Perspectives on Alzheimer’s Disease Treatment Based on Counteracting Oxidative Stress
Abstract
1. Introduction
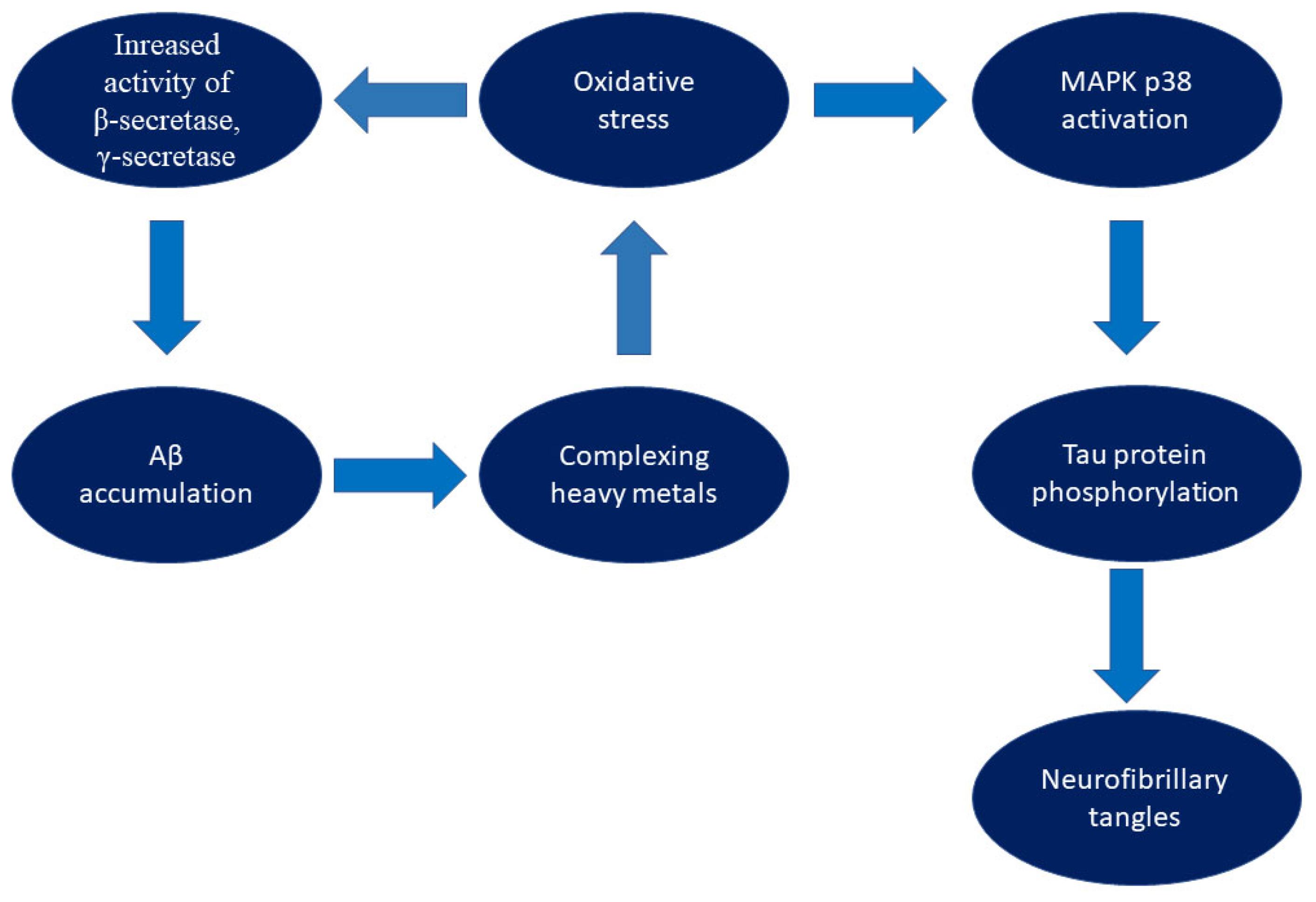
2. The Impact of Oxidative Stress on the Pathophysiology of Alzheimer’s Disease
3. AD Therapies—A Review of Current Research and Therapeutic Approaches
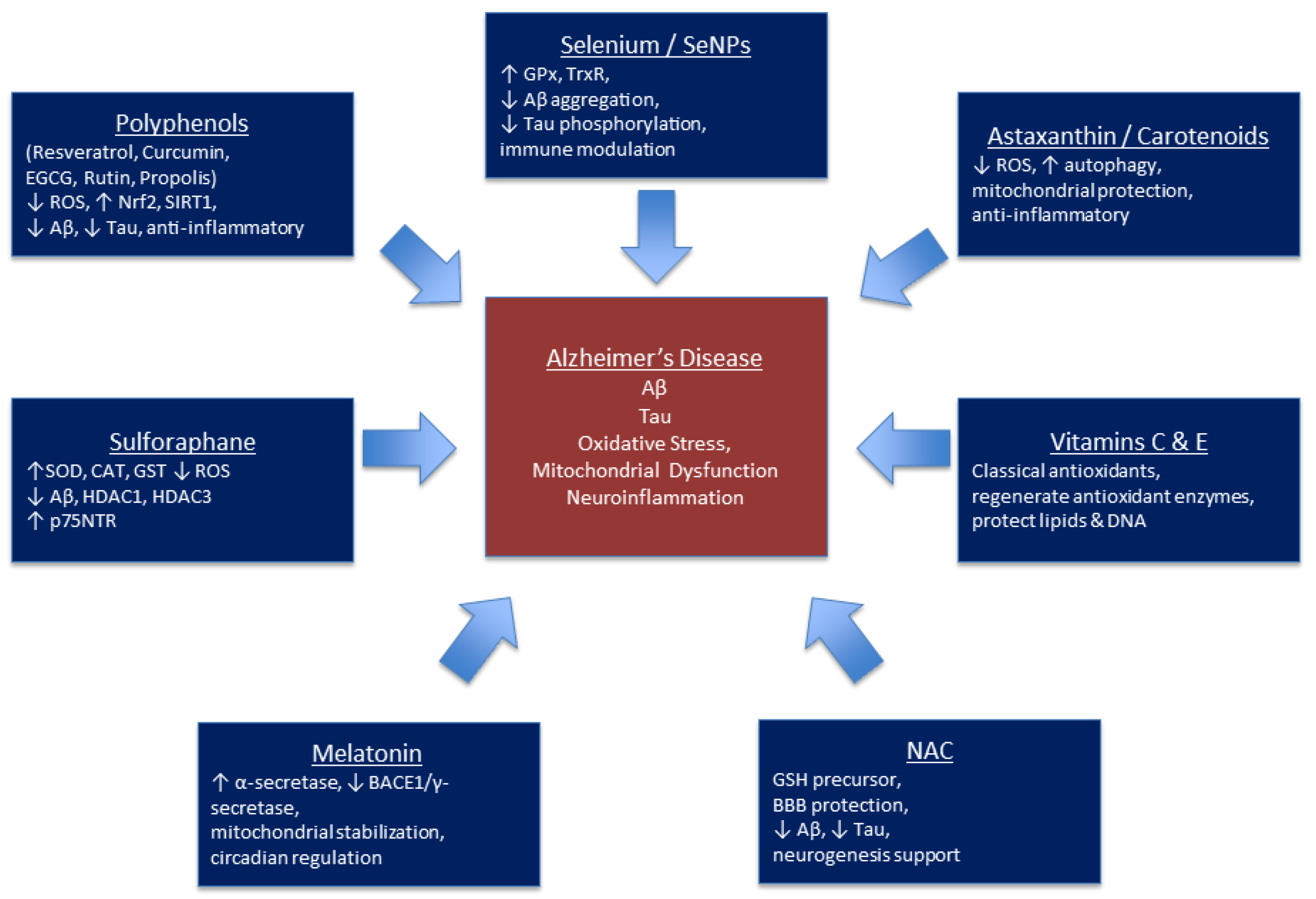
4. Natural Compounds and Diet
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Twiss, E.; McPherson, C.; Weaver, D.F. Global Diseases Deserve Global Solutions: Alzheimer’s Disease. Neurol. Int. 2025, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative Stress, Amyloid-Β Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1345–1367. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of Redox Regulation in Biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719. [Google Scholar] [CrossRef]
- Jones, D.P. Redox Sensing: Orthogonal Control in Cell Cycle and Apoptosis Signalling. J. Intern. Med. 2010, 268, 432–448. [Google Scholar] [CrossRef]
- Calabrò, M.; Rinaldi, C.; Santoro, G.; Crisafulli, C. The Biological Pathways of Alzheimer Disease: A Review. AIMS Neurosci. 2020, 8, 86–132. [Google Scholar] [CrossRef]
- Kołodziejska, R.; Woźniak, A.; Bilski, R.; Wesołowski, R.; Kupczyk, D.; Porzych, M.; Wróblewska, W.; Pawluk, H. Melatonin—A Powerful Oxidant in Neurodegenerative Diseases. Antioxidants 2025, 14, 819. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Mamun, A.A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Ashraf, G.M.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination Drug Therapy for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3272. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Neuroprotective Function of Rasagiline and Selegiline, Inhibitors of Type B Monoamine Oxidase, and Role of Monoamine Oxidases in Synucleinopathies. Int. J. Mol. Sci. 2022, 23, 11059. [Google Scholar] [CrossRef]
- Collins, A.E.; Saleh, T.M.; Kalisch, B.E. Naturally Occurring Antioxidant Therapy in Alzheimer’s Disease. Antioxidants 2022, 11, 213. [Google Scholar] [CrossRef]
- Chen, G.; Su, Y.; Chen, S.; Lin, T.; Lin, X. Polyphenols and Alzheimer’s Disease: A Review on Molecular and Therapeutic Insights with In Silico Support. Food Sci. Nutr. 2025, 13, e70496. [Google Scholar] [CrossRef]
- Hu, L.; Tao, Y.; Jiang, Y.; Qin, F. Recent Progress of Nanomedicine in the Treatment of Alzheimer’s Disease. Front. Cell Dev. Biol. 2023, 11, 1228679. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef] [PubMed]
- La Torre, A.; Lo Vecchio, F.; Angelillis, V.S.; Gravina, C.; D’Onofrio, G.; Greco, A. Reinforcing NRF2 Signaling: Help in the Alzheimer’s Disease Context. Int. J. Mol. Sci. 2025, 26, 1130. [Google Scholar] [CrossRef] [PubMed]
- Plascencia-Villa, G.; Perry, G. Preventive and Therapeutic Strategies in Alzheimer’s Disease: Focus on Oxidative Stress, Redox Metals, and Ferroptosis. Antioxid. Redox Signal. 2020, 34, 591–610. [Google Scholar] [CrossRef]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Role of Oxidative Stress in Alzheimer’s Disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Beiyu, Z.; Rong, Z.; Yi, Z.; Shan, W.; Peng, L.; Meng, W.; Wei, P.; Ye, Y.; Qiumin, Q. Oxidative Stress Is Associated with Aβ Accumulation in Chronic Sleep Deprivation Model. Brain Res. 2024, 1829, 148776. [Google Scholar] [CrossRef]
- Ehrlich, D.; Hochstrasser, T.; Humpel, C. Effects of Oxidative Stress on Amyloid Precursor Protein Processing in Rat and Human Platelets. Platelets 2012, 24, 26–36. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2017, 14, 450–464. [Google Scholar] [CrossRef]
- Roy, R.G.; Mandal, P.K.; Maroon, J.C. Oxidative Stress Occurs Prior to Amyloid AΒ Plaque Formation and TAU Phosphorylation in Alzheimer’s Disease: Role of Glutathione and Metal Ions. ACS Chem. Neurosci. 2023, 14, 2944–2954. [Google Scholar] [CrossRef]
- Squitti, R.; Faller, P.; Hureau, C.; Granzotto, A.; White, A.R.; Kepp, K.P. Copper Imbalance in Alzheimer’s Disease and Its Link with the Amyloid Hypothesis: Towards a Combined Clinical, Chemical, and Genetic Etiology. J. Alzheimer’s Dis. 2021, 83, 23–41. [Google Scholar] [CrossRef]
- Ayton, S.; Portbury, S.; Kalinowski, P.; Agarwal, P.; Diouf, I.; Schneider, J.A.; Morris, M.C.; Bush, A.I. Regional Brain Iron Associated with Deterioration in Alzheimer’s Disease: A Large Cohort Study and Theoretical Significance. Alzheimer’s Dement. 2021, 17, 1244–1256. [Google Scholar] [CrossRef]
- Kheiri, G.; Dolatshahi, M.; Rahmani, F.; Rezaei, N. Role of P38/MAPKs in Alzheimer’s Disease: Implications for Amyloid Beta Toxicity Targeted Therapy. Rev. Neurosci. 2018, 30, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Detka, J.; Płachtij, N.; Strzelec, M.; Manik, A.; Sałat, K. P38α Mitogen-Activated Protein Kinase—An Emerging Drug Target for the Treatment of Alzheimer’s Disease. Molecules 2024, 29, 4354. [Google Scholar] [CrossRef] [PubMed]
- Fułek, M.; Hachiya, N.; Gachowska, M.; Beszłej, J.A.; Bartoszewska, E.; Kurpas, D.; Kurpiński, T.; Adamska, H.; Poręba, R.; Urban, S.; et al. Cellular Prion Protein and Amyloid-Β Oligomers in Alzheimer’s Disease—Are There Connections? Int. J. Mol. Sci. 2025, 26, 2097. [Google Scholar] [CrossRef] [PubMed]
- Peña-Bautista, C.; Tirle, T.; López-Nogueroles, M.; Vento, M.; Baquero, M.; Cháfer-Pericás, C. Oxidative Damage of DNA as Early Marker of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 6136. [Google Scholar] [CrossRef]
- Mizuno, Y.; Abolhassani, N.; Mazzei, G.; Saito, T.; Saido, T.C.; Yamasaki, R.; Kira, J.-I.; Nakabeppu, Y. Deficiency of MTH1 and/or OGG1 Increases the Accumulation of 8-Oxoguanine in the Brain of the AppNL-G-F/NL-G-F Knock-in Mouse Model of Alzheimer’s Disease, Accompanied by Accelerated Microgliosis and Reduced Anxiety-like Behavior. Neurosci. Res. 2021, 177, 118–134. [Google Scholar] [CrossRef]
- Kim, S.; Jung, U.J.; Kim, S.R. Role of Oxidative Stress in Blood–Brain Barrier Disruption and Neurodegenerative Diseases. Antioxidants 2024, 13, 1462. [Google Scholar] [CrossRef]
- Cai, Z.; Qiao, P.-F.; Wan, C.-Q.; Cai, M.; Zhou, N.-K.; Li, Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 63, 1223–1234. [Google Scholar] [CrossRef]
- Kerr, F.; Sofola-Adesakin, O.; Ivanov, D.K.; Gatliff, J.; Perez-Nievas, B.G.; Bertrand, H.C.; Martinez, P.; Callard, R.; Snoeren, I.; Cochemé, H.M.; et al. Direct Keap1-Nrf2 Disruption as a Potential Therapeutic Target for Alzheimer’s Disease. PLoS Genet. 2017, 13, e1006593. [Google Scholar] [CrossRef] [PubMed]
- Akanji, M.A.; Rotimi, D.E.; Elebiyo, T.C.; Awakan, O.J.; Adeyemi, O.S. Redox Homeostasis and Prospects for Therapeutic Targeting in Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2021, 2021, 9971885. [Google Scholar] [CrossRef] [PubMed]
- Casado, Á.; López-Fernández, M.E.; Casado, M.C.; de La Torre, R. Lipid Peroxidation and Antioxidant Enzyme Activities in Vascular and Alzheimer Dementias. Neurochem. Res. 2007, 33, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, Z.; Eren, N.; Orcun, A.; Gokyigit, F.M.; Turgay, F.; Celebi, L.G. Serum Apelin-13 Levels and Total Oxidant/Antioxidant Status of Patients with Alzheimer’s Disease. Aging Med. 2021, 4, 201–205. [Google Scholar] [CrossRef]
- Socha, K.; Klimiuk, K.; Naliwajko, S.K.; Soroczyńska, J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Kochanowicz, J. Dietary Habits, Selenium, Copper, Zinc and Total Antioxidant Status in Serum in Relation to Cognitive Functions of Patients with Alzheimer’s Disease. Nutrients 2021, 13, 287. [Google Scholar] [CrossRef]
- Nie, Y.; Chu, C.; Qin, Q.; Shen, H.; Wen, L.; Tang, Y.; Qu, M. Lipid Metabolism and Oxidative Stress in Patients with Alzheimer’s Disease and Amnestic Mild Cognitive Impairment. Brain Pathol. 2023, 34, e13202. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Reed, T.; Perluigi, M.; De Marco, C.; Coccia, R.; Cini, C.; Sultana, R. Elevated Protein-Bound Levels of the Lipid Peroxidation Product, 4-Hydroxy-2-Nonenal, in Brain from Persons with Mild Cognitive Impairment. Neurosci. Lett. 2006, 397, 170–173. [Google Scholar] [CrossRef]
- Keller, J.N.; Schmitt, F.A.; Scheff, S.W.; Ding, Q.; Chen, Q.; Butterfield, D.A.; Markesbery, W.R. Evidence of Increased Oxidative Damage in Subjects with Mild Cognitive Impairment. Neurology 2005, 64, 1152–1156. [Google Scholar] [CrossRef]
- de Toda, I.M.; Miguélez, L.; Vida, C.; Carro, E.; de La Fuente, M. Altered Redox State in Whole Blood Cells from Patients with Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 71, 153–163. [Google Scholar] [CrossRef]
- Ansari, M.A.; Scheff, S.W. Oxidative Stress in the Progression of Alzheimer Disease in the Frontal Cortex. J. Neuropathol. Exp. Neurol. 2010, 69, 155–167. [Google Scholar] [CrossRef]
- Reed, T.T.; Pierce, W.M.; Markesbery, W.R.; Butterfield, D.A. Proteomic Identification of HNE-Bound Proteins in Early Alzheimer Disease: Insights into the Role of Lipid Peroxidation in the Progression of AD. Brain Res. 2009, 1274, 66–76. [Google Scholar] [CrossRef]
- Chen, J.J.; Thiyagarajah, M.; Song, J.; Chen, C.; Herrmann, N.; Gallagher, D.; Rapoport, M.J.; Black, S.E.; Ramirez, J.; Andreazza, A.C.; et al. Altered Central and Blood Glutathione in Alzheimer’s Disease and Mild Cognitive Impairment: A Meta-Analysis. Alzheimer’s Res. Ther. 2022, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Hervé, V.; Khedher, M.R.B.; Rabanel, J.-M.; Ramassamy, C. Glutathione: An Old and Small Molecule with Great Functions and New Applications in the Brain and in Alzheimer’s Disease. Antioxid. Redox Signal. 2021, 35, 270–292. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.E.; De Souza, J.V.; Gomar, G.G.; Kruk, I.L.; Oliveira, C.S. Glutathione Peroxidase Activity in Alzheimer’s Disease Patients: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2025, 106, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Altunoglu, E.; Guntas, G.; Erdenen, F.; Akkaya, E.; Topac, I.; Irmak, H.; Derici, H.; Yavuzer, H.; Gelisgen, R.; Uzun, H. Ischemia-modified Albumin and Advanced Oxidation Protein Products as Potential Biomarkers of Protein Oxidation in Alzheimer’s Disease. Geriatr. Gerontol. Int. 2014, 15, 872–880. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pandi-Perumal, S.; Cardinali, D.; Poeggeler, B.; Hardeland, R. Melatonin in Alzheimer’s Disease and Other Neurodegenerative Disorders. Behav. Brain Funct. 2006, 2, 15. [Google Scholar] [CrossRef]
- Nous, A.; Engelborghs, S.; Smolders, I. Melatonin Levels in the Alzheimer’s Disease Continuum: A Systematic Review. Alzheimer’s Res. Ther. 2021, 13, 52. [Google Scholar] [CrossRef]
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016, 16, 247–253. [Google Scholar] [CrossRef]
- Li, D.D.; Zhang, Y.H.; Zhang, W.; Zhao, P. Meta-analysis of randomized controlled trials on the efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease. Front. Neurosci. 2019, 13, 472. [Google Scholar] [CrossRef]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2024. Transl. Res. Clin. Interv. 2024, 10, e12465. [Google Scholar] [CrossRef]
- Cummings, J.; Fox, N. Defining disease modifying therapy for Alzheimer’s disease. J. Prev. Alzheimer’s Dis. 2017, 4, 109–115. [Google Scholar] [CrossRef]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment combinations for Alzheimer’s disease: Current and future pharmacotherapy options. J. Alzheimer’s Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef]
- Tong, J.; Meyer, J.H.; Furukawa, Y.; Boileau, I.; Chang, L.-J.; Wilson, A.A.; Houle, S. Distribution of monoamine oxidase proteins in human brain: Implications for brain imaging studies. J. Cereb. Blood Flow Metab. 2013, 33, 863–871. [Google Scholar] [CrossRef]
- Chaurasiya, N.D.; Leon, F.; Muhammad, I.; Tekwani, B.L. Natural Products Inhibitors of Monoamine Oxidases—Potential New Drug Leads for Neuroprotection, Neurological Disorders, and Neuroblastoma. Molecules 2022, 27, 4297. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, M.; Chen, K.; Shih, J.C. Monoamine oxidase inactivation: From pathophysiology to therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Hattori, N.; Kondo, T.; Nomoto, M.; Origasa, H.; Takahashi, R.; Yamamoto, M.; Yanagisawa, N. A Randomized Double-Blind Placebo-Controlled Phase III Trial of Selegiline Monotherapy for Early Parkinson Disease. Clin. Neuropharmacol. 2017, 40, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Takeda, A.; Takeda, S.; Nishimura, A.; Kitagawa, T.; Mochizuki, H.; Nagai, M.; Takahashi, R. Rasagiline monotherapy in early Parkinson’s disease: A phase 3, randomized study in Japan. Park. Relat. Disord. 2019, 60, 146–152. [Google Scholar] [CrossRef]
- Bodkin, J.A.; Amsterdam, J.D. Transdermal selegiline in major depression: A double-blind, placebo-controlled, parallel-group study in outpatients. Am. J. Psychiatry 2002, 159, 1869–1875. [Google Scholar] [CrossRef]
- Basir, H.S.; Mirazi, N.; Komaki, A.; Mohamadpour, B.; Hosseini, A. Selegiline improves cognitive impairment in the rat model of Alzheimer’s disease. Mol. Neurobiol. 2024, 62, 2548–2560. [Google Scholar] [CrossRef]
- Cai, M.; Yang, E.J. Effect of combined electroacupuncture and selegiline treatment in Alzheimer’s disease: An animal model. Front. Pharmacol. 2020, 11, 606480. [Google Scholar] [CrossRef]
- Filip, V.; Kolibás, E. Selegiline in the treatment of Alzheimer’s disease: A long-term randomized placebo-controlled trial. Czech and Slovak Senile Dementia of Alzheimer Type Study Group. J. Psychiatry Neurosci. 1999, 24, 234–243. [Google Scholar] [PubMed Central]
- Alafuzoff, I.; Helisalmi, S.; Heinonen, E.H.; Reinikainen, K.; Hallikainen, M.; Soininen, H.; Koivisto, K. Selegiline treatment and the extent of degenerative changes in brain tissue of patients with Alzheimer’s disease. Eur. J. Clin. Pharmacol. 2000, 55, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, O.; Badinter, F.; Amit, T.; Bar-Am, O.; Youdim, M.B.H. Effect of long-term treatment with rasagiline on cognitive deficits and neuroprotection in aged mice. Neurobiol. Aging 2015, 36, 2628–2636. [Google Scholar] [CrossRef] [PubMed]
- Song, I.U.; Im, J.J.; Jeong, H.; Na, S.H.; Chung, Y.A. Possible neuroprotective effects of rasagiline in Alzheimer’s disease: A SPECT study. Acta Radiol. 2020, 62, 784–790. [Google Scholar] [CrossRef]
- Youdim, M.B.H.; Bar Am, O.; Yogev-Falach, M.; Weinreb, O.; Maruyama, W.; Naoi, M.; Amit, T. Rasagiline: Neurodegeneration, neuroprotection, and mitochondrial permeability transition. J. Neurosci. Res. 2005, 79, 172–179. [Google Scholar] [CrossRef]
- Weinstock, M.; Luques, L.; Poltyrev, T.; Bejar, C.; Shoham, S. Ladostigil prevents age-related glial activation and spatial memory deficits in rats. Neurobiol. Aging 2011, 32, 1069–1078. [Google Scholar] [CrossRef]
- Linial, M.; Stern, A.; Weinstock, M. Effect of ladostigil treatment of aging rats on gene expression in four brain areas associated with regulation of memory. Neuropharm. 2020, 177, 108229. [Google Scholar] [CrossRef]
- Schneider, L.S.; Geffen, Y.; Rabinowitz, J.; Thomas, R.G.; Schmidt, R.; Ropele, S.; Weinstock, M. Low-dose ladostigil for mild cognitive impairment. A phase 2 placebo-controlled clinical trial. Neuropharmacology 2019, 93, e1474–e1484. [Google Scholar] [CrossRef]
- Finberg, J.P.; Gillman, K. Selective inhibitors of monoamine oxidase type B and the “cheese effect”. Int. Rev. Neurobiol. 2011, 100, 169–190. [Google Scholar] [CrossRef]
- Suphanklang, J.; Santimaleeworagun, W.; Supasyndh, O. Combination of Escitalopram and Rasagiline Induced Serotonin Syndrome: A Case Report and Review Literature. J. Med. Assoc. Thai. 2015, 98, 1254–1257. [Google Scholar]
- Nemet, M.; Andrijević, A.; Nedeljkov, Đ.; Andrić, V.; Gavrilović, S. A Case Report on Serotonin Syndrome in a Patient With Parkinson’s Disease: Diagnostic and Management Challenges. Cureus 2023, 15, e36780. [Google Scholar] [CrossRef]
- Hisham, M.; Sivakumar, M.N.; Nandakumar, V.; Lakshmikanthcharan, S. Linezolid and Rasagiline—A culprit for serotonin syndrome. Indian J. Pharmacol. 2016, 48, 91–92. [Google Scholar] [CrossRef]
- Panisset, M.; Chen, J.J.; Rhyee, S.H.; Conner, J.; Mathena, J.; STACCATO Study Investigators. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: Retrospective study (STACCATO). Pharmacotherapy 2014, 34, 1250–1258. [Google Scholar] [CrossRef]
- Yu, T.-W.; Lane, H.-Y.; Lin, C.-H. Novel Therapeutic Approaches for Alzheimer’s Disease: An Updated Review. Int. J. Mol. Sci. 2021, 22, 8208. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Mroczko, B. New Possibilities in the Therapeutic Approach to Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 8902. [Google Scholar] [CrossRef] [PubMed]
- Tolar, M.; Abushakra, S.; Hey, J.A.; Porsteinsson, A.; Sabbagh, M. Aducanumab, Gantenerumab, BAN2401, and ALZ-801—The First Wave of Amyloid-Targeting Drugs for Alzheimer’s Disease with Potential for near Term Approval. Alzheimer’s Res. Ther. 2020, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Nery, E.S.M.; et al. Donanemab in Early Symptomatic Alzheimer Disease. JAMA 2023, 330, 512. [Google Scholar] [CrossRef] [PubMed]
- Harris, E. Alzheimer Drug Lecanemab Gains Traditional FDA Approval. JAMA 2023, 330, 495. [Google Scholar] [CrossRef]
- Panza, F.; Solfrizzi, V.; Seripa, D.; Imbimbo, B.P.; Lozupone, M.; Santamato, A.; Tortelli, R.; Galizia, I.; Prete, C.; Daniele, A.; et al. Tau-Based Therapeutics for Alzheimer’s Disease: Active and Passive Immunotherapy. Immunotherapy 2016, 8, 1119–1134. [Google Scholar] [CrossRef]
- Congdon, E.E.; Sigurdsson, E.M. Tau-Targeting Therapies for Alzheimer Disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef]
- Hoskin, J.L.; Sabbagh, M.N.; Al-Hasan, Y.; Decourt, B. Tau Immunotherapies for Alzheimer’s Disease. Expert Opin. Investig. Drugs 2019, 28, 545–554. [Google Scholar] [CrossRef]
- Choi, M.; Kim, H.; Yang, E.-J.; Kim, H.-S. Inhibition of STAT3 Phosphorylation Attenuates Impairments in Learning and Memory in 5XFAD Mice, an Animal Model of Alzheimer’s Disease. J. Pharmacol. Sci. 2020, 143, 290–299. [Google Scholar] [CrossRef]
- Millot, P.; San, C.; Bennana, E.; Porte, B.; Vignal, N.; Hugon, J.; Paquet, C.; Hosten, B.; Mouton-Liger, F. STAT3 Inhibition Protects against Neuroinflammation and BACE1 Upregulation Induced by Systemic Inflammation. Immunol. Lett. 2020, 228, 129–134. [Google Scholar] [CrossRef]
- Olmos-Alonso, A.; Schetters, S.T.T.; Sri, S.; Askew, K.; Mancuso, R.; Vargas-Caballero, M.; Holscher, C.; Perry, V.H.; Gomez-Nicola, D. Pharmacological Targeting of CSF1R Inhibits Microglial Proliferation and Prevents the Progression of Alzheimer’s-like Pathology. Brain 2016, 139, 891–907. [Google Scholar] [CrossRef]
- Mancuso, R.; Fryatt, G.; Cleal, M.; Obst, J.; Pipi, E.; Monzón-Sandoval, J.; Ribe, E.; Winchester, L.; Webber, C.; Nevado, A.; et al. CSF1R Inhibitor JNJ-40346527 Attenuates Microglial Proliferation and Neurodegeneration in P301S Mice. Brain 2019, 142, 3243–3264. [Google Scholar] [CrossRef]
- Sosna, J.; Philipp, S.; Albay, R.; Reyes-Ruiz, J.M.; Baglietto-Vargas, D.; LaFerla, F.M.; Glabe, C.G. Early Long-Term Administration of the CSF1R Inhibitor PLX3397 Ablates Microglia and Reduces Accumulation of Intraneuronal Amyloid, Neuritic Plaque Deposition and Pre-Fibrillar Oligomers in 5XFAD Mouse Model of Alzheimer’s Disease. Mol. Neurodegener. 2018, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Gejl, M.; Gjedde, A.; Egefjord, L.; Møller, A.; Hansen, S.B.; Vang, K.; Rodell, A.; Brændgaard, H.; Gottrup, H.; Schacht, A.; et al. In Alzheimer’s Disease, 6-Month Treatment with GLP-1 Analog Prevents Decline of Brain Glucose Metabolism: Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Front. Aging Neurosci. 2016, 8, 198350. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chen, P.-K.; Chang, Y.-C.; Chuo, L.-J.; Chen, Y.-S.; Tsai, G.E.; Lane, H.-Y. Benzoate, a D-Amino Acid Oxidase Inhibitor, for the Treatment of Early-Phase Alzheimer Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Psychiatry 2013, 75, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chen, P.-K.; Wang, S.-H.; Lane, H.-Y. Effect of Sodium Benzoate on Cognitive Function among Patients with Behavioral and Psychological Symptoms of Dementia. JAMA Netw. Open 2021, 4, e216156. [Google Scholar] [CrossRef]
- Georgieva, D.; Nikolova, D.; Vassileva, E.; Kostova, B. Chitosan-Based Nanoparticles for Targeted Nasal Galantamine Delivery as a Promising Tool in Alzheimer’s Disease Therapy. Pharmaceutics 2023, 15, 829. [Google Scholar] [CrossRef]
- Roghani, A.K.; Garcia, R.I.; Roghani, A.; Reddy, A.; Khemka, S.; Reddy, R.P.; Pattoor, V.; Jacob, M.; Reddy, P.H.; Sehar, U. Treating Alzheimer’s Disease Using Nanoparticle-Mediated Drug Delivery Strategies/Systems. Ageing Res. Rev. 2024, 97, 102291. [Google Scholar] [CrossRef]
- Liu, L.; He, H.; Du, B.; He, Y. Nanoscale Drug Formulations for the Treatment of Alzheimer’s Disease Progression. RSC Adv. 2025, 15, 4031–4078. [Google Scholar] [CrossRef]
- Bhatia, S.; Singh, M.; Singh, T.; Singh, V. Scrutinizing the Therapeutic Potential of PROTACs in the Management of Alzheimer’s Disease. Neurochem. Res. 2022, 48, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Inuzuka, H.; Liu, J.; Wei, W.; Rezaeian, A.-H. PROTAC Technology for the Treatment of Alzheimer’s Disease: Advances and Perspectives. Acta Mater. Medica 2022, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Meng, L.; Lin, P.; Wu, G. Advancements in PROTAC-Based Therapies for Neurodegenerative Diseases. Future Med. Chem. 2025, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.S.; Price, S.; Wu, M.; Parmar, M.S. Emerging Gene Therapies for Alzheimer’s and Parkinson’s Diseases: An Overview of Clinical Trials and Promising Candidates. Cureus 2024, 16, e67037. [Google Scholar] [CrossRef]
- Morroni, F.; Caccamo, A. Advances and Challenges in Gene Therapy for Alzheimer’s Disease. J. Alzheimer’s Dis. 2024, 101, S417–S431. [Google Scholar] [CrossRef]
- Ortega, A.; Chernicki, B.; Ou, G.; Parmar, M.S. From Lab Bench to Hope: Emerging Gene Therapies in Clinical Trials for Alzheimer’s Disease. Mol. Neurobiol. 2024, 62, 1112–1135. [Google Scholar] [CrossRef]
- Sidiropoulou, G.A.; Metaxas, A.; Kourti, M. Natural antioxidants that act against Alzheimer’s disease through modulation of the NRF2 pathway: A focus on their molecular mechanisms of action. Frontiers 2023, 14, 1664–2392. [Google Scholar] [CrossRef]
- Kong, D.; Yan, Y.; He, X.Y.; Yang, H.; Liang, B.; Wang, J.; He, Y.; Ding, Y.; Yu, H. Effects of Resveratrol on the Mechanisms of Antioxidants and Estrogen in Alzheimer’s Disease. BioMed Res. Int. 2019, 1, 2314–6133. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin—A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.-R.; Brumaghim, J.-L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Grundman, M. Vitamin E and Alzheimer disease: The basis for additional clinical trials. Am. J. Clin. Nutr. 2000, 71, 630–636. [Google Scholar] [CrossRef]
- Wörtwein, G.; Stackman, R.W.; Walsh, T.J. Vitamin E Prevents the Place Learning Deficit and the Cholinergic Hypofunction Induced by AF64A. Exp. Neurol. 1994, 125, 15–21. [Google Scholar] [CrossRef]
- Hamid, M.; Mansoor, S.; Amber, S.; Zahid, S. A quantitative meta-analysis of vitamin C in the pathophysiology of Alzheimer’s disease. Frontiers 2022, 14, 1663–4365. [Google Scholar] [CrossRef]
- Akbari, A.; Jelodar, G.; Nazifi, S.; Sajedianfard, J. An Overview of the Characteristics and Function of Vitamin C in Various Tissues: Relying on its Antioxidant Function. Zahedan J. Res. Med. Sci. 2016, 18, 4037. [Google Scholar] [CrossRef]
- Nowak, D.; Gośliński, M.; Wojtowicz, E.; Przygoński, K. Antioxidant Properties and Phenolic Compounds of Vitamin C-Rich Juices. J. Food Sci. 2018, 83, 2237–2246. [Google Scholar] [CrossRef]
- Singh, N.A.; Mandal, A.K.A.; Khan, Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 2016, 15, 1475–2891. [Google Scholar] [CrossRef]
- Nan, S.; Wang, P.; Zhang, Y.; Fan, J. Epigallocatechin-3-Gallate Provides Protection against Alzheimer’s Disease-Induced Learning and Memory Impairments in Rats. Drug Des. Devel. Ther. 2021, 15, 2013–2024. [Google Scholar] [CrossRef]
- Necip, A.; Demirtas, I.; Tayhan, S.E.; Işık, M.; Bilgin, S.; Turan, İ.F.; İpek, Y.; Beydemir, Ş. Isolation of phenolic compounds from eco-friendly white bee propolis: Antioxidant, wound-healing, and anti-Alzheimer effects. Food Sci. Nutr. 2024, 12, 1928–1939. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Res. Ther. 2012, 4, 1758–9193. [Google Scholar] [CrossRef] [PubMed]
- Nicola, M.A.; Attaai, A.H.; Abdel-Raheem, M.H.; Mohammed, A.F.; Abu-Elhassan, Y.F. Neuroprotective Effects of Rutin against Cuprizone-Induced Multiple Sclerosis in Mice. Inflammopharmacology 2024, 32, 1295–1315. [Google Scholar] [CrossRef] [PubMed]
- Saputri, L.O.; Permatasari, L.; Harahap, H.S.; Rosyidi, R.M.; Rivarti, A.W.; Prihatina, L.M.; Rahayu, Z.; Azariani, W. Potential of Rutin from Rhizophora Mucronata Leaves as a Inhibitor of Kelch-like ECH-Associated Protein 1/Nuclear Factor Erythroid 2 Related Factor 2 Keap1/Nrf2): An in Silico Study for Alzheimer’s Therapy. J. Adv. Pharm. Technol. Res. 2025, 16, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Khan, M.M.; Ahmad, A.; Vaibhav, K.; Ahmad, M.E.; Khan, A.; Ashafaq, M.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. Rutin Prevents Cognitive Impairments by Ameliorating Oxidative Stress and Neuroinflammation in Rat Model of Sporadic Dementia of Alzheimer Type. Neuroscience 2012, 210, 340–352. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Li, L.-J.; Dong, Q.-X.; Zhu, J.; Huang, Y.-R.; Hou, S.-J.; Yu, X.-L.; Liu, R.-T. Rutin Prevents Tau Pathology and Neuroinflammation in a Mouse Model of Alzheimer’s Disease. J. Neuroinflamm. 2021, 18, 131. [Google Scholar] [CrossRef]
- Kim, J. Pre-Clinical Neuroprotective Evidences and Plausible Mechanisms of Sulforaphane in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2929. [Google Scholar] [CrossRef]
- Fahey, J.W.; Liu, H.; Batt, H.; Panjwani, A.A.; Tsuji, P. Sulforaphane and Brain Health: From Pathways of Action to Effects on Specific Disorders. Nutrients 2025, 17, 1353. [Google Scholar] [CrossRef]
- Lee, S.; Choi, B.; Kim, J.; LaFerla, F.M.; Park, J.H.Y.; Han, J.; Lee, K.W.; Kim, J. Sulforaphane Upregulates the Heat Shock Protein Co-Chaperone CHIP and Clears amyloid-Β and TAU in a Mouse Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2018, 62, 1800240. [Google Scholar] [CrossRef]
- Bahn, G.; Park, J.-S.; Yun, U.J.; Lee, Y.J.; Choi, Y.; Park, J.S.; Baek, S.H.; Choi, B.Y.; Cho, Y.S.; Kim, H.K.; et al. NRF2/ARE Pathway Negatively Regulates BACE1 Expression and Ameliorates Cognitive Deficits in Mouse Alzheimer’s Models. Proc. Natl. Acad. Sci. USA 2019, 116, 12516–12523. [Google Scholar] [CrossRef]
- Hou, T.-T.; Yang, H.-Y.; Wang, W.; Wu, Q.-Q.; Tian, Y.-R.; Jia, J.-P. Sulforaphane Inhibits the Generation of Amyloid-Β Oligomer and Promotes Spatial Learning and Memory in Alzheimer’s Disease (PS1V97L) Transgenic Mice. J. Alzheimer’s Dis. 2018, 62, 1803–1813. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Zhan, Z.; Li, X.; Zhou, F.; Xing, A.; Jiang, C.; Chen, Y.; An, L. Beneficial Effects of Sulforaphane Treatment in Alzheimer’s Disease May Be Mediated through Reduced HDAC1/3 and Increased P75NTR Expression. Front. Aging Neurosci. 2017, 9, 121. [Google Scholar] [CrossRef]
- Vu, G.H.; Nguyen, H.D. Molecular Mechanisms of Sulforaphane in Alzheimer’s Disease: Insights from an in-Silico Study. Silico Pharmacol. 2024, 12, 96. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, D.; Wu, W.; Shah, S.Z.A.; Lai, M.; Yang, D.; Li, J.; Guan, Z.; Li, W.; Gao, H.; et al. Melatonin Regulates Mitochondrial Dynamics and Alleviates Neuron Damage in Prion Diseases. Aging 2020, 12, 11139–11151. [Google Scholar] [CrossRef]
- Spinedi, E.; Cardinali, D.P. Neuroendocrine-Metabolic Dysfunction and Sleep Disturbances in Neurodegenerative Disorders: Focus on Alzheimer’s Disease and Melatonin. Neuroendocrinology 2018, 108, 354–364. [Google Scholar] [CrossRef]
- Steinbach, M.J.; Denburg, N.L. Melatonin in Alzheimer’s Disease: Literature Review and Therapeutic Trials. J. Alzheimer’s Dis. 2024, 101, S193–S204. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 1010–1031. [Google Scholar] [CrossRef] [PubMed]
- Tiong, Y.L.; Ng, K.Y.; Koh, R.Y.; Ponnudurai, G.; Chye, S.M. Melatonin Prevents Oxidative Stress-Induced Mitochondrial Dysfunction and Apoptosis in High Glucose-Treated Schwann Cells via Upregulation of BCL2, NF-ΚB, MTOR, WNT Signalling Pathways. Antioxidants 2019, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, X.; Li, S.; Sun, B.; Hang, C. Melatonin Treatment Regulates SIRT3 Expression in Early Brain Injury (EBI) Due to Reactive Oxygen Species (ROS) in a Mouse Model of Subarachnoid Hemorrhage (SAH). Med. Sci. Monit. 2018, 24, 3804–3814. [Google Scholar] [CrossRef]
- Fan, R.; Peng, X.; Xie, L.; Dong, K.; Ma, D.; Xu, W.; Shi, X.; Zhang, S.; Chen, J.; Yu, X.; et al. Importance of Bmal1 in Alzheimer’s Disease and Associated Aging-related Diseases: Mechanisms and Interventions. Aging Cell 2022, 21, e13704. [Google Scholar] [CrossRef]
- Furtado, A.; Astaburuaga, R.; Costa, A.; Duarte, A.C.; Gonçalves, I.; Cipolla-Neto, J.; Lemos, M.C.; Carro, E.; Relógio, A.; Santos, C.R.A.; et al. The Rhythmicity of Clock Genes Is Disrupted in the Choroid Plexus of the APP/PS1 Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 77, 795–806. [Google Scholar] [CrossRef]
- Thangwong, P.; Jearjaroen, P.; Tocharus, C.; Govitrapong, P.; Tocharus, J. Melatonin Suppresses Inflammation and Blood—Brain Barrier Disruption in Rats with Vascular Dementia Possibly by Activating the SIRT1/PGC-1α/PPARγ Signaling Pathway. Inflammopharmacology 2023, 31, 1481–1493. [Google Scholar] [CrossRef]
- Song, J.; Whitcomb, D.J.; Kim, B.C. The Role of Melatonin in the Onset and Progression of Type 3 Diabetes. Mol. Brain 2017, 10, 35. [Google Scholar] [CrossRef]
- Burillo, J.; Marqués, P.; Jiménez, B.; González-Blanco, C.; Benito, M.; Guillén, C. Insulin Resistance and Diabetes Mellitus in Alzheimer’s Disease. Cells 2021, 10, 1236. [Google Scholar] [CrossRef]
- Mehramiz, M.; Porter, T.; Laws, S.M.; Rainey-Smith, S.R. Sleep, Sirtuin 1 and Alzheimer’s Disease: A Review. Aging Brain 2022, 2, 100050. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, T.; Lee, T.H. Cellular Mechanisms of Melatonin: Insight from Neurodegenerative Diseases. Biomolecules 2020, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Park, J.H.; Kim, D.W.; Park, J.; Choi, S.Y.; Kim, I.H.; Cho, J.H.; Lee, T.-K.; Lee, J.C.; Lee, C.-H.; et al. Melatonin Improves Cognitive Deficits via Restoration of Cholinergic Dysfunction in a Mouse Model of Scopolamine-Induced Amnesia. ACS Chem. Neurosci. 2017, 9, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Cachán-Vega, C.; Vega-Naredo, I.; Potes, Y.; Bermejo-Millo, J.C.; Rubio-González, A.; García-González, C.; Antuña, E.; Bermúdez, M.; Gutiérrez-Rodríguez, J.; Boga, J.A.; et al. Chronic Treatment with Melatonin Improves Hippocampal Neurogenesis in the Aged Brain and Under Neurodegeneration. Molecules 2022, 27, 5543. [Google Scholar] [CrossRef]
- Alkandari, A.F.; Madhyastha, S.; Rao, M.S. N-Acetylcysteine Amide against Aβ-Induced Alzheimer’s-like Pathology in Rats. Int. J. Mol. Sci. 2023, 24, 12733. [Google Scholar] [CrossRef]
- Ontawong, A.; Nehra, G.; Maloney, B.J.; Vaddhanaphuti, C.S.; Bauer, B.; Hartz, A.M.S. N-Acetylcysteine Attenuates AΒ-Mediated Oxidative Stress, Blood–Brain Barrier Leakage, and Renal Dysfunction in 5xFAD Mice. Int. J. Mol. Sci. 2025, 26, 4352. [Google Scholar] [CrossRef]
- Tenório, M.C.d.S.; Graciliano, N.G.; Moura, F.A.; de Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, S.; Zargooshnia, S.; Asl, S.S.; Komaki, A.; Sarihi, A. Influence of N-Acetyl Cysteine on Beta-Amyloid-Induced Alzheimer’s Disease in a Rat Model: A Behavioral and Electrophysiological Study. Brain Res. Bull. 2017, 131, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Joy, T.; Rao, M.S.; Madhyastha, S. N-Acetyl Cysteine Supplement Minimize TAU Expression and Neuronal Loss in Animal Model of Alzheimer’s Disease. Brain Sci. 2018, 8, 185. [Google Scholar] [CrossRef]
- Yulug, B.; Altay, O.; Li, X.; Hanoglu, L.; Cankaya, S.; Lam, S.; Velioglu, H.A.; Yang, H.; Coskun, E.; Idil, E.; et al. Combined Metabolic Activators Improve Cognitive Functions in Alzheimer’s Disease Patients: A Randomised, Double-Blinded, Placebo-Controlled Phase-II Trial. Transl. Neurodegener. 2023, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Martínez, S.M.L.; Moisés, F.P.P.; Bitzer-Quintero, O.K.; Ramírez-Jirano, J.; Delgado-Lara, D.L.C.; Trujillo, I.C.; Jasso, J.H.T.; Salazar-Flores, J.; Torres-Sánchez, E.D. Effect of N-Acetyl Cysteine as an Adjuvant Treatment in Alzheimer’s Disease. Brain Sci. 2025, 15, 164. [Google Scholar] [CrossRef]
- Babalola, J.A.; Lang, M.; George, M.; Stracke, A.; Tam-Amersdorfer, C.; Itxaso, I.; Lucija, D.; Tadic, J.; Schilcher, I.; Loeffler, T.; et al. Astaxanthin Enhances Autophagy, Amyloid Beta Clearance and Exerts Anti-Inflammatory Effects in in Vitro Models of Alzheimer’s Disease-Related Blood Brain Barrier Dysfunction and Inflammation. Brain Res. 2023, 1819, 148518. [Google Scholar] [CrossRef]
- Liu, N.; Lyu, X.; Zhang, X.; Zhang, F.; Chen, Y.; Li, G. Astaxanthin Attenuates Cognitive Deficits in Alzheimer’s Disease Models by Reducing Oxidative Stress via the SIRT1/PGC-1α Signaling Pathway. Cell Biosci. 2023, 13, 173. [Google Scholar] [CrossRef]
- Magadmi, R.; Nassibi, S.; Kamel, F.; Al-Rafiah, A.R.; Bakhshwin, D.; Jamal, M.; Alsieni, M.; Burzangi, A.S.; Zaher, M.a.F.; Bendary, M. The Protective Effect of Astaxanthin on Scopolamine—Induced Alzheimer’s Model in Mice. Neurosciences 2024, 29, 103–112. [Google Scholar] [CrossRef]
- Chik, M.W.; Affandi, M.M.R.M.M.; Hazalin, N.A.M.N.; Singh, G.K.S. Astaxanthin Nanoemulsion Improves Cognitive Function and Synaptic Integrity in Streptozotocin-Induced Alzheimer’s Disease Model. Metab. Brain Dis. 2025, 40, 136. [Google Scholar] [CrossRef]
- Balendra, V.; Singh, S.K. Therapeutic Potential of Astaxanthin and Superoxide Dismutase in Alzheimer’s Disease. Open Biol. 2021, 11, 210013. [Google Scholar] [CrossRef]
- Yazdi, F.R.; Taghizadeh, F.; Parvari, S.; Javanbakht, P.; Mojaverrostami, S.; Zarini, D.; Kashani, I.R. Astaxanthin as an Adjunct Therapy in Alzheimer’s Disease and Multiple Sclerosis: Neuroprotective Mechanisms and Future Perspective. Nutr. Neurosci. 2025, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Utomo, N.P.; Pinzon, R.T.; Latumahina, P.K.; Damayanti, K.R.S. Astaxanthin and Improvement of Dementia: A Systematic Review of Current Clinical Trials. Cereb. Circ. Cogn. Behav. 2024, 7, 100226. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.A.; Kamel, M.A.; Osman, M.Y.; Osman, H.M.; Elblehi, S.S.; Mahmoud, S.A. Ameliorative Effects of Astaxanthin on Brain Tissues of Alzheimer’s Disease-like Model: Cross Talk between Neuronal-Specific microRNA-124 and Related Pathways. Mol. Cell. Biochem. 2021, 476, 2233–2249. [Google Scholar] [CrossRef] [PubMed]
- Asl, F.D.; Mousazadeh, M.; Azimzadeh, M.; Ghaani, M.R. Mesoporous Selenium Nanoparticles for Therapeutic Goals: A Review. J. Nanoparticle Res. 2022, 24, 210. [Google Scholar] [CrossRef]
- Pyka, P.; Garbo, S.; Fioravanti, R.; Jacob, C.; Hittinger, M.; Handzlik, J.; Zwergel, C.; Battistelli, C. Selenium-Containing Compounds: A New Hope for Innovative Treatments in Alzheimer’s Disease and Parkinson’s Disease. Drug Discov. Today 2024, 29, 104062. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E. Unravelling the in Vitro and in Vivo Potential of Selenium Nanoparticles in Alzheimer’s Disease: A Bioanalytical Review. Talanta 2023, 269, 125519. [Google Scholar] [CrossRef]
- Seo, Y.; Gang, G.; Kim, H.K.; Kim, Y.; Kang, S.; Kim, H.; Lee, S.G.; Go, G.-w. Effect of MIND diet on cognitive function in elderly: A narrative review with emphasis on bioactive food ingredients. Food Sci. Biotechnol. 2024, 33, 297–306. [Google Scholar] [CrossRef]
- Morris, M.C.; Wang, Y.; Barnes, L.L.; Bennett, D.A.; Dawson-Hughes, B.; Booth, S.L. Nutrients and bioactives in green leafy vegetables and cognitive decline: Prospective study. Neurology 2018, 90, 214–222. [Google Scholar] [CrossRef]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.B.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef]
- Omar, S.H. Mediterranean and MIND Diets Containing Olive Biophenols Reduces the Prevalence of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 2797. [Google Scholar] [CrossRef]
- Saleh, R.N.M.; Minihane, A.M. Fish, n-3 fatty acids, cognition and dementia risk: Not just a fishy tale. Proc. Nutr. Soc. 2022, 81, 27–40. [Google Scholar] [CrossRef]
- Hoscheidt, S.; Sanderlin, A.H.; Baker, L.D.; Jung, Y.; Lockhart, S.; Kellar, D.; Whitlow, C.T.; Hanson, A.J.; Friedman, S.; Register, T.; et al. Mediterranean and Western diet effects on Alzheimer’s disease biomarkers, cerebral perfusion, and cognition in mid-life: A randomized trial. Alzheimer’s Dement. 2021, 18, 457–468. [Google Scholar] [CrossRef]
- Puranik, N.; Kumari, M.; Tiwari, S.; Dhakal, T.; Song, M. Resveratrol as a Therapeutic Agent in Alzheimer’s Disease: Evidence from Clinical Studies. Nutrients 2025, 17, 2557. [Google Scholar] [CrossRef]
- Appiah, D.; Ingabire-Gasana, E.; Appiah, L.; Yang, J. The Relation of Serum Vitamin C Concentrations with Alzheimer’s Disease Mortality in a National Cohort of Community-Dwelling Elderly Adults. Nutrients 2024, 16, 1672. [Google Scholar] [CrossRef]
- Khan, S.; Jatala, F.H.; Muti, A.; Afza, N.; Noor, A.; Mumtaz, S.; Zafar, S. Therapeutic Potential of Nitrogen-Doped Rutin-Bound Glucose Carbon Dots for Alzheimer’s Disease. Yale J. Biol. Med. 2024, 97, 153–164. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Faramarzi, A.; Salarvandian, S.; Zarei, R.; Heidari, M.; Salehian, F.; Esmaeilpour, K. Melatonin Supplementation in Alzheimer’s Disease: The Potential Role in Neurogenesis. Mol. Neurobiol. 2025, 1–23. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Levels in AD Patients | References |
|---|---|---|
| Total Antioxidant Status (TAS) | Decreased | [34,35] |
| MDA | Increased | [36,37,38,39,40] |
| 8-OHdG | Increased | [41] |
| SOD | Decreased | [33,36,40] |
| GSH | Decreased | [33,42,43] |
| CAT | Decreased | [33] |
| Increased | [39] | |
| GPx | Decreased | [33,36,40,44] |
| Advanced oxidation protein products (AOPP) | Increased | [45] |
| Ischemia-modified albumin (IMA) | Increased | [45] |
| Ferric reducing antioxidant power (FRAP) | Decreased | [45] |
| Prooxidant–antioxidant balance (PAB) | Increased | [45] |
| Melatonin | Decreased | [46,47] |
| Drug | Mechanism of Action | Effectiveness | Comments | References |
|---|---|---|---|---|
| Selegiline | Irreversible MAO-B inhibitor. Reduces dopamine breakdown and hydrogen peroxide production, lowering oxidative stress—neuroprotective effect. | Improvement of cognitive function in preclinical trials. Moderate efficacy in humans. | At doses above 10 mg/day may cause the “cheese effect”. Metabolized to amphetamine derivatives. | [59,60,61,62] |
| Rasagiline | Irreversible MAO-B inhibitor. In addition to reducing dopamine breakdown, it increases catalase activity. | Potentially more advanced effects than selegiline, beneficial outcomes in studies. | In combination with serotonin reuptake inhibitors may cause serotonin syndrome. | [63,64,65] |
| Ladostigil | Multifunctional drug combining cholinesterase and MAO-B inhibition. Exhibits anti-inflammatory, anti-apoptotic effects, reduces microglial activation and oxidative stress. | Mixed results in clinical studies. | Experimental drug. | [66,67,68] |
| Therapeutic Strategy | Mechanism of Action | Current Status | References |
|---|---|---|---|
| Anti-amyloid antibodies (Aducanumab, Lecanemab, Donanemab) | Promote clearance of amyloid-β plaques and prevent aggregation | FDA-approved/Phase 3 clinical trials | [75,77,78,79] |
| Tau-targeted therapies (immunotherapies, antisense oligonucleotides, aggregation inhibitors) | Reduce tau phosphorylation, aggregation, and propagation | Preclinical/Early clinical trials | [74,80,81,82] |
| Neuroinflammation modulators (CSF1R inhibitors, STAT3 inhibitors, GLP-1 receptor agonists) | Modulate microglial and astrocytic activity, suppress pro-inflammatory cascades | Preclinical/Ongoing clinical trials | [74,83,84,85,86,87,88] |
| Mitochondrial stabilizers and redox modulators (e.g., sodium benzoate, NMDAR modulators) | Counteract excitotoxicity, stabilize mitochondrial bioenergetics, reduce ROS | Preclinical/Pilot clinical studies | [74,89,90] |
| Nanoparticle-based delivery systems | Enhance CNS penetration and bioavailability of therapeutic compounds | Preclinical/Translational research | [12,91,92,93] |
| Proteolysis-targeting chimeras (PROTACs) | Induce selective degradation of pathogenic proteins (e.g., tau, Aβ-related targets) | Preclinical concept/Early-stage development | [75,94,95,96] |
| Gene therapies | Deliver protective or restorative genes via viral vectors (mainly AAV) to enhance neuroprotection, modulate amyloid/tau pathology, and improve synaptic function | Phase 1/2 clinical trials; preliminary results show safety, biomarker improvements | [97,98,99] |
| Antioxidant Agent | Antioxidant Mechanism | Influence on AD | References |
|---|---|---|---|
| Resveratrol | Increases activity of antioxidant enzymes (SOD, GSH-Px, CAT) while reducing MDA levels | Neuroprotective effect, reduction in oxidative stress | [101] |
| Curcumin | Reduces MDA concentration, enhances total antioxidant capacity (TAC), scavenges reactive nitrogen and oxygen species, regulates enzymes and chelates metals, stabilizes ROS | Reduction in oxidative stress | [102,103] |
| Vitamin E | Scavenges free radicals, inhibits hydrogen peroxide production, improves performance in the water maze | Neuroprotection, reduced neuronal damage | [104,105] |
| Vitamin C | Counters ROS, regenerates antioxidant enzymes, scavenges oxygen and nitrogen radicals, donates hydrogen atoms to lipid radicals, quenches singlet oxygen, removes molecular oxygen, regenerates tocopherol from its oxidized form | Reduction in oxidative stress, enhanced neuronal protection | [106,107] |
| EGCG | Protects against t-butyl hydroperoxide, 6-hydroxydopamine, iron ions, UV radiation, hydrogen peroxide, 3-hydroxykynurenine; lowers TBARS, lipid hydroperoxides, 4-HNE, MDA; increases GPx and GSH activity | Protection against synaptic degeneration | [109,110] |
| Propolis | Scavenges free radicals, strong reducing capacity | Reduced neurotoxicity | [111] |
| Rutin | Binds Keap1 and blocks its interaction with Nrf2; increases activity of antioxidant enzymes (SOD, GPx, CAT) | Reduction in oxidative stress, decreased pro-inflammatory cytokines, inhibition of tau pathology, improved cognitive function in animal models | [112,113,114] |
| Sulforaphane | Activates Nrf2–ARE pathway, increases expression of SOD, CAT, GST; reduces HDAC1/3; enhances histone acetylation; epigenetic effects | Reduction in Aβ and tau, improved cognition, decreased oxidative stress, enhanced synaptic plasticity and blood–brain barrier integrity | [117,121,122] |
| Melatonin | Scavenges free radicals, activates Nrf2, stabilizes mitochondria, regulates PI3K/AKT/mTOR, SIRT1/FOXO, MAPK/ERK pathways | Reduction in Aβ and tau, improved sleep and mitochondrial function, circadian rhythm regulation, neurogenesis and cholinergic protection | [7,128,136] |
| NAC | Thiol group donor, GSH precursor, reduces 4-HNE, improves BBB integrity, stabilizes synapses, anti-inflammatory effect | Reduction in Aβ and phosphorylated tau, improved cognition, enhanced neurogenesis, glutathione support, synergistic effect with other antioxidants | [139,140,144] |
| Astaxanthin | Reduces ROS, activates Nrf2 pathway, lowers mTOR, stimulates autophagy, regulates miRNA-124, supports mitochondria | Improved memory, reduced Aβ and tau, anti-inflammatory and anti-apoptotic effects, enhanced neuroplasticity | [146,147,149] |
| Compound/Approach | Molecular Targets/Mechanisms | Experimental Models | Key Outcomes | Limitations/Translational Issues |
|---|---|---|---|---|
| Resveratrol | ↓ ROS, ↑ SIRT1, modulation of Aβ and tau, anti-inflammatory | in vitro, in vivo, early clinical trials | Neuroprotection, memory improvement, activation of antioxidant pathways | Low bioavailability, rapid metabolism |
| Curcumin | Antioxidant, anti-inflammatory, anti-amyloid | in vitro, in vivo, small clinical trials | Reduction in Aβ, improved mitochondrial function | Very low bioavailability, inconsistent clinical outcomes |
| Rutin | ROS scavenging, mitochondrial stabilization, anti-inflammatory | in vitro, in vivo | Memory improvement, reduced oxidative stress | No clinical data, poor solubility |
| Vitamins C and E | Classical antioxidants (ROS scavenging) | Animal models, clinical trials | Protective effects in models, mixed clinical results | Inconsistent clinical efficacy, limited effectiveness as monotherapy |
| Melatonin | Antioxidant, circadian rhythm regulation, mitochondrial modulation | in vitro, in vivo, clinical studies | Neuroprotection, improved sleep and memory | Variable dosing and bioavailability, inconclusive clinical evidence |
| Selenium and SeNPs | Support of selenoproteins (GPx, TrxR), ↓ ROS, inhibition of Aβ and tau, immunomodulation | in vitro, in vivo | Ebselen, SeNPs, and SELENOW improve cognition, reduce tau pathology | Mainly preclinical data, potential toxicity, lack of large clinical trials |
| MAO-B inhibitors (selegiline, rasagiline, ladostigil) | Reduction in ROS via MAO-B inhibition, neuroprotective effects | Animal models, clinical use | Cognitive improvement, indirect antioxidant effects | Established drugs, significant side effects and interactions |
| Novel approaches (nanoparticles, immunotherapy, mitochondrial/gene therapy) | Targeted drug delivery, redox pathway modulation, immunomodulation | in vitro, in vivo, preliminary clinical studies | Improved BBB penetration, synergistic effects, innovative strategies | Early-stage research, lack of large-scale clinical validation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilski, R.; Dąbkowski, S.; Kozieł, I.; Kozicki, M.; Małachowska, A.; Przygocki, M.; Tyska, O. Perspectives on Alzheimer’s Disease Treatment Based on Counteracting Oxidative Stress. Biomolecules 2025, 15, 1345. https://doi.org/10.3390/biom15091345
Bilski R, Dąbkowski S, Kozieł I, Kozicki M, Małachowska A, Przygocki M, Tyska O. Perspectives on Alzheimer’s Disease Treatment Based on Counteracting Oxidative Stress. Biomolecules. 2025; 15(9):1345. https://doi.org/10.3390/biom15091345
Chicago/Turabian StyleBilski, Rafał, Stanisław Dąbkowski, Igor Kozieł, Michał Kozicki, Anna Małachowska, Mikołaj Przygocki, and Oliwia Tyska. 2025. "Perspectives on Alzheimer’s Disease Treatment Based on Counteracting Oxidative Stress" Biomolecules 15, no. 9: 1345. https://doi.org/10.3390/biom15091345
APA StyleBilski, R., Dąbkowski, S., Kozieł, I., Kozicki, M., Małachowska, A., Przygocki, M., & Tyska, O. (2025). Perspectives on Alzheimer’s Disease Treatment Based on Counteracting Oxidative Stress. Biomolecules, 15(9), 1345. https://doi.org/10.3390/biom15091345






