P.M.; et al. Oxidases and peroxidases in cardiovascular and lung disease: New concepts in reactive oxygen species signaling. Free Radic. Biol. Med. 2011, 51, 1271–1288. [Google Scholar] [CrossRef] [Green Version]
- Infanger, D.W.; Sharma, R.V.; Davisson, R.L. NADPH oxidases of the brain: Distribution, regulation, and function. Antioxid. Redox Signal. 2006, 8, 1583–1596. [Google Scholar] [CrossRef]
- Cross, A.R.; Jones, O.T. Enzymic mechanisms of superoxide production. Biochim. Biophys. Acta 1991, 1057, 281–298. [Google Scholar] [CrossRef]
- Celardo, I.; De Nicola, M.; Mandoli, C.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Ce(3)+ ions determine redox-dependent anti-apoptotic effect of cerium oxide nanoparticles. ACS Nano 2011, 5, 4537–4549. [Google Scholar] [CrossRef]
- Pulido-Reyes, G.; Rodea-Palomares, I.; Das, S.; Sakthivel, T.S.; Leganes, F.; Rosal, R.; Seal, S.; Fernández-Piñas, F. Untangling the biological effects of cerium oxide nanoparticles: The role of surface valence states. Sci. Rep. 2015, 5, 15613. [Google Scholar] [CrossRef]
- Wang, G.; Sarkar, P.; Peterson, J.R.; Anrather, J.; Pierce, J.P.; Moore, J.M.; Feng, J.; Zhou, P.; Milner, T.A.; Pickel, V.M.; et al. COX-1-derived PGE2 and PGE2 type 1 receptors are vital for angiotensin II-induced formation of reactive oxygen species and Ca2+ influx in the subfornical organ. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1451–H1461. [Google Scholar] [CrossRef]
- He, F.; Cao, Y.P.; Che, F.Y.; Yang, L.H.; Xiao, S.H.; Liu, J. Inhibitory effects of edaravone in beta-amyloid-induced neurotoxicity in rats. Biomed. Res. Int. 2014, 2014, 370368. [Google Scholar] [CrossRef]
- Shibuta, S.; Varathan, S.; Kamibayashi, T.; Mashimo, T. Small temperature variations alter edaravone-induced neuroprotection of cortical cultures exposed to prolonged hypoxic episodes. Br. J. Anaesth. 2010, 104, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Hu, P.; Xu, Y.; Cheng, T.; Wei, C.; Pan, L.; Shi, J. Simultaneous blood–brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS Nano 2018, 12, 6794–6805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, H.; Bao, G. Physical principles of nanoparticle cellular endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9 (Suppl. 1), 51–63. [Google Scholar] [Green Version]
- Akinc, A.; Battaglia, G. Exploiting endocytosis for nanomedicines. Cold Spring Harb. Perspect. Biol. 2013, 5, a016980. [Google Scholar] [CrossRef] [PubMed]
- Dan, M.; Wen, H.; Shao, A.; Xu, L. Silver Nanoparticle Exposure Induces Neurotoxicity in the rat hippocampus without increasing the blood-brain barrier permeability. J. Biomed. Nanotechnol. 2018, 14, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, S. Role of phosphate on stability and catalase mimetic activity of cerium oxide nanoparticles. Colloids Surf. B Biointerfaces 2015, 132, 78–84. [Google Scholar] [CrossRef]
- Singh, S.; Dosani, T.; Karakoti, A.S.; Kumar, A.; Seal, S.; Self, W.T. A phosphate-dependent shift in redox state of cerium oxide nanoparticles and its effects on catalytic properties. Biomaterials 2011, 32, 6745–6753. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Tsuruta, R.; Kasaoka, S.; Fujimoto, K.; Tanaka, R.; Oda, Y.; Nanba, M.; Igarashi, M.; Yuasa, M.; Yoshikawa, T.; et al. In vivo real-time measurement of superoxide anion radical with a novel electrochemical sensor. Free Radic. Biol. Med. 2009, 47, 1039–1048. [Google Scholar] [CrossRef]
- Ono, T.; Tsuruta, R.; Fujita, M.; Aki, H.S.; Kutsuna, S.; Kawamura, Y.; Wakatsuki, J.; Aoki, T.; Kobayashi, C.; Kasaoka, S.; et al. Xanthine oxidase is one of the major sources of superoxide anion radicals in blood after reperfusion in rats with forebrain ischemia/reperfusion. Brain Res. 2009, 1305, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, T.; Döhring, J.; Reuter, S.; Finke, C.; Rohr, A.; Brauer, H.; Deuschl, G.; Jansen, O. Selective neuronal vulnerability of human hippocampal CA1 neurons: Lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J. Cereb. Blood Flow Metab. 2015, 35, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, T.; Wulff, P. The hippocampus in aging and disease: From plasticity to vulnerability. Neuroscience 2015, 309, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, P.M.; Berger, R.P.; Bayr, H.; Wagner, A.K.; Jenkins, L.W.; Clark, R.S.B. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: Diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr. Opin. Crit. Care 2008, 14, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Cenini, G.; Butterfield, D.A. Biomarkers of oxidative stress in neurodegenerative diseases. Mol. Basis Oxidative Stress 2013, 359–376. [Google Scholar]
- Sela, H.; Cohen, H.; Elia, P.; Zach, R.; Karpas, Z.; Zeiri, Y. Spontaneous penetration of gold nanoparticles through the blood brain barrier (BBB). J. Nanobiotechnol. 2015, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Heckman, K.L.; Erlichman, J.; Reed, K.; Skeels, M. Application of mass spectrometry to characterize localization and efficacy of nanoceria in vivo. In Advancements of Mass Spectrometry in Biomedical Research. Advances in Experimental Medicine and Biology; Darie, C., Ed.; Springer Nature: Basel, Switzerland, 2014; Volume 806, pp. 569–579. [Google Scholar]
- Sawada, H. Clinical efficacy of edaravone for the treatment of amyotrophic lateral sclerosis. Expert Opin. Pharmacother. 2017, 18, 735–738. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Sack-Zschauer, M.; Karaman-Aplak, E.; Wyrich, C.; Das, S.; Schubert, T.; Meyer, H.; Janiak, C.; Seal, S.; Stahl, W.; Brenneisen, P. Efficacy of different compositions of cerium oxide nanoparticles in tumor-stroma interaction. J. Biomed. Nanotechnol. 2017, 13, 1735–1746. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Meehan, K.; Whiting, M.D.; Dillon, C.E.; Hockey, K.; Brewer, M. Antioxidant Nanoparticles. In Nanomedicine in Health and Disease; Hunter, R.J., Preedy, V.R., Eds.; CRC Press: New York, NY, USA, 2017; pp. 100–122. [Google Scholar]
- Dowding, J.M.; Song, W.; Bossy, K.; Karakoti, A.; Kumar, A.; Kim, A.; Bossy, B.; Seal, S.; Ellisman, M.H.; Perkins, G.; et al. Cerium oxide nanoparticles protect against Aβ-induced mitochondrial fragmentation and neuronal cell death. Cell Death Differ. 2014, 21, 1622. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Madler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef]
- Rubio, L.; Marcos, R.; Hernández, A. Nanoceria acts as antioxidant in tumoral and transformed cells. Chem. Biol. Interact. 2018, 291, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Gutbier, S.; May, P.; Berthelot, S.; Krishna, A.; Trefzer, T.; Behbehani, M.; Efremova, L.; Delp, J.; Gstraunthaler, G.; Waldmann, T.; et al. Major changes of cell function and toxicant sensitivity in cultured cells undergoing mild, quasi-natural genetic drift. Arch. Toxicol. 2018, 92, 3487–3503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Citric Acid/EDTA Ratio | Size in Solution DLS (nm) | Polydispersity | Crystallite Size via XRD (nm) | Zeta Potential (mV) |
|---|---|---|---|---|
| 100/0 | 7.8 | 0.374 | 2.0 | −20.8 |
| 70/30 | 3.8 | 0.309 | 2.3 | −20.4 |
| 60/40 | 2.6 | 0.198 | 2.4 | −18.7 |
| 50/50 | 2.7 | 0.188 | 2.4 | −21.5 |
| 40/60 | 2.9 | 0.162 | 2.5 | −18.3 |
| 30/70 | 3.0 | 0.188 | 2.5 | −23.0 |
| 20/80 | 3.5 | 0.160 | 2.4 | −9.1 |
| 0/100 | 2.4 | 0.230 | 2.1 | −15.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estevez, A.Y.; Ganesana, M.; Trentini, J.F.; Olson, J.E.; Li, G.; Boateng, Y.O.; Lipps, J.M.; Yablonski, S.E.R.; Donnelly, W.T.; Leiter, J.C.; et al. Antioxidant Enzyme-Mimetic Activity and Neuroprotective Effects of Cerium Oxide Nanoparticles Stabilized with Various Ratios of Citric Acid and EDTA. Biomolecules 2019, 9, 562. https://doi.org/10.3390/biom9100562
Estevez AY, Ganesana M, Trentini JF, Olson JE, Li G, Boateng YO, Lipps JM, Yablonski SER, Donnelly WT, Leiter JC, et al. Antioxidant Enzyme-Mimetic Activity and Neuroprotective Effects of Cerium Oxide Nanoparticles Stabilized with Various Ratios of Citric Acid and EDTA. Biomolecules. 2019; 9(10):562. https://doi.org/10.3390/biom9100562
Chicago/Turabian StyleEstevez, Ana Y., Mallikarjunarao Ganesana, John F. Trentini, James E. Olson, Guangze Li, Yvonne O. Boateng, Jennifer M. Lipps, Sarah E. R. Yablonski, William T. Donnelly, James C. Leiter, and et al. 2019. "Antioxidant Enzyme-Mimetic Activity and Neuroprotective Effects of Cerium Oxide Nanoparticles Stabilized with Various Ratios of Citric Acid and EDTA" Biomolecules 9, no. 10: 562. https://doi.org/10.3390/biom9100562
APA StyleEstevez, A. Y., Ganesana, M., Trentini, J. F., Olson, J. E., Li, G., Boateng, Y. O., Lipps, J. M., Yablonski, S. E. R., Donnelly, W. T., Leiter, J. C., & Erlichman, J. S. (2019). Antioxidant Enzyme-Mimetic Activity and Neuroprotective Effects of Cerium Oxide Nanoparticles Stabilized with Various Ratios of Citric Acid and EDTA. Biomolecules, 9(10), 562. https://doi.org/10.3390/biom9100562






