Poly(N-isopropylacrylamide-co-2-((diethylamino)methyl)-4-formyl-6-methoxyphenyl acrylate) Environmental Functional Copolymers: Synthesis, Characterizations, and Grafting with Amino Acids
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Instrumentations and Measurements
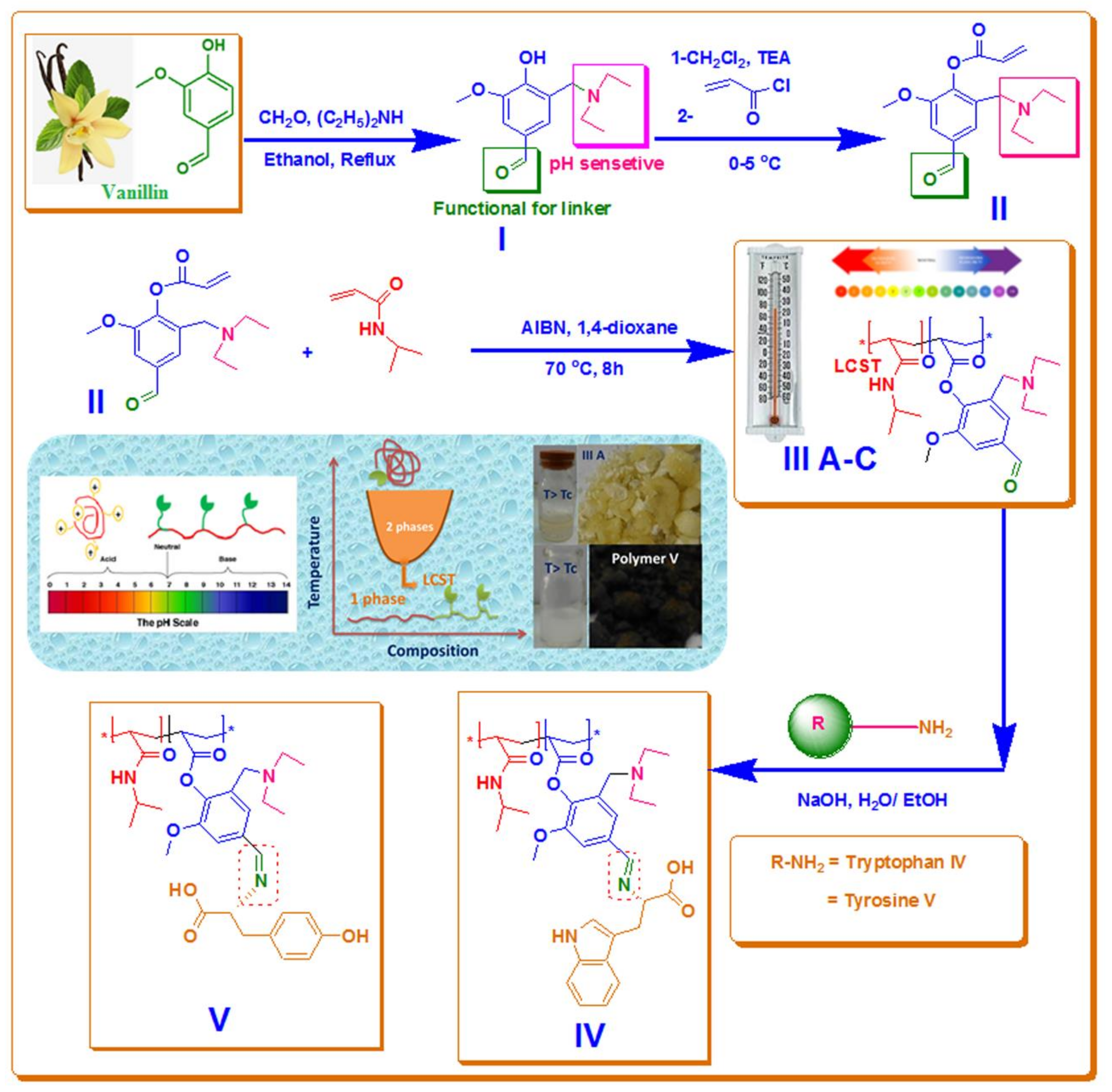
2.3. Synthesis of 2-((diethylamino)methyl)-4-formyl-6-methoxyphenyl acrylate (II) (DEMAVA)
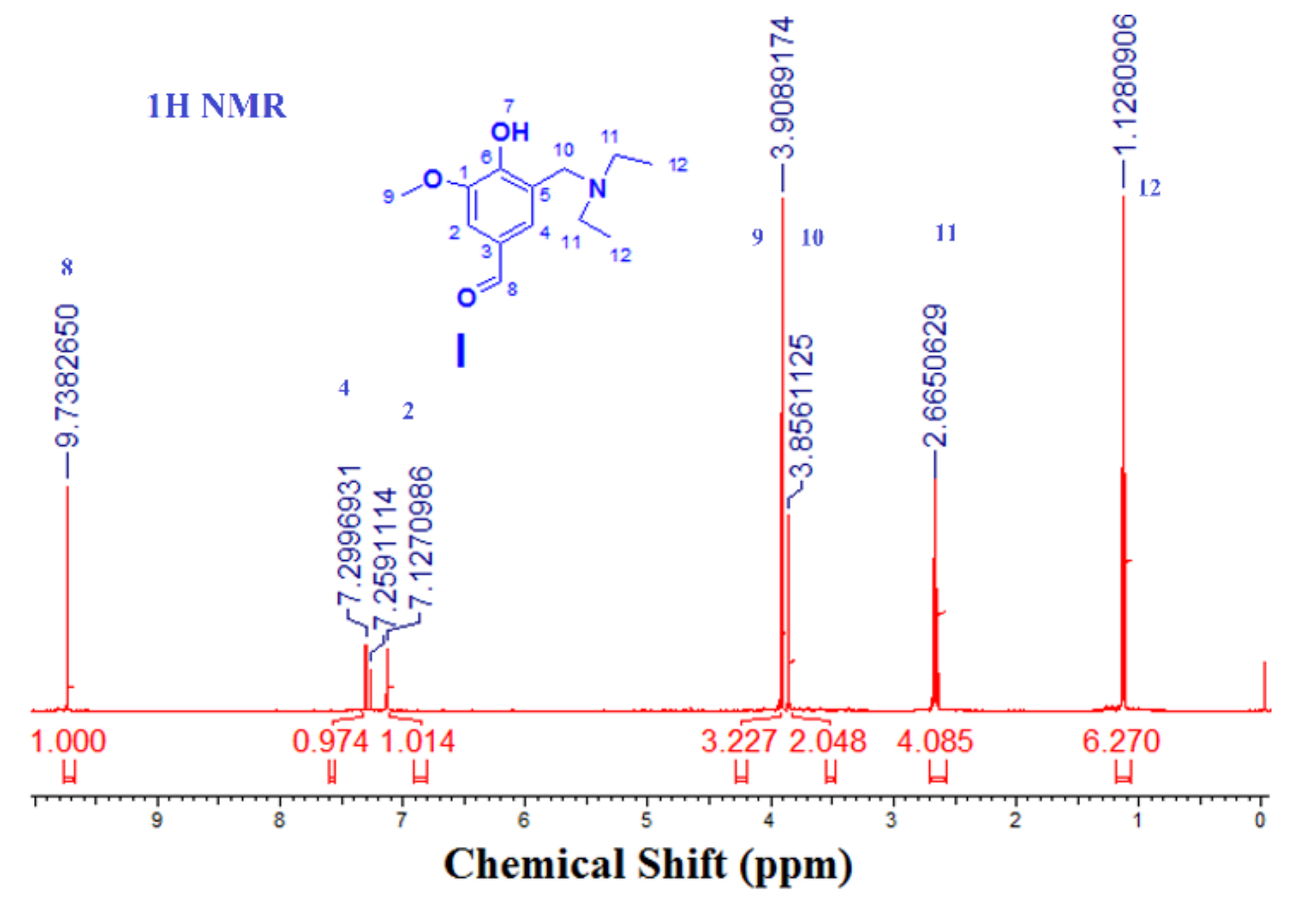
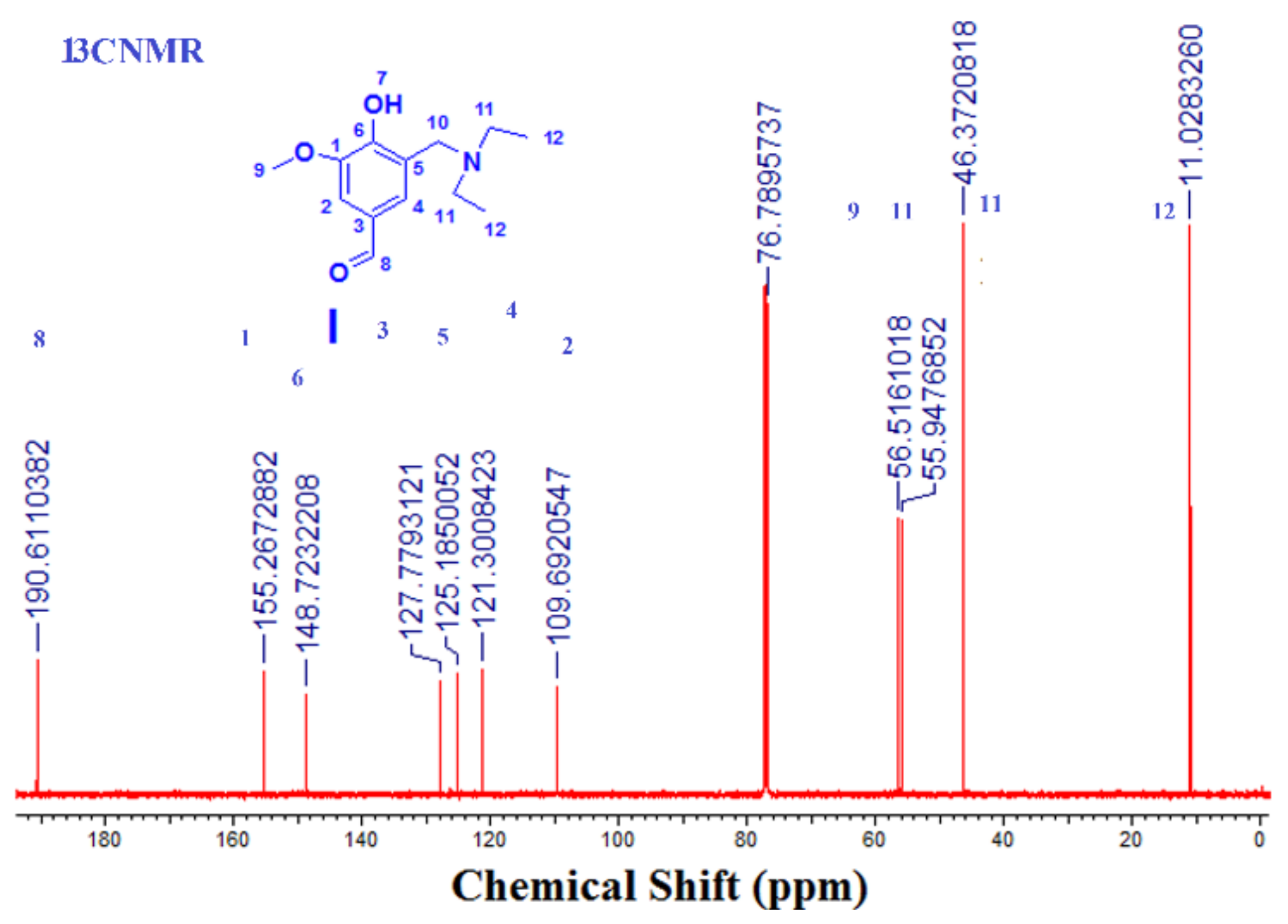
2.3.1. Step 1: Preparation of 3-((diethylamino)methyl)-4-hydroxy-5-methoxy-benzaldehyde) (I)
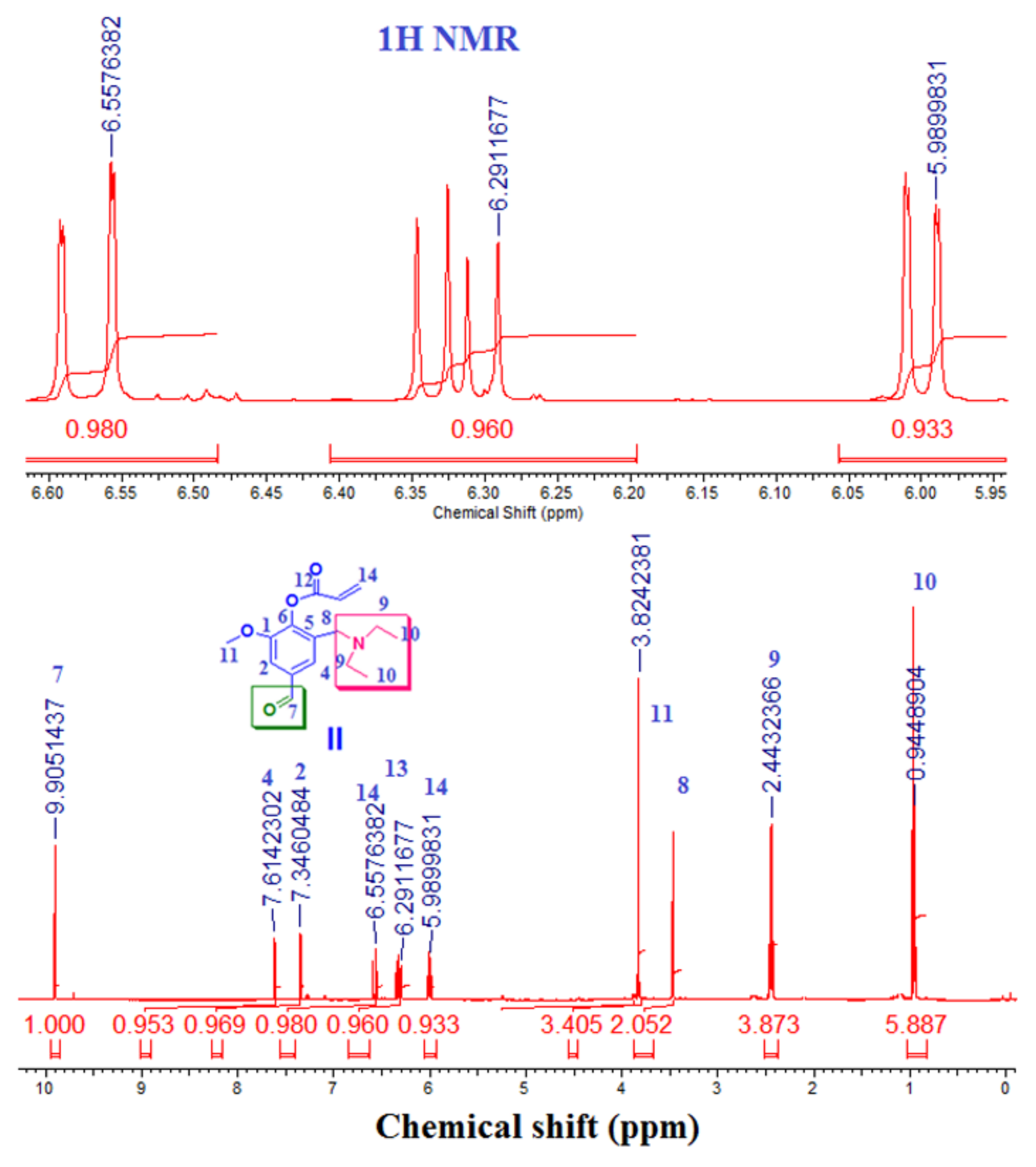
2.3.2. Step 2: Preparation of 2-((diethylamino)methyl)-4-formyl-6-methoxyphenyl acrylate) (II) (DEAMVA)
2.4. Preparation of Poly (NIPAAm-co-DEAMVA) with 5, 10 and 15 mol % of DEAMVA (IIIa–c)
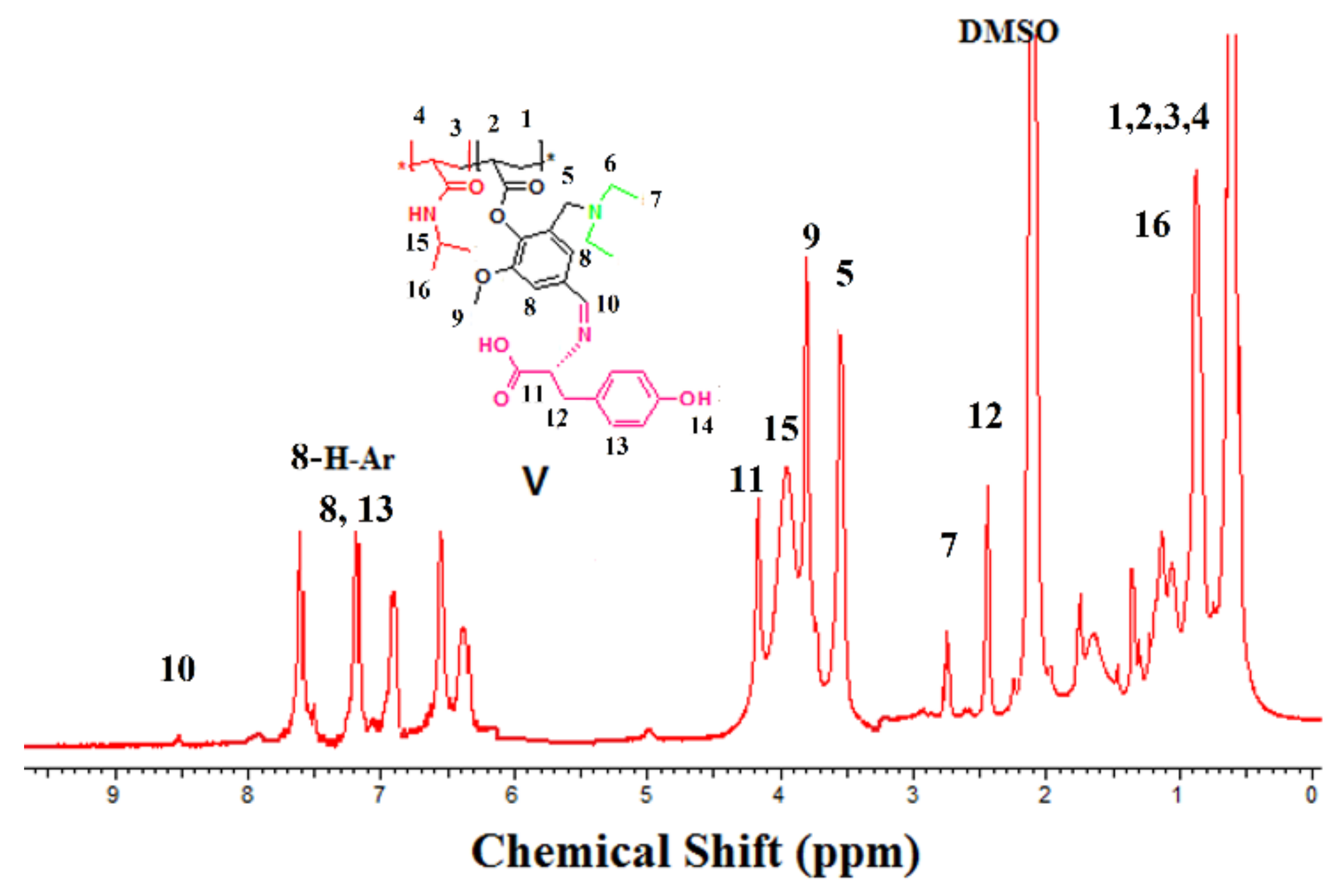
2.5. Synthesis of Grafted 15 mol % Poly (NIPAAm-co-DEAMVA) with Tryptophan and Tyrosine (IV–V)
2.6. Synthesis of Grafted 15 mol % Poly (NIPAAm-co-DEAMVA)-g-tyrosine as a Function of Time
3. Results and Discussion
3.1. Synthesis of Monomer, Copolymers and Grafted Copolymers
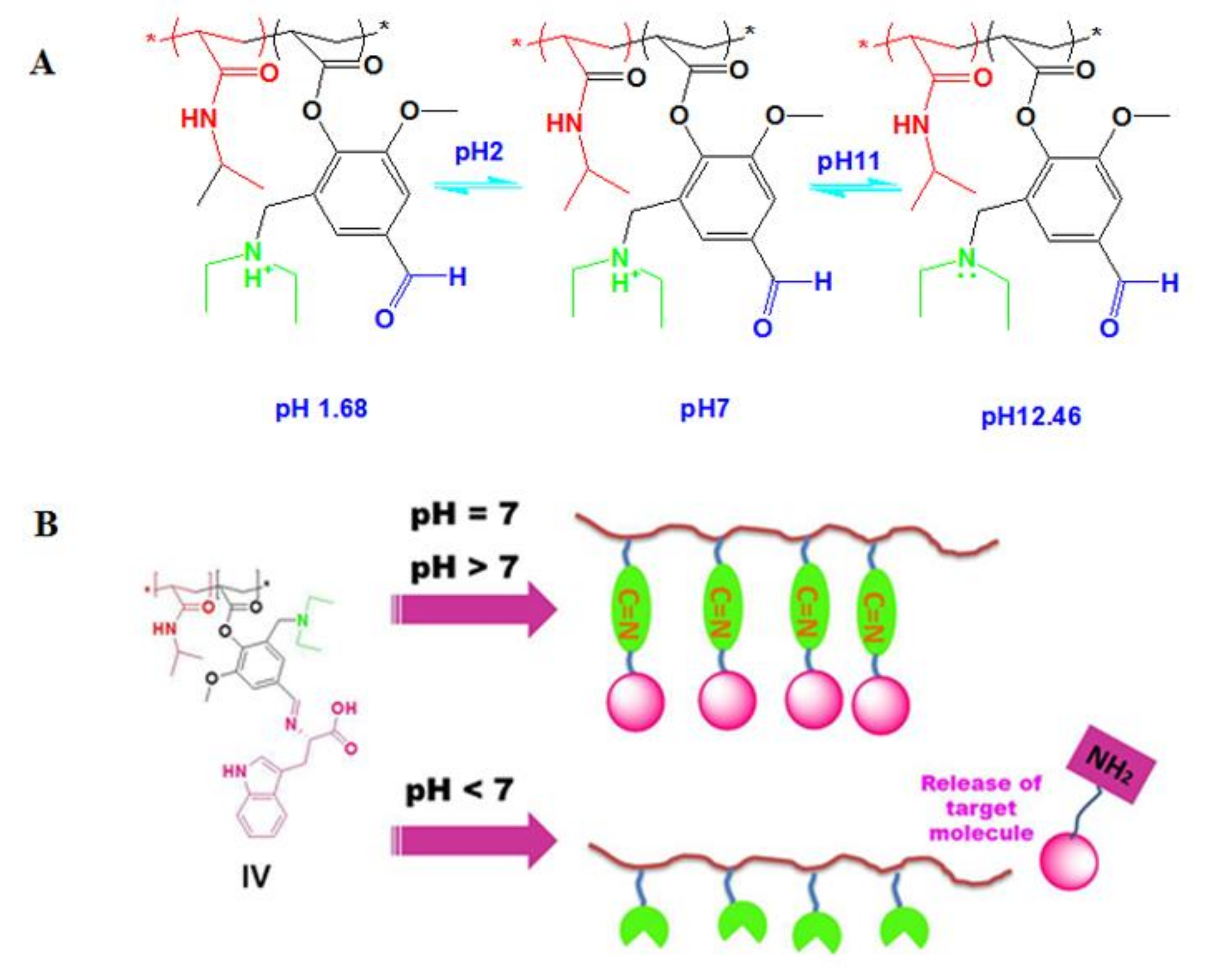
3.1.1. Step 1: Is the formation of (3-[(diethylamino) methyl)-4-hydroxy-5-methoxy-benzaldehyde) (I)
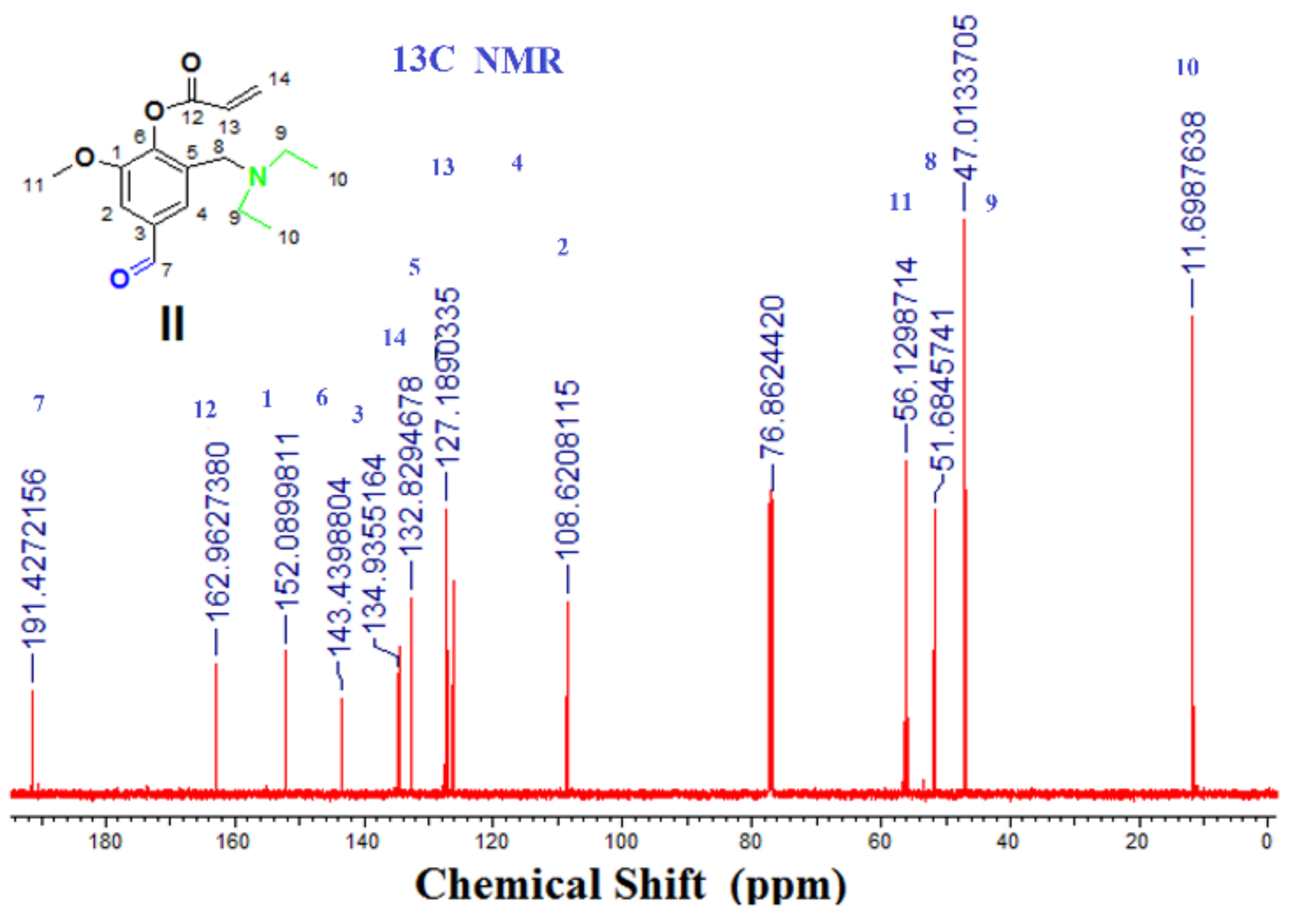
3.2.2. Step 2: Is the formation of 2-[(diethylamino)methyl]-4-formyl-6-methoxyphenyl acrylate (DEMAVA) (II)
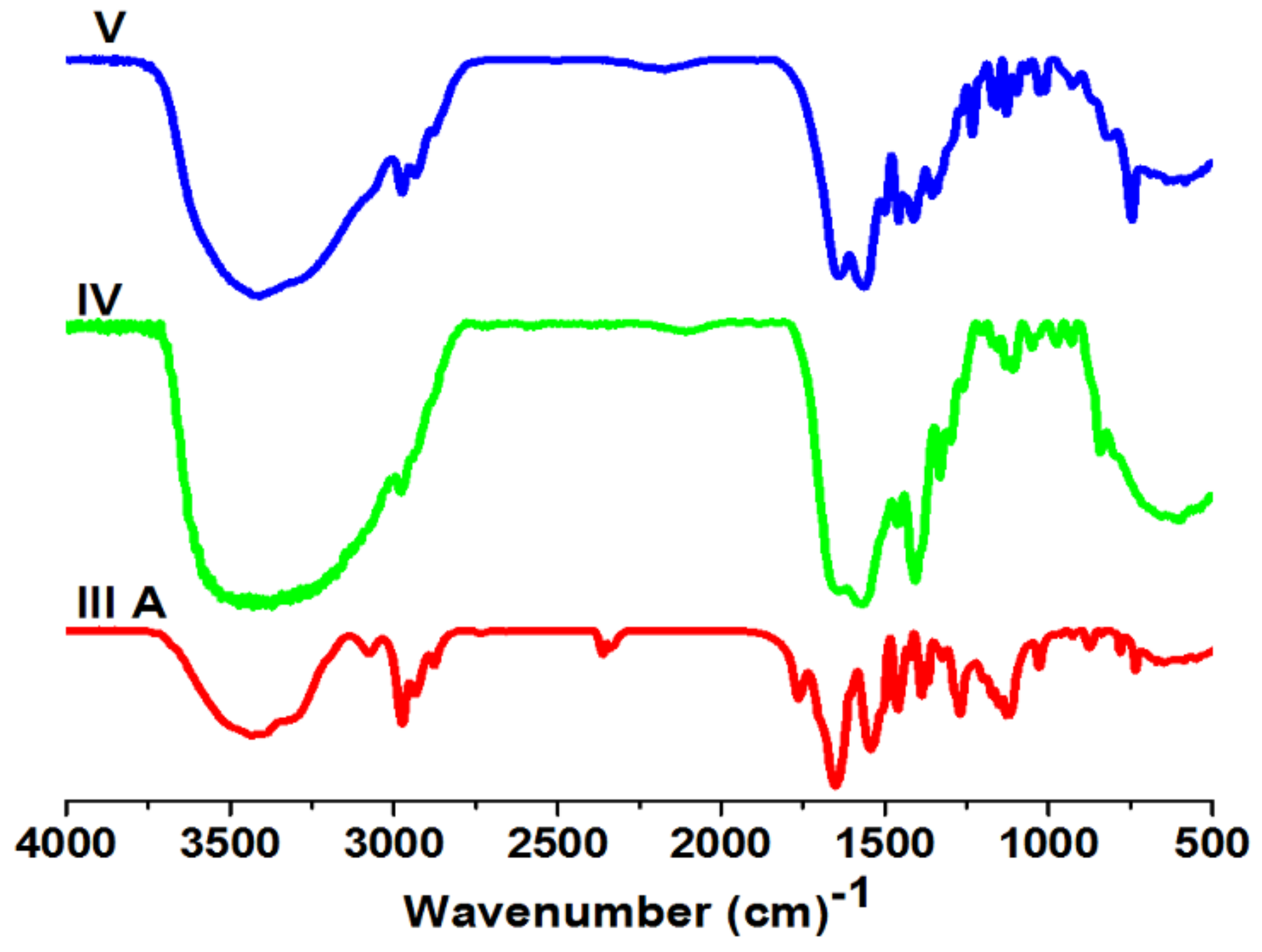
3.2. Polymer Characterization
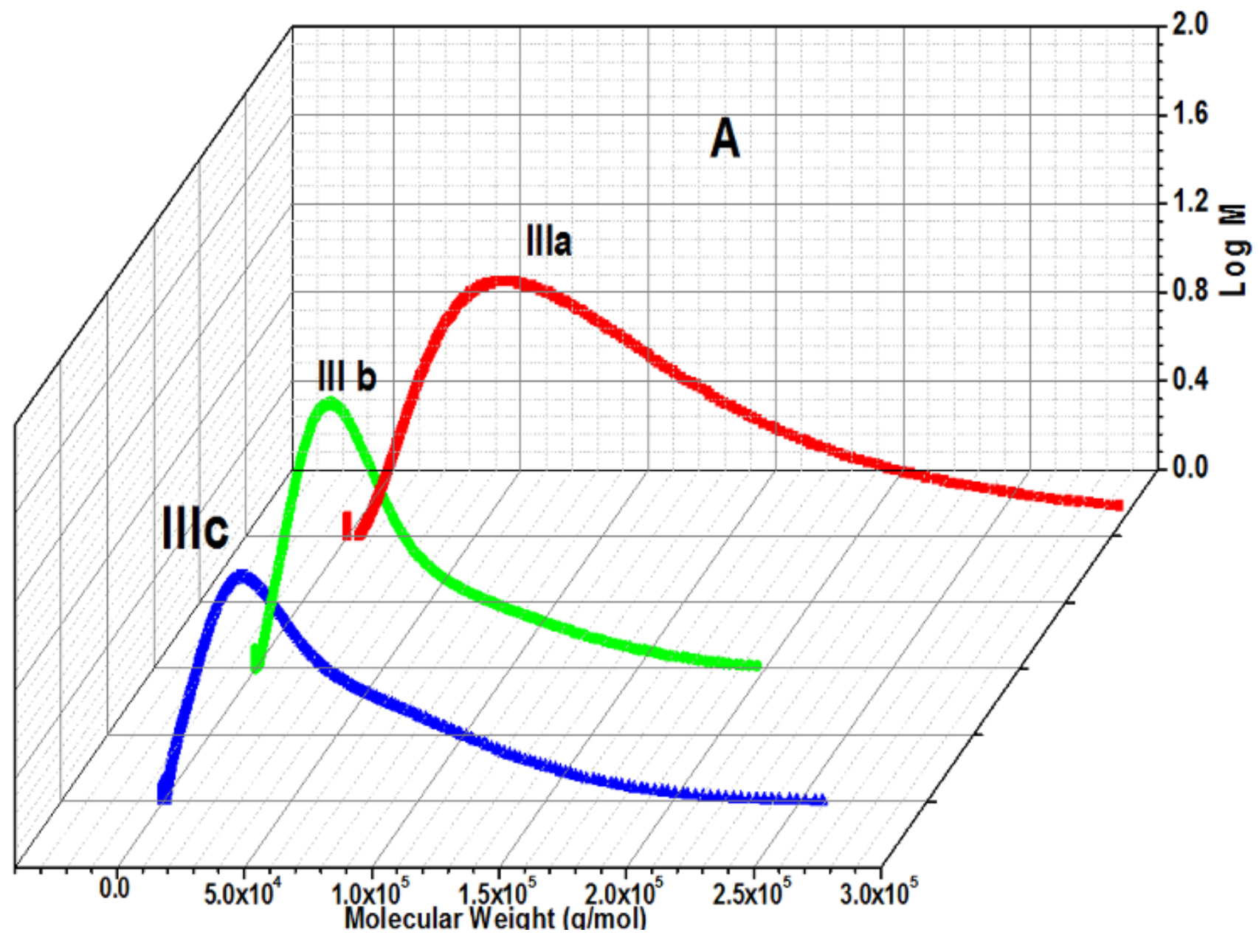
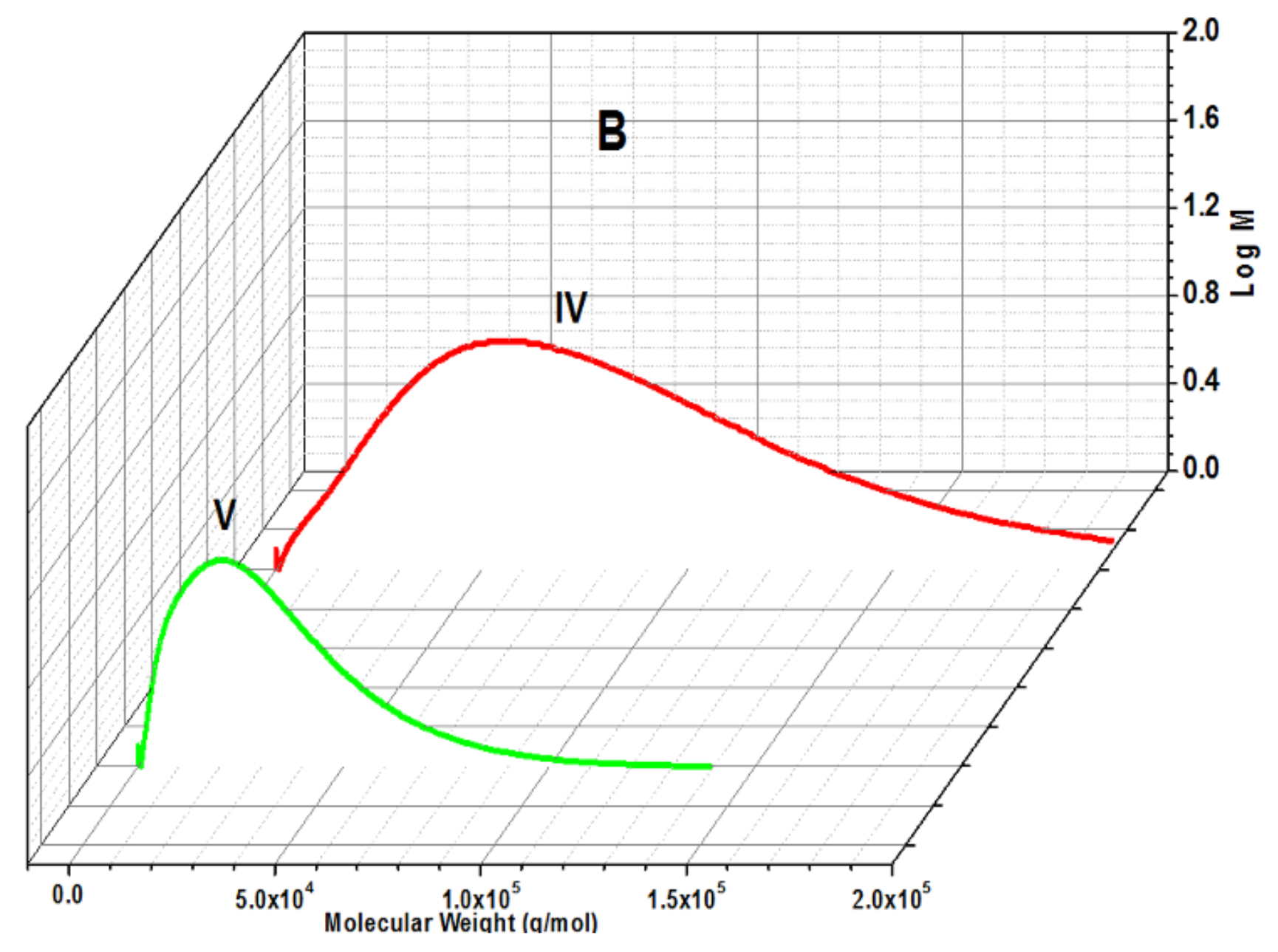
Molecular Weight
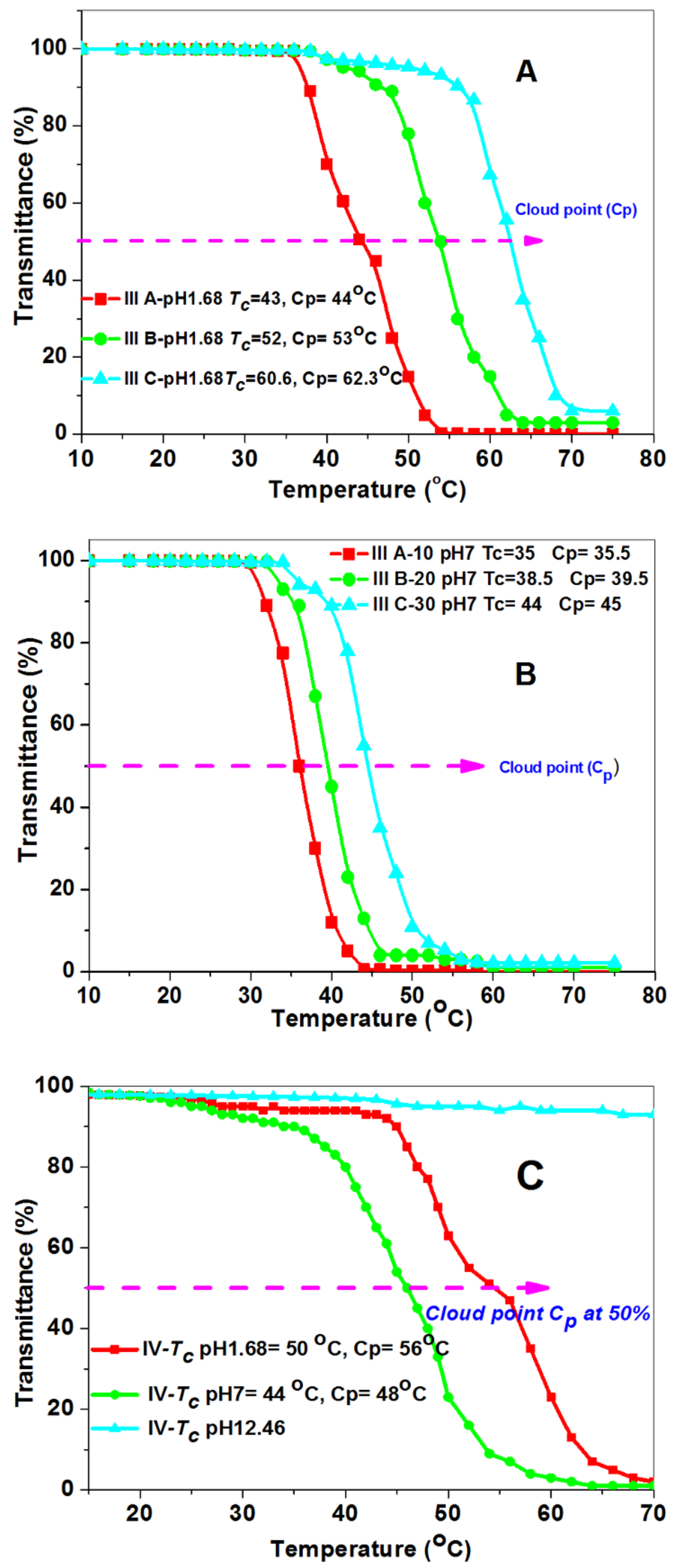
3.3. Study of the Phase Separation of Poly (NIPAAm-Co-DEAMVA) and Grafted Polymers
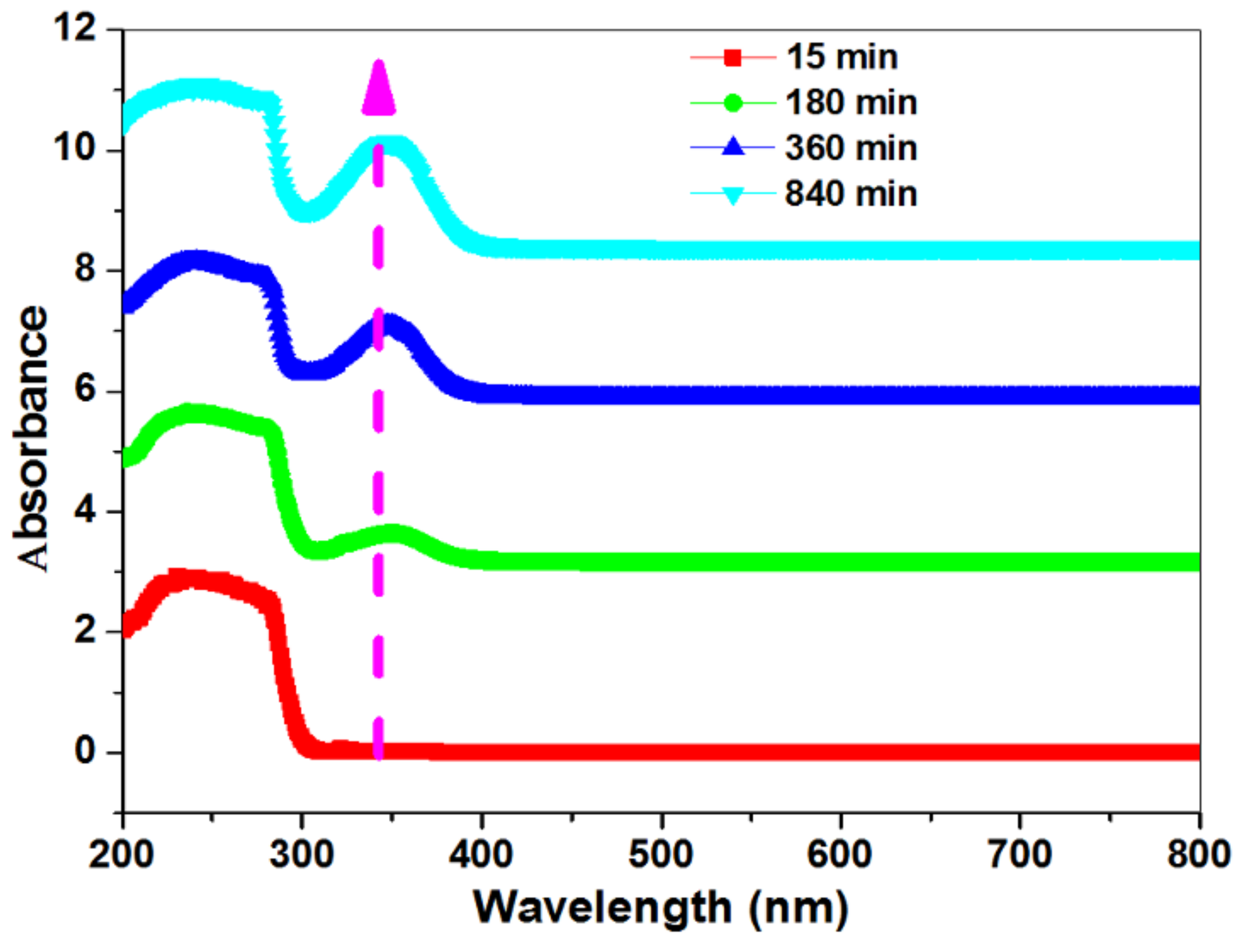
3.4. Determination of the Conversion of poly (NIPAAm-Co-DEAMVA) to Poly (NIPAAm-Co-DEAMVA)-g-Tryptophan
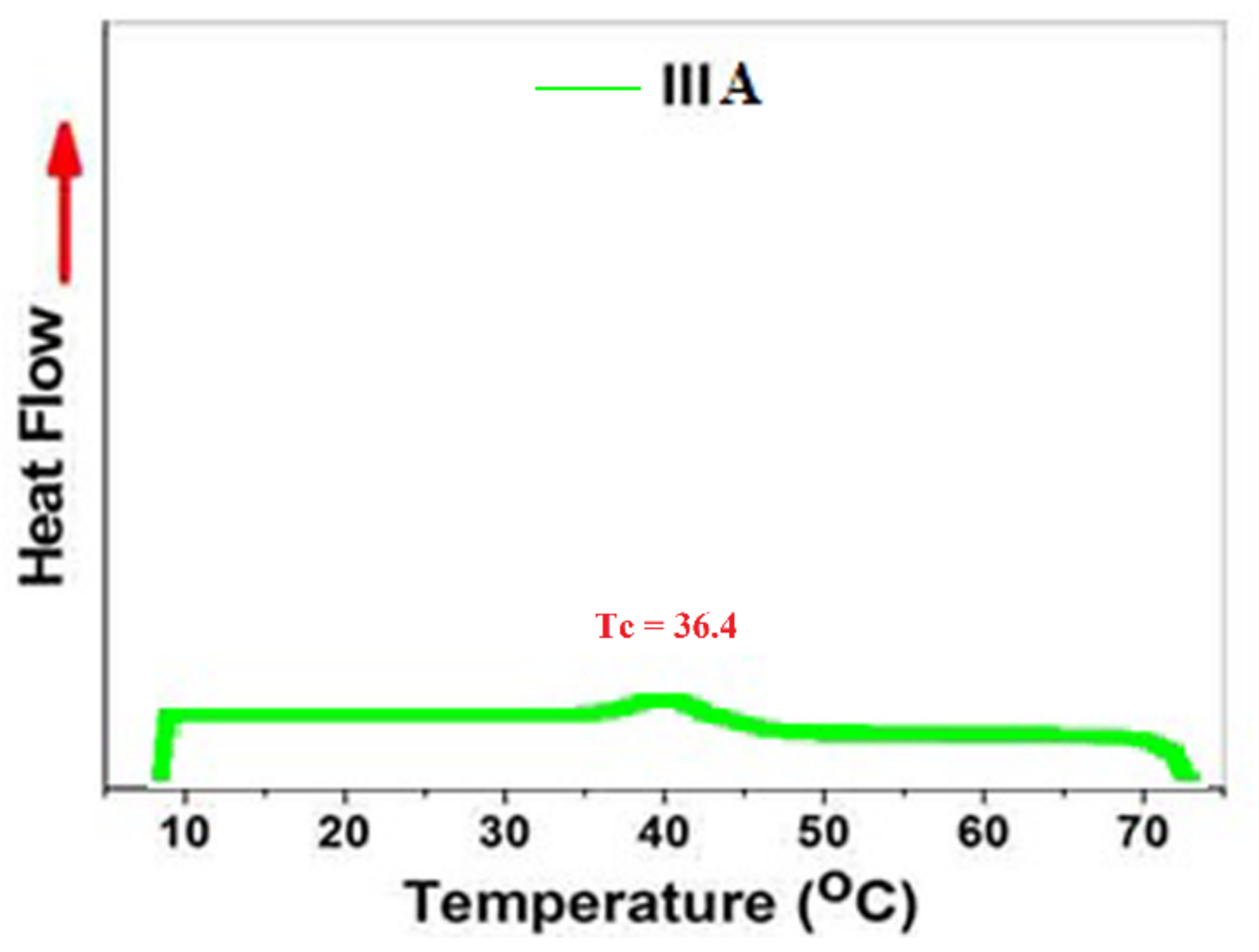
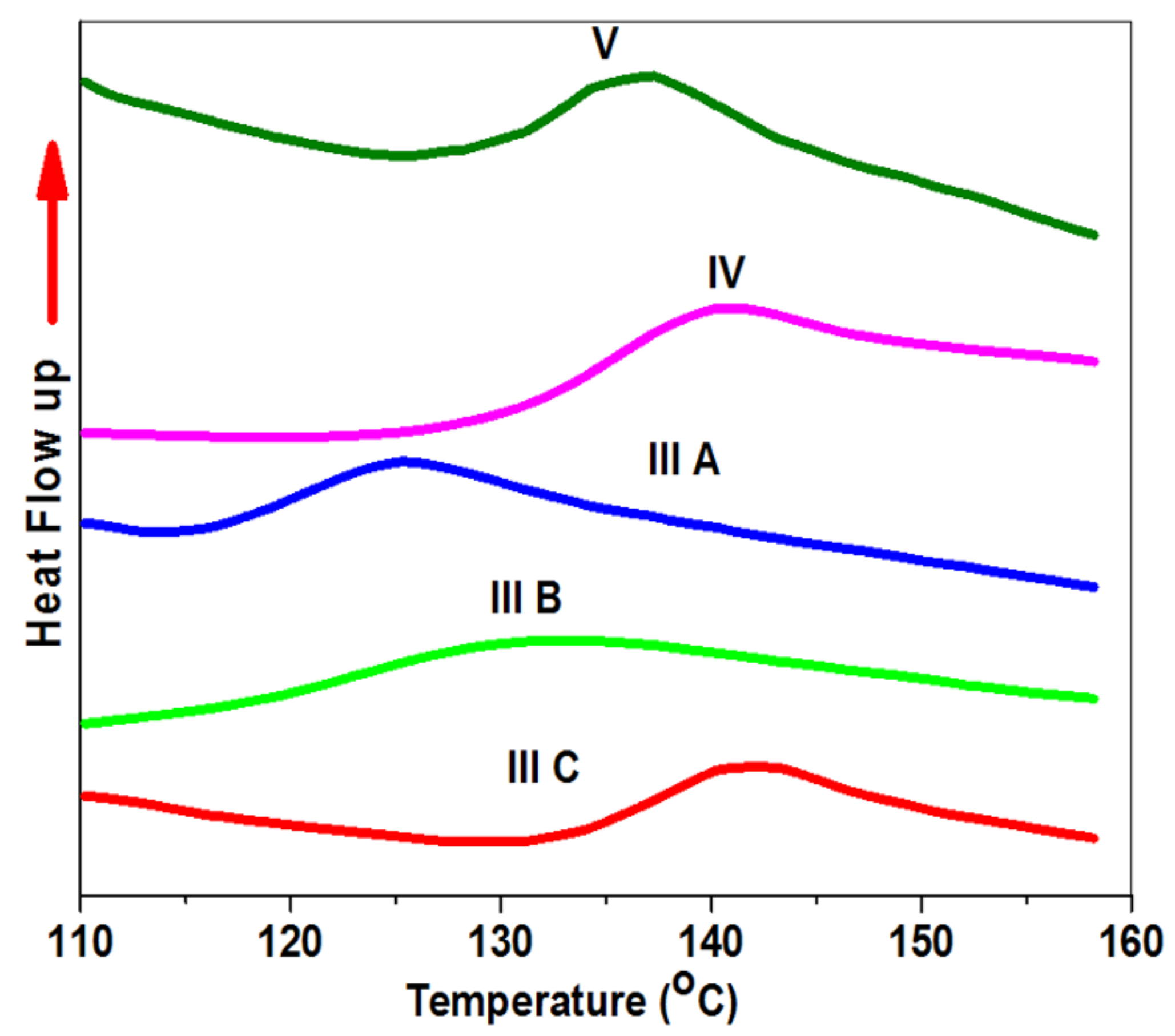
3.5. The Thermal Properties
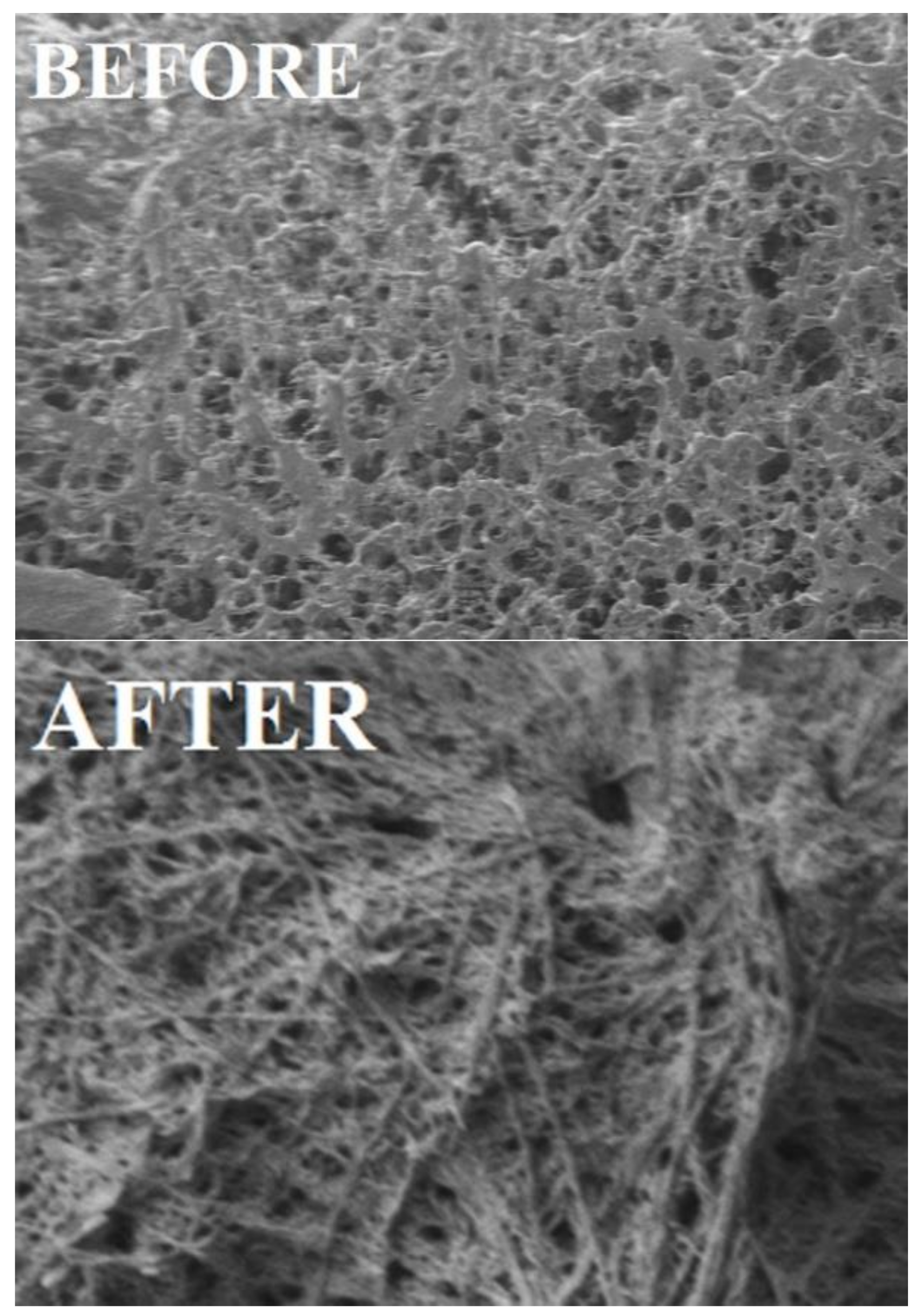
3.6. Morphological Studies
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Young, J.K.; Yukiko, T.M. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef]
- Abdelaty, M.S.A.; Kuckling, D. Synthesis and Characterization of New Functional Photo Cross-Linkable Smart Polymers Containing Vanillin Derivatives. Gels 2016, 2, 1–13. [Google Scholar] [CrossRef]
- Sato, E.; Masuda, Y.; Kadota, J.; Nishiyama, T.; Horibe, H. Dual stimuli-responsive homopolymers: Thermo- and photo-responsive properties of coumarin-containing polymers in organic solvents. Eur. Polym. J. 2015, 69, 605–615. [Google Scholar] [CrossRef]
- Sun, H.; Kabb, C.P.; Dai, Y.; Hil, M.R.; Ghiviriga, I.; Bapat, A.P.; Sumerlin, BS. Macromolecular metamorphosis via stimulus-induced transformations of polymer architecture. Nat. Chem. 2017, 9, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Christopher, P.K.; Sumerlin, B.S. Thermally-labile segmented hyperbranched copolymers: Using reversible-covalent chemistry to investigate the mechanism of self-condensing vinyl copolymerization. Chem. Sci. 2014, 5, 4646–4655. [Google Scholar] [CrossRef]
- Abdelaty, M.S.A. Environmental Functional Photo-Cross-Linked Hydrogel Bilayer Thin Films from Vanillin. J. Polym. Environ. 2018, 26, 2243–2256. [Google Scholar] [CrossRef]
- Abdelaty, M.S.A. Preparation and Characterization of New Environmental Functional Polymers Based on Vanillin and N-isopropylacrylamide for Post Polymerization. J. Polym. Environ. 2018, 26, 636–646. [Google Scholar] [CrossRef]
- Ramkissoon-Ganorkar, C.; Baudys, M.; Kim, S.W. Effect of ionic strength on the loading efficiency of the model polypeptide/protein drugs in pH-/temperature-sensitive polymers. J. Biomater. Sci. Polym. Ed. 2000, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.K.; Kim, S.Y.; Kim, S.J.; Lee, Y.M. pH/temperature-responsive semi-IPN hydrogels composed of alginate and poly(N-isopropylacrylamide). J. Appl. Polym. Sci. 2002, 83, 1128–1139. [Google Scholar] [CrossRef]
- Benrebouh, A.; Avoce, D.; Zhu, X.X. Thermo- and pH-sensitive polymers containing cholic acid derivatives. Polymer 2001, 42, 4031–4038. [Google Scholar] [CrossRef]
- Ning, L.; Min, Y.; Maolin, Z.; Jiuqiang, L.; Hongfei, H. Radiation synthesis and characterization of polyDMAEMA hydrogel. Radiat. Phys. Chem. 2001, 61, 69–73. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Chen, D.; Liu, H.; Kobayashib, T.; Yu, H. Multiresponsive Reversible Gels Based on a Carboxylic Azo Polymer. J. Mater. Chem. 2010, 20, 3610–3614. [Google Scholar] [CrossRef]
- Pasparakisa, G.; Vamvakaki, M. Multiresponsive polymers: Nano-sized assemblies, stimuli-sensitive gels and smart surfaces. Polym. Chem. 2011, 2, 1234. [Google Scholar] [CrossRef]
- Menglian, W.; Yongfeng, G.; Xue, L.; Michael, J.S. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Xia, F.; Ge, H.; Hou, Y.; Sun, T.; Chen, L.; Zhang, G.; Jiang, L. Multiresponsive Surfaces Change Between Superhydrophilicity and Superhydrophobicity. Adv. Mater. 2007, 19, 2520–2524. [Google Scholar] [CrossRef]
- Bousquet, A.; Ibarboure, E.; Papon, E.; Labrugère, C.; Rodriguez-Hernandez, J. Structured multistimuli-responsive functional polymer surfaces obtained by interfacial diffusion of amphiphilic block copolymers. J. Polym. Sci. A 2010, 48, 1952–1961. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli-responsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Shibayama, M.; Tanaka, T. Volume phase transition and related phenomena of polymer gels. Adv. Polym. Sci. 1993, 109, 1–62. [Google Scholar] [CrossRef]
- Kurata, K.; Dobashi, A.J. Novel Temperature- and pH- Responsive Linear Polymers and Crosslinked Hydrogels Comprised of Acidic l-Amino Acid Derivatives. Macromol. Sci. 2004, 41, 143–164. [Google Scholar] [CrossRef]
- Elsila, J.E.; de Leon, N.P.; Plows, F.L.; Buseck, P.R.; Zare, R.N. Extracts of impact breccia samples from Sudbury, Gardnos, and Ries impact craters and the effects of aggregation on C60 detection. Geochim. Cosmochim. Acta 2005, 69, 2891–2899. [Google Scholar] [CrossRef]
- Gan, L.H.; Gan, Y.Y.; Roshan, D.G. Poly (N-acryloyl-N′-propylpiperazine): A New Stimuli-Responsive Polymer. Macromolecules 2000, 33, 7893–7897. [Google Scholar] [CrossRef]
- Zeinab, M.; Mina, H.; Khalil, F. Synthesis of Bio-Based Polyamide/Acid-Functionalized Multiwalled Carbon Nanotube Nanocomposites Using Vanillin. Polym.-Plast. Technol. Eng. 2018, 57, 1367–1376. [Google Scholar] [CrossRef]
- Maxence, F.; Bernard, B.; Sylvain, C. Vanillin, a key-intermediate of biobased polymers. Eur. Polym. J. 2015, 68, 488–502. [Google Scholar] [CrossRef]
- Huanyu, Z.; Xueyong, Y.; Jinyong, Z.; Jianping, D.; Youping, W. Biomass Vanillin-Derived Polymeric Microspheres Containing Functional Aldehyde Groups: Preparation, Characterization, and Application as Adsorbent. ACS Appl. Mater. Interfaces 2016, 4, 2753–2763. [Google Scholar] [CrossRef]
- Ananda, S.A.; Ashfaqur, R. Vanillin-Based Polymers—Part II: Synthesis of Schiff Base Polymers of Divanillin and Their Chelation with Metal Ions. ISRN Polym. Sci. 2012, 2012, 532171. [Google Scholar] [CrossRef]
- Ananda, S.A.; Rocio, G.-O.; Audie, K.T. Vanillin-based polymers: IV. Hydrovanilloin epoxy resins. J. Appl. Polym. Sci. 2018. [Google Scholar] [CrossRef]
- Zou, Y.; Brooks, D.E.; Kizhakkedathu, J.N. A Novel Functional Polymer with Tunable LCST. Macromolecules 2008, 41, 5393–5405. [Google Scholar] [CrossRef]
- Heredia, K.L.; Maynard, H.D. Synthesis of protein-polymer conjugates. Org. Biomol. Chem. 2007, 5, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Hagiwara, M.; Ishazone, T. Synthesis of Thermally Sensitive Water-Soluble Polymethacrylates by Living Anionic Polymerizations of Oligo(ethylene glycol) Methyl Ether Methacrylates. Macromolecules 2003, 36, 8312–8319. [Google Scholar] [CrossRef]
- Abdelaty, M.S.A. Environmental functional photo-cross-linked hydrogel bilayer thin films from vanillin (part 2): Temperature responsive layer A, functional, temperature and pH layer B. Polym. Bull. 2018, 75, 4837–4858. [Google Scholar] [CrossRef]
- Emma, R.L.B.; Zeyun, X.; Luke, A.C. Amino Acid Functional Polymers: Biomimetic Polymer Design Enabling Catalysis, Chiral Materials, and Drug Delivery. Aust. J. Chem. 2016, 69, 705–716. [Google Scholar] [CrossRef]
- Sun, H.; Gao, C. Facile Synthesis of Multiamino Vinyl Poly (amino acid)s for Promising Bioapplications. Biomacromolecules 2010, 11, 3609–3616. [Google Scholar] [CrossRef] [PubMed]
- Bauri, K.; Pant, S.; Roy, S.G.; De, P. Dual pH and temperature responsive helical copolymer libraries with pendant chiral leucinemoieties. Polym. Chem. 2013, 4, 4052–4060. [Google Scholar] [CrossRef]
- Bauri, K.; Roy, S.G.; Pant, S.; De, P. Controlled synthesis of amino acid-based pH-responsive chiral polymers and self-assembly of their block copolymers. Langmuir 2013, 29, 2764–2774. [Google Scholar] [CrossRef] [PubMed]
- Saswati, G.R.; Priyadarsi, D. pH Responsive Polymers with Amino Acids in the Side Chains and Their Potential Applications. J. Appl. Polym. Sci. 2014, 130, 41084. [Google Scholar] [CrossRef]
- Ladmiral, V.; Charlot, A.; Semsarilarc, M.; Armes, S.P. Synthesis and characterization of poly(amino acid)- stabilized diblock copolymer nano-objects. Polym. Chem. 2015, 6, 1805–1816. [Google Scholar] [CrossRef]
- Hideharu, M.; Ikumi, K.; Shoko, S.; Takeshi, E. Proline-Based Block Copolymers Displaying Upper and Lower Critical Solution Temperatures. Macromolecules 2010, 43, 1289–1298. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Takani, M.; Yamauchi, O. Metal complexes of amino acids and amino acid side chain groups. Structures and properties. Dalton Trans. 2009, 14, 7854–7869. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Xin, Y.; Yuan, J. Schiff’s base as a stimuli-responsive linker in polymer chemistry. Polym. Chem. 2012, 3, 3045–3055. [Google Scholar] [CrossRef]
- Aftabuddin, M.D.; Kundu, S. Hydrophobic, Hydrophilic, and Charged Amino Acid Networks within Protein. Biophys. J. 2007, 93, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Dipti, S.; Dirk, K.; Veena, K.; Veena, C.; Hans-Jürgen, A.; Amit Kumar, D. Studies on copolymerization of N-isopropylacrylamide with poly(ethylene glycol) methacrylate. Eur. Polym. J. 2008, 44, 2962–2970. [Google Scholar] [CrossRef]
- Dondoni, A.; Marra, A. Recent applications of thiol–ene coupling as a click process for glycoconjugation. Chem. Soc. Rev. 2012, 41, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, G.S.; Welington Ferreira, M.; Roberto, F. Glass transition and thermal stability of poly(N-isopropylacrylamide) gels and some of their copolymers with acrylamide. Polym. Degrad. Stab. 1998, 61, 275–281. [Google Scholar] [CrossRef]


| Polymer | Yield (%) | 1H NMR DEAMVA (mol %) | Conversion (%) | Mn a (g/mol) 104 | Ð b | Tgc (°C) | Tc d (°C) | ||
|---|---|---|---|---|---|---|---|---|---|
| pH 7 | pH 1.68 | pH 12.46 | |||||||
| IIIa | 83 | 3.55 | - | 15,340 | 1.98 | 122 | 35 | 43 | - |
| IIIb | 82 | 7.26 | - | 12,260 | 2.27 | 127 | 38.5 | 52 | - |
| IIIc | 76 | 11.36 | - | 10,580 | 2.46 | 137 | 44 | 60 | - |
| IV | 80 | - | 84 | 7750 | 1.87 | 136 | 50 | 44 | - |
| V | 81 | - | 79 | 10,270 | 2.12 | 133 | 42 | 42 | - |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelaty, M.S.A. Poly(N-isopropylacrylamide-co-2-((diethylamino)methyl)-4-formyl-6-methoxyphenyl acrylate) Environmental Functional Copolymers: Synthesis, Characterizations, and Grafting with Amino Acids. Biomolecules 2018, 8, 138. https://doi.org/10.3390/biom8040138
Abdelaty MSA. Poly(N-isopropylacrylamide-co-2-((diethylamino)methyl)-4-formyl-6-methoxyphenyl acrylate) Environmental Functional Copolymers: Synthesis, Characterizations, and Grafting with Amino Acids. Biomolecules. 2018; 8(4):138. https://doi.org/10.3390/biom8040138
Chicago/Turabian StyleAbdelaty, Momen S. A. 2018. "Poly(N-isopropylacrylamide-co-2-((diethylamino)methyl)-4-formyl-6-methoxyphenyl acrylate) Environmental Functional Copolymers: Synthesis, Characterizations, and Grafting with Amino Acids" Biomolecules 8, no. 4: 138. https://doi.org/10.3390/biom8040138
APA StyleAbdelaty, M. S. A. (2018). Poly(N-isopropylacrylamide-co-2-((diethylamino)methyl)-4-formyl-6-methoxyphenyl acrylate) Environmental Functional Copolymers: Synthesis, Characterizations, and Grafting with Amino Acids. Biomolecules, 8(4), 138. https://doi.org/10.3390/biom8040138





