Combination of Transcriptomic, Proteomic, and Metabolomic Analysis Reveals the Ripening Mechanism of Banana Pulp
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Banana Maturation Characters Determination
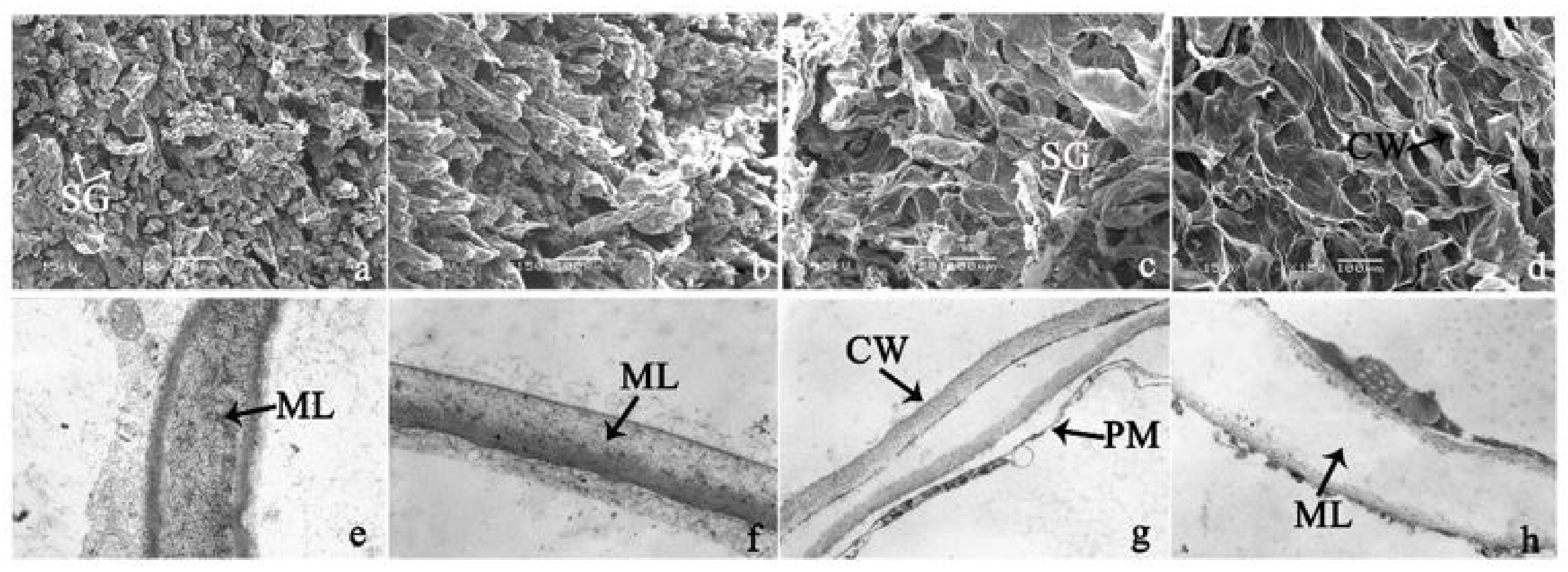
2.3. Scanning Electron Microscopy and Transmission Electron Microscopy Analysis
2.4. RNA Extraction and Digital Gene-Expression Profiling
2.5. Quantitative Real-Time PCR Validation
2.6. Protein Extraction, 2-D Gel Electrophoresis, Gel Staining, and Image Analysis
2.7. In-Gel Tryptic Digestion, MS Analysis, and Database Searching
2.8. Correlation Analysis of Transcriptomics and Proteomics
2.9. Primary Metabolic Profiling
2.10. Least Squares Discriminant Analysis
2.11. Statistical Analysis
3. Results
3.1. Phenotype Change of Ripening Banana Fruit
3.2. Digital Gene-Expression Profiling of Specific Genes of Banana Fruit
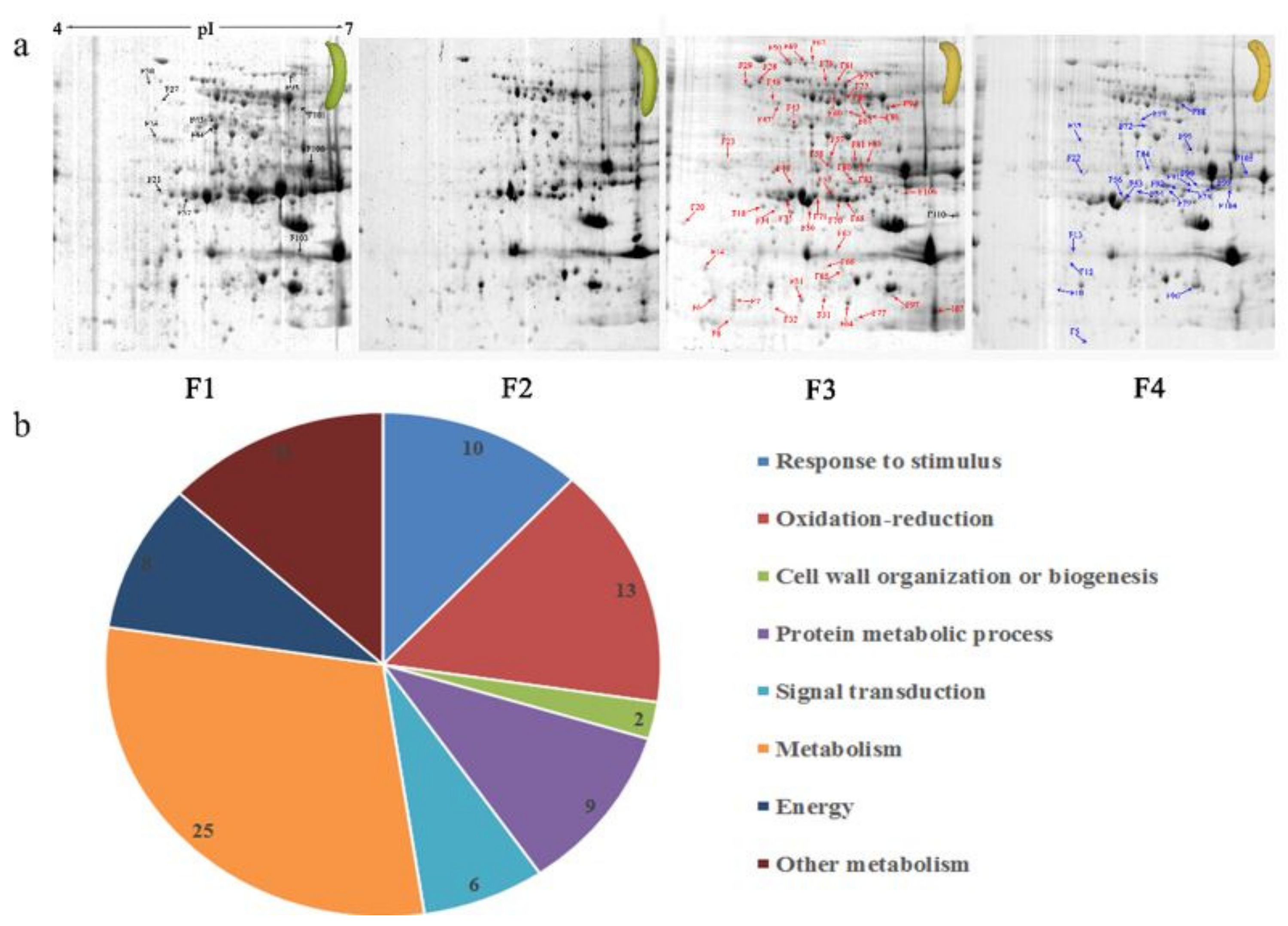
3.3. Two-DE Analysis of Banana Pulp
3.4. Correlation Analysis of Proteomics and Transcriptomics
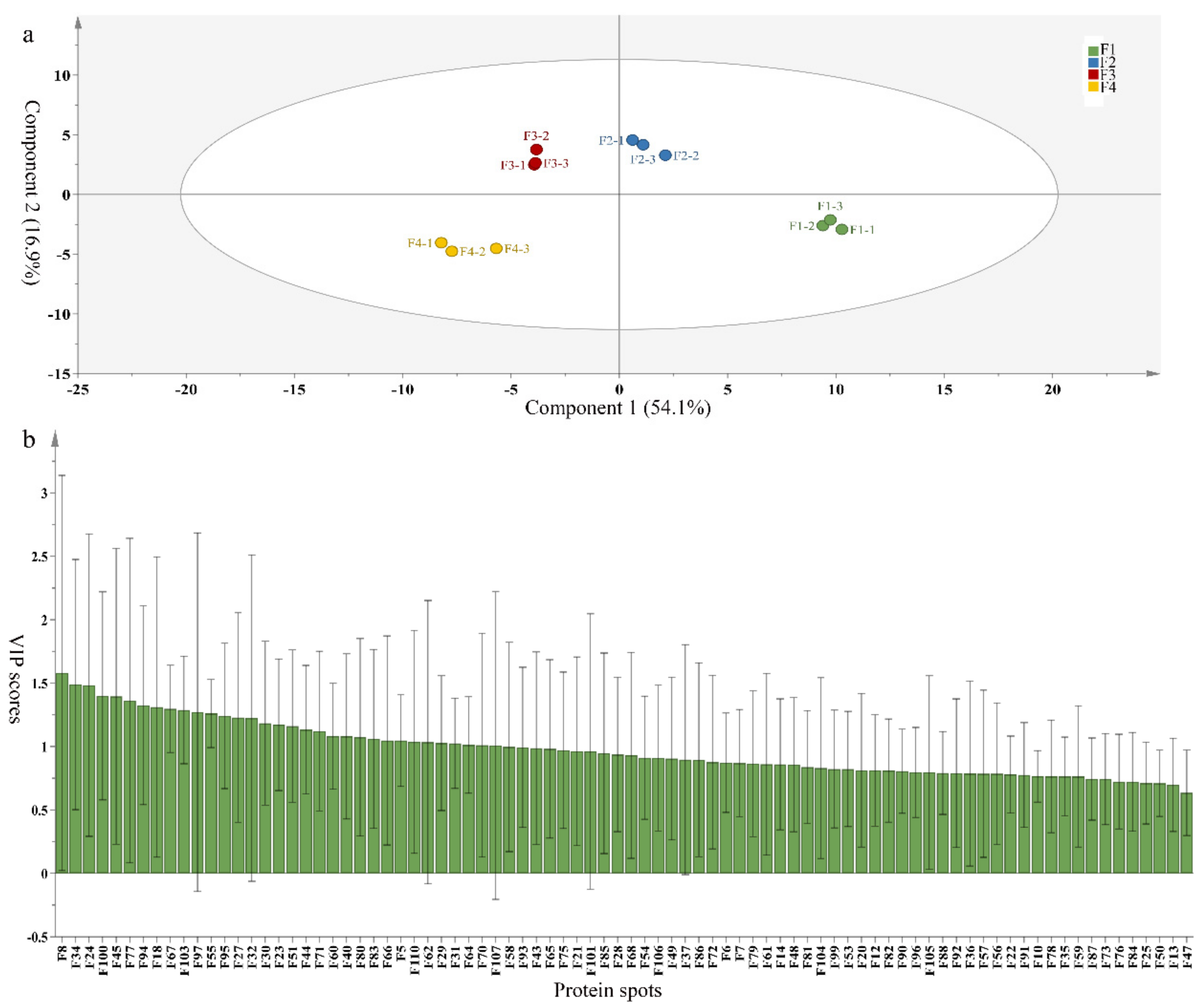
3.5. Partial Least Squares Discriminant Analysis of Differentially Accumulated Proteins
3.6. Differentially Accumulated Primary Metabolites in the Process of Banana pulp Ripening
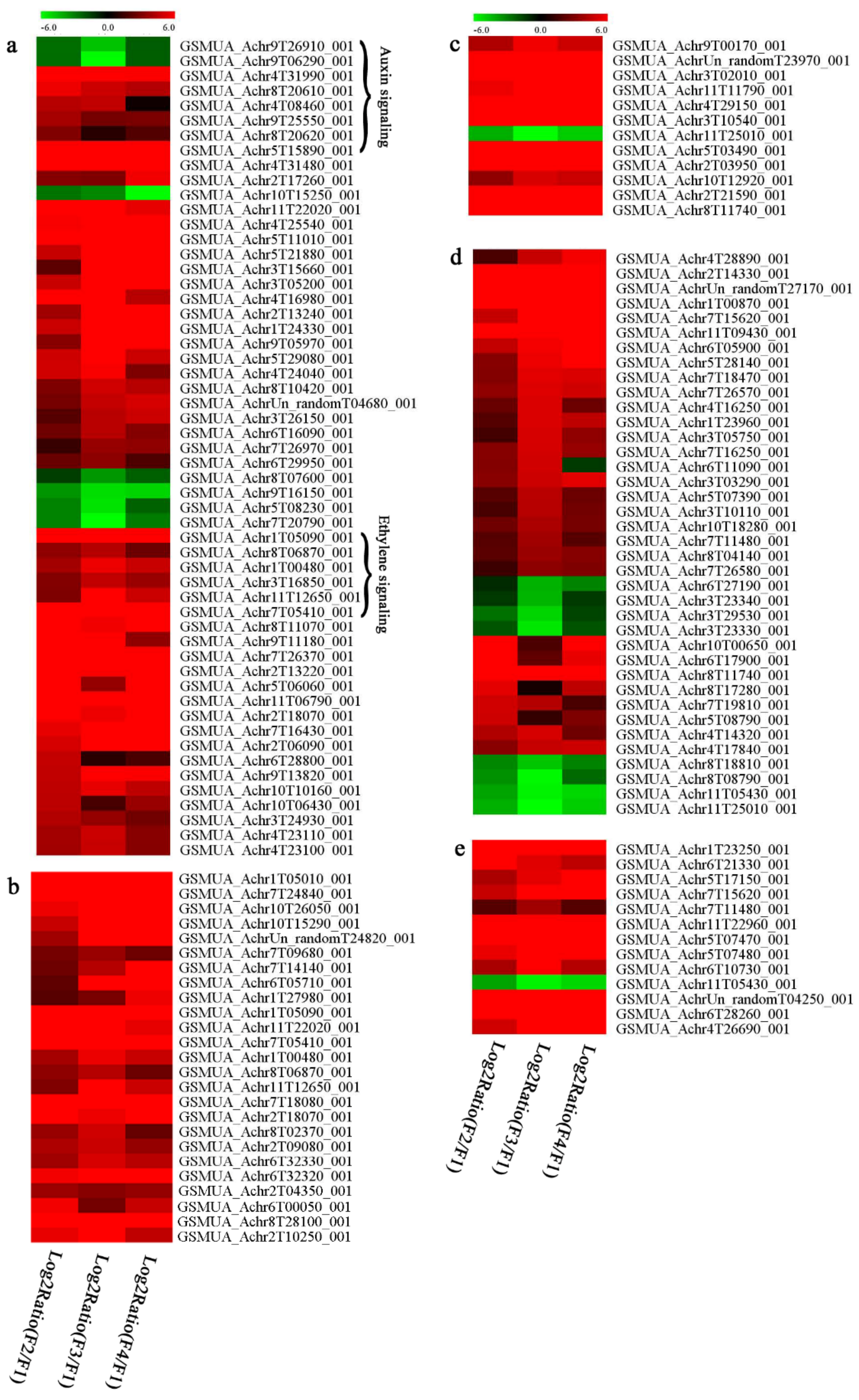
3.7. Differentially Expressed Signal Transduction and Transcriptional Regulation Related Genes and Proteins During Banana Pulp Softening
3.8. Metabolism Related Genes and Proteins were Differentially Expressed During Banana Pulp Softening
3.9. Oxidation-Reduction Process and Protein Metabolism Related Genes and Proteins were Differentially Expressed During Banana Pulp Softening
4. Discussion
4.1. Signal Transduction and Regulation During Banana Pulp Ripening
4.2. Pulp Softening Played a Vital Role During Banana Ripening
4.3. Energy Metabolism and Protein Metabolism were Involved in Banana Pulp Ripening
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agopian, R.G.D.; Peroni-Okita, F.H.G.; Soares, C.A.; Mainardi, J.A.; do Nascimento, J.R.O.; Cordenunsi, B.R.; Lajolo, F.M.; Purgatto, E. Low temperature induced changes in activity and protein levels of the enzymes associated to conversion of starch to sucrose in banana fruit. Postharvest. Biol. Technol. 2011, 62, 133–140. [Google Scholar] [CrossRef]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena—An overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Clendennen, S.K.; May, G.D. Differential gene expression in ripening banana fruit. Plant Physiol. 1997, 115, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Cordenunsi-Lysenko, B.R.; Nascimento, J.R.O.; Castro-Alves, V.C.; Purgatto, E.; Fabi, J.P.; Peroni-Okyta, F.H.G. The Starch Is (Not) Just Another Brick in the Wall: The Primary Metabolism of Sugars During Banana Ripening. Front. Plant Sci. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Elitzur, T.; Yakir, E.; Quansah, L.; Zhangjun, F.; Vrebalov, J.T.; Khayat, E.; Giovannoni, J.J.; Friedman, H. Banana MaMADS transcription factors are necessary for fruit ripening and molecular tools to promote shelf-life and food security. Plant Physiol. 2016, 171, 380. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Huang, S.; Dong, T.; Yang, Q.; Yi, G. Analysis of resistant starch degradation in postharvest ripening of two banana cultivars: Focus on starch structure and amylases. Postharvest. Biol. Technol. 2016, 119, 1–8. [Google Scholar] [CrossRef]
- Han, Y.C.; Kuang, J.F.; Chen, J.Y.; Liu, X.C.; Xiao, Y.Y.; Fu, C.C.; Wang, J.N.; Wu, K.Q.; Lu, W.J. Banana Transcription Factor MaERF11 Recruits Histone Deacetylase MaHDA1 and Represses the Expression of MaACO1 and Expansins during Fruit Ripening. Plant Physiol. 2016, 171, 1070–1084. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Zhao, Y.P.; Yang, J.L.; Lu, F.; Jia, Y.X.; Yang, B. Metabolomic analyses of banana during postharvest senescence by 1H-high resolution-NMR. Food Chem. 2017, 218, 406–412. [Google Scholar] [CrossRef]
- Duan, X.W.; Cheng, G.P.; Yang, E.; Yi, C.; Ruenroengklin, N.; Lu, W.J.; Luo, Y.B.; Jiang, Y.M. Modification of pectin polysaccharides during ripening of postharvest banana fruit. Food Chem. 2008, 111, 144–149. [Google Scholar] [CrossRef]
- Trivedi, P.K.; Nath, P. MaExp1, an ethylene-induced expansin from ripening banana fruit. Plant Sci. 2004, 167, 1351–1358. [Google Scholar] [CrossRef]
- Xiao, Y.Y.; Chen, J.Y.; Kuang, J.F.; Shan, W.; Xie, H.; Jiang, Y.M.; Lu, W.J. Banana ethylene response factors are involved in fruit ripening through their interactions with ethylene biosynthesis genes. J. Exp. Bot. 2013, 64, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.-F.; Chen, J.-Y.; Liu, X.-C.; Han, Y.-C.; Xiao, Y.-Y.; Shan, W.; Tang, Y.; Wu, K.-Q.; He, J.-X.; Lu, W.-J. The transcriptional regulatory network mediated by banana (Musa acuminata) dehydration-responsive element binding (MaDREB) transcription factors in fruit ripening. New Phytol. 2017, 214, 762–781. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.-Q.; Ba, L.-J.; Shan, W.; Xiao, Y.-Y.; Lu, W.-J.; Kuang, J.-F.; Chen, J.-Y. A banana R2R3-MYB transcription factor MaMYB3 is involved in fruit ripening through modulation of starch degradation by repressing starch degradation-related genes and MabHLH6. Plant J. 2018, 96, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wu, F.; Jiang, G.; Xiao, L.; Li, Z.; Duan, X.; Jiang, Y. Genome-wide identification, characterization and expression analysis of NF-Y gene family in relation to fruit ripening in banana. Postharvest. Biol. Technol. 2019, 151, 98–110. [Google Scholar] [CrossRef]
- Shan, W.; Guo, Y.-F.; Wei, W.; Chen, J.-Y.; Lu, W.-J.; Yuan, D.-B.; Su, X.-G.; Kuang, J.-F. Banana MaBZR1/2 associate with MaMPK14 to modulate cell wall modifying genes during fruit ripening. Plant Cell Rep. 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.H.; Lakhwani, D.; Pathak, S.; Gupta, P.; Bag, S.K.; Nath, P.; Trivedi, P.K. Transcriptome analysis of ripe and unripe fruit tissue of banana identifies major metabolic networks involved in fruit ripening process. BMC Plant Biol. 2014, 14, 316. [Google Scholar] [CrossRef]
- Gapper, N.E.; Giovannoni, J.J.; Watkins, C.B. Understanding development and ripening of fruit crops in an ‘omics’ era. Hortic. Res. 2014, 1, 14034. [Google Scholar] [CrossRef] [PubMed]
- Toledo, T.T.; Nogueira, S.B.; Cordenunsi, B.R.; Gozzo, F.C.; Pilau, E.J.; Lajolo, F.M.; do Nascimento, J.R.O. Proteomic analysis of banana fruit reveals proteins that are differentially accumulated during ripening. Postharvest. Biol. Technol. 2012, 70, 51–58. [Google Scholar] [CrossRef]
- D’Hont, A.; Denoeud, F.; Aury, J.M.; Baurens, F.C.; Carreel, F.; Garsmeur, O.; Noel, B.; Bocs, S.; Droc, G.; Rouard, M.; et al. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 2012, 488, 213. [Google Scholar] [CrossRef]
- Yun, Z.; Li, T.; Gao, H.; Zhu, H.; Gupta, V.K.; Jiang, Y.; Duan, X. Integrated Transcriptomic, Proteomic, and Metabolomics Analysis Reveals Peel Ripening of Harvested Banana under Natural Condition. Biomolecules 2019, 9, 167. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, J.Y.; Zhu, H.; Qu, H.X.; You, S.L.; Duan, X.W.; Jiang, Y.M. Proteomic Analysis of Differentially Expressed Proteins Involved in Peel Senescence in Harvested Mandarin Fruit. Front. Plant Sci. 2016, 7, 725. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Jian, Q.J.; Chen, F.; Wang, Y.; Gong, L.; Duan, X.W.; Yang, B.; Jiang, Y.M. Influence of Butylated Hydroxyanisole on the Growth, Hyphal Morphology, and the Biosynthesis of Fumonisins in Fusarium proliferaturn. Front. Microbiol. 2016, 7, 1038. [Google Scholar] [PubMed]
- Mortazavi, A.; Williams, B.A.; Mccue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Shi, D.D.; Wu, Q.X.; Zhang, Z.K.; Qu, H.X.; Jiang, Y.M. Sodium para-aminosalicylate delays pericarp browning of litchi fruit by inhibiting ROS-mediated senescence during postharvest storage. Food Chem. 2019, 278, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Yun, Z.; Zhang, D.D.; Yang, C.W.; Zhu, H.; Jiang, Y.M.; Duan, X.W. Proteomic analysis of differentially expressed proteins involved in ethylene-induced chilling tolerance in harvested banana fruit. Front. Plant Sci. 2015, 6, 845. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Yun, Z.; Gao, H.J.; Liu, P.; Liu, S.Z.; Luo, T.; Jin, S.; Xu, Q.; Xu, J.; Cheng, Y.J.; Deng, X.X. Comparative proteomic and metabolomic profiling of citrus fruit with enhancement of disease resistance by postharvest heat treatment. BMC Plant Biol. 2013, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Yun, Z.; Wu, Q.X.; Zhang, Z.K.; Liu, S.M.; Shi, X.Q.; Duan, X.W.; Jiang, Y.M. Proteomic profiling of 24-epibrassinolide-induced chilling tolerance in harvested banana fruit. J. Proteomics 2018, 187, 1–12. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Roy, S.; Sengupta, D.N. A Ser/Thr protein kinase phosphorylates MA-ACS1 (Musa acuminata 1-aminocyclopropane-1-carboxylic acid synthase 1) during banana fruit ripening. Planta 2012, 236, 491–511. [Google Scholar] [CrossRef]
- Kitagawa, M.; Ito, H.; Shiina, T.; Nakamura, N.; Inakuma, T.; Kasumi, T.; Ishiguro, Y.; Yabe, K.; Ito, Y. Characterization of tomato fruit ripening and analysis of gene expression in F1 hybrids of the ripening inhibitor (rin) mutant. Physiol. Plantarum 2005, 123, 331–338. [Google Scholar] [CrossRef]
- Elitzur, T.; Vrebalov, J.; Giovannoni, J.J.; Goldschmidt, E.E.; Friedman, H. The regulation of MADS-box gene expression during ripening of banana and their regulatory interaction with ethylene. J. Exp. Bot. 2010, 61, 1523–1535. [Google Scholar] [CrossRef]
- Hagen, G. Auxin signal transduction. Essays Biochem. 2015, 58, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zeng, W.F.; Niu, L.; Lu, Z.H.; Liu, H.; Cui, G.C.; Zhu, Y.Q.; Chu, J.F.; Li, W.P.; Fang, W.C.; et al. PpYUC11, a strong candidate gene for the stony hard phenotype in peach (Prunus persica L. Batsch), participates in IAA biosynthesis during fruit ripening. J. Exp. Bot. 2015, 66, 7031–7044. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Ding, Y.D.; Chang, J.W.; Sun, X.H.; Zhang, L.; Wei, Q.J.; Cheng, Y.J.; Chen, L.L.; Xu, J.; Deng, X.X. Comprehensive insights on how 2,4-dichlorophenoxyacetic acid retards senescence in post-harvest citrus fruits using transcriptomic and proteomic approaches. J. Exp. Bot. 2014, 65, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, L.; Jiang, Y.; Chen, J. Changes in Metabolisms of Antioxidant and Cell Wall in Three Pummelo Cultivars during Postharvest Storage. Biomolecules 2019, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Goulao, L.F.; Oliveira, C.M. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Miedes, E.; Suslov, D.; Vandenbussche, F.; Kenobi, K.; Ivakov, A.; Van Der Straeten, D.; Lorences, E.P.; Mellerowicz, E.J.; Verbelen, J.P.; Vissenberg, K. Xyloglucan endotransglucosylase/hydrolase (XTH) overexpression affects growth and cell wall mechanics in etiolated Arabidopsis hypocotyls. J. Exp. Bot. 2013, 64, 2481–2497. [Google Scholar] [CrossRef]
- Fan, Z.; Kuang, J.; Fu, C.; Shan, W.; Han, Y.; Xiao, Y.; Ye, Y.; Lu, W.; Lakshmanan, P.; Duan, X. The Banana Transcriptional Repressor MaDEAR1 Negatively Regulates Cell Wall-Modifying Genes Involved in Fruit Ripening. Front. Plant Sci. 2016, 7, 1021. [Google Scholar] [CrossRef] [PubMed]
- Legay, S.; Guerriero, G.; Deleruelle, A.; Lateur, M.; Evers, D.; Andre, C.M.; Hausman, J.F. Apple russeting as seen through the RNA-seq lens: Strong alterations in the exocarp cell wall. Plant Mol. Biol. 2015, 88, 21–40. [Google Scholar] [CrossRef]
- Catala, C.; Rose, J.K.C.; Bennett, A.B. Auxin-regulated genes encoding cell wall-modifying proteins are expressed during early tomato fruit growth. Plant Physiol. 2000, 122, 527–534. [Google Scholar] [CrossRef]
- Ding, S.Y.; Liu, Y.S.; Zeng, Y.N.; Himmel, M.E.; Baker, J.O.; Bayer, E.A. How Does Plant Cell Wall Nanoscale Architecture Correlate with Enzymatic Digestibility? Science 2012, 338, 1055–1060. [Google Scholar] [CrossRef]
- Su, W.-L.; Liu, N.; Mei, L.; Luo, J.; Zhu, Y.-J.; Liang, Z. Global Transcriptomic Profile Analysis of Genes Involved in Lignin Biosynthesis and Accumulation Induced by Boron Deficiency in Poplar Roots. Biomolecules 2019, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Deluc, L.; Barrieu, F.; Marchive, C.; Lauvergeat, V.; Decendit, A.; Richard, T.; Carde, J.P.; Merillon, J.M.; Hamdi, S. Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol. 2006, 140, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Matthews, M.A.; Shackel, K.A. Seasonal pattern of apoplastic solute accumulation and loss of cell turgor during ripening of Vitis vinifera fruit under field conditions. J. Exp. Bot. 2009, 60, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Mut, P.; Bustamante, C.; Martinez, G.; Alleva, K.; Sutka, M.; Civello, M.; Amodeo, G. A fruit-specific plasma membrane aquaporin subtype PIP1;1 is regulated during strawberry (Fragaria x ananassa) fruit ripening. Physiol. Plant. 2008, 132, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Alleva, K.; Marquez, M.; Villarreal, N.; Mut, P.; Bustamante, C.; Bellati, J.; Martínez, G.; Civello, M.; Amodeo, G. Cloning, functional characterization, and co-expression studies of a novel aquaporin (FaPIP2;1) of strawberry fruit. J. Exp. Bot. 2010, 61, 3935. [Google Scholar] [CrossRef] [PubMed]
- He, W.D.; Gao, J.; Dou, T.X.; Shao, X.H.; Bi, F.C.; Sheng, O.; Deng, G.M.; Li, C.Y.; Hu, C.H.; Liu, J.H. Early Cold-Induced Peroxidases and Aquaporins Are Associated with High Cold Tolerance in Dajiao (Musa spp. ‘Dajiao’). Front. Plant Sci. 2018, 9, 282. [Google Scholar] [CrossRef]
- Mainardi, J.A.; Purgatto, E.; Junior, A.V.; Bastos, W.A.; Cordenunsi, B.R.; Do Nascimento, J.R.O.; Lajolo, F.M. Effects of ethylene and 1-methylcyclopropene (1-MCP) on gene expression and activity profile of alpha-1,4-glucan-phosphorylase during banana ripening. J. Agric. Food Chem. 2006, 54, 7294–7299. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, C.; Wu, W.; Li, X.; Zhang, C.; Fang, J. Enzyme activities and gene expression of starch metabolism provide insights into grape berry development. Hortic. Res. 2017, 4, 17018. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.J.; Wang, X.; Yu, O.; Tang, J.J.; Gu, X.G.; Wan, X.C.; Fang, C.B. Metabolic profiling of strawberry (Fragariaxananassa Duch.) during fruit development and maturation. J. Exp. Bot. 2011, 62, 1103–1118. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, J.; Jiang, Y.; Zeng, J.; Zhou, X.; Hua, Y.; Yang, B. Morin as a Preservative for Delaying Senescence of Banana. Biomolecules 2018, 8, 52. [Google Scholar] [CrossRef]
- D’Ambrosio, C.; Arena, S.; Rocco, M.; Verrillo, F.; Novi, G.; Viscosi, V.; Marra, M.; Scaloni, A. Proteomic analysis of apricot fruit during ripening. J. Proteom. 2013, 78, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Wang, W.H.; Cai, J.H.; Zhang, Y.R.; Qin, G.Z.; Tian, S.P. Tomato nuclear proteome reveals the involvement of specific E2 ubiquitin-conjugating enzymes in fruit ripening. Genome Biol. 2014, 15, 548. [Google Scholar] [CrossRef] [PubMed]
- Rouhier, N.; Lemaire, S.D.; Jacquot, J.P. The role of glutathione in photosynthetic organisms: Emerging functions for glutaredoxins and glutathionylation. Annu. Rev. Plant Biol. 2008, 59, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Dergousova, E.A.; Petrushanko, I.Y.; Klimanova, E.A.; Mitkevich, V.A.; Ziganshin, R.H.; Lopina, O.D.; Makarov, A.A. Effect of Reduction of Redox Modifications of Cys-Residues in the Na,K-ATPase α1-Subunit on Its Activity. Biomolecules 2017, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Faurobert, M.; Mihr, C.; Bertin, N.; Pawlowski, T.; Negroni, L.; Sommerer, N.; Causse, M. Major proteome variations associated with cherry tomato pericarp development and ripening. Plant Physiol. 2007, 143, 1327–1346. [Google Scholar] [CrossRef] [PubMed]

| Sample Name | Protein Accumulation | Protein Name | Protein Mass (kDa) | Isoelectric Point | Pep Count | Protein Score | |
|---|---|---|---|---|---|---|---|
| Response to Stimulus (10) | |||||||
| F6 |  | Glycine cleavage system H protein | 16.89 | 4.99 | 4 | 142 | |
| F31 |  | Whole genome shotgun sequence of line PN40024 | 14.19 | 4.93 | 14 | 270 | |
| F103 |  | Thaumatin-like protein | 20.31 | 4.98 | 6 | 173 | |
| F80 |  | Lichenase | 36.43 | 8.83 | 12 | 386 | |
| F25 | 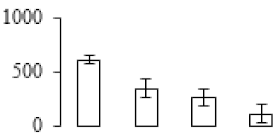 | DNA-damage-repair/toleration protein DRT102 | 45.68 | 7.93 | 11 | 106 | |
| F58 | 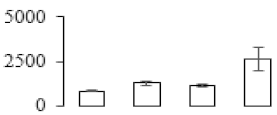 | Cysteine synthase | 34.05 | 5.27 | 16 | 304 | |
| F95 | 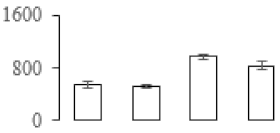 | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase | 61.21 | 6.05 | 11 | 109 | |
| F55 | 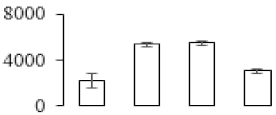 | Triosephosphate isomerase | 33.14 | 7.75 | 19 | 337 | |
| F110 | 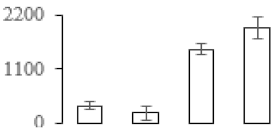 | Cysteine proteinase inhibitor 12 | 23.08 | 6.66 | 10 | 159 | |
| F13 |  | Thaumatin-like protein | 20.31 | 4.98 | 5 | 98.2 | |
| Oxidation-Reduction (13) | |||||||
| F32 | 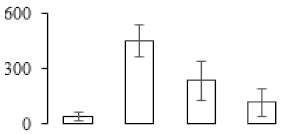 | 1,2-dihydroxy-3-keto-5-methylthiopentene dioxygenase 2 | 23.40 | 4.92 | 7 | 67.3 | |
| F37 | 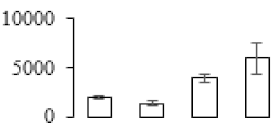 | l-Ascorbate peroxidase | 27.40 | 4.94 | 9 | 102 | |
| F43 |  | Polyphenol oxidase | 62.51 | 6.71 | 19 | 335 | |
| F44 | 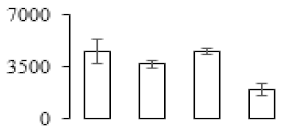 | Polyphenol oxidase | 62.51 | 6.71 | 21 | 430 | |
| F49 | 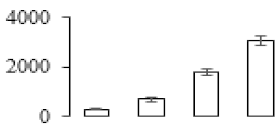 | Heat shock cognate 70 kDa protein | 71.30 | 4.83 | 21 | 216 | |
| F50 | 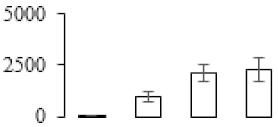 | Heat shock cognate 70 kDa protein | 53.44 | 4.81 | 22 | 146 | |
| F51 | 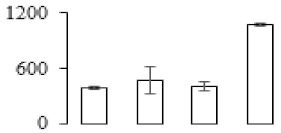 | Heat shock cognate 70 kDa protein | 71.30 | 4.83 | 12 | 59 | |
| F53 | 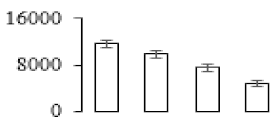 | l-Ascorbate peroxidase | 27.48 | 5.20 | 16 | 464 | |
| F56 | 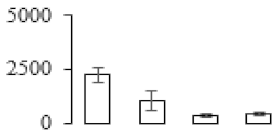 | l-Ascorbate peroxidase | 27.48 | 5.20 | 11 | 73.6 | |
| F57 | 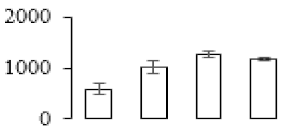 | Enoyl-[acyl-carrier-protein] reductase [NADH] | 40.66 | 8.93 | 9 | 97.5 | |
| F68 |  | l-Ascorbate peroxidase | 27.48 | 5.20 | 8 | 128 | |
| F70 | 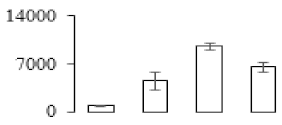 | glutaredoxin | 25.67 | 9.36 | 10 | 63.2 | |
| F45 | 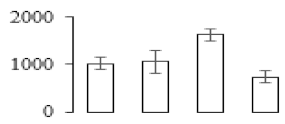 | Succinyl-CoA ligase [GDP-forming] subunit beta | 45.32 | 5.86 | 19 | 170 | |
| Cell Wall Organization or Biogenesis (2) | |||||||
| F47 | 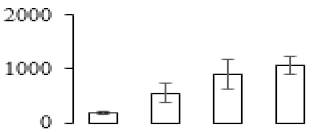 | 3-isopropylmalate dehydrogenase | 107.41 | 6.27 | 17 | 62.8 | |
| F73 | 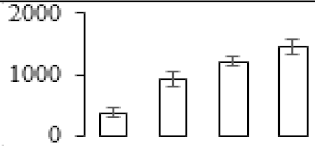 | UTP–glucose-1-phosphate uridylyltransferase | 51.49 | 5.54 | 10 | 125 | |
| Protein Metabolic Process (9) | |||||||
| F48 | 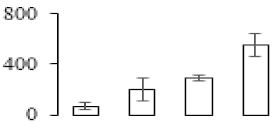 | 26S protease regulatory subunit 6A homolog | 45.53 | 4.95 | 21 | 316 | |
| F106 |  | Proteasome subunit alpha type-6 | 27.53 | 6.26 | 15 | 306 | |
| F107 |  | Proteasome subunit alpha type-6 | 27.53 | 6.26 | 11 | 83.2 | |
| F18 | 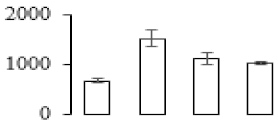 | 20 kDa chaperonin | 27.29 | 7.60 | 7 | 74 | |
| F29 | 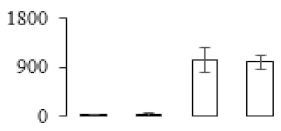 | Protein disulfide-isomerase | 56.62 | 4.51 | 12 | 97.3 | |
| F34 | 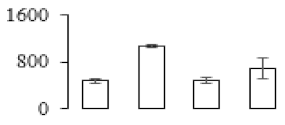 | 20 kDa chaperonin | 27.29 | 7.6 | 11 | 150 | |
| F72 | 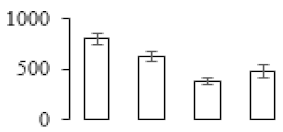 | Intracellular protease 1 | 42.03 | 5.39 | 10 | 206 | |
| F96 |  | 17.2 kDa class II heat shock protein | 17.63 | 6.55 | 6 | 104 | |
| F35 | 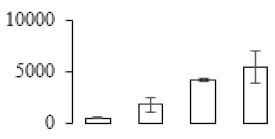 | Cysteine proteinase 2 | 27.95 | 6.29 | 6 | 81.1 | |
| Signal Transduction (6) | |||||||
| F14 | 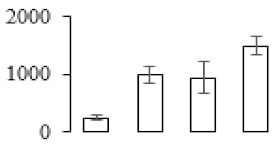 | Ribulose bisphosphate carboxylase/oxygenase activase 1 | 52.07 | 5.37 | 16 | 341 | |
| F20 |  | Ribulose bisphosphate carboxylase/oxygenase activase 1 | 52.07 | 5.37 | 12 | 98.2 | |
| F21 |  | Ribulose bisphosphate carboxylase/oxygenase activase 1 | 52.07 | 5.37 | 14 | 95.8 | |
| F23 |  | Plastid-lipid-associated protein | 34.45 | 5.07 | 14 | 156 | |
| F87 | 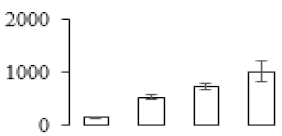 | IAA-amino acid hydrolase ILR1-like 1 | 47.59 | 5.80 | 17 | 122 | |
| F93 |  | Auxin-induced protein PCNT115 | 43.92 | 8.01 | 15 | 157 | |
| Metabolism (25) | |||||||
| F86 |  | S-adenosylmethionine synthase 5 | 43.70 | 5.88 | 21 | 456 | |
| F88 | 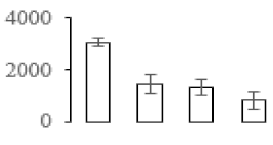 | S-adenosylmethionine synthase 1 | 51.78 | 5.94 | 14 | 68.2 | |
| F22 |  | Glucose-1-phosphate adenylyltransferase large subunit 1 | 52.13 | 7.49 | 10 | 60.1 | |
| F61 | 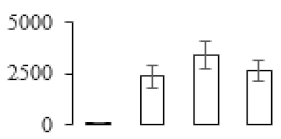 | Granule-bound starch synthase 1 | 68.53 | 7.24 | 32 | 546 | |
| F64 | 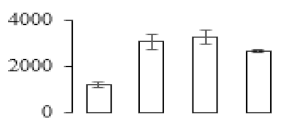 | Granule-bound starch synthase 1 | 68.53 | 7.24 | 17 | 70.2 | |
| F65 | 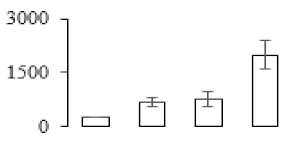 | Granule-bound starch synthase 1 | 68.53 | 7.24 | 14 | 99.6 | |
| F67 | 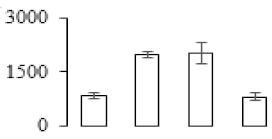 | Granule-bound starch synthase 1 | 68.53 | 7.24 | 13 | 83.1 | |
| F75 | 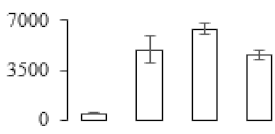 | Granule-bound starch synthase 1 | 68.53 | 7.24 | 21 | 259 | |
| F84 | 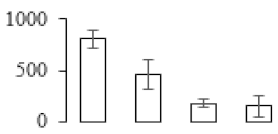 | Thiazole biosynthetic enzyme | 9.61 | 4.24 | 6 | 105 | |
| F85 | 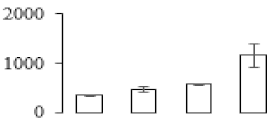 | Phosphoglycerate kinase | 50.16 | 9.23 | 11 | 239 | |
| F76 | 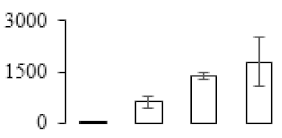 | Putative Acidic endochitinase | 19.57 | 4.86 | 2 | 72.6 | |
| F82 | 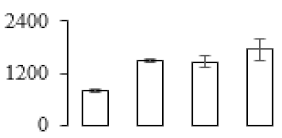 | Putative Acidic endochitinase | 19.57 | 4.86 | 2 | 111 | |
| F94 |  | Putative Acidic endochitinase | 19.57 | 4.86 | 3 | 66.9 | |
| F97 | 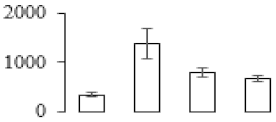 | Putative Acidic endochitinase | 19.57 | 4.86 | 2 | 153 | |
| F10 |  | Putative Acidic endochitinase | 19.57 | 4.86 | 3 | 148 | |
| F104 |  | Putative Acidic endochitinase | 19.57 | 4.86 | 3 | 121 | |
| F54 | 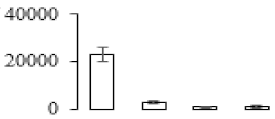 | Putative Acidic endochitinase | 19.57 | 4.86 | 2 | 113 | |
| F78 | 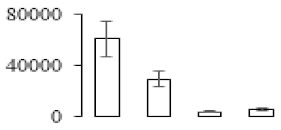 | Putative Acidic endochitinase | 19.57 | 4.86 | 2 | 179 | |
| F79 | 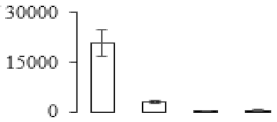 | Putative Acidic endochitinase | 19.57 | 4.86 | 2 | 115 | |
| F90 | 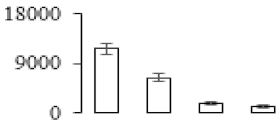 | Putative Acidic endochitinase | 19567.44 | 4.86 | 3 | 98.3 | |
| F91 | 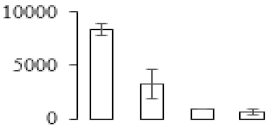 | Putative Acidic endochitinase | 19.57 | 4.86 | 2 | 112 | |
| F92 | 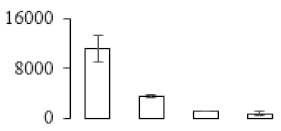 | Putative Acidic endochitinase | 19.57 | 4.86 | 2 | 82.2 | |
| F99 | 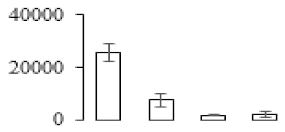 | Putative Acidic endochitinase | 19.57 | 4.86 | 2 | 108 | |
| F100 | 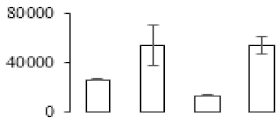 | Endochitinase | 34.14 | 6.67 | 5 | 241 | |
| F83 | 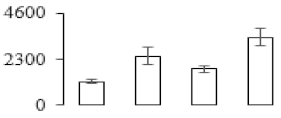 | Endochitinase CH5B | 36.81 | 8.51 | 1 | 116 | |
| Energy (8) | |||||||
| F5 | 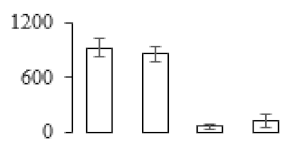 | 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase | 41.13 | 9.35 | 9 | 60.7 | |
| F59 |  | Phosphoglycerate kinase | 42.52 | 5.09 | 6 | 93.5 | |
| F60 | 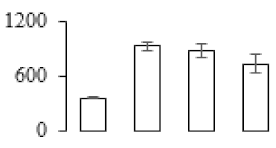 | Enolase | 48.25 | 5.74 | 10 | 128 | |
| F62 | 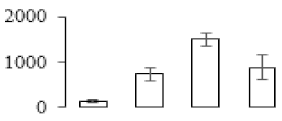 | ATP synthase subunit beta | 59.54 | 6.09 | 11 | 64.8 | |
| F81 | 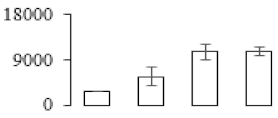 | ATP synthase subunit alpha | 55.80 | 7.02 | 13 | 69.1 | |
| F77 |  | kinesin motor domain containing protein | 146.25 | 7.36 | 20 | 68.6 | |
| F101 | 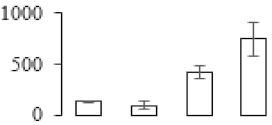 | 6-phosphofructokinase 2 | 51.86 | 6.23 | 17 | 135 | |
| F105 | 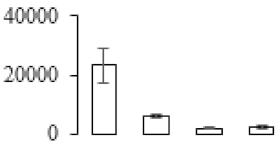 | Pyrophosphate—fructose 6-phosphate 1-phosphotransferase subunit alpha | 68.63 | 7.52 | 13 | 59.6 | |
| Other Metabolism (11) | |||||||
| F28 | 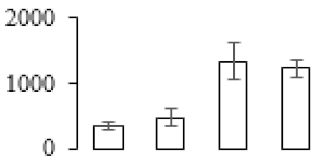 | Putative Vacuolar protein sorting-associated protein 35 | 90.05 | 5.47 | 14 | 61.9 | |
| F36 | 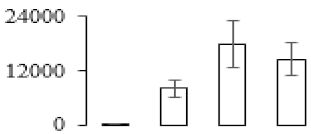 | Germin-like protein 12-1 | 25.23 | 5.99 | 4 | 228 | |
| F40 | 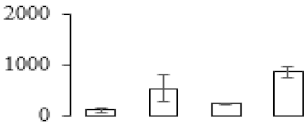 | sugar transporter superfamily | 68.45 | 8.07 | 11 | 60.8 | |
| F66 | 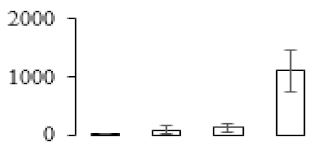 | phospholipid-transporting ATPase 9 | 126.37 | 5.90 | 15 | 59 | |
| F7 | 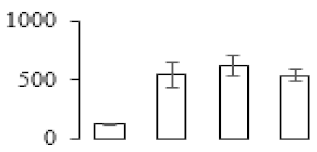 | Pentatricopeptide repeat-containing protein | 45. 40 | 8.03 | 11 | 67.1 | |
| F71 | 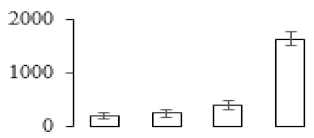 | Pathogen-related protein | 26.60 | 5.40 | 16 | 252 | |
| F8 |  | Glycine-rich RNA-binding protein 2 | 15.49 | 7.51 | 7 | 123 | |
| F12 | 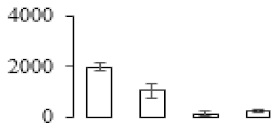 | Putative disease resistance protein RGA1 | 113.61 | 7.93 | 18 | 68.8 | |
| F24 | 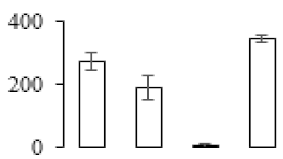 | Sec-independent protein translocase protein tatA/E homolog | 15.12 | 10.11 | 7 | 60.8 | |
| F27 | 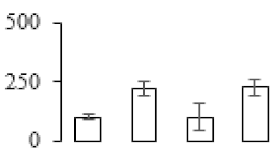 | Putative DNA repair protein RAD23-3 | 43.27 | 4.54 | 9 | 85.2 | |
| F30 |  | Formin-like protein 6 | 23.65 | 7.95 | 9 | 62.6 | |
| Compound Name | Accumulation | RT (min) | ||
|---|---|---|---|---|
| F2/F1 | F3/F1 | F4/F1 | ||
| Sugars | ||||
| Lactulose | 0.00 * | 6.34 | 1.26 | 53.06 |
| α-d-Mannopyranoside | 1.21 | 1.69 * | 3.73 * | 30.69 |
| α-l-Galactopyranose- | 155.74 * | 295.90 * | 680.69 * | 28.63 |
| d-(+)-Turanose | 4.43 * | 5.69 * | 2.68 | 52.36 |
| d-(-)-Fructopyranose | 34.35 * | 83.76 | 174.39 * | 27.11 |
| Ribitol | 1.81 * | 1.71 * | 1.82 * | 23.04 |
| α-d-Glucofuranosyl benzenesulfonate | 0.33 | 1.16 | 0.00* | 8.17 |
| Organic Acids | ||||
| Butanoic acid | 0.80 | 0.68 | 1.47 | 15.97 |
| 10,12-Tricosadiynoic acid | 0.80 | 0.72 | 0.91 | 58.78 |
| Propanoic acid | 2.59 | 0.61 | 1.33 | 55.98 |
| Ethanedioic acid | 0.00* | 0.00* | 1.18 | 4.39 |
| 2-Butenedioic acid | 2.27 | 3.77 | 1.57 | 10.01 |
| Butanedioic acid | 3.82 * | 5.09 | 7.94 * | 14.66 |
| 1,2,3-Propanetricarboxylic acidster | 3.88 | 3.75 | 7.43 | 26.29 |
| Benzenemethano | 2.48 | 1.56 | 1.06 | 4.47 |
| Fatty Acids | ||||
| Hexadecanoic acid | 0.69 | 0.63 | 0.75 | 52.00 |
| Dehydroabietic acid | 0.78 | 0.99 | 2.67 | 49.62 |
| Octadecanoic acid | 0.81 | 0.95 | 1.19 | 46.68 |
| Arachidonic acid | 0.82 | 1.04 | 0.80 * | 18.94 |
| 9,12,15-Octadecatrienoic acid | 2.75 | 2.08* | 1.57 | 48.96 |
| Octadecanoic acid | 1.52 | 0.99* | 1.22 | 53.67 |
| Hexadecanoic acid | 0.69 | 1.14 | 1.88 | 34.71 |
| Alkanes | ||||
| Trisiloxane | 0.65 * | 1.06 * | 1.07 | 9.00 |
| Silane | 0.74 | 0.89 | 1.04 | 17.55 |
| Alcohols | ||||
| 6-Amino-1-hexanol | 0.31 | 0.37 | 1.24 * | 12.47 |
| Myo-Inositol | 2.26 * | 1.95 * | 3.40 * | 36.47 |
| Glycerol | 1.28 * | 1.33 * | 1.35 | 7.62 |
| Isoborneol | 2.95 | 1.11 | 1.45 | 13.92 |
| Borneol | 0.31 | 0.33 | 2.07 | 30.19 |
| D-Pinitol | 1.12 | 1.54 | 1.38 | 27.62 |
| Alkali | ||||
| Benzoylamide | 0.63 | 1.09 | 0.59 | 23.43 |
| 5-Methanesulfonyl | 1.02 | 1.27 | 1.41 | 31.57 |
| Aldehydes | ||||
| 9,12-Octadecadienal | 0.43 | 1.20 | 0.77 | 46.26 |
| Others | ||||
| 4,5-Dihydrobenzo[1,2-c:3,4-c’]bis [1,2,5]oxadiazole-1,6-dioxide | 3.36 | 4.42 | 0.00 | 6.51 |
| 1,3-Benzoxazol | 1.01 | 1.26 * | 0.99 | 16.22 |
| 1-[4-(2,2-Difluoroacetyl)piperazin-1-yl]-2,2-difluoroethanone | 0.00 * | 0.00 * | 0.00 * | 17.39 |
| (Bicyclopentylidene-2-yloxy)trimethylsilane | 0.00 * | 0.00 * | 0.00 * | 29.92 |
| 10-Acetoxy-2-hydroxy | 0.45 | 0.36 | 1.25 | 30.29 |
| 6-Dimethyl(chloromethyl)silyloxypentadecane | 0.94 | 1.75 | 2.78 | 50.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Yun, Z.; Wu, Q.; Qu, H.; Duan, X.; Jiang, Y. Combination of Transcriptomic, Proteomic, and Metabolomic Analysis Reveals the Ripening Mechanism of Banana Pulp. Biomolecules 2019, 9, 523. https://doi.org/10.3390/biom9100523
Li T, Yun Z, Wu Q, Qu H, Duan X, Jiang Y. Combination of Transcriptomic, Proteomic, and Metabolomic Analysis Reveals the Ripening Mechanism of Banana Pulp. Biomolecules. 2019; 9(10):523. https://doi.org/10.3390/biom9100523
Chicago/Turabian StyleLi, Taotao, Ze Yun, Qixian Wu, Hongxia Qu, Xuewu Duan, and Yueming Jiang. 2019. "Combination of Transcriptomic, Proteomic, and Metabolomic Analysis Reveals the Ripening Mechanism of Banana Pulp" Biomolecules 9, no. 10: 523. https://doi.org/10.3390/biom9100523
APA StyleLi, T., Yun, Z., Wu, Q., Qu, H., Duan, X., & Jiang, Y. (2019). Combination of Transcriptomic, Proteomic, and Metabolomic Analysis Reveals the Ripening Mechanism of Banana Pulp. Biomolecules, 9(10), 523. https://doi.org/10.3390/biom9100523








