NLRP3 Inflammasome in Stress-Related Neuropsychiatric Disorders: Mechanisms of Neuron–Microglia–Astrocyte Crosstalk, HPA Axis Dysregulation, and Therapeutic Perspective
Abstract
1. Introduction
2. Neuroendocrine and Neuroimmune Mechanisms in the Pathophysiology of Stress-Related Disorders
2.1. The Dynamics of the HPA Axis and Mechanisms of Neuroimmunological Dysregulation in Response to Stress
2.1.1. Dynamics of the HPA Axis in the Stress Response
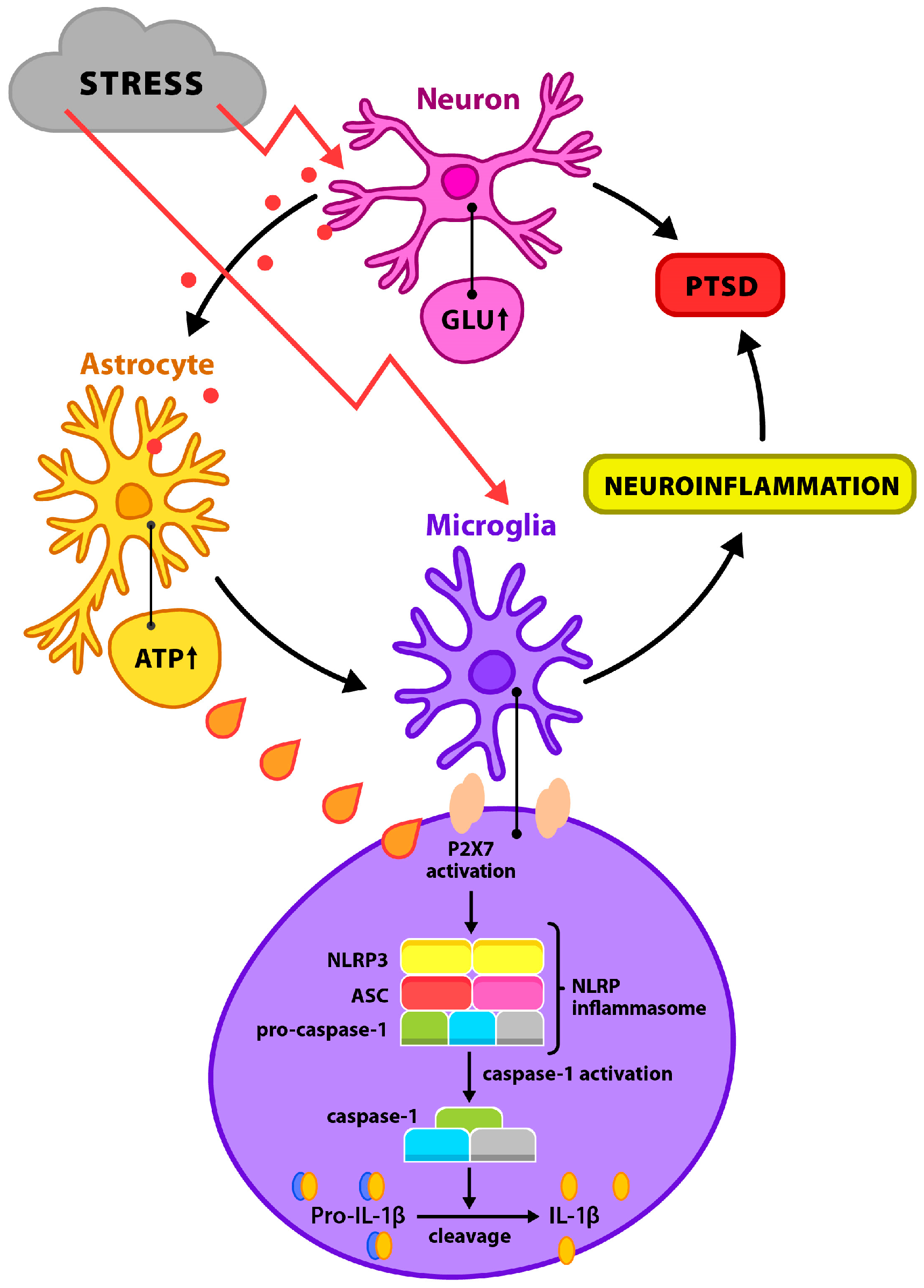
2.1.2. Neuroimmunological Dysregulation and Microglial–Inflammasome Pathways
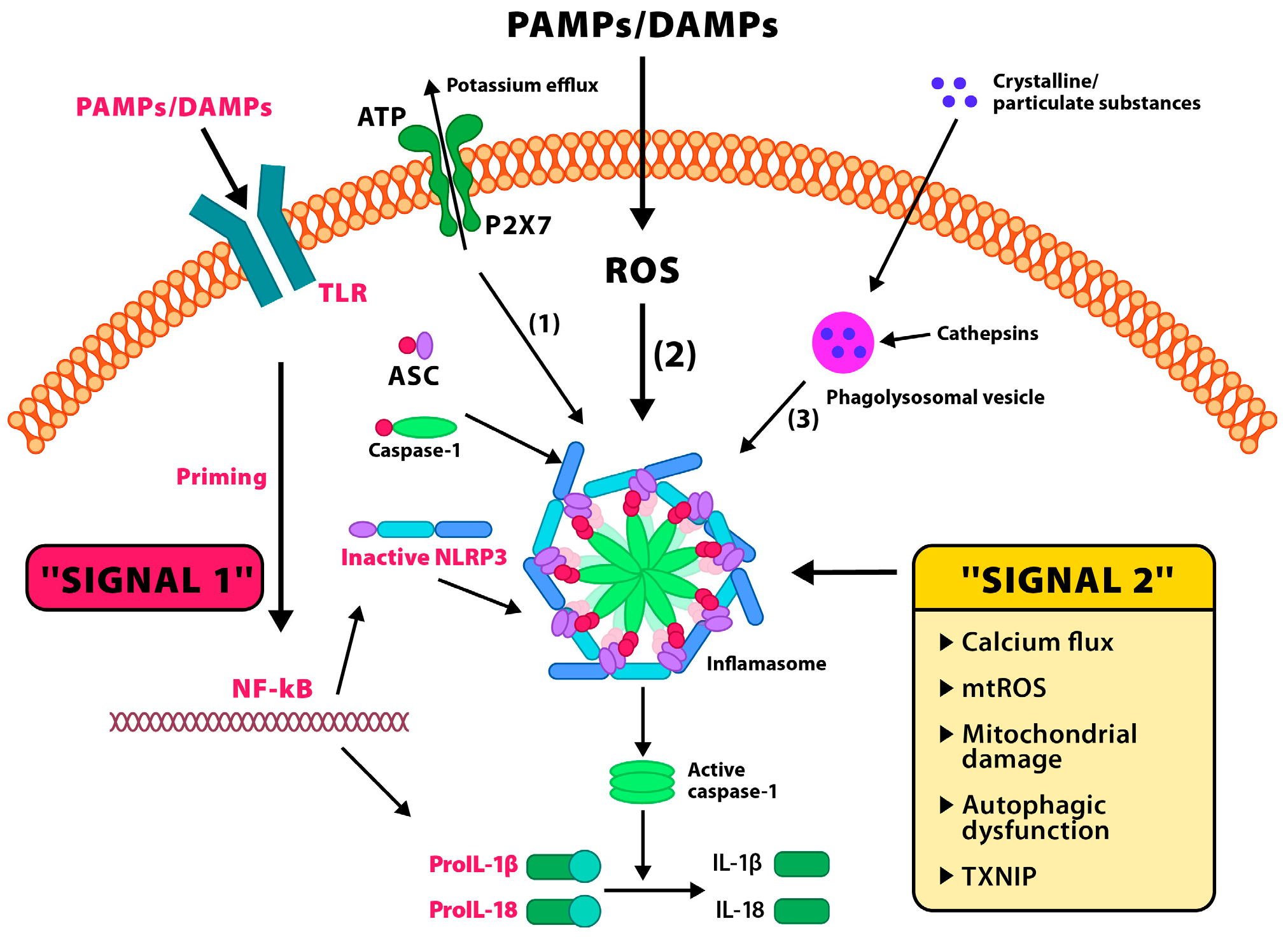
2.2. The NLRP3 Inflammasome as a Molecular Mediator of the Neuroinflammatory Response to Stress: Activation Mechanisms, Interaction with the HPA Axis, and Therapeutic Implications
2.2.1. Activation Mechanisms of the NLRP3 Inflammasome and Its Role in Modulating the Neuroinflammatory Response
2.2.2. Interactions Between the NLRP3 Inflammasome and HPA Axis Regulation: Opportunities for Therapeutic Modulation
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | Central Nervous System |
| NLRP3 | NOD-Like Receptor Pyrin Domain-Containing Protein 3 |
| HPA axis | Hypothalamic–Pituitary–Adrenal Axis |
| DAMPs | Damage-Associated Molecular Patterns |
| IL-1β | Interleukin 1 β |
| IL-18 | Interleukin 18 |
| PTSD | Post-Traumatic Stress Disorder |
| MR | Mineralocorticoid Receptor |
| GR | Glucocorticoid Receptor |
| SNS | Sympathetic Nervous System |
| IL-6 | Interleukin 6 |
| TNF-α | Tumor Necrosis Factor Alpha |
| IL-1α | Interleukin-1 -α |
| BBB | Blood–Brain Barrier |
| IFN-γ | Interferon Gamma |
| CRH | Corticotropin-Releasing Hormone |
| ACTH | Adrenocorticotropic Hormone |
| TLRs | Toll-Like Receptors |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| MAPK | Mitogen-Activated Protein Kinase |
| ATP | Adenosine Triphosphate |
| BNST | Bed Nucleus of the Stria Terminalis |
| BDNF | Brain-Derived Neurotrophic Factor |
| CREB | cAMP Response Element-Binding Protein |
| NMDA receptor | N-Methyl-D-Aspartate Receptor |
| AMPA receptor | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid Receptor |
| CaM-KII | Calcium/Calmodulin-Dependent Protein Kinase II |
| LTP | Long-Term Potentiation |
| GLT-1 | Glutamate Transporter 1 |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| PVN | Paraventricular Nucleus (of the hypothalamus) |
| GSDMD | Gasdermin D |
| MC2R | Melanocortin 2 Receptor |
| AP-1 | Activator Protein 1 |
| ASC | Apoptosis-Associated Speck-Like Protein Containing a CARD |
| PYD | Pyrin Domain |
| CARD | Caspase Activation and Recruitment Domain |
| TLR4 | Toll-Like Receptor 4 |
| ROS | Reactive Oxygen Species |
| TXNIP | Thioredoxin-Interacting Protein |
| NEK7 | NIMA-Related Kinase 7 |
| TREM2 | Triggering Receptor Expressed on Myeloid Cells 2 |
| CX3CR1 | CX3C Chemokine Receptor 1 |
| CRS | Chronic Restraint Stress |
| GSDMD-N | N-terminal of Gasdermin D |
| HDAC2 | Histone Deacetylase 2 |
| mtDNA | Mitochondrial DNA |
| PAMPs | Pathogen-Associated Molecular Patterns |
| LPS | Lipopolysaccharide |
| MCC950 (CRID3) | MCC950 (Cytokine Release Inhibitory Drug 3) |
| AMPK | AMP-Activated Protein Kinase |
| mtROS | Mitochondrial Reactive Oxygen Species |
| β-HB | Beta-Hydroxybutyrate |
| CRP | C-Reactive Protein |
| TLR | Toll-Like Receptor |
| HMGB1 | High Mobility Group Box 1 |
| PRRs | Pattern Recognition Receptors |
| LRR | Leucine-Rich Repeat |
References
- Ge, Y.; Zhou, L.; Fu, Y.; He, L.; Chen, Y.; Li, D.; Xie, Y.; Yang, J.; Wu, H.; Dai, H. Caspase-2 is a condensate-mediated deubiquitinase in protein quality control. Nat. Cell Biol. 2024, 26, 1943–1957. [Google Scholar] [CrossRef]
- Sah, A.; Singewald, N. The (neuro)inflammatory system in anxiety disorders and PTSD: Potential treatment targets. Pharmacol. Ther. 2025, 269, 108825. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lu, J.; Zhao, Z.; Zhang, Z.; Yang, W.; Zhang, G. Research progress on inflammation and immune dysregulation in PTSD. Brain Behav. 2025, 15, e70633. [Google Scholar] [CrossRef]
- Toader, C.; Serban, M.; Munteanu, O.; Covache-Busuioc, R.A.; Enyedi, M.; Ciurea, A.V.; Tataru, C.P. From synaptic plasticity to neurodegeneration: BDNF as a transformative target in medicine. Int. J. Mol. Sci. 2025, 26, 4271. [Google Scholar] [CrossRef]
- Chavan, P.; Kitamura, T.; Sakaguchi, M. Memory processing by hippocampal adult-born neurons. Neurobiol. Learn Mem. 2025, 220, 108062. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, S.; Zhang, X.; Zhang, S.; Chen, L.; Wang, W.; Chen, N.; Yan, J. Inflammasomes in neurodegenerative diseases. Transl. Neurodegener. 2024, 13, 65. [Google Scholar] [CrossRef]
- Joshi, D.C.; Zhang, C.L.; Mathur, D.; Li, A.; Kaushik, G.; Sheng, Z.H.; Chiu, S.Y. Tripartite crosstalk between cytokine IL-1β, NMDA-R and misplaced mitochondrial anchor in neuronal dendrites is a novel pathway for neurodegeneration in inflammatory diseases. J. Neurosci. 2022, 42, 7318–7329. [Google Scholar] [CrossRef]
- Zengeler, K.E.; Hollis, A.; Deutsch, T.C.J.; Samuels, J.D.; Ennerfelt, H.; Moore, K.A.; Steacy, E.J.; Sabapathy, V.; Sharma, R.; Patel, M.K.; et al. Inflammasome signaling in astrocytes modulates hippocampal plasticity. Immunity 2025, 58, 1519–1535.e11. [Google Scholar] [CrossRef]
- Reschke, R.; Sullivan, R.J.; Lipson, E.J.; Enk, A.H.; Gajewski, T.F.; Hassel, J.C. Targeting molecular pathways to control immune checkpoint inhibitor toxicities. Trends Immunol. 2025, 46, 61–73. [Google Scholar] [CrossRef]
- Mohammad, Z.B.; Yudin, S.C.Y.; Goldberg, B.J.; Serra, K.L.; Klegeris, A. Exploring neuroglial signaling: Diversity of molecules implicated in microglia-to-astrocyte neuroimmune communication. Rev. Neurosci. 2024, 36, 91–117. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhang, H.; Shang, Y.; Wu, S.; Jin, T. NLRP3 inflammasome: From drug target to drug discovery. Drug Discov. Today 2025, 30, 104375. [Google Scholar] [CrossRef]
- Yu, H.; Ren, K.; Jin, Y.; Zhang, L.; Liu, H.; Huang, Z.; Zhang, Z.; Chen, X.; Yang, Y.; Wei, Z. Mitochondrial DAMPs: Key mediators in neuroinflammation and neurodegenerative disease pathogenesis. Neuropharmacology 2025, 264, 110217. [Google Scholar] [CrossRef]
- Hu, J.; Li, H.; Wang, X.; Cheng, H.; Zhu, G.; Yang, S. Novel mechanisms of Anshen Dingzhi prescription against PTSD: Inhibiting DCC to modulate synaptic function and inflammatory responses. J. Ethnopharmacol. 2024, 333, 118425. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Wu, X.; Zhao, Z.; Wang, S.; Wang, H.; Qin, X. Xiaoyaosan against depression through suppressing LPS mediated TLR4/NLRP3 signaling pathway in “microbiota-gut-brain” axis. J. Ethnopharmacol. 2024, 335, 118683. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Li, W.; Chen, P.; Wang, L.; Bao, X.; Huang, R.; Liu, G.; Chen, X. Microglial NLRP3 inflammasome-mediated neuroinflammation and therapeutic strategies in depression. Neural Regen. Res. 2024, 19, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, G.V.; Vekaria, H.J.; Hartz, A.M.S.; Bauer, B.; Hubbard, W.B. Oxidative stress alters mitochondrial homeostasis in isolated brain capillaries. Fluids Barriers CNS 2024, 21, 81. [Google Scholar] [CrossRef]
- Wadan, A.S.; Ahmed, M.A.; Moradikor, N. Mapping brain neural networks in stress brain connectivity. Prog. Brain Res. 2025, 291, 239–251. [Google Scholar] [CrossRef] [PubMed]
- González, I.; VanderZwaag, J.; Deslauriers, J.; Tremblay, M.È. Ultrastructural features of psychological stress resilience in the brain: A microglial perspective. Open Biol. 2024, 14, 240079. [Google Scholar] [CrossRef]
- Duarte, Y.; Quintana-Donoso, D.; Moraga-Amaro, R.; Dinamarca, I.; Lemunao, Y.; Cárdenas, K.; Bahamonde, T.; Barrientos, T.; Olivares, P.; Navas, C.; et al. The role of astrocytes in depression, its prevention, and treatment by targeting astroglial gliotransmitter release. Proc. Natl. Acad. Sci. USA 2024, 121, e2307953121. [Google Scholar] [CrossRef]
- Yontar, G.; Mutlu, E.A. Neutrophil-to-lymphocyte, platelet-to-lymphocyte ratios and systemic immune-inflammation index in patients with post-traumatic stress disorder. BMC Psychiatry 2024, 24, 966. [Google Scholar] [CrossRef]
- Munshi, S.; Alarbi, A.M.; Zheng, H.; Kuplicki, R.; Burrows, K.; Figueroa-Hall, L.K.; Victor, T.A.; Aupperle, R.L.; Khalsa, S.S.; Paulus, M.P. Increased expression of ER stress, inflammasome activation, and mitochondrial biogenesis-related genes in peripheral blood mononuclear cells in major depressive disorder. Mol. Psychiatry 2025, 30, 574–586. [Google Scholar] [CrossRef]
- Dow, C.T.; Obaid, L. Proposing Bromo-Epi-Androsterone (BEA) for Post-Traumatic Stress Disorder (PTSD). Cells 2025, 14, 1120. [Google Scholar] [CrossRef]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef]
- Feldman, N.; Rotter-Maskowitz, A.; Okun, E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res. Rev. 2015, 24, 29–39. [Google Scholar] [CrossRef]
- Chen, M.Y.; Ye, X.J.; He, X.H.; Ouyang, D.Y. The signaling pathways regulating NLRP3 inflammasome activation. Inflammation 2021, 44, 1229–1245. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Liberal, J.; Martins, J.D.; Silva, A.; Neves, B.M.; Cruz, M.T. Inflammasome in dendritic cells immunobiology: Implications to diseases and therapeutic strategies. Curr. Drug Targets 2017, 18, 1003–1018. [Google Scholar] [CrossRef]
- Mackay, A.; Velcicky, J.; Gommermann, N.; Mattes, H.; Janser, P.; Wright, M.; Dubois, C.; Brenneisen, S.; Ilic, S.; Vangrevelinghe, E.; et al. Discovery of NP3-253, a potent brain penetrant inhibitor of the NLRP3 inflammasome. J. Med. Chem. 2024, 67, 20780–20798. [Google Scholar] [CrossRef]
- Soltani Khaboushan, A.; Yazdanpanah, N.; Rezaei, N. Neuroinflammation and proinflammatory cytokines in epileptogenesis. Mol. Neurobiol. 2022, 59, 1724–1743. [Google Scholar] [CrossRef] [PubMed]
- Lecca, D.; Jung, Y.J.; Scerba, M.T.; Hwang, I.; Kim, Y.K.; Kim, S.; Modrow, S.; Tweedie, D.; Hsueh, S.C.; Liu, D.; et al. Role of chronic neuroinflammation in neuroplasticity and cognitive function: A hypothesis. Alzheimers Dement. 2022, 18, 2327–2340. [Google Scholar] [CrossRef]
- Wellington, N.J.; Bouças, A.P.; Lagopoulos, J.; Quigley, B.L.; Kuballa, A.V. Molecular pathways of ketamine: A systematic review of immediate and sustained effects on PTSD. Psychopharmacology 2025, 242, 1197–1243. [Google Scholar] [CrossRef] [PubMed]
- Quigley, B.L.; Wellington, N.; Levenstein, J.M.; Dutton, M.; Bouças, A.P.; Forsyth, G.; Gallay, C.C.; Hajishafiee, M.; Treacy, C.; Lagopoulos, J.; et al. Circulating biomarkers and neuroanatomical brain structures differ in older adults with and without post-traumatic stress disorder. Sci. Rep. 2025, 15, 7176. [Google Scholar] [CrossRef]
- Maes, M.; Almulla, A.F.; Zhou, B.; Algon, A.A.A.; Sodsai, P. In major dysmood disorder, physiosomatic, chronic fatigue and fibromyalgia symptoms are driven by immune activation and increased immune-associated neurotoxicity. Sci. Rep. 2024, 14, 7344. [Google Scholar] [CrossRef]
- Rachayon, M.; Jirakran, K.; Sodsai, P.; Sughondhabirom, A.; Maes, M. T cell activation and deficits in T regulatory cells are associated with major depressive disorder and severity of depression. Sci. Rep. 2024, 14, 11177. [Google Scholar] [CrossRef]
- Maes, M.; Jirakran, K.; Vasupanrajit, A.; Niu, M.; Zhou, B.; Stoyanov, D.S.; Tunvirachaisakul, C. The recurrence of illness (ROI) index is a key factor in major depression that indicates increasing immune-linked neurotoxicity and vulnerability to suicidal behaviors. Psychiatry Res. 2024, 339, 116085. [Google Scholar] [CrossRef]
- Li, P.; Gao, Y.; Tao, Z.; Mu, Z.; Du, S.; Zhao, X. PANoptosis: Cross-Talk Among Apoptosis, Necroptosis, and Pyroptosis in Neurological Disorders. Inflamm. Res. 2025, 18, 8131–8140. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Lv, X.J.; Guo, Q.H.; Lv, S.S.; Lv, N.; Xu, W.D.; Yu, J.; Zhang, Y.Q. Asymmetric activation of microglia in the hippocampus drives anxiodepressive consequences of trigeminal neuralgia in rodents. Br. J. Pharmacol. 2023, 180, 1090–1113. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, Y.; Shen, M.; Wang, C.; Dong, B.; Xie, K.; Yu, Y.; Yu, Y. Spinal NLRP3 inflammasome activation mediates IL-1β release and contributes to remifentanil-induced postoperative hyperalgesia by regulating NMDA receptor NR1 subunit phosphorylation and GLT-1 expression in rats. Mol. Pain 2022, 18, 17448069221093016. [Google Scholar] [CrossRef]
- Wan, T.; Li, X.; Fu, M.; Gao, X.; Li, P.; Guo, W. NLRP3-dependent pyroptosis: A candidate therapeutic target for depression. Front. Cell Neurosci. 2022, 16, 863426. [Google Scholar] [CrossRef] [PubMed]
- Ghaffaripour Jahromi, G.; Razi, S.; Rezaei, N. NLRP3 inflammatory pathway. Can we unlock depression? Brain Res. 2024, 1822, 148644. [Google Scholar] [CrossRef]
- Yang, X.J.; Zhao, B.C.; Li, J.; Shi, C.; Song, Y.Q.; Gao, X.Z.; Jiang, H.L.; Yu, Q.Y.; Liang, X.C.; Feng, S.X.; et al. Serum NLRP3 inflammasome and BDNF: Potential biomarkers differentiating reactive and endogenous depression. Front. Psychiatry 2022, 13, 814828. [Google Scholar] [CrossRef]
- Pellegrini, C.; Antonioli, L.; Calderone, V.; Colucci, R.; Fornai, M.; Blandizzi, C. Microbiota-gut-brain axis in health and disease: Is NLRP3 inflammasome at the crossroads of microbiota-gut-brain communications? Prog. Neurobiol. 2020, 191, 101806. [Google Scholar] [CrossRef]
- Komleva, Y.K.; Lopatina, O.L.; Gorina, I.V.; Shuvaev, A.N.; Chernykh, A.; Potapenko, I.V.; Salmina, A.B. NLRP3 deficiency-induced hippocampal dysfunction and anxiety-like behavior in mice. Brain Res. 2021, 1752, 147220. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fang, Y.; Zhang, Y.; Song, M.; Zhang, X.; Ding, X.; Yao, H.; Chen, M.; Sun, Y.; Ding, J.; et al. Microglial NLRP3 inflammasome activates neurotoxic astrocytes in depression-like mice. Cell Rep. 2022, 41, 111532. [Google Scholar] [CrossRef]
- Xu, Q.; Sun, L.; Chen, Q.; Jiao, C.; Wang, Y.; Li, H.; Xie, J.; Zhu, F.; Wang, J.; Zhang, W.; et al. Gut microbiota dysbiosis contributes to depression-like behaviors via hippocampal NLRP3-mediated neuroinflammation in a postpartum depression mouse model. Brain Behav. Immun. 2024, 119, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, X.; Tang, C. Estrogen-immuno-neuromodulation disorders in menopausal depression. J. Neuroinflamm. 2024, 21, 159. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, J.; Peng, R.; Yu, H.; He, Y.; Zhou, Q.; Wang, Y.; Xie, P. NLRP3 in the dorsal raphe nucleus manipulates the depressive-like behaviors. Brain Res. Bull. 2025, 227, 111405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Y.; Liu, P.; Lv, H.; Guan, M.; Cong, J.; Wang, Y.; Xu, Y. Metformin attenuated depressive-like behaviors by suppressing TRPV1/NLRP3 mediated neuroinflammation in the hypothalamus of allergic rhinitis mice. Neuroscience 2025, 571, 52–61. [Google Scholar] [CrossRef]
- Ferreira, F.B.; Kaufmann, F.N.; Bastos, C.R.; Xavier, J.; Aniszewski, S.; Molina, M.L.; Lara, D.R.; Jansen, K.; da Silva, R.A.; Souza, L.D.M.; et al. The gain-of-function variant in the NLRP3 gene predicts the effectiveness of brief psychotherapy but not the risk of major depression. Behav. Brain Res. 2025, 481, 115413. [Google Scholar] [CrossRef]
- Mokhtari, T.; Irandoost, E.; Sheikhbahaei, F. Stress, pain, anxiety, and depression in endometriosis-Targeting glial activation and inflammation. Int. Immunopharmacol. 2024, 132, 111942. [Google Scholar] [CrossRef]
- Ye, T.; Tao, W.Y.; Chen, X.Y.; Jiang, C.; Di, B.; Xu, L.L. Mechanisms of NLRP3 inflammasome activation and the development of peptide inhibitors. Cytokine Growth Factor Rev. 2023, 74, 1–13. [Google Scholar] [CrossRef]
- Qin, Z.; Shi, D.D.; Li, W.; Cheng, D.; Zhang, Y.D.; Zhang, S.; Tsoi, B.; Zhao, J.; Wang, Z.; Zhang, Z.J. Berberine ameliorates depression-like behaviors in mice via inhibiting NLRP3 inflammasome-mediated neuroinflammation and preventing neuroplasticity disruption. J. Neuroinflamm. 2023, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Lang, W.; Wang, C.; Wen, J.; Chen, M.; Wei, D.; Jiang, Y.; Li, X.; Wei, P.; Jin, G.; Zhu, Q. Targeting NEK7 modulates pyroptosis and gut microbiota to alleviate depression-like behavior in rats. Int. Immunopharmacol. 2025, 162, 115148. [Google Scholar] [CrossRef] [PubMed]
- Tetik, M.; Direk, N.; Uzgan, B.Ö.; Aykaç, C.; Ekinci, B.; Yaraş, T.; Kuruoğlu, A.; Özel, F.; Ermiş, Ç.; Alkın, T.; et al. Associations Between Blood Levels of NLRP3 Inflammasome Components and Obsessive Compulsive Disorder. Noro Psikiyatr Ars. 2023, 60, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 inflammasome: A sensor for metabolic stress and danger signals. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Frank, M.G.; Fonken, L.K.; Annis, J.L.; Watkins, L.R.; Maier, S.F. Stress disinhibits microglia via down-regulation of CD200R: A mechanism of neuroinflammatory priming. Brain Behav. Immun. 2019, 80, 47–57. [Google Scholar] [CrossRef]
- Frank, M.G.; Fonken, L.K.; Watkins, L.R.; Maier, S.F. Acute stress induces chronic neuroinflammatory, microglial and behavioral priming: A role for potentiated NLRP3 inflammasome activation. Brain Behav. Immun. 2020, 89, 32–42. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Franklin, T.; Iwata, M.; Duman, R.S. Integrating neuro-immune systems in the neurobiology of depression. Nat. Rev. Neurosci. 2016, 17, 497–511. [Google Scholar] [CrossRef]
- Ma, J.; Tian, Y.; Du, C.; Zhu, Y.; Huang, W.; Ding, C.; Wei, P.; Yi, X.; Lin, Z.; Fang, W. Cerium-doped Prussian blue biomimetic nanozyme as an amplified pyroptosis inhibitor mitigate Aβ oligomer-induced neurotoxicity in Alzheimer’s disease. J. Nanobiotechnol. 2025, 23, 181. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2020, 5, 17023. [Google Scholar] [CrossRef]
- Ogłodek, E. Changes in the Serum Levels of Cytokines: IL-1β, IL-4, IL-8 and IL-10 in Depression with and without Posttraumatic Stress Disorder. Brain Sci. 2022, 12, 387. [Google Scholar] [CrossRef]
- Koirala, R.; Aass, H.C.D.; Søegaard, E.G.I.; Dhakal, H.P.; Ojha, S.P.; Hauff, E.; Thapa, S.B. Association of pro-inflammatory cytokines with trauma and post-traumatic stress disorder visiting a tertiary care hospital in Kathmandu. PLoS ONE 2023, 18, e0281125. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Gururajan, A.; van de Wouw, M.; Boehme, M.; Becker, T.; O’Connor, R.; Bastiaanssen, T.F.S.; Moloney, G.M.; Lyte, J.M.; Ventura Silva, A.P.; Merckx, B.; et al. Resilience to chronic stress is associated with the response of the NLRP3 inflammasome and its epigenetic regulation in the hippocampus. Brain Behav. Immun. 2019, 80, 583–594. [Google Scholar] [CrossRef]
- Mamik, M.K.; Power, C. Inflammasomes in neurological diseases: Emerging pathogenic and therapeutic concepts. Brain 2017, 140, 2273–2285. [Google Scholar] [CrossRef]
- Li, H.; Guan, Y.; Liang, B.; Ding, P.; Hou, X.; Wei, W.; Ma, Y. Therapeutic potential of MCC950, a specific inhibitor of NLRP3 inflammasome. Eur. J. Pharmacol. 2022, 928, 175091. [Google Scholar] [CrossRef] [PubMed]
- Kirbas Cilingir, E.; Seven, E.S.; Zhou, Y.; Walters, B.M.; Mintz, K.J.; Pandey, R.R.; Wikramanayake, A.H.; Chusuei, C.C.; Vanni, S.; Graham, R.M.; et al. Metformin derived carbon dots: Highly biocompatible fluorescent nanomaterials as mitochondrial targeting and blood-brain barrier penetrating biomarkers. J. Colloid Interface Sci. 2021, 592, 485–497. [Google Scholar] [CrossRef]
- Shang, S.; Wang, L.; Lu, X. β-Hydroxybutyrate enhances astrocyte glutamate uptake through EAAT1 expression regulation. Mol. Cell Neurosci. 2024, 131, 103959. [Google Scholar] [CrossRef]
- D’Alessio, L.; Mesarosova, L.; Anink, J.J.; Kochen, S.; Solís, P.; Oddo, S.; Konopka, H.; Iyer, A.M.; Mühlebner, A.; Lucassen, P.J.; et al. Reduced expression of the glucocorticoid receptor in the hippocampus of patients with drug-resistant temporal lobe epilepsy and comorbid depression. Epilepsia 2020, 61, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-J.; Hu, T.; Zhou, X.-N.; Zhang, T.; Guo, J.-H.; Wang, M.-Y.; Wu, Y.-L.; Su, W.-J.; Jiang, C.-L. The involvement of NLRP3 inflammasome in CUMS-induced AD-like pathological changes and related cognitive decline in mice. J. Neuroinflamm. 2023, 20, 112. [Google Scholar] [CrossRef]
- Catorce, M.N.; Gevorkian, G. LPS-induced murine neuroinflammation model: Main features and suitability for pre-clinical assessment of nutraceuticals. Curr. Neuropharmacol. 2016, 14, 155–164. [Google Scholar] [CrossRef]
- Maurya, R.; Sharma, A.; Naqvi, S. Decoding NLRP3 Inflammasome Activation in Alzheimer’s Disease: A Focus on Receptor Dynamics. Mol. Neurobiol. 2025, 62, 10792–10812. [Google Scholar] [CrossRef]
- Herder, C.; Zhu, A.; Schmitt, A.; Spagnuolo, M.C.; Kulzer, B.; Roden, M.; Hermanns, N.; Ehrmann, D. Associations between biomarkers of inflammation and depressive symptoms—Potential differences between diabetes types and symptom clusters of depression. Transl. Psychiatry 2025, 15, 9. [Google Scholar] [CrossRef]
- Du, X.; Zou, S.; Yue, Y.; Fang, X.; Wu, Y.; Wu, S.; Wang, H.; Li, Z.; Zhao, X.; Yin, M.; et al. Peripheral Interleukin-18 is negatively correlated with abnormal brain activity in patients with depression: A resting-state fMRI study. BMC Psychiatry 2022, 22, 531. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Kitaoka, S.; Tanaka, K.; Segi-Nishida, E.; Imoto, Y.; Ogawa, A.; Nakano, F.; Tomohiro, A.; Nakayama, K.; Taniguchi, M.; et al. The innate immune receptors TLR2/4 mediate repeated social defeat stress-induced social avoidance through prefrontal microglial activation. Neuron 2018, 99, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, S.S.; Roa Diaz, S.; Peralta, S.; Nomura, M.; King, C.D.; Ceyhan, K.E.; Lin, A.; Bhaumik, D.; Foulger, A.C.; Shah, S.; et al. β-hydroxybutyrate is a metabolic regulator of proteostasis in the aged and Alzheimer disease brain. Cell Chem. Biol. 2025, 32, 174–191.e8. [Google Scholar] [CrossRef]
- Jo, E.K.; Kim, J.K.; Shin, D.M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Xu, G.; Zhan, F. Minocycline alleviates lipopolysaccharide-induced cardiotoxicity by suppressing the NLRP3/Caspase-1 signaling pathway. Sci. Rep. 2024, 14, 21180. [Google Scholar] [CrossRef]
- Dong, Y.; Li, S.; Lu, Y.; Li, X.; Liao, Y.; Peng, Z.; Li, Y.; Hou, L.; Yuan, Z.; Cheng, J. Stress-induced NLRP3 inflammasome activation negatively regulates fear memory in mice. J. Neuroinflamm. 2020, 17, 205. [Google Scholar] [CrossRef]
- Yang, L.; Xing, W.; Shi, Y.; Hu, M.; Li, B.; Hu, Y.; Zhang, G. Stress-induced NLRP3 inflammasome activation and myelin alterations in the hippocampus of PTSD rats. Neuroscience 2024, 555, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Gong, W.; Wang, S.; Zhang, D.; Chen, B.; Li, X.; Wu, X.; Cui, L.; Feng, Y.; Verkhratsky, A.; et al. Leptin Attenuates Fear Memory by Inhibiting Astrocytic NLRP3 Inflammasome in Post-traumatic Stress Disorder Model. Neurochem. Res. 2023, 48, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Grzesińska, A.; Ogłodek, E.A. Involvement of Matrix Metalloproteinases (MMP-2 and MMP-9), Inflammasome NLRP3, and Gamma-Aminobutyric Acid (GABA) Pathway in Cellular Mechanisms of Neuroinflammation in PTSD. Int. J. Mol. Sci. 2025, 26, 5662. [Google Scholar] [CrossRef] [PubMed]
- Dahlmanns, M.; Dahlmanns, J.; Savaskan, N.; Steiner, H.; Yakubov, E. Glial Glutamate Transporter-Mediated Plasticity: System xc-/xCT/SLC7A11 and EAAT1/2 in Brain Diseases. Front. Biosci. 2023, 28, 57. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, R.; Shahrokhi Nejad, S.; Falah Tafti, M.; Karimi, Z.; Sadr, S.R.; Ramadhan Hussein, D.; Talebian, N.; Esmaeilpour, K. Microglial activation as a hallmark of neuroinflammation in Alzheimer’s disease. Metab. Brain Dis. 2025, 40, 207. [Google Scholar] [CrossRef]
- Grzesińska, A.D. The Involvement of the Endocannabinoid, Glutamatergic, and GABAergic Systems in PTSD. Int. J. Mol. Sci. 2025, 26, 5929. [Google Scholar] [CrossRef]
- Ogłodek, E.A.; Just, M.J.; Szromek, A.R.; Araszkiewicz, A. Assessing the serum concentration levels of NT-4/5, GPX-1, TNF-α, and L-arginine as biomediators of depression severity in first depressive episode patients with and without posttraumatic stress disorder. Pharmacol. Rep. 2017, 69, 1049–1058. [Google Scholar] [CrossRef]
- Ogłodek, E.A. Changes in the Serum Concentration Levels of Serotonin, Tryptophan and Cortisol among Stress-Resilient and Stress-Susceptible Individuals after Experiencing Traumatic Stress. Int. J. Environ. Res. Public Health 2022, 19, 16517. [Google Scholar] [CrossRef]
| Inhibitor | Molecular Target/Mechanism | Preclinical Evidence | Clinical Evidence/Translational Notes |
|---|---|---|---|
| MCC950 (CRID3) | Binds to NACHT domain of NLRP3, blocks ATP hydrolysis and oligomerization | Reduces IL-1β/IL-18 in hippocampus and amygdala, reverses depressive/anxiety-like behaviors in CRS and SPS models | No approved human use; Phase I safety data limited; hepatotoxicity concerns require further study |
| Metformin | Activates AMPK, reduces mtROS, inhibits TXNIP–NLRP3 interaction | Improves neurogenesis, reduces NLRP3/caspase-1 in hippocampus of stressed mice; benefits in metabolic-inflammation comorbidity | Approved T2DM drug; epidemiological links to reduced depression risk; no targeted NLRP3 trials |
| β-HB | Stabilizes mitochondrial membrane potential, inhibits NEK7–NLRP3 interaction | Restores dendritic spine morphology, reduces anxiety-like behavior in stress-induced depression models | Endogenous ketone; ketogenic diets tested in epilepsy and mood disorders; no direct NLRP3-focused trials |
| Minocycline | Inhibits microglial activation, reduces ASC/NLRP3 expression, lowers TNF-α and IL-6 | Attenuates neuroinflammation, improves cognition and mood in rodent stress models | Small clinical trials show antidepressant effect in high-CRP depression; broader trials needed |
| OLT1177 (Dapansutrile) | Selective NLRP3 inhibitor; prevents ASC speck formation | Cardiovascular and neuroprotective benefits in preclinical ischemia models | Phase II in gout and heart failure; potential repurposing for neuropsychiatric inflammation |
| CY-09 | Blocks ATP binding to NLRP3 NACHT domain | Effective in reducing IL-1β release in LPS/ATP-stimulated microglia | No human trials; promising BBB penetration profile in animal models |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźny-Rasała, I.; Ogłodek, E.A. NLRP3 Inflammasome in Stress-Related Neuropsychiatric Disorders: Mechanisms of Neuron–Microglia–Astrocyte Crosstalk, HPA Axis Dysregulation, and Therapeutic Perspective. Biomolecules 2025, 15, 1344. https://doi.org/10.3390/biom15091344
Woźny-Rasała I, Ogłodek EA. NLRP3 Inflammasome in Stress-Related Neuropsychiatric Disorders: Mechanisms of Neuron–Microglia–Astrocyte Crosstalk, HPA Axis Dysregulation, and Therapeutic Perspective. Biomolecules. 2025; 15(9):1344. https://doi.org/10.3390/biom15091344
Chicago/Turabian StyleWoźny-Rasała, Izabela, and Ewa Alicja Ogłodek. 2025. "NLRP3 Inflammasome in Stress-Related Neuropsychiatric Disorders: Mechanisms of Neuron–Microglia–Astrocyte Crosstalk, HPA Axis Dysregulation, and Therapeutic Perspective" Biomolecules 15, no. 9: 1344. https://doi.org/10.3390/biom15091344
APA StyleWoźny-Rasała, I., & Ogłodek, E. A. (2025). NLRP3 Inflammasome in Stress-Related Neuropsychiatric Disorders: Mechanisms of Neuron–Microglia–Astrocyte Crosstalk, HPA Axis Dysregulation, and Therapeutic Perspective. Biomolecules, 15(9), 1344. https://doi.org/10.3390/biom15091344






