Cross-Sectional Associations between Homoarginine, Intermediate Phenotypes, and Atrial Fibrillation in the Community—The Gutenberg Health Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Biomarker Measurement
2.3. Statistical Methods
- Healthy controls with no cardiovascular risk factors (N = 689);
- Individuals with at least one cardiovascular risk factor but without AF (N = 2943);
- Participants with AF (N = 124).
3. Results
3.1. Baseline Data
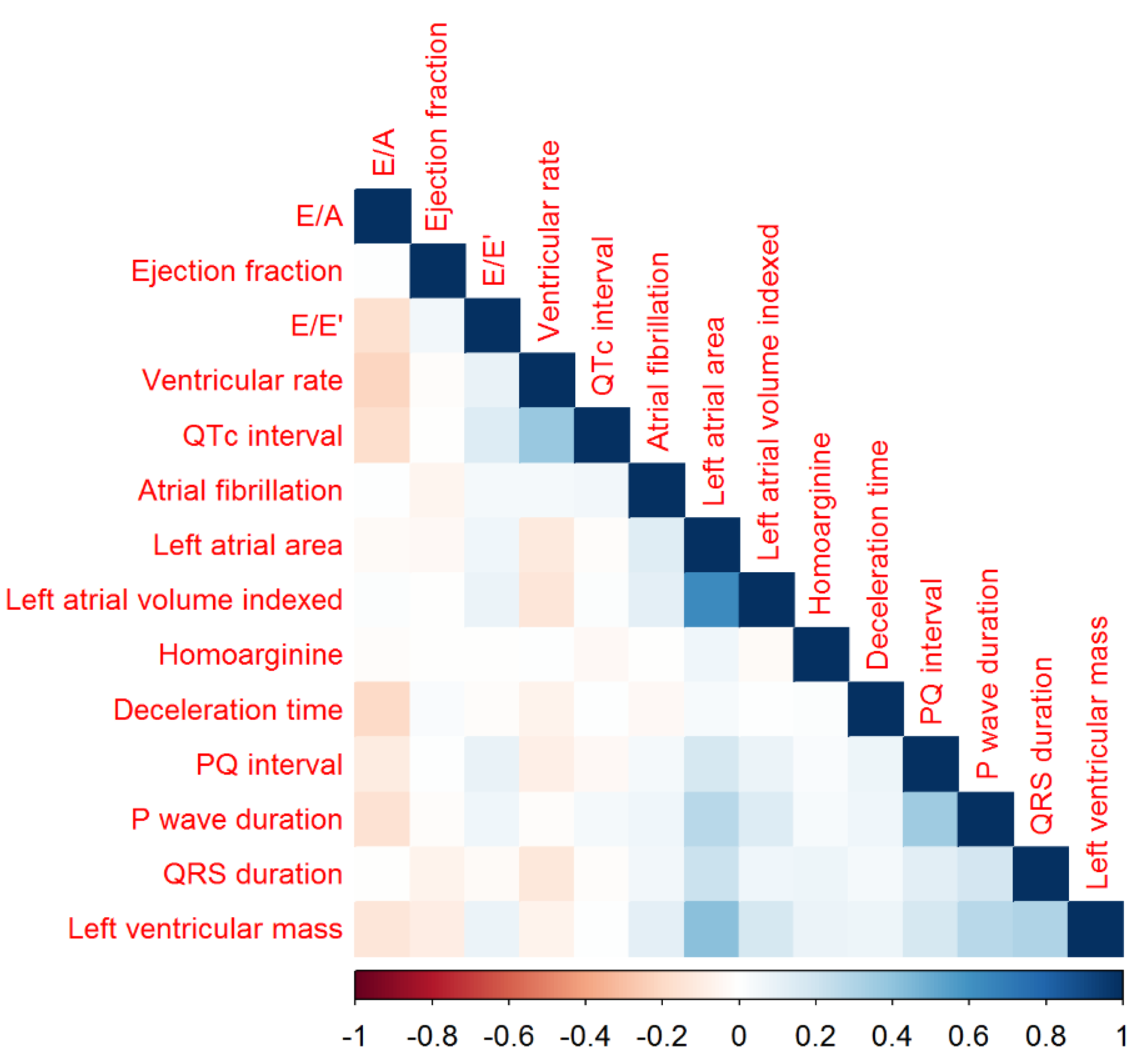
3.2. Homoarginine, Electrocardiographic and Echocardiographic Variables
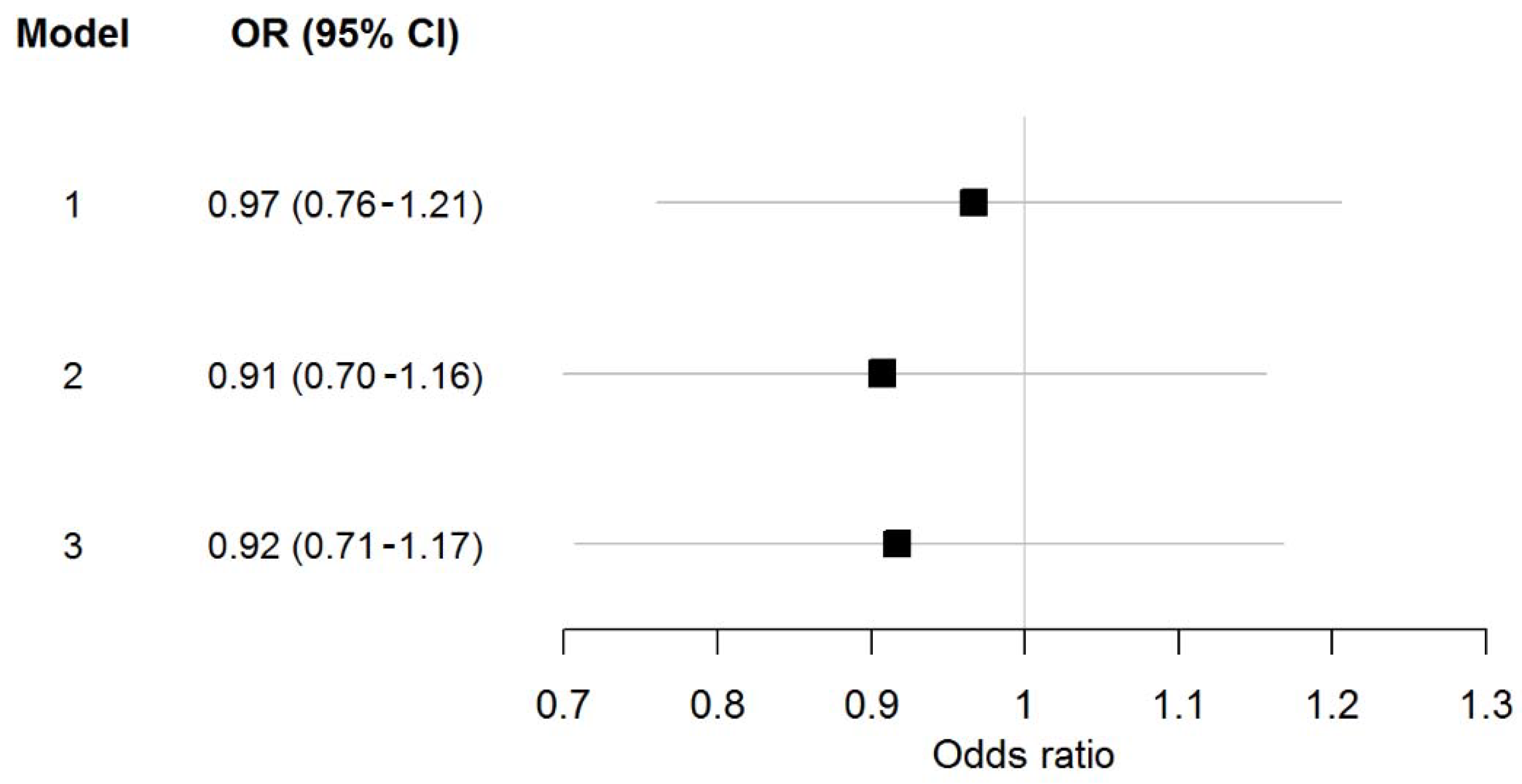
3.3. Homoarginine and Atrial Fibrillation
4. Discussion
4.1. Homoarginine and Electrocardiographic and Echocardiographic Variables
4.2. Homoarginine and Atrial Fibrillation
4.3. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miyasaka, Y.; Barnes, M.E.; Gersh, B.J.; Cha, S.S.; Bailey, K.R.; Abhayaratna, W.P.; Seward, J.B.; Tsang, T.S. Secular Trends in Incidence of Atrial Fibrillation in Olmsted county, Minnesota, 1980 to 2000, and Implications on the Projections for Future Prevalence. Circulation 2006, 114, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of Diagnosed Atrial Fibrillation in Adults: National Implications for Rhythm Management and Stroke prevention: The AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Stefansdottir, H.; Aspelund, T.; Gudnason, V.; Arnar, D.O. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace 2011, 13, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Wang, T.J.; Leip, E.P.; Larson, M.G.; Levy, D.; Vasan, R.S.; D’Agostino, R.B.; Massaro, J.M.; Beiser, A.; Wolf, P.A.; et al. Lifetime Risk for Development of Atrial Fibrillation: The Framingham Heart Study. Circulation 2004, 110, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Levy, D.; Vasan, R.S.; Leip, E.P.; Wolf, P.A.; D’Agostino, R.B.; Murabito, J.M.; Kannel, W.B.; Benjamin, E.J. Temporal relations of Atrial Fibrillation and Congestive Heart Failure and Their Joint Influence on Mortality: The Framingham Heart Study. Circulation 2003, 107, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Shinbane, J.S.; Wood, M.A.; Jensen, D.N.; Ellenbogen, K.A.; Fitzpatrick, A.P.; Scheinman, M.M. Tachycardia-Induced Cardiomyopathy: A Review of Animal Models and Clinical Studies. J. Am. Coll. Cardiol. 1997, 29, 709–715. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, Y.; Barnes, M.E.; Gersh, B.J.; Cha, S.S.; Bailey, K.R.; Seward, J.B.; Iwasaka, T.; Tsang, T.S. Coronary Ischemic Events after First Atrial Fibrillation: Risk and Survival. Am. J. Med. 2007, 120, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Ringborg, A.; Nieuwlaat, R.; Lindgren, P.; Jonsson, B.; Fidan, D.; Maggioni, A.P.; Lopez-Sendon, J.; Stepinska, J.; Cokkinos, D.V.; Crijns, H.J. Costs of atrial fibrillation in five European countries: Results from the Euro Heart Survey on atrial fibrillation. Europace 2008, 10, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Korantzopoulos, P.; Kolettis, T.M.; Galaris, D.; Goudevenos, J.A. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int. J. Cardiol. 2007, 115, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, Z.; Goette, A.; Mera, F.; Honeycutt, C.; Feterik, K.; Wilcox, J.N.; Dudley, S.C., Jr.; Harrison, D.G.; Langberg, J.J. Downregulation of Endocardial Nitric Oxide Synthase Expression and Nitric Oxide Production in Atrial Fibrillation: Potential Mechanisms for Atrial Thrombosis and Stroke. Circulation 2002, 106, 2854–2858. [Google Scholar] [CrossRef] [PubMed]
- Ramuschkat, M.; Appelbaum, S.; Atzler, D.; Zeller, T.; Bauer, C.; Ojeda, F.M.; Sinning, C.R.; Hoffmann, B.; Lackner, K.J.; Boger, R.H.; et al. ADMA, subclinical changes and atrial fibrillation in the general population. Int. J. Cardiol. 2016, 203, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Kitakaze, M.; Sato, H.; Asanuma, H.; Funaya, H.; Koretsune, Y.; Hori, M. Plasma Levels of Nitrite/Nitrate and Platelet cGMP Levels Are Decreased in Patients with Atrial Fibrillation. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3191–3195. [Google Scholar] [CrossRef] [PubMed]
- Nikitovic, D.; Zacharis, E.A.; Manios, E.G.; Malliaraki, N.E.; Kanoupakis, E.M.; Sfiridaki, K.I.; Skalidis, E.I.; Margioris, A.N.; Vardas, P.E. Plasma Levels of Nitrites/Nitrates in Patients with Chronic Atrial Fibrillation are Increased after Electrical Restoration of Sinus Rhythm. J. Interv. Card. Electrophysiol. 2002, 7, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, L.E.; Li, H.; Poulos, T.L.; Griffith, O.W. Structural Characterization and Kinetics of Nitric-Oxide Synthase Inhibition by novel N5-(Iminoalkyl)- and N5-(Iminoalkenyl)-ornithines. J. Biol. Chem. 2003, 278, 46789–46797. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; Gore, M.O.; Ayers, C.R.; Choe, C.U.; Boger, R.H.; de Lemos, J.A.; McGuire, D.K.; Schwedhelm, E. Homoarginine and Cardiovascular Outcome in the Population-Based Dallas Heart Study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2501–2507. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; Schwedhelm, E.; Choe, C.U. l-Homoarginine and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Marz, W.; Meinitzer, A.; Drechsler, C.; Pilz, S.; Krane, V.; Kleber, M.E.; Fischer, J.; Winkelmann, B.R.; Bohm, B.O.; Ritz, E.; et al. Homoarginine, Cardiovascular Risk, and Mortality. Circulation 2010, 122, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Meinitzer, A.; Gaksch, M.; Grubler, M.; Verheyen, N.; Drechsler, C.; Hartaigh, B.O.; Lang, F.; Alesutan, I.; Voelkl, J.; et al. Homoarginine in the renal and cardiovascular systems. Amino Acids 2015, 47, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Meinitzer, A.; Tomaschitz, A.; Drechsler, C.; Ritz, E.; Krane, V.; Wanner, C.; Boehm, B.O.; Marz, W. Low homoarginine concentration is a novel risk factor for heart disease. Heart 2011, 97, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Myers, A.; Farhat, M.; Cathapermal, S.; Ramwell, P.W. Effect of N-substituted arginine compounds on blood pressure in anesthetized rats. J. Pharmacol. Exp. Ther. 1992, 261, 875–878. [Google Scholar] [PubMed]
- Radomski, M.W.; Palmer, R.M.; Moncada, S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. USA 1990, 87, 5193–5197. [Google Scholar] [CrossRef] [PubMed]
- Seppala, I.; Oksala, N.; Jula, A.; Kangas, A.J.; Soininen, P.; Hutri-Kahonen, N.; Marz, W.; Meinitzer, A.; Juonala, M.; Kahonen, M.; et al. The biomarker and causal roles of homoarginine in the development of cardiometabolic diseases: An observational and Mendelian randomization analysis. Sci. Rep. 2017, 7, 1130. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Yanchev, G.R.; Kayacelebi, A.A.; Hanff, E.; Bledau, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; Weissenborn, K.; Bauersachs, J.; et al. The role of L-arginine/L-homoarginine/nitric oxide pathway for aortic distensibility and intima-media thickness in stroke patients. Amino Acids 2017, 49, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; Baum, C.; Ojeda, F.; Keller, T.; Cordts, K.; Schnabel, R.B.; Choe, C.U.; Lackner, K.J.; Munzel, T.; Boger, R.H.; et al. Low Homoarginine Levels in the Prognosis of Patients with Acute Chest Pain. J. Am. Heart Assoc. 2016, 5, e002565. [Google Scholar] [CrossRef] [PubMed]
- Tober, K.M.L.; Runcie, A.; Willox, L.; Talwar, D.; Kinsella, J.; Quasim, T. Asymmetric dimethylarginine, homoarginine levels and atrial fibrillation in oesophagectomy patients. Crit. Care Med. 2013. [Google Scholar] [CrossRef]
- Barnes, M.E.; Miyasaka, Y.; Seward, J.B.; Gersh, B.J.; Rosales, A.G.; Bailey, K.R.; Petty, G.W.; Wiebers, D.O.; Tsang, T.S. Left Atrial Volume in the Prediction of First Ischemic Stroke in an Elderly Cohort without Atrial Fibrillation. Mayo Clin. Proc. 2004, 79, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Moller, J.E.; Hillis, G.S.; Oh, J.K.; Seward, J.B.; Reeder, G.S.; Wright, R.S.; Park, S.W.; Bailey, K.R.; Pellikka, P.A. Left Atrial Volume: A Powerful Predictor of Survival after Acute Myocardial Infarction. Circulation 2003, 107, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Osranek, M.; Bursi, F.; Bailey, K.R.; Grossardt, B.R.; Brown, R.D., Jr.; Kopecky, S.L.; Tsang, T.S.; Seward, J.B. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three-decade follow-up. Eur. Heart J. 2005, 26, 2556–2561. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.S.; Barnes, M.E.; Bailey, K.R.; Leibson, C.L.; Montgomery, S.C.; Takemoto, Y.; Diamond, P.M.; Marra, M.A.; Gersh, B.J.; Wiebers, D.O.; et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin. Proc. 2001, 76, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.S.; Barnes, M.E.; Gersh, B.J.; Takemoto, Y.; Rosales, A.G.; Bailey, K.R.; Seward, J.B. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J. Am. Coll. Cardiol. 2003, 42, 1199–1205. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Levy, D.; Garrison, R.J.; Savage, D.D.; Kannel, W.B.; Castelli, W.P. Prognostic Implications of Echocardiographically Determined Left Ventricular Mass in the Framingham Heart Study. N. Engl. J. Med. 1990, 322, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Swamy, R.S.; Lang, R.M. Echocardiographic Quantification of Left Ventricular Mass: Prognostic Implications. Curr. Cardiol. Rep. 2010, 12, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Edelmann, F.; Meinitzer, A.; Gelbrich, G.; Doner, U.; Dungen, H.D.; Tomaschitz, A.; Kienreich, K.; Gaksch, M.; Duvinage, A.; et al. Associations of Methylarginines and Homoarginine with Diastolic Dysfunction and Cardiovascular Risk Factors in Patients with Preserved Left Ventricular EjectionFfraction. J. Card. Fail. 2014, 20, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Meinitzer, A.; Pilz, S.; Krane, V.; Tomaschitz, A.; Ritz, E.; Marz, W.; Wanner, C. Homoarginine, heart failure, and sudden cardiac death in haemodialysis patients. Eur. J. Heart Fail. 2011, 13, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; McAndrew, D.J.; Cordts, K.; Schneider, J.E.; Zervou, S.; Schwedhelm, E.; Neubauer, S.; Lygate, C.A. Dietary Supplementation with Homoarginine Preserves Cardiac Function in a Murine Model of Post-Myocardial Infarction Heart Failure. Circulation 2017, 135, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Shimano, M.; Shibata, R.; Inden, Y.; Yoshida, N.; Uchikawa, T.; Tsuji, Y.; Murohara, T. Reactive oxidative metabolites are associated with atrial conduction disturbance in patients with atrial fibrillation. Heart Rhythm 2009, 6, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Barouch, L.A.; Harrison, R.W.; Skaf, M.W.; Rosas, G.O.; Cappola, T.P.; Kobeissi, Z.A.; Hobai, I.A.; Lemmon, C.A.; Burnett, A.L.; O’Rourke, B.; et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 2002, 416, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar] [PubMed]
- Chowdhary, S.; Vaile, J.C.; Fletcher, J.; Ross, H.F.; Coote, J.H.; Townend, J.N. Nitric Oxide and Cardiac Autonomic Control in Humans. Hypertension 2000, 36, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, A.; Okamoto, L.E.; Raj, S.R.; Diedrich, A.; Shibao, C.A.; Robertson, D.; Biaggioni, I. Nitric Oxide and Regulation of Heart Rate in Patients with Postural Tachycardia Syndrome and Healthy Subjects. Hypertension 2013, 61, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Tommasi, S.; Elliot, D.J.; Da Boit, M.; Gray, S.R.; Lewis, B.C.; Mangoni, A.A. Homoarginine and inhibition of human arginase activity: Kinetic characterization and biological relevance. Sci. Rep. 2018, 8, 3697. [Google Scholar] [CrossRef] [PubMed]
- Stamboul, K.; Lorin, J.; Lorgis, L.; Guenancia, C.; Beer, J.C.; Touzery, C.; Rochette, L.; Vergely, C.; Cottin, Y.; Zeller, M. Atrial Fibrillation Is Associated with a Marker of Endothelial Function and Oxidative Stress in Patients with Acute Myocardial Infarction. PLoS ONE 2015, 10, e0131439. [Google Scholar] [CrossRef] [PubMed]
- Samman Tahhan, A.; Sandesara, P.B.; Hayek, S.S.; Alkhoder, A.; Chivukula, K.; Hammadah, M.; Mohamed-Kelli, H.; O’Neal, W.T.; Topel, M.; Ghasemzadeh, N.; et al. Association between oxidative stress and atrial fibrillation. Heart Rhythm 2017, 14, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Santulli, G.; Reiken, S.R.; Yuan, Q.; Osborne, B.W.; Chen, B.X.; Marks, A.R. Mitochondrial oxidative stress promotes atrial fibrillation. Sci. Rep. 2015, 5, 11427. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Santulli, G.; Santamaria, M.; Barbieri, M.; Sacra, C.; Paolisso, P.; D’Amico, F.; Testa, N.; Caporaso, I.; Paolisso, G.; et al. Effects of Alpha Lipoic Acid on Multiple Cytokines and Biomarkers and Recurrence of Atrial Fibrillation within 1 Year of Catheter Ablation. Am. J. Cardiol. 2017, 119, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- D’Ascia, S.L.; D’Ascia, C.; Marino, V.; Lombardi, A.; Santulli, R.; Chiariello, M.; Santulli, G. Cardiac resynchronisation therapy response predicts occurrence of atrial fibrillation in non-ischaemic dilated cardiomyopathy. Int. J. Clin. Pract. 2011, 65, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; D’Ascia, S.L.; D’Ascia, C. Development of Atrial Fibrillation in Recipients of Cardiac Resynchronization Therapy: The Role of Atrial Reverse Remodelling. Can. J. Cardiol. 2012, 28, 245. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Larson, M.G.; Yamamoto, J.F.; Sullivan, L.M.; Pencina, M.J.; Meigs, J.B.; Tofler, G.H.; Selhub, J.; Jacques, P.F.; Wolf, P.A.; et al. Relations of Biomarkers of Distinct Pathophysiological Pathways and Atrial Fibrillation Incidence in the Community. Circulation 2010, 121, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Wild, P.S.; Wilde, S.; Ojeda, F.M.; Schulz, A.; Zeller, T.; Sinning, C.R.; Kunde, J.; Lackner, K.J.; Munzel, T.; et al. Multiple biomarkers and atrial fibrillation in the general population. PLoS ONE 2014, 9, e112486. [Google Scholar] [CrossRef] [PubMed]
- Faller, K.M.E.; Atzler, D.; McAndrew, D.J.; Zervou, S.; Whittington, H.J.; Simon, J.N.; Aksentijevic, D.; Ten Hove, M.; Choe, C.U.; Isbrandt, D.; et al. Impaired cardiac contractile function in arginine:glycine amidinotransferase knockout mice devoid of creatine is rescued by homoarginine but not creatine. Cardiovasc. Res. 2018, 114, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Figueiras, A.; Domenech-Massons, J.M.; Cadarso, C. Regression models: Calculating the confidence interval of effects in the presence of interactions. Stat. Med. 1998, 17, 2099–2105. [Google Scholar] [CrossRef]
| Total Sample (N = 3761) | No Atrial Fibrillation (N = 3606) | Atrial Fibrillation (N = 124) | p-Value | |
|---|---|---|---|---|
| Age (years) | 56.0 (46.0, 65.0) | 55.0 (46.0, 64.0) | 67.0 (60.0, 72.0) | <0.001 |
| Males, N (%) | 1946 (51.7) | 1836 (50.9) | 91 (73.4) | <0.001 |
| Heart rate (bpm) | 68.0 (61.5, 76.0) | 68.0 (61.5, 75.5) | 69.0 (59.5, 79.0) | 0.72 |
| (a) Cardiovascular risk factors and diseases | ||||
| Body mass index (kg/m2) | 26.6 (24.0, 29.8) | 26.5 (23.9, 29.7) | 28.2 (25.3, 32.3) | <0.001 |
| Active smoker, N (%) | 693 (18.5) | 675 (18.8) | 13 (10.5) | 0.027 |
| Diabetes, N (%) | 290 (7.7) | 272 (7.5) | 16 (12.9) | 0.043 |
| Dyslipidemia, N (%) | 1059 (28.2) | 1003 (27.8) | 47 (36.7) | 0.019 |
| Family history of myocardial infarction, number (%) | 678 (18.0) | 647 (17.9) | 25 (20.2) | 0.61 |
| Hypertension, N (%) | 1952 (51.9) | 1846 (51.2) | 87 (70.2) | <0.001 |
| Heart failure, N (%) | 754 (20.1) | 682 (19.0) | 61 (49.2) | <0.001 |
| (b) Biomarkers | ||||
| Homoarginine (µmol/L) | 2.0 (1.5, 2.5) | 2.0 (1.5, 2.5) | 1.9 (1.5, 2.5) | 0.56 |
| Creatinine (mg/dL) | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.1) | <0.001 |
| Total Sample (N = 3761) | No Atrial Fibrillation (N = 3606) | Atrial Fibrillation (N = 124) | p-Value | |
|---|---|---|---|---|
| (a) Electrocardiographic variables | ||||
| Ventricular heart rate (bpm) | 61 (55, 67) | 61 (55, 67) | 65 (54, 77) | <0.001 |
| QRS duration (msec) | 94 (88, 102) | 94 (88, 102) | 96 (92, 109) | <0.001 |
| QTc interval (msec) | 420 (404, 435) | 419 (404, 435) | 430 (407, 450) | <0.001 |
| (b) Echocardiographic variables | ||||
| Left atrial area (cm2) | 18 (16, 21) | 18 (16, 21) | 23 (19, 28) | <0.001 |
| E/E’ | 7.0 (5.8, 8.7) | 7.0 (5.7, 8.7) | 7.8 (6.4, 9.8) | <0.001 |
| Deceleration time (msec) | 225 (194, 260) | 225 (194, 260) | 213 (173, 257) | 0.0084 |
| Left ventricular ejection fraction (%) | 64. (60, 68) | 64 (60, 68) | 61 (57, 68) | <0.001 |
| Left ventricular mass (g) | 153 (125, 185) | 152 (123, 183) | 202 (157, 241) | <0.001 |
| Left atrial area indexed by body surface area (cm2/m2) | 10 (9, 11) | 10 (9, 11) | 11 (10, 14) | <0.001 |
| Clinical Variables | Beta (95% CI) | Beta per SD (95% CI) | p Value |
|---|---|---|---|
| PQ interval | −0.24 (−1.14, 0.66) | −0.20 (−0.96, 0.55) | 0.6 |
| P wave duration | −0.38 (−0.86, 0.10) | −0.32 (−0.72, 0.08) | 0.12 |
| Ventricular rate | 0.30 (−0.09, 0.69) | 0.25 (−0.08, 0.58) | 0.14 |
| QRS duration | −0.10 (−0.60, 0.40) | −0.08 (−0.50, 0.34) | 0.7 |
| QTc interval | 0.35 (−0.53, 1.24) | 0.30 (−0.45, 1.05) | 0.43 |
| Left atrial area | −0.15 (−0.28, −0.02) | −0.12 (−0.23, −0.02) | 0.024 |
| E/A | −0.01 (−0.03, −0.003) | −0.01 (−0.02, −0.003) | 0.013 |
| E/E’ | 0.05 (−0.05, 0.15) | 0.04 (−0.04, 0.12) | 0.3 |
| Deceleration time | 0.76 (−1.24, 2.75) | 0.64 (−1.05, 2.33) | 0.46 |
| Left ventricular ejection fraction | 0.19 (−0.06, 0.44) | 0.16 (−0.05, 0.37) | 0.13 |
| Left ventricular mass | −1.44 (−2.88, 0.00) | −1.21 (−2.43, 0.004) | 0.051 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niekamp, C.; Atzler, D.; Ojeda, F.M.; Sinning, C.R.; Lackner, K.J.; Böger, R.H.; Munzel, T.; Beutel, M.E.; Schmidtmann, I.; Pfeiffer, N.; et al. Cross-Sectional Associations between Homoarginine, Intermediate Phenotypes, and Atrial Fibrillation in the Community—The Gutenberg Health Study. Biomolecules 2018, 8, 86. https://doi.org/10.3390/biom8030086
Niekamp C, Atzler D, Ojeda FM, Sinning CR, Lackner KJ, Böger RH, Munzel T, Beutel ME, Schmidtmann I, Pfeiffer N, et al. Cross-Sectional Associations between Homoarginine, Intermediate Phenotypes, and Atrial Fibrillation in the Community—The Gutenberg Health Study. Biomolecules. 2018; 8(3):86. https://doi.org/10.3390/biom8030086
Chicago/Turabian StyleNiekamp, Christoph, Dorothee Atzler, Francisco M. Ojeda, Christoph R. Sinning, Karl J. Lackner, Rainer H Böger, Thomas Munzel, Manfred E. Beutel, Irene Schmidtmann, Norbert Pfeiffer, and et al. 2018. "Cross-Sectional Associations between Homoarginine, Intermediate Phenotypes, and Atrial Fibrillation in the Community—The Gutenberg Health Study" Biomolecules 8, no. 3: 86. https://doi.org/10.3390/biom8030086
APA StyleNiekamp, C., Atzler, D., Ojeda, F. M., Sinning, C. R., Lackner, K. J., Böger, R. H., Munzel, T., Beutel, M. E., Schmidtmann, I., Pfeiffer, N., Leuschner, A., Blankenberg, S., Wild, P. S., Zeller, T., Schwedhelm, E., & Schnabel, R. B. (2018). Cross-Sectional Associations between Homoarginine, Intermediate Phenotypes, and Atrial Fibrillation in the Community—The Gutenberg Health Study. Biomolecules, 8(3), 86. https://doi.org/10.3390/biom8030086









