Role of Glucagon-like Peptide-1 Receptor Agonists (GLP-1RAs) in Patients with Chronic Heart Failure
Abstract
1. Introduction
Literature Search Strategy
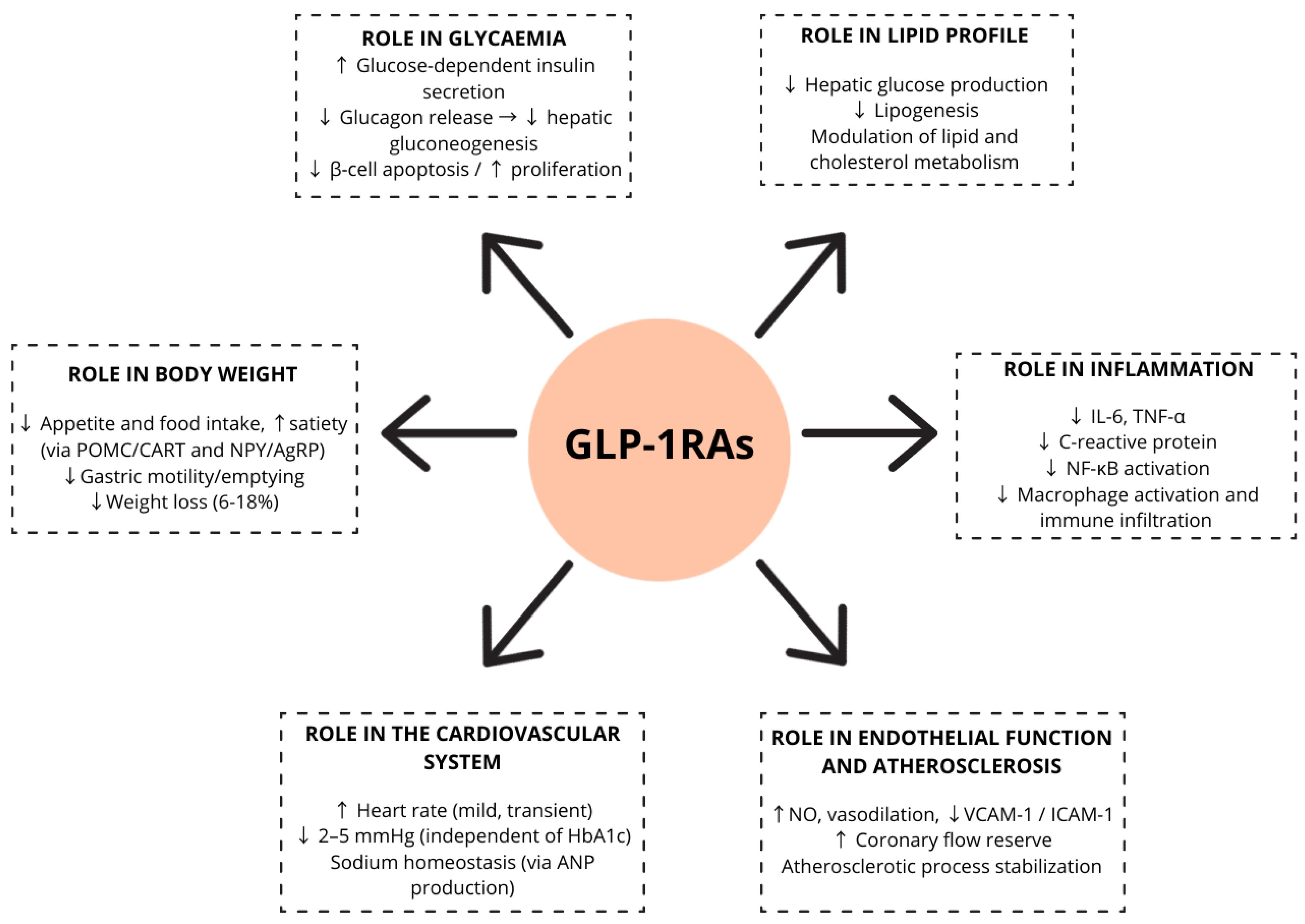
2. GLP-1 Physiology and Mechanism of Action of Its Analogues
2.1. Role of GLP-1 in Glycaemia
2.2. Role of GLP-1 in Body Weight
2.3. Role of GLP-1 in Lipid Profile
2.4. Role of GLP-1 in Inflammation
2.5. Role of GLP-1 in Blood Pressure (BP)
2.6. Role of GLP-1 in Heart Rate (HR)
2.7. Role of GLP-1 in Endothelial Function and Atherosclerosis
3. General Cardiovascular Effects of GLP-1RAs
GLP-1RAs Cardiovascular Outcome Trials Results:
4. GLP-1RAs and Chronic Heart Failure (HF)
4.1. GLP-1RAs and Heart Failure with Reduced Ejection Fraction (HFrEF)
4.2. GLP-1RAs and Heart Failure with Preserved Ejection Fraction (HFpEF)
5. Potential Mechanisms of GLP-1RAs in Heart Failure
6. Gaps in Knowledge and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| BP | Blood Pressure |
| cAMP | Cyclic adenosine monophosphate |
| CI | Confidence Interval |
| CNS | Central Nervous System |
| CV | Cardiovascular |
| CVOT | Cardiovascular Outcome Trials |
| DPP-4 | Dipeptidyl-peptidase-4 |
| EAT | Epicardial Adipose Tissue |
| eGFR | Estimated Glomerular Filtration Rate |
| GIP | Glucose-dependent Insulinotropic Polypeptide |
| GLP-1 | Glucagon-like peptide-1 |
| GLP-1 RAs | Glucagon-like peptide-1 receptor agonists |
| HF | Heart Failure |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| HFpEF | Heart Failure with Preserved Ejection Fraction |
| HHF | Hospitalization for Heart Failure |
| HR | Heart Rate |
| KCCQ-CSS | Kansas City Cardiomyopathy Questionnaire—Clinical Summary Score |
| LVEF | Left Ventricle Ejection Fraction |
| MACE | Major Adverse Cardiovascular Event |
| RAAS | Renin–Angiotensin–Aldosteron System |
| RCT | Randomized Clinical Trials |
| T2D | Type 2 Diabetes Mellitus |
| VT | Ventricular Tachycardia |
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Khan, M.S.; Shahid, I.; Bennis, A.; Rakisheva, A.; Metra, M.; Butler, J. Global epidemiology of heart failure. Nat. Rev. Cardiol. 2024, 21, 717–734. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Januzzi, J.L.; Bruemmer, D.; Butalia, S.; Green, J.B.; Horton, W.B.; Knight, C.; Levi, M.; Rasouli, N.; Richardson, C.R. Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care 2022, 45, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Krim, S.; Ventura, H. The Bi-directional Impact of Two Chronic Illnesses: Heart Failure and Diabetes—A review of the Epidemiology and Outcomes. Card. Fail. Rev. 2015, 1, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Guglin, M.; Lynch, K.; Krischer, J. Heart Failure as a Risk Factor for Diabetes Mellitus. Cardiology 2014, 129, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Benn, M.; Marott, S.C.W.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Obesity increases heart failure incidence and mortality: Observational and Mendelian randomization studies totalling over 1 million individuals. Cardiovasc. Res. 2021, 118, 3576–3585. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Wilcox, T.; De Block, C.; Schwartzbard, A.Z.; Newman, J.D. Diabetic Agents, From Metformin to SGLT2 Inhibitors and GLP1 Receptor Agonists. JACC 2020, 75, 1956–1974. [Google Scholar] [CrossRef]
- Heuvelman, V.D.; Van Raalte, D.H.; Smits, M.M.; Smits, M.M. Cardiovascular effects of GLP-1 receptor agonists: From mechanistic studies in humans to clinical outcomes. Cardiovasc. Res. 2020, 116, 916–930. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 1–29. [Google Scholar] [CrossRef]
- Bu, T.; Sun, Z.; Pan, Y.; Deng, X.; Yuan, G. Glucagon-Like Peptide-1: New Regulator in Lipid Metabolism. Diabetes Metab. J. 2024, 48, 354–372. [Google Scholar] [CrossRef]
- Sun, F.; Wu, S.; Wang, J.; Guo, S.; Chai, S.; Yang, Z.; Li, L.; Zhang, Y.; Ji, L.; Zhan, S. Effect of Glucagon-like Peptide-1 Receptor Agonists on Lipid Profiles Among Type 2 Diabetes: A Systematic Review and Network Meta-analysis. Clin. Ther. 2015, 37, 225–241.e8. [Google Scholar] [CrossRef]
- Hachuła, M.; Kosowski, M.; Ryl, S.; Basiak, M.; Okopień, B. Impact of Glucagon-Like Peptide 1 Receptor Agonists on Biochemical Markers of the Initiation of Atherosclerotic Process. Int. J. Mol. Sci. 2024, 25, 1854. [Google Scholar] [CrossRef] [PubMed]
- Bendotti, G.; Montefusco, L.; Lunati, M.E.; Usuelli, V.; Pastore, I.; Lazzaroni, E.; Assi, E.; Seelam, A.J.; El Essawy, B.; Jang, J.; et al. The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists. Pharmacol. Res. 2022, 182, 106320. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Hullon, D.; Subeh, G.K.; Volkova, Y.; Janiec, K.; Trach, A.; Mnevets, R. The role of glucagon-like peptide-1 receptor (GLP-1R) agonists in enhancing endothelial function: A potential avenue for improving heart failure with preserved ejection fraction (HFpEF). Cardiovasc. Diabetol. 2025, 24, 70. [Google Scholar] [CrossRef]
- Bray, J.J.H.; Foster-Davies, H.; Salem, A.; Hoole, A.L.; Obaid, D.R.; Halcox, J.P.J.; Stephens, J.W. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomised controlled trials. Diabetes, Obes. Metab. 2021, 23, 1806–1822. [Google Scholar] [CrossRef]
- El Aziz, M.S.A.; Kahle, M.; Meier, J.J.; Nauck, M.A. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes Obes. Metab. 2017, 19, 216–227. [Google Scholar] [CrossRef]
- Pauza, A.G.; Thakkar, P.; Tasic, T.; Felippe, I.; Bishop, P.; Greenwood, M.P.; Rysevaite-Kyguoliene, K.; Ast, J.; Broichhagen, J.; Hodson, D.J.; et al. GLP1R Attenuates Sympathetic Response to High Glucose via Carotid Body Inhibition. Circ. Res. 2022, 130, 694–707. [Google Scholar] [CrossRef]
- Gaspari, T.; Welungoda, I.; E Widdop, R.; Simpson, R.W.; E Dear, A. The GLP-1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE−/− mouse model. Diabetes Vasc. Dis. Res. 2013, 10, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Platt, M.J.; Shibasaki, T.; E Quaggin, S.; Backx, P.H.; Seino, S.; A Simpson, J.; Drucker, D.J. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat. Med. 2013, 19, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wu, S.; Guo, S.; Yu, K.; Yang, Z.; Li, L.; Zhang, Y.; Quan, X.; Ji, L.; Zhan, S. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetes Res. Clin. Pract. 2015, 110, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, K.; Bidel, Z.; Nazarzadeh, M.; Copland, E.; Canoy, D.; Ramakrishnan, R.; Pinho-Gomes, A.; Woodward, M.; Chalmers, J.; Teo, K.; et al. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: An individual participant-level data meta-analysis. Lancet 2021, 397, 1625–1636. [Google Scholar] [CrossRef]
- Subaran, S.C.; Sauder, M.A.; Chai, W.; Jahn, L.A.; Fowler, D.E.; Aylor, K.W.; Basu, A.; Liu, Z. GLP-1 at physiological concentrations recruits skeletal and cardiac muscle microvasculature in healthy humans. Clin. Sci. 2014, 127, 163–170. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef]
- Kaur, S.; A Rose, R. New insights into the effects of glucagon-like peptide-1 on heart rate and sinoatrial node function. Cardiovasc. Res. 2024, 120, 1367–1368. [Google Scholar] [CrossRef]
- Lorenz, M.; Lawson, F.; Owens, D.; Raccah, D.; Roy-Duval, C.; Lehmann, A.; Perfetti, R.; Blonde, L. Differential effects of glucagon-like peptide-1 receptor agonists on heart rate. Cardiovasc. Diabetol. 2017, 16, 6. [Google Scholar] [CrossRef]
- Lau, K.; Malik, A.; Foroutan, F.; Buchan, T.A.; Daza, J.F.; Sekercioglu, N.; Orchanian-Cheff, A.; Alba, A.C. Resting Heart Rate as an Important Predictor of Mortality and Morbidity in Ambulatory Patients With Heart Failure: A Systematic Review and Meta-Analysis. J. Card. Fail. 2021, 27, 349–363. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Køber, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef]
- Chen, W.R.; Chen, Y.D.; Tian, F.; Yang, N.; Cheng, L.Q.; Hu, S.Y.; Wang, J.; Yang, J.J.; Wang, S.F.; Gu, X.F. Effects of Liraglutide on Reperfusion Injury in Patients With ST-Segment–Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.S.; Vasques-Nóvoa, F.; Borges-Canha, M.; Leite, A.R.; Sharma, A.; Carvalho, D.; Packer, M.; Zannad, F.; Leite-Moreira, A.; Ferreira, J.P. Risk of adverse events with liraglutide in heart failure with reduced ejection fraction: A post hoc analysis of the FIGHT trial. Diabetes Obes. Metab. 2023, 25, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef]
- Wei, R.; Ma, S.; Wang, C.; Ke, J.; Yang, J.; Li, W.; Liu, Y.; Hou, W.; Feng, X.; Wang, G.; et al. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am. J. Physiol. Metab. 2016, 310, E947–E957. [Google Scholar] [CrossRef]
- Chai, W.; Dong, Z.; Wang, N.; Wang, W.; Tao, L.; Cao, W.; Liu, Z. Glucagon-Like Peptide 1 Recruits Microvasculature and Increases Glucose Use in Muscle via a Nitric Oxide–Dependent Mechanism. Diabetes 2012, 61, 888–896. [Google Scholar] [CrossRef]
- Krasner, N.M.; Ido, Y.; Ruderman, N.B.; Cacicedo, J.M.; Bauer, P.M. Glucagon-Like Peptide-1 (GLP-1) Analog Liraglutide Inhibits Endothelial Cell Inflammation through a Calcium and AMPK Dependent Mechanism. PLoS ONE 2014, 9, e97554. [Google Scholar] [CrossRef]
- Nyström, T.; Gutniak, M.K.; Zhang, Q.; Zhang, F.; Holst, J.J.; Ahrén, B.; Sjöholm, Å. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am. J. Physiol. Metab. 2004, 287, E1209–E1215. [Google Scholar] [CrossRef]
- Gejl, M.; Søndergaard, H.M.; Stecher, C.; Bibby, B.M.; Møller, N.; Bøtker, H.E.; Hansen, S.B.; Gjedde, A.; Rungby, J.; Brock, B. Exenatide Alters Myocardial Glucose Transport and Uptake Depending on Insulin Resistance and Increases Myocardial Blood Flow in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2012, 97, E1165–E1169. [Google Scholar] [CrossRef]
- A Read, P.; Khan, F.Z.; Dutka, D.P. Cardioprotection against ischaemia induced by dobutamine stress using glucagon-like peptide-1 in patients with coronary artery disease. Heart 2011, 98, 408–413. [Google Scholar] [CrossRef]
- Turner, R.; Holman, R.; Cull, C.; Stratton, I.; Matthews; Frighi, V.; Manley, S.; Neil, A.; McElroy, K.; Wright, D.; et al. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Page, R.L., II; O’Bryant, C.L.; Cheng, D.; Dow, T.J.; Ky, B.; Stein, C.M.; Spencer, A.P.; Trupp, R.J.; Lindenfeld, J.; American Heart Association Clinical Pharmacology; et al. Drugs That May Cause or Exacerbate Heart Failure: A scientific statement from the American heart association. Circulation 2016, 134, e32–e69. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Seo, G.H.; Jung, C.H.; Kim, M.-K.; Jin, S.-M.; Hwang, Y.-C.; Lee, B.-W.; Kim, J.H. Increased Risk of Hospitalization for Heart Failure with Newly Prescribed Dipeptidyl Peptidase-4 Inhibitors and Pioglitazone Using the Korean Health Insurance Claims Database. Diabetes Metab. J. 2015, 39, 247–252. [Google Scholar] [CrossRef]
- ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Balapattabi, K.; Beverly, E.A.; Briggs Early, K.; Bruemmer, D.; Das, S.R.; Echouffo-Tcheugui, J.B.; Ekhlaspour, L.; et al. 10. Cardiovascular disease and risk management: Standards of care in diabetes—2025. Diabetes Care 2025, 48, S207–S238. [Google Scholar] [CrossRef]
- Cintra, R.M.; Nogueira, A.C.; Bonilha, I.; Luchiari, B.M.; Coelho-Filho, O.R.; Coelho, O.R.; Schwartzmann, P.; Muscellie, E.; Nadruz, W.; Carvalho, L.S.F.; et al. Glucose-lowering Drugs and Hospitalization for Heart Failure: A Systematic Review and Additive-effects Network Meta-analysis With More Than 500 000 Patient-years. J. Clin. Endocrinol. Metab. 2021, 106, 3060–3067. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Sattar, N.; Rosenstock, J.; Ramasundarahettige, C.; Pratley, R.; Lopes, R.D.; Lam, C.S.; Khurmi, N.S.; Heenan, L.; Del Prato, S.; et al. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Uriel, N.; Gonzalez-Costello, J.; Mignatti, A.; Morrison, K.A.; Nahumi, N.; Colombo, P.C.; Jorde, U.P. Adrenergic Activation, Fuel Substrate Availability, and Insulin Resistance in Patients With Congestive Heart Failure. JACC: Hear. Fail. 2013, 1, 331–337. [Google Scholar] [CrossRef]
- Sokos, G.G.; Nikolaidis, L.A.; Mankad, S.; Elahi, D.; Shannon, R.P. Glucagon-Like Peptide-1 Infusion Improves Left Ventricular Ejection Fraction and Functional Status in Patients With Chronic Heart Failure. J. Card. Fail. 2006, 12, 694–699. [Google Scholar] [CrossRef]
- Margulies, K.B.; Hernandez, A.F.; Redfield, M.M.; Givertz, M.M.; Oliveira, G.H.; Cole, R.; Mann, D.L.; Whellan, D.J.; Kiernan, M.S.; Felker, G.M.; et al. Effects of Liraglutide on Clinical Stability Among Patients With Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 316, 500–508. [Google Scholar] [CrossRef]
- Lepore, J.J.; Olson, E.; Demopoulos, L.; Haws, T.; Fang, Z.; Barbour, A.M.; Fossler, M.; Davila-Roman, V.G.; Russell, S.D.; Gropler, R.J. Effects of the Novel Long-Acting GLP-1 Agonist, Albiglutide, on Cardiac Function, Cardiac Metabolism, and Exercise Capacity in Patients With Chronic Heart Failure and Reduced Ejection Fraction. JACC: Hear. Fail. 2016, 4, 559–566. [Google Scholar] [CrossRef]
- Jorsal, A.; Kistorp, C.; Holmager, P.; Tougaard, R.S.; Nielsen, R.; Hänselmann, A.; Nilsson, B.; Møller, J.E.; Hjort, J.; Rasmussen, J.; et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)—A multicentre, double-blind, randomised, placebo-controlled trial. Eur. J. Hear. Fail. 2016, 19, 69–77. [Google Scholar] [CrossRef]
- Neves, J.S.; Leite, A.R.; Mentz, R.J.; Holman, R.R.; Zannad, F.; Butler, J.; Packer, M.; Ferreira, J.P. Cardiovascular outcomes with exenatide in type 2 diabetes according to ejection fraction: The EXSCEL trial. Eur. J. Hear. Fail. 2024, 27, 540–551. [Google Scholar] [CrossRef]
- Kamel, R.; Leroy, J.; Vandecasteele, G.; Fischmeister, R. Cyclic nucleotide phosphodiesterases as therapeutic targets in cardiac hypertrophy and heart failure. Nat. Rev. Cardiol. 2022, 20, 90–108. [Google Scholar] [CrossRef]
- Prausmüller, S.; Weidenhammer, A.; Heitzinger, G.; Spinka, G.; Goliasch, G.; Arfsten, H.; Mawgoud, R.A.; Gabler, C.; Strunk, G.; Hengstenberg, C.; et al. Obesity in heart failure with preserved ejection fraction with and without diabetes: Risk factor or innocent bystander? Eur. J. Prev. Cardiol. 2023, 30, 1247–1254. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Petrie, M.C.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Hovingh, G.K.; Kitzman, D.W.; Møller, D.V.; Treppendahl, M.B.; Verma, S.; et al. Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes. N. Engl. J. Med. 2024, 390, 1394–1407. [Google Scholar] [CrossRef]
- Shah, S.J.; Sharma, K.; A Borlaug, B.; Butler, J.; Davies, M.; Kitzman, D.W.; Petrie, M.C.; Verma, S.; Patel, S.; Chinnakondepalli, K.M.; et al. Semaglutide and diuretic use in obesity-related heart failure with preserved ejection fraction: A pooled analysis of the STEP-HFpEF and STEP-HFpEF-DM trials. Eur. Heart J. 2024, 45, 3254–3269. [Google Scholar] [CrossRef]
- Pratley, R.E.; Tuttle, K.R.; Rossing, P.; Rasmussen, S.; Perkovic, V.; Nielsen, O.W.; Mann, J.F.; MacIsaac, R.J.; Kosiborod, M.N.; Kamenov, Z.; et al. Effects of Semaglutide on Heart Failure Outcomes in Diabetes and Chronic Kidney Disease in the FLOW Trial. JACC 2024, 84, 1615–1628. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Deanfield, J.; Pratley, R.; A Borlaug, B.; Butler, J.; Davies, M.J.; Emerson, S.S.; E Kahn, S.; Kitzman, D.W.; Lingvay, I.; et al. Semaglutide versus placebo in patients with heart failure and mildly reduced or preserved ejection fraction: A pooled analysis of the SELECT, FLOW, STEP-HFpEF, and STEP-HFpEF DM randomised trials. Lancet 2024, 404, 949–961. [Google Scholar] [CrossRef]
- Siddiqui, H.F.; Waqas, S.A.; Batool, R.M.; Salim, H.; Minhas, A.M.K.; Hasni, S.F.; Alsaid, A.; Sannino, A.; Afzal, A.M.; Khan, M.S. The effect of GLP-1 receptor agonists on cardiac remodeling in heart failure patients with preserved and reduced ejection fraction: A systematic review and meta-analysis. Heart Fail. Rev. 2025, 30, 991–1004. [Google Scholar] [CrossRef]
- Huixing, L.; Di, F.; Daoquan, P. Effect of Glucagon-like Peptide-1 Receptor Agonists on Prognosis of Heart Failure and Cardiac Function: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin. Ther. 2023, 45, 17–30. [Google Scholar] [CrossRef]
- Avogaro, A.; Azzolina, D.; Gregori, D.; De Kreutzenberg, S.; Fadini, G.P.; Mannucci, E. The effect of GLP-1 receptor agonists on N-terminal pro-brain natriuretic peptide. A scoping review and metanalysis. Int. J. Cardiol. 2022, 357, 123–127. [Google Scholar] [CrossRef]
- Yagi, K.; Imamura, T.; Tada, H.; Chujo, D.; Liu, J.; Shima, Y.; Ohbatake, A.; Miyamoto, Y.; Okazaki, S.; Ito, N.; et al. Diastolic cardiac function improvement by liraglutide is mainly body weight reduction dependent but independently contributes to B-type natriuretic peptide reduction in patients with type 2 diabetes with preserved ejection fraction. J. Diabetes Res. 2021, 2021, 8838026. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Yang, Q.; Cheng, X.-Y.; Ding, J.-C.; Hu, P.-F. Beyond weight loss: The potential of glucagon-like peptide-1 receptor agonists for treating heart failure with preserved ejection fraction. Heart Fail. Rev. 2024, 30, 17–38. [Google Scholar] [CrossRef]
- Packer, M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. JACC 2018, 71, 2360–2372. [Google Scholar] [CrossRef]
- Packer, M.; Zile, M.R.; Kramer, C.M.; Baum, S.J.; Litwin, S.E.; Menon, V.; Ge, J.; Weerakkody, G.J.; Ou, Y.; Bunck, M.C.; et al. Tirzepatide for Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2025, 392, 427–437. [Google Scholar] [CrossRef]
- Packer, M. SGLT2 inhibitors: Role in protective reprogramming of cardiac nutrient transport and metabolism. Nat. Rev. Cardiol. 2023, 20, 443–462. [Google Scholar] [CrossRef]
- Pandey, A.K.; Bhatt, D.L.; Pandey, A.; Marx, N.; Cosentino, F.; Pandey, A.; Verma, S. Mechanisms of benefits of sodium-glucose cotransporter 2 inhibitors in heart failure with preserved ejection fraction. Eur. Heart J. 2023, 44, 3640–3651. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M. Potential Mechanisms of the Protective Effects of the Cardiometabolic Drugs Type-2 Sodium–Glucose Transporter Inhibitors and Glucagon-like Peptide-1 Receptor Agonists in Heart Failure. Int. J. Mol. Sci. 2024, 25, 2484. [Google Scholar] [CrossRef]
- Ng, A.C.T.; Delgado, V.; Borlaug, B.A.; Bax, J.J. Diabesity: The combined burden of obesity and diabetes on heart disease and the role of imaging. Nat. Rev. Cardiol. 2020, 18, 291–304. [Google Scholar] [CrossRef]
- Daskalopoulos, E.P.; Dufeys, C.; Beauloye, C.; Bertrand, L.; Horman, S. AMPK in Cardiovascular Diseases; Springer: Cham, Switzerland, 2016; pp. 179–201. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Jaroenlapnopparat, A.; Colak, S.C.; Yu, C.-C.; Xanthavanij, N.; Wang, T.-H.; See, X.Y.; Lo, S.-W.; Ko, A.; Chang, Y.-C.; et al. Glucagon-Like Peptide-1 Receptor Agonists and Gastrointestinal Adverse Events: A Systematic Review and Meta-Analysis. Gastroenterology 2025. [Google Scholar] [CrossRef]
- Cai, C.X.; Hribar, M.; Baxter, S.; Goetz, K.; Swaminathan, S.S.; Flowers, A.; Brown, E.N.; Toy, B.; Xu, B.; Chen, J.; et al. Semaglutide and Nonarteritic Anterior Ischemic Optic Neuropathy. JAMA Ophthalmol. 2025, 143, 304–314. [Google Scholar] [CrossRef] [PubMed]
| ELIXA (n = 6068) | LEADER (n = 9340) | SUSTAIN-6 (n = 3297) | EXSCEL (n = 14,752) | Harmony Outcomes (n = 9463) | REWIND (n = 9901) | PIONEER 6 (n = 3183) | AMPLITUDE-O (n = 4076) | |
|---|---|---|---|---|---|---|---|---|
| Drug | Lixisenatide | Liraglutide | Semaglutide | Exenatide | Albiglutide | Dulaglutide | Semaglutide | Efpeglenatide |
| Age | 60.6 (±9.6) | 64 (±7.2) | 64.6 (±7.4) | 62.0 (±6) | 64 (±8.7) | 66.2 (±6.5) | 66 (±7) | 64.5 (±8.2) |
| Sex (male) | 4207 (69%) | 6003 (64%) | 2002 (61%) | 9149 (62%) | 6569 (69%) | 5312 (54%) | 2176 (68%) | 2732 (67%) |
| Follow-up (median, years) | 2.1 | 3.8 | 2.1 | 3.2 | 1.6 | 5.4 | 1.3 | 1.81 |
| BMI (kg/m2) | 30.1 (±5.6) | 32.5 (±6.3) | 32.8 (±6.2) | 32.7 (±6.4) | 32.3 (±5.9) | 32.3 (±5.7) | 32.3 (±6.5) | 32.7 (±6.2) |
| HbA1c, % | 7.7 (±1.3) | 8.7 (±1.6) | 8.7 (±1.5) | 8.1 (±1.0) | 8.7 (±1.5) | 7.3 (±1.1) | 8.2 (±1.6) | 8.9 (±1.5) |
| eGFR, mL/min per 1.73 m2 | 78 (±21) | 80 (NR) | 80 (61–92) | 77 (61–92) | 79 (±25) | 77 (±23) | 74 (±21) | 72 (±22) |
| Previous CVD, % | 100% | 81% | 83% | 73% | 100% | 31% | 85% | 90% |
| HF history, % | 22% | 18% | 24% | 16% | 20% | 9% | NR | 18% |
| SGLT2i use, % | NR | NR | <1% | 1% | 6% | <1% | 10% | 15% |
| 3-point MACE | 1.02 (0.89–1.17) | 0.87 (0.78–0.97) | 0.74 (0.58–0.95) | 0.91 (0.83–1.00) | 0.78 (0.68–0.90) | 0.88 (0.79–0.99) | 0.79 (0.57–1.11) | 0.73 (0.58–0.92) |
| CV death | 0.98 (0.78–1.22) | 0.78 (0.66–0.93) | 0.98 (0.65–1.48) | 0.88 (0.76–1.02) | 0.93 (0.73–1.19) | 0.91 (0.78–1.06) | 0.49 (0.27–0.92) | 0.72 (0.50–1.03) |
| Nonfatal MI | 1.03 (0.87–1.22) | 0.86 (0.73–1.00) | 0.74 (0.51–1.08) | 0.97 (0.85–1.10) | 0.75 (0.61–0.90) | 0.96 (0.79–1.16) | 1.18 (0.73–1.90) | 0.78 (0.55–1.10) |
| Nonfatal Stroke | 1.12 (0.79–1.58) | 0.86 (0.71–1.06) | 0.61 (0.38–0.99) | 0.85 (0.70–1.03) | 0.86 (0.66–1.14) | 0.76 (0.61–0.95) | 0.74 (0.35–1.57) | 0.80 (0.48–1.31) |
| HF hospitalizations | 0.96 (0.75–1.23) | 0.87 (0.73–1.05) | 1.11 (0.77–1.61) | 0.94 (0.78–1.13) | NA | 0.93 (0.77–1.12) | 0.86 (0.48–1.55) | 0.61 (0.38–0.98) |
| All-cause mortality | 0.94 (0.78–1.13) | 0.85 (0.74–0.97) | 1.05 (0.74–1.50) | 0.86 (0.77–0.97) | 0.95 (0.79–1.16) | 0.90 (0.80–1.01) | 0.51 (0.31–0.84) | 0.78 (0.58–1.06) |
| STEP HFpEF (n = 529) | STEP HFpEF DM (n = 616) | SUMMIT (n = 731) | FIGHT (n = 300) | Lepore et al. (n = 82) | LIVE (n = 241) | |
|---|---|---|---|---|---|---|
| Drug | Semaglutide | Semaglutide | Tirzepatide | Liraglutide | Albiglutide | Liraglutide |
| Age | 69 (62–75) | 69 (62–74) | 65.5 (±10.5) | 61 (52–68) | 56 (±10) | 65 (±9.2) |
| Sex (male) | 43.9% | 58.7% | 45.1% | 80% | 74% | 89% |
| Follow-up (weeks) | 52 | 52 | 104 | 25 | 12 | 24 |
| BMI (kg/m2) | 37.0 (33.7–41.4) | 36.9 (33.6–41.5) | 38.3 (±6.4) | 32 (26–37) | 31 (±7) | 28 (±3.8) |
| T2D (%) | 0% | 100% | 47.8% | 59% | 0% | 32% |
| eGFR, mL/min/m2 | NR | NR | 64.5 (±23.7) | NR | NR | 79 (±20) |
| NT-proBNP, pg/mL | 450.8 (218.2–1015.0) | 477.8 (251.2–969.2) | 196 (56–488) | 1936 (1075–4231) | 89.6 (±23.2) • | 413 (208–926) |
| LVEF, % | 57.0 (50.0–60.0) | 57.0 (50.0–61.0) | 61.0 (6.5) | 25% (20–33) | 31% (1.6) | 33.7% (7.6) |
| Change in Quality of Life * | 7.8 (4.8 to 10.9) | 7.3 (4.1 to 10.4) | 6.9 (3.3 to 10.6) | 0.6 (−4.5 to 5.8) | 2.5 (4.8), p = 0.61 | −1.6 (−5.3, 2.0) |
| Body weight loss, % †† | –10.7 (–11.9 to –9.4) | –6.4 (–7.6 to −5.2) | −11.6 (−12.9 to −10.4) | −1.8 (−3.9 to 0.3) | −1.6 (0.4), p = 0.003 | −0.8 (−1.1, −0.4) |
| Change in 6-MWT distance | 20.3 (8.6 to 32.1) | 14.3 (3.7 to 24.9) | 18.3 (9.9 to 26.7) | 5 (−29 to 39) | 9 (±16), p = 0.58 | 24 (2 to 47), p = 0.04 |
| HF event | 0.08 (0.00 to 0.42) ** | 0.40 (0.15 to 0.92) ** | 0.62 (0.41 to 0.95) † | 146 vs. 156, p = 0.31 ¶ | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llongueras-Espí, P.; García-Romero, E.; Comín-Colet, J.; González-Costello, J. Role of Glucagon-like Peptide-1 Receptor Agonists (GLP-1RAs) in Patients with Chronic Heart Failure. Biomolecules 2025, 15, 1342. https://doi.org/10.3390/biom15091342
Llongueras-Espí P, García-Romero E, Comín-Colet J, González-Costello J. Role of Glucagon-like Peptide-1 Receptor Agonists (GLP-1RAs) in Patients with Chronic Heart Failure. Biomolecules. 2025; 15(9):1342. https://doi.org/10.3390/biom15091342
Chicago/Turabian StyleLlongueras-Espí, Pasqual, Elena García-Romero, Josep Comín-Colet, and José González-Costello. 2025. "Role of Glucagon-like Peptide-1 Receptor Agonists (GLP-1RAs) in Patients with Chronic Heart Failure" Biomolecules 15, no. 9: 1342. https://doi.org/10.3390/biom15091342
APA StyleLlongueras-Espí, P., García-Romero, E., Comín-Colet, J., & González-Costello, J. (2025). Role of Glucagon-like Peptide-1 Receptor Agonists (GLP-1RAs) in Patients with Chronic Heart Failure. Biomolecules, 15(9), 1342. https://doi.org/10.3390/biom15091342






