LRRK2-Mediated Neuroinflammation-Induced Neuronal Dysfunctions in a Parkinson’s and Alzheimer’s Disease Cellular Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Mixed Glial Primary Culture
2.2. Aβ1–42 Fibril and α-Syn Pff Generation
2.3. Compound and Cell Treatment
2.4. Dopaminergic and Cholinergic Differentiation from Human iPSCs
2.5. RNA Extraction, Retro-Transcription, and Real-Time PCR
2.6. RNA Sequencing and Analysis
2.7. Immunofluorescence
2.8. Propidium Iodide Staining
2.9. Reactive Oxygen Species (ROS) Detection
2.10. Immunocytochemistry and Neuronal Morphometric Measurement
2.11. Cell Lysis and Western Blotting
2.12. Statistical Analysis
3. Results
3.1. Generation and Characterization of hiPSC-Derived Neurons
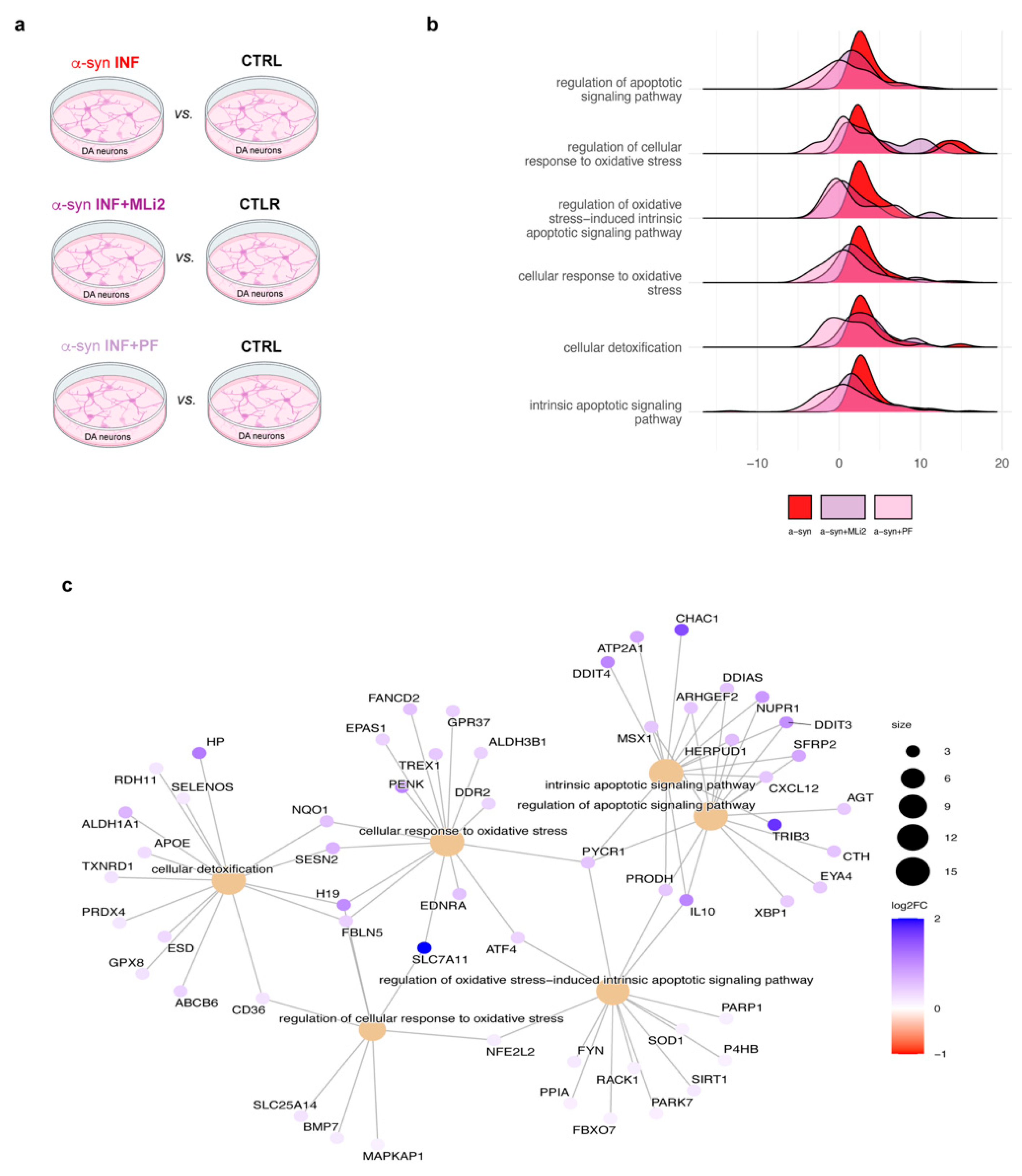
3.2. Transcriptomic Profiles of hiPSC-Derived Neurons Exposed to Inflamed Glial Media
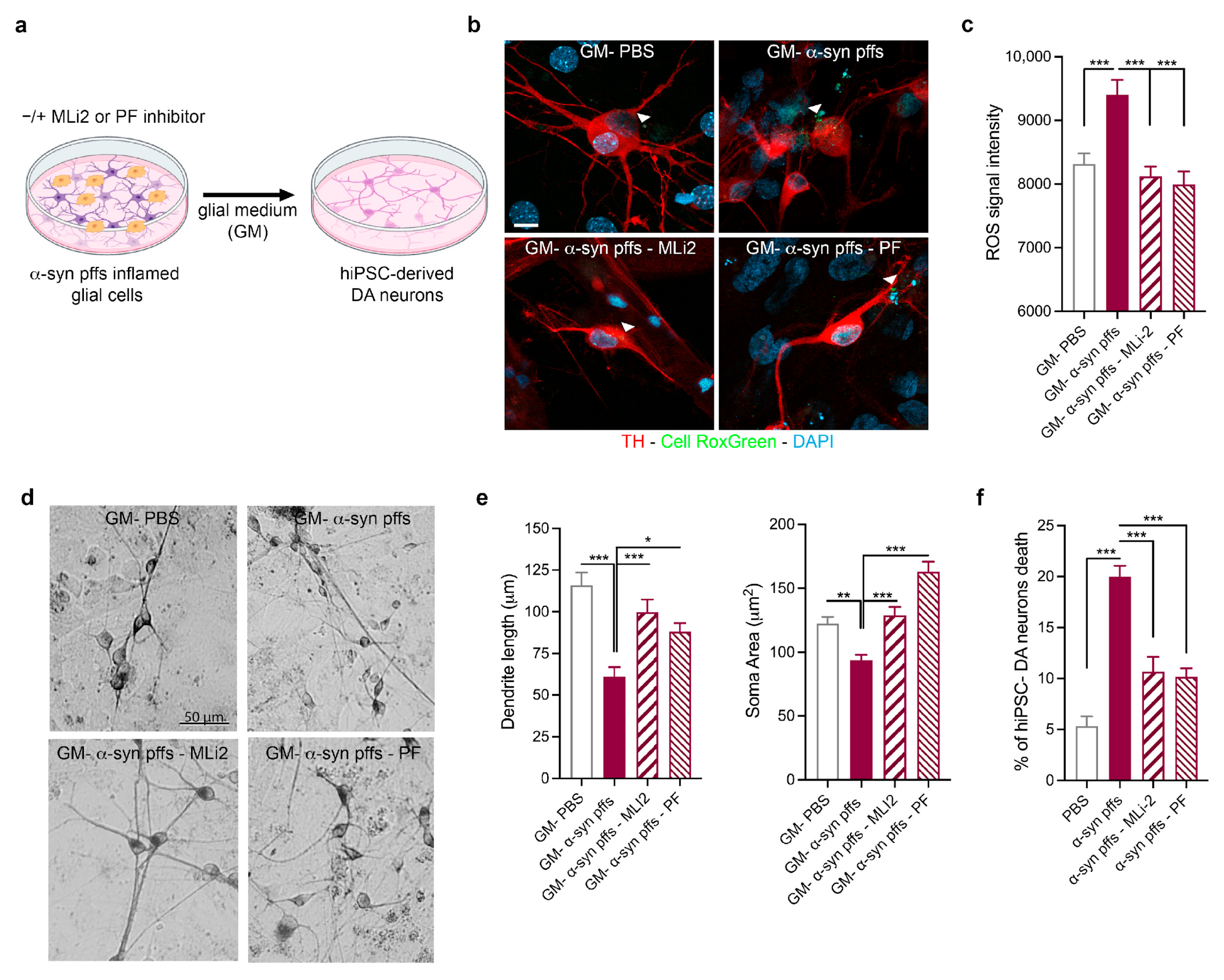
3.3. Validation of the Effect of LRRK2-Mediated α-Syn Pff Neuroinflammation on hiPSC-Derived DA Neurons
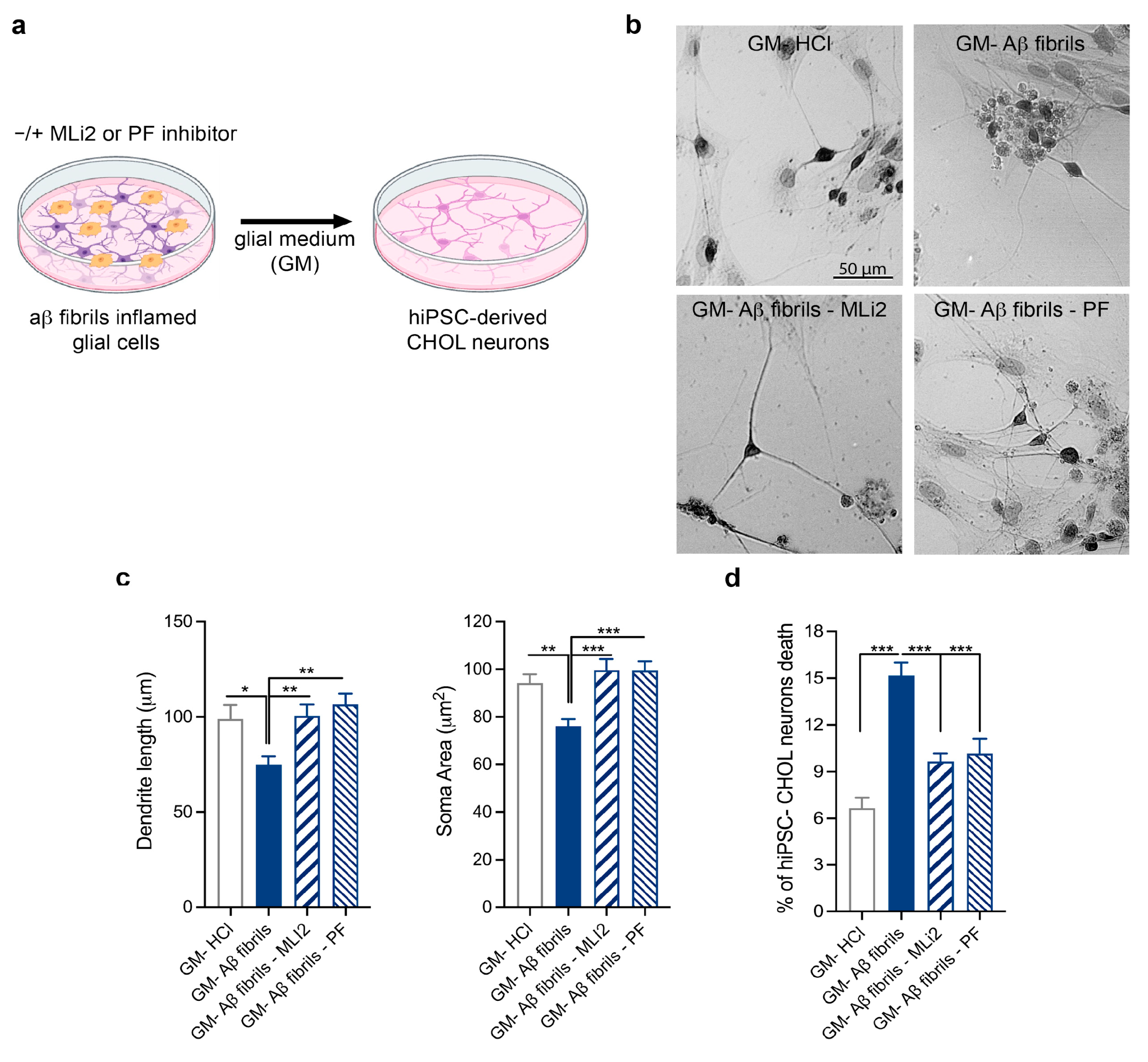
3.4. Validation of the Effect of LRRK2-Mediated Aβ Fibril Neuroinflammation on hiPSC-Derived CHOL Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NDs | Neurodegenerative diseases |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| LRRK2 | Leucine-Rich Repeat Kinase 2 |
| α-syn pffs | a-synuclein pre-formed fibrils |
| Aβ1–42 fibrils | Amyloid beta fibrils |
| LPS | Lipopolysaccharides |
| EDTA | Ethylenediaminetetraacetic acid |
| hiPSCs | Human induced pluripotent stem cells |
| DA | Dopaminergic |
| CHOL | Cholinergic |
| RNA-Seq | RNA sequencing |
| pSer935-LRRK2 | Phosphoserine 935 LRRK2 |
| IL-1β | Interleukin-1 beta |
| ROS | Reactive Oxygen Species |
| PBS | Phosphate-buffered saline |
| BDNF | Brain-derived neurotrophic factor |
| GDNF | Glial-derived neurotrophic factor |
| IGF-1 | Insulin-like growth factor 1 |
| cAMP | Cyclic adenosine monophosphate |
| DAPT | N-[1-(3,5-Difluorophenyl)ethyl]-N-[2-(3,5-difluorophenyl)acetyl]-l-alanyl-l-Phenylalanyl glycine |
| ChAT | Choline acetyltransferase |
| VAChT | Vesicular acetylcholine transporter |
| TH | Tyrosine hydroxylase |
| NANOG | Nanog Homeobox |
| Oct-4 | octamer-binding transcription factor 4 |
| ASCL1 | Achaete-scute homolog 1 |
| DLX2 | Distal-Less Homeobox 2 |
| FOXG1 | Forkhead box G1 |
| LHX8 | LIM Homeobox 8 |
| NKX2 | NK2 Homeobox 1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HPRT | Hypoxanthine Phosphoribosyltransferase 1 |
| GM | Glial medium |
References
- Adamu, A.; Li, S.; Gao, F.; Xue, G. The role of neuroinflammation in neurodegenerative diseases: Current understanding and future therapeutic targets. Front. Aging Neurosci. 2024, 16, 1347987. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Mucke, L. Inflammation in neurodegenerative disease—A double-edged sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef]
- Russo, M.V.; McGavern, D.B. Inflammatory neuroprotection following traumatic brain injury. Science 2016, 353, 783–785. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Russo, I.; Bubacco, L.; Greggio, E. LRRK2 and neuroinflammation: Partners in crime in Parkinson’s disease? J. Neuroinflamm. 2014, 11, 52. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Ising, C.; Heneka, M.T. Functional and structural damage of neurons by innate immune mechanisms during neurodegeneration. Cell Death Dis. 2018, 9, 120. [Google Scholar] [CrossRef]
- Russo, I.; Bubacco, L.; Greggio, E. LRRK2 as a target for modulating immune system responses. Neurobiol. Dis. 2022, 169, 105724. [Google Scholar] [CrossRef] [PubMed]
- Marín, I. The Parkinson disease gene LRRK2: Evolutionary and structural insights. Mol. Biol. Evol. 2006, 23, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Mutti, V.; Carini, G.; Filippini, A.; Castrezzati, S.; Giugno, L.; Gennarelli, M.; Russo, I. LRRK2 Kinase Inhibition Attenuates Neuroinflammation and Cytotoxicity in Animal Models of Alzheimer’s and Parkinson’s Disease-Related Neuroinflammation. Cells 2023, 12, 1799. [Google Scholar] [CrossRef]
- Filippini, A.; Salvi, V.; Dattilo, V.; Magri, C.; Castrezzati, S.; Veerhuis, R.; Bosisio, D.; Gennarelli, M.; Russo, I. LRRK2 Kinase Inhibition Attenuates Astrocytic Activation in Response to Amyloid β1-42 Fibrils. Biomolecules 2023, 13, 307. [Google Scholar] [CrossRef]
- Russo, I.; Kaganovich, A.; Ding, J.; Landeck, N.; Mamais, A.; Varanita, T.; Biosa, A.; Tessari, I.; Bubacco, L.; Greggio, E.; et al. Transcriptome analysis of LRRK2 knock-out microglia cells reveals alterations of inflammatory- and oxidative stress-related pathways upon treatment with α-synuclein fibrils. Neurobiol. Dis. 2019, 129, 67–78. [Google Scholar] [CrossRef]
- Russo, I.; Berti, G.; Plotegher, N.; Bernardo, G.; Filograna, R.; Bubacco, L.; Greggio, E. Leucine-rich repeat kinase 2 positively regulates inflammation and down-regulates NF-κB p50 signaling in cultured microglia cells. J. Neuroinflamm. 2015, 12, 230. [Google Scholar] [CrossRef]
- Pajarillo, E.; Kim, S.; Digman, A.; Dutton, M.; Son, D.-S.; Aschner, M.; Lee, E. The role of microglial LRRK2 kinase in manganese-induced inflammatory neurotoxicity via NLRP3 inflammasome and RAB10-mediated autophagy dysfunction. J. Biol. Chem. 2023, 299, 104879. [Google Scholar] [CrossRef]
- Nyarko-Danquah, I.; Pajarillo, E.; Kim, S.; Digman, A.; Multani, H.K.; Ajayi, I.; Son, D.-S.; Aschner, M.; Lee, E. Microglial Sp1 induced LRRK2 upregulation in response to manganese exposure, and 17β-estradiol afforded protection against this manganese toxicity. Neurotoxicology 2024, 103, 105–114. [Google Scholar] [CrossRef]
- Filippini, A.; Mutti, V.; Faustini, G.; Longhena, F.; Ramazzina, I.; Rizzi, F.; Kaganovich, A.; Roosen, D.A.; Landeck, N.; Duffy, M.; et al. Extracellular clusterin limits the uptake of α-synuclein fibrils by murine and human astrocytes. Glia 2021, 69, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Kriks, S.; Shim, J.-W.; Piao, J.; Ganat, Y.M.; Wakeman, D.R.; Xie, Z.; Carrillo-Reid, L.; Auyeung, G.; Antonacci, C.; Buch, A.; et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011, 480, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Bono, F.; Savoia, P.; Guglielmi, A.; Gennarelli, M.; Piovani, G.; Sigala, S.; Leo, D.; Espinoza, S.; Gainetdinov, R.R.; Devoto, P.; et al. Role of Dopamine D2/D3 Receptors in Development, Plasticity, and Neuroprotection in Human iPSC-Derived Midbrain Dopaminergic Neurons. Mol. Neurobiol. 2018, 55, 1054–1067. [Google Scholar] [CrossRef]
- Bono, F.; Mutti, V.; Devoto, P.; Bolognin, S.; Schwamborn, J.C.; Missale, C.; Fiorentini, C. Impaired dopamine D3 and nicotinic acetylcholine receptor membrane localization in iPSCs-derived dopaminergic neurons from two Parkinson’s disease patients carrying the LRRK2 G2019S mutation. Neurobiol. Aging 2021, 99, 65–78. [Google Scholar] [CrossRef]
- Bono, F.; Mutti, V.; Savoia, P.; Barbon, A.; Bellucci, A.; Missale, C.; Fiorentini, C. Nicotine prevents alpha-synuclein accumulation in mouse and human iPSC-derived dopaminergic neurons through activation of the dopamine D3- acetylcholine nicotinic receptor heteromer. Neurobiol. Dis. 2019, 129, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Krajka, V.; Naujock, M.; Pauly, M.G.; Stengel, F.; Meier, B.; Stanslowsky, N.; Klein, C.; Seibler, P.; Wegner, F.; Capetian, P. Ventral Telencephalic Patterning Protocols for Induced Pluripotent Stem Cells. Front. Cell Dev. Biol. 2021, 9, 716249. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Risso, D.; Ngai, J.; Speed, T.P.; Dudoit, S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol. 2014, 32, 896–902. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Gao, H.-M.; Hong, J.-S. Why neurodegenerative diseases are progressive: Uncontrolled inflammation drives disease progression. Trends Immunol. 2008, 29, 357–365. [Google Scholar] [CrossRef]
- Castro-Gomez, S.; Heneka, M.T. Innate immune activation in neurodegenerative diseases. Immunity 2024, 57, 790–814. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Riederer, P.; Narabayashi, H.; Fujita, K.; Nagatsu, T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett. 1994, 165, 208–210. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Kim, M.E.; Lee, J.S. Mechanisms and Emerging Regulators of Neuroinflammation: Exploring New Therapeutic Strategies for Neurological Disorders. Curr. Issues Mol. Biol. 2024, 47, 8. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Tago, H.; McGeer, E.G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 1987, 79, 195–200. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhou, X.-W.; Wang, J.-Z. The dual roles of cytokines in Alzheimer’s disease: Update on interleukins, TNF-α, TGF-β and IFN-γ. Transl. Neurodegener. 2016, 5, 7. [Google Scholar] [CrossRef]
- Perez-Nievas, B.G.; Serrano-Pozo, A. Deciphering the Astrocyte Reaction in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.M.; Roy, A.; Varshney, M.; Kumar, A. Mapping reactive astrogliosis in Parkinson’s brain with astroglial tracers BU99008 and Deprenyl: New insights from a multi-marker postmortem study. Alzheimers Dement. 2025, 21, e14488. [Google Scholar] [CrossRef]
- Daher, J.P.L.; Abdelmotilib, H.A.; Hu, X.; Volpicelli-Daley, L.A.; Moehle, M.S.; Fraser, K.B.; Needle, E.; Chen, Y.; Steyn, S.J.; Galatsis, P.; et al. Leucine-rich Repeat Kinase 2 (LRRK2) Pharmacological Inhibition Abates α-Synuclein Gene-induced Neurodegeneration. J. Biol. Chem. 2015, 290, 19433–19444. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Sequence (5′-3′) |
|---|---|
| NANOG | Forward: TGCAAGAACTCTCCAACATCCT Reverse: ATTGCTATTCTTCGGCCAGTT |
| OCT4 | Forward: GACAGGGGGAGGGGAGGAGCTAGG Reverse: CTTCCCTCCAACCAGTTGCCCCAAAC |
| ASCL1 | Forward: CATCTCCCCCAACTACTCCA Reverse: AACGCCACTGACAAGAAAGC |
| DLX2 | Forward: GCACATGGGTTCCTACCAGT Reverse: AACGCCACTGACAAGAAAGC |
| FOXG1 | Forward: TGTTGACTCAGAACTCGCTGG Reverse: CTGCTCTGCGAAGTCATTGAC |
| LHX8 | Forward: CAAGCACAATTTGCTCAGGA Reverse: CTGCTCTGCGAAGTCATTGAC |
| NKX2 | Forward: GACACCATGAGGAACAGC Reverse: ACAGGTACTTCTGTTGCTTG |
| ChAT | Forward: GAGTACTGGCTGAATGACATG Reverse: AGTACACCAGAGATGAGGCT |
| VACHT | Forward: TACCCTACGGAGAGCGAAGA Reverse: CTGTAGAGGCGAACATGACG |
| GAPDH | Forward: GTTGTGGATCTGACATGCCG Reverse: GGTGGAAGAATGGGAGTTGC |
| HPRT | Forward: GGTGAAAAGGACCTCTCGAAG Reverse: GCTTTTCCACTTTCGCTGATG |
| ACTIN-β | Forward: CCTCTATGCCAACACAGTGC Reverse: CCTGCTTGCTGATCCACATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutti, V.; Carini, G.; Marizzoni, M.; Filippini, A.; Bono, F.; Fiorentini, C.; Saleri, S.; De Cillis, F.; Cattaneo, A.; Gennarelli, M.; et al. LRRK2-Mediated Neuroinflammation-Induced Neuronal Dysfunctions in a Parkinson’s and Alzheimer’s Disease Cellular Model. Biomolecules 2025, 15, 1322. https://doi.org/10.3390/biom15091322
Mutti V, Carini G, Marizzoni M, Filippini A, Bono F, Fiorentini C, Saleri S, De Cillis F, Cattaneo A, Gennarelli M, et al. LRRK2-Mediated Neuroinflammation-Induced Neuronal Dysfunctions in a Parkinson’s and Alzheimer’s Disease Cellular Model. Biomolecules. 2025; 15(9):1322. https://doi.org/10.3390/biom15091322
Chicago/Turabian StyleMutti, Veronica, Giulia Carini, Moira Marizzoni, Alice Filippini, Federica Bono, Chiara Fiorentini, Samantha Saleri, Floriana De Cillis, Annamaria Cattaneo, Massimo Gennarelli, and et al. 2025. "LRRK2-Mediated Neuroinflammation-Induced Neuronal Dysfunctions in a Parkinson’s and Alzheimer’s Disease Cellular Model" Biomolecules 15, no. 9: 1322. https://doi.org/10.3390/biom15091322
APA StyleMutti, V., Carini, G., Marizzoni, M., Filippini, A., Bono, F., Fiorentini, C., Saleri, S., De Cillis, F., Cattaneo, A., Gennarelli, M., Martini, P., & Russo, I. (2025). LRRK2-Mediated Neuroinflammation-Induced Neuronal Dysfunctions in a Parkinson’s and Alzheimer’s Disease Cellular Model. Biomolecules, 15(9), 1322. https://doi.org/10.3390/biom15091322







