Variations of Alkaloid Accumulation and Gene Transcription in Nicotiana tabacum
Abstract
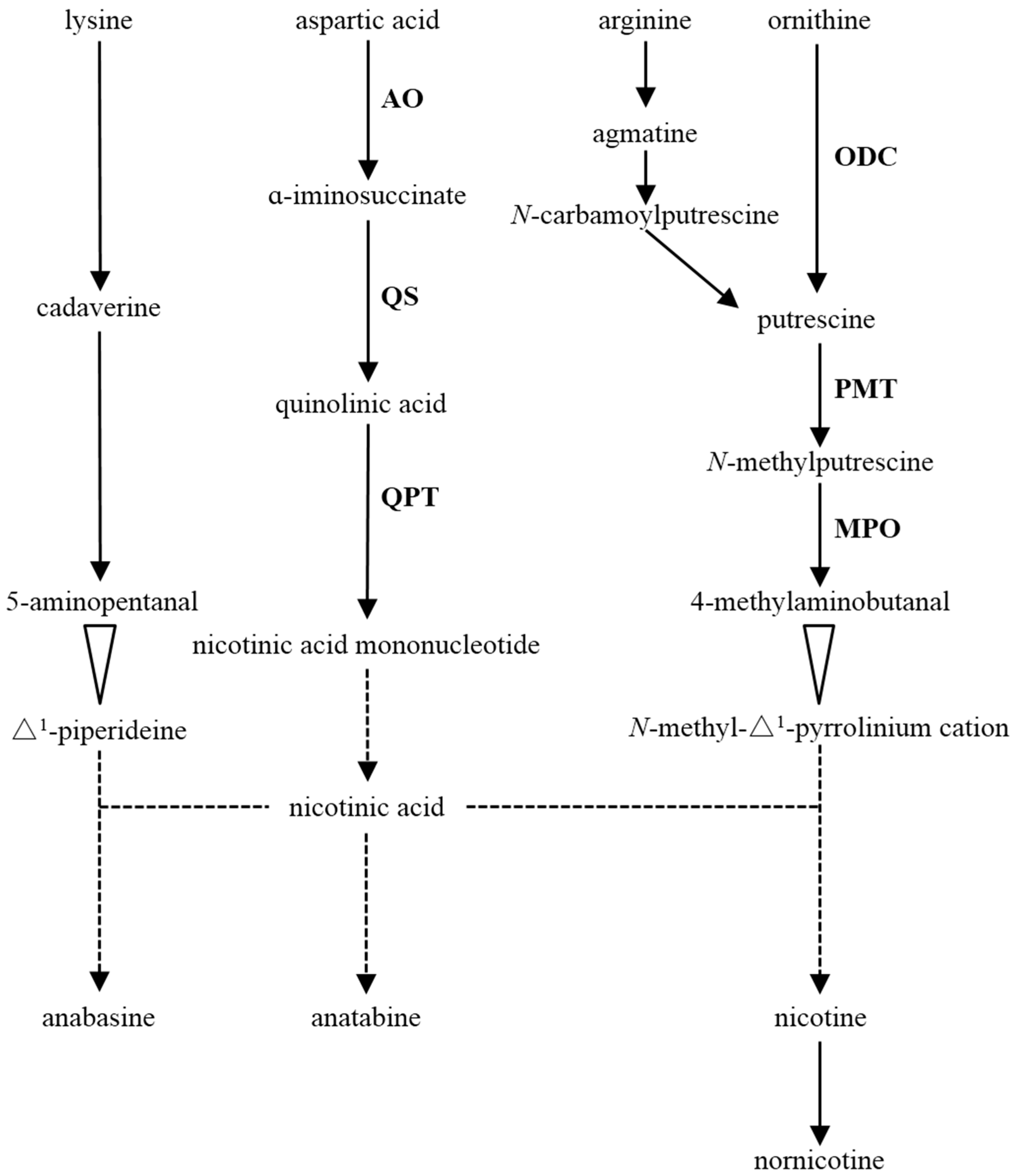
1. Introduction
2. Materials and Methods
2.1. Plant Materials
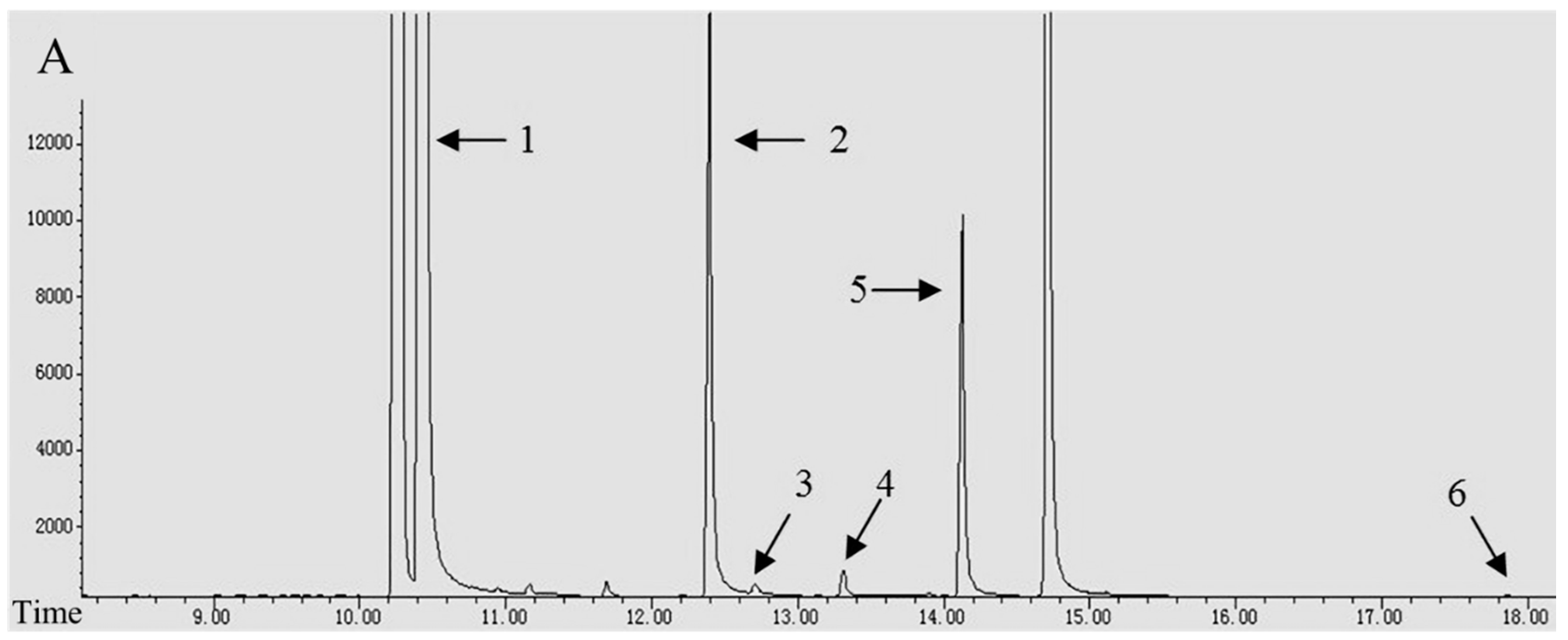
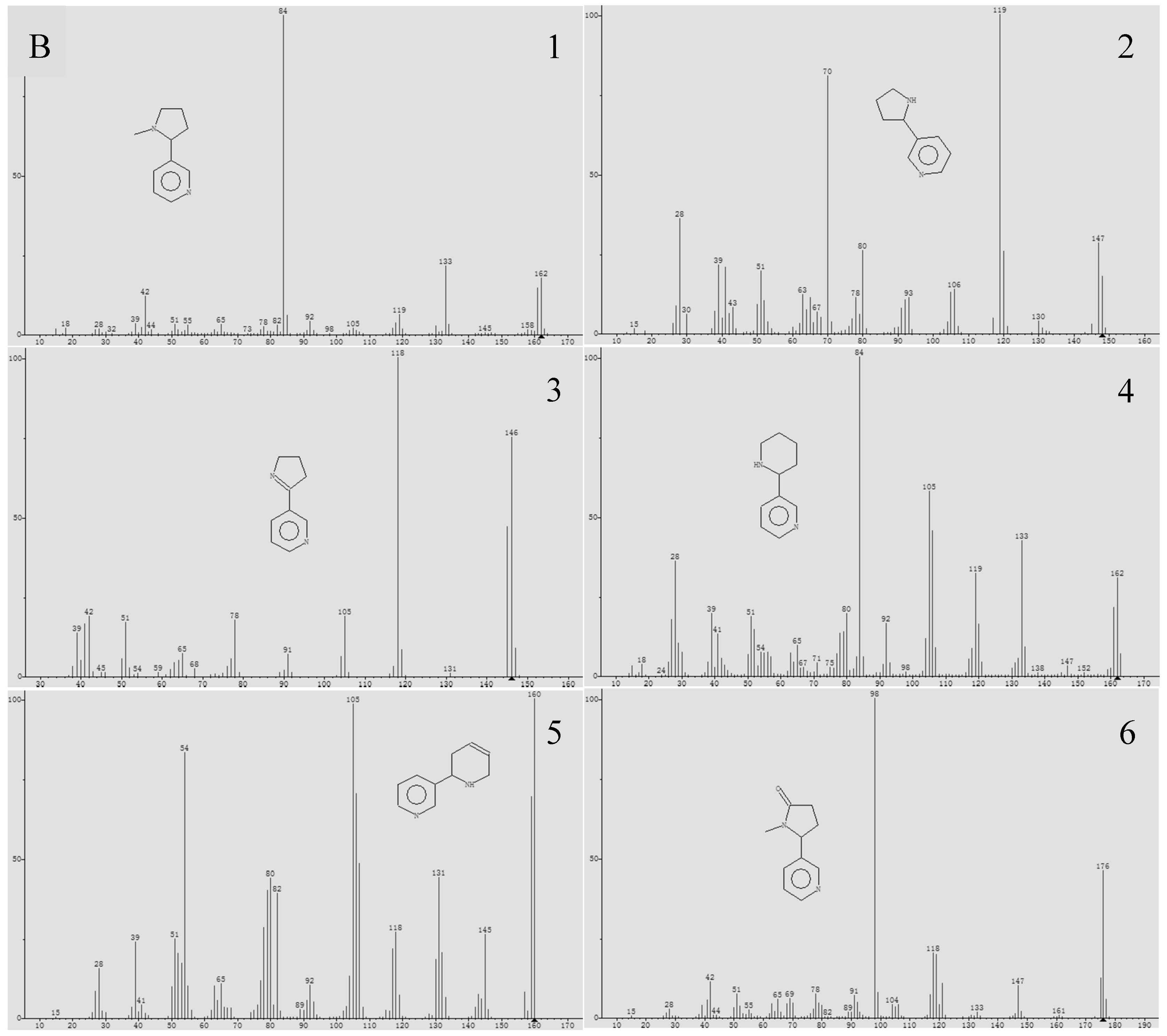
2.2. Alkaloid Analysis
2.3. Quantitative Real Time PCR Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. The Composition and Content of Alkaloids
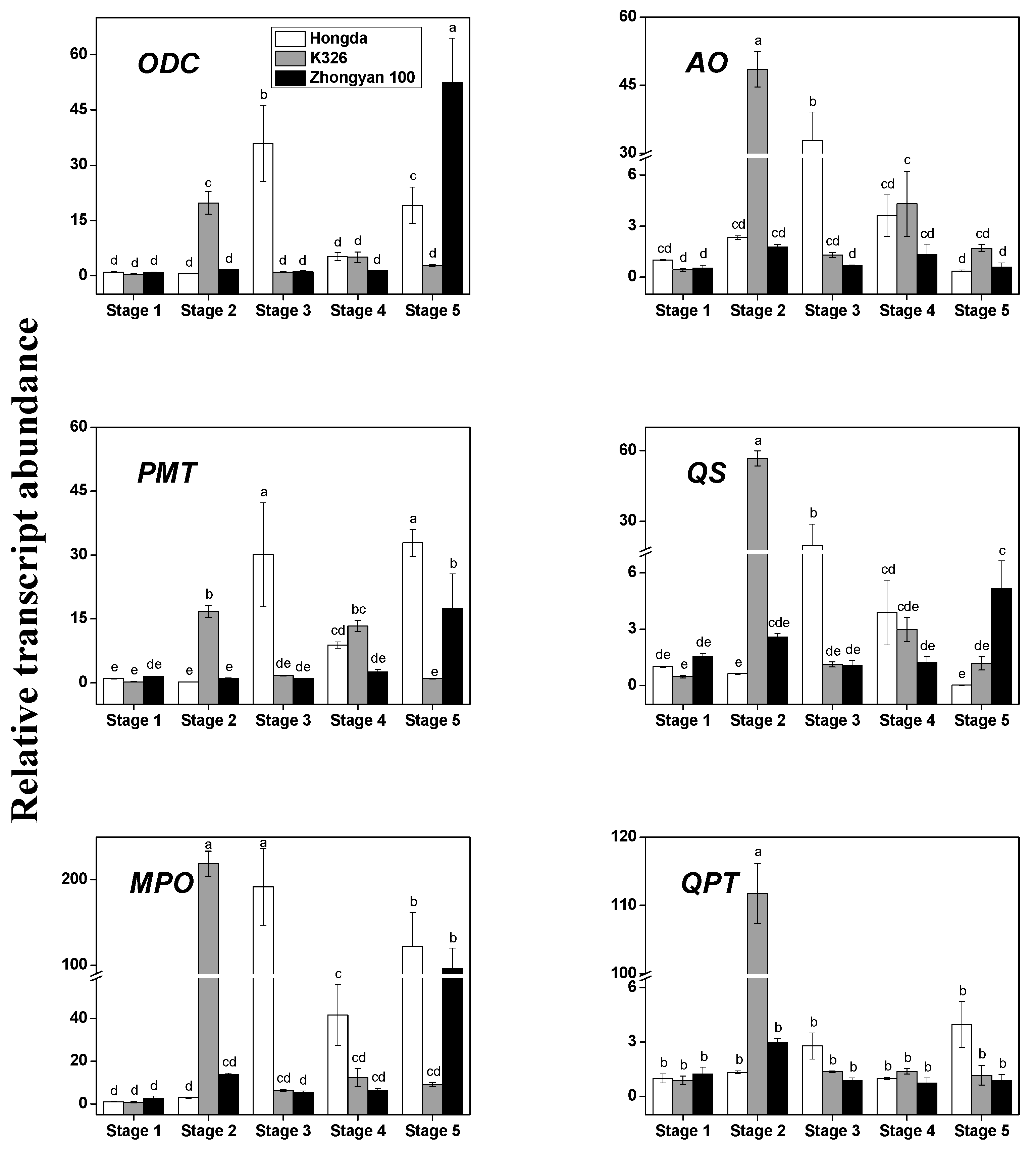
3.2. The Transcription Levels of the Key Structural Genes of Alkaloid Biosynthesis
3.3. Variance Analysis of Genetic Effects
3.4. The Correlation among Different Alkaloids
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Luca, V.; St-Peirre, B. The cell and developmental biology of alkaloid biosynthesis. Trends Plant Sci. 2000, 5, 168–173. [Google Scholar] [CrossRef]
- Wang, J.M.; Sheehan, M.; Brookman, H.; Timko, M.P. Characterization of cDNAs differentially expressed in roots of tobacco (Nicotiana tabacum cv Burley 21) during the early stages of alkaloid biosynthesis. Plant Sci. 2000, 158, 19–32. [Google Scholar] [CrossRef]
- Kutchan, T.M. Alkaloid biosynthesis-The basis for metabolic engineering of medicinal plants. Plant Cell 1995, 7, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Kajikawa, M.; Hashimoto, T. Clustered transcription factor genes regulate nicotine biosynthesis in tobacco. Plant Cell 2010, 22, 3390–3409. [Google Scholar] [CrossRef] [PubMed]
- Nayyatip, S.; Thaichana, P.; Buayairaksa, M.; Tuntiwechapikul, W.; Meepowpan, P.; Nuntasaen, N.; Pompimon, W. Aristolactam-Type Alkaloids from Orophea enterocarpa and Their Cytotoxicities. Int. J. Mol. Sci. 2012, 13, 5010–5018. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.B.; Liu, B.Z.; Lin, P.; Su, Q.D. Fast analysis of nicotine related alkaloids in tobacco and cigarette smoke by megabore capillary gas chromatography. J. Chromatogr. A 2003, 1017, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Hibi, N.; Higashiguchi, S.; Hashimoto, T.; Yamada, Y. Gene expression in tobacco low-nicotine mutants. Plant Cell 1994, 6, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Siminszky, B.; Gavilano, L.; Bowen, S.W.; Dewey, R.E. Conversion of nicotine to nornicotine in Nicotiana tabacum is mediated by CYP82E4, a cytochrome P450 monooxygenase. Proc. Natl. Acad. Sci. USA 2005, 102, 14919–14924. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.T.; Tilleman, S.; Šwiątek, A.; De Sutter, V.; Rischer, H.; Vanhoutte, I.; Van Onckelen, H.; Hilson, P.; Inzé, D.; Oksman-Caldentey, K.; Goossens, A. Functional characterization of genes involved in pyridine alkaloid biosynthesis in tobacco. Phytochemistry 2007, 68, 2773–2785. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Z.; Huang, Y.J.; Liu, G.S.; Zhao, M.Q.; Bush, L.P. Alkaloid content and proportion in Chinese tobacco and cigarettes. Acta Tabacaria Sin. 2001, 2, 8–12. [Google Scholar]
- Lin, G.H.; Zhou, J.H.; Fan, Q.F.; Yang, H.Q.; Cao, R.X. Effects of topping technology on leaf yield, quality and alkaloid content of flue-cured tobacco. Chin. Tob. Sci. 2002, 4, 8–12. [Google Scholar]
- Lian, Y.Y.; Wang, Y.B.; Qiu, J.; Zhang, Z.F.; Cao, J.M.; Ning, Y.; Yue, S.; Niu, P. Analysis on major alkaloids contents and constituent proportions of flue-cured tobacco from different tobacco production regions. Chin. Tob. Sci. 2008, 29, 6–9. [Google Scholar]
- Shi, Q.M.; Li, C.J.; Zhang, F.S. Nicotine synthesis in Nicotiana tabacum L. induced by mechanical wounding is regulated by auxin. J. Exp. Bot. 2006, 57, 2899–2907. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, F.; Zhou, G.J.; Chu, G.H.; Huang, F.F.; Wang, Q.M.; Jin, L.F.; Lin, F.C.; Yang, J. Genetic variation in alkaloid accumulation in leaves of Nicotiana. J. Zhejiang Univ. Sci. B 2013, 14, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Wang, R.; Meng, J.J.; Bi, Y.P.; Xu, P.L.; Guo, F.; Wan, S.B.; He, Q.W.; Li, X.G. Overexpression of Arabidopsis CBF1 gene in transgenic tobacco alleviates photoinhibition of PSII and PSI during chilling stress under low irradiance. J. Plant Physiol. 2010, 167, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Analytic methods for seed models with genotype × environment interactions. Acta Genet. Sin. 1996, 23, 56–58. [Google Scholar] [PubMed]
- Chen, G.B.; Zhu, Z.X.; Zhang, F.T.; Zhu, J. Quantitative genetic analysis station for the genetic analysis of complex traits. Chin. Sci. Bull. 2012, 57, 2721–2726. [Google Scholar] [CrossRef]
- Miller, R.G. The jackknife: A review. Biometrika 1974, 61, 1–15. [Google Scholar]
- Zador, E.; Jones, D. The biosynthesis of a novel nicotine alkaloid in the trichomes of Nicotiana stocktonii. Plant Physiol. 1986, 82, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, S.J.; Johnson, R.; Hamill, J.D. Analysis of wound-induced gene expression in Nicotiana species with contrasting alkaloid profiles. Funct. Plant Biol. 2004, 31, 721–729. [Google Scholar] [CrossRef]
- Yan, X.F.; Chen, S.X. Regulation of plant glucosinolate metabolism. Planta 2007, 226, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.J.; Sun, J.L.; Wang, J.; Jia, Y.H.; Zhang, X.Y.; Pang, B.Y.; Sun, J.; Du, X.M. Genetic effects and heterosis of the fibre colour and quality of brown cotton (Gossypium hirsutum). Plant Breed. 2011, 130, 450–456. [Google Scholar] [CrossRef]
| Variety | Site | Developmental Stage | Sampling Leaf Position | Sampling Time (yy/mm/dd) |
|---|---|---|---|---|
| Hongda | Xiangyun county, Yunnan Province, China | Rosette stage | Middle part | 11/06/20 |
| Vigorous growing stage | Middle part | 11/07/06 | ||
| Lower leaves maturing stage | 4–6 | 11/07/23 | ||
| Middle leaves maturing stage | 11–13 | 11/08/09 | ||
| Upper leaves maturing stage | 16–18 | 11/08/25 | ||
| K326 | Zunyi county, Guizhou Province, China | Rosette stage | Middle part | 11/06/20 |
| Vigorous growing stage | Middle part | 11/07/06 | ||
| Lower leaves maturing stage | 4–6 | 11/07/23 | ||
| Middle leaves maturing stage | 11–13 | 11/08/09 | ||
| Upper leaves maturing stage | 16–18 | 11/08/25 | ||
| Zhongyan 100 | Xiangxian county, Henan Province, China | Rosette stage | Middle part | 11/06/20 |
| Vigorous growing stage | Middle part | 11/07/06 | ||
| Lower leaves maturing stage | 4–6 | 11/07/23 | ||
| Middle leaves maturing stage | 11–13 | 11/08/09 | ||
| Upper leaves maturing stage | 16–18 | 11/08/25 |
| Gene Name | Forward (F) or Reverse (R) | Sequence | Reference |
|---|---|---|---|
| ODC | F | ACTGTGTTTGGGCCCACTTG | [4] |
| R | CCATATTAGGAAAAACCAGC | ||
| PMT | F | ATTGGACCAAGATCGAGTC | [4] |
| R | ATTACTGCAGAATTCTCCTAC | ||
| MPO | F | CAGTGATGTTACTGAAACTA | [4] |
| R | ATAGGCGAGGAGGACTCATG | ||
| AO | F | TTAACAAAGTCATCCGTCGG | [4] |
| R | ATTTAGTCTTGAGGTAGACC | ||
| QS | F | AATCACTGCTTGATGGTATC | [4] |
| R | ACTGGCAAGTTCTTGGACTC | ||
| QPT | F | GACGCATTCCGTGAAAGCAC | [4] |
| R | AAGTAATGGCGCTCATGCTC | ||
| 26S-RNA | F | GAAGAAGGTCCCAAGGGTTC | [16] |
| R | TCTCCCTTTAACACCAACGG |
| Variety | Developmental Stage | Nicotine (mg g−1 DW) | Nornicotine (μg g−1 DW) | Myosmine (μg g−1 DW) | Anabasine (μg g−1 DW) | Anatabine (μg g−1 DW) | Cotinine (μg g−1 DW) | Total Alkaloids (mg g−1 DW) |
|---|---|---|---|---|---|---|---|---|
| Hongda | Rosette stage | 7.31 ± 0.35 g | 47.12 ± 2.55 f | 3.95 ± 0.48 f | 6.21 ± 0.73 g | 97.38 ± 9.06 h | 5.96 ± 1.25 e | 7.47 ± 0.36 g |
| Vigorous growing stage | 6.22 ± 0.49 g | 37.77 ± 4.07 f | 3.39 ± 0.58 f | 8.53 ± 1.25 g | 180.58 ± 32.41 gh | 4.58 ± 0.40 e | 6.46 ± 0.52 g | |
| Lower leaves maturing stage | 7.59 ± 0.66 g | 54.03 ± 0.94 f | 3.70 ± 0.06 f | 10.48 ± 1.00 g | 286.95 ± 45.29 g | 6.15 ± 1.04 e | 7.95 ± 0.66 g | |
| Middle leaves maturing stage | 11.70 ± 0.22 f | 124.77 ± 23.88 e | 12.22 ± 1.54 de | 27.75 ± 2.17 f | 607.87 ± 60.02 f | 34.39 ± 2.54 c | 12.50 ± 0.24 f | |
| Upper leaves maturing stage | 21.85 ± 2.20 cd | 321.10 ± 11.40 cd | 17.10 ± 3.22 d | 76.72 ± 7.22 c | 1307.54 ± 126.89 c | 8.25 ± 0.49 e | 23.58 ± 2.32 cd | |
| K326 | Rosette stage | 5.77 ± 0.26 g | 47.52 ± 2.65 f | 4.04 ± 1.69 f | 5.63 ± 0.19 g | 104.41 ± 8.28 h | 5.71 ± 1.95 e | 5.94 ± 0.26 g |
| Vigorous growing stage | 11.34 ± 1.06 f | 119.31 ± 20.41 e | 9.38 ± 1.64 e | 13.35 ± 0.17 g | 273.91 ± 39.38 g | 7.71 ± 1.21 e | 11.76 ± 1.10 f | |
| Lower leaves maturing stage | 22.97 ± 1.69 c | 347.05 ± 34.48 c | 27.54 ± 2.90 c | 33.99 ± 2.69 ef | 738.93 ± 38.97 ef | 33.05 ± 5.25 c | 24.15 ± 1.69 c | |
| Middle leaves maturing stage | 20.75 ± 0.73 d | 358.34 ± 16.00 c | 36.42 ± 5.64 b | 49.28 ± 5.06 d | 933.56 ± 187.86 d | 72.70 ± 12.28 a | 22.20 ± 0.67 cd | |
| Upper leaves maturing stage | 38.82 ± 0.51 a | 1055.81 ± 94.16 a | 58.41 ± 3.52 a | 128.18 ± 8.66 a | 2029.32 ± 179.12 a | 43.67 ± 2.29 b | 42.13 ± 0.72 a | |
| Zhongyan 100 | Rosette stage | 7.25 ± 0.12 g | 49.03 ± 3.81 f | 3.82 ± 0.42 f | 6.87 ± 0.85 g | 102.53 ± 8.67 h | 6.28 ± 1.17 e | 7.42 ± 0.12 g |
| Vigorous growing stage | 5.73 ± 0.68 g | 46.02 ± 2.48 f | 2.46 ± 0.22 f | 6.67 ± 0.94 g | 133.08 ± 14.44 h | 2.45 ± 0.20 e | 5.92 ± 0.69 g | |
| Lower leaves maturing stage | 18.11 ± 0.59 e | 280.40 ± 52.63 d | 16.43 ± 0.97 d | 33.27 ± 1.38 ef | 702.27 ± 34.30 f | 19.01 ± 2.94 d | 19.16 ± 0.67 e | |
| Middle leaves maturing stage | 20.29 ± 1.13 d | 369.45 ± 15.49 c | 23.11 ± 1.52 c | 42.67 ± 3.35 de | 873.33 ± 60.32 de | 42.32 ± 9.34 b | 21.65 ± 1.19 d | |
| Upper leaves maturing stage | 27.38 ± 3.12 b | 897.02 ± 28.12 b | 27.49 ± 8.15 c | 104.20 ± 21.69 b | 1881.15 ± 67.92 b | 25.55 ± 5.22 d | 30.32 ± 3.17 b |
| Trait | Developmental Stage | ODC | PMT | MPO | AO | QS | QPT |
|---|---|---|---|---|---|---|---|
| Vigorous Growing Stage | |||||||
| Nicotine | Vigorous growing stage | 0.991 | 0.993 | 0.992 | 0.998 | 0.994 | 0.996 |
| Nornicotine | 0.999 | 0.999 | 0.999 | 0.995 | 0.998 | 0.997 | |
| Myosmine | 0.984 | 0.986 | 0.986 | 0.994 | 0.988 | 0.991 | |
| Anabasine | 0.948 | 0.951 | 0.95 | 0.966 | 0.954 | 0.959 | |
| Anatabine | 0.925 | 0.929 | 0.928 | 0.947 | 0.933 | 0.939 | |
| Cotinine | 0.893 | 0.898 | 0.897 | 0.919 | 0.903 | 0.91 | |
| Total alkaloids | 0.991 | 0.992 | 0.992 | 0.997 | 0.993 | 0.995 | |
| Nicotine | Lower leaves maturing stage | 0.777 | 0.77 | 0.772 | 0.736 | 0.763 | 0.752 |
| Nornicotine | 0.714 | 0.706 | 0.708 | 0.668 | 0.698 | 0.686 | |
| Myosmine | 0.873 | 0.867 | 0.868 | 0.84 | 0.862 | 0.853 | |
| Anabasine | 0.567 | 0.558 | 0.56 | 0.514 | 0.549 | 0.534 | |
| Anatabine | 0.604 | 0.596 | 0.598 | 0.553 | 0.587 | 0.573 | |
| Cotinine | 0.902 | 0.897 | 0.899 | 0.873 | 0.893 | 0.885 | |
| Total alkaloids | 0.772 | 0.765 | 0.766 | 0.73 | 0.758 | 0.746 | |
| Nicotine | Middle leaves maturing stage | 0.582 | 0.573 | 0.575 | 0.53 | 0.564 | 0.55 |
| Nornicotine | 0.51 | 0.501 | 0.503 | 0.456 | 0.492 | 0.476 | |
| Myosmine | 0.916 | 0.911 | 0.912 | 0.889 | 0.907 | 0.899 | |
| Anabasine | 0.771 | 0.764 | 0.766 | 0.73 | 0.757 | 0.754 | |
| Anatabine | 0.682 | 0.674 | 0.676 | 0.635 | 0.666 | 0.653 | |
| Cotinine | 0.99 | 0.998 | 0.988 | 0.979 | 0.986 | 0.983 | |
| Total alkaloids | 0.586 | 0.577 | 0.579 | 0.534 | 0.569 | 0.554 | |
| Nicotine | Upper leaves maturing stage | 0.963 | 0.96 | 0.961 | 0.944 | 0.957 | 0.952 |
| Nornicotine | 0.705 | 0.697 | 0.699 | 0.66 | 0.69 | 0.677 | |
| Myosmine | 0.982 | 0.979 | 0.98 | 0.968 | 0.977 | 0.973 | |
| Anabasine | 0.872 | 0.867 | 0.868 | 0.84 | 0.862 | 0.853 | |
| Anatabine | 0.697 | 0.689 | 0.691 | 0.651 | 0.682 | 0.669 | |
| Cotinine | 0.897 | 0.892 | 0.893 | 0.868 | 0.887 | 0.879 | |
| Total alkaloids | 0.951 | 0.947 | 0.948 | 0.93 | 0.944 | 0.938 | |
| Parameter | Nicotine | Nornicotine | Myosmine | Anabasine | Anatabine | Cotinine | Total Alkaloids |
|---|---|---|---|---|---|---|---|
| VV/VP | 0.147 ** | 0.135 ** | 0.262 ** | 0.046 ** | 0.048 ** | 0.186 ** | 0.141 ** |
| VD/VP | 0.683 ** | 0.655 ** | 0.468 ** | 0.865 ** | 0.887 ** | 0.611 ** | 0.700 ** |
| VVD/VP | 0.153 ** | 0.204 ** | 0.231 ** | 0.053 ** | 0.050 ** | 0.170 ** | 0.144 ** |
| Ve/VP | 0.018 | 0.006 | 0.038 * | 0.036 | 0.016 * | 0.033 ** | 0.016 |
| Trait | Nicotine | Nornicotine | Myosmine | Anabasine | Anatabine | Cotinine | Total Alkaloids |
|---|---|---|---|---|---|---|---|
| Nicotine | 0.857 ** | 0.801 ** | 0.810 ** | 0.864 ** | 0.558 ** | 0.886 ** | |
| 0.878 ** | 0.838 ** | 0.827 ** | 0.881 ** | 0.583 ** | 0.898 ** | ||
| Nornicotine | 0.900 ** | 0.775 ** | 0.843 ** | 0.895 ** | 0.483 ** | 0.868 ** | |
| 0.946 ** | 0.809 ** | 0.872 ** | 0.915 ** | 0.501 ** | 0.888 ** | ||
| 0.682 ** | |||||||
| Myosmine | 0.832 ** | 0.809 ** | 0.698 ** | 0.750 ** | 0.657 ** | 0.807 ** | |
| 0.903 ** | 0.873 ** | 0.753 ** | 0.784 ** | 0.687 ** | 0.840 ** | ||
| 0.809 ** | 0.768 ** | ||||||
| Anabasine | 0.818 ** | 0.851 ** | 0.768 ** | 0.877 ** | 0.422 ** | 0.821 ** | |
| 0.917 ** | 0.947 ** | 0.865 ** | 0.899 ** | 0.451 ** | 0.838 ** | ||
| 0.626 ** | 0.778 ** | 0.803 ** | |||||
| Anatabine | 0.803 ** | 0.865 ** | 0.741 ** | 0.774 ** | 0.487 ** | 0.876 ** | |
| 0.973 ** | 0.981 ** | 0.939 ** | 0.954 ** | 0.509 ** | 0.892 ** | ||
| 0.666 ** | 0.861 ** | 0.709 ** | 0.663 ** | ||||
| Cotinine | 0.851 ** | 0.817 ** | 0.854 ** | 0.780 ** | 0.734 ** | 0.557 ** | |
| 0.535 ** | 0.404 ** | 0.638 ** | 0.420 ** | 0.513 ** | 0.581 ** | ||
| 0.569 ** | 0.616 ** | 0.736 ** | 0.623 ** | 0.636 ** | |||
| Total Alkaloids | 0.828 ** | 0.902 ** | 0.831 ** | 0.820 ** | 0.805 ** | 0.848 ** | |
| 0.947 ** | 0.950 ** | 0.906 ** | 0.922 ** | 0.977 ** | 0.532 ** | ||
| 0.848 ** | 0.712 ** | 0.819 ** | 0.644 ** | 0.688 ** | 0.585 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Tian, Y.-X.; Zhang, F.; Chen, Q.; Zhang, Y.; Luo, Y.; Wang, X.-R.; Lin, F.-C.; Yang, J.; Tang, H.-R. Variations of Alkaloid Accumulation and Gene Transcription in Nicotiana tabacum. Biomolecules 2018, 8, 114. https://doi.org/10.3390/biom8040114
Sun B, Tian Y-X, Zhang F, Chen Q, Zhang Y, Luo Y, Wang X-R, Lin F-C, Yang J, Tang H-R. Variations of Alkaloid Accumulation and Gene Transcription in Nicotiana tabacum. Biomolecules. 2018; 8(4):114. https://doi.org/10.3390/biom8040114
Chicago/Turabian StyleSun, Bo, Yu-Xiao Tian, Fen Zhang, Qing Chen, Yong Zhang, Ya Luo, Xiao-Rong Wang, Fu-Cheng Lin, Jun Yang, and Hao-Ru Tang. 2018. "Variations of Alkaloid Accumulation and Gene Transcription in Nicotiana tabacum" Biomolecules 8, no. 4: 114. https://doi.org/10.3390/biom8040114
APA StyleSun, B., Tian, Y.-X., Zhang, F., Chen, Q., Zhang, Y., Luo, Y., Wang, X.-R., Lin, F.-C., Yang, J., & Tang, H.-R. (2018). Variations of Alkaloid Accumulation and Gene Transcription in Nicotiana tabacum. Biomolecules, 8(4), 114. https://doi.org/10.3390/biom8040114








