Longitudinal Study of Delta-Aminolevulinate Dehydratase Activity and Oxidative Profile in Healthy Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
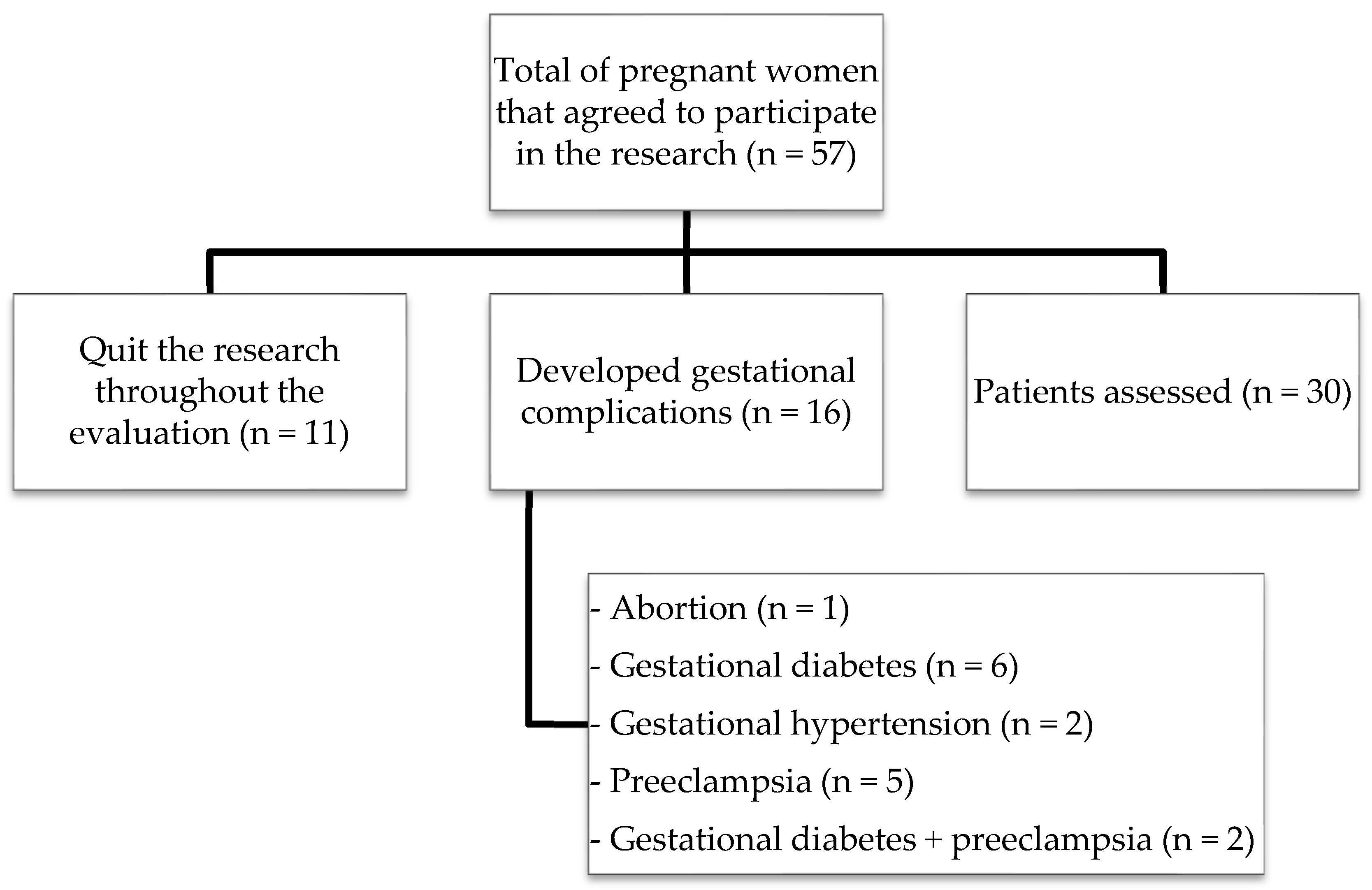
2.1. Study Population
2.2. Sample Collection
2.3. Clinical and Hematological Profile
2.4. Biochemical Profile
2.5. Oxidative Profile
2.6. Statistical Analysis
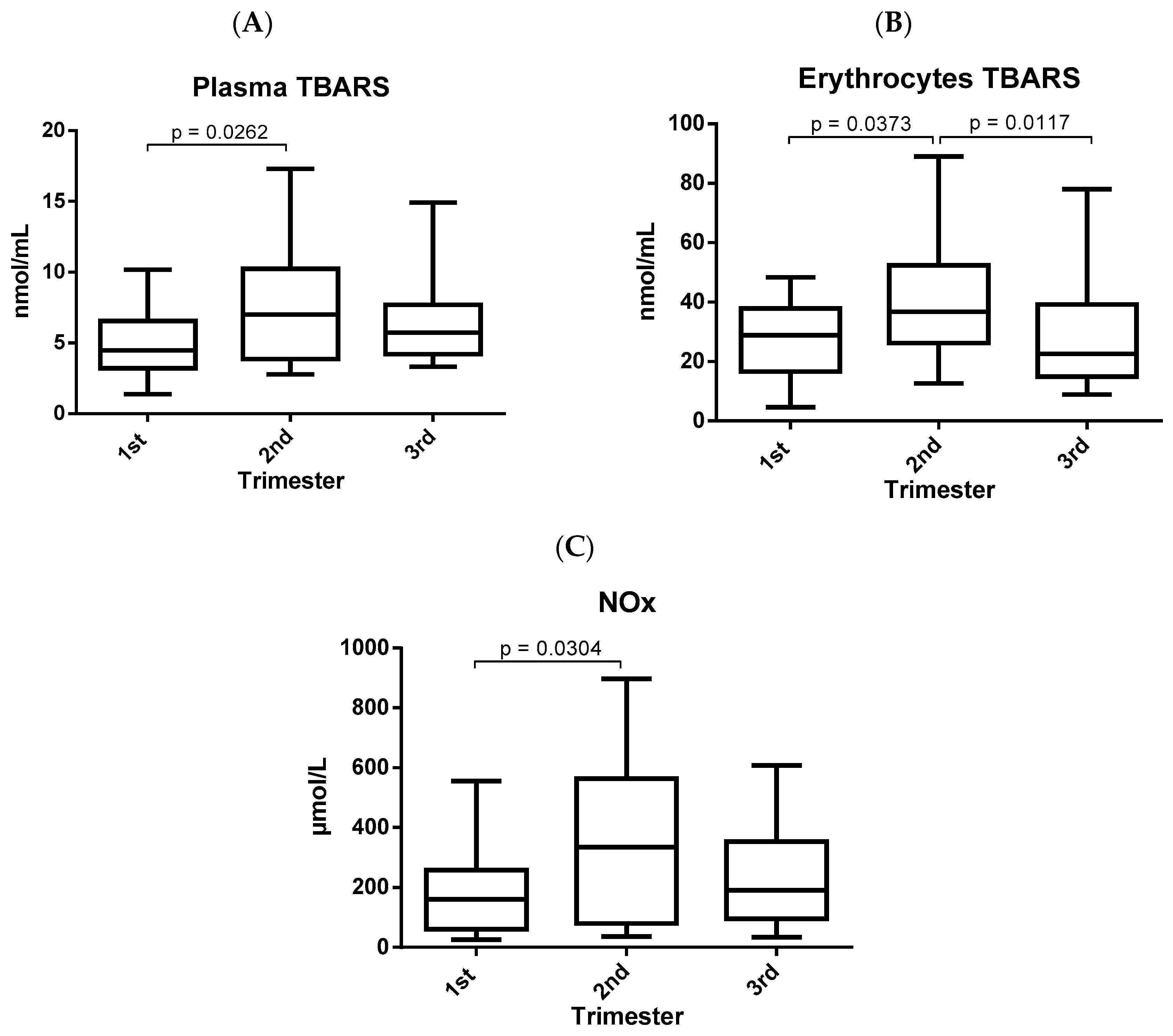
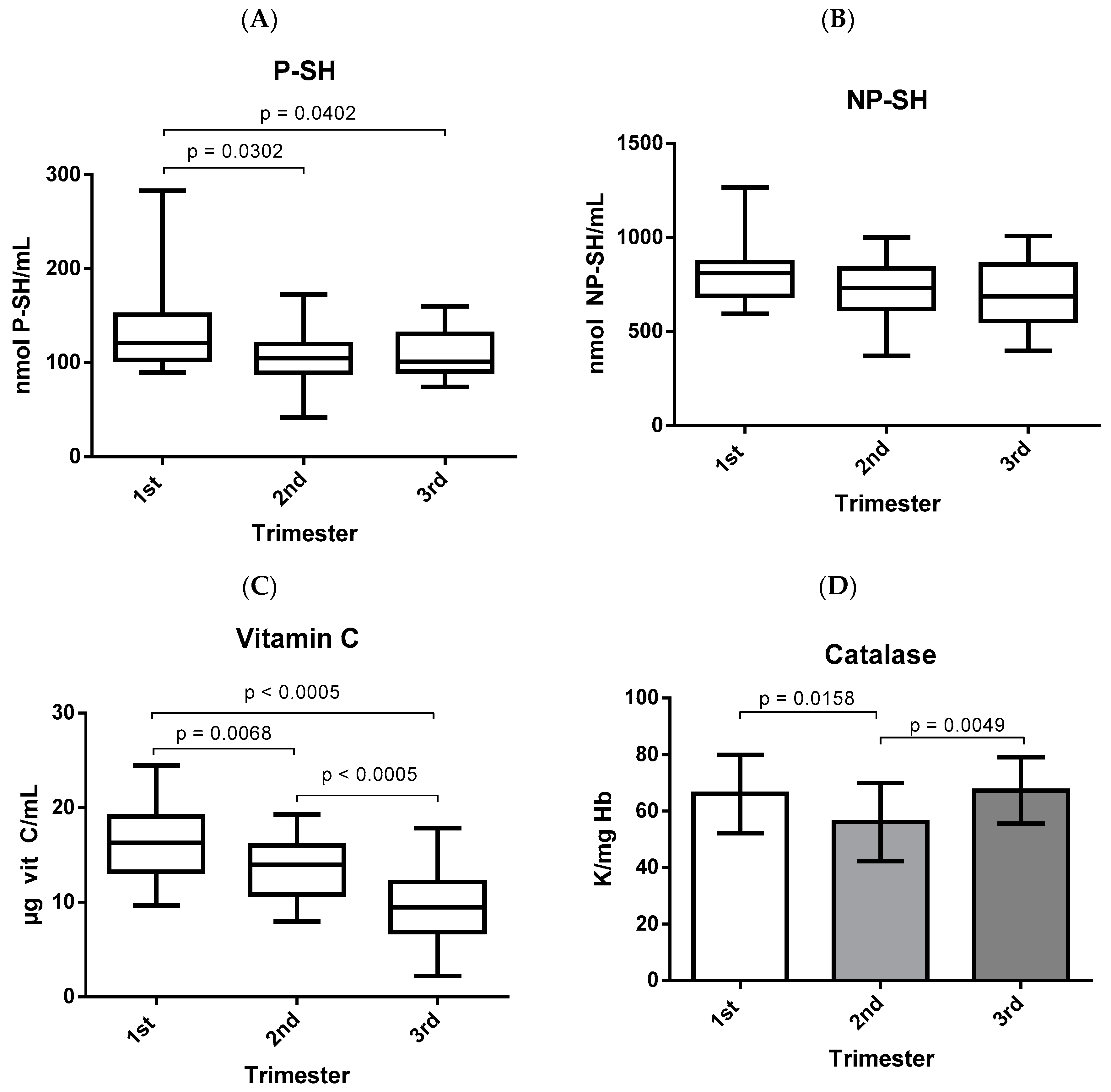
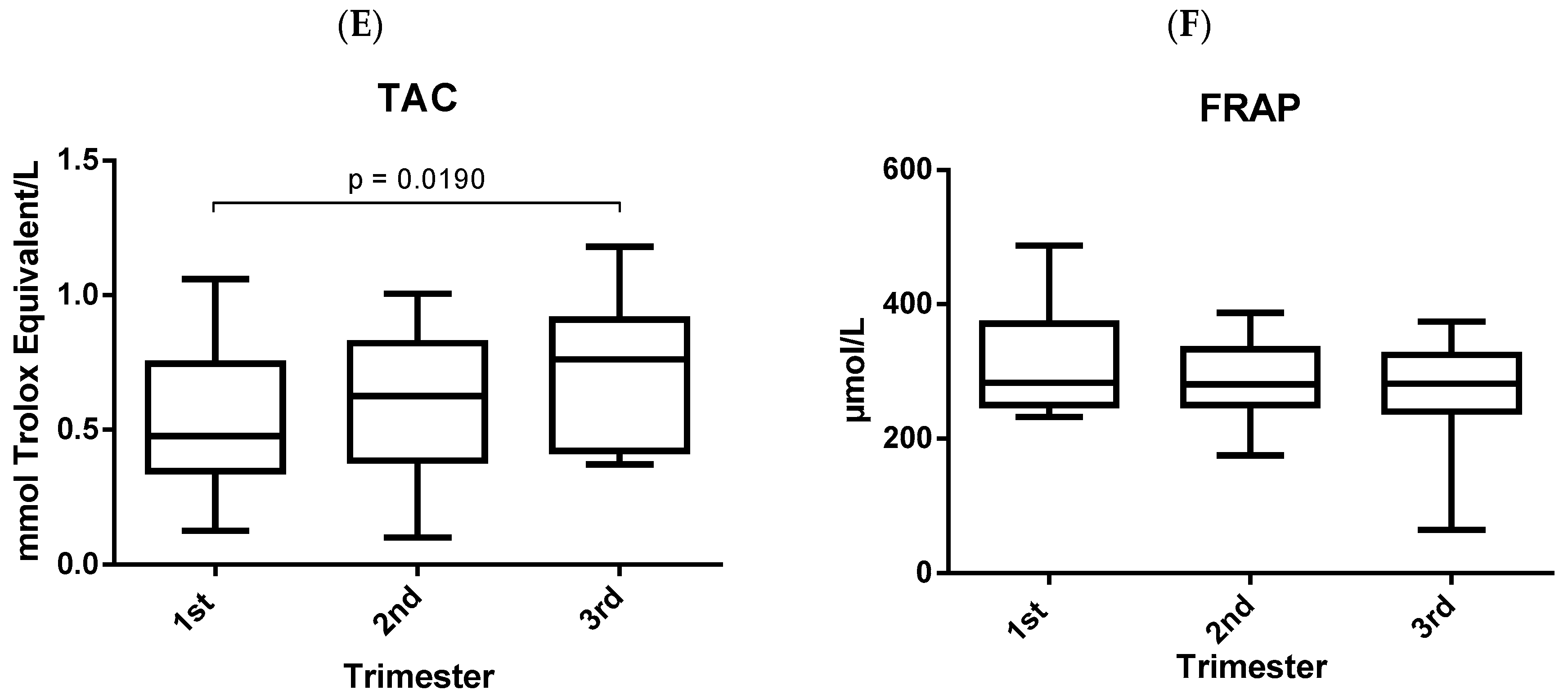
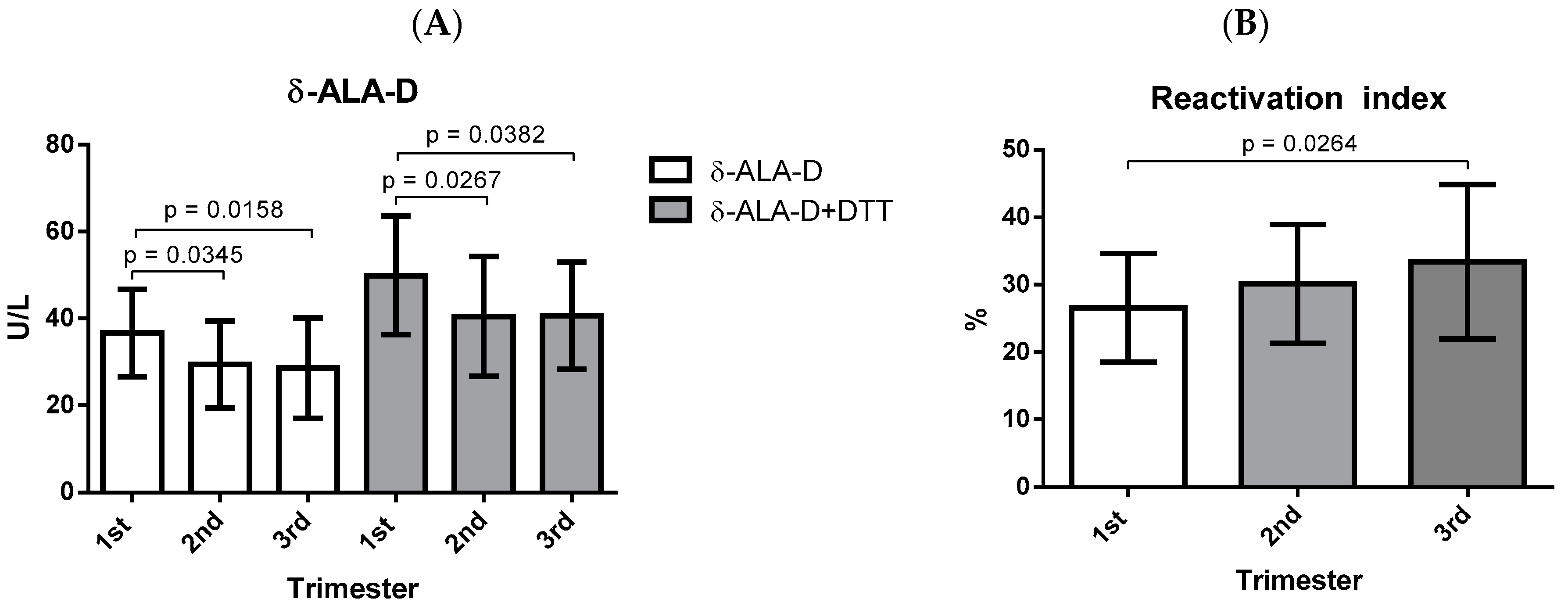
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ribas, J.T.; Belló, C.; Ito, C.A.S.; Mine, J.C.; Vellosa, J.C.R. Metabolic and inflammatory changes in pregnancy. J. Basic Appl. Pharm. Sci. 2015, 36, 181–188. Available online: http://seer.fcfar.unesp.br/rcfba/index.php/rcfba/article/view/230/134 (accessed on 23 October 2018).
- Herrero-Mercado, M.; Waliszewski, S.M.; Caba, M.; Martínez-Valenzuela, C.; Gómez Arroyo, S.; Villalobos Pietrini, R.; Cantú Martínez, P.C.; Hernández-Chalate, F. Organochlorine pesticide gradient levels among maternal adiposetissue, maternal blood serum and umbilical blood serum. Bull. Environ. Contam. Toxicol. 2011, 86, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agarwal, A.; Sharma, R.K. The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstet. Gynecol. Surv. 2005, 60, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.J.; Clifton, V.L.; Hodyl, N.A. Differential effects of docosahexaenoic acid on preterm and term placental pro-oxidant/antioxidant balance. Reproduction 2013, 146, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 2010, 31, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Stangenberg, S.; Nguyen, L.T.; Chen, H.; Al-Odat, I.; Killingsworth, M.C.; Gosnell, M.F.; Anwer, A.G.; Goldys, E.M.; Pollock, C.A.; Saad, S. Oxidative stress, mitochondrial perturbations and fetal programming of renal disease induced by maternal smoking. Int. J. Biochem. Cell Biol. 2015, 64, 81–90. [Google Scholar] [CrossRef]
- Marseglia, A.; D’Angelo, G.; Manti, S.; Arrigo, T.; Barberi, I.; Reiter, R.J.; Gitto, E. Oxidative stress-mediated aging during the fetal and perinatal periods. Oxid. Med. Cell Longev. 2014, 2014, 358–375. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28–48. [Google Scholar] [CrossRef]
- Mert, I.; Oruc, A.S.; Yuksel, S.; Cakar, E.S.; Buyukkagnici, U.; Karaer, A.; Danisman, N. Role of oxidative stress in preeclampsia and intrauterine growth restriction. J. Obstet. Gynaecol. Res. 2012, 38, 658–664. [Google Scholar] [CrossRef]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Piedrafita, G.; Keller, M.A.; Ralser, M. The Impact of Non-Enzymatic Reactions and Enzyme Promiscuity on Cellular Metabolism during (Oxidative) Stress Conditions. Biomolecules 2015, 5, 2101–2122. [Google Scholar] [CrossRef]
- Abuja, P.M.; Albertini, R. Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin. Chim. Acta 2001, 306, 1–17. [Google Scholar] [CrossRef]
- Elliot, M.G. Oxidative stress and the evolutionary origins of preeclampsia. J. Reprod. Immunol. 2016, 114, 75–80. [Google Scholar] [CrossRef]
- Zanini, D.; Pelinson, L.P.; Schmatz, R.; Pereira, L.B.; Martins, C.C.; Baldissareli, J.; Amaral, G.P.; Soares, F.A.; Reetz, L.G.B.; do Araújo, M.C.; et al. D-Aminolevulinate dehydratase activity in lung cancer patients and its relationship with oxidative stress. Biomed. Pharmacother. 2014, 68, 603–609. [Google Scholar] [CrossRef] [PubMed]
- De Lucca, L.; Rodrigues, F.; Jantsch, L.B.; Kober, H.; Neme, W.S.; Gallarreta, F.M.P.; Gonçalves, T.L. Delta-aminolevulinate dehydratase activity and oxidative stress markers in preeclampsia. Biomed. Pharmacother. 2016, 19, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Alpoim, P.N.; Perucci, L.O.; Godoi, L.C.; Goulart, C.O.L.; Dusse, L.M.S. Oxidative stress markers and thrombomodulin plasma levels in women with early and late severe preeclampsia. Clin. Chim. Acta 2018, 483, 234–238. [Google Scholar] [CrossRef]
- Peskin, A.V.; Pace, P.E.; Behring, J.B.; Paton, L.N.; Soethoudt, M.; Bachschmid, M.M.; Winterbourn, C.C. Glutathionylation of the Active Site Cysteines of Peroxiredoxin 2 and Recycling by Glutaredoxin. J. Biol. Chem. 2016, 291, 3053–3062. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hampton, M.B. Redox biology: Signaling via a peroxiredoxin sensor. Nat. Chem. Biol. 2015, 11, 5–6. [Google Scholar] [CrossRef]
- Rocha, J.B.T.; Saraiva, R.A.; Garcia, S.C.; Gravina, F.S.; Nogueira, C.W. Aminolevulinate dehydratase (d-ALA-D) as marker protein of intoxication with metals and other pro-oxidant situations. Toxicol. Res. (Camb.) 2012, 1, 85–102. [Google Scholar] [CrossRef]
- Rodrigues, F.; De Lucca, L.; Neme, W.S.; Gonçalves, T.L. Influence of gestational diabetes on the activity of δ-aminolevulinate dehydratase and oxidative stress biomarkers. Redox Rep. 2017, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, D.; Ciofani, G.; Pierdomenico, S.D.; Giamberardino, M.A.; Cuccurullo, F. Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxides in human plasma. Free Radic. Biol. Med. 2001, 31, 331–335. [Google Scholar] [CrossRef]
- Tatsch, E.; Bochi, G.V.; Pereira Rda, S.; Kober, H.; Agertt, V.A.; de Campos, M.M.; Gomes, P.; Duarte, M.M.; Moresco, R.N. A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin. Biochem. 2011, 44, 348–350. [Google Scholar] [CrossRef]
- Boyne, A.F.; Ellman, G.L. A methodology for analysis of tissue sulfhydryl components. Anal. Biochem. 1972, 46, 639–653. [Google Scholar] [CrossRef]
- Jacques-Silva, M.C.; Nogueira, C.W.; Broch, L.C.; Flores, E.M.; Rocha, J.B. Dyphenyldiselenide and ascorbic acid changes deposition of selenium and brain of mice. Pharmacol. Toxicol. 2001, 88, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F.; Davies, M.J.; Webster, N.R. Ascorbyl radical formation in patients with sepsis: Effect of ascorbate loading. Free Radic. Biol. Med. 1996, 20, 139–143. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Berlin, A.; Schaller, K. European standardized method for the determination of δ–aminolevulinic dehydratase activity in blood. Z. Klin. Chem. Klin. Biochem. 1974, 12, 389–390. Available online: http://edoc.hu-berlin.de/oa/degruyter/cclm.1974.12.8.389.pdf (accessed on 30 August 2016).
- Bukhari, S.A.; Rajoka, M.I.; Ibrahim, Z.; Jalal, F.; Rana, S.M.; Nagra, S.A. Oxidative stress elevated DNA damage and homocysteine level in normal pregnant women in a segment of Pakistani population. Mol. Biol. Rep. 2011, 38, 2703–2710. [Google Scholar] [CrossRef]
- Ghneim, H.K.; Alshebly, M.M. Biochemical Markers of Oxidative Stress in Saudi Women with Recurrent Miscarriage. J. Korean Med. Sci. 2016, 31, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidou, C.; Kaindl, A.M. Neuronal death and oxidative stress in the developing brain. Antioxid. Redox Signal. 2011, 14, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Gad, A.; Salilew-Wondim, D.; Prastowo, S.; Held, E.; Hoelker, M.; Rings, F.; Tholen, E.; Neuhoff, C.; Looft, C.; et al. Bovine embryo survival under oxidative-stress conditions is associated with activity of the NRF2-mediated oxidative-stress-response pathway. Mol. Reprod. Dev. 2014, 81, 497–513. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. The chemistry of free radicals and related reactive species. In Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2007; pp. 30–78. [Google Scholar]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef]
- Grotto, D.; Valentini, J.; Boeira, S.; Paniz, C.; Maria, L.S.; Vicentini, J.; Moro, A.; Charão, M.; Garcia, S.C. Evaluation of the stability of the oxidative stress plasmatic biomarker—Malondialdehyde. Quim. Nova 2008, 2, 275–279. [Google Scholar] [CrossRef]
- Yüksel, S.; Yiğit, A.A. Malondialdehyde and nitric oxide levels and catalase, superoxide dismutase, and glutathione peroxidase levels in maternal blood during different trimesters of pregnancy and in the cord blood of newborns. Turk. J. Med. Sci. 2015, 45, 454–945. [Google Scholar] [CrossRef]
- Lappas, M.; Hiden, U.; Desoye, G.; Froehlich, J.; Hauguel-de Mouzon, S.; Jawerbaum, A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid. Redox Signal. 2011, 15, 3061–3100. [Google Scholar] [CrossRef]
- Niki, E. Do antioxidants impair signaling by reactive oxygen species and lipid oxidation products. FEBS Lett. 2012, 586, 3767–3770. [Google Scholar] [CrossRef]
- Huang, P.L.; Huang, Z.; Mashimo, H.; Bloch, K.D.; Moskowitz, M.A.; Bevan, J.A.; Fishman, M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 1995, 377, 239–242. [Google Scholar] [CrossRef]
- Van Den Oever, I.A.; Raterman, H.G.; Nurmohamed, M.T.; Simsek, S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. Oxf. 2010, 2010, 792393. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S. Nitrite: A Physiological Store of Nitric Oxide and Modulator of Mitochondrial Function. Redox Biol. 2013, 1, 40–44. [Google Scholar] [CrossRef]
- Soccal, R.M.; de Carvalho, J.A.; Bochi, G.V.; Moresco, R.N.; da Silva, J.E. Nitric oxide levels in HIV-infected, untreated patients and HIV-infected patients receiving antiretroviral therapy. Biomed. Pharmacother. 2016, 79, 302–307. [Google Scholar] [CrossRef]
- Dotan, Y.; Lichtenberg, D.; Pinchuk, I. Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Prog. Lipid Res. 2004, 43, 200–227. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994, 233, 380–385. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, X. Rapid and thiol-specific high-throughput assay for simultaneous relative quantification of total thiols, protein thiols, and nonproteinthiols in cells. Anal. Chem. 2015, 87, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Atiba, A.S.; Abbiyesuku, F.M.; Niran-atiba, T.A.; Oparinde, D.P.; Ajose, O.A.; Akindele, R.A. Free radical attack on membrane lipid and antioxidant vitamins in the course of pre-eclamptic pregnancy. Ethiop. J. Health Sci. 2014, 24, 35–42. [Google Scholar] [CrossRef]
- Boni, A.; Pugliese, C.; Cláudio, C.C.; Patin, R.V.; Oliveira, F.L.C. Antioxidant vitamins and prevention of atherosclerosis in childhood. Rev. Paul. Pediatr. 2010, 28, 373–380. [Google Scholar] [CrossRef]
- Gaetani, G.F.; Galiano, S.; Canepa, L.; Ferraris, A.M.; Kirkman, H.N. Catalase and glutathione peroxidase are equally active in detoxification of hydrogen peroxide in human erythrocytes. Blood 1989, 73, 334–339. Available online: http://www.bloodjournal.org/content/bloodjournal/73/1/334.full.pdf (accessed on 5 November 2018).
- Goyal, M.M.; Basak, A. Human catalase: Looking for complete identity. Protein Cell 2010, 1, 888–897. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.K.B. Total antioxidant capacity: A biomarker in biomedical and nutritional studies. J. Cell Mol. Biol. 2008, 7, 1–15. Available online: https://pdfs.semanticscholar.org/3659/e3495ae01e32c4de95df380d8a3c8bd2f741.pdf (accessed on 5 November 2018).
- Gabriel, D.; Pivetta, L.; Folmer, V.; Soares, J.C.M.; Augusti, G.R.; Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Human erythrocyte d-aminolevulinate dehydratase inhibition by monosaccharides is not mediated by oxidation of enzyme sulfhydryl groups. Cell Biol. Int. 2005, 29, 669–674. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Bland, J.M.; Robertson, W.B.; Brosens, I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 1983, 4, 397–413. [Google Scholar] [CrossRef]
- Wilson, M.L.; Goodwin, T.M.; Pan, V.L.; Ingles, S.A. Molecular epidemiology of preeclampsia. Obstet. Gynecol. Surv. 2003, 58, 39–65. [Google Scholar] [CrossRef] [PubMed]
| Parameter | 1st Trimester (n = 30) | 2nd Trimester (n = 30) | 3rd Trimester (n = 30) |
|---|---|---|---|
| Age (years) | 26.72 ± 4.18 | - | - |
| Gestational age (weeks) | 13 (12–14) | 22 (21–23) | 30 (30–31) |
| Weight (kg) | 63.77 ± 10.47 | 67.49 ± 10.07 | 71.54 ± 10.63 * |
| BMI (kg/m2) | 23.94 ± 3.79 | 25.36 ± 3.78 | 26.89 ± 4.02 * |
| Systolic pressure (mmHg) | 110 (100–120) | 110 (100–110) | 110 (100–110) |
| Diastolic pressure (mmHg) | 60 (60–70) | 60 (60–70) | 60 (60–70) |
| Erythrocytes (106/mm3) | 4.19 ± 0.32 | 4.07 ± 0.33 | 3.99 ± 0.39 |
| Hemoglobin (g/dL) | 12.39 ± 0.93 | 12.12 ± 0.95 | 11.94 ± 1.06 |
| Hematocrit (%) | 36.69 ± 2.94 | 35.83 ± 2.93 | 35.29 ± 3.14 |
| Platelets (103/mm3) | 235.9 ± 46.6 | 225.9 ± 47.8 | 219.7 ± 49.1 |
| Leukocytes (/mm3) | 8999 ± 1991 | 9307 ± 1889 | 9852 ± 2922 |
| Glucose (mg/dL) | 82.60 ± 7.70 | 80.80 ± 6.46 | 79.23 ± 7.26 |
| Urinary creatinine (mg/dL) | 92.75 (44.44–131.20) | 70.38 (41.63–99.13) | 70.50 (46.50–100.80) |
| Urinary albumin (mg/L) | 4.55 (2.79–14.20) | 4.715 (1.99–7.68) | 7.72 (3.58–11.39) |
| Urinary protein (mg/L) | 4.35 (1.17–5.65) | 3.40 (1.42–5.50) | 5.50 (2.57–6.53) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lucca, L.; Jantsch, L.B.; Vendrame, S.A.; Stein, C.d.S.; Klein, V.C.G.; Soares, K.B.; Gallarreta, F.M.P.; Moresco, R.N.; Gonçalves, T.d.L. Longitudinal Study of Delta-Aminolevulinate Dehydratase Activity and Oxidative Profile in Healthy Pregnant Women. Biomolecules 2019, 9, 18. https://doi.org/10.3390/biom9010018
de Lucca L, Jantsch LB, Vendrame SA, Stein CdS, Klein VCG, Soares KB, Gallarreta FMP, Moresco RN, Gonçalves TdL. Longitudinal Study of Delta-Aminolevulinate Dehydratase Activity and Oxidative Profile in Healthy Pregnant Women. Biomolecules. 2019; 9(1):18. https://doi.org/10.3390/biom9010018
Chicago/Turabian Stylede Lucca, Leidiane, Letícia Bigolin Jantsch, Silmara Ana Vendrame, Carolina dos Santos Stein, Vanessa Cristina Grólli Klein, Karina Biaggio Soares, Francisco Maximiliano Pancich Gallarreta, Rafael Noal Moresco, and Thissiane de Lima Gonçalves. 2019. "Longitudinal Study of Delta-Aminolevulinate Dehydratase Activity and Oxidative Profile in Healthy Pregnant Women" Biomolecules 9, no. 1: 18. https://doi.org/10.3390/biom9010018
APA Stylede Lucca, L., Jantsch, L. B., Vendrame, S. A., Stein, C. d. S., Klein, V. C. G., Soares, K. B., Gallarreta, F. M. P., Moresco, R. N., & Gonçalves, T. d. L. (2019). Longitudinal Study of Delta-Aminolevulinate Dehydratase Activity and Oxidative Profile in Healthy Pregnant Women. Biomolecules, 9(1), 18. https://doi.org/10.3390/biom9010018




