Bio-Molecular Applications of Recent Developments in Optical Tweezers
Abstract
1. Introduction
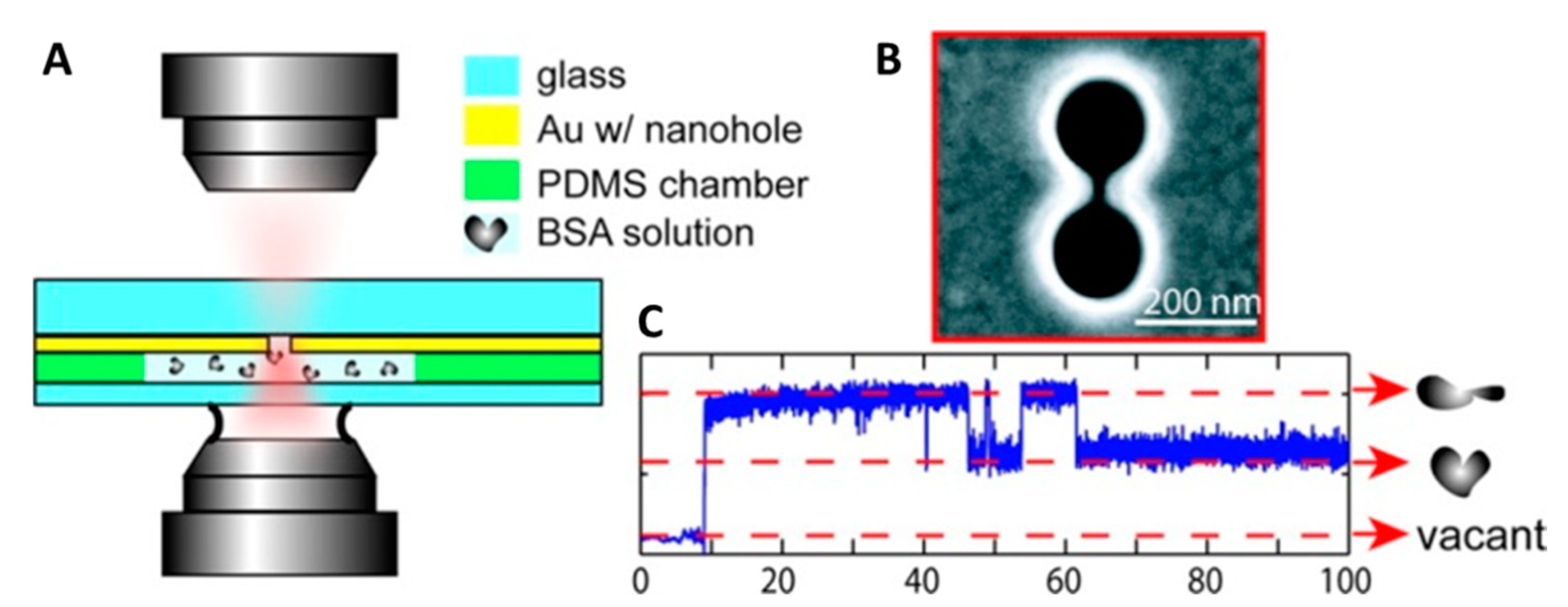
2. Plasmonic Optical Tweezers
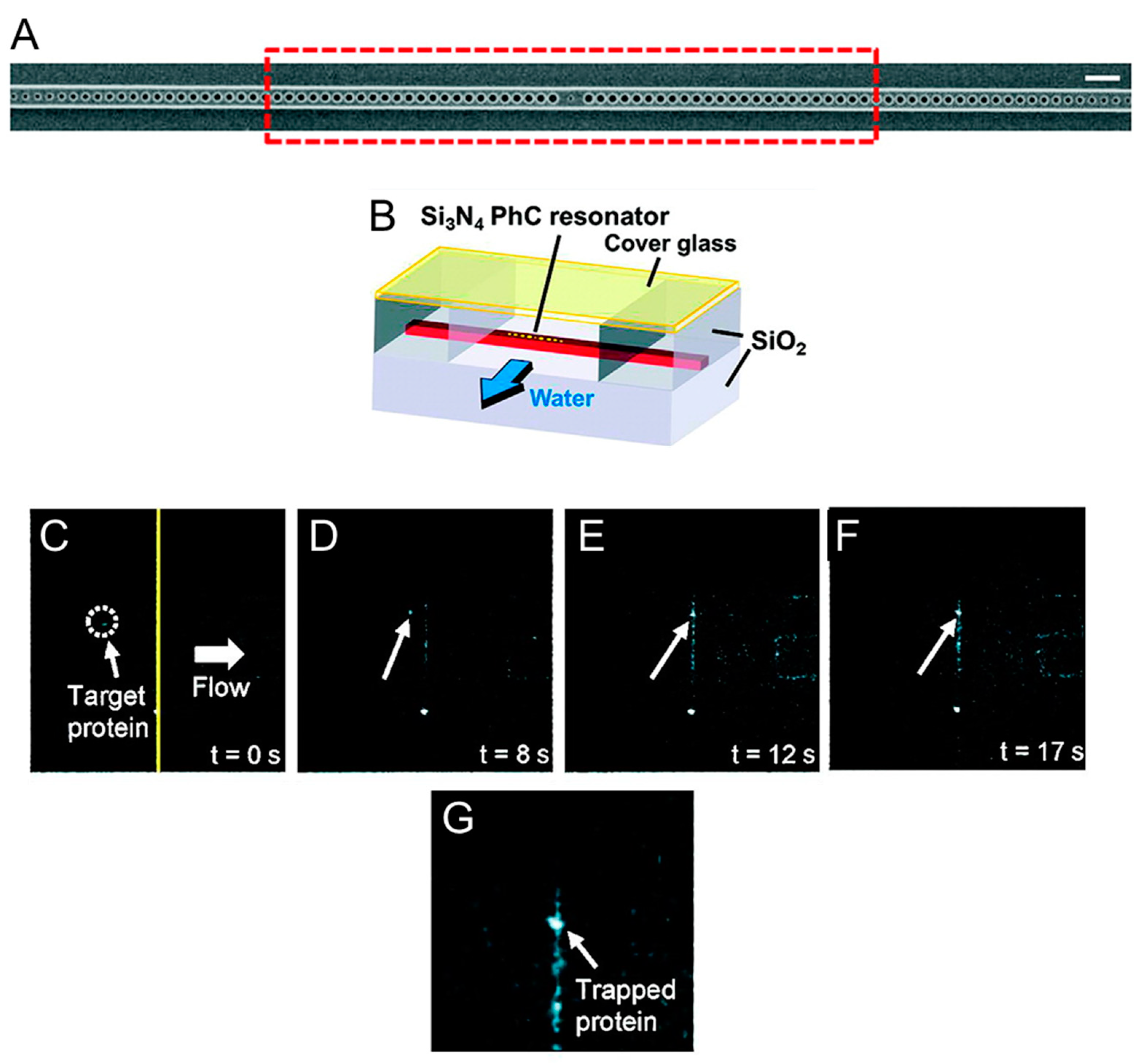
3. Photonic Crystal Optical Tweezers
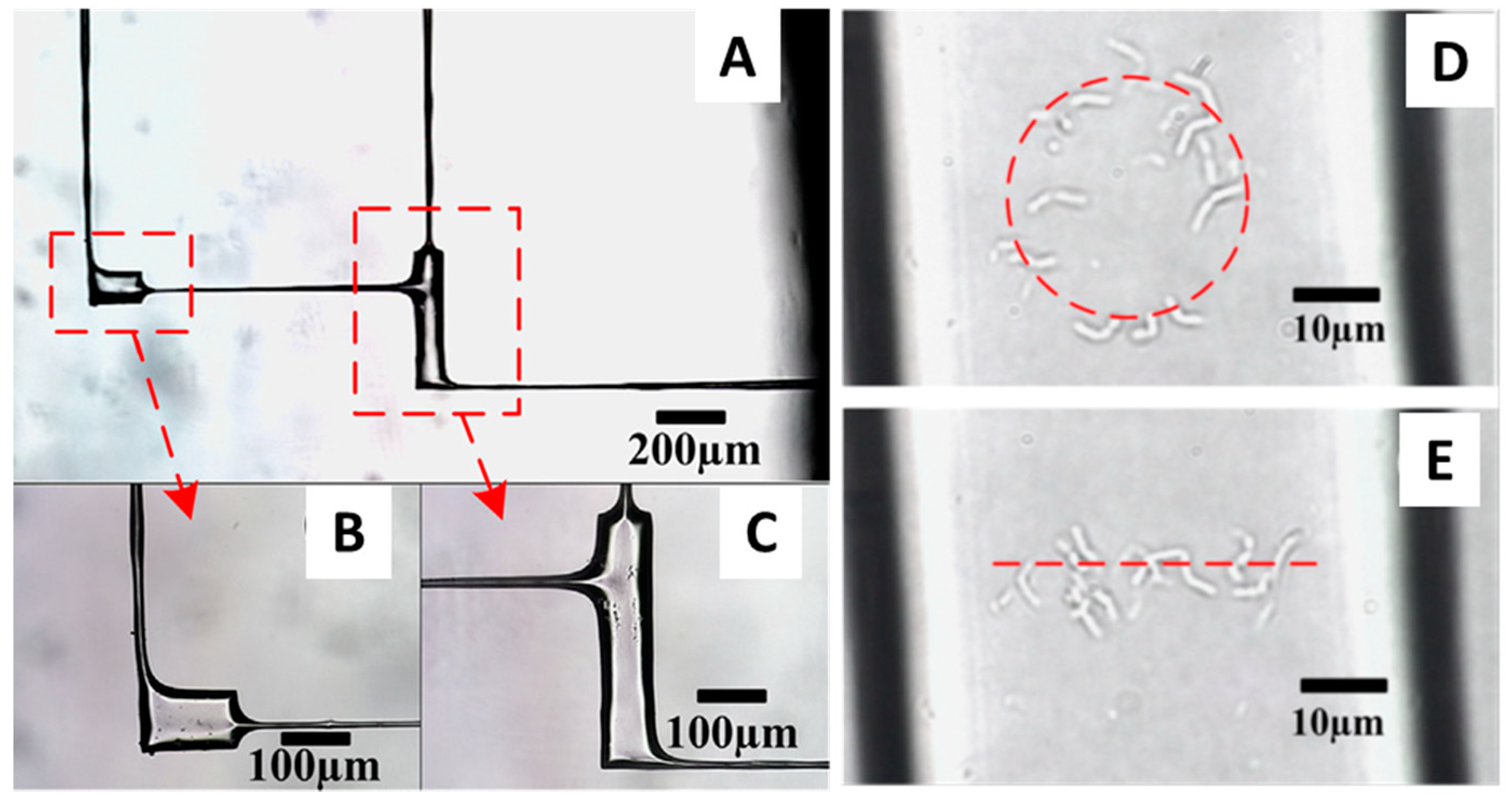
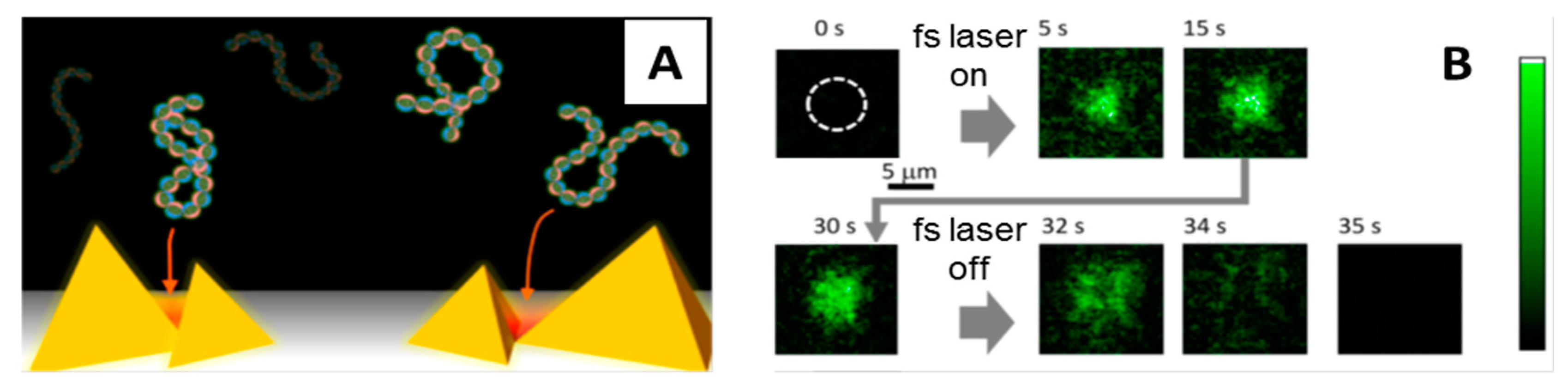
4. Femtosecond Optical Tweezers
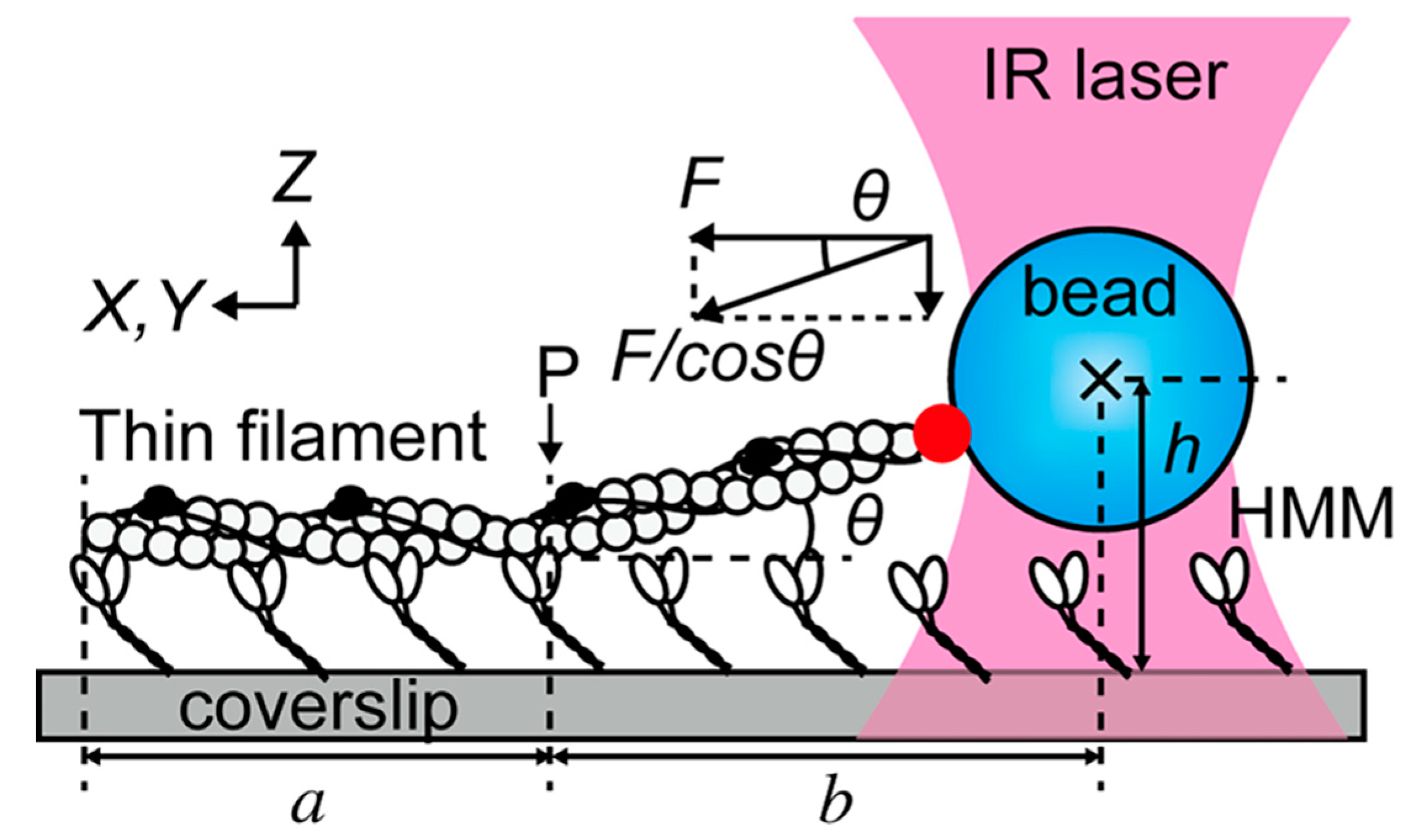
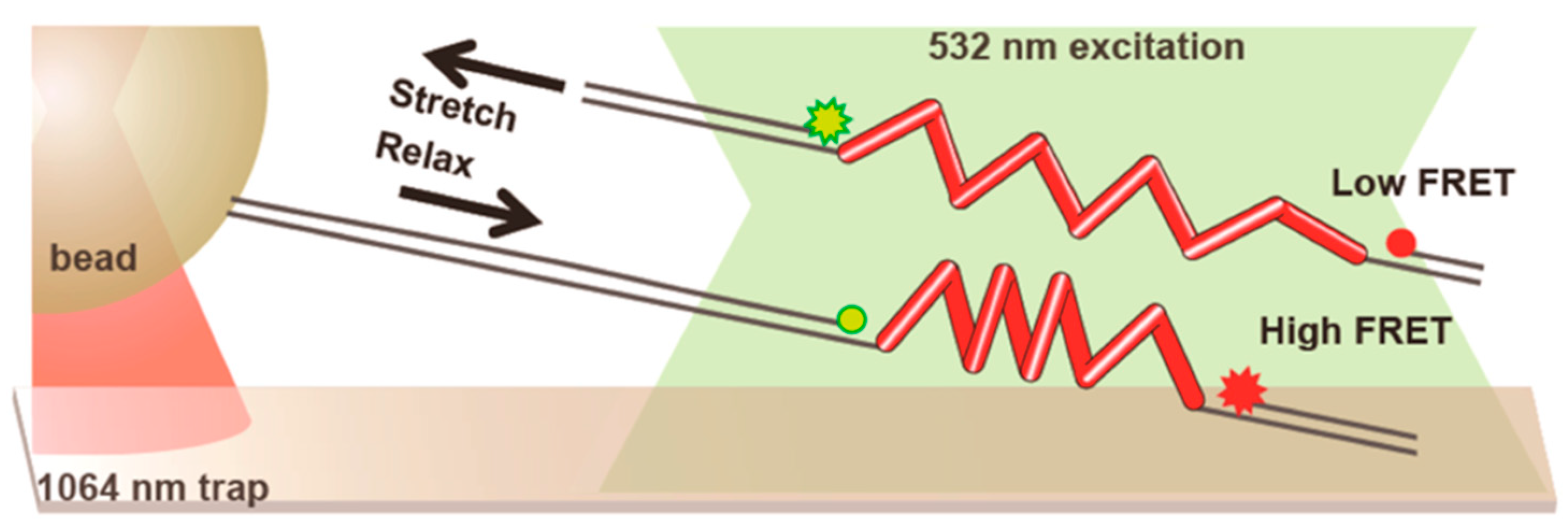
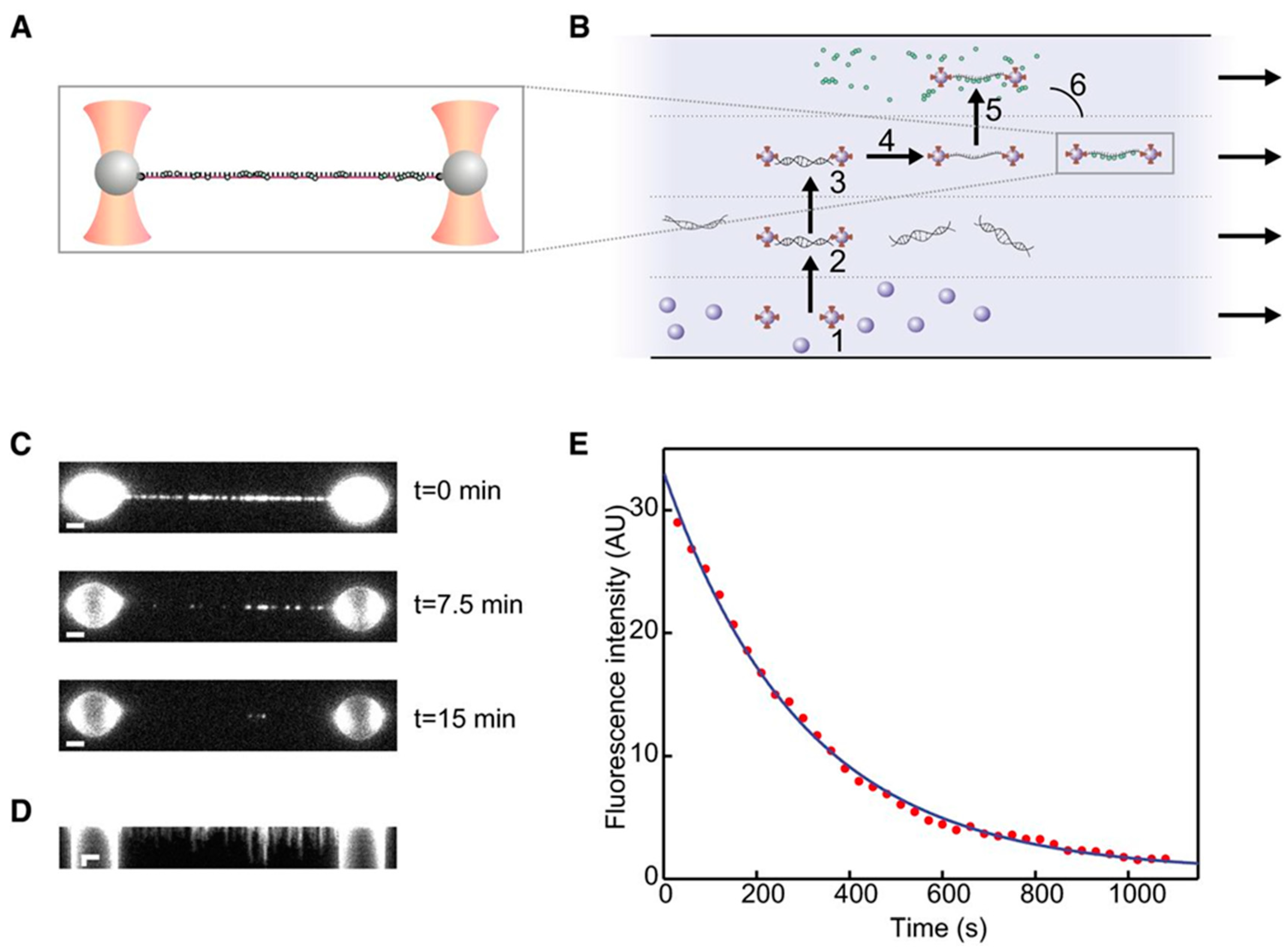
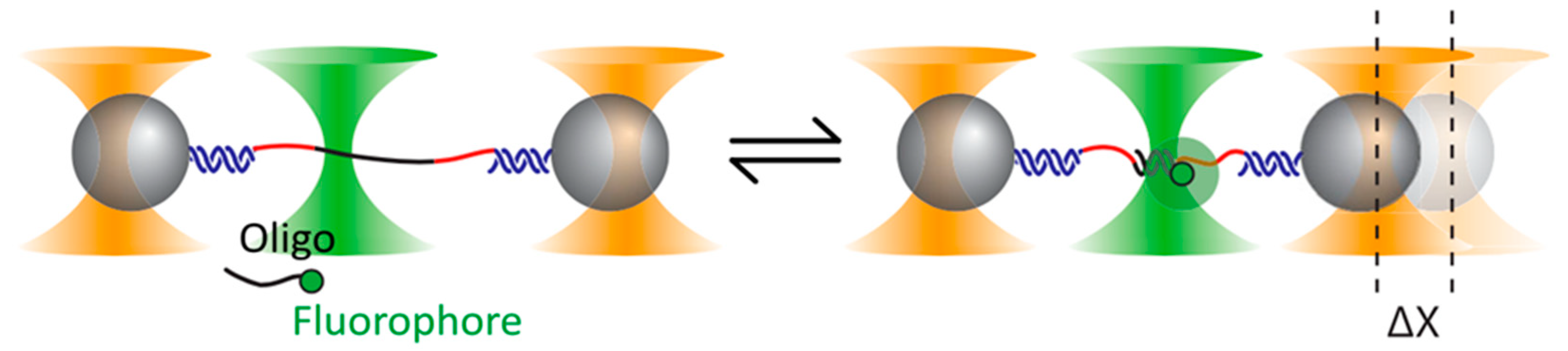
5. Optical Tweezers Combined with Fluorescence
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ashkin, A.; Dziedzic, J.M. Optical trapping and manipulation of viruses and bacteria. Science 1987, 235, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A.; Dziedzic, J.M.; Bjorkholm, J.; Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett. 1986, 11, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A.; Dziedzic, J.M.; Yamane, T. Optical trapping and manipulation of single cells using infrared laser beams. Nature 1987, 330, 769. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, K.; Schmidt, C.F.; Schnapp, B.J.; Block, S.M. Direct observation of kinesin stepping by optical trapping interferometry. Nature 1993, 365, 721–727. [Google Scholar] [CrossRef]
- Kishino, A.; Yanagida, T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature 1988, 334, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Ritchie, K.; Merkel, R. Sensitive force technique to probe molecular adhesion and structural linkages at biological interfaces. Biophys. J. 1995, 68, 2580–2587. [Google Scholar] [CrossRef]
- Smith, S.B.; Finzi, L.; Bustamante, C. Direct mechanical measurements of the elasticity of single DNA-molecules by using magnetic beads. Science 1992, 258, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, M.; Pavone, F.S. Interrogating biology with force: Single molecule high-resolution measurements with optical tweezers. Biophys. J. 2013, 105, 1293–1303. [Google Scholar] [CrossRef]
- Moffitt, J.R.; Chemla, Y.R.; Izhaky, D.; Bustamante, C. Differential detection of dual traps improves the spatial resolution of optical tweezers. Proc. Natl. Acad. Sci. USA 2006, 103, 9006–9011. [Google Scholar] [CrossRef]
- Carter, A.R.; King, G.M.; Ulrich, T.A.; Halsey, W.; Alchenberger, D.; Perkins, T.T. Stabilization of an optical microscope to 0.1 nm in three dimensions. Appl. Opt. 2007, 46, 421–427. [Google Scholar] [CrossRef]
- Chakraborty, A.; Meng, C.A.; Block, S.M. Observing Single RNA Polymerase Molecules Down to Base-Pair Resolution. In Optical Tweezers: Methods and Protocols; Gennerich, A., Ed.; Springer New York: New York, NY, USA, 2017; pp. 391–409. [Google Scholar]
- Seifert, U. Stochastic thermodynamics, fluctuation theorems and molecular machines. Rep. Prog. Phys. 2012, 75, 58. [Google Scholar] [CrossRef] [PubMed]
- Ciliberto, S. Experiments in stochastic thermodynamics: Short history and perspectives. Phys. Rev. X 2017, 7, 26. [Google Scholar] [CrossRef]
- Milic, B.; Andreasson, J.O.L.; Hogan, D.W.; Block, S.M. Intraflagellar transport velocity is governed by the number of active KIF17 and KIF3AB motors and their motility properties under load. Proc. Natl. Acad. Sci. USA 2017, 114, E6830–E6838. [Google Scholar] [CrossRef]
- Brunnbauer, M.; Dombi, R.; Ho, T.H.; Schliwa, M.; Rief, M.; Okten, Z. Torque generation of kinesin motors is governed by the stability of the neck domain. Mol. Cell 2012, 46, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Lisica, A.; Engel, C.; Jahnel, M.; Roldan, E.; Galburt, E.A.; Cramer, P.; Grill, S.W. Mechanisms of backtrack recovery by RNA polymerases I and II. Proc. Natl. Acad. Sci. USA 2016, 113, 2946–2951. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, T.; Lemishko, K.M.; de Lorenzo, S.; Somoza, Á.; Ritort, F.; Pérez, E.M.; Ibarra, B. Dynamics of individual molecular shuttles under mechanical force. Nat. Commun. 2018, 9, 4512. [Google Scholar] [CrossRef]
- Bryant, Z.; Stone, M.D.; Gore, J.; Smith, S.B.; Cozzarelli, N.R.; Bustamante, C. Structural transitions and elasticity from torque measurements on DNA. Nature 2003, 424, 338–341. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, C.Z.; Zhang, X.H.; Springer, T.A. A mechanically stabilized receptor–ligand flex-bond important in the vasculature. Nature 2010, 466, 992. [Google Scholar] [CrossRef]
- Heidarsson, P.O.; Naqvi, M.M.; Otazo, M.R.; Mossa, A.; Kragelund, B.B.; Cecconi, C. Direct single-molecule observation of calcium-dependent misfolding in human neuronal calcium sensor-1. Proc. Natl. Acad. Sci. USA 2014, 111, 13069–13074. [Google Scholar] [CrossRef]
- Alemany, A.; Mossa, A.; Junier, I.; Ritort, F. Experimental free-energy measurements of kinetic molecular states using fluctuation theorems. Nat. Phys. 2012, 8, 688–694. [Google Scholar] [CrossRef]
- Jahn, M.; Tych, K.; Girstmair, H.; Steinmassl, M.; Hugel, T.; Buchner, J.; Rief, M. Folding and domain interactions of three orthologs of Hsp90 studied by single-molecule force spectroscopy. Structure 2018, 26, 96. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, C.; Shank, E.A.; Bustamante, C.; Marqusee, S. Direct observation of the three-state folding of a single protein molecule. Science 2005, 309, 2057–2060. [Google Scholar] [CrossRef] [PubMed]
- Bechtluft, P.; van Leeuwen, R.G.H.; Tyreman, M.; Tomkiewicz, D.; Nouwen, N.; Tepper, H.L.; Driessen, A.J.M.; Tans, S.J. Direct observation of chaperone-induced changes in a protein folding pathway. Science 2007, 318, 1458–1461. [Google Scholar] [CrossRef]
- Gao, Y.; Sirinakis, G.; Zhang, Y.L. Highly anisotropic stability and folding kinetics of a single coiled coil protein under mechanical tension. J. Am. Chem. Soc. 2011, 133, 12749–12757. [Google Scholar] [CrossRef] [PubMed]
- Caldarini, M.; Sonar, P.; Valpapuram, I.; Tavella, D.; Volonte, C.; Pandini, V.; Vanoni, M.A.; Aliverti, A.; Broglia, R.A.; Tiana, G.; et al. The complex folding behavior of HIV-1-protease monomer revealed by optical-tweezer single-molecule experiments and molecular dynamics simulations. Biophys. Chem. 2014, 195, 32–42. [Google Scholar] [CrossRef]
- Smith, S.B.; Cui, Y.J.; Bustamante, C. Overstretching B-DNA: The elastic response of individual double-stranded and single-stranded DNA molecules. Science 1996, 271, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Smith, C.L.; Saha, A.; Grill, S.W.; Mihardja, S.; Smith, S.B.; Cairns, B.R.; Peterson, C.L.; Bustamantel, C. DNA translocation and loop formation mechanism of chromatin remodeling by SWI/SNF and RSC. Mol. Cell 2006, 24, 559–568. [Google Scholar] [CrossRef]
- Mossa, A.; Manosas, M.; Forns, N.; Huguet, J.M.; Ritort, F. Dynamic force spectroscopy of DNA hairpins: I. Force kinetics and free energy landscapes. J. Stat. Mech. Theory Exp. 2009. [Google Scholar] [CrossRef]
- Manosas, M.; Mossa, A.; Forns, N.; Huguet, J.M.; Ritort, F. Dynamic force spectroscopy of DNA hairpins: II. Irreversibility and dissipation. J. Stat. Mech. Theory Exp. 2009. [Google Scholar] [CrossRef]
- Bongini, L.; Melli, L.; Lombardi, V.; Bianco, P. Transient kinetics measured with force steps discriminate between double-stranded DNA elongation and melting and define the reaction energetics. Nucleic Acids Res. 2014, 42, 3436–3449. [Google Scholar] [CrossRef]
- Li, P.T.X.; Bustamante, C.; Tinoco, I. Real-time control of the energy landscape by force directs the folding of RNA molecules. Proc. Natl. Acad. Sci. USA 2007, 104, 7039–7044. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, D.B.; Woodside, M.T. Probing the structural dynamics of proteins and nucleic acids with optical tweezers. Curr. Opin. Struct. Biol. 2015, 34, 43–51. [Google Scholar] [CrossRef]
- Farre, A.; van der Horst, A.; Blab, G.A.; Downing, B.P.B.; Forde, N.R. Stretching single DNA molecules to demonstrate high-force capabilities of holographic optical tweezers. J. Biophotonics 2010, 3, 224–233. [Google Scholar] [CrossRef]
- Baker, J.E.; Badman, R.P.; Wang, M.D. Nanophotonic trapping: Precise manipulation and measurement of biomolecular arrays. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Zhou, J.; Zhu, W.S.; Manley, P.W.; Wang, Y.K.; Hood, T.; Wylie, A.; Xie, X.S. Imaging the intracellular distribution of tyrosine kinase inhibitors in living cells with quantitative hyperspectral stimulated Raman scattering. Nat. Chem. 2014, 6, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Enger, J.; Goksor, M.; Ramser, K.; Hagberg, P.; Hanstorp, D. Optical tweezers applied to a microfluidic system. Lab Chip 2004, 4, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Teh, S.Y.; Lee, A.; Kim, H.H.; Lee, C.; Shung, K.K. Single beam acoustic trapping. Appl. Phys. Lett. 2009, 95, 3. [Google Scholar] [CrossRef]
- Grigorenko, A.N.; Roberts, N.W.; Dickinson, M.R.; Zhang, Y. Nanometric optical tweezers based on nanostructured substrates. Nat. Photonics 2008, 2, 365–370. [Google Scholar] [CrossRef]
- Marago, O.M.; Jones, P.H.; Gucciardi, P.G.; Volpe, G.; Ferrari, A.C. Optical trapping and manipulation of nanostructures. Nat. Nanotechnol. 2013, 8, 807–819. [Google Scholar] [CrossRef]
- Chen, Y.F.; Serey, X.; Sarkar, R.; Chen, P.; Erickson, D. Controlled photonic manipulation of proteins and other nanomaterials. Nano Lett. 2012, 12, 1633–1637. [Google Scholar] [CrossRef]
- Shoji, T.; Tsuboi, Y. Plasmonic optical tweezers toward molecular manipulation: Tailoring plasmonic nanostructure, light source, and resonant trapping. J. Phys. Chem. Lett. 2014, 5, 2957–2967. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.L.; Righini, M.; Quidant, R. Plasmon nano-optical tweezers. Nat. Photonics 2011, 5, 349–356. [Google Scholar] [CrossRef]
- Novotny, L.; Bian, R.X.; Xie, X.S. Theory of nanometric optical tweezers. Phys. Rev. Lett. 1997, 79, 645–648. [Google Scholar] [CrossRef]
- Wang, K.; Schonbrun, E.; Steinvurzel, P.; Crozier, K.B. Trapping and rotating nanoparticles using a plasmonic nano-tweezer with an integrated heat sink. Nat. Commun. 2011, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.L.; Gordon, R.; Pang, Y.J.; Eftekhari, F.; Quidant, R. Self-induced back-action optical trapping of dielectric nanoparticles. Nat. Phys. 2009, 5, 915–919. [Google Scholar] [CrossRef]
- Pang, Y.J.; Gordon, R. Optical trapping of 12 nm dielectric spheres using double-nanoholes in a gold film. Nano Lett. 2011, 11, 3763–3767. [Google Scholar] [CrossRef]
- Zhang, W.H.; Huang, L.N.; Santschi, C.; Martin, O.J.F. Trapping and sensing 10 nm metal nanoparticles using plasmonic dipole antennas. Nano Lett. 2010, 10, 1006–1011. [Google Scholar] [CrossRef]
- Righini, M.; Ghenuche, P.; Cherukulappurath, S.; Myroshnychenko, V.; de Abajo, F.J.G.; Quidant, R. Nano-optical trapping of Rayleigh particles and Escherichia coli bacteria with resonant optical antennas. Nano Lett. 2009, 9, 3387–3391. [Google Scholar] [CrossRef]
- Huang, L.; Maerkl, S.J.; Martin, O.J.F. Integration of plasmonic trapping in a microfluidic environment. Opt. Express 2009, 17, 6018–6024. [Google Scholar] [CrossRef]
- Pang, Y.J.; Gordon, R. Optical trapping of a single protein. Nano Lett. 2012, 12, 402–406. [Google Scholar] [CrossRef]
- Kotnala, A.; Gordon, R. Double nanohole optical tweezers visualize protein p53 suppressing unzipping of single DNA-hairpins. Biomed. Opt. Express 2014, 5, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.H.J.; Moore, S.D.; Schmidt, B.S.; Klug, M.; Lipson, M.; Erickson, D. Optical manipulation of nanoparticles and biomolecules in sub-wavelength slot waveguides. Nature 2009, 457, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, D.; Dell’Olio, F.; Krauss, T.F.; Ciminelli, C. Photonic and plasmonic nanotweezing of nano- and microscale particles. Appl. Spectrosc. 2017, 71, 367–390. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Serey, X.; Erickson, D. Nanomanipulation using silicon photonic crystal resonators. Nano Lett. 2010, 10, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Joannopoulos, J.D.; Villeneuve, P.R.; Fan, S.H. Photonic crystals: Putting a new twist on light. Nature 1997, 386, 143–149. [Google Scholar] [CrossRef]
- Yablonovitch, E. Inhibited spontaneous emission in solid-state physics and electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef] [PubMed]
- Yablonovitch, E.; Gmitter, T.J. Photonic band-structure—The face-centered-cubic case. J. Opt. Soc. Am. A-Opt. Image Sci. Vis. 1990, 7, 1792–1800. [Google Scholar] [CrossRef]
- Lin, S.Y.; Hu, J.J.; Kimerling, L.; Crozier, K. Design of nanoslotted photonic crystal waveguide cavities for single nanoparticle trapping and detection. Opt. Lett. 2009, 34, 3451–3453. [Google Scholar] [CrossRef]
- Serey, X.; Mandal, S.; Chen, Y.F.; Erickson, D. DNA Transport and delivery in thermal gradients near optofluidic resonators. Phys. Rev. Lett. 2012, 108, 5. [Google Scholar] [CrossRef]
- Jing, P.F.; Wu, J.D.; Liu, G.W.; Keeler, E.G.; Pun, S.H.; Lin, L.Y. Photonic crystal optical tweezers with high efficiency for live biological samples and viability characterization. Sci. Rep. 2016, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Krishan, A. Rapid flow cytofluorometric analysis of mammalian-cell cycle by propidium iodide staining. J. Cell Biol. 1975, 66, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Keeler, E.G.; Wu, J.; Jing, P.; Lin, L.Y. MEMS Resonator and Photonic Crystal Integration for Enhanced Cellular Mass Sensing. In Proceedings of the Optics in the Life Sciences, Vancouver, BC, Canada, 12 April 2015; p. BW1A.6. [Google Scholar]
- Jiang, Y.Q.; Narushima, T.; Okamoto, H. Nonlinear optical effects in trapping nanoparticles with femtosecond pulses. Nat. Phys. 2010, 6, 1005–1009. [Google Scholar] [CrossRef]
- Muramatsu, M.; Shen, T.F.; Chiang, W.Y.; Usman, A.; Masuhara, H. Picosecond motional relaxation of nanoparticles in femtosecond laser trapping. J. Phys. Chem. C 2016, 120, 5251–5256. [Google Scholar] [CrossRef]
- Xing, Q.R.; Mao, F.L.; Lu, C.; Wang, Q.Y. Numerical modeling and theoretical analysis of femtosecond laser tweezers. Opt. Laser Technol. 2004, 36, 635–639. [Google Scholar] [CrossRef]
- Malmqvist, L.; Hertz, H.M. Second-harmonic generation in optically trapped nonlinear particles with pulsed lasers. Appl. Opt. 1995, 34, 3392–3397. [Google Scholar] [CrossRef]
- Agate, B.; Brown, C.T.A.; Sibbett, W.; Dholakia, K. Femtosecond optical tweezers for in-situ control of two-photon fluorescence. Opt. Express 2004, 12, 3011–3017. [Google Scholar] [CrossRef]
- Chiang, W.Y.; Usman, A.; Masuhara, H. Femtosecond Pulse-width dependent trapping and directional ejection dynamics of dielectric nanoparticles. J. Phys. Chem. C 2013, 117, 19182–19188. [Google Scholar] [CrossRef]
- Usman, A.; Chiang, W.Y.; Masuhara, H. Femtosecond trapping efficiency enhanced for nano-sized silica spheres. In Optical Trapping and Optical Micromanipulation Ix; Dholakia, K., Spalding, G.C., Eds.; Spie-Int Soc Optical Engineering: Bellingham, DC, USA, 2012; Volume 8458. [Google Scholar]
- Zhou, M.; Yang, H.F.; Di, J.; Zhao, E.L. Manipulation on human red blood cells with femtosecond optical tweezers. Chin. Opt. Lett. 2008, 6, 919–921. [Google Scholar] [CrossRef]
- Mao, F.L.; Xing, Q.R.; Wang, K.; Lang, L.Y.; Wang, Z.; Chai, L.; Wang, Q.Y. Optical trapping of red blood cells and two-photon excitation-based photodynamic study using a femtosecond laser. Opt. Commun. 2005, 256, 358–363. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.Y.; Qu, S.L. Living cell manipulation in a microfluidic device by femtosecond optical tweezers. Opt. Lasers Eng. 2014, 55, 150–154. [Google Scholar] [CrossRef]
- Roxworthy, B.J.; Toussaint, K.C. Femtosecond-pulsed plasmonic nanotweezers. Sci. Rep. 2012, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Saitoh, J.; Kitamura, N.; Nagasawa, F.; Murakoshi, K.; Yamauchi, H.; Ito, S.; Miyasaka, H.; Ishihara, H.; Tsuboi, Y. Permanent Fixing or Reversible Trapping and Release of DNA micropatterns on a gold nanostructure using continuous-wave or femtosecond-pulsed near-infrared laser light. J. Am. Chem. Soc. 2013, 135, 6643–6648. [Google Scholar] [CrossRef] [PubMed]
- Best, R.B.; Paci, E.; Hummer, G.; Dudko, O.K. Pulling direction as a reaction coordinate for the mechanical unfolding of single molecules. J. Phys. Chem. B 2008, 112, 5968–5976. [Google Scholar] [CrossRef] [PubMed]
- Avdoshenko, S.M.; Makarov, D.E. Reaction coordinates and pathways of mechanochemical transformations. J. Phys. Chem. B 2016, 120, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Dame, R.T.; Noom, M.C.; Wuite, G.J.L. Bacterial chromatin organization by H-NS protein unravelled using dual DNA manipulation. Nature 2006, 444, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Inman, J.T.; Smith, B.Y.; Hall, M.A.; Forties, R.A.; Jin, J.; Sethna, J.P.; Wang, M.D. DNA Y structure: A versatile, multidimensional single molecule assay. Nano Lett. 2014, 14, 6475–6480. [Google Scholar] [CrossRef] [PubMed]
- Elms, P.J.; Chodera, J.D.; Bustamante, C.; Marqusee, S. The molten globule state is unusually deformable under mechanical force. Proc. Natl. Acad. Sci. USA 2012, 109, 3796–3801. [Google Scholar] [CrossRef]
- Heidarsson, P.O.; Valpapuram, I.; Camilloni, C.; Imparato, A.; Tiana, G.; Poulsen, F.M.; Kragelund, B.B.; Cecconi, C. A highly compliant protein native state with a spontaneous-like mechanical unfolding pathway. J. Am. Chem. Soc. 2012, 134, 17068–17075. [Google Scholar] [CrossRef]
- Hohlbein, J.; Gryte, K.; Heilemann, M.; Kapanidis, A.N. Surfing on a new wave of single-molecule fluorescence methods. Phys. Biol. 2010, 7, 22. [Google Scholar] [CrossRef]
- Hellenkamp, B.; Schmid, S.; Doroshenko, O.; Opanasyuk, O.; Kuhnemuth, R.; Adariani, S.R.; Ambrose, B.; Aznauryan, M.; Barth, A.; Birkedal, V.; et al. Precision and accuracy of single-molecule FRET measurements-a multi-laboratory benchmark study. Nat. Methods 2018, 15, 669. [Google Scholar] [CrossRef] [PubMed]
- Shabestari, M.H.; Meijering, A.E.C.; Roos, W.H.; Wuite, G.J.L.; Peterman, E.J.G. Recent advances in biological single-molecule applications of optical tweezers and fluorescence microscopy. In Single-Molecule Enzymology: Nanomechanical Manipulation and Hybrid Methods; Spies, M., Chemla, Y.R., Eds.; Elsevier Academic Press Inc: San Diego, CA, USA, 2017; Volmue 582, pp. 85–119. [Google Scholar]
- Chemla, Y.R. High-resolution, hybrid optical trapping methods, and their application to nucleic acid processing proteins. Biopolymers 2016, 105, 704–714. [Google Scholar] [CrossRef]
- Cordova, J.C.; Das, D.K.; Manning, H.W.; Lang, M.J. Combining single-molecule manipulation and single-molecule detection. Curr. Opin. Struct. Biol. 2014, 28, 142–148. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, M.A.; Kapitein, L.C.; van Mameren, J.; Schmidt, C.F.; Peterman, E.J.G. Combining optical trapping and single-molecule fluorescence spectroscopy: Enhanced photobleaching of fluorophores. J. Phys. Chem. B 2004, 108, 6479–6484. [Google Scholar] [CrossRef]
- Roy, R.; Hohng, S.; Ha, T. A practical guide to single-molecule FRET. Nat. Methods 2008, 5, 507–516. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.; Kulkarni, P.; Weninger, K. Single Molecule FRET: A powerful tool to study intrinsically disordered proteins. Biomolecules 2018, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Ishii, S.; Kawai, M.; Ishiwata, S.; Suzuki, M. Estimation of actomyosin active force maintained by tropomyosin and troponin complex under vertical forces in the in vitro motility assay system. PLoS ONE 2018, 13, e0192558. [Google Scholar] [CrossRef] [PubMed]
- Reinemann, D.N.; Sturgill, E.G.; Das, D.K.; Degen, M.S.; Voros, Z.; Hwang, W.; Ohi, R.; Lang, M.J. Collective force regulation in anti-parallel microtubule gliding by dimeric Kif15 kinesin motors. Curr. Biol. 2017, 27, 2810. [Google Scholar] [CrossRef] [PubMed]
- Kudalkar, E.M.; Scarborough, E.A.; Umbreit, N.T.; Zelter, A.; Gestaut, D.R.; Riffle, M.; Johnson, R.S.; MacCoss, M.J.; Asbury, C.L.; Davis, T.N. Regulation of outer kinetochore Ndc80 complex-based microtubule attachments by the central kinetochore Mis12/MIND complex. Proc. Natl. Acad. Sci. USA 2015, 112, E5583–E5589. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-T.; Ha, T. Probing single helicase dynamics on long nucleic acids through fluorescence-force measurement. In Optical Tweezers; Springer: Berlin, Germany, 2017; pp. 295–316. [Google Scholar]
- Brenner, M.D.; Zhou, R.B.; Conway, D.E.; Lanzano, L.; Gratton, E.; Schwartz, M.A.; Ha, T. Spider silk peptide is a compact, linear nanospring ideal for intracellular tension sensing. Nano Lett. 2016, 16, 2096–2102. [Google Scholar] [CrossRef]
- Lee, S.; Hohng, S. An optical trap combined with three-color FRET. J. Am. Chem. Soc. 2013, 135, 18260–18263. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, I.; Moschetti, T.; Candelli, A.; Garcin, E.B.; Modesti, M.; Pellegrini, L.; Wuite, G.J.L.; Peterman, E.J.G. Two distinct conformational states define the interaction of human RAD51-ATP with single-stranded DNA. Embo J. 2018, 37, 13. [Google Scholar] [CrossRef] [PubMed]
- Forget, A.L.; Kowalczykowski, S.C. Single-molecule imaging of DNA pairing by RecA reveals a three-dimensional homology search. Nature 2012, 482, 423. [Google Scholar] [CrossRef]
- van Mameren, J.; Vermeulen, K.; Wuite, G.J.L.; Peterman, E.J.G. A polarized view on DNA under tension. J. Chem. Phys. 2018, 148, 9. [Google Scholar] [CrossRef] [PubMed]
- Whitley, K.D.; Comstock, M.J.; Chemla, Y.R. Ultrashort nucleic acid duplexes exhibit long wormlike chain behavior with force-dependent edge effects. Phys. Rev. Lett. 2018, 120, 6. [Google Scholar] [CrossRef]
- Ganim, Z.; Rief, M. Mechanically switching single-molecule fluorescence of GFP by unfolding and refolding. Proc. Natl. Acad. Sci. USA 2017, 114, 11052–11056. [Google Scholar] [CrossRef] [PubMed]
- Duesterberet, V.K.; Fischer-Hwangt, I.T.; Pereet, C.F.; Hogan, D.W.; Block, S.M. Observation of long-range tertiary interactions during ligand binding by the TPP riboswitch aptamer. eLife 2015, 4, 17. [Google Scholar] [CrossRef]
- Suksombat, S.; Khafizov, R.; Kozlov, A.G.; Lohman, T.M.; Chemla, Y.R. Structural dynamics of E. coli single-stranded DNA binding protein reveal DNA wrapping and unwrapping pathways. eLife 2015, 4, 23. [Google Scholar] [CrossRef]
- Whitley, K.D.; Comstock, M.J.; Chemla, Y.R. High-resolution optical tweezers combined with single-molecule confocal microscopy. In Single-Molecule Enzymology: Nanomechanical Manipulation and Hybrid Methods; Spies, M., Chemla, Y.R., Eds.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2017; Volume 582, pp. 137–169. [Google Scholar]
- Sirinakis, G.; Ren, Y.X.; Gao, Y.; Xi, Z.Q.; Zhang, Y.L. Combined versatile high-resolution optical tweezers and single-molecule fluorescence microscopy. Rev. Sci. Instrum. 2012, 83, 9. [Google Scholar] [CrossRef]
- Leijnse, N.; Oddershede, L.B.; Bendix, P.M. Helical buckling of actin inside filopodia generates traction. Proc. Natl. Acad. Sci. USA 2015, 112, 136–141. [Google Scholar] [CrossRef]
- Podlipec, R.; Strancar, J. Cell-scaffold adhesion dynamics measured in first seconds predicts cell growth on days scale—Optical tweezers study. Acs Appl. Mater. Interfaces 2015, 7, 6782–6791. [Google Scholar] [CrossRef]
- Pang, Y.J.; Song, H.N.; Kim, J.H.; Hout, X.M.; Cheng, W. Optical trapping of individual human immunodeficiency viruses in culture fluid reveals heterogeneity with single-molecule resolution. Nat. Nanotechnol. 2014, 9, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Paliwal, A.; Tomar, M.; Gupta, V. Detection of Neisseria meningitidis using surface plasmon resonance based DNA biosensor. Biosens. Bioelectron. 2016, 78, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Shih, J.S. Surface acoustic wave immunosensors based on immobilized C60-proteins. Sens. Actuator B Chem. 2007, 121, 522–529. [Google Scholar] [CrossRef]
- Duhr, S.; Braun, D. Why molecules move along a temperature gradient. Proc. Natl. Acad. Sci. USA 2006, 103, 19678–19682. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhary, D.; Mossa, A.; Jadhav, M.; Cecconi, C. Bio-Molecular Applications of Recent Developments in Optical Tweezers. Biomolecules 2019, 9, 23. https://doi.org/10.3390/biom9010023
Choudhary D, Mossa A, Jadhav M, Cecconi C. Bio-Molecular Applications of Recent Developments in Optical Tweezers. Biomolecules. 2019; 9(1):23. https://doi.org/10.3390/biom9010023
Chicago/Turabian StyleChoudhary, Dhawal, Alessandro Mossa, Milind Jadhav, and Ciro Cecconi. 2019. "Bio-Molecular Applications of Recent Developments in Optical Tweezers" Biomolecules 9, no. 1: 23. https://doi.org/10.3390/biom9010023
APA StyleChoudhary, D., Mossa, A., Jadhav, M., & Cecconi, C. (2019). Bio-Molecular Applications of Recent Developments in Optical Tweezers. Biomolecules, 9(1), 23. https://doi.org/10.3390/biom9010023




