Assembly Mechanisms of Specialized Core Particles of the Proteasome
Abstract
:1. Introduction
2. Results
2.1. β4-Independent Incorporation of β5i on the α-Ring during Immunoproteasome Assembly
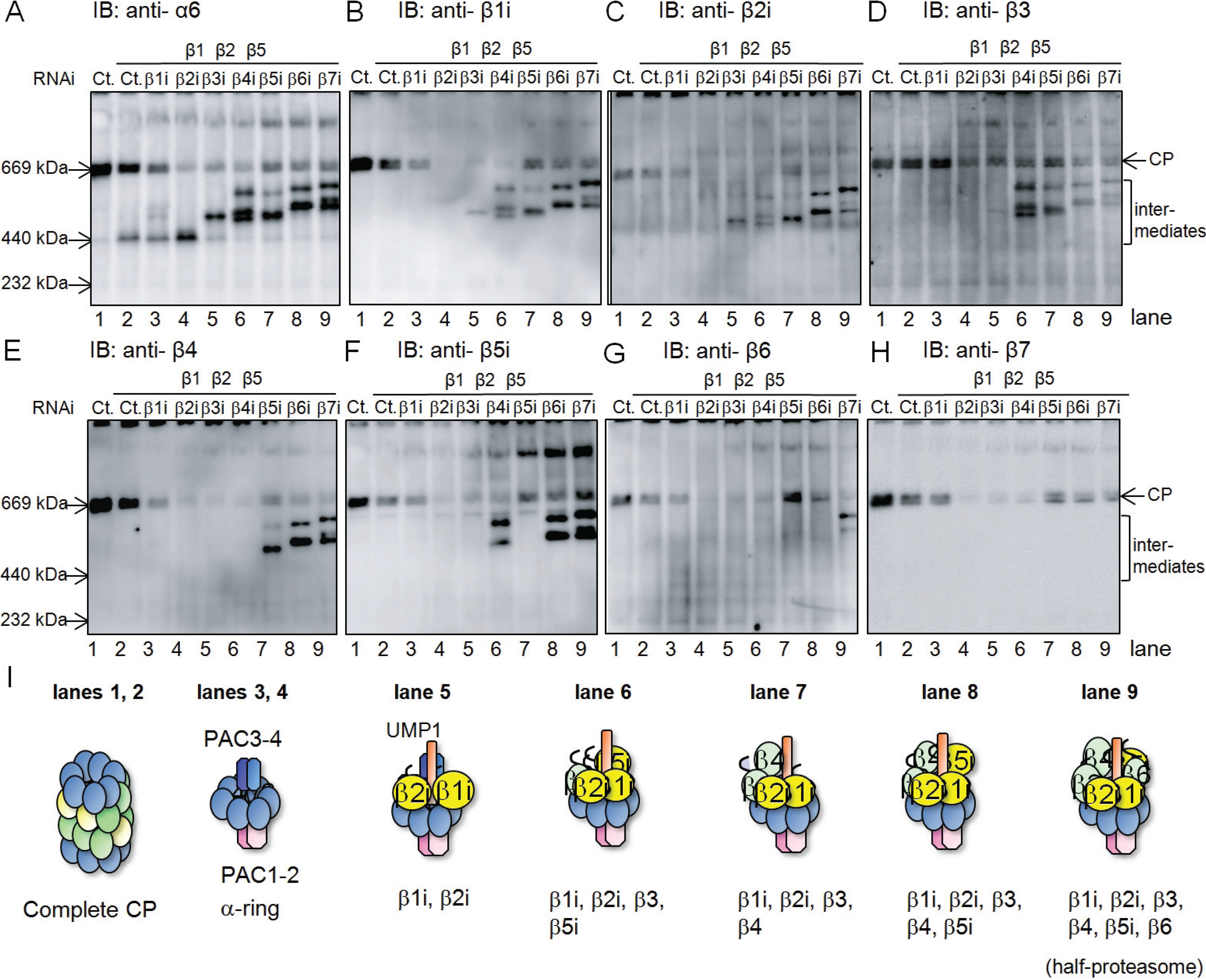
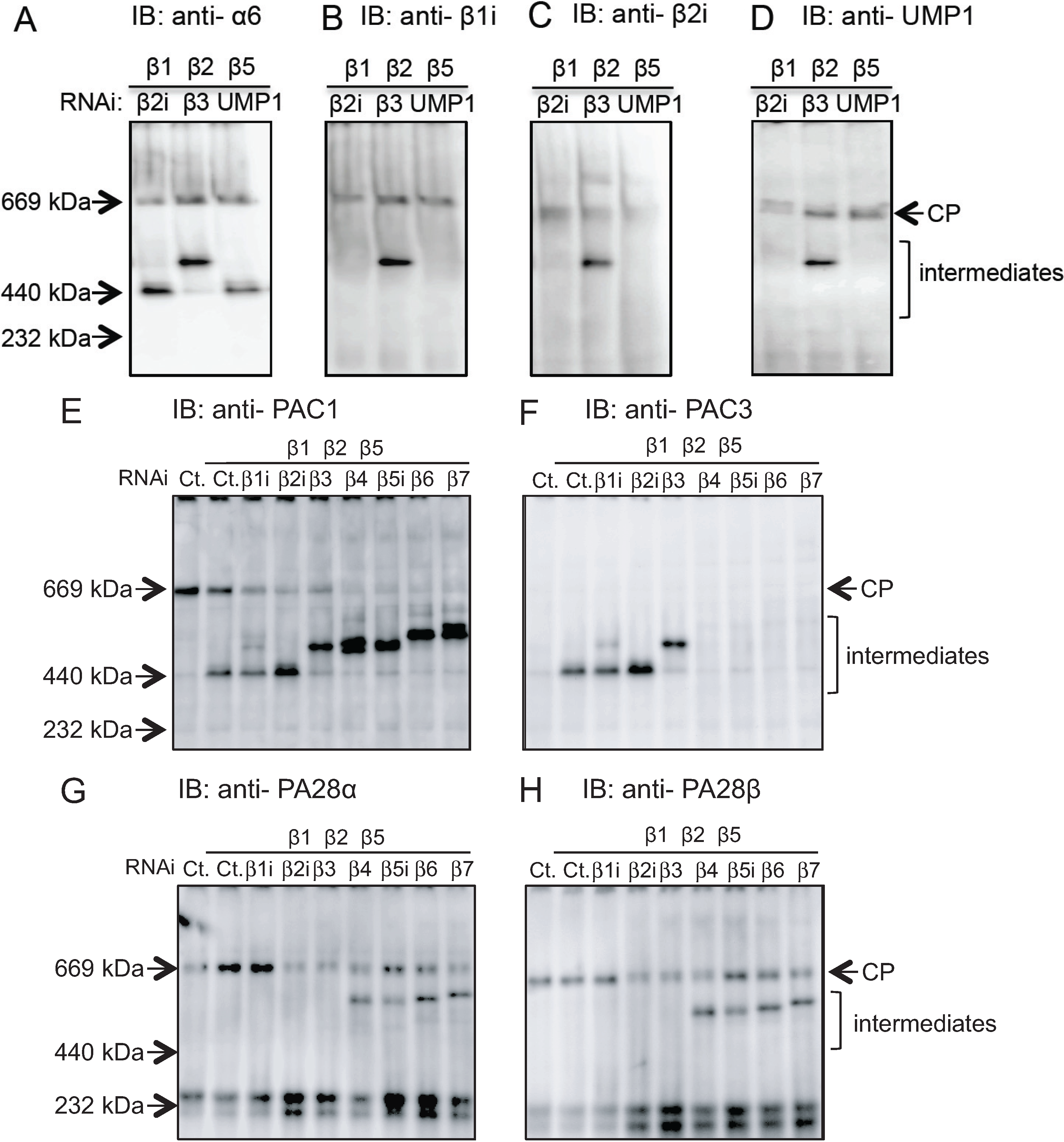
2.2. Conserved Roles of Assembly Chaperones during Immunoproteasome Biogenesis
2.3. Earlier Incorporation of β5i Is Independent of β1i and β2i
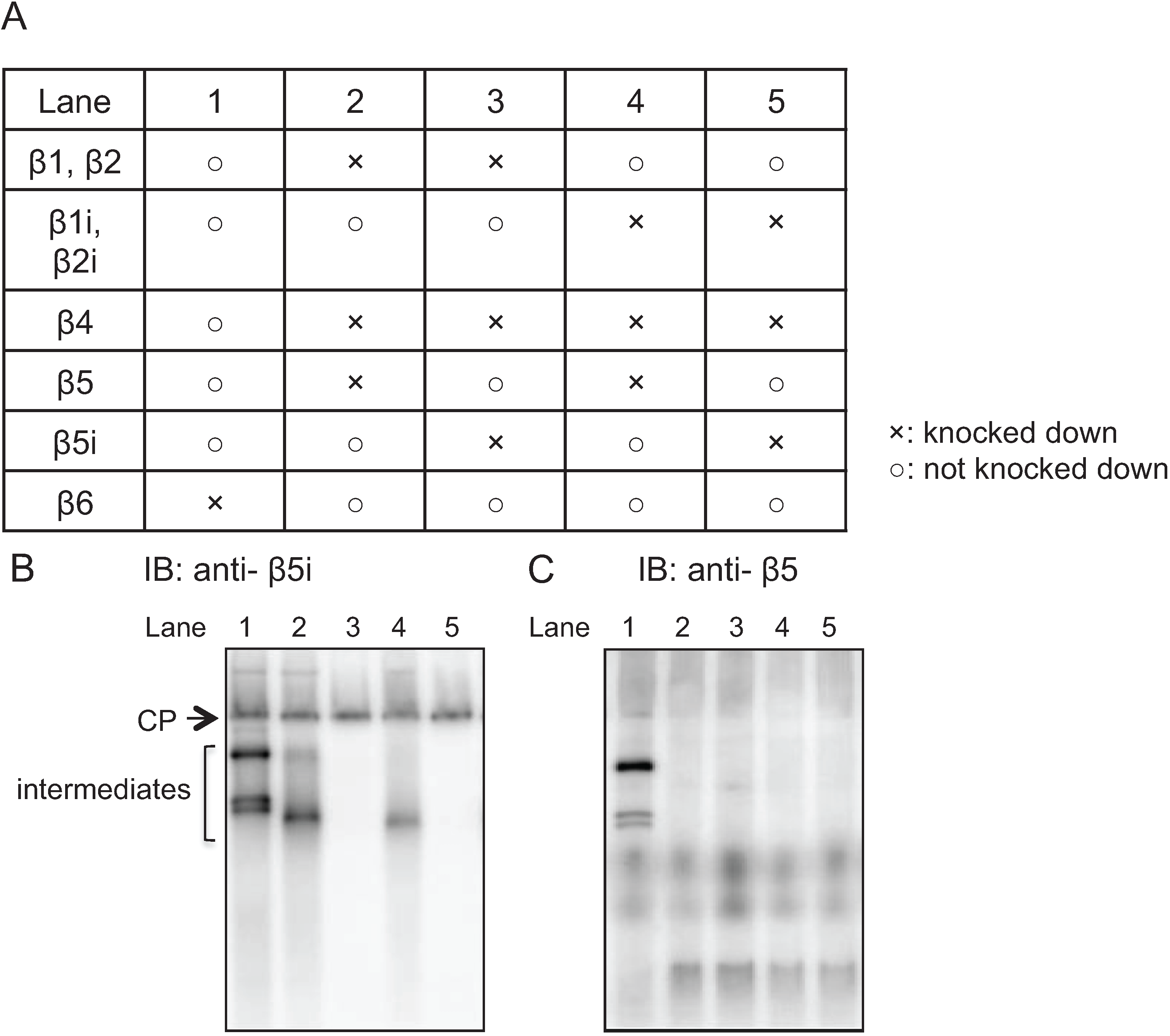
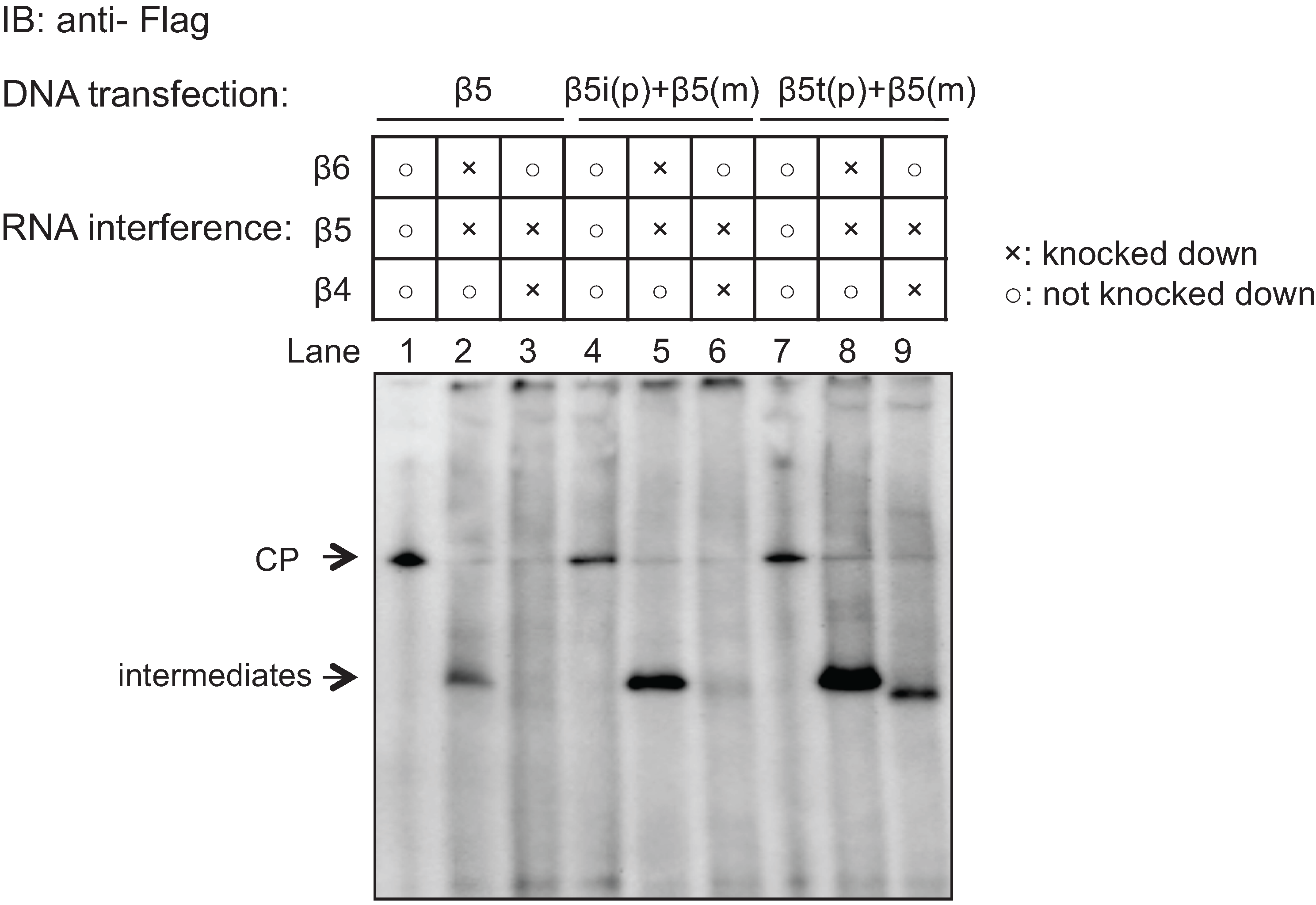
2.4. β5t Can Also Be Incorporated before β4 during CP Assembly
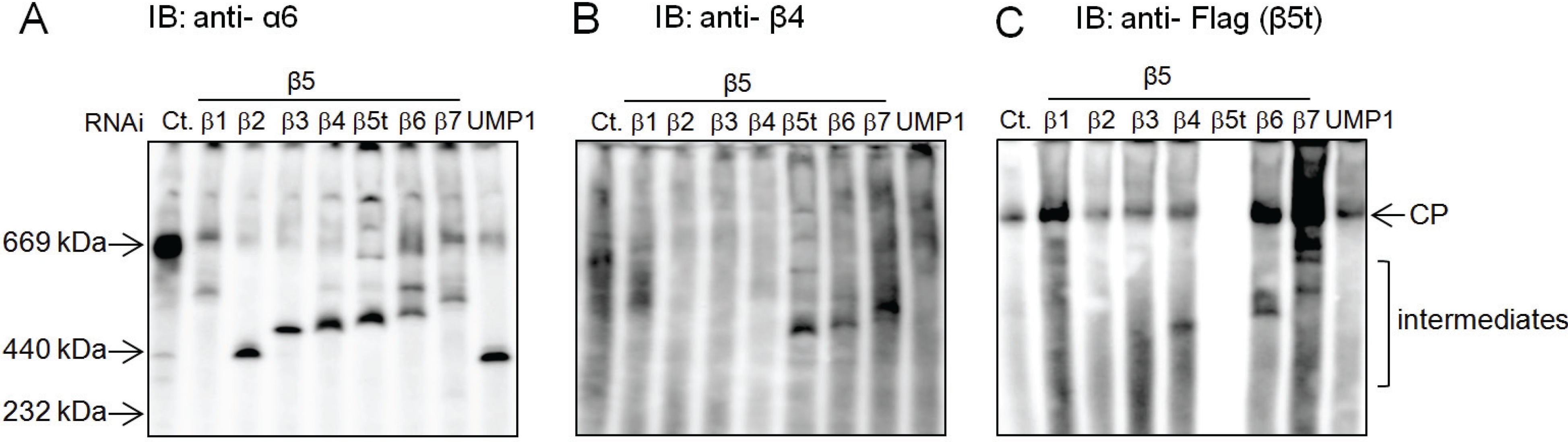
2.5. Role of the Propeptides of β5i and β5t in the Earlier Incorporation
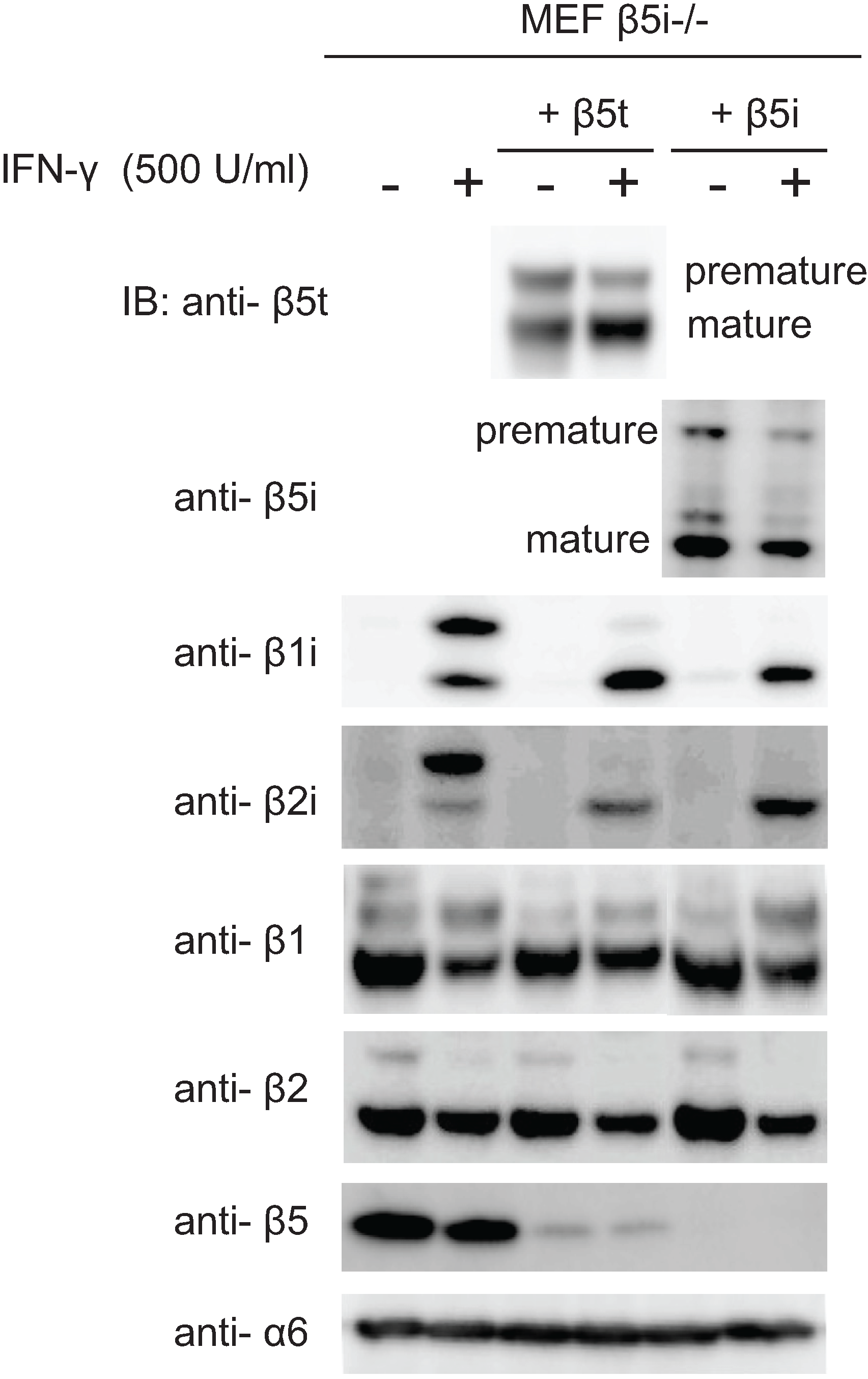
2.6. Maturation of β5t Is Largely Dependent on IFN-γ
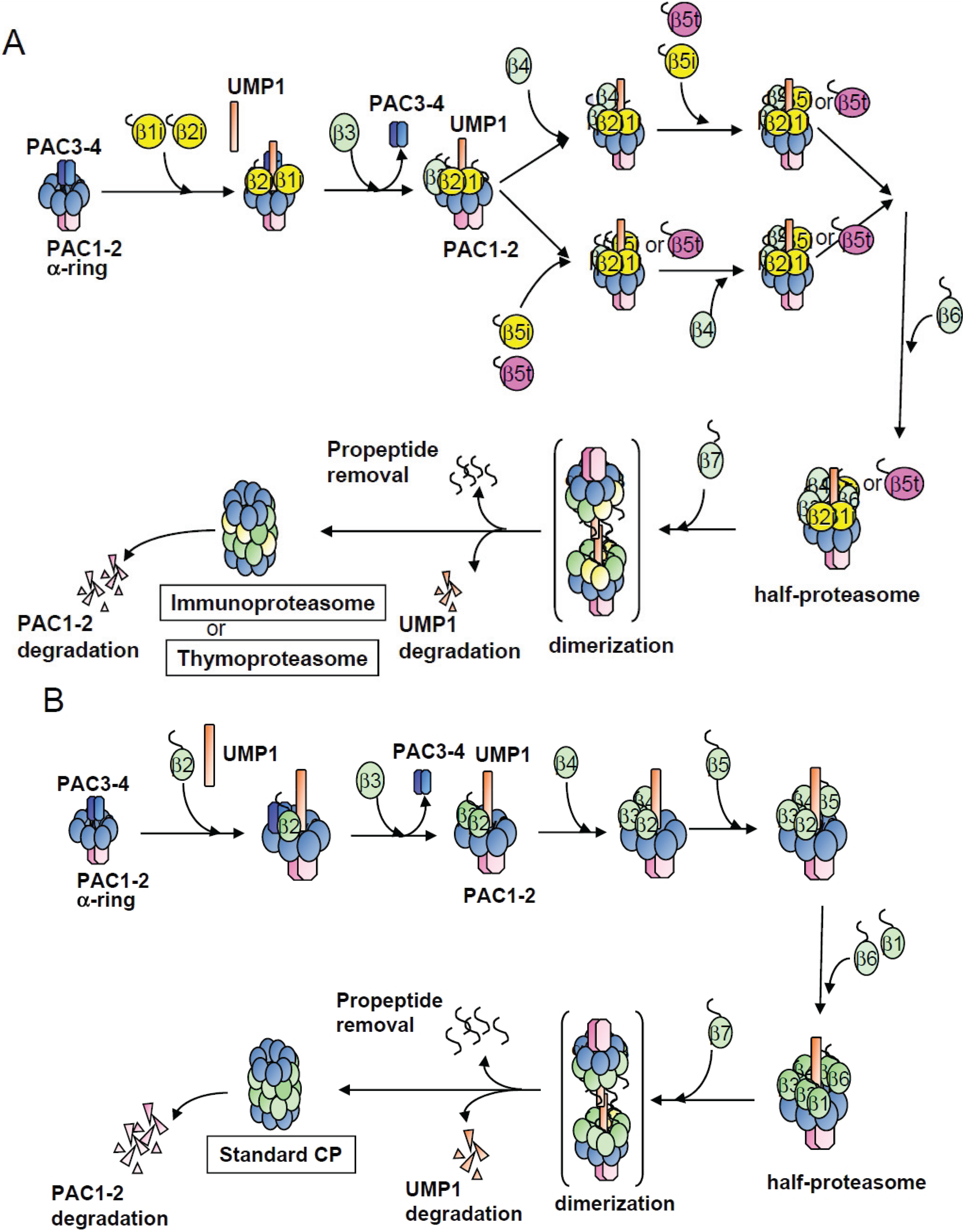
3. Discussion
4. Experimental
4.1. Cell Culture
4.2. DNA Constructs
4.3. RNA Interference
4.4. Protein Extraction, Immunological Analysis and Antibodies
| Name | Sequence | Supplier |
|---|---|---|
| Human β1i | 5'-CCGGUGUGGACCAUCGAGUCAUCUU-3' | Invitrogen |
| Human β2i | 5'-GGACGCAUGUGUGAUCACAAAGACU-3' | Invitrogen |
| Human β3 | 5'-AUAAGGUUUGAUCUGCCGACCUUCC-3' | Invitrogen |
| Human β4 | 5'-UAGUCCAUGUAAUACAGCGCUGGCC-3' | Invitrogen |
| Human β5i | 5'-GGACUCGGCUCUCAGGAAAUAUGUU-3 | Invitrogen |
| Human β6 | 5'-AAUACAGGAUUGUAGACAGCAUUGC-3' | Invitrogen |
| Human β7 | 5'-GCAUGCGAGUGCUGUACUACC-3' | Sigma |
| Human UMP1 | 5'-AAGACGCUGAACCUGCUGCACUGCC-3 | Invitrogen |
| Human β1 | 5'-AUAGGUGUCAGCUUGUCAGUCACUC-3 | Invitrogen |
| Human β2 | 5'-ACAUAAGGCAACUUAUCAGUUGAUC-3' | Invitrogen |
| Human β5 | 5'-UGAUAGAGAUCAACCCAUACCUGCU-3' | Invitrogen |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baumeister, W.; Walz, J.; Zuhl, F.; Seemuller, E. The proteasome: Paradigm of a self-compartmentalizing protease. Cell 1998, 92, 367–380. [Google Scholar] [CrossRef]
- Glickman, M.H.; Ciechanover, A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar]
- Köhler, A.; Cascio, P.; Leggett, D.S.; Woo, K.M.; Goldberg, A.L.; Finley, D. The axial channel of the proteasome core particle is gated by the Rpt2 atpase and controls both substrate entry and product release. Mol. Cell. 2001, 7, 1143–1152. [Google Scholar] [CrossRef]
- Groll, M.; Ditzel, L.; Löwe, J.; Stock, D.; Bochtler, M.; Bartunik, H.D.; Huber, R. Structure of 20S proteasome from yeast at 2.4 Å resolution. Nature 1997, 386, 463–471. [Google Scholar] [CrossRef]
- Unno, M.; Mizushima, T.; Morimoto, Y.; Tomisugi, Y.; Tanaka, K.; Yasuoka, N.; Tsukihara, T. The structure of the mammalian 20S proteasome at 2.75 Å resolution. Structure 2002, 10, 609–618. [Google Scholar] [CrossRef]
- Heinemeyer, W.; Fischer, M.; Krimmer, T.; Stachon, U.; Wolf, D.H. The active sites of the eukaryotic 20S proteasome and their involvement in subunit precursor processing. J. Biol. Chem. 1997, 272, 25200–25209. [Google Scholar] [CrossRef]
- Bar-Nun, S.; Glickman, M.H. Proteasomal AAA-ATPases: Structure and function. Biochim. Biophys. Acta 2012, 1823, 67–82. [Google Scholar] [CrossRef]
- Murata, S.; Yashiroda, H.; Tanaka, K. Molecular mechanisms of proteasome assembly. Nat. Rev. Mol. Cell. Biol. 2009, 10, 104–115. [Google Scholar] [CrossRef]
- Frentzel, S.; Pesold-Hurt, B.; Seelig, A.; Kloetzel, P.M. 20S proteasomes are assembled via distinct precursor complexes. Processing of LMP2 and LMP7 proproteins takes place in 13–16 S preproteasome complexes. J. Mol. Biol. 1994, 236, 975–981. [Google Scholar] [CrossRef]
- Nandi, D.; Woodward, E.; Ginsburg, D.B.; Monaco, J.J. Intermediates in the formation of mouse 20S proteasomes: Implications for the assembly of precursor beta subunits. EMBO J. 1997, 16, 5363–5375. [Google Scholar] [CrossRef]
- Hirano, Y.; Hendil, K.B.; Yashiroda, H.; Iemura, S.; Nagane, R.; Hioki, Y.; Natsume, T.; Tanaka, K.; Murata, S. A heterodimeric complex that promotes the assembly of mammalian 20S proteasomes. Nature 2005, 437, 1381–1385. [Google Scholar] [CrossRef]
- Hirano, Y.; Hayashi, H.; Iemura, S.; Hendil, K.B.; Niwa, S.; Kishimoto, T.; Kasahara, M.; Natsume, T.; Tanaka, K.; Murata, S. Cooperation of multiple chaperones required for the assembly of mammalian 20S proteasomes. Mol. Cell. 2006, 24, 977–984. [Google Scholar] [CrossRef]
- Murata, S. Multiple chaperone-assisted formation of mammalian 20S proteasomes. IUBMB Life 2006, 58, 344–348. [Google Scholar] [CrossRef]
- Li, X.; Kusmierczyk, A.R.; Wong, P.; Emili, A.; Hochstrasser, M. Beta-subunit appendages promote 20S proteasome assembly by overcoming an Ump1-dependent checkpoint. EMBO J. 2007, 26, 2339–2349. [Google Scholar] [CrossRef]
- Le Tallec, B.; Barrault, M.B.; Courbeyrette, R.; Guérois, R.; Marsolier-Kergoat, M.C.; Peyroche, A. 20S proteasome assembly is orchestrated by two distinct pairs of chaperones in yeast and in mammals. Mol. Cell. 2007, 27, 660–674. [Google Scholar] [CrossRef]
- Yashiroda, H.; Mizushima, T.; Okamoto, K.; Kameyama, T.; Hayashi, H.; Kishimoto, T.; Niwa, S.; Kasahara, M.; Kurimoto, E.; Sakata, E.; et al. Crystal structure of a chaperone complex that contributes to the assembly of yeast 20S proteasomes. Nat. Struct. Mol. Biol. 2008, 15, 228–236. [Google Scholar] [CrossRef]
- Kusmierczyk, A.R.; Kunjappu, M.J.; Funakoshi, M.; Hochstrasser, M. A multimeric assembly factor controls the formation of alternative 20S proteasomes. Nat. Struct. Mol. Biol. 2008, 15, 237–244. [Google Scholar] [CrossRef]
- Heinemeyer, W.; Ramos, P.C.; Dohmen, R.J. The ultimate nanoscale mincer: Assembly, structure and active sites of the 20S proteasome core. Cell. Mol. Life Sci. 2004, 61, 1562–1578. [Google Scholar]
- Hirano, Y.; Kaneko, T.; Okamoto, K.; Bai, M.; Yashiroda, H.; Furuyama, K.; Kato, K.; Tanaka, K.; Murata, S. Dissecting beta-ring assembly pathway of the mammalian 20S proteasome. EMBO J. 2008, 27, 2204–2213. [Google Scholar] [CrossRef]
- Ramos, P.C.; Marques, A.J.; London, M.K.; Dohmen, R.J. Role of C-terminal extensions of subunits beta2 and beta7 in assembly and activity of eukaryotic proteasomes. J. Biol. Chem. 2004, 279, 14323–14330. [Google Scholar] [CrossRef]
- Ramos, P.C.; Hockendorff, J.; Johnson, E.S.; Varshavsky, A.; Dohmen, R.J. Ump1p is required for proper maturation of the 20S proteasome and becomes its substrate upon completion of the assembly. Cell 1998, 92, 489–499. [Google Scholar] [CrossRef]
- Tanaka, K.; Kasahara, M. The MHC class I ligand-generating system: Roles of immunoproteasomes and the interferon-gamma-inducible proteasome activator PA28. Immunol. Rev. 1998, 163, 161–176. [Google Scholar] [CrossRef]
- Murata, S.; Sasaki, K.; Kishimoto, T.; Niwa, S.; Hayashi, H.; Takahama, Y.; Tanaka, K. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science 2007, 316, 1349–1353. [Google Scholar] [CrossRef]
- Seifert, U.; Bialy, L.P.; Ebstein, F.; Bech-Otschir, D.; Voigt, A.; Schröter, F.; Prozorovski, T.; Lange, N.; Steffen, J.; Rieger, M.; et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 2010, 142, 613–624. [Google Scholar] [CrossRef]
- Murata, S.; Takahama, Y.; Tanaka, K. Thymoproteasome: Probable role in generating positively selecting peptides. Curr. Opin. Immunol. 2008, 20, 192–196. [Google Scholar] [CrossRef]
- Chen, P.; Hochstrasser, M. Autocatalytic subunit processing couples active site formation in the 20S proteasome to completion of assembly. Cell 1996, 86, 961–972. [Google Scholar] [CrossRef]
- Griffin, T.A.; Nandi, D.; Cruz, M.; Fehling, H.J.; Kaer, L.V.; Monaco, J.J.; Colbert, R.A. Immunoproteasome assembly: Cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J. Exp. Med. 1998, 187, 97–104. [Google Scholar]
- Nil, A.; Firat, E.; Sobek, V.; Eichmann, K.; Niedermann, G. Expression of housekeeping and immunoproteasome subunit genes is differentially regulated in positively and negatively selecting thymic stroma subsets. Eur. J. Immunol. 2004, 34, 2681–2689. [Google Scholar] [CrossRef]
- Chondrogianni, N.; Tzavelas, C.; Pemberton, A.J.; Nezis, I.P.; Rivett, A.J.; Gonos, E.S. Overexpression of proteasome beta5 assembled subunit increases the amount of proteasome and confers ameliorated response to oxidative stress and higher survival rates. J. Biol. Chem. 2005, 280, 11840–11850. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bai, M.; Zhao, X.; Sahara, K.; Ohte, Y.; Hirano, Y.; Kaneko, T.; Yashiroda, H.; Murata, S. Assembly Mechanisms of Specialized Core Particles of the Proteasome. Biomolecules 2014, 4, 662-677. https://doi.org/10.3390/biom4030662
Bai M, Zhao X, Sahara K, Ohte Y, Hirano Y, Kaneko T, Yashiroda H, Murata S. Assembly Mechanisms of Specialized Core Particles of the Proteasome. Biomolecules. 2014; 4(3):662-677. https://doi.org/10.3390/biom4030662
Chicago/Turabian StyleBai, Minghui, Xian Zhao, Kazutaka Sahara, Yuki Ohte, Yuko Hirano, Takeumi Kaneko, Hideki Yashiroda, and Shigeo Murata. 2014. "Assembly Mechanisms of Specialized Core Particles of the Proteasome" Biomolecules 4, no. 3: 662-677. https://doi.org/10.3390/biom4030662
APA StyleBai, M., Zhao, X., Sahara, K., Ohte, Y., Hirano, Y., Kaneko, T., Yashiroda, H., & Murata, S. (2014). Assembly Mechanisms of Specialized Core Particles of the Proteasome. Biomolecules, 4(3), 662-677. https://doi.org/10.3390/biom4030662






