α-Tocopheryl Succinate-Based Polymeric Nanoparticles for the Treatment of Head and Neck Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Copolymers and Nanoparticles
2.2. Cell Culture and Treatment
2.3. Cell Viability Assay
2.4. Reactive Species Quantification (Total ROS)
2.5. Superoxide Anion Detection
2.6. Western Blot
2.7. Annexin V Detection by Indirect Immunofluorescence
2.8. Cytokine Quantification
2.9. In Vitro Angiogenesis
2.10. Wound Healing Assay
2.11. Statistical Analysis
2.12. Ethics Approval and Consent to Participate
3. Results
3.1. α-Tocopheryl Succinate In Vitro Release
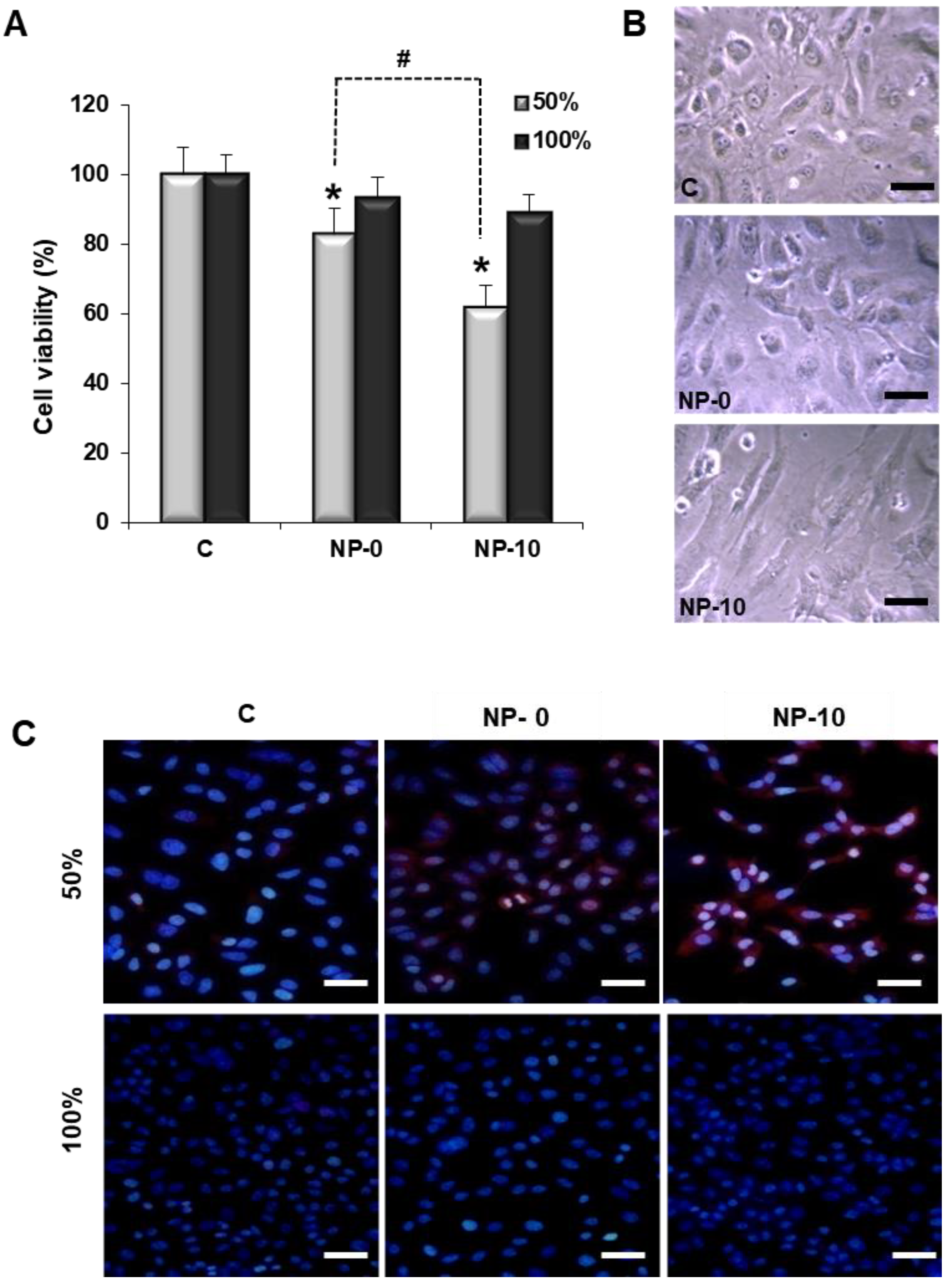
3.2. Reduction of Endothelial Cell Viability
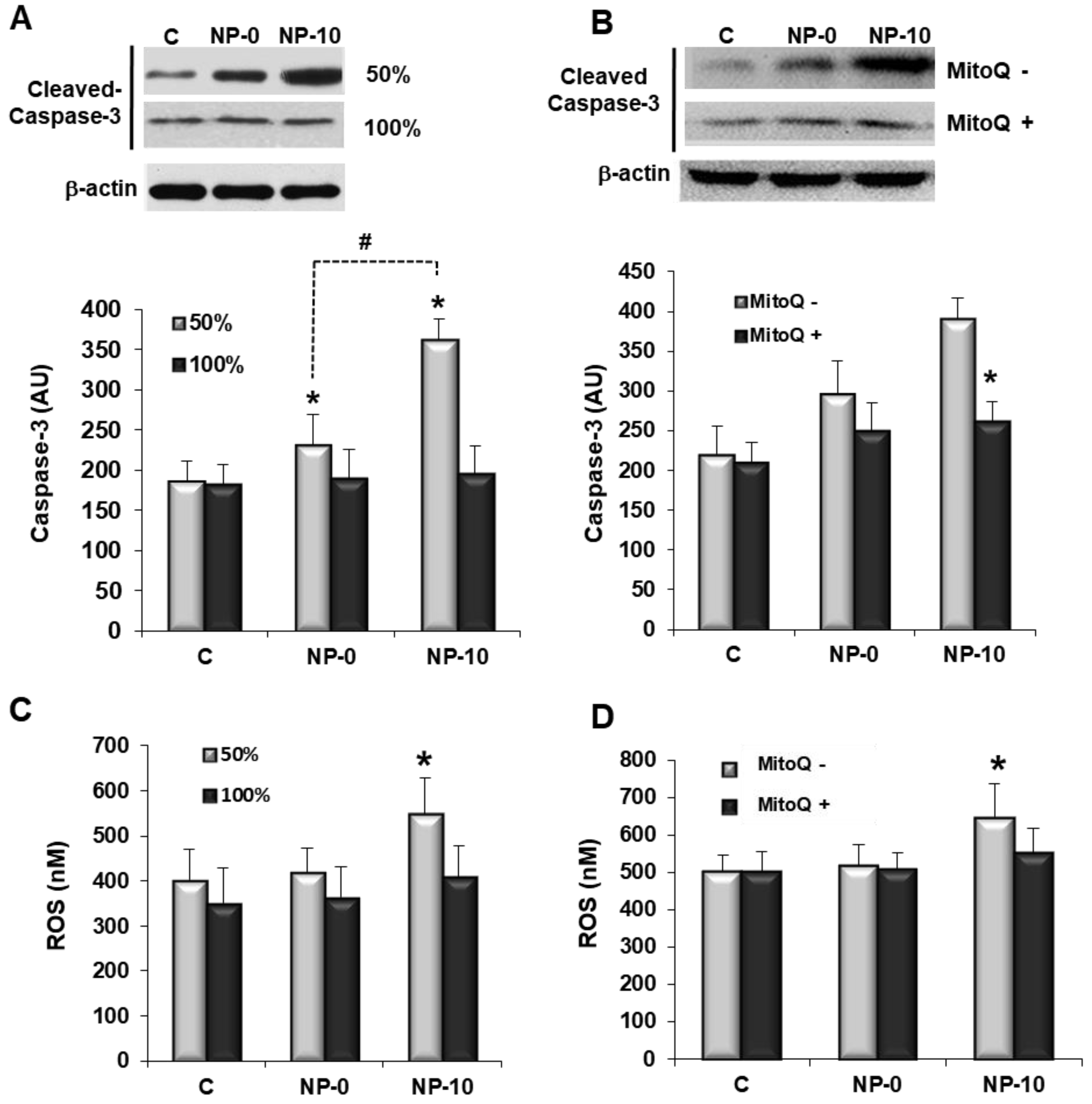
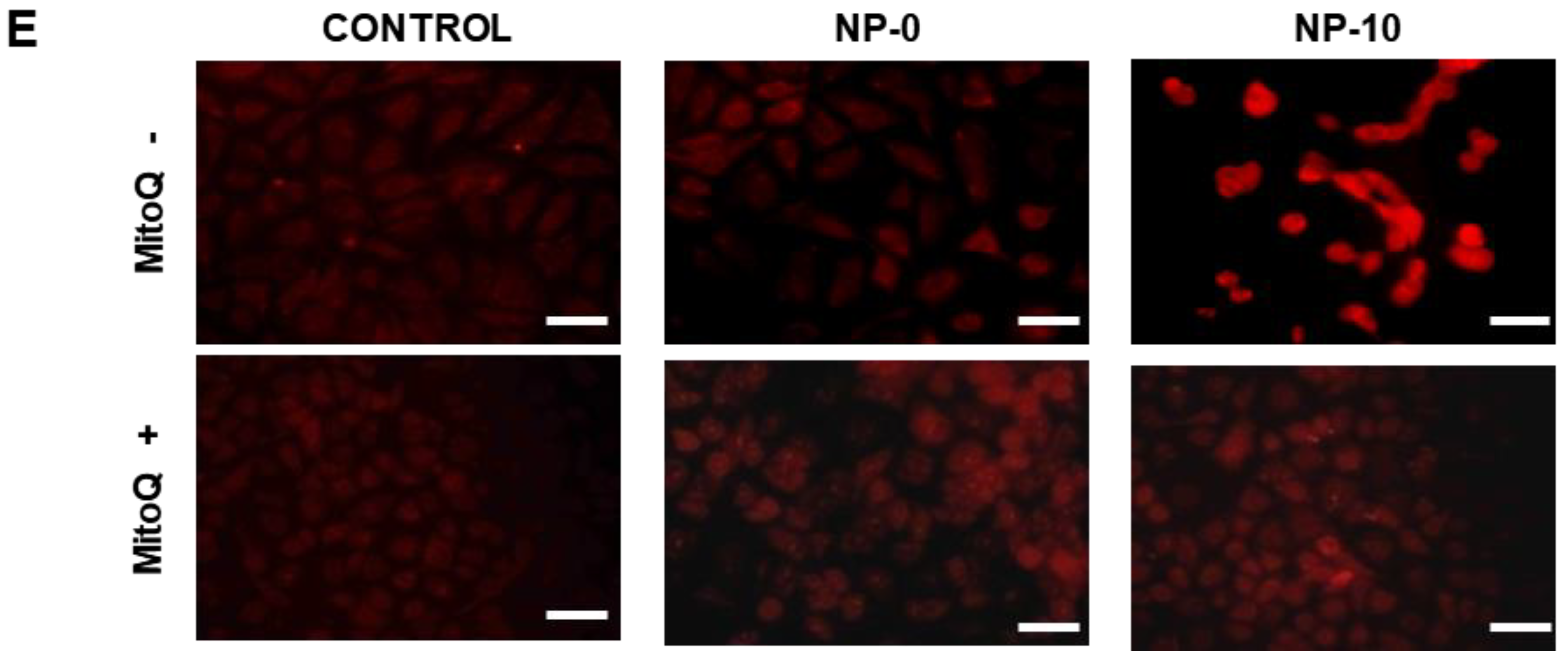
3.3. Apoptosis Induction and Oxidative Stress in Endothelial Cells
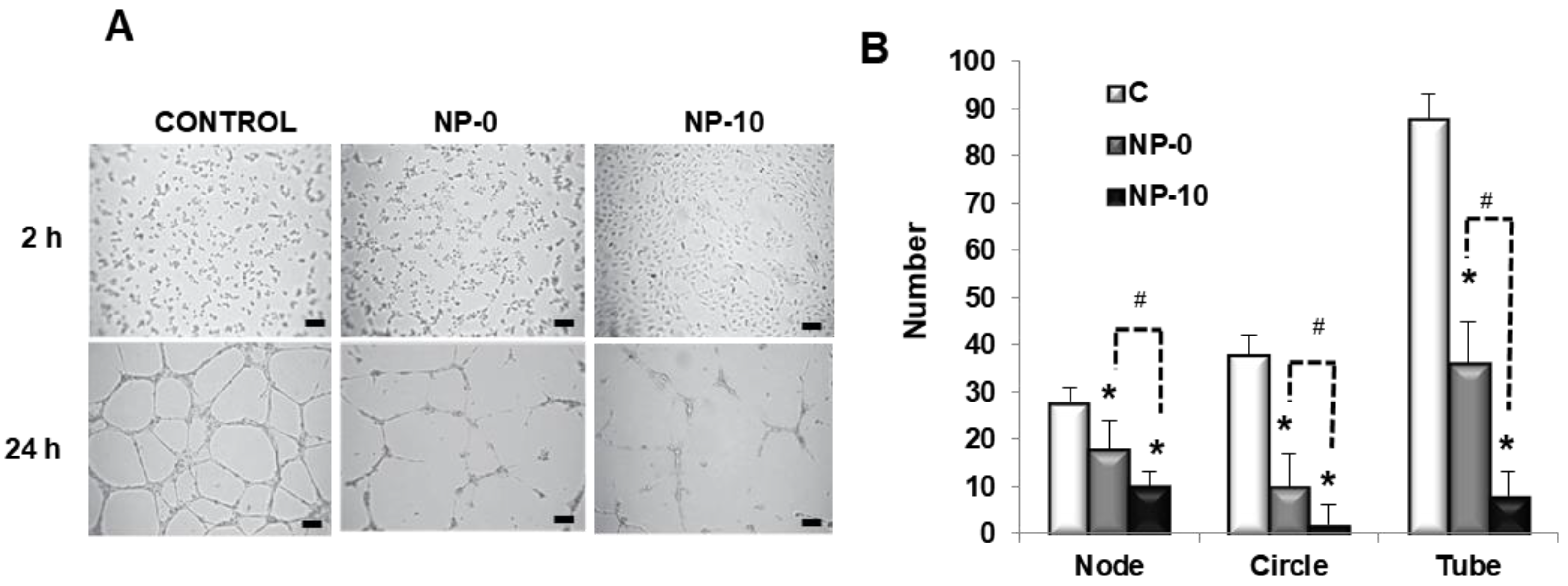
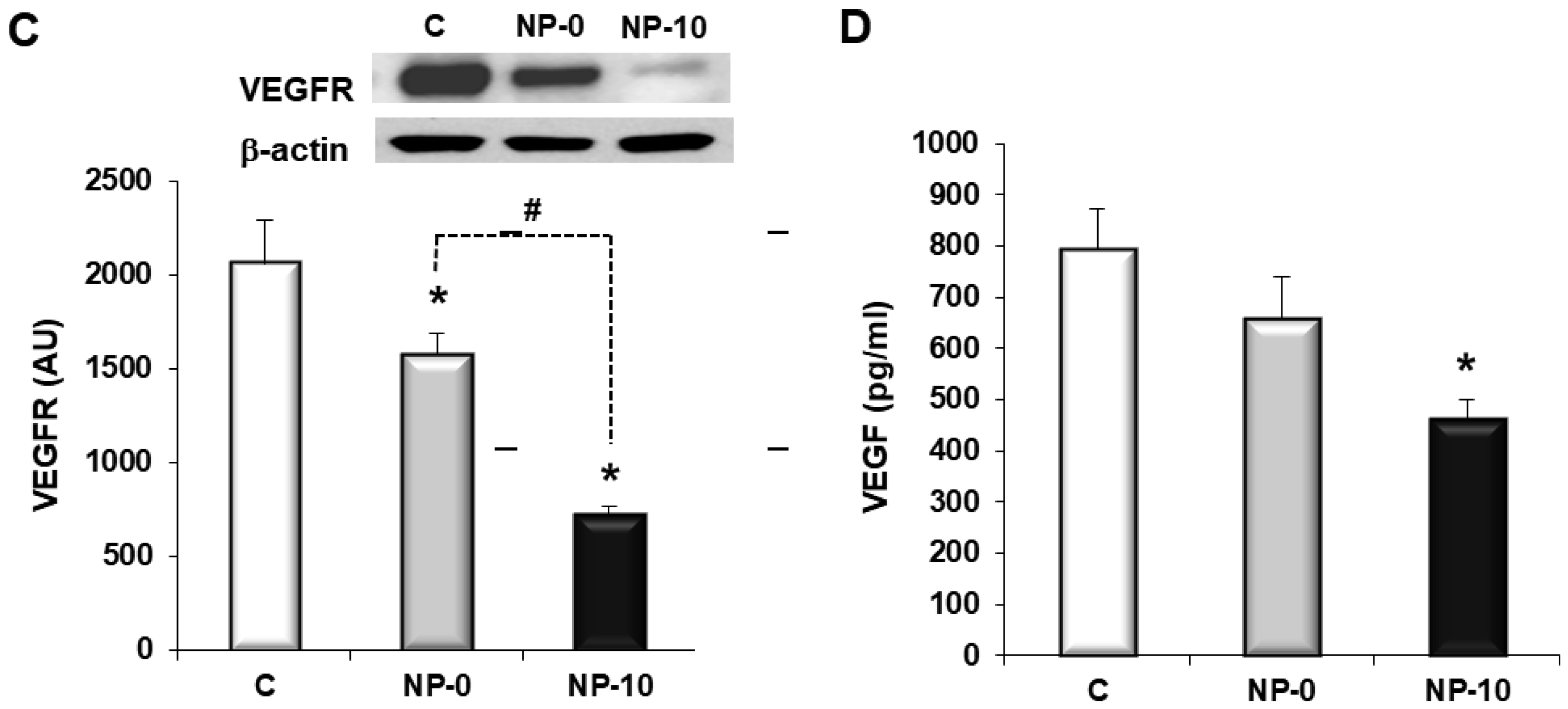
3.4. In Vitro Antiangiogenic Activity of Endothelial Cells
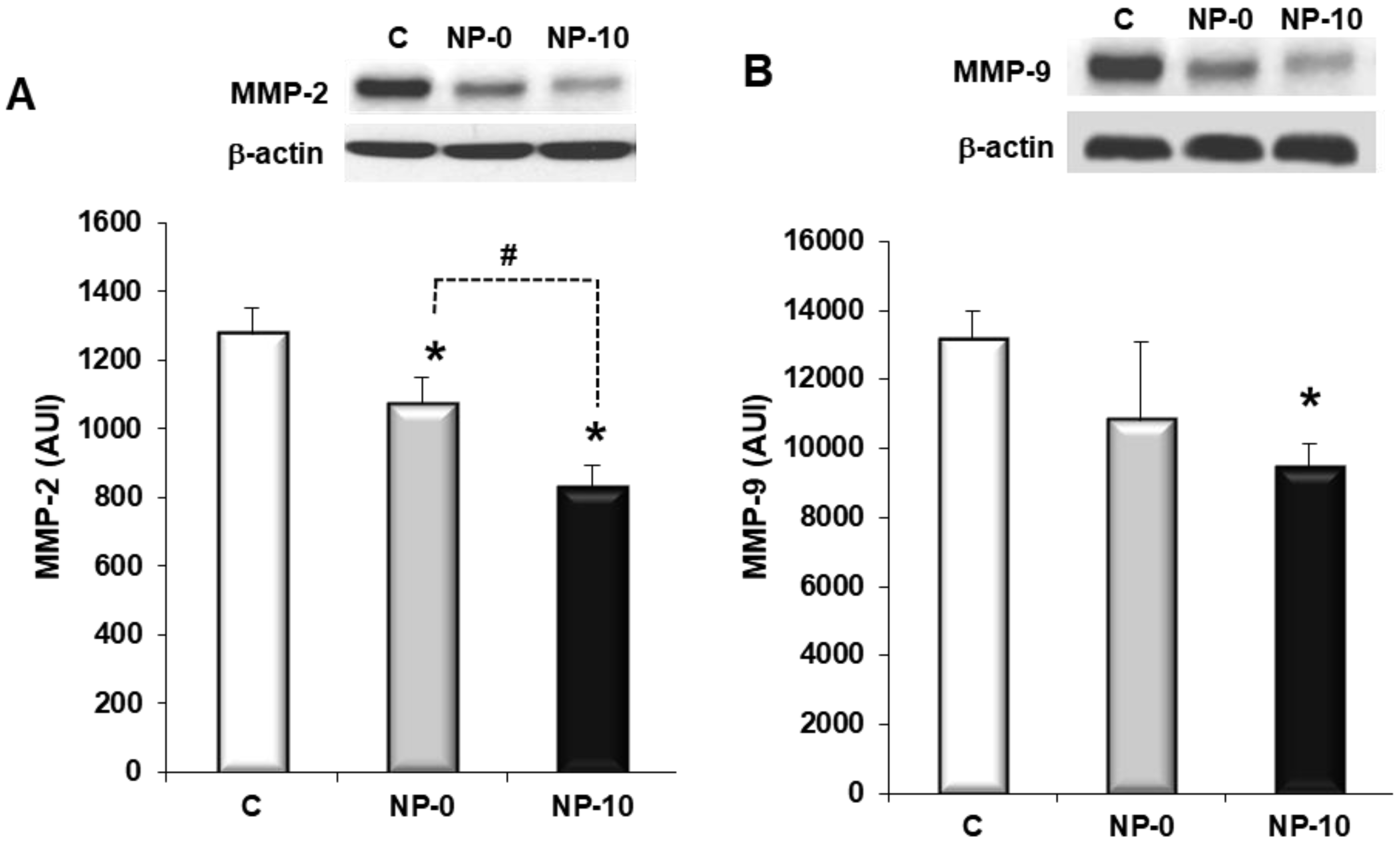
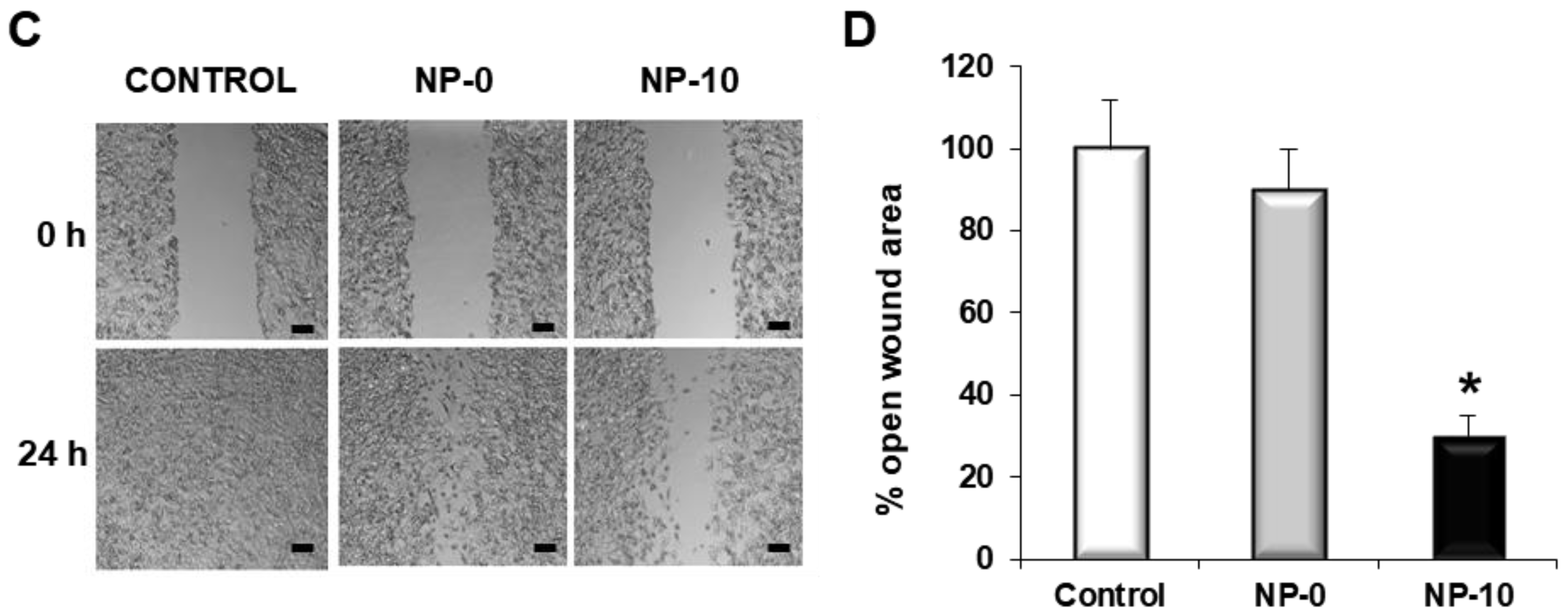
3.5. Inhibition of Matrix Metalloproteinases Expression and Cell Migration of Hypopharynx Carcinoma Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teknos, T.N.; Islam, M.; Arenberg, D.A.; Pan, Q.; Carskadon, S.L.; Abarbanell, A.M.; Marcus, B.; Paul, S.; Vandenberg, C.D.; Carron, M.; et al. The effect of tetrathiomolybdate on cytokine expression, angiogenesis, and tumor growth in squamous cell carcinoma of the head and neck. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Lathers, D.M.R.; Young, M.R.I. Increased aberrance of cytokine expression in plasma of patients with more advanced squamous cell carcinoma of the head and neck. Cytokine 2004, 25, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Malhotra, P.S.; Thomas, G.R.; Ondrey, F.G.; Duffey, D.C.; Smith, C.W.; Enamorado, I.; Yeh, N.T.; Kroog, G.S.; Rudy, S.; et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin. Cancer Res. 1999, 5, 1369–1379. [Google Scholar] [PubMed]
- Ruokolainen, H.; Pääkkö, P.; Turpeenniemi-Hujanen, T. Tissue and circulating immunoreactive protein for MMP-2 and TIMP-2 in head and neck squamous cell carcinoma—Tissue immunoreactivity predicts aggressive clinical course. Mod. Pathol. 2006, 19, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Kieran, M.W.; Kalluri, R.; Cho, Y.J. The VEGF pathway in cancer and disease: Responses, resistance, and the path forward. Cold Spring Harb. Perspect. Med. 2012, 2, a006593. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Galanopoulos, N.; Lavertu, P.; Fu, P.; Gibson, M.; Argiris, A.; Rezaee, R.; Zender, C.; Wasman, J.; Machtay, M.; et al. Phase II study of bevacizumab in combination with docetaxel and radiation in locally advanced squamous cell carcinoma of the head and neck. Head Neck 2015, 37, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.-F.; Swettenham, E.; Eliasson, J.; Wang, X.-F.; Gold, M.; Medunic, Y.; Stantic, M.; Low, P.; Prochazka, L.; Witting, P.K. Vitamin E analogues inhibit angiogenesis by selective induction of apoptosis in proliferating endothelial cells: The role of oxidative stress. Cancer Res. 2007, 67, 11906–11913. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.-F.; Neuzil, J. Vitamin E analogues as prototypic mitochondria-targeting anti-cancer agents. In Mitochondria: The Anti-Cancer Target for the Third Millennium; Springer: Berlin, Germany, 2014; pp. 151–181. [Google Scholar]
- Dong, L.-F.; Low, P.; Dyason, J.C.; Wang, X.-F.; Prochazka, L.; Witting, P.K.; Freeman, R.; Swettenham, E.; Valis, K.; Liu, J. α-tocopheryl succinate induces apoptosis by targeting ubiquinone-binding sites in mitochondrial respiratory complex II. Oncogene 2008, 27, 4324–4335. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Dalen, H.; Andera, L.; Nègre-Salvayre, A.; Augé, N.; Sticha, M.; Lloret, A.; Terman, A.; Witting, P.K.; Higuchi, M.; et al. Mitochondria play a central role in apoptosis induced by α-tocopheryl succinate, an agent with antineoplastic activity: Comparison with receptor-mediated pro-apoptotic signaling. Biochemistry 2003, 42, 4277–4291. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tamai, H.; Ishisaka, R.; Kanno, T.; Arita, K.; Kobuchi, H.; Utsumi, K. Mechanism of α-tocopheryl succinate-induced apoptosis of promyelocytic leukemia cells. Free. Radic. Res. 2000, 33, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Rohlena, J.; Dong, L.F.; Kluckova, K.; Zobalova, R.; Goodwin, J.; Tilly, D.; Stursa, J.; Pecinova, A.; Philimonenko, A.; Hozak, P.; et al. Mitochondrially targeted α-tocopheryl succinate is antiangiogenic: Potential benefit against tumor angiogenesis but caution against wound healing. Antioxid. Redox Signal. 2011, 15, 2923–2935. [Google Scholar] [CrossRef] [PubMed]
- Palao-Suay, R.; Aguilar, M.R.; Parra-Ruiz, F.J.; Fernández-Gutiérrez, M.; Parra, J.; Sánchez-Rodríguez, C.; Sanz-Fernández, R.; Rodrigáñez, L.; Román, J.S. Anticancer and antiangiogenic activity of surfactant-free nanoparticles based on self-assembled polymeric derivatives of vitamin E: Structure-activity relationship. Biomacromolecules 2015, 16, 1566–1581. [Google Scholar] [CrossRef] [PubMed]
- Palao-Suay, R.; Aguilar, M.R.; Parra-Ruiz, F.J.; Maji, S.; Hoogenboom, R.; Rohner, N.A.; Thomas, S.N.; Román, J.S. Enhanced bioactivity of α-tocopheryl succinate based block copolymer nanoparticles by reduced hydrophobicity. Macromol. Biosci. 2016, 16, 1824–1837. [Google Scholar] [CrossRef] [PubMed]
- Palao-Suay, R.; Aguilar, M.R.; Parra-Ruiz, F.J.; Maji, S.; Hoogenboom, R.; Rohner, N.A.; Thomas, S.N.; San Román, J. α-TOS-based RAFT block copolymers and their NPs for the treatment of cancer. Polym. Chem. 2016, 7, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Palao-Suay, R.; Rodrigáñez, L.; Aguilar, M.R.; Sánchez-Rodríguez, C.; Parra, F.; Fernández, M.; Parra, J.; Riestra-Ayora, J.; Sanz-Fernández, R.; Román, J.S. Mitochondrially targeted nanoparticles based on α-TOS for the selective cancer treatment. Macromol. Biosci. 2016, 16, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Martín-Saldaña, S.; Palao-Suay, R.; Aguilar, M.R.; Ramírez-Camacho, R.; San Román, J. Polymeric nanoparticles loaded with dexamethasone or α-tocopheryl succinate to prevent cisplatin-induced ototoxicity. Acta Biomater. 2017, 53, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Mead, L.E.; Moore, D.B.; Woodard, W.; Fenoglio, A.; Yoder, M.C. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 2005, 105, 2783–2786. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-R.; Jeong, H.E.; Joo, H.J.; Choi, S.-C.; Park, C.-Y.; Kim, J.-H.; Choi, J.-H.; Cui, L.-H.; Hong, S.J.; Chung, S.; et al. Intrinsic FGF2 and FGF5 promotes angiogenesis of human aortic endothelial cells in 3D microfluidic angiogenesis system. Sci. Rep. 2016, 6, 28832. [Google Scholar] [CrossRef] [PubMed]
- Kelso, G.F.; Porteous, C.M.; Coulter, C.V.; Hughes, G.; Porteous, W.K.; Ledgerwood, E.C.; Smith, R.A.J.; Murphy, M.P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J. Boil. Chem. 2001, 276, 4588–4596. [Google Scholar] [CrossRef] [PubMed]
- El-Kenawi, A.E.; El-Remessy, A.B. Angiogenesis inhibitors in cancer therapy: Mechanistic perspective on classification and treatment rationales. Br. J. Pharmacol. 2013, 170, 712–729. [Google Scholar] [CrossRef] [PubMed]
- Van Hinsbergh, V.W.M.; Koolwijk, P. Endothelial sprouting and angiogenesis: Matrix metalloproteinases in the lead. Cardiovasc. Res. 2008, 78, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Shing, Y. Angiogenesis. J. Biol. Chem. 1992, 267, 10931–10934. [Google Scholar] [PubMed]
- Rafii, S.; Lyden, D.; Benezra, R.; Hattori, K.; Heissig, B. Vascular and haematopoietic stem cells: Novel targets for anti-angiogenesis therapy? Nat. Rev. Cancer 2002, 2, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, W.G.; Liu, H.K.; Qi, G.Y.; Wang, Q.; Sun, X.R.; Chen, B.Q.; Liu, J.R. γ-Tocotrienol inhibits angiogenesis of human umbilical vein endothelial cell induced by cancer cell. J. Nutr. Biochem. 2011, 22, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Heinke, J.; Wehofsits, L.; Zhou, Q.; Zoeller, C.; Baar, K.M.; Helbing, T.; Laib, A.; Augustin, H.; Bode, C.; Patterson, C.; et al. BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ. Res. 2008, 103, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Dilda, P.J. Mitochondria as targets in angiogenesis inhibition. Mol. Asp. Med. 2010, 31, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Stapelberg, M.; Gellert, N.; Swettenham, E.; Tomasetti, M.; Witting, P.K.; Procopio, A.; Neuzil, J. Alpha-tocopheryl succinate inhibits malignant mesothelioma by disrupting the fibroblast growth factor autocrine loop mechanism and the role of oxidative stress. J. Biol. Chem. 2005, 280, 25369–25376. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, J.; Swettenham, E.; Wang, X.-F.; Dong, L.-F.; Stapelberg, M. A-tocopheryl succinate inhibits angiogenesis by disrupting paracrine FGF2 signalling. FEBS Lett. 2007, 581, 4611–4615. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Guo, Y.; Gu, X. [anticancer mechanisms of vitamin E succinate]. Ai Zheng Aizheng Chin. J. Cancer 2009, 28, 1114–1118. [Google Scholar] [CrossRef]
- Shibata, A.; Nakagawa, K.; Sookwong, P.; Tsuduki, T.; Oikawa, S.; Mlyazawa, T. δ-Tocotrienol suppresses VEGF induced angiogenesis whereas α-Tocopherol does not. J. Agric. Food. Chem. 2009, 57, 8696–8704. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist 2004, 9, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Bourboulia, D.; Stetler-Stevenson, W.G. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin. Cancer Biol. 2010, 20, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Devy, L.; Huang, L.; Naa, L.; Yanamandra, N.; Pieters, H.; Frans, N.; Chang, E.; Tao, Q.; Vanhove, M.; Lejeune, A.; et al. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 2009, 69, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
| NP | Drug | Load (% w/w) | Dh (nm) | PDI | ζ (mV) | EE (%) |
|---|---|---|---|---|---|---|
| NP-0 | - | - | 134.3 ± 9.2 | 0.128 ± 0.022 | −3.0 ± 0.2 | - |
| NP-10 | α-TOS | 10 | 164.3 ± 4.9 | 0.177 ± 0.042 | −18.2 ± 0.6 | 72 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Rodríguez, C.; Palao-Suay, R.; Rodrigáñez, L.; Aguilar, M.R.; Martín-Saldaña, S.; San Román, J.; Sanz-Fernández, R. α-Tocopheryl Succinate-Based Polymeric Nanoparticles for the Treatment of Head and Neck Squamous Cell Carcinoma. Biomolecules 2018, 8, 97. https://doi.org/10.3390/biom8030097
Sánchez-Rodríguez C, Palao-Suay R, Rodrigáñez L, Aguilar MR, Martín-Saldaña S, San Román J, Sanz-Fernández R. α-Tocopheryl Succinate-Based Polymeric Nanoparticles for the Treatment of Head and Neck Squamous Cell Carcinoma. Biomolecules. 2018; 8(3):97. https://doi.org/10.3390/biom8030097
Chicago/Turabian StyleSánchez-Rodríguez, Carolina, Raquel Palao-Suay, Laura Rodrigáñez, María Rosa Aguilar, Sergio Martín-Saldaña, Julio San Román, and Ricardo Sanz-Fernández. 2018. "α-Tocopheryl Succinate-Based Polymeric Nanoparticles for the Treatment of Head and Neck Squamous Cell Carcinoma" Biomolecules 8, no. 3: 97. https://doi.org/10.3390/biom8030097
APA StyleSánchez-Rodríguez, C., Palao-Suay, R., Rodrigáñez, L., Aguilar, M. R., Martín-Saldaña, S., San Román, J., & Sanz-Fernández, R. (2018). α-Tocopheryl Succinate-Based Polymeric Nanoparticles for the Treatment of Head and Neck Squamous Cell Carcinoma. Biomolecules, 8(3), 97. https://doi.org/10.3390/biom8030097





