Latent Toxoplasma gondii Infection Does Not Modulate Immune Aging in a Cross-Sectional Working-Age Population Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Dortmund Vital Study
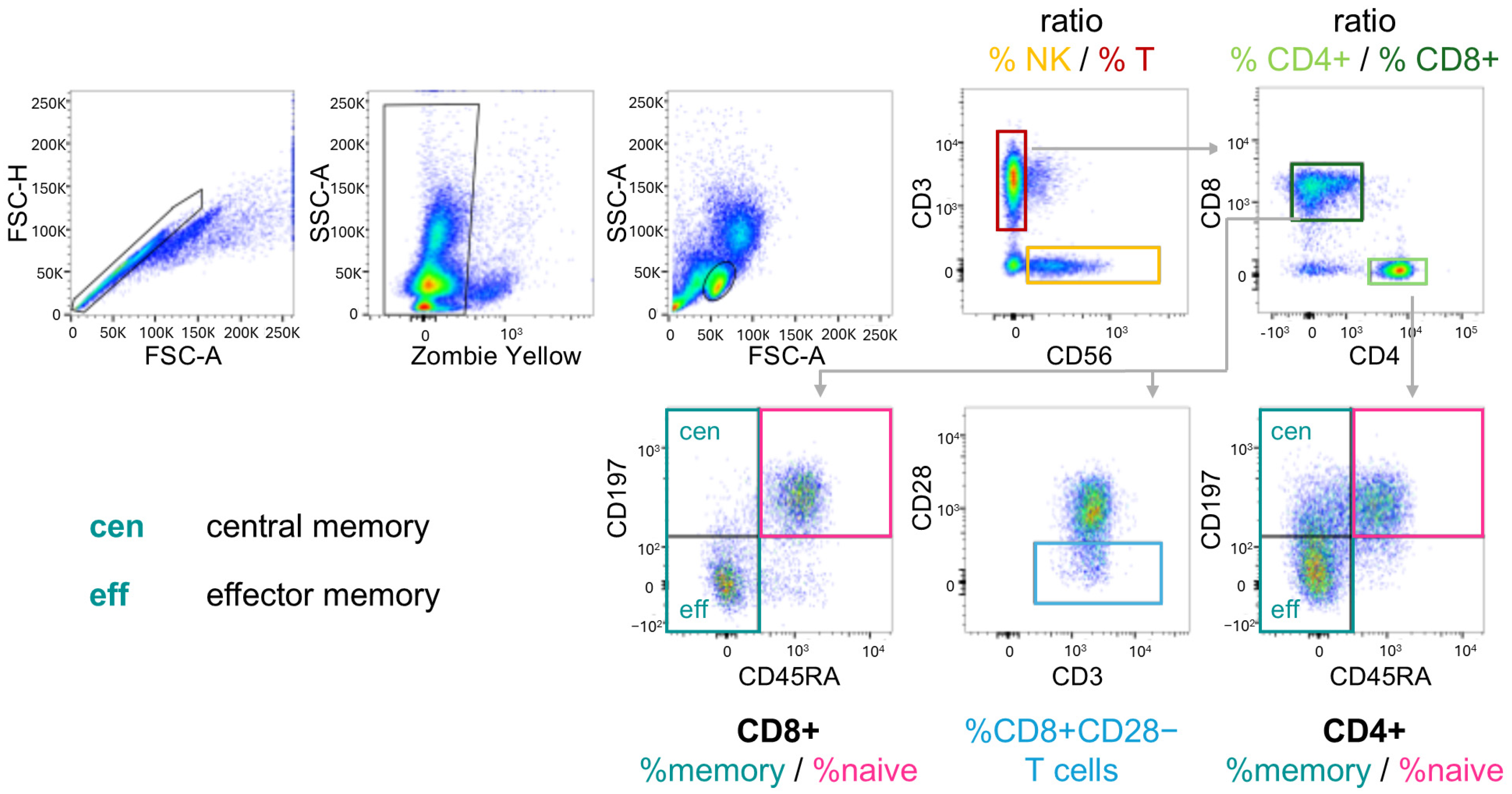
2.2. Immune Cell Subpopulations
2.3. T. gondii Antibody Levels
2.4. Data Analysis and Statistics
3. Results
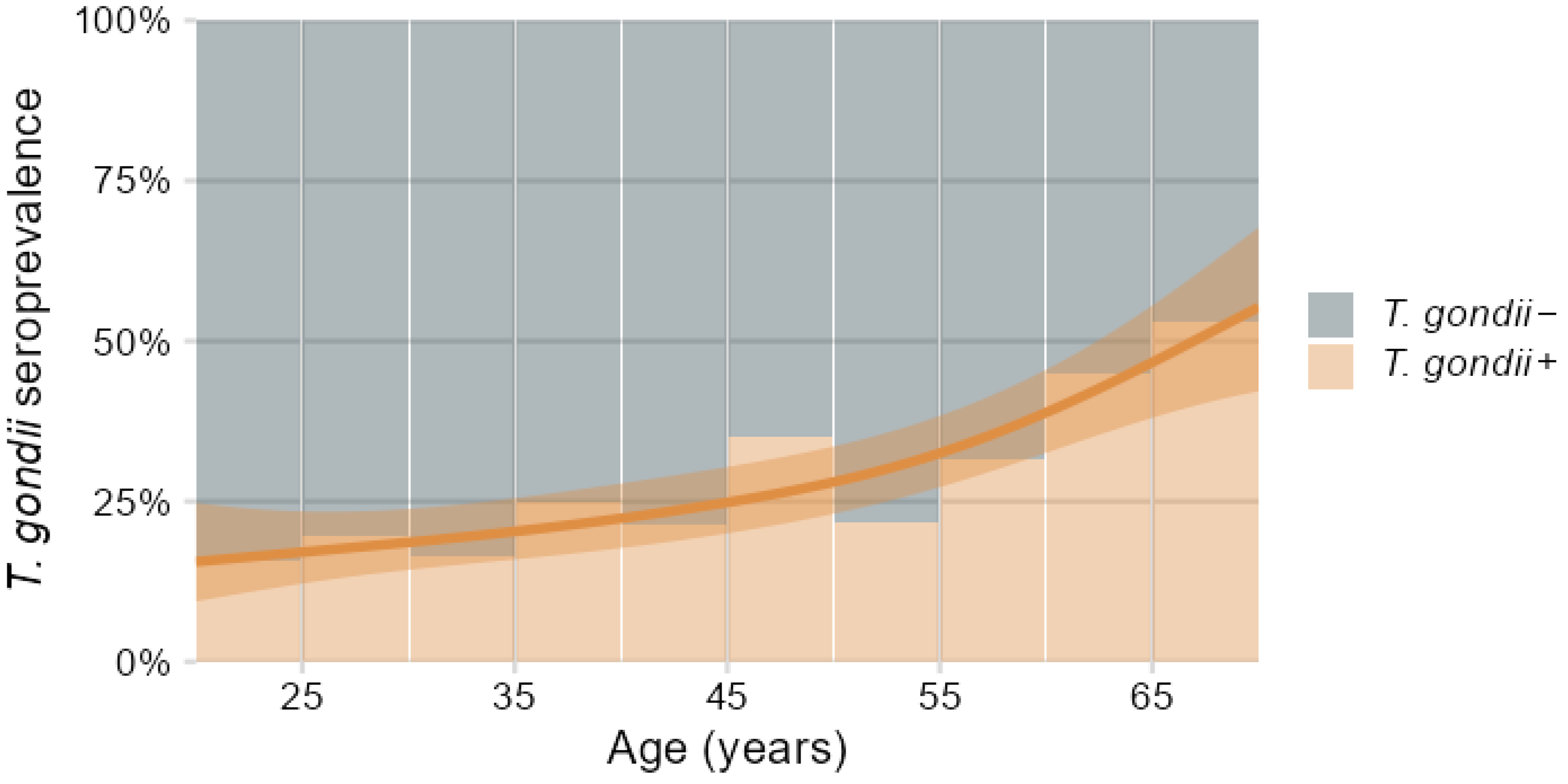
3.1. Demographics and T. gondii Seroprevalence
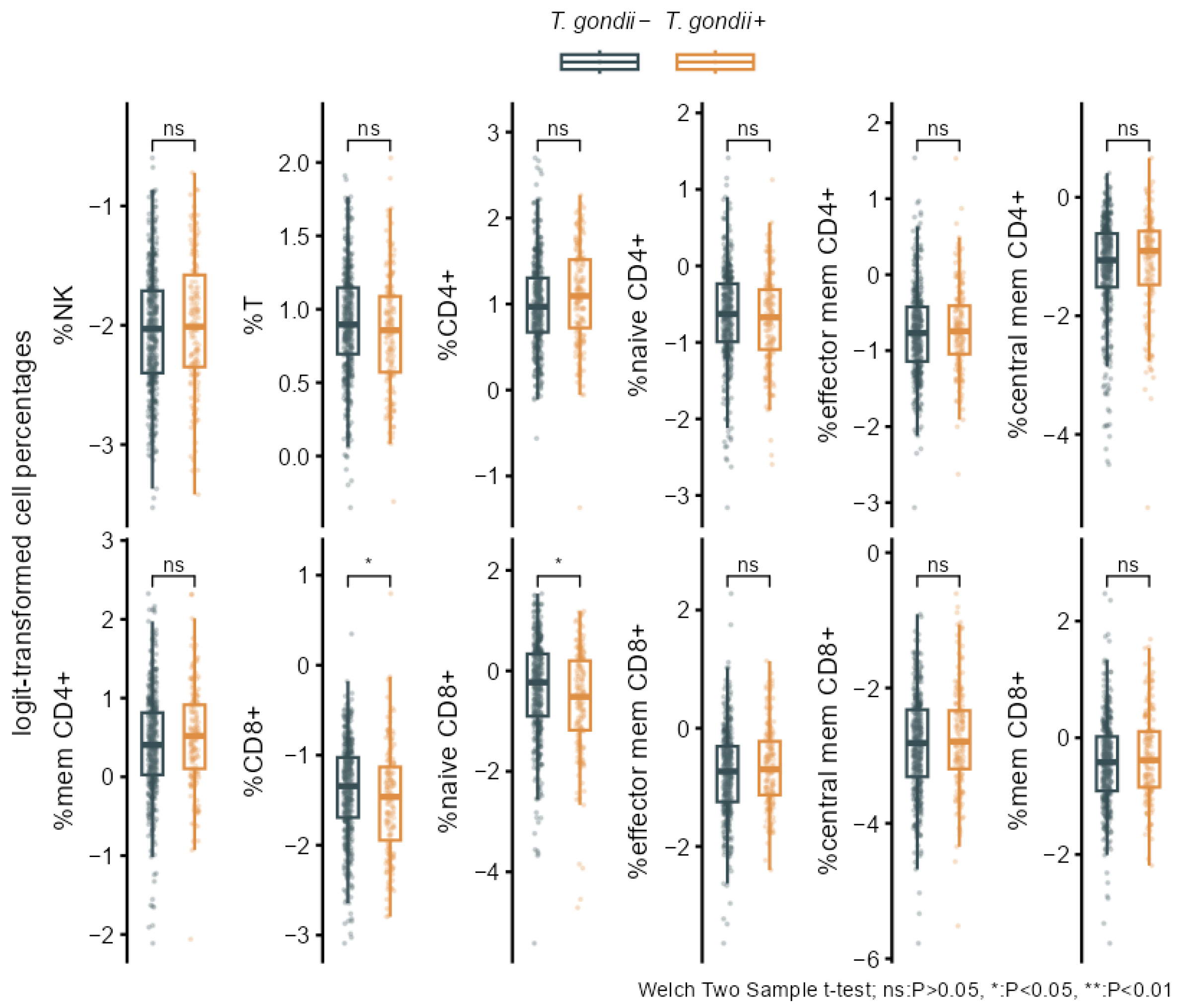
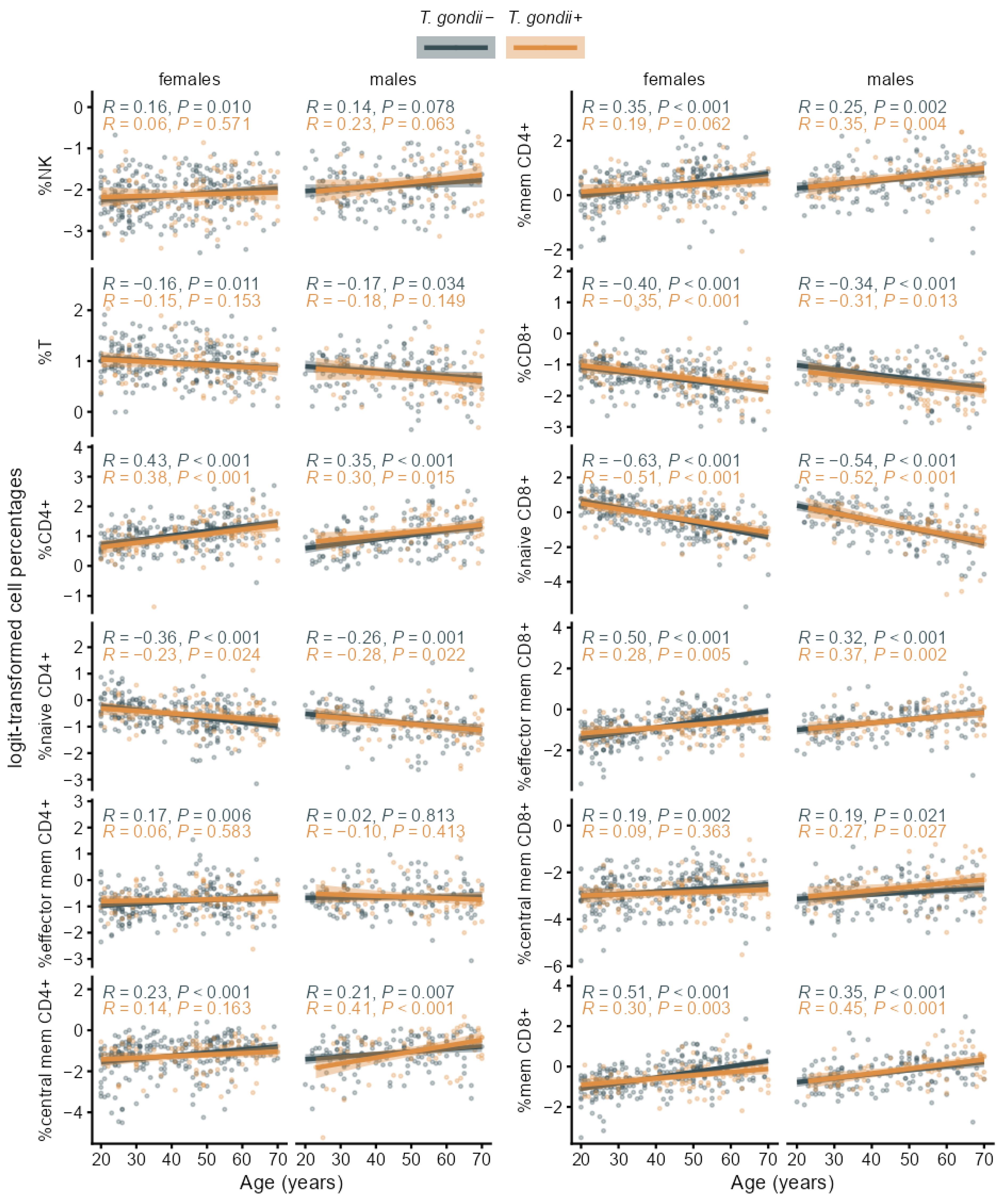
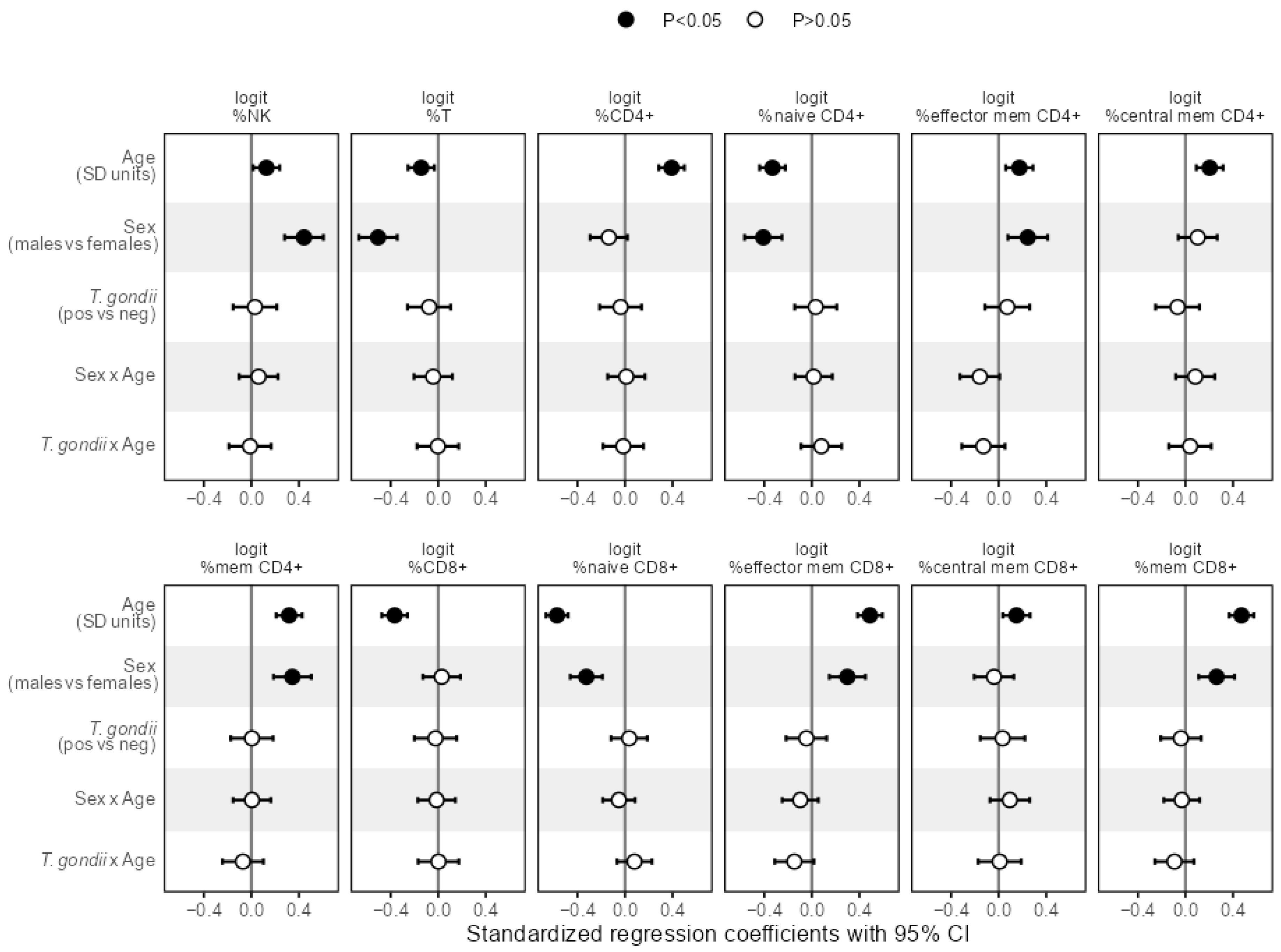
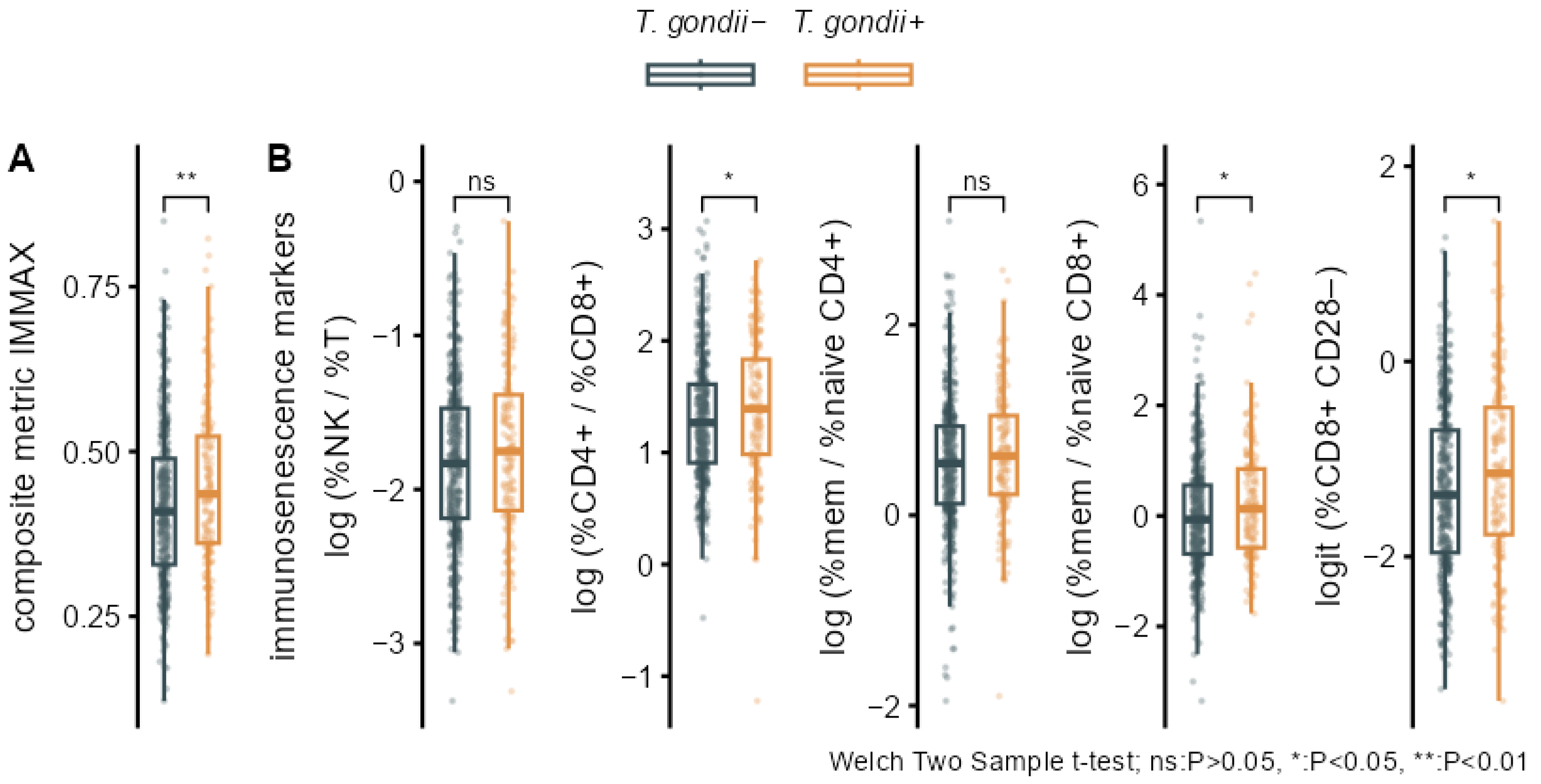
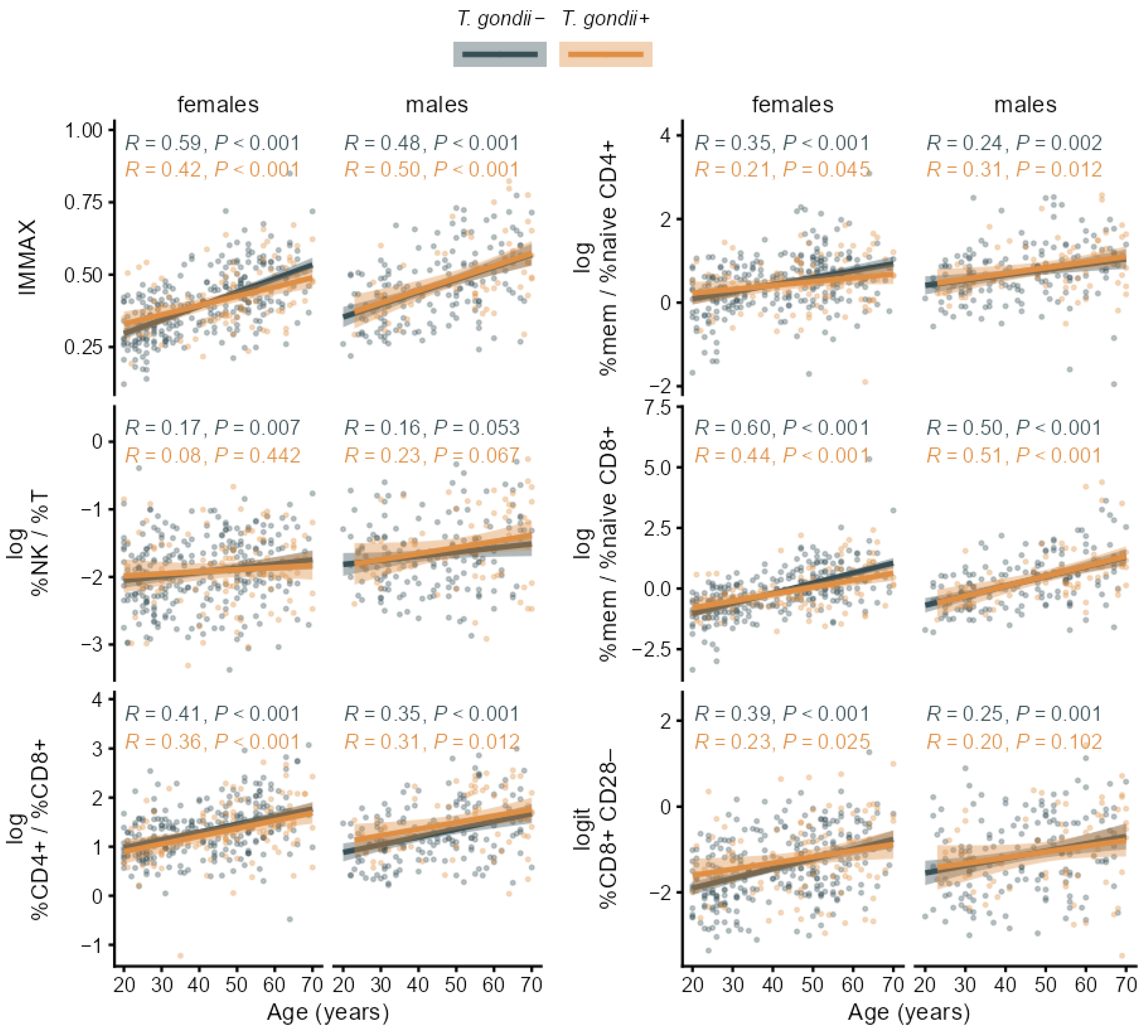
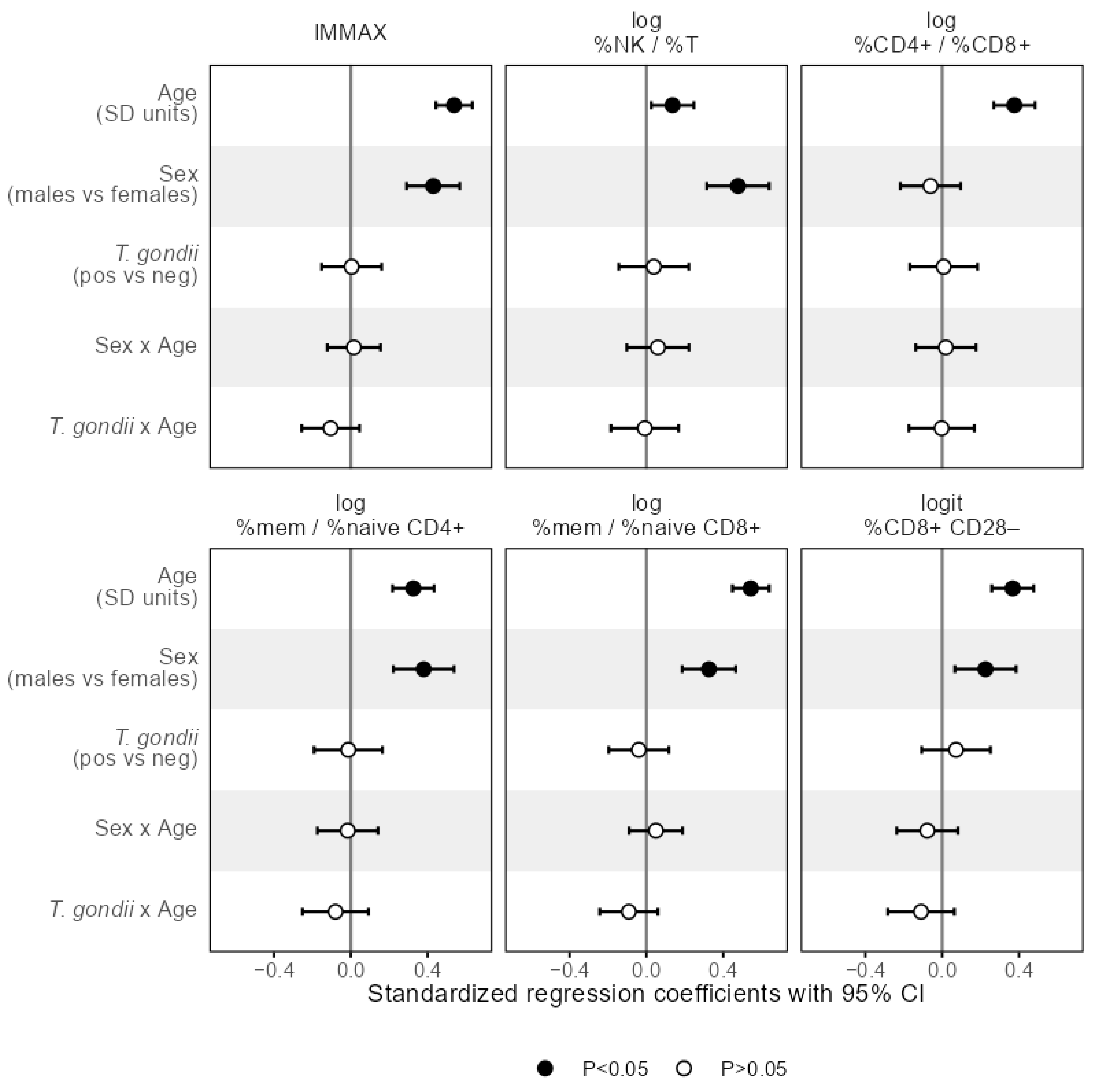
3.2. Associations of T. gondii Status with Immunosenescence Markers
4. Discussion
Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANCOVA | Analysis of co-variance |
| CD4+ | CD4 positive T-cells |
| CD8+ | CD8 positive T-cells |
| CD8+ CD28− | CD28 negative CD8 positive T-cells |
| CMV | Cytomegalovirus |
| CI | Confidence Interval |
| DVS | Dortmund Vital Study |
| ELISA | Enzyme-Linked Immuno-Sorbent Assay |
| IMMAX | Composite metric IMMune Age indeX |
| IgG | Immunoglobulin G antibody |
| NK | Natural Killer cells |
| PBMC | Peripheral Blood Mononuclear Cells |
| q | p-value corrected for false discovery rate in multiple testing |
| SD | Standard Deviation |
| T | T-cells |
| T. gondii | Toxoplasma gondii |
| T. gondii+ | T. gondii seropositive |
| T. gondii− | T. gondii seronegative |
| %cen mem | Percentage of central memory T-cells |
| %eff mem | Percentage of effector memory T-cells |
| %mem | Percentage of memory T-cells |
| %naive | Percentage of naïve T-cells |
Appendix A
| Logit-Transformed Cell Percentages | T. gondii− N = 423 1 | T. gondii+ N = 161 1 | P 2 | q-Value 2 | ∆adj (95% CI) 3 | Padj 3 |
|---|---|---|---|---|---|---|
| %NK | −2.05 (0.51) | −1.99 (0.55) | 0.226 | 0.271 | 0.03 (−0.15, 0.21) | 0.750 |
| %T | 0.90 (0.37) | 0.84 (0.39) | 0.065 | 0.243 | −0.08 (−0.26, 0.10) | 0.386 |
| %CD4+ | 1.01 (0.52) | 1.09 (0.56) | 0.130 | 0.251 | −0.04 (−0.21, 0.13) | 0.648 |
| %naive CD4+ | −0.64 (0.62) | −0.71 (0.60) | 0.219 | 0.271 | 0.05 (−0.12, 0.22) | 0.571 |
| %eff mem CD4+ | −0.76 (0.59) | −0.71 (0.57) | 0.340 | 0.371 | 0.04 (−0.14, 0.23) | 0.661 |
| %cen mem CD4+ | −1.19 (0.85) | −1.13 (0.87) | 0.465 | 0.465 | −0.06 (−0.24, 0.12) | 0.543 |
| %mem CD4+ | 0.41 (0.67) | 0.50 (0.64) | 0.104 | 0.250 | −0.01 (−0.18, 0.16) | 0.907 |
| %CD8+ | −1.38 (0.54) | −1.50 (0.60) | 0.037 | 0.222 | −0.02 (−0.20, 0.15) | 0.798 |
| %naive CD8+ | −0.37 (1.00) | −0.62 (1.05) | 0.012 | 0.141 | 0.05 (−0.10, 0.20) | 0.509 |
| %eff mem CD8+ | −0.76 (0.74) | −0.66 (0.69) | 0.146 | 0.251 | −0.08 (−0.25, 0.09) | 0.351 |
| %cen mem CD8+ | −2.85 (0.73) | −2.75 (0.78) | 0.177 | 0.266 | 0.04 (−0.15, 0.22) | 0.685 |
| %mem CD8+ | −0.43 (0.76) | −0.31 (0.76) | 0.081 | 0.243 | −0.06 (−0.22, 0.11) | 0.489 |
References
- Robert-Gangneux, F.; Dardé, M.-L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef]
- Jones, J.L.; Kruszon-Moran, D.; Wilson, M.; McQuillan, G.; Navin, T.; McAuley, J.B. Toxoplasma gondii Infection in the United States: Seroprevalence and Risk Factors. Am. J. Epidemiol. 2001, 154, 357–365. [Google Scholar] [CrossRef]
- Colzato, L.; Zhang, W.; Beste, C.; Stock, A.-K. Dissociating direct and indirect effects: A theoretical framework of how latent toxoplasmosis affects cognitive profile across the lifespan. Neurobiol. Aging 2021, 102, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Galvis, C.A.; Cardona-Londoño, K.Y.; Orrego-Cardozo, M.; Elcoroaristizabal-Martín, X. Toxoplasma gondii infection and peripheral-blood gene expression profiling of older people reveals dysregulation of cytokines and identifies hub genes as potential therapeutic targets. Heliyon 2022, 8, e10576. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef]
- Pawelec, G.; Bronikowski, A.; Cunnane, S.C.; Ferrucci, L.; Franceschi, C.; Fülöp, T.; Gaudreau, P.; Gladyshev, V.N.; Gonos, E.S.; Gorbunova, V.; et al. The conundrum of human immune system “senescence”. Mech. Ageing Dev. 2020, 192, 111357. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, B.; Alu, A.; Hong, W.; Lei, H.; He, X.; Shi, H.; Cheng, P.; Yang, X. Immunosenescence: Signaling pathways, diseases and therapeutic targets. Signal Transduct. Target. Ther. 2025, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wong, G.; Hwang, Y.Y.; Larbi, A. The untwining of immunosenescence and aging. Semin. Immunopathol. 2020, 42, 559–572. [Google Scholar] [CrossRef]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells 2022, 11, 359. [Google Scholar] [CrossRef]
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: Emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef]
- Fard, M.T.; Savage, K.M.; Stough, C.K. Peripheral inflammation marker relationships to cognition in healthy older adults—A systematic review. Psychoneuroendocrinology 2022, 144, 105870. [Google Scholar] [CrossRef]
- Appay, V.; Sauce, D. Naive T cells: The crux of cellular immune aging? Exp. Gerontol. 2014, 54, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Rodríguez, V.; Herrero-Fernández, I.; Castro, M.J.; Castillo, A.; Rosado-Sánchez, I.; Galvá, M.I.; Ramos, R.; Olivas-Martínez, I.; Bulnes-Ramos, Á.; Cañizares, J.; et al. Immunological features beyond CD4/CD8 ratio values in older individuals. Aging 2021, 13, 13443–13459. [Google Scholar] [CrossRef] [PubMed]
- Ligotti, M.E.; Aiello, A.; Accardi, G.; Aprile, S.; Bonura, F.; Bulati, M.; Gervasi, F.; Giammanco, G.M.; Pojero, F.; Zareian, N.; et al. Analysis of T and NK cell subsets in the Sicilian population from young to supercentenarian: The role of age and gender. Clin. Exp. Immunol. 2021, 205, 198–212. [Google Scholar] [CrossRef]
- Ramasubramanian, R.; Meier, H.C.S.; Vivek, S.; Klopack, E.; Crimmins, E.M.; Faul, J.; Nikolich-Žugich, J.; Thyagarajan, B. Evaluation of T-cell aging-related immune phenotypes in the context of biological aging and multimorbidity in the Health and Retirement Study. Immun. Ageing 2022, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.J.; Lalinde Ruiz, N.; Llano León, M.; Martínez Enríquez, L.; Montilla Velásquez, M.d.P.; Ortiz Aguirre, J.P.; Rodríguez Bohórquez, O.M.; Velandia Vargas, E.A.; Hernández, E.D.; Parra López, C.A. Immunosenescence Study of T Cells: A Systematic Review. Front. Immunol. 2021, 11, 604591. [Google Scholar] [CrossRef]
- Brzezińska, A.; Magalska, A.; Szybińska, A.; Sikora, E. Proliferation and apoptosis of human CD8+CD28+ and CD8+CD28− lymphocytes during aging. Exp. Gerontol. 2004, 39, 539–544. [Google Scholar] [CrossRef]
- Fagnoni, F.F.; Vescovini, R.; Passeri, G.; Bologna, G.; Pedrazzoni, M.; Lavagetto, G.; Casti, A.; Franceschi, C.; Passeri, M.; Sansoni, P. Shortage of circulating naive CD8+ T cells provides new insights on immunodeficiency in aging. Blood 2000, 95, 2860–2868. [Google Scholar] [CrossRef]
- Vescovini, R.; Fagnoni, F.F.; Telera, A.R.; Bucci, L.; Pedrazzoni, M.; Magalini, F.; Stella, A.; Pasin, F.; Medici, M.C.; Calderaro, A.; et al. Naïve and memory CD8 T cell pool homeostasis in advanced aging: Impact of age and of antigen-specific responses to Cytomegalovirus. AGE 2014, 36, 625–640. [Google Scholar] [CrossRef]
- Huff, W.X.; Kwon, J.H.; Henriquez, M.; Fetcko, K.; Dey, M. The Evolving Role of CD8+CD28− Immunosenescent T Cells in Cancer Immunology. Int. J. Mol. Sci. 2019, 20, 2810. [Google Scholar] [CrossRef]
- Alpert, A.; Pickman, Y.; Leipold, M.; Rosenberg-Hasson, Y.; Ji, X.; Gaujoux, R.; Rabani, H.; Starosvetsky, E.; Kveler, K.; Schaffert, S.; et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 2019, 25, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Z.; Kumar, S.; Botey-Bataller, J.; Zoodsma, M.; Ehsani, A.; Zhan, Q.; Alaswad, A.; Zhou, L.; Grondman, I.; et al. Single-cell immune aging clocks reveal inter-individual heterogeneity during infection and vaccination. Nat. Aging 2025, 5, 607–621. [Google Scholar] [CrossRef]
- Rizzo, L.B.; Swardfager, W.; Maurya, P.K.; Graiff, M.Z.; Pedrini, M.; Asevedo, E.; Cassinelli, A.C.; Bauer, M.E.; Cordeiro, Q.; Scott, J.; et al. An immunological age index in bipolar disorder: A confirmatory factor analysis of putative immunosenescence markers and associations with clinical characteristics. Int. J. Methods Psychiatr. Res. 2018, 27, e1614. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, J.; Oh, H.; Wyss-Coray, T. Measuring biological age using omics data. Nat. Rev. Genet. 2022, 23, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, K.; Wu, J.; Zhu, Y. The immunosenescence clock: A new method for evaluating biological age and predicting mortality risk. Ageing Res. Rev. 2025, 104, 102653. [Google Scholar] [CrossRef]
- Henrickson, S.E. Is your immune system over the hill? Sci. Immunol. 2019, 4, eaax8198. [Google Scholar] [CrossRef]
- Dolan, M.; Libby, K.A.; Ringel, A.E.; van Galen, P.; McAllister, S.S. Ageing, immune fitness and cancer. Nat. Rev. Cancer 2025, 25, 848–872. [Google Scholar] [CrossRef]
- Franceschi, C.; Olivieri, F.; Moskalev, A.; Ivanchenko, M.; Santoro, A. Toward precision interventions and metrics of inflammaging. Nat. Aging 2025, 5, 1441–1454. [Google Scholar] [CrossRef]
- Foster, M.A.; Bentley, C.; Hazeldine, J.; Acharjee, A.; Nahman, O.; Shen-Orr, S.S.; Lord, J.M.; Duggal, N.A. Investigating the potential of a prematurely aged immune phenotype in severely injured patients as predictor of risk of sepsis. Immun. Ageing 2022, 19, 60. [Google Scholar] [CrossRef]
- Lord, J.M.; Veenith, T.; Sullivan, J.; Sharma-Oates, A.; Richter, A.G.; Greening, N.J.; McAuley, H.J.C.; Evans, R.A.; Moss, P.; Moore, S.C.; et al. Accelerated immune ageing is associated with COVID-19 disease severity. Immun. Ageing 2024, 21, 6. [Google Scholar] [CrossRef]
- Bröde, P.; Claus, M.; Gajewski, P.D.; Getzmann, S.; Golka, K.; Hengstler, J.G.; Wascher, E.; Watzl, C. Calibrating a Comprehensive Immune Age Metric to Analyze the Cross Sectional Age-Related Decline in Cardiorespiratory Fitness. Biology 2022, 11, 1576. [Google Scholar] [CrossRef] [PubMed]
- Bröde, P.; Claus, M.; Gajewski, P.D.; Getzmann, S.; Wascher, E.; Watzl, C. From Immunosenescence to Aging Types–Establishing Reference Intervals for Immune Age Biomarkers by Centile Estimation. Int. J. Mol. Sci. 2023, 24, 13186. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Rieker, J.A.; Athanassiou, G.; Bröde, P.; Claus, M.; Golka, K.; Hengstler, J.G.; Kleinsorge, T.; Nitsche, M.A.; Reinders, J.; et al. A Systematic Analysis of Biological, Sociodemographic, Psychosocial, and Lifestyle Factors Contributing to Work Ability Across the Working Life Span: Cross-sectional Study. JMIR Form. Res. 2023, 7, e40818. [Google Scholar] [CrossRef]
- Claus, M.; Bröde, P.; Urlaub, D.; Wolfsdorff, N.; Watzl, C. Investigation of the relationship between Immune Age and Vaccination against SARS-CoV-2. Eur. J. Immunol. 2022, 52, 168. [Google Scholar] [CrossRef]
- Davies, M.; Denise, H.; Day, M.; Henson, S.M.; Scotton, C.J.; Harries, L.W. Immune age is correlated with decreased TCR clonal diversity and antibody response to SARS-CoV-2. Sci. Rep. 2025, 15, 19883. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Maecker, H.T.; Brodin, P.; Nygaard, U.C.; Lyu, S.C.; Davis, M.M.; Nadeau, K.C.; Andorf, S. Aging and CMV discordance are associated with increased immune diversity between monozygotic twins. Immun. Ageing 2021, 18, 5. [Google Scholar] [CrossRef]
- Pawelec, G. Latent CMV makes older adults less naive. eBioMedicine 2022, 77, 103887. [Google Scholar] [CrossRef]
- Pawelec, G.; Derhovanessian, E. Role of CMV in immune senescence. Virus Res. 2011, 157, 175–179. [Google Scholar] [CrossRef]
- Khan, I.A.; Moretto, M. Immune responses to Toxoplasma gondii. Curr. Opin. Immunol. 2022, 77, 102226. [Google Scholar] [CrossRef]
- Khan, I.A.; Ouellette, C.; Chen, K.; Moretto, M. Toxoplasma: Immunity and Pathogenesis. Curr. Clin. Microbiol. Rep. 2019, 6, 44–50. [Google Scholar] [CrossRef]
- Moretto, M.M.; Chen, J.; Meador, M.; Phan, J.; Khan, I.A. A Lower Dose of Infection Generates a Better Long-Term Immune Response against Toxoplasma gondii. ImmunoHorizons 2023, 7, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Gazzinelli, R.; Xu, Y.; Hieny, S.; Cheever, A.; Sher, A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 1992, 149, 175–180. [Google Scholar] [CrossRef]
- Chu, H.H.; Chan, S.-W.; Gosling, J.P.; Blanchard, N.; Tsitsiklis, A.; Lythe, G.; Shastri, N.; Molina-París, C.; Robey, E.A. Continuous Effector CD8+ T Cell Production in a Controlled Persistent Infection Is Sustained by a Proliferative Intermediate Population. Immunity 2016, 45, 159–171. [Google Scholar] [CrossRef]
- Khan, I.A.; Hwang, S.; Moretto, M. Toxoplasma gondii: CD8 T Cells Cry for CD4 Help. Front. Cell. Infect. Microbiol. 2019, 9, 136. [Google Scholar] [CrossRef]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence and human vaccine immune responses. Immun. Ageing 2019, 16, 25. [Google Scholar] [CrossRef]
- Eraghi, A.T.; Garweg, J.G.; Pleyer, U. The role of age in ocular toxoplasmosis: Clinical signs of immunosenescence and inflammaging. Front. Med. 2024, 11, 1311145. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wang, W.; Wang, Q.; Xu, J.; Dai, G.; Bai, Y.; Zhang, J. Activity Evaluation and Mode of Action of ICA Against Toxoplasma gondii In Vitro. Biomolecules 2025, 15, 202. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Getzmann, S.; Bröde, P.; Burke, M.; Cadenas, C.; Capellino, S.; Claus, M.; Genç, E.; Golka, K.; Hengstler, J.G.; et al. Impact of Biological and Lifestyle Factors on Cognitive Aging and Work Ability in the Dortmund Vital Study: Protocol of an Interdisciplinary, Cross-sectional, and Longitudinal Study. JMIR Res. Protoc. 2022, 11, e32352. [Google Scholar] [CrossRef]
- Getzmann, S.; Golka, K.; Bröde, P.; Reinders, J.; Kadhum, T.; Hengstler, J.G.; Wascher, E.; Gajewski, P.D. Chronic Toxoplasma gondii Infection Modulates Hearing Ability across the Adult Life Span. Life 2024, 14, 194. [Google Scholar] [CrossRef] [PubMed]
- Claus, M.; Dychus, N.; Ebel, M.; Damaschke, J.; Maydych, V.; Wolf, O.T.; Kleinsorge, T.; Watzl, C. Measuring the immune system: A comprehensive approach for the analysis of immune functions in humans. Arch. Toxicol. 2016, 90, 2481–2495. [Google Scholar] [CrossRef]
- Cossarizza, A.; Chang, H.-D.; Radbruch, A.; Abrignani, S.; Addo, R.; Akdis, M.; Andrä, I.; Andreata, F.; Annunziato, F.; Arranz, E.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (third edition). Eur. J. Immunol. 2021, 51, 2708–3145. [Google Scholar] [CrossRef]
- van den Boogaart, K.G.; Tolosana-Delgado, R. Analyzing Compositional Data with R; Springer: Berlin/Heidelberg, Germany, 2013; p. 255. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Calabrò, A.; Accardi, G.; Aiello, A.; Caruso, C.; Candore, G. Sex and gender affect immune aging. Front. Aging 2023, 4, 1272118. [Google Scholar] [CrossRef]
- Hirokawa, K.; Utsuyama, M.; Hayashi, Y.; Kitagawa, M.; Makinodan, T.; Fulop, T. Slower immune system aging in women versus men in the Japanese population. Immun. Ageing 2013, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, P.D.; Falkenstein, M.; Hengstler, J.G.; Golka, K. Toxoplasma gondii impairs memory in infected seniors. Brain Behav. Immun. 2014, 36, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Mendy, A.; Vieira, E.R.; Albatineh, A.N.; Gasana, J. Immediate rather than delayed memory impairment in older adults with latent toxoplasmosis. Brain Behav. Immun. 2015, 45, 36–40. [Google Scholar] [CrossRef]
- Song, G.; Zhao, Q.; Chen, H.; Li, M.; Zhang, Z.; Qu, Z.; Yang, C.; Lin, X.; Ma, W.; Standlee, C.R. Toxoplasma gondii seropositivity and cognitive functioning in older adults: An analysis of cross-sectional data of the National Health and Nutrition Examination Survey 2011–2014. BMJ Open 2024, 14, e071513. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, P.D.; Bröde, P.; Claus, M.; Golka, K.; Hengstler, J.G.; Reinders, J.; Watzl, C.; Wascher, E.; Getzmann, S. Changes of cognitive functions and proinflammatory cytokines across the lifespan in latent Toxoplasma gondii infection. Brain Behav. Immun. Health 2025, 49, 101105. [Google Scholar] [CrossRef]
- Stock, A.-K.; Dajkic, D.; Köhling, H.L.; von Heinegg, E.H.; Fiedler, M.; Beste, C. Humans with latent toxoplasmosis display altered reward modulation of cognitive control. Sci. Rep. 2017, 7, 10170. [Google Scholar] [CrossRef]
- Stock, A.-K.; Heintschel von Heinegg, E.; Köhling, H.-L.; Beste, C. Latent Toxoplasma gondii infection leads to improved action control. Brain Behav. Immun. 2014, 37, 103–108. [Google Scholar] [CrossRef]
- Eberhard, J.N.; Shallberg, L.A.; Winn, A.; Chandrasekaran, S.; Giuliano, C.J.; Merritt, E.F.; Willis, E.; Konradt, C.; Christian, D.A.; Aldridge, D.L.; et al. Immune targeting and host-protective effects of the latent stage of Toxoplasma gondii. Nat. Microbiol. 2025, 10, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, S.; Zhou, W.; Schüssler-Fiorenza Rose, S.M.; Sailani, M.R.; Contrepois, K.; Avina, M.; Ashland, M.; Brunet, A.; Snyder, M. Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nat. Med. 2020, 26, 83–90. [Google Scholar] [CrossRef]
- Andreou, D.; Steen, N.E.; Jørgensen, K.N.; Ueland, T.; Wortinger, L.A.; Mørch-Johnsen, L.; Drabløs, I.; Calkova, T.; Yolken, R.H.; Andreassen, O.A.; et al. Increased Herpes simplex virus 1, Toxoplasma gondii and Cytomegalovirus antibody concentrations in severe mental illness. Transl. Psychiatry 2024, 14, 498. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G. Human T Cell Aging and the Impact of Persistent Viral Infections. Front. Immunol. 2013, 4, 271. [Google Scholar] [CrossRef] [PubMed]
- Gelderman, A.H.; Grimley, P.M.; Lunde, M.N.; Rabson, A.S. Toxoplasma gondii and Cytomegalovirus: Mixed Infection by a Parasite and a Virus. Science 1968, 160, 1130–1132. [Google Scholar] [CrossRef]
- Pomeroy, C.; Kline, S.; Jordan, M.C.; Filice, G.A. Reactivation of Toxoplasma gondii by Cytomegalovirus Disease in Mice: Antimicrobial Activities of Macrophages. J. Infect. Dis. 1989, 160, 305–311. [Google Scholar] [CrossRef]
- Ross, D.S.; Jones, J.L.; Lynch, M.F. Toxoplasmosis, Cytomegalovirus, Listeriosis, and Preconception Care. Matern. Child. Health J. 2006, 10, 189–193. [Google Scholar] [CrossRef]
- Frye, M.A.; Coombes, B.J.; McElroy, S.L.; Jones-Brando, L.; Bond, D.J.; Veldic, M.; Romo-Nava, F.; Bobo, W.V.; Singh, B.; Colby, C.; et al. Association of Cytomegalovirus and Toxoplasma gondii Antibody Titers with Bipolar Disorder. JAMA Psychiatry 2019, 76, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Gratama, J.W.; Fridell, E.; Lenkei, R.; Oosterveer, M.A.P.; Ljungström, I.; Tanke, H.J.; Linde, A. Correlation between Cytomegalovirus and Toxoplasma gondii Serology and Lymphocyte Phenotypes in Peripheral Blood and Cord Blood. Scand. J. Infect. Dis. 1989, 21, 611–616. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 9 April 2025).
| Antigen | Clone | Fluorochrome | Company | Dilution 1/x |
|---|---|---|---|---|
| CD3 | UCHT1 | BV510 | BD Horizon™ (Franklin Lakes, NJ, USA) | 400 |
| live/dead | zombie Yellow | Biolegend (San Diego, CA, USA) | 1000 | |
| CD8 | RPA-T8 | FITC | BD Pharmingen™ (Franklin Lakes, NJ, USA) | 200 |
| CD28 | CD28.2 | PerCP-Cy™ 5.5 | BD Pharmingen™ | 100 |
| CD57 | NK-1 | PE | BD Pharmingen™ | 800 |
| CD56 | B159 | PE-CF594 | BD Pharmingen™ | 100 |
| CD197 (CCR7) | 150503 | Alexa Fluor® 647 | BD Pharmingen™ | 50 |
| CD4 | RPA-T4 | APC-H7 | BD Pharmingen™ | 100 |
| CD45RA | HI100 | Alexa Fluor® 700 | BD Pharmingen™ | 400 |
| Index i | Predictor xi | Coefficient ci |
|---|---|---|
| 0 | 1 (constant) | 0.024798 |
| 1 | log (%NK/%T) | 0.154033 |
| 2 | log (%mem/%naive CD4+) | 0.236631 |
| 3 | log (%CD4+/%CD8+) | 0.063959 |
| 4 | log (%mem/%naive CD8+) | 0.235855 |
| 5 | logit (%CD8+ CD28−) | 0.227827 |
| Characteristics | T. gondii− N = 423 (72%) 1 | T. gondii+ N = 161 (28%) 1 | P 2 | q-Value 2 | ∆adj (95% CI) 3 | Padj 3 |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Sex | 0.406 | 0.406 | ||||
| female | 268 (74%) | 96 (26%) | ||||
| male | 155 (70%) | 65 (30%) | ||||
| Age (years) | 42 (14) | 49 (14) | <0.001 | <0.001 | ||
| Immunosenescence biomarkers | ||||||
| IMMAX | 0.42 (0.12) | 0.45 (0.12) | 0.007 | 0.029 | −0.02 (−0.17, 0.14) | 0.823 |
| log (%NK/%T) | −1.83 (0.53) | −1.76 (0.58) | 0.173 | 0.197 | 0.04 (−0.14, 0.22) | 0.676 |
| log (%CD4+/%CD8+) | 1.29 (0.56) | 1.41 (0.62) | 0.048 | 0.077 | 0.01 (−0.16, 0.18) | 0.928 |
| log (%mem/%naive CD4+) | 0.54 (0.68) | 0.63 (0.65) | 0.159 | 0.197 | −0.03 (−0.20, 0.14) | 0.730 |
| log (%mem/%naive CD8+) | 0.00 (1.01) | 0.23 (1.06) | 0.023 | 0.061 | −0.06 (−0.21, 0.10) | 0.460 |
| logit (%CD8+ CD28−) | −1.32 (0.85) | −1.14 (0.92) | 0.034 | 0.067 | 0.05 (−0.13, 0.22) | 0.600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Bröde, P.; Claus, M.; Getzmann, S.; Golka, K.; Hengstler, J.G.; Reinders, J.; Wascher, E.; Watzl, C.; Gajewski, P.D. Latent Toxoplasma gondii Infection Does Not Modulate Immune Aging in a Cross-Sectional Working-Age Population Study. Biomolecules 2026, 16, 55. https://doi.org/10.3390/biom16010055
Bröde P, Claus M, Getzmann S, Golka K, Hengstler JG, Reinders J, Wascher E, Watzl C, Gajewski PD. Latent Toxoplasma gondii Infection Does Not Modulate Immune Aging in a Cross-Sectional Working-Age Population Study. Biomolecules. 2026; 16(1):55. https://doi.org/10.3390/biom16010055
Chicago/Turabian StyleBröde, Peter, Maren Claus, Stephan Getzmann, Klaus Golka, Jan G. Hengstler, Jörg Reinders, Edmund Wascher, Carsten Watzl, and Patrick D. Gajewski. 2026. "Latent Toxoplasma gondii Infection Does Not Modulate Immune Aging in a Cross-Sectional Working-Age Population Study" Biomolecules 16, no. 1: 55. https://doi.org/10.3390/biom16010055
APA StyleBröde, P., Claus, M., Getzmann, S., Golka, K., Hengstler, J. G., Reinders, J., Wascher, E., Watzl, C., & Gajewski, P. D. (2026). Latent Toxoplasma gondii Infection Does Not Modulate Immune Aging in a Cross-Sectional Working-Age Population Study. Biomolecules, 16(1), 55. https://doi.org/10.3390/biom16010055










