1. Introduction
Inhibitory immune checkpoints play an essential role in the control and limitation of T-cell activation and functionality. When functioning properly, these control mechanisms can prevent autoimmunity, excessive inflammation, and tissue damage. Tumor cells exploit inhibitory immune checkpoints to develop defense mechanisms against the host’s immune system. Together with the infiltration of regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and type 2-activated tumor-associated macrophages (TAMs), the presence of inhibitory immune checkpoints creates a strong resistance that limits the effective elimination of tumor cells by the induction of a functional unresponsiveness [
1].
Blockade of inhibitory immune checkpoints, such as CTLA-4 and PD-1/PD-L1, has changed the classical approach to treat cancer [
2]. Since the FDA approval of therapeutics targeting CTLA-4 and PD1/PD-L1 substantial interest raised for ‘next generation’ immune checkpoints such as LAG-3 (CD223) [
3]. As currently reported in the literature, LAG-3 expression has been detected on the surface of activated T cells, Tregs, B cells, natural killer (NK) cells, and plasmacytoid dendritic cells (DCs) [
4]. LAG-3 is known to downregulate T-cell responses via interaction with major histocompatibility complex class-II (MHC-II), present on tumor cells or antigen-presenting cells like DCs [
5]. Additionally, three novel LAG-3 ligands have been reported: galectin-3, liver sinusoidal endothelial cell lectin (LSECtin), and fibrinogen-like protein 1 (FGL1) which are abundantly present in the tumor environment to help regulate and effectively abolish anti-tumor immunity of CD8
+ T cells [
6,
7,
8]. LAG-3 expression is often observed on tumor-infiltrating lymphocytes (TILs) and is associated with shorter progression-free survival in patients treated with anti-PD-1 therapy, suggesting independence of these immune evasion pathways [
9,
10]. Preclinical research demonstrated that monoclonal antibody (mAb) mediated LAG-3 blockade activates antigen-specific T cells at the tumor site, ultimately leading to a disruption in tumor growth [
11,
12,
13]. The latter instigated the development of LAG-3 blocking moieties suited for the human setting and recently resulted in the execution of phase I/II clinical trials by Bristol Myers-Squibb (BMS), Novartis, Merck and Macrogenics, making LAG-3 the third inhibitory immune checkpoint targeted with an antagonistic mAb in the clinic [
14,
15].
Unfortunately, many patients fail to respond to immune checkpoint inhibition (ICI). For example, an objective response to single agent blockade with the anti-PD-1 mAb nivolumab is observed in 21% of patients with renal cell carcinoma [
16] and 45% with advanced melanoma [
17]. Correlative studies that performed immunohistochemistry (IHC) on tissue biopsies suggest that PD-L1 expression observed on tumor-infiltrating immune cells or on cancer cells could serve as a predictive marker for PD-1 blockade treatment, resulting in the development of complementary or companion diagnostics for some treatment indications [
18]. Nevertheless, detection of PD-L1 expression in IHC of tumor biopsies is no guarantee for therapy response since some patients with clear positivity can fail therapy while patients with no apparent PD-L1 expression can benefit from PD-L1 blocking treatment [
19]. The latter can be explained by the high heterogeneity of inhibitory immune checkpoint expression in primary tumors and metastases that are furthermore likely to change over time. Additionally, IHC relies on biopsies, which are tempospatially-restricted samples of ever evolving heterogeneous tumor masses. Consequently, IHC is a less suitable technique to stratify patients or predict therapy outcome. Preclinical studies evaluating noninvasive imaging using positron-emission tomography (PET) or single-photon emission computed tomography (SPECT) with radiolabeled antibodies to CTLA-4, PD-1, or PD-L1, suggest that this imaging method could be a valuable approach to improve patient selection [
20,
21]. Early-phase clinical trial results corroborate these preclinical findings, showing that pretreatment PET signals are more reliable to predict therapy response than IHC-based evaluation [
22,
23].
The use of nanobodies (Nbs), the recombinant variable domains (VHH) of heavy-chain only antibodies (HCAbs) that are naturally present in animals from the
Camelidae family, is increasingly explored in the field of immuno-oncology [
24]. Their small size and unique epitope targeting possibilities make them very interesting to target proteins present in dense tissues like the tumor environment [
24]. Additionally, they can be easily engineered and produced in prokaryotic systems or yeast cells [
25,
26]. Nbs have been studied for molecular imaging of inhibitory immune checkpoints like PD-1/PD-L1 and CTLA-4 in the tumor environment [
27,
28,
29,
30,
31]. Overall, preclinical molecular imaging studies show that Nbs are excellent tools to visualize the heterogenic expression of inhibitory immune checkpoints. Moreover, Xing Y. et al. conducted a phase I clinical study evaluating the use of
99mTechnetium (
99mTc) labeled Nbs for SPECT imaging of PD-L1 in patients with non-small cell lung cancer, showing that it is safe and feasible to image PD-L1 levels in the tumor as soon as 2 h after injection [
29].
These results encourage the development of Nbs for molecular imaging of the inhibitory immune checkpoint receptor LAG-3. As this requires substantial preclinical evaluation, we developed and validated Nbs targeting mouse LAG-3 (moLAG-3) as probes for SPECT imaging. After alpaca immunization and biopanning via phage display, 114 Nbs were identified to bind recombinant LAG-3 protein. Bacterial extracts of these Nbs were further analyzed for binding to moLAG-3 using ELISA, flow cytometry and off-rate analysis using surface plasmon resonance (SPR). As such, nine moLAG-3 binding Nbs were selected. These Nbs were bacterially produced and purified, tested in flow cytometry, and their affinity was analyzed using SPR. Next, we labeled them with 99mTc, after which the biodistribution was assessed in immunocompetent and moLAG-3 gene-deficient mice by SPECT/CT imaging and dissection analyses. Subsequently, we evaluated the specific targeting of these Nbs in mice harboring tumors modified to overexpress mouse LAG-3.
2. Materials and Methods
2.1. Reagents
Specific binding of hemagglutinin (HA) tagged Nbs from small-scale productions was detected by ELISA and flow cytometry using an anti-HA antibody (Biolegend, clone 16B12). For flow cytometry a phycoerythrin (PE) labeled anti-mouse IgG antibody (BD biosciences, clone A85-1) was used to visualize Nb binding. For ELISA, the primary anti-HA antibody (Biolegend, clone 16B12) and the secondary anti-mouse IgG1κ (alkaline phosphatase conjugated) was used to detect mouse LAG-3 (moLAG-3) binding Nbs. An anti-His antibody (AbD Serotec, clone AD1.1.10), was used in flow cytometry to detect binding of purified hexahistidine (His6) tagged Nbs from large-scale productions. A PerCP-eFluor 710 or PE labeled antibody specific for moLAG-3 (Biolegend, clone eBioC9B7W) was used in flow cytometry to evaluate moLAG-3 expression on cells. The antibodies used to discriminate immune populations in flow cytometry were CD45.2-APC-eF780 (BD Biosciences, clone 104), CD11b-AF700 (BD Biosciences, clone M1/70), Ly6G-AF647 (BD Biosciences, clone 1AB), MHCII-PE-Dazzle 594 (Biolegend, clone M5/114.15.2), CD11c-AF488 (BD Biosciences, clone HL3), F4/80-BV421 (BD Biosciences, clone 6F12), Ly6C-PE-Cy7 (BD Biosciences, clone AL-21), CD19-FITC or AF647 (BD Biosciences, clone 1D3), CD335-AF647 (Biolegend, clone 29A1.4), CD160-PE-CF59 (BD Biosciences, clone CNX46-3 4), CD3-PE-Cy7 (Biolegend, clone 17A2), CD4-AF700 (BD Biosciences, clone RM4-5), CD8a-BV450 (BD Biosciences, clone 53-67), CD25-BB515 (BD Biosciences, clone PC61). Recombinant mouse and human LAG-3-Fc fusion proteins (RnDsystems, 3328-L3 and 2319-L3) were used for immunization, biopanning, ELISA, and SPR. T-activator CD3/CD28 dynabeads (11456D, Thermo Fisher Scientific) were used to stimulate spleen suspensions.
2.2. Isolation of LAG-3-Specific Nbs
Two llamas were subcutaneously immunized six times weekly, each time with a mixture containing 100 μg recombinant mouse and human LAG-3-Fc proteins (RnDsystems, cat. 3328-L3 and 2319-L3). Gerbu LQ#3000 was used as adjuvant. On the day 40, blood was collected for lymphocyte preparation from which total RNA was isolated. Using oligo(dT) primers, cDNA was synthesized from VHH encoding sequences using PCR. These amplicons were used as a source to create two Nb-phage display libraries in the pMECS phagemids as described previously [
32]. These libraries were phage-displayed and subjected to four rounds of biopanning on moLAG-3 recombinant protein that was immobilized on immunosorbent plastic. Small-scale freeze-thaw periplasmic extracts of individual clones were made and tested in ELISA on moLAG-3 and control-Fc fusion proteins. Different moLAG-3-specific Nb clones were identified by sequencing.
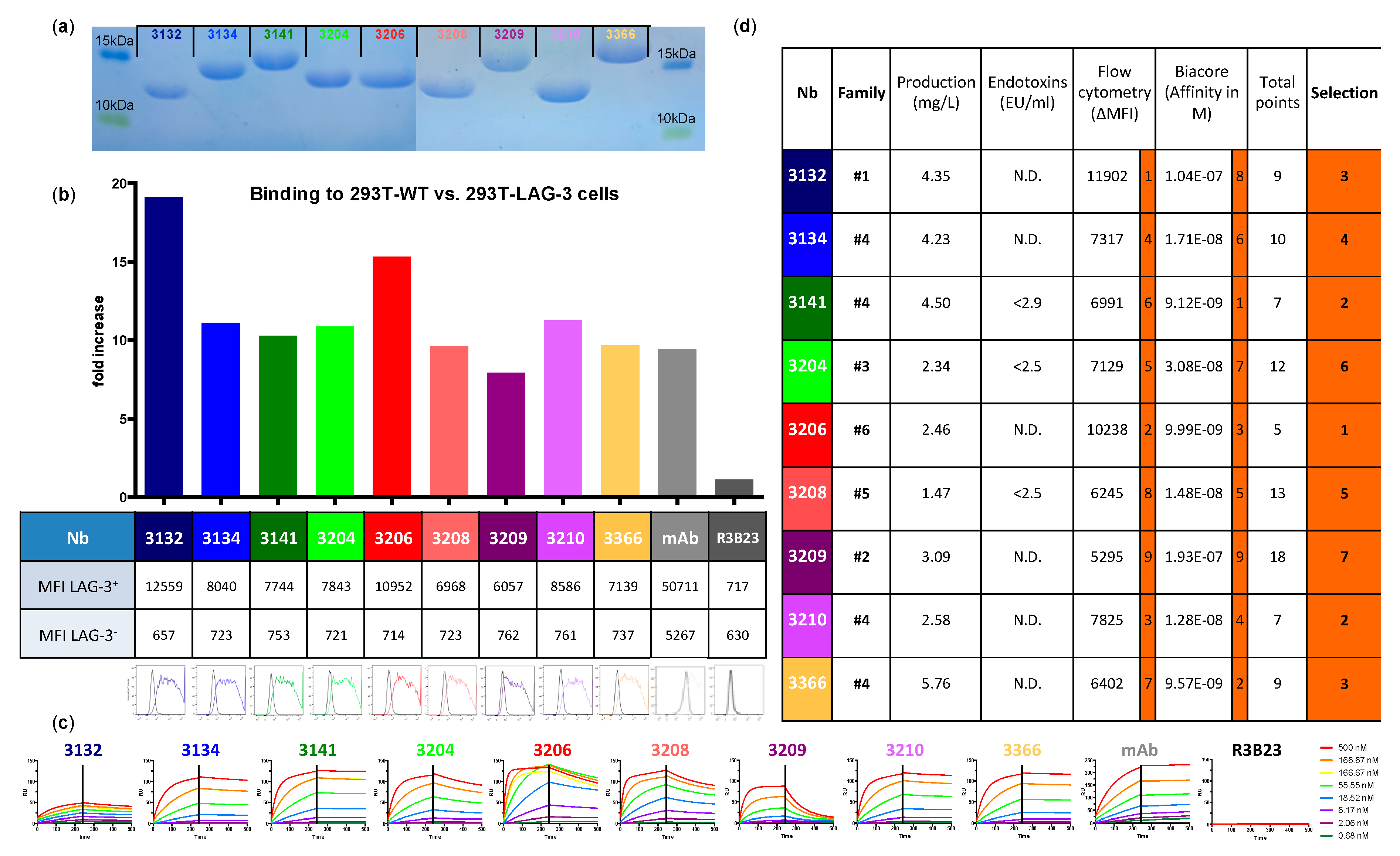
2.3. Bacterial Production, Purification, and Quality Control of Nbs
Nb production and purification is detailed elsewhere [
32]. Briefly, the sequences of selected Nbs, were PCR amplified using PCR with primers A6E (5′ GAT GTG CAG CTG CAG GAG TCT GGG GGA GG 3′) and PMCF (5′ CTA GTG CGG CCG CTG AGG AGA CGG TGA CCT GGG T 3′). cDNA of selected Nbs was cloned into PstI/BstEII digested pHEN6 plasmid to encode a C-terminal HIS
6 tag. Selected Nb clones in pHEN6 were transformed into WK6
Escherichia coli (
E. coli) for large-scale production. Each clone was cultured in 1 L supplemented terrific broth (TB) medium. Periplasmic extracts were generated by osmotic shock with Tris EDTA sucrose (TES) solution. The extracts were incubated with 1 mL of HisPur™ Ni-NTA Resin (ThermoFisher, Asse, Belgium) on a shaker for 1 h at 4 °C. After imidazole elution, the suspension was loaded on a size exclusion chromatography (SEC) column (HiLoad Superdex 200 pg, GE Healthcare, Machelen, Belgium), mounted on an AKTA avant high performance liquid chromatographer (HPLC) (GE Healthcare). Purity was evaluated on a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, followed by staining with Coomassie blue. The presence of lipopolysaccharide (LPS) was measured using a limulus amebocyte lysate (LAL)-test (Endosafe
® nexgen-PTS™, Charles River, Ecully, France). Moreover, Nb R3B23, specific for 5T2 M-proteins [
33], was produced as well and used during the study as negative control.
2.4. Evaluation of Affinity/Kinetics
The affinity for moLAG-3 of purified Nbs and the off-rate of Nbs in periplasmic extracts was performed using a Biacore T200 device (GE Healthcare, Machelen, Belgium). The measurements were performed at 25 °C using Hepes-buffered saline (HBS; 0.01 M HEPES pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% Tween 20) as running buffer. Briefly, recombinant, moLAG-3 protein was diluted to 10 μg/mL in 10 mM Na-acetate pH 5.5 and immobilized on a CM5 sensor chip using linkage chemistry with 1-(3-(dimethylamino)propyl)-3-ethylcarbodi-imide (EDC) and N-hydroxy-succinimide (NHS). Free EDC-NHS linkers present on the chip were neutralized with 1 M elthanolamine-HCl pH 8.5. Three dilutions of periplasmic extracts and seven dilutions of purified Nbs were analyzed at a flow rate of 10 µL/min. The association was set to 240 s and the dissociation to 300 s for each dilution. After each cycle, the chip was regenerated twice for 30 s using 100 mM Glycine HCl pH2.0. The measured response units from the flow channel on which moLAG-3 protein was immobilized was subtracted from the response units measured on a flow channel without protein. Additionally, the signal from the blank (HBS only) was subtracted from each measurement. As a result, the off-rate value koff for Nbs in periplasmic extracts and the equilibrium dissociation constant KD for purified Nbs were calculated using the Biacore T200 evaluation software (GE Healthcare, Machelen, Belgium).
2.5. Mice
Female, 6 to 12-week-old C57BL/6, B6.129S2-Lag3tm1Doi/J (moLAG-3 gene-deficient or knock-out (KO)) and Swiss Nude Crl:NU(Ico) Foxn1nu (immunodeficient) mice were purchased from Charles River (Ecully, France). All experiments using mice were approved by the Ethical Committee for laboratory animals of the Vrije Universiteit Brussel and executed in accordance to the European guidelines for animal experimentation (ethical dossier number 15-214-1).
2.6. Cell Lines
The TC-1 mouse lung epithelial cell line was kindly provided by T.C. Wu (Johns Hopkins University, Baltimore, MD, USA). The culture medium consisted of RPMI 1640 medium (Sigma-Aldrich, Zwijndrecht, Belgium), supplemented with 10% FCI serum (Harlan, Horst, The Netherlands), 2 mmol/L l-glutamine (L-Glu; Sigma-Aldrich, Zwijndrecht, Belgium), 100 U/mL penicillin, 100 µg/mL streptomycin (PS; Sigma-Aldrich, Zwijndrecht, Belgium), 1 mmol/L sodium pyruvate, and non-essential amino acids (Sigma-Aldrich, Zwijndrecht, Belgium), 12.5 mM d(+)-glucose, 1 mM Geneticin (G418), 5 mM hepes and 50 μM β-mercaptoethanol. Human embryonal kidney (HEK) 293T cells were obtained from the American Type Culture Collection (ATCC, Molsheim Cedex, France) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, Zwijndrecht, Belgium) supplemented with 10% FBS (Harlan, Horst, The Netherlands), l-Glu, and PS.
2.7. Production of Lentiviral Vectors and Transduction of Cells
The transfer plasmid encoding moLAG-3 was generated using Gibson cloning. Briefly, gBlocks were designed to contain the coding sequence for moLAG-3, nucleotide 355 to 1920 from NM_008479.2, and purchased at Integrated DNA Technologies (IDT). The sequences were flanked by 20 nucleotides that allow creating overhangs with the donor lentiviral transfer vector pHR’, which together with the packaging plasmid pCMVΔR8.9 and the VSV.G encoding plasmid pMD.G, was a gift from D. Trono (University of Geneva, Switzerland). Accordingly, the production of lentiviral vectors and the transduction of HEK293T cells and TC-1 cells was performed as described [
34]. HEK293T and TC-1 cells transduced with lentiviral vectors harboring moLAG-3 are referred to as 293T-LAG-3 cells and TC-1-LAG-3 cells respectively. Non-transduced cells are referred to as wild type (WT) cells.
2.8. Flow Cytometry
The procedure for staining of cell surface markers was previously described [
35]. Flow cytometry experiments were performed on the LSRFortessa and FACSCelesta flow cytometer (BD Biosciences). Data were analyzed with FACSDiva (BD Biosciences) or FlowJo X
® (Tree star, Inc., Ashland, OR, USA) software.
2.9. Inoculation of Tumor Cells
Swiss nude Crl:NU(Ico) Foxn1nu mice were subcutaneously injected with 5 × 10
5 TC-1-WT and TC-1-LAG-3 cells at their left and right flank respectively. The well-being of the mice was examined daily. Tumor growth was measured every other day using an electronic caliper and the tumor volume was calculated using the formula: (length × width
2)/2 [
28].
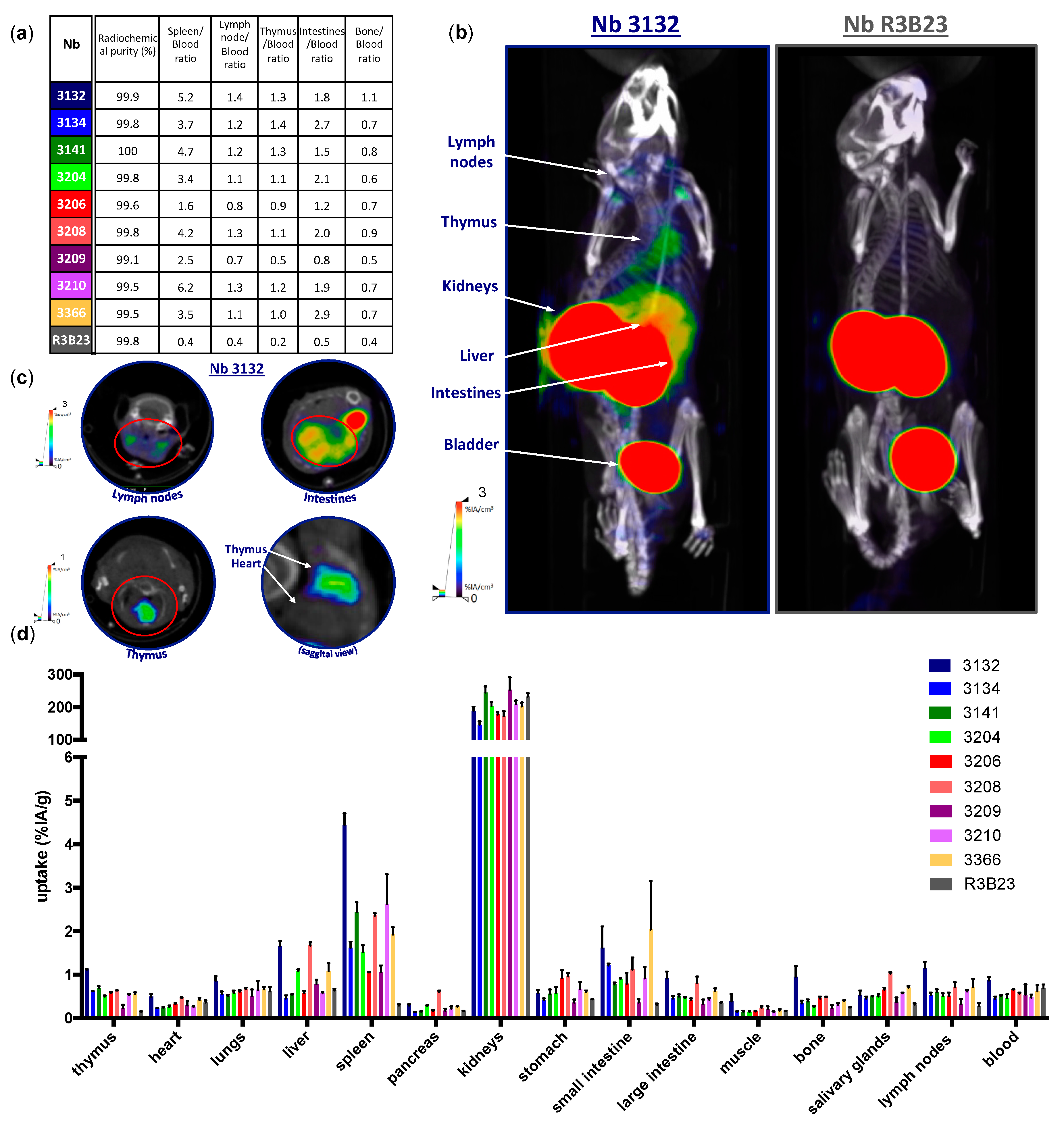
2.10. 99mTc-Nb Labeling, Pinhole SPECT-Micro-CT Imaging and Image Analysis
The labeling of Nbs with
99mTc was performed as previously described [
36]. To summarize,
99mTc-tricarbonyl [
99mTc(H
2O)
3(CO)
3]
99m was complexed with the C-terminal HIS
6-tag of the Nbs. The former was generated using the Isolink
® labelling kit (Mallinckrodt Medical BV, Petten, The Netherlands). The
99mTc-Nb solution was subjected to two purification steps, i.e., gel filtration on a NAP-5 column (GE Healthcare, Machelen, Belgium) pre-equilibrated with PBS to remove uncomplexed (
99mTc(H
2O)
3(CO)
3)
+ and filtration through a 0.22 μm filter (Millipore, Haren, Belgium) to remove aggregates. Labeling efficiency was determined by instant thin-layer chromatography (iTLC) before and after purification, with 100% acetone as the mobile phase. Uncomplexed
99mTc(H
2O)
3(CO)
3 migrates with the mobile phase and separates from the complexed fraction (
99mTc-labeled Nb). The ratio between uncomplexed and complexed fraction determines the radiochemical purity. Imaging was performed as described previously [
31]. Mice used for imaging experiments, were injected intravenously with 100 µL of
99mTc-labeled Nbs (5 µg) with on average 51.1 ± 16.7 MBq of injected activity, 1 h before pinhole SPECT-micro-CT imaging. SPECT-CT imaging was performed using a Vector
+ scanner from MiLABS. SPECT imaging was performed with a 1.5 mm 75-pinhole general purpose collimator, in spiral mode with six bed positions, the total body SPECT scan time was 15 min, 150 s per position. The total CT-scan time was 139 s, set to 60 kV and 615 mA. After imaging, mice were sacrificed and organs were isolated to evaluate the organ-specific uptake of the tracer using a γ-counter (Cobra Inspector 5003, Canberra, Packard, Illinois, IL, USA). The tissue/organ uptake was corrected for decay and calculated as the percentage of injected activity per gram tissue (%IA/g). Image analysis was performed using HOROS medical imaging viewer (LGPL license at Horosproject.org) and AMIDE (Medical Image Data Examiner software, UCLA, California, CA, USA).
2.11. Single Cell Preparation of Organs and Tumors
Single cell suspensions of lymph nodes, spleens, and implanted tumors were prepared according to the protocols of Miltenyi Biotec. Briefly, tumors were cut in pieces of approximately 5 mm and transferred to gentleMACS C tubes containing 5 mL ice-cold RPMI 1640 supplemented with 1000 U/mL DNAse I. The tumors were homogenized on the gentleMACS dissociator. The suspension was filtered through a 70 μm nylon filter (BD Falcon) and centrifuged for 5 min at 1500 rpm. Erythrocytes were lysed by resuspending the cell pellet with 5 mL red blood cell lysis buffer (0.16 M NH4Cl, 0.17 M Tris, pH 7.2). After 3 min incubation, the buffer was neutralized by adding 10 mL phosphate buffered saline (PBS, Sigma-Aldrich). Cells were centrifuged and counted (CasyTon, Innovatis, Reutlingen, Germany) and prepared for flow cytometry staining and analysis. Lymph nodes and spleens were prepared by mincing the organ through a 70 μm nylon filter in 5 mL PBS containing 1000 U/mL DNAse I (StemCell). Cells were centrifuged and incubated with 5 mL red blood cell lysis buffer. After 3 min of incubation, the buffer was neutralized by adding 10 mL PBS. Cells were centrifuged and counted (CasyTon) and prepared for flow cytometry staining and analysis.
2.12. Immunohistochemistry
Organs were incubated in 4% formalin for 24 h before transfer to the tissue processing apparatus Histokinette (Leica TP 1028) where the tissues are paraffin embedded. Slices of 3 μm were prepared with the microtome and baked at 60 °C before staining. Hematoxylin eosin saffron (HES) staining was performed following standard protocols. Stained sections were mounted with mounting media (Pertex, for histolab) and scanned with an Aperio digital pathology slide scanner (Leica Aperio CS2). Immunofluorescence (IF) staining of moLAG-3 was performed using an anti-LAG-3 antibody (Abcam, EPR20294-77). Therefore, the sections were deparaffinized, rehydrated and antigen retrieval was performed in citrate buffer pH 6.0 using the 2100 Retriever (Aptum Biologics). Slices were washed with PBS, blocked with serum free Dako Protein block (Agilent Technologies), and incubated overnight with the primary anti-LAG-3 antibody at 4 °C in a humidity chamber. After three wash steps with PBS, the secondary antibody (anti-rabbit AF477/Cy5, Jackson Laboratories) was added for 1 h. After three washes in PBS, the sections were mounted with fluorescent mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Agilent Technologies). For spleen sections, an autofluorescence quenching kit was used to lower the autofluorescence of red blood cells (Vector TrueVIEW, SP-8400). The stained sections were evaluated with the EVOS FL Auto fluorescence microscope (Life Technologies, Asse, Belgium).
2.13. Statistics
All statistical analyses were performed with GraphPad Prism software. All data are represented as mean ± standard deviation (SD). p-values were calculated using a Kruskal–Wallis test followed by a Dunn’s post hoc test or using a two-way Anova followed by a Tukey post hoc test. The asterisks in the figures indicate statistical significance as follows: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; n.s. not significant.
4. Discussion
Immune checkpoint therapy has become an important pillar in cancer therapy with mAbs targeting CTLA-4 and PD-1/PD-L1 showing clinical benefit in patient subgroups with solid tumors. Despite its unseen success, a significant number of patients do not respond to anti-CTLA-4 and/or PD-1/PD-L1 therapy, and inhibition of more recently described inhibitory receptors such as LAG-3 is actively investigated in the clinic as an alternative or additional treatment [
10,
14,
15]. As the list of immune checkpoint inhibitors and their combination regimens grow, there is an increasing need for easy-to-implement strategies that can predict patient response and that allow monitoring of patient responses during therapy. Given the current prizes of immune checkpoint drugs, the development of predictive markers is not only clinically relevant but also an economic necessity.
Detection of expression of immune checkpoints has mainly been performed by IHC. However, IHC provides a static picture at time of biopsy and is therefore a less suitable technique to predict therapy outcome even if the tumor is accessible. Limitations of IHC for predictive testing have been extensively discussed by Broos et al. [
21]. In contrast to IHC, molecular imaging is a noninvasive technique that can be performed repeatedly and allows quantification of tumor characteristics irrespective of the location of the tumor lesions. It was recently shown that PET is able to detect expression of the immune checkpoint receptor PD-1 and its ligand PD-L1, and that PET images correlated better with therapy outcome than IHC data [
22,
23].
Different moieties can be used for noninvasive molecular imaging. However, it is generally accepted that small antigen binding moieties will generate fast and high-contrast images, as radiolabeled nanobodies were shown to be cleared from the blood as fast as 20–30 min after intravenous injection [
38,
39]. As LAG-3 blocking mAbs are currently tested in 28 different clinical trials for the treatment of cancer, we evaluated Nbs specific for the inhibitory immune checkpoint LAG-3 and used these for noninvasive imaging of moLAG-3.
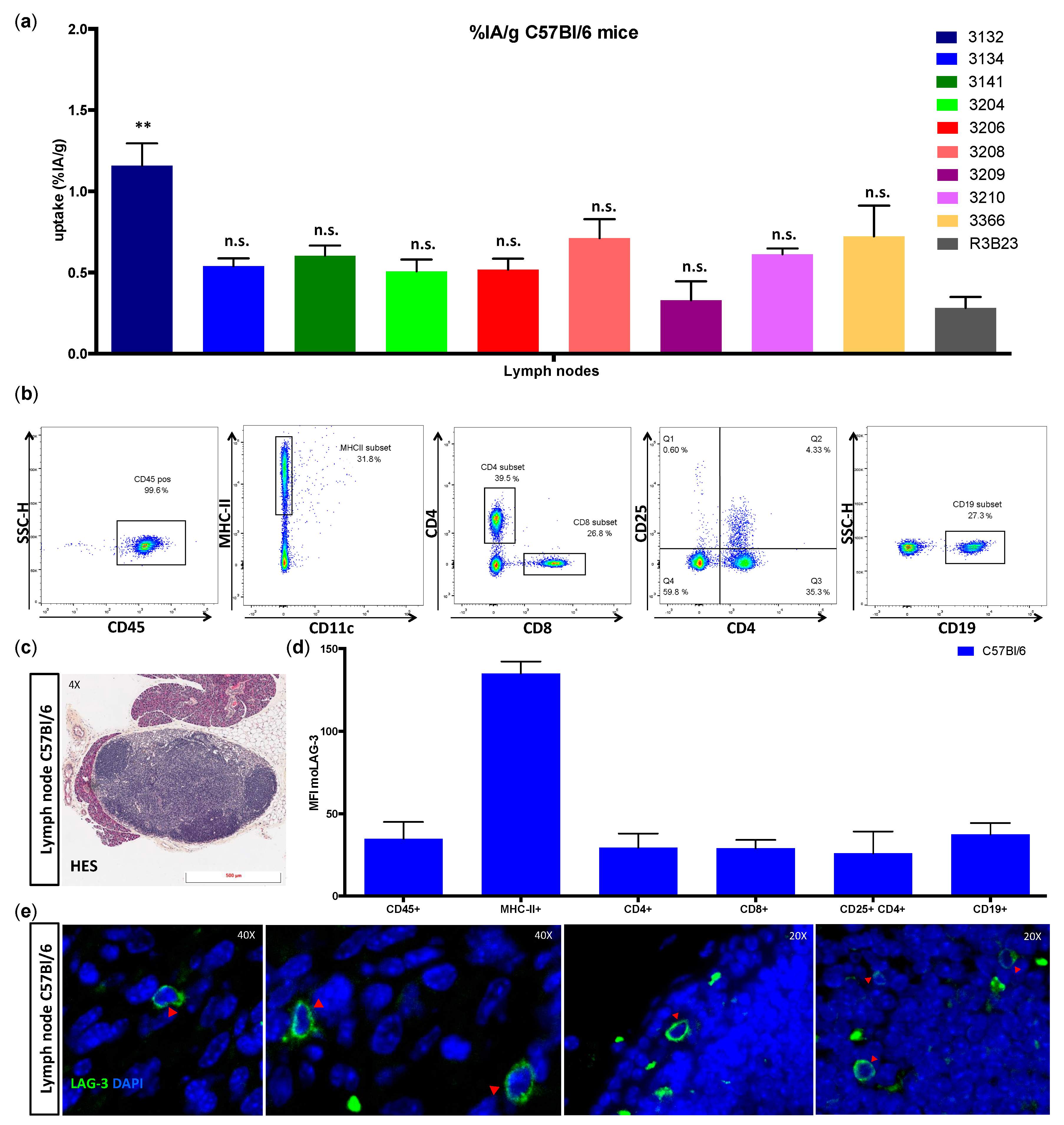
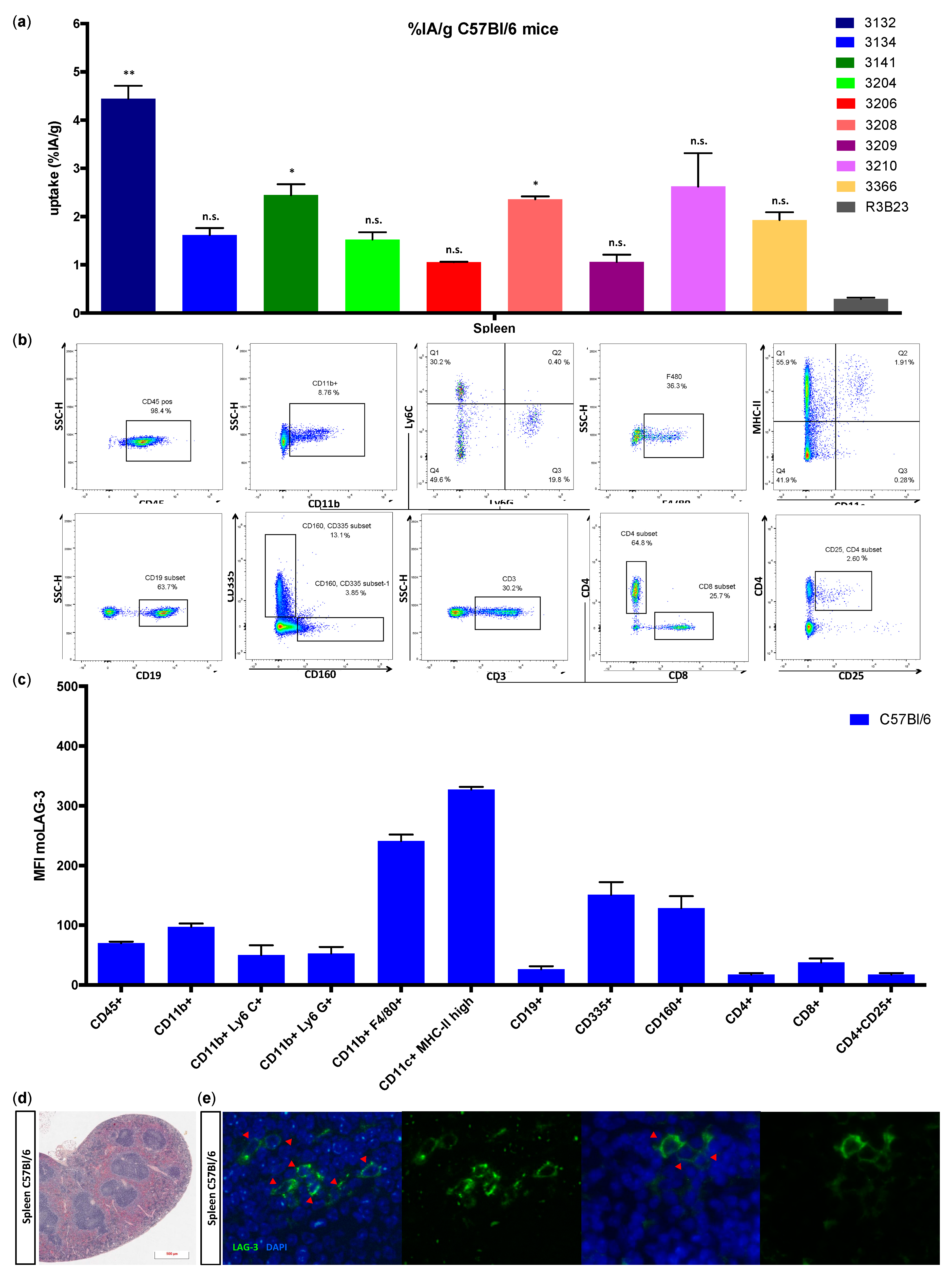
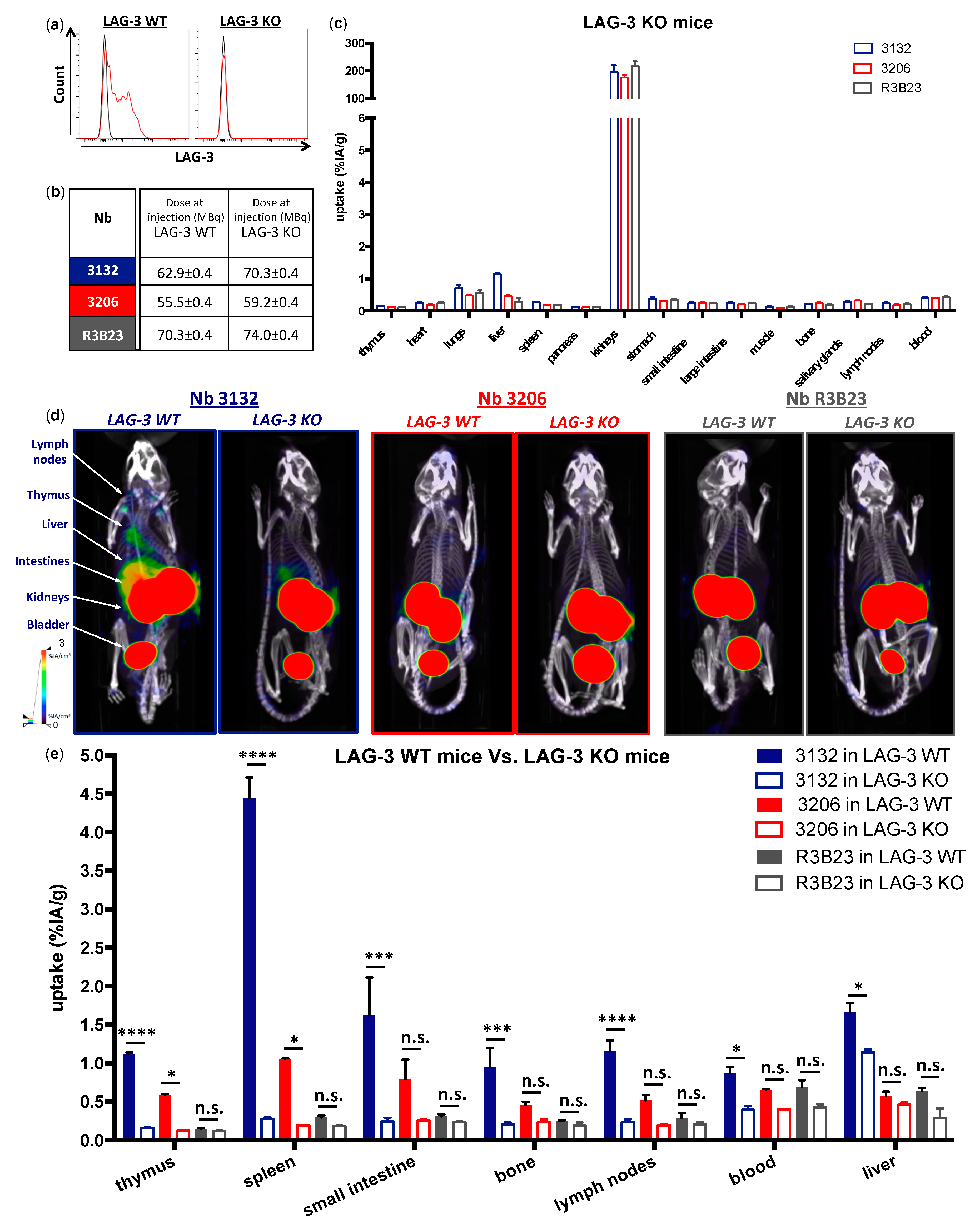
We showed that lymphocytes, residing in the lymph nodes of naive and healthy C57BL/6 mice, express low levels of moLAG-3. In contrast, lymph node residing antigen presenting cells expressed higher levels of moLAG-3. Within the spleen, the immune cell population (CD45
+) with the highest moLAG-3 expression could be detected on antigen-presenting cells, like dendritic cells and macrophages, and on NK and NKT cells [
4]. Moreover, very low amounts of moLAG-3 are expressed on CD19
+ B cells, and CD4
+ and CD8
+ T cells, which is comparable to the low levels found on lymphocytes isolated from lymph nodes. Nevertheless, out of nine different
99mTc-labeled anti-moLAG-3 Nbs, Nb 3132 was still able to detect and visualize low levels of moLAG-3 expressing immune cells residing in the spleen and lymph nodes. Although the affinity of Nb 3132 for moLAG-3 is rather low, injection of
99mTc-labeled Nb 3206, with a 10-fold higher affinity for moLAG-3, was less able to detect and image moLAG-3 expressing cells in the spleen and lymph nodes. Interestingly, when evaluating our Nbs for binding on moLAG-3 expressing cells using flow cytometry, Nb 3132 showed the highest binding of all Nbs. This observation could mean that recombinant moLAG-3 coated chips for SPR or membrane expressed LAG-3 for flow cytometry analysis are not completely identical and thus could lead to under or overestimation of Nb affinity. Moreover, the affinity of Nbs is only one of the parameters used to predict its potential for imaging purposes. Vaneycken et al. showed that the dissociation rate of Nbs did not correlate with tumor uptake since their Nb with the slowest dissociation rate targeted poorly to tumors, while another Nb, with a fast dissociation rate, targeted very well [
40]. Nevertheless, the specific uptake for
99mTc-labeled anti-moLAG-3 Nb 3132 and 3206 was further validated using LAG-3 KO mice, where no uptake could be detected in organs like spleen and lymph nodes, expect for the liver. However, the uptake of
99mTc-labeled Nb 3132 in the liver of LAG-3 KO was significantly lower compared than in LAG-3 WT mice, suggesting that accumulation was partly due to specific binding. To this date, no supportive data could be found in literature describing LAG-3 expression in the liver. Several studies have reported non-specific uptake of radiolabeled Nbs in the liver, explaining the remaining liver uptake in LAG-3 KO mice [
39]. Unfortunately, the nonspecific accumulation in the liver could obscure possible liver metastases and such accumulation should be taken into account when selecting Nbs for the noninvasive imaging of human LAG-3 since these are most likely to reach the clinic compared to moLAG-3 targeting Nbs.
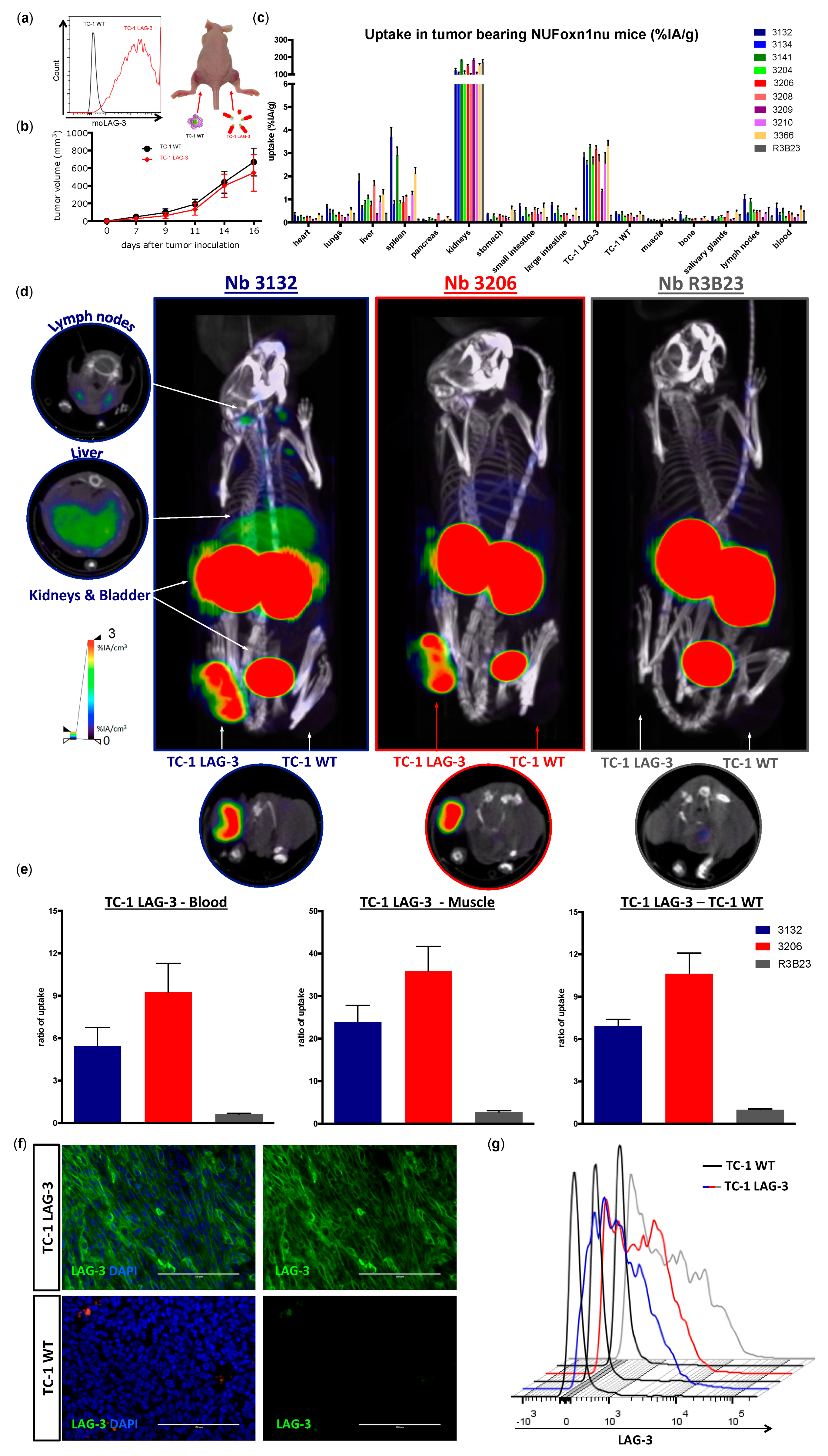
Next, we evaluated the potential of the
99mTc-labeled Nbs to detect moLAG-3 present in dense tissues like tumors. For this purpose, we used an artificial model where mice, harboring a subcutaneous tumor that was lentivirally modified to overexpress moLAG-3, are imaged using our
99mTc-labeled Nbs. Moreover, athymic nude mice were used for these experiments in order to prevent rejection of the lentiviral transformed TC-1 tumors. The uptake of Nb 3132 and 3206 in the moLAG-3 expressing tumors was comparable and with high contrast levels as fast as 1 h after injection. This shows that Nbs are excellent candidate molecules for the visualization of immune checkpoints within the tumor environment, like previously described in Broos et al. using PD-L1 targeting Nbs [
31]. Moreover, in view of repeated imaging, which could be interesting for follow up of the immune checkpoint status during therapy, it is important to note that Nbs are weakly immunogenic because of their high similarity with human variable heavy chain fragments properties (reviewed by [
24]).
These results allow us to further investigate the potential of noninvasive LAG-3 detection within the tumor environment using the selected Nb 3132. Furthermore, we will also continue with the development of human LAG-3 specific Nbs for the translation of this approach to the clinic. Taken together we are confident that small imaging agents, such as the here presented LAG-3 targeting Nbs, could be used as a diagnostic tool to noninvasively evaluate LAG-3 expression before and after immunotherapy and to adapt treatments accordingly.