Non-Curcuminoids from Turmeric and Their Potential in Cancer Therapy and Anticancer Drug Delivery Formulations
Abstract
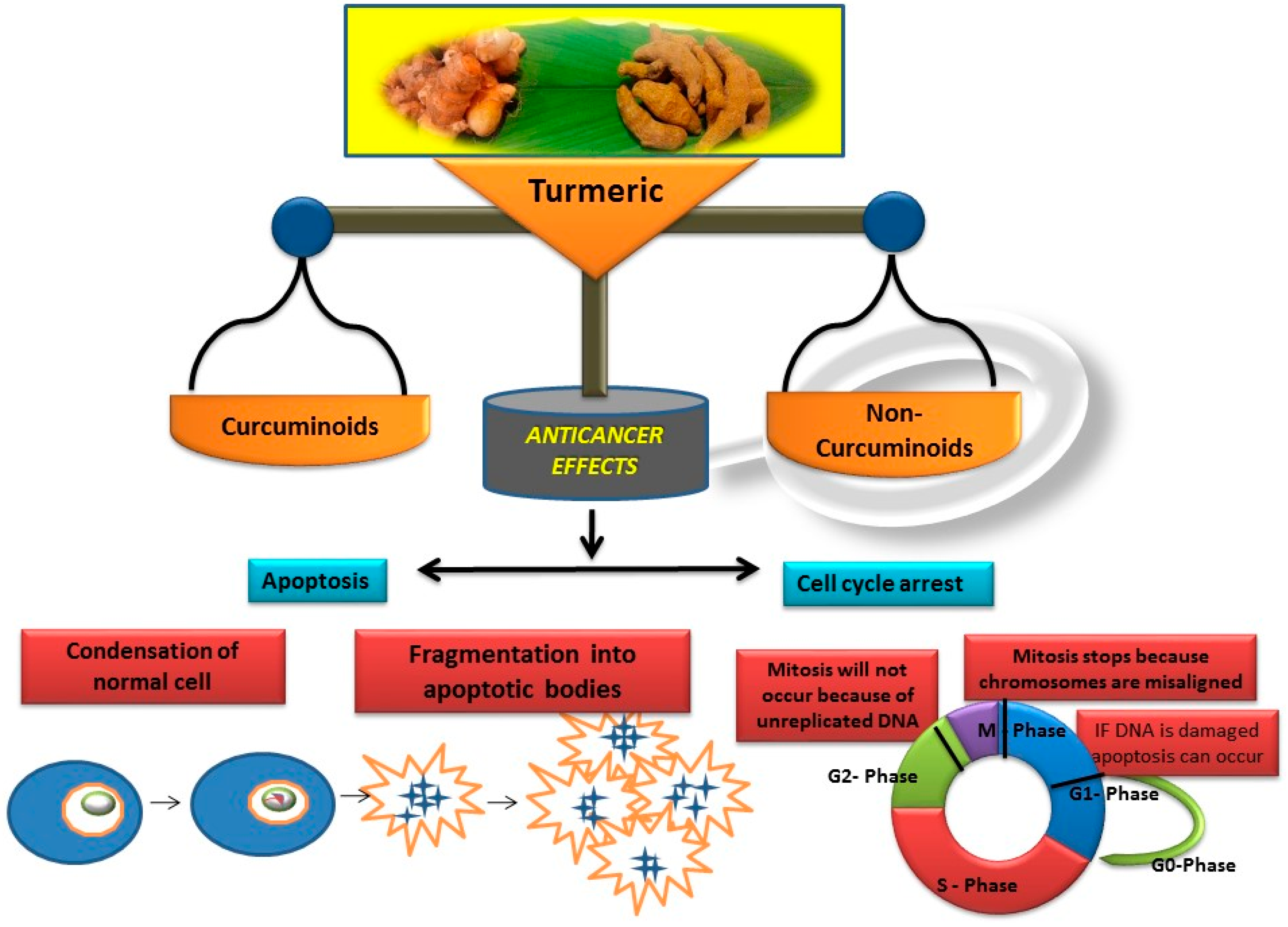
1. Introduction
2. Identification of Non-Curcuminoids
3. Chemical Constituents of Non-Curcuminoids from Turmeric
4. Non-Curcuminoids as Anticancer Agents
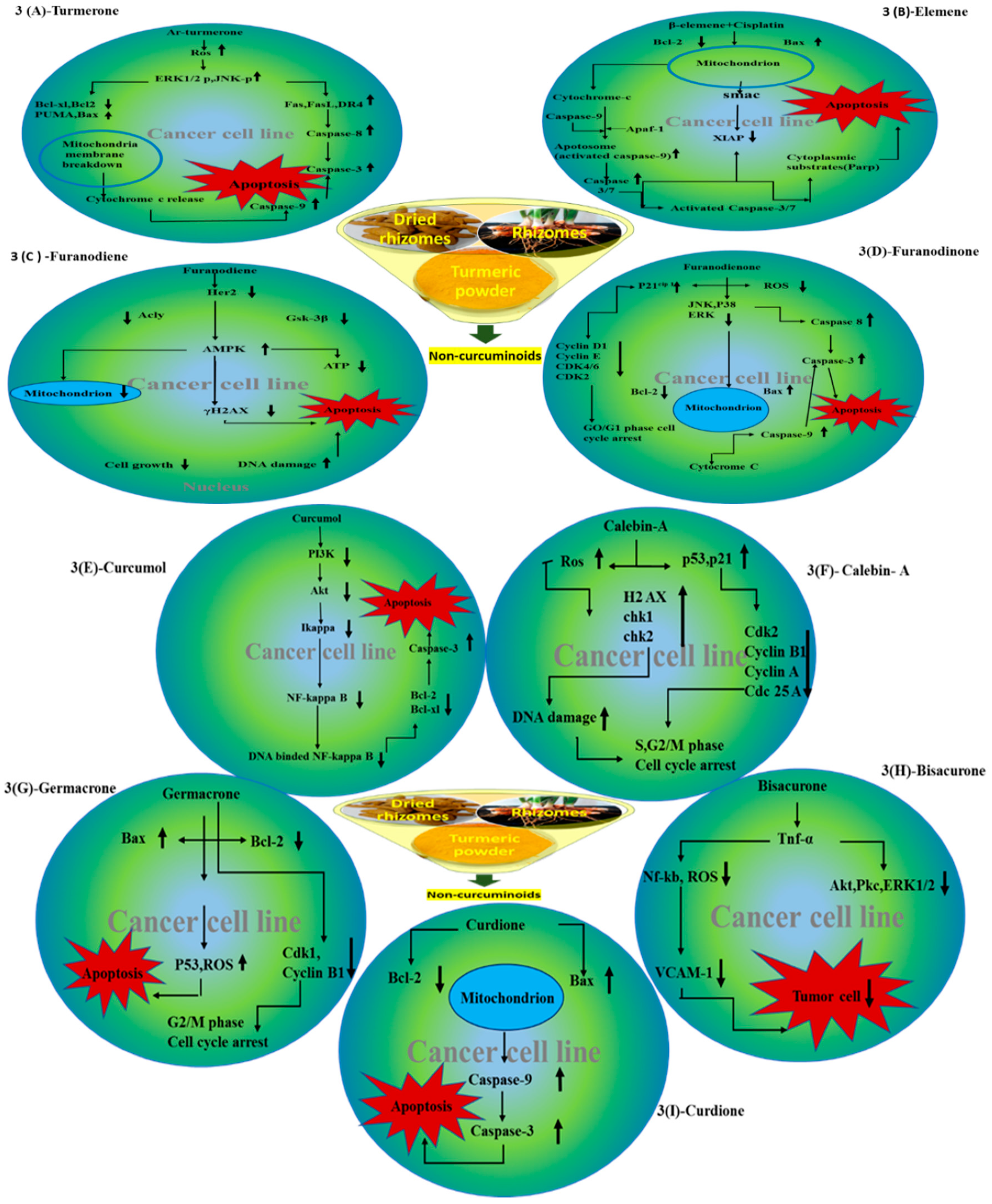
4.1. Turmerones
4.2. Elemene
4.2.1. β-Elemene
4.2.2. δ-Elemene
4.3. Furanodiene
4.4. Furanodienone
4.5. Curcumol
4.6. Cyclocurcumin
4.7. Calebin A
4.8. Germacrone
4.9. Bisacurone
4.10. Curdione
5. Critical Analysis of the In-Vivo Effects of Non-Curcuminoids
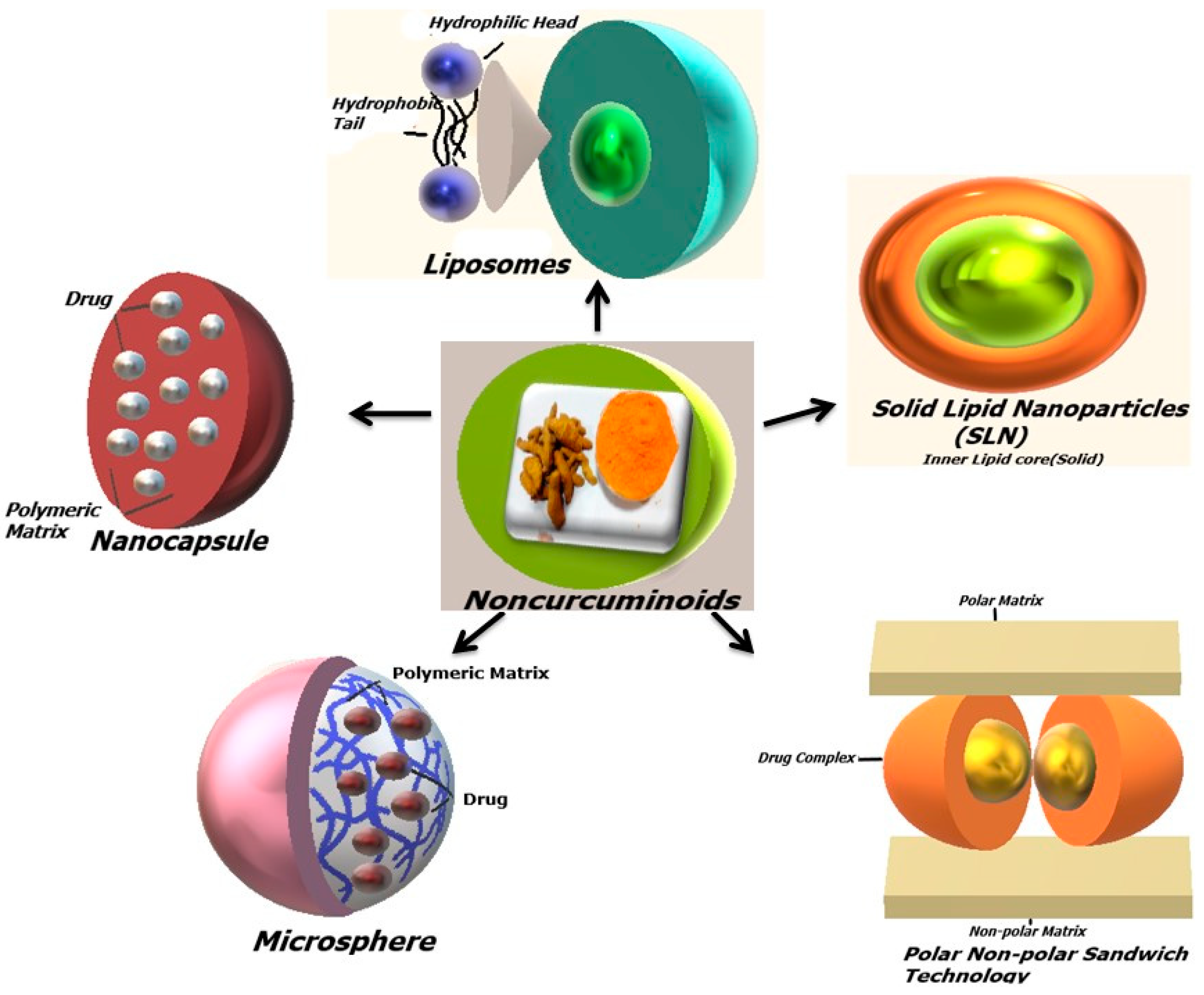
6. Biological Activities of Non-Curcuminoids in Different Drug Delivery Formulations
6.1. Cocrystals
6.2. Liposomes
6.3. Solid Lipid Nanoparticles
6.4. Nanocapsules
6.5. Microcapsule/Microsphere
6.6. Microemulsion and Nanoemulsion
6.7. Complete Natural Turmeric Matrix Formulation
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACLY | ATP citrate lyase |
| ACP | Acidic phosphatase |
| ADR | Adriamycin |
| AICAR | (5-Aminoimidazole-4-carboxamide-1-b-4-ribofuranoside |
| Akt | Protein kinase B |
| AMPK | Adenosine monophosphate-activated protein kinase |
| AMPKa | AMP-activated protein kinase |
| Ar-turmerone | Aromatic turmerone |
| ATM | Ataxia telangiectasia mutated |
| ATO | Arsenic trioxide |
| Bax | Bcl 2-associated X protein |
| Bcl | B cell lymphoma 2 |
| BCRP | Breast cancer resistance protein |
| Bid | BH3-interacting domain death agonist |
| BIP | Binding immunoglobulin protein |
| Cdc | Cell division cycle |
| CDKI | Cyclin-dependent kinases inhibitors |
| cFLIP | Cellular FLICE-like inhibitory protein |
| CHK2 | Checkpoint kinase 2 |
| cMet | Tyrosine-protein kinase Met |
| CNTM | Complete natural turmeric matrix |
| COX-2 | Cyclooxygenase -2 |
| CREB | Camp-response element |
| CTR | Calcitonin receptor |
| DC | Dendritic cells |
| DNA | Deoxyribonucleic acid |
| DNMT1 | DNA (cytosine-5)-methyltransferase 1 |
| DOXR | Doxorubicin |
| DR4 | Death receptor 4 |
| ED50 | Effective dose—50% |
| EGFR | Epithelial growth factor |
| ELISA | Enzyme-linked immunosorbent assay |
| EMT | Epithelial mesenchymal transition |
| ER | Endoplasmic reticulum |
| ERK | Extracellular regulated kinase |
| ER | α-Estrogen response |
| ETME | N-(β-Elemene-13-yl) tryptophan methyl ester |
| FACS | Fluorescence activated cell sorting |
| FAS | First apoptosis signal |
| FITC | Fluorescein isothiocyanate |
| FN | Furanodiene |
| GBM | Glioblastoma multiform |
| GFAP | Glial fibrillary acidic protein |
| GSCs | Glioblastoma stem cells |
| GSK | 3-Glycogen synthase kinase |
| HCC | Hepatocellular carcinoma |
| HER | Human epithelial growth receptor |
| HIF-1 | Hypoxia-inducible factors |
| HSC | Hepatic stellate cells |
| HUVEC | Human umbilical vein endothelial cells |
| IAPs | Inhibitor of apoptosis protein |
| IARC | The international agency for research on cancer |
| IC50 | Half-maximal inhibitory concentration |
| IGF-1R | Insulin-like factor-1 receptor |
| IL2 | Interleukin 2 |
| JAK | JANUS-activated kinases |
| JNK | c Jun N terminal kinase |
| LDH | Lactate dehydrogenase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen activated protein kinase |
| MDR | Multi-resistance protein gene |
| MKK | Mitogen-activated protein kinase kinase |
| MMP-9 | Matrix metalloproteinase -9 |
| MMP | Mitochondrial membrane potential |
| mRNA | Messenger ribonucleic acid |
| Myc | Myelocytomatosis |
| NAC | N-acetyl cysteine |
| NF-κB | Nuclear factor kappa light chain enhancer of activated B cells |
| NFATc-1 | Nuclear factor of activated T-cells, cytoplasmic 1 |
| NLC | Nanostructured lipid carriers |
| NSCLC | Non-small cell lung cancer cell |
| N-WASP | Neural Wiskott-Aldrich syndrome protein |
| PARP | Poly (ADP-ribose) polymerase; PARP |
| PCNA | Protein cell nuclear antigen |
| P-gp | Permeability glycoprotein |
| Phosphor-cdc2 | Phosphorylated cell division cycle 2 |
| PKC | Protein kinase C |
| PLGA | Poly (lactic-co-glycolic acid) |
| PUMA | p 53 upregulated modulator of apoptosis |
| RANKL | Receptor activator of nuclear factor kappa-Β ligand |
| ROS | Reactive oxygen species |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SHH | Sonic hedgehog |
| SLN | Solid lipid nanoparticles |
| STAT | Signal transducer and activator of transcription |
| TAM | Tamoxifen |
| TMZ | Temozolomide |
| TNF | Tumor necrosis factor |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| TRAP | Tartrate-resistant acid phosphatase |
| VCAM | Vascular cell adhesion molecules |
| VEGF | Vascular endothelial growth factor |
| WST-1 | Water soluble tetrazolium salts-1 |
| z-DEVD-fmk | Z-D (OMe)E (OMe)VD(OMe) fluoromethylketone |
References
- Huang, J.; Zhao, D.; Liu, Z.; Liu, F. Repurposing psychiatric drugs as anticancer agents. Cancer Lett. 2018, 419, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Chemotherapy and Dietary Phytochemical Agents. Chemother. Res. Pract. 2012, 2012, 282570. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhang, R.; Wu, F.; Zhai, L.; Wang, K.; Xiao, M.; Xie, T.; Sui, X. Non-apoptotic Cell Death in Malignant Tumor Cells and Natural Compounds. Cancer Lett. 2018, 420, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.N.; Rajashekhar, R.N.; Kaiser, J. Spicy Anticancer spices: A review. J. Int. Pharm. Pharm. Sci. 2015, 11, 1–6. [Google Scholar]
- Chavani, S.S.; Damale, M.M.; Shamkuwari, P.B.; Pawar, D.P. Traditional medicinal plants for anticancer activity. Int. J. Curr. Pharm. Res. 2013, 5, 50–54. [Google Scholar]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. Am. Assoc. Pharm. Sci. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef]
- Attokaran, M. Natural Food Flavors and Colorants; John Wiley &Sons Inc.: Hoboken, NJ, USA, 2011; pp. 391–398. [Google Scholar]
- Jacob, J.N. Comparative studies in relation to the structure and biochemical properties of the active compounds in the volatile and nonvolatile fractions of turmeric (C. longa) and ginger (Z. officinale). Stud. Nat. Prod. Chem. 2016, 48, 1–10. [Google Scholar]
- Srinivasan, K.R. The coloring matter in turmeric. Curr. Sci. 1952, 21, 311–312. [Google Scholar]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits antiinflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2016, 7, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Vyas, K. The cure is in the roots: Turmeric. J. Nutr. Disord. Ther. 2015, 5, 163. [Google Scholar]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, X.; Zhang, J.; Zhang, X.; Martin, R.C.G. Hepatic protection and anticancer activity of curcuma: A potential chemopreventive strategy against hepatocellular carcinoma. Int. J. Oncol. 2014, 44, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.; Pelletier, J. Curcumin-biological and medicinal properties. J. Pharm. 1815, 2, 1–50. [Google Scholar]
- Anderson, A.M.; Mitchell, M.S.; Mohan, R.S. Isolation of curcumin from turmeric. J. Chem. Educ. 2000, 77, 359–360. [Google Scholar] [CrossRef]
- Milobedzka, J.; Kostanecki, S.; Lampe, V. Notes on Curcumins. Ber. Deutsch. Chem. Ges. 1910, 43, 2163–2170. [Google Scholar]
- Lampe, V.; Milobedeska, J. Studien uber curcumin. Ber. Deutsch. Chem. Ges. 1913, 46, 2235–2240. [Google Scholar] [CrossRef]
- Bharat, B.A.; Indra, D.B.; Haruyo, I.; Kwang, S.A.; Gautam, S.; Santosh, K.S.; Chitra, N.; Navindra, S.; Shishir, S. Curcumin—Biological and Medicinal Properties. J. Pharm. 1815, 2, 24. [Google Scholar]
- Kuttan, R.; Bhanumathy, P.; Nirmala, K.; George, M.C. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett. 1985, 29, 197–202. [Google Scholar] [CrossRef]
- Malingre, T.R. Curcuma xanthorrhiza roxb., temoe lawak, aLs plant met galdrijrende werking. Pharm. Weekbl. 1975, 110, 601–606. [Google Scholar]
- Ohshiro, M.; Kuroyanagi, M.; Ueno, M.A. Structures of sesquiterpenes from Curcuma longa. Phytochemistry 1990, 29, 2201–2205. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Prasad, S.; Yuan, W.; Li, S.; Aggarwal, B.B. Identification of a novel compound (β-sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: Comparison with curcumin. Investig. New Drugs 2015, 33, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.T.; Zhang, M.; Zhang, C.F.; Wang, Z.T. Alkaloid and sesquiterpenes from the root tuber of Curcuma longa. Acta Pharm. Sin. 2008, 43, 724–727. [Google Scholar]
- Li, W.; Wang, S.; Feng, J.; Xiao, Y. Structure elucidation and NMR assignments for curcuminoids from the rhizomes of Curcuma longa. Magn. Reson. Chem. 2009, 47, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Prasad, V.; Pal, R.; Singh, S. Standardization and stability studies of neuroprotective lipid soluble fraction obtained from Curcuma longa. J. Pharm. Biomed. Anal. 2007, 44, 1079–1086. [Google Scholar] [CrossRef]
- Kojima, H.; Yanai, T.; Toyota, A.; Hanani, E.; Saiki, Y. Essential oil constituents from Curcuma aromatica, C. longa and C. xanthorrhiza rhizomes. In Towards Natural Medicine Research in the 21st Century; Elsevier: Amsterdam, The Netherlands, 1998; pp. 531–539. [Google Scholar]
- Chen, J.J.; Tsai, C.S.; Hwang, T.L.; Shieh, P.C.; Chen, J.F.; Sung, P.J. Sesquiterpenes from the rhizome of Curcuma longa with inhibitory activity on superoxide generation and elastase release by neutrophils. Food Chem. 2010, 119, 974–980. [Google Scholar] [CrossRef]
- Cho, B.G.; Kwak, E.K.; Chung, B.H.; Cho, W.J.; Cheon, S.H. Synthesis of sesquiterpene derivatives as potential antitumor agents; Elemane Derivative. Arch. Pharm. Res. 1999, 22, 575–578. [Google Scholar] [CrossRef]
- Li, S.; Yuan, W.; Deng, G.; Wang, P.; Yang, P. Chemical composition and product quality control of turmeric (Curcuma longa L.). Pharm. Crops 2011, 2, 28–54. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Ben, C.L.; Chan, B.C.L.; Hon, P.M.; Mavis, Y.H.; Lee, M.Y.H.; Fung, K.P.; Leung, P.C.; Lau, C.B.S. Evaluation of in vitro antiproliferative and immunomodulatory activities of compounds isolated from Curcuma longa. Food Chem. Toxicol. 2010, 48, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, J.; Ji, M. Induction of apoptosis by ar-turmerone on various cell lines. Indian J. Med. Microbiol. 2004, 14, 253–256. [Google Scholar]
- Yue, G.G.L.; Cheng, S.W.; Yu, H.; Xu, Z.S.; Lee, J.K.M.; Hon, P.M.; Lee, M.Y.H.; Kennelly, E.J.; Deng, G.; Yeung, S.K.; et al. The role of turmerones on curcumin transportation and P-glycoprotein activities in intestinal Caco-2 Cells. J. Med. Food 2012, 15, 242–252. [Google Scholar] [CrossRef]
- Sun, P.Y.; Young, K.H. Aromatic-turmerone attenuates invasion and expression of MMP-9 and COX-2 through inhibition of NF-kB activation in TPA-induced breast. J. Cancer Cells 2012, 113, 3653–3662. [Google Scholar]
- Yonggang, T.; Yiming, M.; Heying, Z.; Cheng, S.; Qiushi, W.; Xianghong, Y.; Wei, Z.; Huawei, Z.; Shan, F. Maturation and upregulation of functions of murine dendritic cells (DCs) under the influence of purified aromatic-turmerone (AR). Hum. Vaccin. Immunother. 2012, 8, 1416–1424. [Google Scholar] [PubMed]
- Cheng, H.B.; Wu, L.C.; Hsieh, Y.C.; Wu, C.H.; Chan, Y.J.; Chang, L.H.; Chang, C.M.J.; Hsu, S.L.; Teng, C.L.; Wu, C.C. Supercritical carbon dioxide extraction of aromatic turmerone from Curcuma longa L induces apoptosis through reactive oxygen species-triggered intrinsic and extrinsic pathways in human hepatocellular carcinoma HepG2 cells. J. Agric. Food Chem. 2012, 60, 9620–9630. [Google Scholar] [CrossRef]
- Kim, D.; Suh, Y.; Lee, H.; Lee, Y. Immune activation and antitumor response of ar-turmerone on P388D1 lymphoblast cell implanted tumors. Int. J. Mol. Med. 2013, 31, 386–392. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Jiang, L.; Kwok, H.F.; Lee, J.K.M.; Chan, K.M.; Fung, K.P.; Leung, P.C.; Lau, C.B.S. Turmeric ethanolic extract possesses stronger inhibitory activities on colon tumor growth than curcumin—The importance of turmerones. J. Funct. Foods 2016, 22, 565–577. [Google Scholar] [CrossRef]
- Liu, X.L.; Yang, C.; Zhang, W.H.; Zhou, G.; Ma, X.T.; Lin, B.; Zhang, M.; Zhou, Y.; Feng, T.T. Construction of turmerone moti-fused spiropyrrolidine oxindoles and their biological evaluation for anticancer activities. Tetrahedron Lett. 2016, 57, 1385–1389. [Google Scholar] [CrossRef]
- Oh, W.G.; Bail, K.U.; Jung, S.; Ahn, B.Z. The role of substituents of ar-turmerone for its anticancer activity. Arch. Pharm. Res. 1992, 15, 256–262. [Google Scholar] [CrossRef]
- Baik, K.U.; Lung, S.H.; Ahn, B.Z. Recognition of pharmacophore of ar-turmerone for its anticancer activity. Arch. Pharm. Res. 1993, 16, 254–256. [Google Scholar] [CrossRef]
- Paek, S.H.; Kim, G.J.; Jeong, H.S.; Yum, S.K. Ar-turmerone and atlantone induce internucleosomal DNA fragmentation associated with programmed cell death in human myeloid leukemia HL-60 cells. Arch. Pharm. Res. 1996, 19, 91–94. [Google Scholar] [CrossRef]
- Mukunthan, K.S.; Satyan, R.S.; Patel, T.N. Pharmacological evaluation of phytochemicals from south Indian black turmeric (Curcuma caesia Roxb.) to target cancer apoptosis. J. Ethnopharmacol. 2017, 209, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Xianping, W.; Linlin, Y.; Shu, Z. The antitumor activity of elemene is associated with apoptosis. Chin. J. Cancer Res. 1997, 9, 83–88. [Google Scholar]
- Wu, X.S.; Xiea, T.; Lina, J.; Fana, H.Z.; Huang-Fua, H.J.; Nia, L.F.; Yan, H.F. An investigation of the ability of elemene to pass through the blood-brain barrier and its effect on brain carcinomas. J. Pharm. Pharmacol. 2009, 61, 1653–1656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, G.; Li, X.; Huang, F.; Zhao, J.; Ding, H.; Cunningham, C.; Coad, J.E.; Flynn, D.C.; Reed, E.; Li, Q.Q. Antitumor effect of β-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell. Mol. Life Sci. 2005, 62, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Wangi, G.; Zhangi, M.; Cuff, C.F.; Huang, L.; Reed, E. β-elemene, a novel plant-derived antineoplastic agent, increases cisplatin chemosensitivity of lung tumor cells by triggering apoptosis. Oncol. Rep. 2009, 22, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Liua, J.; Hub, X.J.; Jina, B.; Qua, X.J.; Houa, K.Z.; Liua, Y.P. β-elemene induces apoptosis as well as protective autophagy in human non-small-cell lung cancer A549 cells. J. Pharm. pharmacol. 2011, 64, 146–153. [Google Scholar]
- Li, L.; Xu, L.; Qu, X.; Zhao, M.; Yu, P.; Kang, J.; Liu, Y.; Hu, X. Cbl-regulated Akt and ERK signals are involved in β-elemene-induced cell apoptosis in lung cancer cells. Mol. Med. Rep. 2011, 4, 1243–1246. [Google Scholar]
- Zhao, S.Y.; Wu, J.; Zheng, F.; Tang, Q.; Yang, L.J.; Li, L.; Wu, W.Y.; Hann, S.S. β-elemene inhibited expression of DNA methyltransferase 1 through activation of ERK1/2 and AMPKa signalling pathways in human lung cancer cells: The role of Sp1. J. Cell. Mol. Med. 2015, 19, 630–641. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, L.; Qu, X.; Zhao, M.; Jin, B.; Kang, J.; Liu, Y.; Hu, X. Synergistic antitumor effect of β-elemene and etoposide is mediated via induction of cell apoptosis and cell cycle arresting non-small cell lung carcinoma cells. Mol. Med. Rep. 2011, 4, 1189–1193. [Google Scholar] [PubMed]
- Liu, J.S.; He, S.C.; Zhang, Z.L.; Chen, R.; Fan, L.; Qiu, G.L.; Chang, S.; Li, L.; Che, X.M. Anticancer effects of β-elemene in gastric cancer cells and its potential underlying proteins: A proteomic study. Oncol. Rep. 2014, 32, 2635–2647. [Google Scholar] [CrossRef] [PubMed]
- Qina, Y.; Guoa, Y.; Weia, W.; Wang, B.; Jin, H.; Sun, J.; Qia, X.; Ren, S.; Zuo, Y. Antitumor effect of β-elemene in murine hepatocellular carcinoma cell line H22 depends on the level of c-Met downregulation. Biomed. Prev. Nutr. 2012, 2, 91–98. [Google Scholar] [CrossRef]
- Zhan, Y.H.; Liu, J.; Qu, X.J.; Hou, K.Z.; Wang, K.F.; Liu, Y.P.B.; Wu, B. β-elemene induces apoptosis in human renal-cell carcinoma 786-0 cells through inhibition of MAPK/ERK and PI3K/Akt/mTOR signaling pathways. Asian Pac. J. Cancer Prev. 2012, 13, 2739–2744. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Li, Q.Q.; Zhao, J.; Li, J.M.; Cuff, C.F.; Reed, E. β-elemene and taxanes synergistically induce cytotoxicity and inhibit proliferation in ovarian cancer and other tumor cells. Anticancer Res. 2013, 33, 929–940. [Google Scholar] [PubMed]
- Zhang, Y.; Mu, X.D.; Li, E.Z. The Role of E3 Ubiquitin ligase Cbl proteins in β-elemene reversing multi-drug resistance of human gastric adenocarcinoma cells: Int. J. Mol. Sci. 2013, 14, 10075–10089. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.Q.; Ding, X.; Jia, Y.C.; Huang, C.X.; Wang, Y.Z.; Xu, Y.H. Antitumor effect of β-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Lett. 2008, 264, 27–134. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, L.; Zhao, Y. β-elemene enhances both radio sensitivity and chemo sensitivity of glioblastoma cells through the inhibition of the ATM signaling pathway. Oncol. Rep. 2015, 34, 943–951. [Google Scholar] [CrossRef]
- Zhu, T.; Li, X.; Luo, L. Reversion of malignant phenotypes of human glioblastoma cells by β-elemene through β-catenin-mediated regulation of stemness-differentiation and epithelial-to-mesenchymal transition-related molecules. J. Trans. Med. 2015, 13, 356. [Google Scholar] [CrossRef]
- Xu, L.; Tao, S.; Wang, X.; Yu, Z.; Wang, M.; Chen, D.; Jing, Y.; Dong, J. The synthesis and antiproliferative effects of β-elemene derivatives with mTOR inhibition activity. Bioorgan. Med. Chem. Lett. 2006, 14, 5351–5356. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, G.; Zhang, Y.; Zhu, H.; Ren, Y.; Shen, Y.M. Synthesis and in vitro antiproliferative activity of β-elemene monosubstituted derivatives in HeLa cells mediated through arrest of cell cycle at the G1 phase. Bioorgan. Med. Chem. 2009, 17, 1118–1124. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wang, G.; Huang, F.; Li, J.M.; Cuff, C.F.; Reed, E. Sensitization of lung cancer cells to cisplatin by b-elemene is mediated through blockade of cell cycle progression: Antitumor efficacies of β-elemene and its synthetic analogs. Med. Oncol. 2013, 30, 488. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Lee, R.X.; Liang, H.; Zhong, Y. Anticancer activity of β-Elemene and its synthetic analogs in human malignant brain tumor cells. Anticancer Res. 2013, 33, 65–76. [Google Scholar] [PubMed]
- Yua, Z.; Wu, F.; Chen, L.; Li, Q.; Wang, C.; Dong, J.; Xie, S.Q. ETME, a novel β-elemene derivative, synergizes with arsenic trioxide in inducing apoptosis and cell cycle arrest in hepatocarcinoma cells via a p53-dependent pathway. Acta Pharmacol. Sin. B 2014, 4, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, T.; Xu, S.; Zhang, P.; Lin, A.; Wu, L.; Yao, H.; Xie, W.; Zhu, Z.; Xu, J. Discovery of novel antitumor nitric oxide-donating β-elemene hybrids through inhibiting the PI3K/Akt pathway. Eur. J. Med. Chem. 2017, 135, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, T.; Xu, S.; Lin, A.; Yao, H.; Xie, W.; Zhu, Z.; Xu, J. Novel hybrids of natural β-elemene bearing isopropanolaminemoieties: Synthesis, enhanced anticancer profile, and improved aqueous solubility. Fitote 2017, 120, 117–125. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Hu, D. Furanodienone induces G0/G1 arrest and causes apoptosis via the ROS/MAPKs-mediated caspase-dependent pathway in human colorectal cancer cells: A study in vitro and in vivo. Cell Death Dis. 2017, 8, e2815. [Google Scholar] [CrossRef]
- Tang, C.Y.; Zhu, L.X.; Yu, J.D.; Chen, Z.; Gu, M.C.; Mu, C.F.; Liu, Q.; Xiong, Y. Effect of β-elemene on the kinetics of intracellular transport of d-luciferin potassium salt (ABC substrate) in doxorubicin-resistant breast cancer cells and the associated molecular mechanism. Eur. J. Pharm. Sci. 2018, 120, 20–29. [Google Scholar] [CrossRef]
- Guo, H.Q.; Zhang, G.N.; Wang, Y.J.; Zhang, Y.K.; Sodani, K.; Talele, T.T.; Charles, R.; Ashbey, C.R.; Chen, Z.S. β-elemene, a compound derived from Rhizoma zedoariae, reverses multidrug resistance mediated by the ABCB1 transporter. Oncol. Rep. 2014, 31, 858–866. [Google Scholar] [CrossRef]
- Kataria, D.; Chahal, K.K.; Kumar, A.; Singh, R. Antifungal Activity and Molecular Docking Studies with Sesquiterpenoids from Daucus carota. Pestic. Res. J. 2017, 29, 188–195. [Google Scholar]
- Wang, X.S.; Yang, W.; Tao, S.J.; Li, K.; Li, M.; Dong, J.H.; Wang, M.W. Effect of delta-elemene on Hela cell lines by apoptosis induction. Yakugaku Zasshi 2006, 126, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yang, F.Q.; Li, S.P.; Gao, J.L.; Hu, G.; Lao, S.C.; Conceicao, L.E.; Fung, K.P.; Wang, Y.T.; Simon, L.M.Y. Furanodiene induces G2/M cell cycle arrest and apoptosis through MAPK signaling and mitochondria-caspase pathway in human hepatocellular carcinoma cells. Cancer Biol. Ther. 2007, 6, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Wang, X.; Li, Y.; Sun, X.; Tai, W.; Li, T.; Guo, T. Induction of apoptosis by furanodiene in HL60 leukemia cells through activation of TNFR1 signaling pathway. Cancer Lett. 2008, 271, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Ba, Z.Z.; Zheng, Y.P.; Zhang, H.; Sun, X.Y.; Lin, D.H. Potential anticancer activity of furanodiene. Chin. J. Cancer Res. 2009, 21, 154–158. [Google Scholar] [CrossRef]
- Sun, X.Y.; Zheng, Y.P.; Lin, D.H.; Zhang, H.; Zhao, F. Potential anticancer activities of furanodiene, A sesquiterpene from Curcuma wenyujin. Am. J. Chin. Med. 2009, 37, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Peia, L.; Liua, S.; Zhenga, J.; Chen, X. A sensitive method for determination of furanodiene in rat plasma using liquid chromatography/tandem mass spectrometry and its application to a pharmacokinetic study. Biomed. Chromatogr. 2011, 26, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Zhonga, Z.; Danga, Y.; Yuan, X.; Guo, W.; Lia, Y.; Tana, W.; Cuib, J.; Lua, J.; Zhanga, Q.; Chena, X.; et al. Furanodiene, a natural product, inhibits breast cancer growth both in vitro and in vivo. Cell. Physiol. Biochem. 2012, 30, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.F.; Hoi, P.M.; Wua, G.S.; Xua, Z.T.; Tana, W.; Chena, X.P.; Cuic, L.; Wuc, T.; Wang, Y.T. Anti-angiogenic effect of furanodiene on HUVECs in vitro and on zebrafish in vivo. J. Ethnopharmacol. 2012, 141, 721–727. [Google Scholar] [CrossRef]
- Xu, W.S.; Dang, Y.Y.; Guo, J.J.; Wu, G.S.; Lu, J.J.; Chen, X.P.; Wang, Y.T. Furanodiene induces endoplasmic reticulum stress and presents antiproliferative activities in lung cancer cells. Evid. Based Complement. Altern. Med. 2012, 2012, 426521. [Google Scholar] [CrossRef]
- Zhong, Z.F.; Li, Y.B.; Wang, S.P.; Tan, W.; Chen, X.P.; Chen, M.W.; Wang, Y.T. Furanodiene enhances tamoxifen-induced growth inhibitory activity of ERa-positive breast cancer cells in a PPARg independent manner. J. Cell. Biochem. 2012, 113, 2643–2651. [Google Scholar] [CrossRef]
- Zhong, Z.; Tan, W.; Chen, X.; Wang, Y. Furanodiene, a natural small molecule suppresses metastatic breast cancer cell migration and invasion in vitro. Eur. J. Pharm. 2014, 737, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.F.; Tan, W.; Qiang, W.W.; Scofield, V.L.; Tian, K.; Wang, C.M.; Qiang, W.A.; Wang, Y.T. Furanodiene alters mitochondrial function in doxorubicin-resistant MCF-7 human breast cancer cells in an AMPK-dependent manner. Mol. Biosyst. 2016, 12, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.F.; Tan, W.; Tian, K.; Yu, H.; Qiang, W.A.; Wang, Y.T. Combined effects of furanodiene and doxorubicin on the migration and invasion of MDA-MB-231 breast cancer cells in vitro. Oncol. Rep. 2016, 37, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Zhu, G.Y.; Shen, X.L.; Chu, J.H.; Yu, Z.L.; Fong, W.F. Furanodienone inhibits cell proliferation and survival by suppressing ERα signaling in human breast cancer MCF-7 cells. J. Cell. Biochem. 2011, 112, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Zhu, G.Y.; Shen, X.L.; Chu, J.H.; Yu, Z.L.; Fong, W.F. Furanodienone induces cell cycle arrest and apoptosis by suppressing EGFR/HER2 signaling in HER2-overexpressing human breast cancer cells. Cancer Chemother. Pharmacol. 2011, 68, 1315–1323. [Google Scholar] [CrossRef]
- Jiang, Z.; Jacob, J.A.; Loganathachetti, D.S.; Nainangu, P.; Chen, B. β-Elemene: Mechanistic studies on cancer cell interaction and its chemosensitization effect, Mini review. Front. Pharmacol. 2017, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Z.; Chen, T. Curcumol induces apoptosis via caspases-independent mitochondrial pathway in human lung adenocarcinoma ASTC-a-1 cells. Med. Oncol. 2011, 28, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Dang, Y.Y.; Lu, J.J.; Wang, Y.T. Anticancer activities of terpenoids isolated from Rhizoma Curcumae—A review. J. Ethnopharm. 2012, 143, 406–411. [Google Scholar]
- Chen, G.; Wang, Y.; Li, M.; Xu, T.; Wang, X.; Hong, B.; Niu, Y. Curcumol induces HSC-T6 cell death through suppression of Bcl-2: Involvement of PI3K and NF-κB pathways. Eur. J. Pharm. Sci. 2014, 65, 21–28. [Google Scholar] [CrossRef]
- Wang, J.; Huang, F.; Bai, Z.; Chi, B.; Wu, J.; Chen, X. Curcumol Inhibits Growth and Induces Apoptosis of Colorectal Cancer LoVo Cell Line via IGF-1R and p38 MAPK Pathway. Int. J. Mol. Sci. 2015, 16, 19851–19867. [Google Scholar] [CrossRef]
- Simon, A.; Allais, D.P.; Duroux, J.L.; Basly, J.P. Inhibitory effect of curcuminoids on MCF-7 cell proliferation and structure-activity relationships. Cancer Lett. 1998, 129, 111–116. [Google Scholar] [CrossRef]
- Adhikary, R.; Barnes, C.A.; Trampel, R.L.; Wallace, S.J. Photoinduced trans-to-cis isomerization of cyclocurcumin. J. Phys. Chem. B 2011, 115, 10707–10714. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bora, U. Molecular Docking Studies of Curcumin Natural Derivatives with DNA Topoisomerase I and II-DNA Complexes. Interdiscip. Sci. Comput. Life Sci. 2014, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Han, Y.; Liu, J.; Zhang, J.; Li, F.; Wang, Y.; Liu, X.; Yao, L. Calebin A induces apoptosis and modulates MAPK family activity in drug resistant human cancer cell. Eur. J. Pharmacol. 2008, 591, 252–258. [Google Scholar] [CrossRef]
- Liou, W.S.; Lin, C.; Lee, P.S. Calebin A induces cell cycle arrest in human colon cancer cells and xenografts in nude mice. J. Funct. Foods 2016, 26, 781–791. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Prasad, S.; Majeed, M.; Aggarwal, B.B. Calebin A downregulates osteoclastogenesis through suppression of RANKL signaling. Arch. Biochem. Biophys. 2016, 593, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Xiuping, C.; Zangfen, Z.; Xu, Z.; Zhou, K.; Wu, T.; Cui, L.; Wang, Y. Germacrone inhibits the proliferation of breast cancer cell lines by inducing cell cycle arrest and promoting cell apoptosis. Eur. J. Pharmacol. 2011, 667, 50–55. [Google Scholar]
- Bin, F.; Wei, W.; Yunyi, L. Antitumor effect of germacrone on human hepatoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Eur. J. Pharmacol. 2012, 698, 95–102. [Google Scholar]
- Liu, Y.Y.; Zheng, Q.; Fang, B.; Wang, W.; Ma, F.Y.; Roshan, S.; Banafa, A.; Chen, M.J.; Chang, J.L.; Deng, X.M.; et al. Germacrone induces apoptosis in human hepatoma hepG2 cells through inhibition of the JAK2/STAT3 signaling pathway. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 339–345. [Google Scholar] [CrossRef]
- Xie, X.H.; Zhao, H.; Hu, Y.Y.; Gu, X.D. Germacrone reverses adriamycin resistance through cell apoptosis in multidrug-resistant breast cancer cells. Exp. Ther. Med. 2014, 8, 1611–1615. [Google Scholar] [CrossRef]
- Lim, M.S.; Choung, S.Y.; Jeong, K.W. Germacrone inhibits estrogen receptor α-mediated transcription in MCF-7 breast cancer cells. Phytother. Res. 2016, 30, 2036–2043. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.B.; Wu, J.; Chen, W.; Feng, Y.; Shen, Z. Novel anticancer agents based on germacrone: Design, synthesis, biological activity, docking studies and MD simulations. RSC Adv. 2017, 7, 3760. [Google Scholar] [CrossRef]
- Sripathi, R.; Ravi, S. Molecular Docking Studies of the Constituents Present in the Essential Oil of Plectranthus hadiensis against Bacterial Proteins. Int. J. Chem. Sci. 2017, 15, 185. [Google Scholar]
- Suna, D.I.; Nizamutdinova, I.T.; Kim, Y.M.; Cai, X.F.; Lee, J.J.; Kang, S.S.; Kim, Y.S.; Kang, K.M.; Chai, G.Y.; Chang, K.C.; et al. Bisacurone inhibits adhesion of inflammatory monocytes or cancer cells to endothelial cells through down-regulation of VCAM-1 expression. Int. Immunopharmacol. 2008, 8, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Oh, O.J.; Min, H.Y.; Lee, S.K. Inhibition of inducible prostaglandin E2 production and cyclooxy-genase-2 expression by curdione from Curcuma zedoaria. Arch. Pharm. Res. 2007, 30, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Sun, F.; Chen, X. Impact of Fixed-Dose Combination of germacrone, curdione, and furanodiene on breast cancer cell proliferation. Cell J. 2013, 15, 160–165. [Google Scholar]
- Li, J.; Bian, W.H.; Wan, J.; Zhou, J.; Lin, Y.; Wang, J.R.; Wang, Z.X.; Shen, Q.; Wang, K.M. Curdione inhibits proliferation of MCF-7 cells by inducing apoptosis. Asian Pac. J. Cancer Prev. 2014, 15, 9997–10001. [Google Scholar] [CrossRef]
- Li, J.; Mao, C.; Li, L.; Ji, D.; Yin, F.; Lang, Y.; Lua, T.; Xiao, Y.Q.; Li, L. Pharmacokinetics and liver distribution study of unbound curdione and curcumol in rats by microdialysis coupled with rapid resolution liquid chromatography (RRLC) and tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 95, 146–150. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Grabarska, A.; Łuszczki, J.; Czernicka, L.; Nowosadzka, E.; Gumbarewicz, E.; Jarząb, A.; Audo, G.; Upadhyay, S.; Głowniak, K.; et al. Superior anticancer activity is demonstrated by total extract of Curcuma longa L. as opposed to individual curcuminoids separated by centrifugal partition chromatography. Phytother. Res. 2018, 32, 933–942. [Google Scholar] [CrossRef]
- Mahmood, K.; Zia, K.M.; Zuber, M.; Salman, M.; Anjum, M.N. Recent developments in curcumin and curcumin based polymeric materials for biomedical applications: A review. Int. J. Biol. Macromol. 2015, 81, 877–890. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ (accessed on 23 February 2018).
- Chang, Z.; Gao, M.; Zhang, W.; Song, L.; Jia, Y.; Qin, Y. Beta-elemene treatment is associated with improved outcomes of patients with esophageal squamous cell carcinoma. Surg. Oncol. 2017, 26, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Peng, X.X.; Sun, R.; Li, J.; Zhan, X.R.; Wu, L.J.; Xie, T. Systematic Review of β-Elemene injection as adjunctive treatment for lung cancer. Chin. J. Integr. Med. 2012, 18, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.; Ghaisas, S.; Vaidya, A.; Vaidya, R.; Kamat, D.V.; Bhagwat, A.N.; Bhide, S. Early Human Safety Study of Turmeric Oil (Curcuma longa Oil) Administered Orally in Healthy Volunteers. J. Assoc. Phys. Indian 2003, 51, 1055–1060. [Google Scholar]
- Haiyee, Z.A.; Saim, N.; Said, M.; Illias, R.M.; Mustapha, W.A.W.; Hassan, O. Characterization of cyclodextrin complexes with turmeric oleoresin. Food Chem. 2009, 114, 459–465. [Google Scholar] [CrossRef]
- Gopi, S.; Jacob, J.; Varma, K.; Jude, S.; Amalraj, A.; Arundhathy, C.A.; George, R.; Sreeraj, T.R.; Divya, C.; Kunnumakkara, A.B.; et al. Comparative oral absorption of curcumin in a natural turmeric matrix with two other curcumin formulations: An open-label parallel arm study. Phytother. Res. 2017, 31, 1883–1891. [Google Scholar] [CrossRef]
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A novel highly bioavailable curcumin formulation improves symptoms and diagnostic indicators in rheumatoid arthritis patients: A randomized, double-blind, placebo-controlled, two-dose, three-arm, and parallel-group study. J. Med. Food 2017, 20, 1022–1030. [Google Scholar] [CrossRef]
- Sanphui, P.; Bolla, G. Curcumin—A biological wonder molecule: A crystal engineering point of review. Cryst. Growth Des. 2018, 18, 5690–5711. [Google Scholar] [CrossRef]
- Wong, S.N.; Hu, S.; Ng, W.W.; Xu, X.; Lai, K.L.; Lee, W.Y.T.; Chow, A.H.L.; Sun, C.C.; Chow, S.F. Cocrystallization of curcumin with benzenediols and benzenetriols via rapid solvent removal. Cryst. Growth Des. 2018, 18, 5534–5546. [Google Scholar] [CrossRef]
- Chen, M.; Wang, S.; Tan, M. Applications of nanoparticles in herbal medicine: Zedoary turmeric oil and its active compound β-elemene. Am. J. Chin. Med. 2011, 39, 1093–1102. [Google Scholar] [CrossRef]
- Li, G.; Lin, D.H.; Xie, X.X.; Qin, L.F.; Wang, J.T.; Liu, K. Uptake and transport of furanodiene in Caco-2 cell monolayers: A comparison study between furanodiene and furanodiene loaded PLGA nanoparticles. Chin. J. Nat. Med. 2013, 11, 49–55. [Google Scholar] [CrossRef]
- Zhang, J.; He, Y.; Jiang, J.; Li, M.; Jin, C.; Wang, L. In vitro and in vivo evaluation of folate mediated pegylated nanostructured lipid carriers for the efficient delivery of furanodiene. Drug Dev. Ind. Pharm. 2017, 43, 1520–5762. [Google Scholar] [CrossRef]
- Pan, M.H.; Chiou, Y.S.; Kalyanam, N.; Ho, C.T.; Ding, B. Preparation of Calebin A Liposomes and its Antiproliferation in Human Cancer Cells. J. Anal. Pharm. Res. 2017, 5, 00137. [Google Scholar] [CrossRef]
- Natrajan, D.; Srinivasan, S.; Sundar, K.; Ravindran, A. Formulation of essential oil-loaded chitosan alginate nanocapsules. J. Food Drug Anal. 2015, 23, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wei, Z.; Zhao, Y. In vitro and in vivo anti-tumor characterizations of β-elemene-loaded nanoemulsion. J. Nanomed. Nanotechnol. 2016, 12, 449–575. [Google Scholar] [CrossRef]
- Amalraj, A.; Jude, S.; Varma, K.; Jacob, J.; Gopi, S.; Luwafemi, O.S.; Thomas, S. Preparation of a novel bioavailable curcuminoid formulation (Cureit™) using Polar-Nonpolar-Sandwich (PNS) technology and its characterization and applications. Mater. Sci. Eng. C 2017, 75, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; George, R.; Thomas, M.; Jude, S. A pilot cross-over study to assess the human bioavailability of “Cureit” a bioavailable curcumin in complete natural matrix. Asian J. Pharm. Technol. Innov. 2015, 3, 92–96. [Google Scholar]
- Gopi, S.; George, R.; Jude, S.; Sriraam, V.T. Cell culture study on the cytotoxic effects of “Cureit”—A novel bio available curcumin-anticancer effects. J. Chem. Pharm. Res. 2014, 6, 96–100. [Google Scholar]
- Gopi, S.; George, R.; Sriraam, V.T. Anti-oxidant potential of “Cureit”—A novel bioavailable Curcumin Formulation. Asian J. Pharm. Technol. Innov. 2014, 2, 123–127. [Google Scholar]
- Gopi, S.; George, R.; Sriraam, V.T. Cell culture study on the effects of “cureit” hyaluronidase inhibition—Anti aging effects. Int. J. Curr. Res. 2014, 6, 8473–8474. [Google Scholar]

| Name | IUPAC Name | Structure | Molecular Weight (g/mol) | Molecular Formula | Physical and Chemical Properties |
|---|---|---|---|---|---|
| Ar-turmerone | 6S-2-methyl-6-(4-methylphenyl) hept-2-en-4-one | 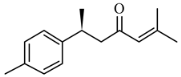 | 216.324 | C15H20O | Appearance: Yellowish oil Boiling point: 326 °C Melting point: 122 °C Density: 0.945 g/cm3 Solubility: Hexane, petroleum ether, ethanol |
| β-Elemene | (1S,2S,4R)-1-ethenyl-1-methyl-2,4-bis(prop-1-en-2-yl) cyclohexane | 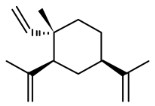 | 204.357 | C15H24 | Appearance: Colorless to yellow clear liquid Boiling point: 252 °C Melting point: 98 °C Density: 0.862 g/cm3 Solubility: Alcohol |
| γ- Elemene | (1S,2S)-1-ethenyl-1-methyl-2-propan-2-ylidene-2-prop-1-en-2-yl) cyclohexane | 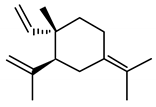 | 204.357 | C15H24 | Appearance: Clear and colorless liquid Boiling point: 258.2 ± 40 °C at 760 mmHg Melting point: 101.3 ± 22.2 °C Density: 0.9 ± 0.1 g/cm3 Solubility: Alcohol |
| Furanodiene | (5E,9E)-3,6,10-trimethyl-4,7,8,11-tetrahydrocyclodeca(b) furan | 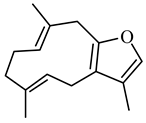 | 216.324 | C15H20O | Appearance: White powder Boiling point: 309.6 ± 11 °C at 760 mmHg Melting point: 137.1 ± 6.1 °C Density:0.9 ± 0.1 g/cm3 Solubility: Partially soluble in water |
| Calebin A | (E-4-(4-hydroxy-3-methoxyphenyl)-2-oxobut-3-enyl)E-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate) | 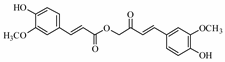 | 384.384 | C21H20O7 | Appearance: White powder Boiling point: 592.1 ± 50.0 °C at 760 mmHg Melting point: 207.9 ± 23.6 °C Density:1.3 ± 0.1 g/cm3 Solubility: Sparingly soluble in water |
| Germacrone | (3E,7E)-3,7-dimethyl-10-propan-2-ylidenecyclodeca-3,7-dien-1-one | 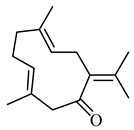 | 218.34 | C15H22O | Appearance: White crystalline powder Boiling point:666.52 °C Melting point:347.53 °C Density:0.9 ± 0.1 g/cm3 Solubility: Methanol |
| Cyclocurcumin | (E)-2-(4-hydroxy-3-methoxyphenyl-6-(4-hydrox-3-methoxystyryl)-2H-pyra-4(3H)-one |  | 368.38 | C21H20O6 | Appearance: Yellow powder Boiling point:571.9 ± 50.0 °C at 760 mmHg Melting point: 202.6 ± 23.6 °C Density:1.4 ± 0.1 g/cm3 Solubility: Ethanol and concentrated acetic acid |
| Bisacurone | (6S)-6-((1R,4S,5S)-4,5-dihydroxy-4-methylcyclohex-2-en-1-yl) |  | 254.354 | C15H24O3 | Appearance: Bright orange powder Boiling point: 377.7 ± 42.0 °C at 760 mmHg Melting point: 196.4 ± 24.4 °C Density: 1.1 ± 0.1 g/cm3 Solubility: Acetone and ethanol |
| Curcumol | (1S,2S,5S,9S)-9-isopropyl-2-methyl-6-methylene-11-oxatricyclo(6.2.1.01,5)undecan-8-ol | 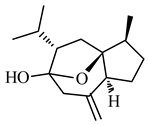 | 236.355 | C15H24O2 | Appearance: White crystals Boiling point:334.5 °C Melting point:134.69 C Density:1.06 g/cm3 Solubility: DMSO in ethanol |
| Non-Curcuminoids | Various Anticancer Studies | References |
|---|---|---|
| Turmerone | Unlike curcuminoids, turmerone brought apoptosis due to the distortion caused to the mitochondria in the intrinsic pathway with activation in procaspase-3 cleavage | [33] |
| Ar-turmerone | Ar-turmerone brought apoptosis on various cell lines such as K562, RBL-2H3, L1210, and U937 in time and dose-dependent manner. | [34] |
| Ar-turmerone facilitated curcumin transport through heterogeneous human epithelial colorectal adenocarcinoma (Caco 2) cells and inhibited the efflux of rhodamine-123 and permeability glycoprotein multidrug resistant gene messenger ribonucleic acid (Pgp (MDRI gene) mRna) expression levels. | [35] | |
| Ar-turmerone suppressed 12-O-tetradecanoylphorbol-13-acetate (TPA)- induced up-regulation of matrix metalloproteinase -9 (MMP-9) and cyclooxygenase-2 (COX-2) expression by blocking nuclear factor kappa light chain enhancer of activated B cells (NF-kB), Phosphoinositide 3-kinase/ protein kinase B (PI3K/Akt) and extracellular regulated kinase (ERK)1/2 signaling in human breast cancer cells and inhibited TPA induced invasion, migration, and colony formation in human breast cancer cells. | [36] | |
| Ar-turmerone was effective on murine dendritic cells (DCs). | [37] | |
| Ar-turmerone showed inhibitory action against hepatocellular carcinoma cells by apoptosis through intracellular reactive oxygen species (ROS) generation-mediated activation of ERK and c Jun N terminal kinase (JNK) kinases. | [38] | |
| Ar-turmerone had cytotoxic effects on Lymphocytic leukemia (L-1210) and myeloid cell line (HL-60) cells with an inhibition rate of 11–12% and apoptosis index 5–6. | [39] | |
| Ar-turmerones enhanced the activities of curcumin in human colon carcinoma (HCT-116), colorectal adenocarcinoma (HT-29) and human umbilical vein endothelial cells (HUVEC) cell lines. | [40] | |
| Ar-turmerone, when motif-fused with spiropyrollidone oxindoles increased the potency in lung cancer and leukemia cells. | [41] | |
| Ar-turmerone and its analogs exhibited cytotoxic activities against L1210 cell. | [42] | |
| Ar-turmerone exhibited apoptosis through changes in morphological characters. | [43] | |
| Ar-turmerone exhibited apoptosis on human myeloid leukemia HL-60 cells. | [44] | |
| Elemene | Elemene can inhibit the tumor growth of various cells, such as ovarian, laryngeal, non-small cell lung, prostrate, melanoma, leukemia, breast, brain, hepatoma, colorectal adenocarcinoma, glioblastoma, and human cervix epithelioid carcinoma cells | [45] |
| Elemene brought apoptosis in HL-60 cell by causing cell cycle arrest between phase transitions from S to G2M phase | [46] | |
| Elemene can pass through the blood brain barrier due to its small size and lipophilic nature which is helpful in brain carcinomas | [47] | |
| β-elemene | The mechanism of action of β-elemene in non-small-cell lung cancer cell (NSCLC) death may be through a mitochondrial release of the cytochrome c-mediated apoptotic pathway. | [48] |
| β-elemene sensitizes the human NSCLC cell lines H460 and A549 to cisplatin via mitochondria mediated intrinsic apoptosis pathway involving B cell lymphoma-2 (Bcl-2) family proteins and IAPs (inhibitor of apoptosis proteins) in a time and dose-dependent manner. | [49] | |
| β-elemene exhibited antitumor effect on NSCLC A549 cells and inhibited the activity of the PI3K/Akt/mammalian target of rapamycin (mTOR)/Ribosomal protein S6 kinase beta-1(p70S6K1) signaling pathway, resulting in apoptosis as well as protective autophagy. | [50] | |
| β-elemene inhibited ERK and Akt activation and upregulation of Casitas B–lineage lymphoma (c-Cb1 and Cb1-b) expression to initiate apoptosis in lung cancer cells. | [51] | |
| β-elemene has potential to inhibit human lung cancer NSCLC cell growth via ERK1/2- and AMP-activated protein kinase (AMPKa)-mediated inhibition of transcription factor Sp1, followed by reduction in DNA (cytosine-5)-methyltransferase 1(DNMT1) protein expression. | [52] | |
| β-elemene in combination with etoposide is beneficial for lung cancer cells. | [53] | |
| β-elemene is a promising therapeutic role in human SGC7901 and MKN45 gastric cancer cells. | [54] | |
| β-elemene and its relation between the expression level of tyrosine-protein kinase Met (c-Met) promoted its anticancer mechanism. | [55] | |
| β-elemene can inhibit the proliferation of RCC 786- 0 cells by the inhibition of mitogen activated protein kinase (MAPK)/ERK and PI3K/Akt/mTOR signaling pathway, thus inducing apoptosis and protective autophagy. | [56] | |
| β-elemene when treated with taxane over ovarian cell lines A2780/CP70 and its parental cell line A2780 showed promising results. | [57] | |
| β-elemene can reverse tumor multi drug resistance (MDR) and enhanced the doxorubicin activity. | [58] | |
| β-elemene showed antiproliferative activity on glioblastoma cells which was supported by phosphorylation of p38 MAPK, cell-cycle arrest in the G0/G1 phase. | [59] | |
| β-elemene inhibited the proliferation and survival of the cell lines of glioblastoma multiforme (GBM) when combined with radiotherapy or temozolomide (TMZ) via inhibition of DNA damage repair. | [60] | |
| β-elemene arrested glioblastoma cells (C6 and U87) in G0/G1 phase of the cell cycle, as well as brought about inhibition in cell proliferation by regulating the glia mutation factor β/mitogen activated protein kinase 3/6/p38 and extracellular signal-regulated kinase 1/2/B cell lymphoma 2/surviving pathways. | [61] | |
| β-elemene enhances susceptibility to cisplatin resistant ovarian cancer cell. | [12] | |
| β-elemene exhibited antiproliferative activities, promoted apoptosis, impaired invasiveness in glioblastoma cells, suppressed the growth of animal xenografts. | [62] | |
| β-elemene and its derivatives containing a piperazine, a morpholine, a tetrahydropyrrole, a thiophenylethylamine, or a cyclohexamine group exhibited proliferative activity in human cervix epitheloid carcinoma HeLa, gastric carcinoma SGC-7901, and leukemia K562 cells. | [63] | |
| β elemene monosubstituted amine, ether and rhenium coordinated complex structure was synthesized and characterized. The in vitro anti-proliferative activity of β-elemene monosubstituted amine and Re (CO)3-β-elemene derivatives in human cervix epitheloid carcinoma HeLa cells were promising. | [64] | |
| β-elemene-enhanced inhibitory effect of cisplatin on lung carcinoma cell proliferation, regulated by a check point kinase (CHK) 2-mediated cell division cycle CDC25C/CDC2/cyclin B1 signaling pathway which leads to the blockade of cell cycle progression at G2/M. | [65] | |
| Arsenic trioxide (ATO) combined with the derivative of β-elemene, N-(β-elemene-13-yl) tryptophan methyl ester (ETME) synergistically enhanced the antiproliferative activity and apoptosis in hepatocellular carcinoma (HCC) cells. | [66] | |
| Novel furoxan-based NO- donating β-elemene hybrids are promising anti-cancer agents. | [67] | |
| Comparison of cytotoxic efficacy of β-elemene and its synthetic analogs [(R or S)–2–((1R,3S,4S)–3–isopropenyl–4–methyl–4–vinylcyclohexyl)–propane–1,2–diol] (Lr-1), [(S)–2–((1R,3S,4S)–3–isopropenyl–4–methyl–4–vinyl–cyclohexyl)–propane–1,2–diol and (R)–2–((1R,3S,4S)–3–isopropenyl–4–methyl–4–vinylcyclohexyl)–propane–1,2–diol] Lr-2) in the brain tumor cell lines A172, CCF-STTG1, and U-87MG proved beneficial. | [68] | |
| β-elemene was reviewed for its anticancer effects on different cancer cells and apoptosis and cell cycle arrest were found to be the major cause behind its anticancer activities β-elemene is influential in altering the functionality and quantity of ABC transporters thereby efficient in doxorubicin resistant breast cancer cells. | [69,70] | |
| The possible mode of anticancer activity of β-elemene in altering MDR through the inhibition of ABCB1 transporter efflux activity was determined through molecular docking study | [71] | |
| Docking studies proved that sesquiterpenes have potential biological activities | [72] | |
| δ-elemene | Increment of p38 MAPK and inducible nitric oxide synthase levels were seen when δ -elemene was treated in NCL-H292 lung cancer cells through activation of the caspase signaling pathway. | [73] |
| Furanodiene | Furanodiene were useful in the treatment of liver diseases. | [74] |
| Furanodiene was influential on human leukemia HL60 cells, evaluated by DNA fragmentation. | [75] | |
| Furanodiene is an effective agent against uterine cervix cancer, and has a protective effect on the immune function. | [76] | |
| Furanodiene induced cell death in dose-dependent manner when analyzed by (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction assay MTT assay, thus effective against uterine cervical cancer growth. | [77] | |
| Bioavailability of furanodiene in rat’s plasma as analyzed with liquid chromatography/tandem mass spectrometry was about 49.0%. | [78] | |
| Furanodiene suppresses breast cancer cell growth both in vitro and in vivo in a dose-dependent manner inducing cell cycle arrest at the G0/G1 phase. | [79] | |
| Furanodiene increases the inhibition activities and apoptosis nature of tamoxifen, thus facilitating the treatment of breast cancer. It was beneficial against proliferation carried out by vascular endothelial growth factor (VEGF). | [80] | |
| Furanodiene showed antiproliferative activity on A549, NIH-H1299, and 95-D lung cancer cells and was useful in combination therapy with paclitaxel. | [81] | |
| Growth inhibitory and pro-apoptosis activity of furanodiene was enhanced and affected Tamoxifen (TAM) by inducing cell cycle arrest and cell apoptosis. | [82] | |
| Furanodiene suppresses proliferation and increase the lactate dehydrogenase (LDH) release in a dose-dependent manner by cell cycle arrest at G0/G1 phase and apoptosis in breast cells. | [83] | |
| Doxorubicin-resistant MCF-7 (MCF-7/DOXR) breast cancer cells, when treated with furanodiene showed an alteration in mitochondrial function as well as adenosine triphosphate (ATP) levels were reduced causing apoptotic cell death. | [84] | |
| The combinational treatment of furanodiene with doxorubicin in breast cancer cells were found promising. | [85] | |
| Furanodienone | Furanodienone inhibited cell cycle proliferation and induced apoptosis in dose-dependent manner by inhibiting estrogen receptor alpha signaling alpha and mRNA expression levels without effecting estrogen receptor (ER) beta. | [86] |
| Furanodienone also triggered apoptosis on human breast cancer cells through epithelial growth factor pathways. | [87] | |
| Furanodienone induced apoptosis through ROS on colorectal carcinoma cells. | [88] | |
| Curcumol | Curcumol effects cell proliferation by RNA synthesis in a concentration-dependent manner in lung cancer. | [89] |
| Curcumol bring apoptosis in nasopharyngeal carcinoma CNE-2 cells by down regulation of NF-kb. | [90] | |
| Curcumol induced apoptosis by suppression of PI3K/Nf-kB pathway on hepatic stellate cells. | [91] | |
| Curcumol exhibited promising anticancer effects when treated on colorectal cancer cells (LOVO). | [92] | |
| Cyclocurcumin | Cyclocurcumin inhibited the proliferation of breast cancer cells. | [12] |
| When combined with curcumin, cyclocurcumin showed nematocidal behavior. | [93] | |
| Trans-to-cis isomerization of cyclocurcumin proved beneficial. | [94] | |
| Through docking study, an insight on the efficiency of cyclocurcumin as therapeutically potential compound for treating various cancers such as ovarian, brain, lymphomas (Hodgkin and non-Hodgkin), lungs, and adrenocortical cancers was provided | [95] | |
| Calebin A | Calebin A inhibited growth and induced apoptosis in drug-resistant human gastric cancer cells reduction in S phase and G2/M phase arrest. | [96] |
| Calebin A inhibited growth in drug-resistant human colon cancer cells by decreasing the expression of cell cycle regulatory protein and increasing the ROS levels, inducing apoptosis. | [97] | |
| Calebin A has wide scope in multiple myeloma and breast cancer cells by suppression of osteoclastogenesis. | [98] | |
| Germacrone | Germacrone inhibited human breast cancer cells by cell cycle arrest and apoptosis with increase in the LDH release and inducing mitochondrial membrane potential depolarization. | [99] |
| Germacrone inhibits human hepatoma cell lines by protein expression of cyclin B1 decrease and activation of cyclin-dependent kinases inhibitors (CDK)1. | [100] | |
| Germacrone shared relation between the JANUS-activated kinases (JAK2)/ Signal transducer and activator of transcription (STAT) 3 signaling pathway and it induced apoptosis in HepG2 cells. | [101] | |
| The combination of germacrone with ADR enhanced the apoptotic effect and resulted in the reduction of anti-apoptotic protein expression levels (Bcl-2) and enhancement of pro-apoptotic protein expression levels (p53 and Bcl 2 associated X protein (Bax) in MCF-7/ADR cells. | [102] | |
| Germacrone is beneficial in combination therapy with other drugs as it potentiates the anti- tumor activity of methotrexate and 5-fluorouracil on ER α-positive breast cancer cells. | [103] | |
| The derivatives of germacrone had inhibitory effects on Bel-7402, HepG2, A549, and HeLa cells. | [104] | |
| Germacrone possess enormous biological activities as proved by molecular docking study | [105] | |
| Bisacurone | Bisacurone inhibits adhesion of cancer cells to endothelial cells through down regulation of vascular cell adhesion molecule (Vcam)1 expression. | [106] |
| Curdione | Curdione inhibited production of prostaglandin (PG), E2 in lipopolysaccharide (LPS)-stimulated mouse machrophages RAW 264.7 through suppression of enzyme COX-2 mRNA expression in a dose-dependent manner. | [107] |
| The three main sesquiterpenes (germacrone, curdione, furanodiene) had proliferative activity on MDA-MB-231 and MCF-7 breast cancer cells, alone or in combination with a fixed-dose-combination. | [108] | |
| Curdione inhibited the proliferation of breast cancer cells in xenograft nude mouse in a dose-dependent manner. | [109] | |
| Additionally, the pharmacokinetic studies of curdione proved promising. | [110] |
| Formulation | Method | Active Ingredient | Major Activities | References |
|---|---|---|---|---|
| Co-crystals | Rapid solvent evaporation | Curcuminoids | Enhance hygroscopicity, stability, dissolution | [120] |
| Rapid solvent evaporation | Turmeric compounds | Enhance solubility | [121] | |
| Liposomal products | Freeze dry method | Turmeric Oil | Anticancer activity | [122] |
| Thin film hydration followed by reverse phase evaporation with high-pressure extrusion | β-elemene | |||
| Pegylated liposomes | Emulsification by ultrasonication and solidification through low temperature | Furonodiene | Anticancer activity | [123,124] |
| Thin film sonication method | Calebin A | Anticancer activity | [125] | |
| Solid lipid nanoparticles | Membrane extrusion and Supersonic film ultrasonic wave dissolving technique | β-elemene | Enhance the bioavailability of active molecules | [122] |
| Nanocapsules | Encapsulation | Ar-turmerone | Antiproliferative activity | [126] |
| Microcapsule/Microsphere | Emulsification internal gelatification technology | Zedoary turmeric oil and β-elemene | Antitumor activity | [122] |
| Microemulsion | High pressure emulsification with phase inversion ultrasound technology | Zedoary turmeric oil and β-elemene | Anticancer agent for lung, breast, gastrointestinal, skin and gynecological cancer cells | [122] |
| Phase inversion method | β-elemene | Anticancer agent for hepatoma 3B cells cancer cells | [127] | |
| PNS Cureit | Polar-nonpolar-sandwich (PNS) technology | Complete natural turmeric matrix | Anti-aging, Anti-rheumatoid activity | [118,119,128,129,130,131,132] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, A.; Amalraj, A.; Jacob, J.; Kunnumakkara, A.B.; Gopi, S. Non-Curcuminoids from Turmeric and Their Potential in Cancer Therapy and Anticancer Drug Delivery Formulations. Biomolecules 2019, 9, 13. https://doi.org/10.3390/biom9010013
Nair A, Amalraj A, Jacob J, Kunnumakkara AB, Gopi S. Non-Curcuminoids from Turmeric and Their Potential in Cancer Therapy and Anticancer Drug Delivery Formulations. Biomolecules. 2019; 9(1):13. https://doi.org/10.3390/biom9010013
Chicago/Turabian StyleNair, Akhila, Augustine Amalraj, Joby Jacob, Ajaikumar B. Kunnumakkara, and Sreeraj Gopi. 2019. "Non-Curcuminoids from Turmeric and Their Potential in Cancer Therapy and Anticancer Drug Delivery Formulations" Biomolecules 9, no. 1: 13. https://doi.org/10.3390/biom9010013
APA StyleNair, A., Amalraj, A., Jacob, J., Kunnumakkara, A. B., & Gopi, S. (2019). Non-Curcuminoids from Turmeric and Their Potential in Cancer Therapy and Anticancer Drug Delivery Formulations. Biomolecules, 9(1), 13. https://doi.org/10.3390/biom9010013





