Monosodium Glutamate (MSG) Renders Alkalinizing Properties and Its Urinary Metabolic Markers of MSG Consumption in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Animals
2.2. Experimental Design
2.3. Urine Analysis
2.4. Sample Preparation for 1H-NMR Spectroscopic Analysis
2.5. 1H-Nuclear Magnetic Resonance Spectroscopic Analysis of Urine
2.6. Data Pre-Processing and Multivariate Statistical Analysis
2.7. Gene Expression Analysis
2.8. Statistical Analysis
3. Results
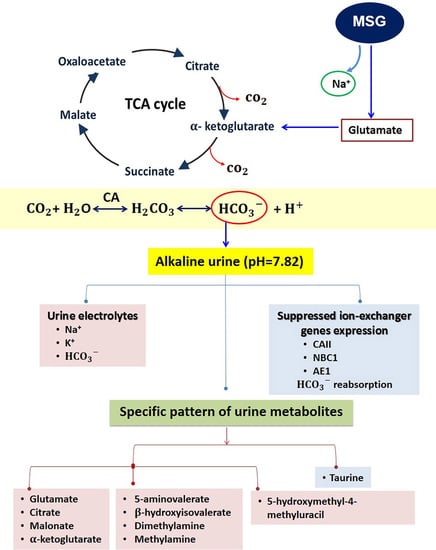
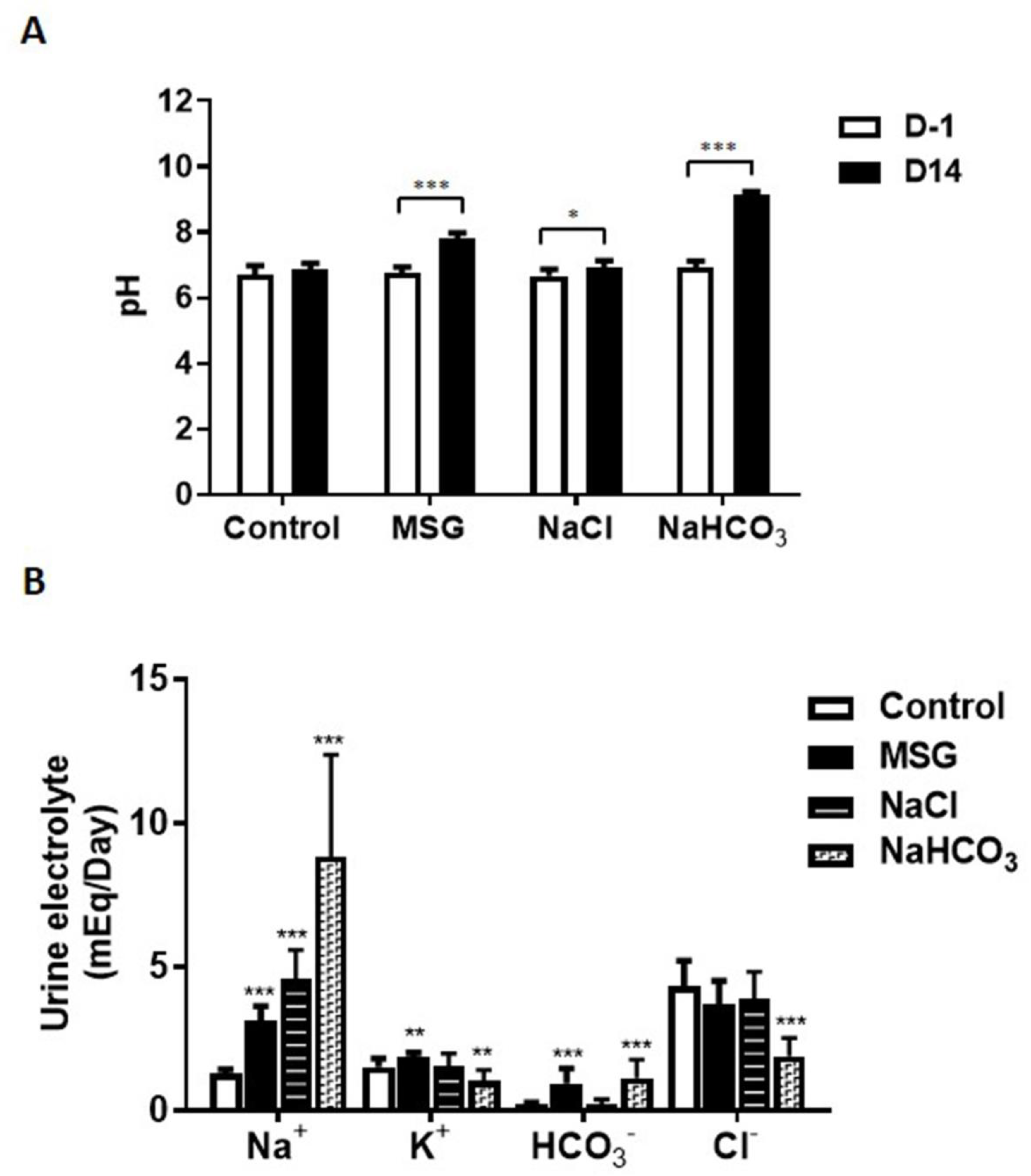
3.1. MSG Exhibits Urine Chemistries Similar to Alkaline Loading
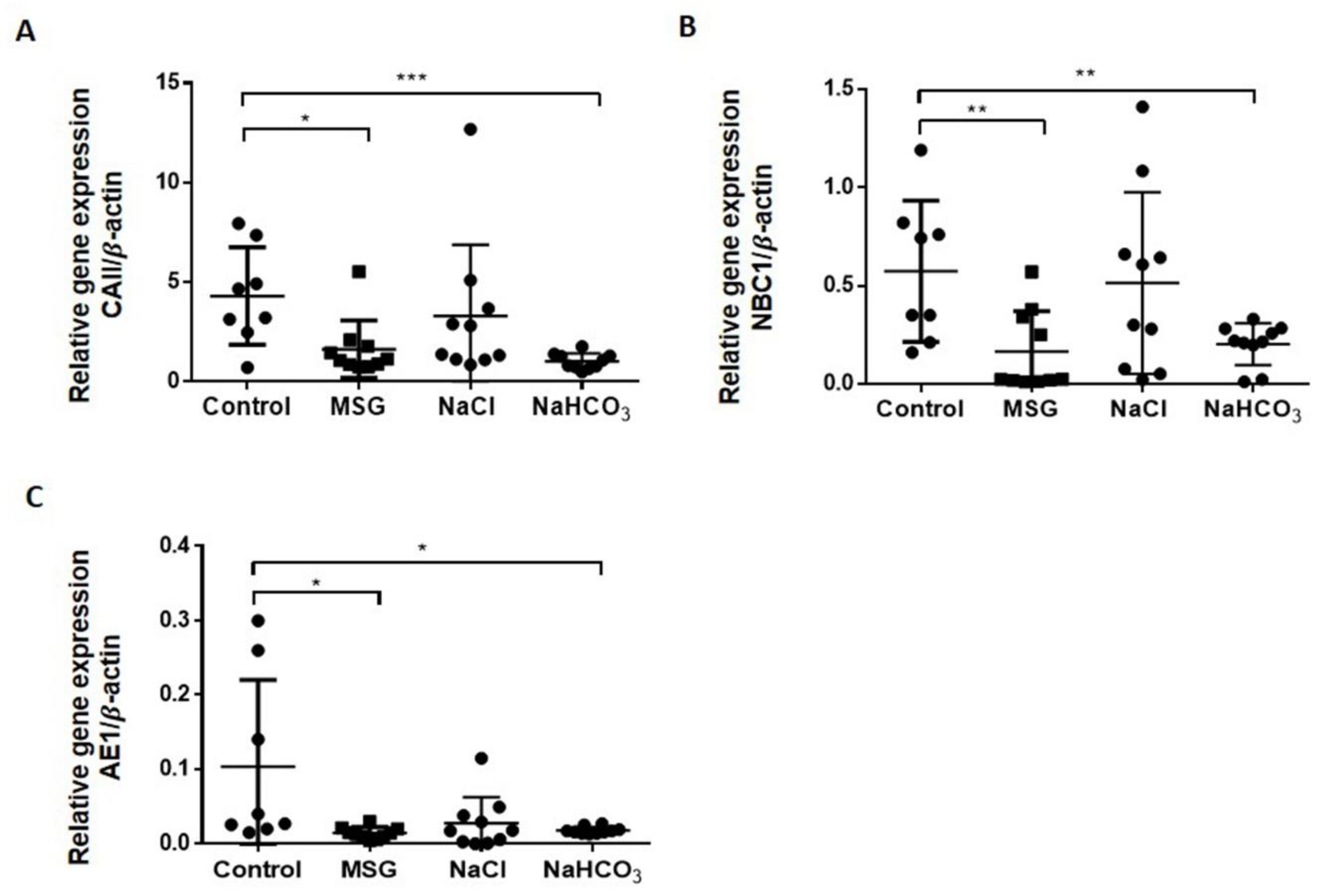
3.2. MSG Suppresses HCO3− Reabsorption Similar to Alkaline Loading
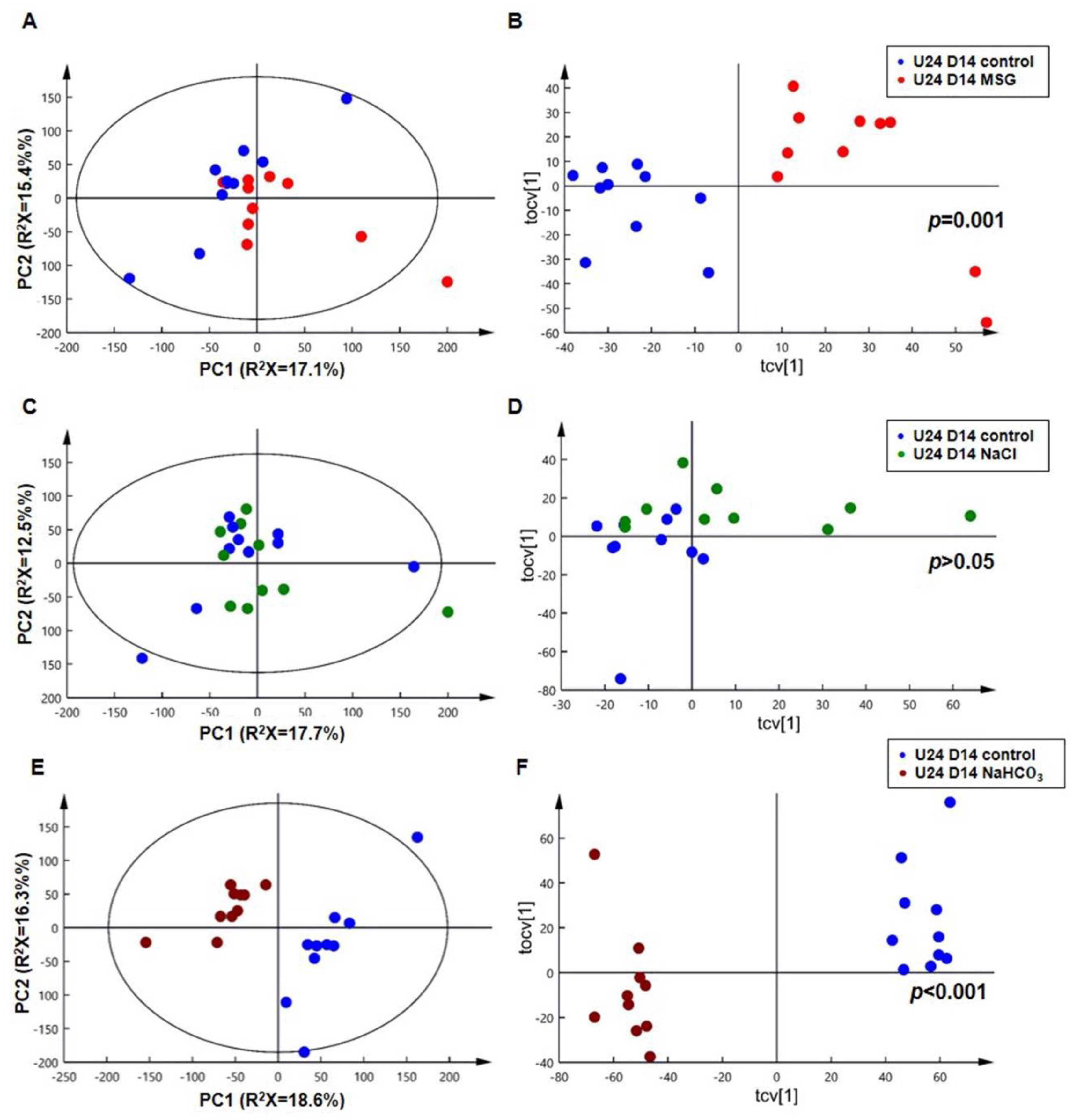
3.3. MSG Shares Common Urinary Metabolic Profile Resembling Alkaline Loading
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beyreuther, K.; Biesalski, H.K.; Fernstrom, J.D.; Grimm, P.; Hammes, W.P.; Heinemann, U.; Kempski, O.; Stehle, P.; Steinhart, H.; Walker, R. Consensus meeting: Monosodium glutamate—An update. Eur. J. Clin. Nutr. 2007, 61, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Lupien, J.R. The safety evaluation of monosodium glutamate. J. Nutr. 2000, 130, 1049S–1052S. [Google Scholar] [CrossRef] [PubMed]
- Insawang, T.; Selmi, C.; Cha’on, U.; Pethlert, S.; Yongvanit, P.; Areejitranusorn, P.; Boonsiri, P.; Khampitak, T.; Tangrassameeprasert, R.; Pinitsoontorn, C.; et al. Monosodium glutamate (MSG) intake is associated with the prevalence of metabolic syndrome in a rural Thai population. Nutr. Metab. (Lond.) 2012, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhao, L.; Daviglus, M.L.; Dyer, A.R.; Van Horn, L.; Garside, D.; Zhu, L.; Guo, D.; Wu, Y.; Zhou, B.; et al. Association of monosodium glutamate intake with overweight in Chinese adults: The INTERMAP Study. Obes. (Silver Spring) 2008, 16, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Du, S.; Xun, P.; Sharma, S.; Wang, H.; Zhai, F.; Popkin, B. Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS). Am. J. Clin. Nutr. 2011, 93, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yuan, B.; Taylor, A.W.; Dai, Y.; Pan, X.; Gill, T.K.; Wittert, G.A. Monosodium glutamate is related to a higher increase in blood pressure over 5 years: Findings from the Jiangsu Nutrition Study of Chinese adults. J. Hypertens. 2011, 29, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Luscombe-Marsh, N.D.; Wittert, G.A.; Yuan, B.; Dai, Y.; Pan, X.; Taylor, A.W. Monosodium glutamate is not associated with obesity or a greater prevalence of weight gain over 5 years: Findings from the Jiangsu Nutrition Study of Chinese adults. Br. J. Nutr. 2010, 104, 457–463. [Google Scholar] [CrossRef]
- Thu Hien, V.T.; Thi Lam, N.; Cong Khan, N.; Wakita, A.; Yamamoto, S. Monosodium glutamate is not associated with overweight in Vietnamese adults. Public Health Nutr. 2013, 16, 922–927. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Bitzer-Quintero, O.K.; Zarate, C.B.; Rodriguez-Reynoso, S.; Larios-Arceo, F.; Velazquez-Brizuela, I.E.; Pacheco-Moises, F.; Rosales-Corral, S.A. Monosodium glutamate-induced damage in liver and kidney: A morphological and biochemical approach. Biomed. Pharmacother. 2006, 60, 86–91. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Tsuneyama, K.; Fujimoto, M.; Salunga, T.L.; Nomoto, K.; An, J.L.; Takano, Y.; Iizuka, S.; Nagata, M.; Suzuki, W.; et al. Monosodium glutamate (MSG): A villain and promoter of liver inflammation and dysplasia. J. Autoimmun. 2008, 30, 42–50. [Google Scholar] [CrossRef]
- Collison, K.S.; Maqbool, Z.; Saleh, S.M.; Inglis, A.; Makhoul, N.J.; Bakheet, R.; Al-Johi, M.; Al-Rabiah, R.; Zaidi, M.Z.; Al-Mohanna, F.A. Effect of dietary monosodium glutamate on trans fat-induced nonalcoholic fatty liver disease. J. Lipid Res. 2009, 50, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, D.; Magami, Y.; Azuma, T.; Inokuchi, H.; Furukawa, M.; Ohyashiki, J.; Yoshimoto, T.; Mizuguchi, J.; Moriyasu, F.; Kawai, K.; et al. Turnover of acinar and islet cells in the pancreas of monosodium glutamate-treated obese mice. Obes. Res. 2003, 11, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Boonnate, P.; Waraasawapati, S.; Hipkaeo, W.; Pethlert, S.; Sharma, A.; Selmi, C.; Prasongwattana, V.; Cha’on, U. Monosodium Glutamate Dietary Consumption Decreases Pancreatic beta-Cell Mass in Adult Wistar Rats. PLoS ONE 2015, 10, e0131595. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Prasongwattana, V.; Cha’on, U.; Selmi, C.; Hipkaeo, W.; Boonnate, P.; Pethlert, S.; Titipungul, T.; Intarawichian, P.; Waraasawapati, S.; et al. Monosodium glutamate (MSG) consumption is associated with urolithiasis and urinary tract obstruction in rats. PLoS ONE 2013, 8, e75546. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.Y.; Fuller, C.; Sakhaee, K.; Preminger, G.M.; Britton, F. Long-term treatment of calcium nephrolithiasis with potassium citrate. J. Urol. 1985, 134, 11–19. [Google Scholar] [CrossRef]
- Mattle, D.; Hess, B. Preventive treatment of nephrolithiasis with alkali citrate—A critical review. Urol. Res. 2005, 33, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Mirrakhimov, A.E.; Ayach, T.; Barbaryan, A.; Talari, G.; Chadha, R.; Gray, A. The Role of Sodium Bicarbonate in the Management of Some Toxic Ingestions. Int. J. Nephrol. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Rouch, J.A.; Burton, B.; Dabb, A.; Brown, V.; Seung, A.H.; Kinsman, K.; Holdhoff, M. Comparison of enteral and parenteral methods of urine alkalinization in patients receiving high-dose methotrexate. J. Oncol. Pharm. Pract. 2017, 23, 3–9. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Fernandez-Fernandez, C.; Mourino-Bayolo, D.; Castro-Quintela, E.; Dominguez-Montero, A. Sodium bicarbonate therapy in patients with metabolic acidosis. Sci. World J. 2014, 2014, 627673. [Google Scholar] [CrossRef]
- Browning, J.J.; Channer, K.S. Hyperkalaemic cardiac arrhythmia caused by potassium citrate mixture. Br. Med. J. (Clin. Res. Ed.) 1981, 283, 1366. [Google Scholar] [CrossRef]
- Wilson, R.G.; Farndon, J.R. Hyperkalaemic cardiac arrhythmia caused by potassium citrate mixture. Br. Med. J. (Clin. Res. Ed.) 1982, 284, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Kim, Y.G.; Yoo, K.H.; Lee, D.G.; Min, G.E.; Lee, H.L. Hyperkalemic cardiac arrhythmia resulting from short-term ingestion of potassium citrate for the management of ureter stones. Urolithiasis 2016, 44, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, O.; Coen, M.; Keun, H.C.; Wang, Y.; Ebbels, T.M.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. High-resolution magic-angle-spinning NMR spectroscopy for metabolic profiling of intact tissues. Nat. Protoc. 2010, 5, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Veselkov, K.A.; Lindon, J.C.; Ebbels, T.M.; Crockford, D.; Volynkin, V.V.; Holmes, E.; Davies, D.B.; Nicholson, J.K. Recursive segment-wise peak alignment of biological (1)h NMR spectra for improved metabolic biomarker recovery. Anal. Chem. 2009, 81, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef]
- Nowik, M.; Lecca, M.R.; Velic, A.; Rehrauer, H.; Brandli, A.W.; Wagner, C.A. Genome-wide gene expression profiling reveals renal genes regulated during metabolic acidosis. Physiol. Genom. 2008, 32, 322–334. [Google Scholar] [CrossRef]
- Wen, C.P.; Hayes, K.C.; Gershoff, S.N. Effects of dietary supplementation of monosodium glutamate on infant monkeys, weanling rats, and suckling mice. Am. J. Clin. Nutr. 1973, 26, 803–813. [Google Scholar] [CrossRef]
- De Groot, A.P.; Feron, V.J.; Immel, H.R. Induction of hyperplasia in the bladder epithelium of rats by a dietary excess of acid or base: Implications for toxicity/carcinogenicity testing. Food Chem. Toxicol. 1988, 26, 425–434. [Google Scholar] [CrossRef]
- Krieger, N.S.; Asplin, J.R.; Frick, K.K.; Granja, I.; Culbertson, C.D.; Ng, A.; Grynpas, M.D.; Bushinsky, D.A. Effect of Potassium Citrate on Calcium Phosphate Stones in a Model of Hypercalciuria. J. Am. Soc. Nephrol. 2015, 26, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Amlal, H.; Chen, Q.; Greeley, T.; Pavelic, L.; Soleimani, M. Coordinated down-regulation of NBC-1 and NHE-3 in sodium and bicarbonate loading. Kidney Int. 2001, 60, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Sabolic, I.; Brown, D.; Gluck, S.L.; Alper, S.L. Regulation of AE1 anion exchanger and H(+)-ATPase in rat cortex by acute metabolic acidosis and alkalosis. Kidney Int. 1997, 51, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Delatour, T.; Douki, T.; Gasparutto, D.; Pouget, J.P.; Ravanat, J.L.; Sauvaigo, S. Hydroxyl radicals and DNA base damage. Mutat. Res. 1999, 424, 9–21. [Google Scholar] [CrossRef]
- Hediger, M.A. Glutamate transporters in kidney and brain. Am. J. Physiol. 1999, 277 Pt 2, F487–F492. [Google Scholar] [CrossRef]
- Brennan, S.; Hering-Smith, K.; Hamm, L.L. Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am. J. Physiol. 1988, 255 Pt 2, F301–F306. [Google Scholar] [CrossRef]
- Kaufman, A.M.; Kahn, T. Complementary role of citrate and bicarbonate excretion in acid-base balance in the rat. Am. J. Physiol. 1988, 255 Pt 2, F182–F187. [Google Scholar] [CrossRef]
- Balagura, S.; Pitts, R.F. Renal Handling of Alpha-Ketoglutarate by the Dog. Am. J. Physiol. 1964, 207, 483–494. [Google Scholar] [CrossRef]
- Balagura, S.; Stone, W.J. Renal tubular secretion of alpha ketoglutarate in dog. Am. J. Physiol. 1967, 212, 1319–1326. [Google Scholar] [CrossRef]
- Cohen, J.J.; Wittmann, E. Renal utilization and excretion of alpha-ketoglutarate in dog: Effect of alkalosis. Am. J. Physiol. 1963, 204, 795–811. [Google Scholar] [CrossRef]
- Ferrier, B.; Martin, M.; Baverel, G. Reabsorption and secretion of alpha-ketoglutarate along the rat nephron: A micropuncture study. Am. J. Physiol. 1985, 248 Pt 2, F404–F412. [Google Scholar] [CrossRef]
- Martin, M.; Ferrier, B.; Baverel, G. Transport and utilization of alpha-ketoglutarate by the rat kidney in vivo. Pflug. Arch. 1989, 413, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.K.; Curry, C.A.; Brown, K.M. Urinary organic anion excretion in response to dietary acid and base loading. J. Am. Soc. Nephrol. 1995, 5, 1624–1629. [Google Scholar] [PubMed]
- Grollman, A.P.; Harrison, H.C.; Harrison, H.E. The renal excretion of citrate. J. Clin. Investig. 1961, 40, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- 3-Hydroxyisovaleric Acid (HMDB0000754). Available online: http://www.hmdb.ca/metabolites/HMDB0000754 (accessed on 3 August 2019).
- Thompson, D.E.; Vivian, V.M. Dietary-induced variations in urinary taurine levels of college women. J. Nutr. 1977, 107, 673–679. [Google Scholar] [CrossRef]
- Bergstrom, J.; Alvestrand, A.; Furst, P.; Lindholm, B. Sulphur amino acids in plasma and muscle in patients with chronic renal failure: Evidence for taurine depletion. J. Intern. Med. 1989, 226, 189–194. [Google Scholar] [CrossRef]
- Chesney, R.W.; Han, X.; Patters, A.B. Taurine and the renal system. J. Biomed. Sci. 2010, 17 (Suppl. 1), S4. [Google Scholar] [CrossRef]
- Paul, M.V.; Abhilash, M.; Varghese, M.V.; Alex, M.; Nair, R.H. Protective effects of alpha-tocopherol against oxidative stress related to nephrotoxicity by monosodium glutamate in rats. Toxicol. Mech. Methods 2012, 22, 625–630. [Google Scholar] [CrossRef]
- Zhu, Y.; Jameson, E.; Parslow, R.A.; Lidbury, I.; Fu, T.; Dafforn, T.R.; Schafer, H.; Chen, Y. Identification and characterization of trimethylamine N-oxide (TMAO) demethylase and TMAO permease in Methylocella silvestris BL2. Environ. Microbiol. 2014, 16, 3318–3330. [Google Scholar] [CrossRef]
- Messenger, J.; Clark, S.; Massick, S.; Bechtel, M. A review of trimethylaminuria: (fish odor syndrome). J. Clin. Aesthet. Dermatol. 2013, 6, 45–48. [Google Scholar]
- Rath, S.; Heidrich, B.; Pieper, D.H.; Vital, M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed]


| Group | Gene | Primer | Oligonucleotide Sequence | Product Size (bp) |
|---|---|---|---|---|
| Internal Control | Beta-actin | Forward | ACAACCTTCTTGCAGCTCCT | 197 |
| Reverse | ACCCATACCCACCATCACAC | |||
| Ion-Exchanger | AE1 | Forward | TCCCGCTACACTCAGGAGAT | 118 |
| Reverse | CAGGGGCATAGCTCTCTTGT | |||
| CAII | Forward | TGCTGGAATGTGTGACCTGG | 101 | |
| Reverse | CTCCCCCTCCGAATTGAAGT | |||
| Na+/K+ ATPase | Forward | CAGCACTCGCTTTCCCTCG | 189 | |
| Reverse | GGCCAGGCAGCCATAGAATA | |||
| H+/K+ ATPase | Forward | CCCCTGAGTACGTGAAGTTCG | 168 | |
| Reverse | CCACAACCACAGCAATGAGTG | |||
| CA IV | Forward | GTCTATGCCCTCAAGCACCA | 114 | |
| Reverse | TTGGGCTCCTTGGCTTGAAT | |||
| NBC1 | Forward | GCTATCCCGGCTTTGCTAGT | 153 | |
| Reverse | GAAGGAGCACACCACCATGA | |||
| NHE3 | Forward | ACTGCTTAATGACGCGGTGA | 160 | |
| Reverse | GAAGGCGAAGATGACACCA | |||
| Rhbg | Forward | TGTCCGCTACAACCACGAAA | 96 | |
| Reverse | TGGAAGCTTGGGTAGCGAAA | |||
| Rhcg | Forward | CTCTTCGGCGTGTTCGTGC | 200 | |
| Reverse | CCTACAGCGCTGAACCCATA | |||
| Pendrin | Forward | AGAACCAGGCCAAATCCAGG | 83 | |
| Reverse | CAAGTCTACGCATGGCCTCA | |||
| H+-ATPase | Forward | TGCCTTCAGTTAGAGAGGCCGTGA | 147 | |
| Reverse | TGCCAAGAAGAGTCTGGGACAAGG | |||
| Glutamate and Glutamine Metabolism | XC-sys | Forward | GCATCGTCCTTTCAAGGTGC | 150 |
| Reverse | AAGAGGTAATACGCCGGGAC | |||
| EAAC1 | Forward | AAACCACGGTGCTCGGTC | 127 | |
| Reverse | ACCGGCGTTTGTGAGGAATC | |||
| Glutaminase | Forward | TGGGCATGATGTGTTGGTCT | 199 | |
| Reverse | TACGCAGCAAACAGGAGGTT | |||
| SNAT3 | Forward | GGAACGGAGTGCTGAACGTG | 83 | |
| Reverse | CTGAAACCACCCCAGAGCAC | |||
| PEPCK | Forward | TGCCATGGCTGCTATGTACC | 89 | |
| Reverse | TTTGGATGCTACGGCATGGT | |||
| TCA Cycle | Citrate synthase | Forward | TGCTACACAGAACCTCAGTTCAC | 243 |
| Reverse | ATCTGACACGTCTTTGCCGA | |||
| Aconitase | Forward | CCTGTACCTGACACTGCTCG | 223 | |
| Reverse | TGTAGTCAGAGGGGTCAGCA | |||
| IDH | Forward | TGCAAAAATATCCCCCGCCT | 144 | |
| Reverse | GCCATCCTTTGGGGTGAAGA |
| Metabolites | Chemical Shift (Multiplicity) | MSG and NaHCO3 Induced Metabolic Changes Compared to Control | The Acid Dissociation Constant (pKa) | |
|---|---|---|---|---|
| (−) Control vs. (+) MSG R2X = 44%, Q2Y = 0.75, p = 0.001 | (−) Control vs. (+) NaHCO3 R2X = 47%, Q2Y = 0.95, p = 0.001 | |||
| 3-carboxy-2-methyl-3-oxopropanamine | 1.08 (d); 2.49 (m); 3.19(m); 3.56 (m); 3.72 (m) | - | (*0.95)(∆3.33 × 10−10) | |
| Beta-hydroxyisovalerate | 1.28 (s) | (*0.77)(∆6.19 × 10−5) | (*0.89)(∆1.83 × 10−7) | pKa1 = 4.55 |
| 5-aminovalerate | 1.68 (m); 2.21 (t); 3.02 (t) | (*0.79)(∆3.35 × 10−5) | (*0.95)(∆1.38 × 10−10) | pKa1 = 4.27, pKa2 = 10.77 |
| 5-hydroxymethyl-4-methyluracil | 1.98 (s); 4.42 (s) | (*0.83)(∆5.84 × 10−6) | (*−0.84)(∆4.15 × 10−6) | pKa1 = 9.87 |
| Glutamate | 2.02 (m); 2.34 (m); 3.76 (m) | (*0.85)(∆2.31 × 10−6) | (*0.88)(∆2.91 × 10−7) | pKa1 = 2.19, pKa2 = 4.25, pKa3 = 9.67 |
| Succinate | 2.41 (s) | - | (*0.87)(∆8.10 × 10−7) | pKa1 = 4.16, pKa2 = 5.61 |
| Alpha-ketoglutarate | 2.44 (t); 3.01 (t) | (*0.75)(∆1.37 × 10−4) | (*0.89)(∆1.46 × 10−7) | pKa1 = 2.47, pKa2 = 4.68 |
| Citrate | 2.54 (d); 2.66 (d) | (*0.87)(∆5.10 × 10−7) | (*0.96)(∆4.63 × 10−11) | pKa1 = 3.14, pKa2 = 4.77, pKa3 = 6.39 |
| Methylamine | 2.61 (s) | (*0.52) (∆0.019) | - | pKa1 = 10.63 |
| Dimethylamine | 2.77 (s) | (*0.71)(∆4.76 × 10−4) | - | pKa1 = 2.36, pKa2 = 10.21 |
| Malonate | 3.11 (s) | (*0.74)(∆2.17 × 10−4) | (*0.97)(∆1.66 × 10−12) | pKa1 = 2.85, pKa2 = 5.70 |
| Choline | 3.21 (s); 3.52 (m); 4.07 (m) | - | (*0.80)(∆2.70 × 10−5) | pKa1 = 13.9 |
| Taurine | 3.25 (t); 3.43 (t) | (*−0.73)(∆2.69 × 10−4) | (*−0.80)(∆2.80 × 10−5) | pKa1 = 1.15, pKa2 = 9.06 |
| Control | MSG | NaCl | NaHCO3 | |
|---|---|---|---|---|
| Urine pH | - | ↑ | - | ↑ |
| Urine Electrolytes (HCO3−) | - | ↑ | - | ↑ |
| Ion exchanger gene expression (HCO3− reabsorption, CAII, NBC1, AE1) | - | ↓ | - | ↓ |
| Urinary metabolic markers Beta-hydroxyisovalerate 5-aminovalerate Glutamate Alpha-ketoglutarate Citrate Malonate | - | ↑ | - | ↑ |
| Taurine | - | ↓ | - | ↓ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahok, K.; Li, J.V.; Phetcharaburanin, J.; Abdul, H.; Wongkham, C.; Thanan, R.; Silsirivanit, A.; Anutrakulchai, S.; Selmi, C.; Cha’on, U. Monosodium Glutamate (MSG) Renders Alkalinizing Properties and Its Urinary Metabolic Markers of MSG Consumption in Rats. Biomolecules 2019, 9, 542. https://doi.org/10.3390/biom9100542
Nahok K, Li JV, Phetcharaburanin J, Abdul H, Wongkham C, Thanan R, Silsirivanit A, Anutrakulchai S, Selmi C, Cha’on U. Monosodium Glutamate (MSG) Renders Alkalinizing Properties and Its Urinary Metabolic Markers of MSG Consumption in Rats. Biomolecules. 2019; 9(10):542. https://doi.org/10.3390/biom9100542
Chicago/Turabian StyleNahok, Kanokwan, Jia V. Li, Jutarop Phetcharaburanin, Hasina Abdul, Chaisiri Wongkham, Raynoo Thanan, Atit Silsirivanit, Sirirat Anutrakulchai, Carlo Selmi, and Ubon Cha’on. 2019. "Monosodium Glutamate (MSG) Renders Alkalinizing Properties and Its Urinary Metabolic Markers of MSG Consumption in Rats" Biomolecules 9, no. 10: 542. https://doi.org/10.3390/biom9100542
APA StyleNahok, K., Li, J. V., Phetcharaburanin, J., Abdul, H., Wongkham, C., Thanan, R., Silsirivanit, A., Anutrakulchai, S., Selmi, C., & Cha’on, U. (2019). Monosodium Glutamate (MSG) Renders Alkalinizing Properties and Its Urinary Metabolic Markers of MSG Consumption in Rats. Biomolecules, 9(10), 542. https://doi.org/10.3390/biom9100542