Combined Radiations: Biological Effects of Mixed Exposures Across the Radiation Spectrum
Abstract
1. Introduction
2. Methods
- (“alpha radiation”[tiab] OR “alpha particles”[MeSH Terms]) AND (“X-rays”[MeSH Terms] OR “X radiation”[tiab]).
- (“simultaneous”[tiab] OR “sequential”[tiab]) AND (“irradiation”[tiab] OR “radiation”[MeSH Terms]).
- “proton therapy”[tiab] AND “targeted radionuclide therapy”[tiab].
- “space radiation”[tiab] OR “simulated galactic cosmic rays”[tiab] OR “GCRsim”[tiab].
3. Results
3.1. Radiobiological Studies
3.1.1. Combinations of Non-Ionizing and Ionizing Radiation
3.1.2. Combinations of Non-Ionizing Radiation Types
3.1.3. Combinations of Ionizing Radiation Types
3.2. Therapeutic Studies
3.3. Space Radiation Studies
4. Discussion
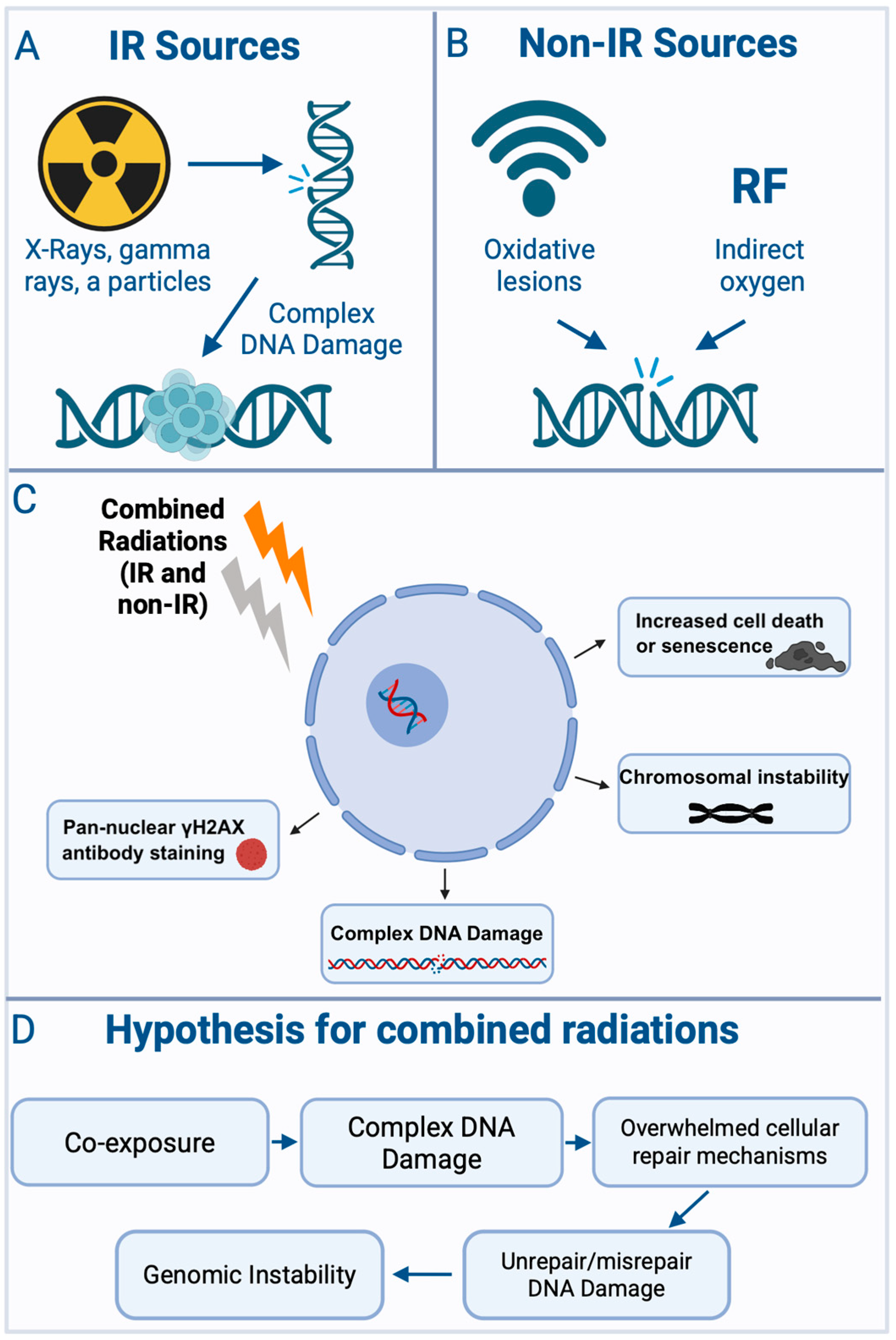
4.1. Mechanistic Basis of Mixed-Radiation Effects
4.1.1. Exposure Sequence Dependence in Mixed-Radiation Exposures
4.1.2. DNA Repair Dependencies, Lesion Interactions, and Temporal Modulation
4.1.3. Cell-Cycle-Specific Interaction Effects
4.1.4. Mechanistic Insights from Non-Ionizing Radiation Combinations
4.1.5. Mechanisms and Functional Consequences of Combined Ionizing Radiation Exposure
4.1.6. Multiscale Biological Effects and Uncommon Exposure Scenarios
4.2. Determinants and Mechanisms of Synergy
4.3. Therapeutic and Protective Strategies
4.4. Space and Environmental Risk Contexts
4.5. Methodological Challenges and Experimental Gaps
4.6. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EURAMET | European Association of National Metrology Institutes |
| Europe PMC | Europe PubMed Central |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
References
- Blanco, Y.; De Diego-Castilla, G.; Viúdez-Moreiras, D.; Cavalcante-Silva, E.; Rodríguez-Manfredi, J.A.; Davila, A.F.; McKay, C.P.; Parro, V. Effects of gamma and electron radiation on the structural integrity of organic molecules and macromolecular biomarkers measured by microarray immunoassays and their astrobiological implications. Astrobiology 2018, 18, 1497–1516. [Google Scholar] [CrossRef] [PubMed]
- Aljabab, S.; Lui, A.; Wong, T.; Liao, J.; Laramore, G.; Parvathaneni, U. A combined neutron and proton regimen for advanced salivary tumors: Early clinical experience. Cureus 2021, 13, e14844. [Google Scholar] [CrossRef]
- Kumar, K.; Moon, B.-H.; Kumar, S.; Angdisen, J.; Kallakury, B.V.S.; Fornace, A.J.; Suman, S. Senolytic agent ABT-263 mitigates low- and high-LET radiation-induced gastrointestinal cancer development in Apc1638N/+ mice. Aging 2025, 17, 97–115. [Google Scholar] [CrossRef]
- Holmberg, M. Lack of synergistic effect between X-ray and UV irradiation on the frequency of chromosome aberrations in PHA-stimulated human lymphocytes in the G1 stage. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1976, 34, 141–147. [Google Scholar] [CrossRef]
- Holmberg, M.; Jonasson, J. Synergistic effect of X-ray and UV irradation on the frequency of chromosome breakage in human lymphocytes. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1974, 23, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Lambert, B.; Hansson, K.; Bui, T.H.; Funes-Cravioto, F.; Lindsten, J.; Holmberg, M.; Strausmanis, R. DNA repair and frequency of X-ray and U.V.-light induced chromosome aberrations in leukocytes from patients with Down’s syndrome. Ann. Hum. Genet. 1976, 39, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, M.; Strausmanis, R. The repair of chromosome aberrations in human lymphocytes after combined irradiation with UV-radiation (254 nm) and X-rays. Mutat. Res. Lett. 1983, 120, 45–50. [Google Scholar] [CrossRef]
- Han, A.; Elkind, M.M. Ultraviolet light and X-ray damage interaction in Chinese hamster cells. Radiat. Res. 1978, 74, 88–100. [Google Scholar] [CrossRef]
- Martignoni, K.D.; Smith, K.C. The synergistic action of ultraviolet and X radiation on mutants of Escherichia coli K-12. Photochem. Photobiol. 1973, 18, 1–8. [Google Scholar] [CrossRef]
- Tyrrell, R.M. The interaction of near U.V. (365 nm) and X-radiations on wild-type and repair-deficient strains of Escherichia coli K-12: Physical and biological measurements. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1974, 25, 373–390. [Google Scholar] [CrossRef]
- Baptist, J.E.; Haynes, R.H. The U.V.-X-ray synergism in Escherichia coli B-r. I. Inhibition by the incorporation of 5-bromouracil and by purine starvation. Photochem. Photobiol. 1972, 16, 459–464. [Google Scholar] [CrossRef]
- Schneider, E.; Kiefer, J. Interaction of ionizing radiation and ultraviolet-light in diploid yeast strains of different sensitivity. Photochem. Photobiol. 1976, 24, 573–578. [Google Scholar] [CrossRef]
- Gentner, N.E.; Werner, M.M. Synergistic interaction between UV and ionizing radiation in wild-type Schizosaccharomyces pombe. Mol. Gen. Genet. MGG 1978, 164, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.F.; Shah, A.R.; Kumta, U.S. Synergistic killing effect in pre-UV-irradiated Micrococcus radiophilus. Photochem. Photobiol. 1975, 22, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.A.; Khaliq, S.; Ahmad, S.; Ashraf, N.; Ghauri, M.A.; Anwar, M.A.; Akhtar, K. Application of combined irradiation mutagenesis technique for hyperproduction of surfactin in Bacillus velezensis strain AF_3B. Int. J. Microbiol. 2025, 2025, 5570585. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Kondo, S. Synergistic effects of P32 decay and ultraviolet irradiation on inactivation of Salmonella. Radiat. Res. 1964, 22, 440. [Google Scholar] [CrossRef]
- Okuda, A. Inhibition of the UV-ionizing radiation synergism in Escherichia coli B/r by liquid holding between the two irradiations. Photochem. Photobiol. 1973, 18, 335–337. [Google Scholar] [CrossRef]
- Neary, G.J.; Bance, D.A.; Cox, R.; Preston, R.J.; Richards, V.; Stephens, M.A.; Stretch, A.; Wilkinson, R.E. A synergistic interaction between U.V. and protons in causing loss of reproductive capacity in Escherichia coli B/r. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1974, 26, 187–192. [Google Scholar] [CrossRef]
- König, A.; Zöller, N.; Kippenberger, S.; Bernd, A.; Kaufmann, R.; Layer, P.G.; Heselich, A. Non-thermal near-infrared exposure photobiomodulates cellular responses to ionizing radiation in human full thickness skin models. J. Photochem. Photobiol. B Biol. 2018, 178, 115–123. [Google Scholar] [CrossRef]
- Zalesskaya, G.A.; Batay, L.E.; Koshlan, I.V.; Nasek, V.M.; Zilberman, R.D.; Milevich, T.I.; Govorun, R.D.; Koshlan, N.A.; Blaga, P. Combined impact of gamma and laser radiation on peripheral blood of rats in vivo. J. Appl. Spectrosc. 2017, 84, 796–803. [Google Scholar] [CrossRef]
- Koreck, A.; Csoma, Z.; Borosgyevi, M.; Ignacz, F.; Bodai, L.; Dobozy, A.; Kemeny, L. Inhibition of immediate type hypersensitivity reaction by combined irradiation with ultraviolet and visible light. J. Photochem. Photobiol. B Biol. 2004, 77, 93–96. [Google Scholar] [CrossRef]
- Cho, S.; Lee, M.J.; Kim, M.S.; Lee, S.; Kim, Y.K.; Lee, D.H.; Lee, C.W.; Cho, K.H.; Chung, J.H. Infrared plus visible light and heat from natural sunlight participate in the expression of MMPs and type I procollagen as well as infiltration of inflammatory cell in human skin in vivo. J. Dermatol. Sci. 2008, 50, 123–133. [Google Scholar] [CrossRef]
- Berg, R.J.; de Gruijl, F.R.; van der Leun, J.C. Interaction between ultraviolet A and ultraviolet B radiations in skin cancer induction in hairless mice. Cancer Res. 1993, 53, 4212–4217. [Google Scholar] [PubMed]
- Johnson, L.E.; Treible, L.M. Hanging under the ledge: Synergistic consequences of UVA and UVB radiation on scyphozoan polyp reproduction and health. PeerJ 2023, 11, e14749. [Google Scholar] [CrossRef] [PubMed]
- Cheang, W.K.; Wong, G.R.; Rahim, A.N.; Kethiravan, D.; Harikrishna, J.A.; Tan, B.C.; Ramakrishnan, N.; Mazumdar, P. Synergistic effects of UV-B and UV-C in suppressing Sclerotinia sclerotiorum infection in tomato plants. J. Crop Health 2024, 76, 1383–1402. [Google Scholar] [CrossRef]
- Probst-Rüd, S.; McNeill, K.; Ackermann, M. Thiouridine residues in tRNAs are responsible for a synergistic effect of UVA and UVB light in photoinactivation of Escherichia coli. Environ. Microbiol. 2017, 19, 434–442. [Google Scholar] [CrossRef]
- Probst-Rüd, S.; Nyangaresi, P.O.; Adeyeye, A.A.; Ackermann, M.; Beck, S.E.; McNeill, K. Synergistic effect of UV-A and UV-C light is traced to UV-induced damage of the transfer RNA. Water Res. 2024, 252, 121189. [Google Scholar] [CrossRef]
- Nyangaresi, P.O.; Qin, Y.; Chen, G.; Zhang, B.; Lu, Y.; Shen, L. Effects of single and combined UV-LEDs on inactivation and subsequent reactivation of E. coli in water disinfection. Water Res. 2018, 147, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, M.; Wu, Z.; Wang, Y.; Blatchley, E.R.; Xie, T.; Qiang, Z. Water disinfection with dual-wavelength (222 + 275 nm) ultraviolet radiations: Microbial inactivation and reactivation. Environ. Sci. Technol. 2025, 59, 1448–1456. [Google Scholar] [CrossRef]
- Beck, S.E.; Ryu, H.; Boczek, L.A.; Cashdollar, J.L.; Jeanis, K.M.; Rosenblum, J.S.; Lawal, O.R.; Linden, K.G. Evaluating UV-C LED disinfection performance and investigating potential dual-wavelength synergy. Water Res. 2017, 109, 207–216. [Google Scholar] [CrossRef]
- Tsenter, I.; Garkusheva, N.; Matafonova, G.; Batoev, V. A novel water disinfection method based on dual-wavelength UV radiation of KrCl (222 nm) and XeBr (282 nm) excilamps. J. Environ. Chem. Eng. 2022, 10, 107537. [Google Scholar] [CrossRef]
- Song, K.; Mohseni, M.; Taghipour, F. Mechanisms investigation on bacterial inactivation through combinations of UV wavelengths. Water Res. 2019, 163, 114875. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, B.; Zhang, H.; Lai, A.C. Inactivation of foodborne pathogenic and spoilage bacteria by single and dual wavelength UV-LEDs: Synergistic effect and pulsed operation. Food Control 2021, 125, 107999. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, W.; Sun, Y.; Sun, Y.; Wang, B.; Liu, M.; Qiu, Z.; Wang, Y.; Sun, Z.; Hu, P.; et al. Effects of multi-wavelength ultraviolet radiation on the inactivation and reactivation of Escherichia coli in recirculating water system. Aquac. Rep. 2025, 41, 102688. [Google Scholar] [CrossRef]
- Nakahashi, M.; Mawatari, K.; Hirata, A.; Maetani, M.; Shimohata, T.; Uebanso, T.; Hamada, Y.; Akutagawa, M.; Kinouchi, Y.; Takahashi, A. Simultaneous Irradiation with Different Wavelengths of Ultraviolet Light has Synergistic Bactericidal Effect on Vibrio parahaemolyticus. Photochem. Photobiol. 2014, 90, 1397–1403. [Google Scholar] [CrossRef]
- Woo, H.; Beck, S.; Boczek, L.; Carlson, K.; Brinkman, N.; Linden, K.; Lawal, O.; Hayes, S.; Ryu, H. Efficacy of inactivation of human enteroviruses by dual-wavelength germicidal ultraviolet (UV-C) light emitting diodes (LEDs). Water 2019, 11, 1131. [Google Scholar] [CrossRef]
- Hull, N.M.; Linden, K.G. Synergy of MS2 disinfection by sequential exposure to tailored UV wavelengths. Water Res. 2018, 143, 292–300. [Google Scholar] [CrossRef]
- Chen, H.; Moraru, C. Synergistic effects of sequential light treatment with 222-nm/405-nm and 280-nm/405-nm wavelengths on inactivation of foodborne pathogens. Appl. Environ. Microbiol. 2023, 89, e00650-23. [Google Scholar] [CrossRef] [PubMed]
- Sollazzo, A.; Shakeri-Manesh, S.; Fotouhi, A.; Czub, J.; Haghdoost, S.; Wojcik, A. Interaction of low and high LET radiation in TK6 cells—Mechanistic aspects and significance for radiation protection. J. Radiol. Prot. 2016, 36, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Guerra Liberal, F.D.C.; Thompson, S.J.; Prise, K.M.; McMahon, S.J. High-LET radiation induces large amounts of rapidly-repaired sublethal damage. Sci. Rep. 2023, 13, 11198. [Google Scholar] [CrossRef]
- Raju, M.R.; Jett, J.H. RBE and OER Variations of Mixtures of Plutonium Alpha Particles and X-Rays for Damage to Human Kidney Cells (T-1). Radiat. Res. 1974, 60, 473. [Google Scholar] [CrossRef]
- Cheng, L.; Brzozowska-Wardecka, B.; Lisowska, H.; Wojcik, A.; Lundholm, L. Impact of ATM and DNA-PK inhibition on gene expression and individual response of human lymphocytes to mixed beams of alpha particles and X-rays. Cancers 2019, 11, 2013. [Google Scholar] [CrossRef]
- Staaf, E.; Deperas-Kaminska, M.; Brehwens, K.; Haghdoost, S.; Czub, J.; Wojcik, A. Complex aberrations in lymphocytes exposed to mixed beams of 241Am alpha particles and X-rays. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 756, 95–100. [Google Scholar] [CrossRef]
- Staaf, E.; Brehwens, K.; Haghdoost, S.; Nievaart, S.; Pachnerova-Brabcova, K.; Czub, J.; Braziewicz, J.; Wojcik, A. Micronuclei in human peripheral blood lymphocytes exposed to mixed beams of X-rays and alpha particles. Radiat. Environ. Biophys. 2012, 51, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Sollazzo, A.; Brzozowska, B.; Cheng, L.; Lundholm, L.; Haghdoost, S.; Scherthan, H.; Wojcik, A. Alpha particles and X rays interact in inducing DNA damage in U2OS cells. Radiat. Res. 2017, 188, 400. [Google Scholar] [CrossRef] [PubMed]
- Staaf, E.; Brehwens, K.; Haghdoost, S.; Czub, J.; Wojcik, A. Gamma-H2AX foci in cells exposed to a mixed beam of X-rays and alpha particles. Genome Integr. 2012, 3, 8. [Google Scholar] [CrossRef]
- Cheng, L.; Brzozowska, B.; Sollazzo, A.; Lundholm, L.; Lisowska, H.; Haghdoost, S.; Wojcik, A. Simultaneous induction of dispersed and clustered DNA lesions compromises DNA damage response in human peripheral blood lymphocytes. PLoS ONE 2018, 13, e0204068. [Google Scholar] [CrossRef] [PubMed]
- Sollazzo, A.; Brzozowska, B.; Cheng, L.; Lundholm, L.; Scherthan, H.; Wojcik, A. Live dynamics of 53BP1 foci following simultaneous induction of clustered and dispersed DNA damage in U2OS cells. Int. J. Mol. Sci. 2018, 19, 519. [Google Scholar] [CrossRef]
- Karimi Roshan, M.; Belikov, S.; Ix, M.; Protti, N.; Balducci, C.; Dodel, R.; Ross, J.A.; Lundholm, L. Fractionated alpha and mixed beam radiation promote stronger pro-inflammatory effects compared to acute exposure and trigger phagocytosis. Front. Cell. Neurosci. 2024, 18, 1440559. [Google Scholar] [CrossRef]
- Lee, U.-S.; Lee, D.-H.; Kim, E.-H. Characterization of γ-H2AX foci formation under alpha particle and X-ray exposures for dose estimation. Sci. Rep. 2022, 12, 3761. [Google Scholar] [CrossRef]
- Staaf, E.; Brehwens, K.; Haghdoost, S.; Pachnerova-Brabcova, K.; Czub, J.; Braziewicz, J.; Nievaart, S.; Wojcik, A. Characterisation of a setup for mixed beam exposures of cells to 241Am alpha particles and X-rays. Radiat. Prot. Dosim. 2012, 151, 570–579. [Google Scholar] [CrossRef] [PubMed]
- McNally, N.J.; De Ronde, J.; Folkard, M. Interaction between X-ray and α-particle damage in V79 cells. Int. J. Radiat. Biol. 1988, 53, 917–920. [Google Scholar] [CrossRef]
- Brooks, A.L.; Newton, G.J.; Shyr, L.-J.; Seiler, F.A.; Scott, B.R. The combined effects of α-particles and X-rays on cell killing and micronuclei induction in lung epithelial cells. Int. J. Radiat. Biol. 1990, 58, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Randers-Pehrson, G.; Geard, C.R.; Brenner, D.J.; Hall, E.J.; Hei, T.K. Interaction between radiation-induced adaptive response and bystander mutagenesis in mammalian cells. Radiat. Res. 2003, 160, 512–516. [Google Scholar] [CrossRef][Green Version]
- Tartas, A.; Lundholm, L.; Scherthan, H.; Wojcik, A.; Brzozowska, B. The order of sequential exposure of U2OS cells to gamma and alpha radiation influences the formation and decay dynamics of NBS1 foci. PLoS ONE 2023, 18, e0286902. [Google Scholar] [CrossRef]
- Akuwudike, P.; López-Riego, M.; Ginter, J.; Cheng, L.; Wieczorek, A.; Życieńska, K.; Łysek-Gładysińska, M.; Wojcik, A.; Brzozowska, B.; Lundholm, L. Mechanistic insights from high resolution DNA damage analysis to understand mixed radiation exposure. DNA Repair 2023, 130, 103554. [Google Scholar] [CrossRef]
- Murthy, M.S.S.; Madhvanath, U.; Subrahmanyam, P.; Rao, B.S.; Reddy, N.M.S. Synergistic effect of simultaneous exposure to 60Co gamma rays and 210Po alpha rays in diploid yeast. Radiat. Res. 1975, 63, 185. [Google Scholar] [CrossRef]
- Bubryak, I.; Vilensky, E.; Naumenko, V.; Grodzisky, D. Influence of combined alpha, beta and gamma radionuclide contamination on the frequency of waxy-reversions in barley pollen. Sci. Total Environ. 1992, 112, 29–36. [Google Scholar] [CrossRef]
- Killion, D.D.; Constantin, M.J. Effects of separate and combined beta and gamma irradiation on the soybean plant. Radiat. Bot. 1974, 14, 91–99. [Google Scholar] [CrossRef]
- Scott, B.R.; Hahn, F.F.; Snipes, M.B.; Newton, G.J.; Eidson, A.F.; Mauderly, J.L.; Boecker, B.B. Predicted and observed early effects of combined alpha and beta lung irradiation. Health Phys. 1990, 59, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Grilj, V.; Broustas, C.G.; Ghandhi, S.A.; Harken, A.D.; Garty, G.; Amundson, S.A. Human transcriptomic response to mixed neutron-photon exposures relevant to an improvised nuclear device. Radiat. Res. 2019, 192, 189. [Google Scholar] [CrossRef] [PubMed]
- Broustas, C.G.; Harken, A.D.; Garty, G.; Amundson, S.A. Identification of differentially expressed genes and pathways in mice exposed to mixed field neutron/photon radiation. BMC Genom. 2018, 19, 504. [Google Scholar] [CrossRef]
- Miller, R.C.; Geard, C.R.; Marino, S.A.; Richards, M.; Randers-Pehrson, G. Oncogenic transformation following sequential irradiations with monoenergetic neutrons and X rays. Radiat. Res. 1991, 125, 338. [Google Scholar] [CrossRef]
- Joiner, M.C.; Bremner, J.C.; Denekamp, J.; Maughan, R.L. The interaction between X-rays and 3 MeV neutrons in the skin of the mouse foot. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1984, 46, 625–638. [Google Scholar] [CrossRef]
- Masuda, K. Effects of 14 MeV neutrons and X-rays, singly or combined on the reproductive capacity of L cells. J. Radiat. Res. 1970, 11, 107–112. [Google Scholar] [CrossRef][Green Version]
- Marples, B.; Skov, K.A. Small doses of high-linear energy transfer radiation increase the radioresistance of chinese hamster V79 cells to subsequent X irradiation. Radiat. Res. 1996, 146, 382. [Google Scholar] [CrossRef] [PubMed]
- McNally, N.J.; De Ronde, J.; Hinchliffe, M. The effect of sequential irradiation with X-rays and fast neutrons on the survival of V79 chinese hamster cells. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1984, 45, 301–310. [Google Scholar] [CrossRef]
- Ngo, F.Q.H.; Han, A.; Elkind, M.M. On the repair of sub-lethal damage in V79 chinese hamster cells resulting from irradiation with fast neutrons or fast neutrons combined with X-rays. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1977, 32, 507–511. [Google Scholar] [CrossRef]
- Hornsey, S.; Andreozzi, U.; Warren, P.R. Sublethal damage in cells of the mouse gut after mixed treatment with X rays and fast neutrons. Br. J. Radiol. 1977, 50, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, A.; Obe, G.; Lisowska, H.; Czub, J.; Nievaart, V.; Moss, R.; Huiskamp, R.; Sauerwein, W. Chromosomal aberrations in peripheral blood lymphocytes exposed to a mixed beam of low energy neutrons and gamma radiation. J. Radiol. Prot. 2012, 32, 261–274. [Google Scholar] [CrossRef]
- Palmqvist, T.; Lopez-Riego, M.; Bucher, M.; Oestreicher, U.; Pojtinger, S.; Giesen, U.; Toma-Dasu, I.; Wojcik, A. Biological effectiveness of combined exposure to neutrons and gamma radiation applied in two orders of sequence: Relevance for biological dosimetry after nuclear emergencies. Radiat. Med. Prot. 2025, 6, 1–10. [Google Scholar] [CrossRef]
- Yuan, Y.; Chai, D.; Zhang, R.; Cheng, J.; Dong, J.; Liu, H.; Zhang, Z.; Dang, X.; Ning, K. Effects of neutron and gamma rays combined irradiation on the transcriptional profile of human peripheral blood. Radiat. Res. 2023, 200, 65–79. [Google Scholar] [CrossRef]
- Suzuki, S. Survival of chinese hamster V79 cells after irradiation with a mixture of neutrons and 60 co g rays: Experimental and theoretical analysis of mixed irradiation. Radiat. Res. 1993, 133, 327. [Google Scholar] [CrossRef]
- Posypanova, G.A.; Ratushnyak, M.G.; Semochkina, Y.P.; Strepetov, A.N. Response of murine neural stem/progenitor cells to gamma-neutron radiation. Int. J. Radiat. Biol. 2022, 98, 1559–1570. [Google Scholar] [CrossRef]
- Wang, C.-K.C.; Zhang, X.; Gifford, I.; Burgett, E.; Adams, V.; Al-Sheikhly, M. Experimental validation of the new nanodosimetry-based cell survival model for mixed neutron and gamma-ray irradiation. Phys. Med. Biol. 2007, 52, N367–N374. [Google Scholar] [CrossRef] [PubMed]
- Railton, R.; Lawson, R.C.; Porter, D. Interaction of γ-ray and neutron effects on the proliferative capacity of chinese hamster cells. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1975, 27, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.D.; DeLuca, P.M.; Pearson, D.W.; Gould, M.N. V79 survival following simultaneous or sequential irradiation by 15-MeV neutrons and 60Co photons. Radiat. Res. 1983, 95, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.D.; DeLuca, P.M.; Gould, M.N. Effect of pulsed dose in simultaneous and sequential irradiation of V-79 cells by 14.8-MeV neutrons and 60Co photons. Radiat. Res. 1984, 99, 591–595. [Google Scholar] [CrossRef]
- Skidmore, W.D.; McHale, C.G. Fluorescence of serum and urine from rats exposed to mixed gamma-neutron radiations. Radiat. Res. 1969, 38, 357. [Google Scholar] [CrossRef]
- Kubasova, T.; Antal, S.; Somosy, Z.; Köteles, G.J. Effects of mixed neutron-gamma irradiation in vivo on the cell surfaces of murine blood cells. Radiat. Environ. Biophys. 1984, 23, 269–277. [Google Scholar] [CrossRef]
- Rodina, A.V.; Semochkina, Y.P.; Vysotskaya, O.V.; Romantsova, A.N.; Strepetov, A.N.; Moskaleva, E.Y. Low dose gamma irradiation pretreatment modulates the sensitivity of CNS to subsequent mixed gamma and neutron irradiation of the mouse head. Int. J. Radiat. Biol. 2021, 97, 926–942. [Google Scholar] [CrossRef] [PubMed]
- Jervis, H.R.; McLaughlin, M.M.; Johnson, M.C. Effect of neutron-gamma radiation on the morphology of the mucosa of the small intestine of germfree and conventional mice. Radiat. Res. 1971, 45, 613–628. [Google Scholar] [CrossRef]
- Iliakis, G.; Ngo, F.Q.; Roberts, W.K.; Youngman, K. Evidence for similarities between radiation damage expressed by beta-araA and damage involved in the interaction effect observed after exposure of V79 cells to mixed neutrons and gamma radiation. Radiat. Res. 1985, 104, 303–316. [Google Scholar] [CrossRef]
- Mason, A.J.; Giusti, V.; Green, S.; Af Rosenschöld, P.M.; Beynon, T.D.; Hopewell, J.W. Interaction between the biological effects of high- and low-LET radiation dose components in a mixed field exposure. Int. J. Radiat. Biol. 2011, 87, 1162–1172. [Google Scholar] [CrossRef]
- Laiakis, E.C.; Canadell, M.P.; Grilj, V.; Harken, A.D.; Garty, G.Y.; Astarita, G.; Brenner, D.J.; Smilenov, L.; Fornace, A.J. Serum lipidomic analysis from mixed neutron/X-ray radiation fields reveals a hyperlipidemic and pro-inflammatory phenotype. Sci. Rep. 2019, 9, 4539. [Google Scholar] [CrossRef]
- Matchuk, O.N.; Yakimova, A.O.; Saburov, V.O.; Koryakin, S.N.; Ivanov, S.A.; Zamulaeva, I.A. Effects of combined action of neutron and proton radiation on the pool of breast cancer stem cells and expression of stemness genes in vitro. Bull. Exp. Biol. Med. 2022, 173, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Ngo, F.Q.; Blakely, E.A.; Tobias, C.A. Sequential exposures of mammalian cells to low- and high-LET radiations. I. Lethal effects following X-ray and neon-ion irradiation. Radiat. Res. 1981, 87, 59–78. [Google Scholar] [CrossRef]
- Ngo, F.Q.; Blakely, E.A.; Tobias, C.A.; Chang, P.Y.; Lommel, L. Sequential exposures of mammalian cells to low- and high-LET radiations. II. As a function of cell-cycle stages. Radiat. Res. 1988, 115, 54–69. [Google Scholar] [CrossRef]
- Bird, R.P.; Zaider, M.; Rossi, H.H.; Hall, E.J.; Marino, S.A.; Rohrig, N. The sequential irradiation of mammalian cells with X rays and charged particles of high LET. Radiat. Res. 1983, 93, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, T.S.; Montoya, V.J.; Afzal, S.M.J.; Parr, S.S.; Curtis, S.B. Response of rat rhabdomyosarcoma tumors to split doses of mixed high- and low-LET radiation. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 1529–1536. [Google Scholar] [CrossRef]
- Mizumoto, M.; Tsuboi, K.; Igaki, H.; Yamamoto, T.; Takano, S.; Oshiro, Y.; Hayashi, Y.; Hashii, H.; Kanemoto, A.; Nakayama, H.; et al. Phase I/II trial of hyperfractionated concomitant boost proton radiotherapy for supratentorial glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 98–105. [Google Scholar] [CrossRef]
- Slater, J.D.; Yonemoto, L.T.; Mantik, D.W.; Bush, D.A.; Preston, W.; Grove, R.I.; Miller, D.W.; Slater, J.M. Proton radiation for treatment of cancer of the oropharynx: Early experience at Loma Linda University Medical Center using a concomitant boost technique. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 494–500. [Google Scholar] [CrossRef]
- Munzenrider, J.E.; Liebsch, N.J. Proton therapy for tumors of the skull base. Strahlenther. Und Onkol. 1999, 175, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Troshina, M.V.; Koryakina, E.V.; Potetnya, V.I.; Solov’ev, A.N.; Saburov, V.O.; Lychagin, A.A.; Ivanov, S.A.; Kaprin, A.D.; Koryakin, S.N. Biological response of chinese hamster B14-150 cells to sequential combined exposure to protons and 12C ions. Bull. Exp. Biol. Med. 2023, 176, 38–41. [Google Scholar] [CrossRef]
- Müller, C.; De Prado Leal, M.; Dominietto, M.D.; Umbricht, C.A.; Safai, S.; Perrin, R.L.; Egloff, M.; Bernhardt, P.; Van Der Meulen, N.P.; Weber, D.C.; et al. Combination of Proton Therapy and Radionuclide Therapy in Mice: Preclinical Pilot Study at the Paul Scherrer Institute. Pharmaceutics 2019, 11, 450. [Google Scholar] [CrossRef]
- Arbuznikova, D.; Klotsotyra, A.; Uhlmann, L.; Domogalla, L.-C.; Steinacker, N.; Mix, M.; Niedermann, G.; Spohn, S.K.B.; Freitag, M.T.; Grosu, A.L.; et al. Exploring the role of combined external beam radiotherapy and targeted radioligand therapy with 177Lu-PSMA-617 for prostate cancer—From bench to bedside. Theranostics 2024, 14, 2560–2572. [Google Scholar] [CrossRef]
- Koryakina, E.V.; Potetnya, V.I.; Troshina, M.V.; Solov’ev, A.N.; Saburov, V.O.; Lychagin, A.A.; Koryakin, S.N.; Ivanov, S.A.; Kaprin, A.D. Post-irradiation recovery of B14-150 fibrosarcoma cells after combined irradiation with low and high linear energy transfer. Bull. Exp. Biol. Med. 2022, 173, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Balakin, V.E.; Rozanova, O.M.; Smirnova, E.N.; Belyakova, T.A.; Shemyakov, A.E.; Strelnikova, N.S. Combined effect of neutron and proton radiations on the growth of solid ehrlich ascites carcinoma and remote effects in mice. Dokl. Biochem. Biophys. 2021, 498, 159–164. [Google Scholar] [CrossRef]
- Koryakin, S.N.; Troshina, M.V.; Koryakina, E.V.; Potetnya, V.I.; Pichkunova, A.A.; Saburov, V.O.; Kazakov, E.I.; Lychagin, A.A.; Ivanov, S.A.; Kaprin, A.D.; et al. The study of effectiveness of combined proton—Neutron irradiation of tumor cells in vitro. Bull. Exp. Biol. Med. 2024, 178, 75–78. [Google Scholar] [CrossRef] [PubMed]
- López-Nieva, P.; González-Vasconcellos, I.; González-Sánchez, L.; Cobos-Fernández, M.A.; Ruiz-García, S.; Sánchez Pérez, R.; Aroca, Á.; Fernández-Piqueras, J.; Santos, J. Differential molecular response in mice and human thymocytes exposed to a combined-dose radiation regime. Sci. Rep. 2022, 12, 3144. [Google Scholar] [CrossRef]
- Gao, F.; Fish, B.L.; Szabo, A.; Schock, A.; Narayanan, J.; Jacobs, E.R.; Moulder, J.E.; Lazarova, Z.; Medhora, M. Enhanced survival from radiation pneumonitis by combined irradiation to the skin. Int. J. Radiat. Biol. 2014, 90, 753–761. [Google Scholar] [CrossRef][Green Version]
- Reinhold, A.; Glasow, A.; Nürnberger, S.; Weimann, A.; Telemann, L.; Stolzenburg, J.-U.; Neuhaus, J.; Berndt-Paetz, M. Ionizing radiation and photodynamic therapy lead to multimodal tumor cell death, synergistic cytotoxicity and immune cell invasion in human bladder cancer organoids. Photodiagnosis Photodyn. Ther. 2025, 51, 104459. [Google Scholar] [CrossRef]
- Luksiene, Z.; Kalvelyte, A.; Supino, R. On the combination of photodynamic therapy with ionizing radiation. J. Photochem. Photobiol. B Biol. 1999, 52, 35–42. [Google Scholar] [CrossRef]
- Bulin, A.-L.; Broekgaarden, M.; Simeone, D.; Hasan, T. Low dose photodynamic therapy harmonizes with radiation therapy to induce beneficial effects on pancreatic heterocellular spheroids. Oncotarget 2019, 10, 2625–2643. [Google Scholar] [CrossRef]
- Wang, G.D.; Nguyen, H.T.; Chen, H.; Cox, P.B.; Wang, L.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J. X-Ray Induced Photodynamic Therapy: A Combination of Radiotherapy and Photodynamic Therapy. Theranostics 2016, 6, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- Montazerabadi, A.R.; Sazgarnia, A.; Bahreyni-Toosi, M.H.; Ahmadi, A.; Aledavood, A. The effects of combined treatment with ionizing radiation and indocyanine green-mediated photodynamic therapy on breast cancer cells. J. Photochem. Photobiol. B Biol. 2012, 109, 42–49. [Google Scholar] [CrossRef]
- Tran, T.A.; Kappelhoff, J.; Jüstel, T.; Anderson, R.R.; Purschke, M. UV emitting nanoparticles enhance the effect of ionizing radiation in 3D lung cancer spheroids. Int. J. Radiat. Biol. 2022, 98, 1484–1494. [Google Scholar] [CrossRef]
- Müller, M.; Wang, Y.; Squillante, M.R.; Held, K.D.; Anderson, R.R.; Purschke, M. UV scintillating particles as radiosensitizer enhance cell killing after X-ray excitation. Radiother. Oncol. 2018, 129, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Kargar, S.; Khoei, S.; Khoee, S.; Shirvalilou, S.; Mahdavi, S.R. Evaluation of the combined effect of NIR laser and ionizing radiation on cellular damages induced by IUdR-loaded PLGA-coated Nano-graphene oxide. Photodiagnosis Photodyn. Ther. 2018, 21, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, H.D.A.; Mendez, L.C.; Stuart, S.R.; Guimarães, R.G.R.; Ramos, C.C.A.; Paula, L.A.D.; Sales, C.P.D.; Chen, A.T.C.; Blasbalg, R.; Baroni, R.H. Implementation of image-guided brachytherapy (IGBT) for patients with uterine cervix cancer: A tumor volume kinetics approach. J. Contemp. Brachytherapy 2016, 4, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Míguez, C.; Jiménez-Ortega, E.; Palma, B.A.; Miras, H.; Ureba, A.; Arráns, R.; Carrasco-Peña, F.; Illescas-Vacas, A.; Leal, A. Clinical implementation of combined modulated electron and photon beams with conventional MLC for accelerated partial breast irradiation. Radiother. Oncol. 2017, 124, 124–129. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Huang, F.; Deng, Y.; Li, B.; Ouyang, R.; Miao, Y.; Sun, Y.; Li, Y. X-ray and NIR light dual-triggered mesoporous upconversion nanophosphor/Bi heterojunction radiosensitizer for highly efficient tumor ablation. Acta Biomater. 2020, 113, 570–583. [Google Scholar] [CrossRef]
- Daneshvar, F.; Salehi, F.; Karimi, M.; Vais, R.D.; Mosleh-Shirazi, M.A.; Sattarahmady, N. Combined X-ray radiotherapy and laser photothermal therapy of melanoma cancer cells using dual-sensitization of platinum nanoparticles. J. Photochem. Photobiol. B Biol. 2020, 203, 111737. [Google Scholar] [CrossRef]
- Zhou, Y.; Kou, J.; Zhang, Y.; Ma, R.; Wang, Y.; Zhang, J.; Zhang, C.; Zhan, W.; Li, K.; Li, X. Magnetic-guided nanocarriers for ionizing/non-ionizing radiation synergistic treatment against triple-negative breast cancer. Biomed. Eng. Online 2024, 23, 67. [Google Scholar] [CrossRef]
- Maruoka, Y.; Nagaya, T.; Sato, K.; Ogata, F.; Okuyama, S.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy with combined exposure of external and interstitial light sources. Mol. Pharm. 2018, 15, 3634–3641. [Google Scholar] [CrossRef] [PubMed]
- Demizu, Y.; Kagawa, K.; Ejima, Y.; Nishimura, H.; Sasaki, R.; Soejima, T.; Yanou, T.; Shimizu, M.; Furusawa, Y.; Hishikawa, Y.; et al. Cell biological basis for combination radiotherapy using heavy-ion beams and high-energy X-rays. Radiother. Oncol. 2004, 71, 207–211. [Google Scholar] [CrossRef]
- Furusawa, Y.; Nakano-Aoki, M.; Matsumoto, Y.; Hirayama, R.; Kobayashi, A.; Konishi, T. Equivalency of the quality of sublethal lesions after photons and high-linear energy transfer ion beams. J. Radiat. Res. 2017, 58, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Kolker, J.D.; Halpern, H.J.; Krishnasamy, S.; Brown, F.; Dohrmann, G.; Ferguson, L.; Hekmatpanah, J.; Mullan, J.; Wollman, R.; Blough, R.; et al. “Instant-mix” whole brain photon with neutron boost radiotherapy for malignant gliomas. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 409–414. [Google Scholar] [CrossRef]
- Laramore, G.E.; Diener-west, M.; Griffin, T.W.; Nelson, J.S.; Griem, M.L.; Thomas, F.J.; Hendrickson, F.R.; Griffin, B.R.; Myrianthopoulos, L.C.; Saxton, J. Randomized neutron dose searching study for malignant gliomas of the brain: Results of an RTOG study. Int. J. Radiat. Oncol. Biol. Phys. 1988, 14, 1093–1102. [Google Scholar] [CrossRef]
- Ando, K.; Koike, S.; Fukuda, N.; Kanehira, C. Independent effect of a mixed-beam regimen of fast neutrons and gamma rays on a murine fibrosarcoma. Radiat. Res. 1984, 98, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Shiba, S.; Shimo, T.; Yamanaka, M.; Yagihashi, T.; Sakai, M.; Ohno, T.; Tokuuye, K.; Omura, M. Increased cell killing effect in neutron capture enhanced proton beam therapy. Sci. Rep. 2024, 14, 28484. [Google Scholar] [CrossRef]
- Kinashi, Y.; Yokomizo, N.; Takahashi, S. DNA double-strand breaks induced by fractionated neutron beam irradiation for boron neutron capture therapy. Anticancer Res. 2017, 37, 1681–1685. [Google Scholar] [CrossRef]
- Phoenix, B.; Green, S.; Hill, M.A.; Jones, B.; Mill, A.; Stevens, D.L. Do the various radiations present in BNCT act synergistically? Cell survival experiments in mixed alpha-particle and gamma-ray fields. Appl. Radiat. Isot. 2009, 67, S318–S320. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Phoenix, B.; Mill, A.J.; Hill, M.; Charles, M.W.; Thompson, J.; Jones, B.; Ngoga, D.; Detta, A.; James, N.D.; et al. The Birmingham Boron Neutron Capture Therapy (BNCT) Project: Developments towards Selective Internal Particle Therapy. Clin. Oncol. 2011, 23, S23–S24. [Google Scholar] [CrossRef][Green Version]
- Okumura, K.; Kinashi, Y.; Kubota, Y.; Kitajima, E.; Okayasu, R.; Ono, K.; Takahashi, S. Relative biological effects of neutron mixed-beam irradiation for boron neutron capture therapy on cell survival and DNA double-strand breaks in cultured mammalian cells. J. Radiat. Res. 2013, 54, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.; Kuthala, N.; Kong, X.; Chiang, C.-S.; Hwang, K.C. Combined gadolinium and boron neutron capture therapies for eradication of head-and-neck tumor using Gd10 B6 nanoparticles under MRI/CT image guidance. JACS Au 2023, 3, 2192–2205. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Y.; Feola, J.M.; Wierzbicki, J. Study of biological effects of varying mixtures of Cf-252 and gamma radiation on the acute radiation syndromes: Relevance to clinical radiotherapy of radioresistant cancer. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 907–914. [Google Scholar] [CrossRef]
- Hada, M.; Meador, J.A.; Cucinotta, F.A.; Gonda, S.R.; Wu, H. Chromosome aberrations induced by dual exposure of protons and iron ions. Radiat. Environ. Biophys. 2007, 46, 125–129. [Google Scholar] [CrossRef]
- Yang, H.; Magpayo, N.; Held, K.D. Targeted and non-targeted effects from combinations of low doses of energetic protons and iron ions in human fibroblasts. Int. J. Radiat. Biol. 2011, 87, 311–319. [Google Scholar] [CrossRef]
- Bennett, P.V.; Cutter, N.C.; Sutherland, B.M. Split-dose exposures versus dual ion exposure in human cell neoplastic transformation. Radiat. Environ. Biophys. 2007, 46, 119–123. [Google Scholar] [CrossRef]
- Zhou, G.; Bennett, P.V.; Cutter, N.C.; Sutherland, B.M. Proton-HZE-particle sequential dual-beam exposures increase anchorage-independent growth frequencies in primary human fibroblasts. Radiat. Res. 2006, 166, 488–494. [Google Scholar] [CrossRef]
- Kiffer, F.; Carr, H.; Groves, T.; Anderson, J.E.; Alexander, T.; Wang, J.; Seawright, J.W.; Sridharan, V.; Carter, G.; Boerma, M.; et al. Effects of 1H +16O charged particle irradiation on short-term memory and hippocampal physiology in a murine model. Radiat. Res. 2018, 189, 53–63. [Google Scholar] [CrossRef]
- Kiffer, F.; Alexander, T.; Anderson, J.; Groves, T.; McElroy, T.; Wang, J.; Sridharan, V.; Bauer, M.; Boerma, M.; Allen, A. Late Effects of 1H + 16O on Short-Term and Object Memory, Hippocampal Dendritic Morphology and Mutagenesis. Front. Behav. Neurosci. 2020, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Sasi, S.P.; Yan, X.; Zuriaga-Herrero, M.; Gee, H.; Lee, J.; Mehrzad, R.; Song, J.; Onufrak, J.; Morgan, J.; Enderling, H.; et al. Different sequences of fractionated low-dose proton and single iron-radiation-induced divergent biological responses in the heart. Radiat. Res. 2017, 188, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Yamazaki, J.; Torres, E.R.S.; Kirchoff, N.; Stagaman, K.; Sharpton, T.; Turker, M.S.; Kronenberg, A. Combined effects of three high-energy charged particle beams important for space flight on brain, behavioral and cognitive endpoints in B6D2F1 female and male mice. Front. Physiol. 2019, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Luitel, K.; Kim, S.B.; Barron, S.; Richardson, J.A.; Shay, J.W. Lung cancer progression using fast switching multiple ion beam radiation and countermeasure prevention. Life Sci. Space Res. 2020, 24, 108–115. [Google Scholar] [CrossRef]
- Lenarczyk, M.; Kronenberg, A.; Mäder, M.; Komorowski, R.; Hopewell, J.W.; Baker, J.E. Exposure to multiple ion beams, broadly representative of galactic cosmic rays, causes perivascular cardiac fibrosis in mature male rats. PLoS ONE 2023, 18, e0283877. [Google Scholar] [CrossRef]
- Suman, S.; Kumar, S.; Kallakury, B.V.S.; Moon, B.-H.; Angdisen, J.; Datta, K.; Fornace, A.J. Predominant contribution of the dose received from constituent heavy-ions in the induction of gastrointestinal tumorigenesis after simulated space radiation exposure. Radiat. Environ. Biophys. 2022, 61, 631–637. [Google Scholar] [CrossRef]
- Bishawi, M.; Lee, F.H.; Abraham, D.M.; Glass, C.; Blocker, S.J.; Cox, D.J.; Brown, Z.D.; Rockman, H.A.; Mao, L.; Slaba, T.C.; et al. Late onset cardiovascular dysfunction in adult mice resulting from galactic cosmic ray exposure. iScience 2022, 25, 104086. [Google Scholar] [CrossRef]
- Burke, M.; Wong, K.; Talyansky, Y.; Mhatre, S.D.; Mitchell, C.; Juran, C.M.; Olson, M.; Iyer, J.; Puukila, S.; Tahimic, C.G.T.; et al. Sexual dimorphism during integrative endocrine and immune responses to ionizing radiation in mice. Sci. Rep. 2024, 14, 7334. [Google Scholar] [CrossRef]
- Simmons, P.; Trujillo, M.; McElroy, T.; Binz, R.; Pathak, R.; Allen, A.R. Evaluating the effects of low-dose simulated galactic cosmic rays on murine hippocampal-dependent cognitive performance. Front. Neurosci. 2022, 16, 908632. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, K.; Grue, K.; Becker, M.; Elizarraras, E.; Frias, E.S.; Halvorsen, A.; Koenig-Zanoff, M.; Frattini, V.; Nimmagadda, H.; Feng, X.; et al. The impact of deep space radiation on cognitive performance: From biological sex to biomarkers to countermeasures. Sci. Adv. 2021, 7, eabg6702. [Google Scholar] [CrossRef]
- Varma, C.; Schroeder, M.K.; Price, B.R.; Khan, K.A.; Curty Da Costa, E.; Hochman-Mendez, C.; Caldarone, B.J.; Lemere, C.A. Long-term, sex-specific effects of GCRsim and gamma irradiation on the brains, hearts, and kidneys of mice with alzheimer’s disease mutations. Int. J. Mol. Sci. 2024, 25, 8948. [Google Scholar] [CrossRef] [PubMed]
- Nemec-Bakk, A.S.; Sridharan, V.; Desai, P.; Landes, R.D.; Hart, B.; Allen, A.R.; Boerma, M. Effects of simulated 5-ion galactic cosmic radiation on function and structure of the mouse heart. Life 2023, 13, 795. [Google Scholar] [CrossRef]
- Puukila, S.; Siu, O.; Rubinstein, L.; Tahimic, C.G.T.; Lowe, M.; Tabares Ruiz, S.; Korostenskij, I.; Semel, M.; Iyer, J.; Mhatre, S.D.; et al. Galactic cosmic irradiation alters acute and delayed species-typical behavior in male and female mice. Life 2023, 13, 1214. [Google Scholar] [CrossRef]
- Sanford, L.D.; Adkins, A.M.; Boden, A.F.; Gotthold, J.D.; Harris, R.D.; Shuboni-Mulligan, D.; Wellman, L.L.; Britten, R.A. Sleep and core body temperature alterations induced by space radiation in rats. Life 2023, 13, 1002. [Google Scholar] [CrossRef]
- McDonald, J.T.; Kim, J.; Farmerie, L.; Johnson, M.L.; Trovao, N.S.; Arif, S.; Siew, K.; Tsoy, S.; Bram, Y.; Park, J.; et al. Space radiation damage rescued by inhibition of key spaceflight associated miRNAs. Nat. Commun. 2024, 15, 4825. [Google Scholar] [CrossRef]
- Wieg, L.; Ciola, J.C.; Wasén, C.C.; Gaba, F.; Colletti, B.R.; Schroeder, M.K.; Hinshaw, R.G.; Ekwudo, M.N.; Holtzman, D.M.; Saito, T.; et al. Cognitive effects of simulated galactic cosmic radiation are mediated by ApoE status, sex, and environment in APP knock-in mice. Int. J. Mol. Sci. 2024, 25, 9379. [Google Scholar] [CrossRef]
- Smits, E.; Reid, F.E.; Tamgue, E.N.; Alvarado Arriaga, P.; Nguyen, C.; Britten, R.A. Sex-dependent changes in risk-taking predisposition of rats following space radiation exposure. Life 2025, 15, 449. [Google Scholar] [CrossRef]
- Klein, P.M.; Parihar, V.K.; Szabo, G.G.; Zöldi, M.; Angulo, M.C.; Allen, B.D.; Amin, A.N.; Nguyen, Q.-A.; Katona, I.; Baulch, J.E.; et al. Detrimental impacts of mixed-ion radiation on nervous system function. Neurobiol. Dis. 2021, 151, 105252. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, S.; Liu, A.; Blackwell, A.A.; Britten, R.A. Multiple decrements in switch task performance in female rats exposed to space radiation. Behav. Brain Res. 2023, 449, 114465. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.W.; Stanbouly, S.; Chieu, B.; Sridharan, V.; Allen, A.R.; Boerma, M. Low dose space radiation-induced effects on the mouse retina and blood-retinal barrier integrity. Acta Astronaut. 2022, 199, 412–419. [Google Scholar] [CrossRef]
- Weiss, M.; Nikisher, B.; Haran, H.; Tefft, K.; Adams, J.; Edwards, J.G. High throughput screen of small molecules as potential countermeasures to galactic cosmic radiation induced cellular dysfunction. Life Sci. Space Res. 2022, 35, 76–87. [Google Scholar] [CrossRef]
- Edwards, S.; Adams, J.; Tchernikov, A.; Edwards, J.G. Low-dose X-ray radiation induces an adaptive response: A potential countermeasure to galactic cosmic radiation exposure. Exp. Physiol. 2024, EP091350. [Google Scholar] [CrossRef]
- Britten, R.A.; Fesshaye, A.; Tidmore, A.; Blackwell, A.A. Similar loss of executive function performance after exposure to low (10 cGy) doses of single (4He) ions and the multi-ion GCRSim beam. Radiat. Res. 2022, 198, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Boutros, S.W.; Zimmerman, B.; Nagy, S.C.; Lee, J.S.; Perez, R.; Raber, J. Amifostine (WR-2721) mitigates cognitive injury induced by heavy ion radiation in male mice and alters behavior and brain connectivity. Front. Physiol. 2021, 12, 770502. [Google Scholar] [CrossRef]
- Mehta, S.K.; Diak, D.M.; Bustos-Lopez, S.; Nelman-Gonzalez, M.; Chen, X.; Plante, I.; Stray, S.J.; Tandon, R.; Crucian, B.E. Effect of simulated cosmic radiation on cytomegalovirus reactivation and lytic replication. Int. J. Mol. Sci. 2024, 25, 10337. [Google Scholar] [CrossRef]
- Blackwell, A.A.; Fesshaye, A.; Tidmore, A.; Lake, I.R.; Wallace, D.G.; Britten, R.A. Rapid loss of fine motor skills after low dose space radiation exposure. Behav. Brain Res. 2022, 430, 113907. [Google Scholar] [CrossRef]
- Raber, J.; Fuentes Anaya, A.; Torres, E.R.S.; Lee, J.; Boutros, S.; Grygoryev, D.; Hammer, A.; Kasschau, K.D.; Sharpton, T.J.; Turker, M.S.; et al. Effects of six sequential charged particle beams on behavioral and cognitive performance in B6D2F1 female and male mice. Front. Physiol. 2020, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Keiser, A.A.; Kramár, E.A.; Dong, T.; Shanur, S.; Pirodan, M.; Ru, N.; Acharya, M.M.; Baulch, J.E.; Limoli, C.L.; Wood, M.A. Systemic HDAC3 inhibition ameliorates impairments in synaptic plasticity caused by simulated galactic cosmic radiation exposure in male mice. Neurobiol. Learn. Mem. 2021, 178, 107367. [Google Scholar] [CrossRef]
- Kumar, K.; Angdisen, J.; Ma, J.; Datta, K.; Fornace, A.J.; Suman, S. Simulated galactic cosmic radiation exposure-induced mammary tumorigenesis in Apcmin/+ mice coincides with activation of ERα-ERRα-SPP1 signaling axis. Cancers 2024, 16, 3954. [Google Scholar] [CrossRef]
- Alaghband, Y.; Klein, P.M.; Kramár, E.A.; Cranston, M.N.; Perry, B.C.; Shelerud, L.M.; Kane, A.E.; Doan, N.-L.; Ru, N.; Acharya, M.M.; et al. Galactic cosmic radiation exposure causes multifaceted neurocognitive impairments. Cell. Mol. Life Sci. 2023, 80, 29. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.I.; Kangas, B.D.; Luc, O.T.; Solakidou, E.; Smith, E.C.; Dawes, M.H.; Ma, X.; Makriyannis, A.; Chatterjee, S.; Dayeh, M.A.; et al. Complex 33-beam simulated galactic cosmic radiation exposure impacts cognitive function and prefrontal cortex neurotransmitter networks in male mice. Nat. Commun. 2023, 14, 7779. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.A.; Blackwell, A.A.; Oltmanns, J.R.O.; Einhaus, R.; Lake, R.; Hein, C.P.; Baulch, J.E.; Limoli, C.L.; Ton, S.T.; Kartje, G.L.; et al. Differential organization of open field behavior in mice following acute or chronic simulated GCR exposure. Behav. Brain Res. 2022, 416, 113577. [Google Scholar] [CrossRef]
- Luitel, K.; Siteni, S.; Barron, S.; Shay, J.W. Simulated galactic cosmic radiation-induced cancer progression in mice. Life Sci. Space Res. 2024, 41, 43–51. [Google Scholar] [CrossRef]
- Yun, S.; Kiffer, F.C.; Bancroft, G.L.; Guzman, C.S.; Soler, I.; Haas, H.A.; Shi, R.; Patel, R.; Lara-Jiménez, J.; Kumar, P.L.; et al. The longitudinal behavioral effects of acute exposure to galactic cosmic radiation in female C57BL/6J mice: Implications for deep space missions, female crews, and potential antioxidant countermeasures. J. Neurochem. 2025, 169, e16225. [Google Scholar] [CrossRef] [PubMed]
- Kiffer, F.C.; Luitel, K.; Tran, F.H.; Patel, R.A.; Guzman, C.S.; Soler, I.; Xiao, R.; Shay, J.W.; Yun, S.; Eisch, A.J. Effects of a 33-ion sequential beam galactic cosmic ray analog on male mouse behavior and evaluation of CDDO-EA as a radiation countermeasure. Behav. Brain Res. 2022, 419, 113677. [Google Scholar] [CrossRef]
- Kumar, K.; Moon, B.-H.; Datta, K.; Fornace, A.J.; Suman, S. Simulated galactic cosmic radiation (GCR)-induced expression of Spp1 coincide with mammary ductal cell proliferation and preneoplastic changes in Apc mouse. Life Sci. Space Res. 2023, 36, 116–122. [Google Scholar] [CrossRef]
- Riego, M.L.; Meher, P.K.; Brzozowska, B.; Akuwudike, P.; Bucher, M.; Oestreicher, U.; Lundholm, L.; Wojcik, A. Chromosomal damage, gene expression and alternative transcription in human lymphocytes exposed to mixed ionizing radiation as encountered in space. Sci. Rep. 2024, 14, 11502. [Google Scholar] [CrossRef]
- Gkikoudi, A.; Manda, G.; Beinke, C.; Giesen, U.; Al-Qaaod, A.; Dragnea, E.-M.; Dobre, M.; Neagoe, I.V.; Sangsuwan, T.; Haghdoost, S.; et al. Synergistic effects of UVB and ionizing radiation on human non-malignant cells: Implications for ozone depletion and secondary cosmic radiation exposure. Biomolecules 2025, 15, 536. [Google Scholar] [CrossRef]
- Holden, S.; Perez, R.; Hall, R.; Fallgren, C.M.; Ponnaiya, B.; Garty, G.; Brenner, D.J.; Weil, M.M.; Raber, J. Effects of acute and chronic exposure to a mixed field of neutrons and photons and single or fractionated simulated galactic cosmic ray exposure on behavioral and cognitive performance in mice. Radiat. Res. 2021, 196, 31–39. [Google Scholar] [CrossRef]
- Furusawa, Y.; Aoki, M.; Durante, M. Simultaneous exposure of mammalian cells to heavy ions and X-rays. Adv. Space Res. 2002, 30, 877–884. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Chaprov, K.; Abaimov, D.A.; Nesterov, M.S.; Pikalov, V.A. Combined irradiation by gamma-rays and carbon-12 nuclei caused hyperlocomotion and change in striatal metabolism of rats. Life Sci. Space Res. 2025, 44, 99–107. [Google Scholar] [CrossRef]
- Anokhina, I.P.; Anokhin, P.K.; Kokhan, V.S. Combined Irradiation by Gamma Rays and Carbon Nuclei Increases the C/EBP-β LIP Isoform Content in the Pituitary Gland of Rats. Dokl. Biol. Sci. 2019, 488, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef] [PubMed]
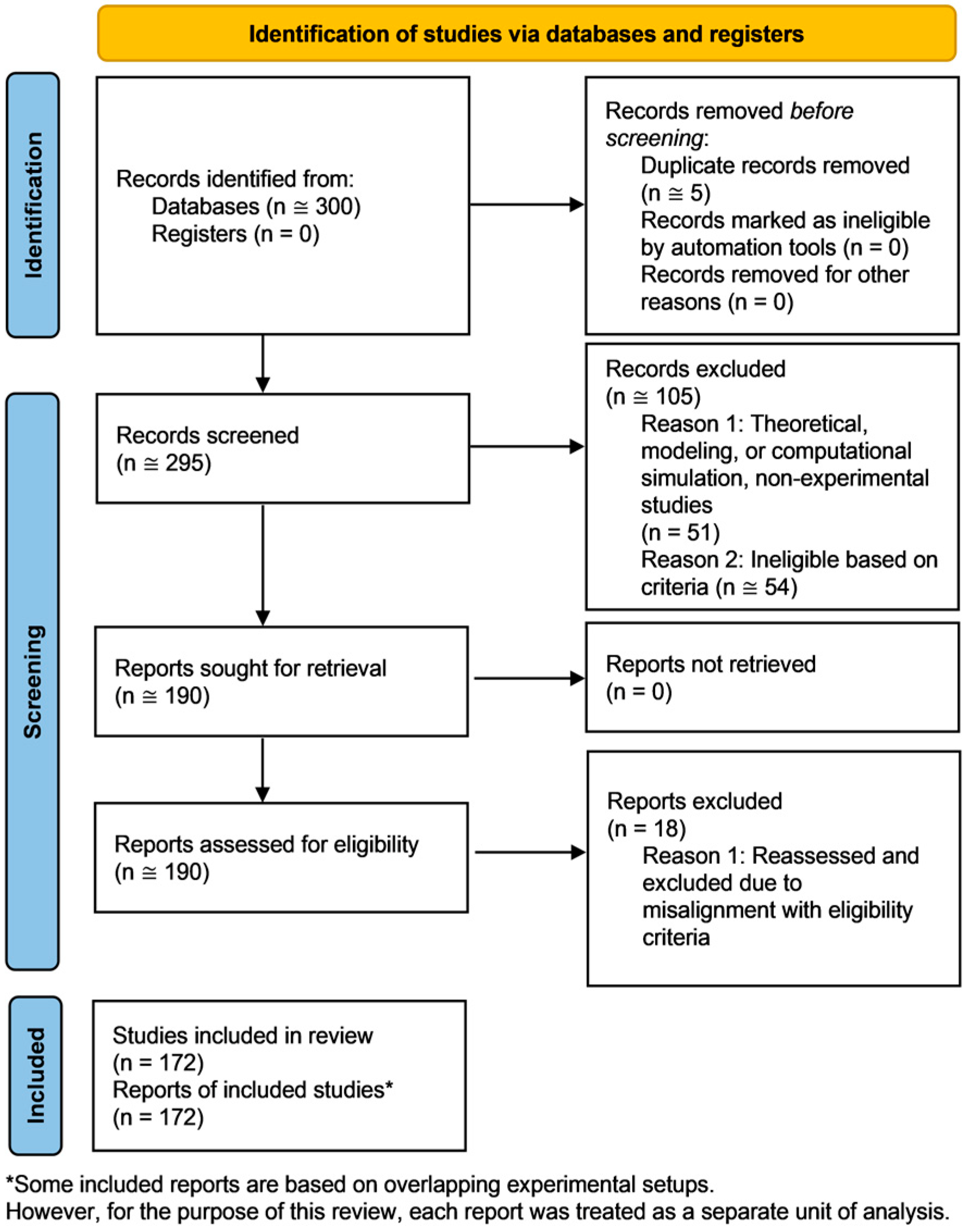
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| Type and Dose | Biological Systems | Biological Endpoints | Effects | Ref. |
|---|---|---|---|---|
| UV-C (254 nm): 70 erg/mm2; X-rays: 150 rad; irradiations 2–6 h post-PHA stimulation | PHA-stimulated human lymphocytes (G1 stage) | Dicentric and chromatid-type chromosome aberrations (first mitosis) | • No synergistic effect observed • Dicentric yields from combined exposures matched X-rays alone • UV induced only chromatid-type aberrations • Suggests G1 cells possess enhanced repair capacity preventing UV–X-ray interaction | [4] |
| UV-C (253.7 nm): 40–100 erg/mm2; X-rays (260 kV): 125–200 rad; exposures ≤ 30 s apart | Human peripheral blood lymphocytes (unstimulated, G0 stage) | Dicentric chromosome formation (cytogenetic analysis) | • ~2-fold synergistic ↑* in dicentrics vs. X-rays alone • Synergy consistent across UV doses and exposure order • Suggests interaction between X-ray-induced breaks and UV-mediated repair inhibition or lesion overlap | [5] |
| UV-C (254 nm): 65–95 erg/mm2; X-rays (260 kVp): 150 rad; exposures given in sequence with <30 s interval | Human peripheral blood leukocytes from healthy donors; human peripheral blood leukocytes from Down’s syndrome patients | Chromosome aberrations (dicentrics); DNA repair synthesis ([3H]thymidine incorporation) | • ↑ Dicentrics after combined UV + X-rays vs. X-rays alone (2× in controls, 27% in Down’s syndrome) • ↓* UV-induced DNA repair synthesis in Down’s syndrome cells, synergism suggests shared repair pathways | [6] |
| UV-C (254 nm): 6 J/m2; X-rays (260 kVp, 0.5 Gy/min): 1.5 Gy; timing between irradiations varied from <30 s to 90 min | Human peripheral blood lymphocytes in G0 phase | Dicentric chromosome yield per cell | • UV followed by X-rays produced a stable 2-fold ↑ in dicentrics across all timing intervals • X-rays followed by UV showed decreasing synergistic effect with ~20 min half-life • Indicates short-lived DNA lesions responsible for early chromosome exchange events | [7] |
| UV-C (254 nm): 100–175 erg/mm2; X-rays (50 kVp): 450–700 rad; applied sequentially within <40 s; some experiments up to 6 h intervals | Chinese hamster V79 cells (synchronous populations in mid-S-phase) | Cell survival (colony-forming ability) | • Combined exposure resulted in additive ↓ in survival, removal of shoulder in survival curves • Order of exposure modulated effect, with UV preceding X-rays causing greater loss of sublethal damage repair capacity | [8] |
| UV-C (254 nm): up to 1000 ergs/mm2; X-rays (50 kVp): up to ~10 krads/min | E. coli K-12 wild-type; DNA repair mutants (uvrA, uvrB, uvrC, recA, recB, recC, exrA, polA) | Cell survival; DNA single-strand break repair (alkaline sucrose gradients) | • ↑ X-ray sensitivity after UV pretreatment (wild-type and uvr mutants) • UV inhibits type III repair of X-ray-induced DNA SSBs • No synergism in recA, recB, recC, exrA mutants | [9] |
| Near-UV (365 nm): up to 2.5× 106 J/m2; X-rays (250 kVp): up to 19.6 krad (2 krad/min); UV administered ~3–5 min before X-rays | E. coli K12 strains (wild-type W3110, polA mutant P3478, recA recB mutant SR111) | Clonogenic survival; single-strand break rejoining (alkaline sucrose gradients); DNA degradation (TCA-insoluble material) | • Pretreatment with 365 nm UV enhanced X-ray lethality (up to 3.4× slope ↑ in survival curves) • Synergism absent in recA recB mutants • 365 nm inhibited type II and III DNA repair • Full-medium incubation partially restored repair in wild-type and recA recB but not in polA | [10] |
| UV-C (254 nm): up to 960 ergs/mm2; X-rays (150 kVp): up to 40 krads | E. coli B/r and Bs-1 strains; 5-BU substituted DNA and purine-starved cells | Cell survival (colony-forming ability) | • ↑ X-ray sensitivity after UV pretreatment in B/r (up to 3×) • No synergism in 5-BU-substituted or purine-starved cells • UV and 5-BU inhibit X-ray repair • No effect in Bs-1 cells | [11] |
| UV-C (254 nm): 1–120 J/m2; X-rays (100 kVp): 6.7–26.6 krad or alpha particles (4.5 MeV, LET 140–180 keV/μm): 4–48 krad | Diploid Saccharomyces cerevisiae strains (wild-type, rad2 mutant, rad9 mutant) | Colony-forming ability | • ↑ Synergistic loss of colony-forming ability when X-rays precede UV • Reduced UV survival shoulder, altered slopes depending on repair genotype • Synergism absent or reduced in rad2 and rad9 mutants | [12] |
| UV-C (254 nm): 170 J/m2; gamma rays: 90 krad; sequential exposures, no delay | Schizosaccharomyces pombe (wild-type 972h−) and rad1 mutant | Inactivation (colony survival); recovery kinetics | • Strong synergistic interaction in wild-type cells • Survival dropped > 100× beyond additive prediction • No synergism in recombinational repair-deficient rad1 mutant • Recovery kinetics depend on second radiation type, not first | [13] |
| UV-C (253.7 nm): 270–810 J/m2; gamma rays: 0.1–0.3 Mrad; exposures ≤ 5 min apart | Micrococcus radiophilus | Survival/colony-forming ability | • Synergistic killing effect with UV pretreatment (3.5× increased gamma ray sensitivity) • Additive effect with gamma pretreatment | [14] |
| UV-B (300 nm): 20, 40, 60, 80, 100, and 120 s exposure time, fixed distance of 50 cm; gamma rays: 500–3000 Gy | Wild-type and mutant Bacillus velezensis strains from soil and oil samples | Functional microbial endpoints: surfactin yield; isoform distribution (MALDI-TOF); antifungal bioactivity (zone of inhibition); emulsification capacity (E24); colony morphology | • Mutant AF-UVγ2500 showed ~2× higher surfactin yield (1.62 g/L vs. 0.85 g/L) • Stronger antifungal activity, 3× isoform abundance • Sequential UV and gamma ray mutagenesis more effective than either treatment alone | [15] |
| Beta particles (32P, internal): time-integrated decay (up to 25 mc/mg P); UV-C (254 nm): 120–530 erg/mm2; exposures sequential with varied timing and order | Salmonella typhimurium strain LM2 | Inactivation (colony-forming ability) | • Strong synergistic effect between 32P decay and UV damage • Reciprocity observed (UV→32P and 32P→UV) • Synergy eliminated by photoreactivation, suggesting interaction between nonlethal DNA lesions (e.g., thymine dimers and 32P-induced strand breaks) within ~25 nucleotide pairs | [16] |
| Electron beam (32 MeV): 21–28 krad; UV-C (254 nm): 450 erg/mm2; exposures ≤ 2 min apart | E. coli B/r | Survival/colony-forming ability | • Synergism observed • Inhibited by 3 h liquid holding between irradiations regardless of order • Attributed to excision repair activity | [17] |
| UV-C (254 nm): 150 ergs/mm2; Protons (LET 20 keV/µm): 8–40 krads | E. coli B/r (wild-type, log-phase) | Survival (loss of reproductive capacity) | • UV pre-exposure sensitized cells to protons • Survival curve slope increased 1.5–1.7× • Synergy not observed when reverse order applied or with other radiation types • Suggests repair system involvement | [18] |
| NIR (600–1400 nm, non-thermal): 360 kJ/m2 (30 min); X-rays (90 kV, 5.23 Gy/min): 1–4 Gy; sequential exposure with NIR pretreatment 30 min before X-rays; temperature-controlled setup | Human full-thickness skin model (primary dermal fibroblasts and keratinocytes) | DNA double-strand breaks (53BP1, γH2AX); cell proliferation (BrdU, Ki-67); apoptosis (TUNEL); morphology (H&E) | • NIR pretreatment delayed repair of X-ray-induced DSBs • Protected fibroblasts from apoptosis • Counteracted X-ray-induced proliferation inhibition in keratinocytes • No morphological disruption observed • Photobiomodulation modulated radiation stress response | [19] |
| Low-intensity laser radiation (670 nm): 5.3–16 J/cm2 over 3–4 days, finished 24 h before gamma irradiation; gamma rays (137Cs): 1 or 3 Gy whole-body; | Wistar rats (peripheral blood in vivo) | Cell counts (RBC, WBC, LYM); enzyme activity (SOD, catalase); blood oxygenation | • No synergistic toxicity • Laser pretreatment ↑ leukocyte counts, antioxidant enzyme activity, and oxygenation • Evidence of radioprotective effects from laser pre-irradiation | [20] |
| Type and Dose | Biological Systems | Biological Endpoints | Effects | Ref. |
|---|---|---|---|---|
| UVA (320–400 nm): 0.5, 1, 2 J/cm2; VIS (395–600 nm): 2, 4, 6, 8 J/cm2; Mixed UVB (5%, 310–320 nm est.), UVA (25%, 320–400 nm), visible light (70%, 395–600 nm) = mUV/VIS: 2, 4, 6, 8 J/cm2 (i.e., 0.1–0.4 J/cm2 UVB) | Human volunteers (ragweed-allergic patients, n = 19); forearm skin tested | Skin prick test (SPT) wheal size; allergen-induced immediate hypersensitivity response | • Only mUV/VIS (not UV-A or VIS alone) caused significant, dose-dependent inhibition of allergen-induced wheal formation (up to 83% at 8 J/cm2) • Inhibition occurred even at suberythematous doses • Suggests synergistic action of UV and VIS light on mast cell-mediated response | [21] |
| UV-B (290–320 nm); UV-A (320–400 nm); VIS (400–740 nm); Near-infrared (predominantly IR-A, 760–1400 nm); combined and filtered exposure (natural sunlight fractions) | Human skin tissue (in vivo biopsies) | Photoaging biomarkers (MMP-1, MMP-9, expression, type I procollagen levels), inflammatory cell infiltration (neutrophils, macrophages) | • Full-spectrum sunlight elicited stronger biomarker changes than UV-filtered or heat-only conditions • Composite effects consistent with contributions from multiple spectral components • Enhanced MMP-1 expression, reduced type I procollagen, inflammatory cell infiltration • Neutrophil recruitment required UV, whereas macrophage infiltration also occurred with visible/IR and heat | [22] |
| UV-A (315–400 nm): 25 J/cm2; UV-B (280–315 nm): 0.014 J/cm2, alone and in combinations over 600 days | Albino hairless mice (SKH:HR1 strain) | Tumor induction (SCC, papillomas, solar keratoses); tumor latency (t50); size-specific incidence | • UV-A and UV-B showed additive effects on SCC induction • No significant synergism or antagonism • Papillomas more frequent under UV-A • UV-B enhanced papilloma induction by UV-A | [23] |
| UV-A (379.68 nm): ~11.65 µW/cm2; UV-B (305.22 nm): ~8.65 µW/cm2; 6 h/day exposure for up to 27 days | Scyphozoan jellyfish polyps (Aurelia sp.) | Asexual reproduction (budding rate), survival (mortality), substrate detachment, tentacle condition (retraction/loss), feeding behavior | • Strong synergistic effect: combined UV-A + UV-B caused 100% mortality by day 21 • No budding, total loss of attachment and feeding • UV-B alone reduced reproduction and health • UV-A alone had minimal impact • Combined exposure drastically worsened all outcomes | [24] |
| UV-B (280–315 nm): 1080–3600 J/m2; UV-C (100–280 nm): 280–930 J/m2 | Sclerotinia sclerotiorum; tomato plants | Sclerotia germination; mycelial growth; ROS production; lipid peroxidation; SOD/CAT activity; disease severity; chlorophyll levels; fruit yield; defense gene expression | • Combination had strongest pathogen suppression and yield/quality improvement • ↑* Defense gene expression and antioxidants | [25] |
| UV-A (365 nm): 49 W/m2; UV-B (311 nm): 8 W/m2; simultaneous exposure for 6–8 h/day | E. coli MG1655 (wild-type and mutant strains) | Colony-forming ability; log10 CFU reduction; whole-genome mutations; tRNA photodamage (UVA absorbance, s4U-mediated) | • Strong synergistic inactivation effect (~100× more than UV-B alone) • Synergy traced to thiouridine (s4U) in tRNA absorbing UV-A and impairing protein synthesis, reducing DNA repair • Mutants with ThiI gene alterations lost synergy, confirming mechanistic link | [26] |
| UV-A (365 nm): 600–1200 mJ/cm2; UV-C (268 nm): 2.5–20 mJ/cm2; sequential exposure with UV-A first, intervals 0–24 h; LED-based setup | E. coli K12 MG1655 (wild type) and SP11 (ThiI mutant) | Inactivation (CFU assay), growth delay (OD600), single-cell division time, tRNA photodamage | • Strong synergistic inactivation in wild type (up to 5.7 log10 reduction with UV-C after UV-A) • Effect absent in ThiI mutant lacking s4U tRNA modification • Synergy linked to UV-A-induced translational arrest via tRNA damage • Effect persists up to 24 h post UV-A | [27] |
| UV-LEDs (267 nm, 275 nm, 310 nm): 0.384 mW/cm2; combined exposures (267/275, 267/310, 275/310 nm) matched for irradiance; fluences of 8.78–23.04 mJ/cm2 for 3–4 log inactivation | E. coli (strain CGMCC 1.3373) in water suspension | Inactivation efficiency (log reduction), photoreactivation and dark repair | • No synergistic effect observed for combined wavelengths • 267 nm UV-LED had highest inactivation efficiency • 275 nm showed strongest resistance to reactivation, likely due to protein damage • Photoreactivation was dominant over dark repair | [28] |
| UV-C (222 nm): 0.32 mW/cm2; UV-C (275 nm): 0.50 mW/cm2; delivered simultaneously for 12–20 s | E. coli ATCC 15597 (bacteria) and PR772 (bacteriophage) in PBS suspension | Log10 microbial inactivation; photoreactivation and dark repair; DNA damage; ROS production | • Marked synergistic inactivation of E. coli (synergism coefficient up to 1.92) • No synergism for PR772 • DWUV suppressed photoreactivation in both organisms • Enhanced ROS and protein damage likely mechanism for synergy in bacteria | [29] |
| UV-C (260 nm): 38–122 mJ/cm2; UV-C (280 nm): 41–89 mJ/cm2; UV-C (260|280 nm): 41–105 mJ/cm2 | E. coli, MS2 coliphage, Human adenovirus type 2 (HAdV2), Bacillus pumilus spores | Inactivation kinetics (log10 reduction), DNA/RNA damage (qPCR) | • No synergistic effect observed • Combined 260|280 nm inactivation matched additive sum of individual wavelengths • No enhanced DNA/RNA damage or energy efficiency • Supports fluence-based independence of dual UV wavelengths | [30] |
| UV-C (222 nm): 1.0–2.4 mJ/cm2; UV-C (282 nm): 0.8–2.1 mJ/cm2; fluences for 5-log inactivation: E. coli 2.4–2.6 mJ/cm2, E. faecalis 3.6–5.4 mJ/cm2 | E. coli and Enterococcus faecalis in synthetic water (pH 6.4–7.0) | Log10 microbial inactivation (CFU count); photoreactivation and dark repair | • Strong time-based synergistic effect in all dual-wavelength combinations (φ = 1.3–3.8) • No dose-based synergy • Complete inactivation achieved rapidly • Synergy linked to combined protein damage and DNA damage mechanisms • No repair observed after DWUV exposure | [31] |
| UVA (365 nm): 1.7–52 J/cm2; UVC (265 nm): 4.2–20 mJ/cm2 | E. coli (ATCC 11229) and coliphage MS2 (ATCC 15597-B1) in PBS suspension | Log10 inactivation; photoreactivation and dark repair; ROS-mediated effects (inferred via scavenger assays) | • UV-A pretreatment significantly enhanced UV-C inactivation of E. coli (up to +2.2 log) • Eliminated photoreactivation by impairing self-repair (via hydroxyl radicals inside cells) • No synergy observed for MS2 • Simultaneous UV-A+UV-C decreased E. coli inactivation (photoreactivation effect) | [32] |
| UVA (369 nm), UVB (288 nm), UVC (271 nm), dual UV (288/271 nm, 369/288 nm, 369/271 nm): 0.75–6.75 mJ/cm2 | E. coli, Staphylococcus epidermidis, S. Typhimurium, Serratia marcescens, Pseudomonas alcaligenes on agar (simulated food surface) | Log10 inactivation (colony count) | • Significant synergistic effect observed only for 288/271 nm (UV-B/UV-C) on E. coli, S. epidermidis, and S. Typhimurium • Synergy ratios 0.20–0.87 • No synergy with 369/271 or 369/288 combinations • Pulsed and continuous modes equally effective at same dose | [33] |
| UVA-LED (365 nm): 3240 mJ/cm2; UVC-LED (275 nm): 375–750 mJ/cm2, applied sequentially in recirculating water system | E. coli (ATCC 8099) in aquaculture recirculating water | Survival (log inactivation), photoreactivation and dark reactivation rates | • Sequential UVA-UVC irradiation produced ~2–3 log higher inactivation than UVC alone • UVA pretreatment enhanced bactericidal efficacy and reduced bacterial reactivation • Higher UVC doses further suppressed reactivation | [34] |
| UV-A (365 nm): 700 W/m2; UV-C (254 nm): 0.7 W/m2; simultaneous irradiation for 6 min in 96-well plate format | Vibrio parahaemolyticus WT and recA/lexA mutants; cultures in LB broth | DNA damage (CPDs, 8-OHdG); log survival (colony-forming ability); SOS-dependent repair capacity | • Simultaneous UV-A+UV-C caused synergistic bactericidal effect (log survival −3.3) vs. additive effects of single/seq. exposure (−2.1) • CPD repair suppressed • Synergy absent in SOS-deficient mutants, implicating RecA/LexA-dependent repair in survival | [35] |
| UV-C doses: 5–20 mJ/cm2; irradiance: 0.194 mW/cm2 (260 nm), 0.314 mW/cm2 (280 nm), 0.473 mW/cm2 (260/280 combined); simultaneous exposure | Enteroviruses (CVA10, Echo30, PV1, EV70) in water suspension; propagated in BGMK cells | Log10 inactivation (infectivity via ICC-RTqPCR) | • No synergistic effect observed • 260 nm alone was most effective • Dual 260/280 nm either matched or underperformed vs. 260 nm • 280 nm less effective overall • Results consistent with nucleic acid absorption peak near 260 nm | [36] |
| UV-C (222 nm): 0–25 mJ/cm2; UV-C (254 nm): 0–25 mJ/cm2; UV-C (255/265/285 nm): 0–25 mJ/cm2 each | MS2 bacteriophage (virus surrogate) in water suspension (host: E. coli Famp) | Log10 inactivation (PFU count) | • Significant synergy observed for LP or excimer lamps followed by LEDs • Enhanced disinfection vs. additive predictions • Reverse sequences less effective • Excimer + LP sequence showed highest energy efficiency • Supports order-dependent synergy in UV-UV disinfection | [37] |
| UVC (222 nm or 280 nm); 405-nm blue light; pretreatment: 30 s (222-nm: 7.1 mJ/cm2, 280-nm: 1.2 mJ/cm2); 405-nm: up to 48 h, 86.4 J/cm2 | E. coli, Listeria monocytogenes, Staphylococcus aureus, S. Typhimurium, Pseudomonas aeruginosa (in vitro) | Survival/colony counts; membrane integrity; ROS generation | • Synergistic bactericidal effect on E. coli, L. monocytogenes, S. Typhimurium • Minor for S. aureus • Antagonistic for P. aeruginosa • Synergy linked to ↑ ROS and membrane damage | [38] |
| Type and Dose | Biological Systems | Biological Endpoints | Effects | Ref. |
|---|---|---|---|---|
| Alpha particles (LET 100–238 keV/µm): 0.1–0.2 Gy; X-rays (80 keV): 0.1–0.6 Gy | Human TK6 cells (wild-type and hMYH knockdown) | Clonogenic survival, mutant frequency | • Mixed beams show synergy in wild-type cells (survival) • MYH− cells resistant to survival loss but show high mutant frequency • Oxidative stress role unclear | [39] |
| Alpha particles (241Am, 2.88 MeV, LET ~129 keV/µm): 0.25–2 Gy; X-rays (225 kVp, 0.59 Gy/min): 0.25–3 Gy; applied sequentially with intervals from 15 min to 6 h | PC-3 prostate cancer cells and U2OS osteosarcoma cells | Clonogenic survival | • Sequential mixed-field exposures showed significant sublethal damage repair • RBESLD ~2.8–3.7 • Repair kinetics similar to X-rays • Order of exposure slightly modulated survival at late timepoints | [40] |
| Alpha particles (2.9 MeV, LET ~140 keV/μm, 11–45% of total dose); X-rays (250 kVp, dose rate 0.1 Gy/min); total dose of 1–10 Gy; sequential exposure (alpha then X-ray) | T-1 human kidney cells; aerobic and hypoxic conditions | Clonogenic survival (aerobic and hypoxic conditions) | • Alpha particle irradiation ↑* RBE (~2.3 at 10% survival) and ↓ OER sharply (~1.0) • Mixed radiation ↓ OER further • Observed trends aligned with theory for mixtures of high- and low-LET radiation | [41] |
| Alpha particles (LET 90.9 keV/μm); X-rays (190 keV); 1:1 dose ratio mixed beam (e.g., 1 Gy = 0.5 Gy alpha particles + 0.5 Gy X-rays); total dose of 0.5–2 Gy; simultaneous exposure using custom irradiation setup | Human peripheral blood lymphocytes (from 4 donors) | DNA damage response gene expression (FDXR, GADD45A, MDM2, BBC3, CDKN1A, XPC, qPCR at 24 h post-exposure) | • Mixed beams induced gene expression levels ≥ alpha alone • Synergy detected in 3 of 4 donors using “envelope of additivity” • FDXR most responsive • ATM inhibition decreased response, indicating role in synergistic effect | [42] |
| Alpha particles (LET 90.92 keV/μm): 0.13–0.54 Gy; X-rays (190 kV): 0.20–0.80 Gy; mixed beams included 0.20X + 0.07 alpha, 0.40X + 0.13 alpha, and 0.40X + 0.27 alpha (doses in Gy); simultaneous exposure via dual-source setup | Human peripheral blood lymphocytes (PBL) from 1 donor | Chromosomal aberrations (simple vs. complex, via FISH in chromosomes 2, 8, and 14) | • Significant synergistic effect at the level of complex aberrations for two highest mixed doses • Linear-quadratic dose–response for complex events • ↑ damage complexity suggests higher-than-additive biological effect | [43] |
| Alpha particles (LET 97–238 keV/μm): 0.13–1.33 Gy; X-rays (190 kV): 0.25–2.00 Gy; mixed-beam doses: 0.38, 0.77, 1.53 Gy; simultaneous exposure using custom dual-source irradiator at 37 °C | Human peripheral blood lymphocytes (1 male donor) | Micronucleus (MN) frequency in binucleated cells; MN size | • All mixed-beam doses showed statistically significant synergistic effects (average 1.8× higher MN than additive prediction) • Linear dose–response • Synergy attributed to impaired repair of X-ray-induced damage by prior alpha exposure | [44] |
| Alpha particles (LET 100–172 keV/μm): 0–1 Gy; X-rays (80 keV): 0–1 Gy (always 1:1 ratio in mixed exposures) | Human osteosarcoma U2OS cells expressing 53BP1-GFP | DNA double-strand break focus formation and decay (53BP1 foci); ATM and p53 activation | • Strong synergistic interaction observed in both small and large DSB foci • Slower focus decay and prolonged ATM/p53 signaling suggest overwhelmed DNA repair • Synergy most pronounced at lower total doses | [45] |
| Alpha particles (LET 100–238 keV/μm): 0.13–0.32 Gy; X-rays: 0.20–0.80 Gy; mixed beams: 25% alpha + 75% X-rays (e.g., 0.53 Gy ≅ 0.13 alpha + 0.40 X); simultaneous exposure using MAX dual-source system | Human VH10 fibroblasts (immortalized) | γ-H2AX focus formation and repair kinetics (IRIF); small vs. large focus quantification | • No dose–response synergy detected at 1 h • Mixed-beam exposure delayed formation and decay of large foci compared to predicted additive effect, indicating a transient impairment of DNA damage response | [46] |
| Alpha particles (0.223 Gy/min, LET ~91 keV/μm); X-rays (0.052–0.068 Gy/min); total dose of 0–2 Gy; 1:1 ratio in mixed beam; exposures via dual-source platform on blood discs (simultaneous delivery) | Human peripheral blood lymphocytes (PBLs, from 3 donors) | DNA damage (alkaline comet assay), repair kinetics, phosphorylated DDR proteins (ATM, DNA-PK, p53), gene expression (qPCR) | • Mixed beams caused significantly more DNA damage than additive prediction (synergy via envelope analysis) • Delayed repair kinetics • Highest activation of DDR proteins and gene expression vs. either radiation alone • Results support synergistic impairment of repair by clustered + dispersed DNA damage | [47] |
| Alpha (LET 100–238 keV/μm); X-rays (peak 80 keV); 0.5 Gy each; simultaneous delivery using a dual-source irradiator | Human osteosarcoma U2OS cells | 53BP1-GFP foci kinetics, area, intensity, mobility (live microscopy), chromatin dynamics | • Mixed-beam foci showed unique dynamic behavior • Intermediate size, highest intensity, low mobility, and persistent signal • Distinct from additive prediction • Results support synergistic effect via impaired repair and complex DSB clustering at chromatin domains | [48] |
| Alpha particles (LET 91 keV/μm); X-rays (190 kV, 3:1 ratio); total dose of 2 Gy, acute or 0.4 Gy × 5 fractionated | Human microglial HMC3 cells; cultured in vitro on Mylar-covered disks | γH2AX foci, CDKN1A and MDM2 expression (qPCR), IL-1β (ELISA), NF-κB/STING phosphorylation, phagocytosis capacity | • Fractionated alpha or alpha + X-ray exposure→stronger pro-inflammatory response and DNA damage signaling than acute • ↑ IL-1β, CDKN1A, MDM2, STING/NF-κB activation, and phagocytosis • Responses returned to baseline by day 14 | [49] |
| Alpha particles (241Am): 0.05–1 Gy; X-rays (150 kVp, 0.356 Gy/min): 0.05–1 Gy | BEAS-2B (human lung epithelial); SVEC4-10EHR1 (mouse endothelial) cells | γ-H2AX foci count, size distribution, and decay (dephosphorylation rate) over 24 h | • Alpha-induced foci were larger and dephosphorylated more slowly than X-ray-induced foci • Radiation type correctly identified in >80% of blinded tests • Individual alpha and X-ray doses estimated within 12% error using mixed-beam exposure data | [50] |
| Alpha particles: 0.166–0.994 Gy; X-rays: 0.25–1.0 Gy; mixed beam 1: 75% X-rays + 25% alpha (0.333–1.327 Gy); mixed beam 2: 50% X-rays + 50% alpha (0.249–0.999 Gy); simultaneous exposure using custom MAX irradiator at 37 °C | Chinese hamster ovary (CHO) cells (AA8) | Clonogenic survival (colony formation assay) | • No synergistic effect observed • Mixed-beam survival data fell within or near predicted additive models • Envelopes of additivity and mathematical modeling confirmed additivity for both mixed-beam conditions | [51] |
| Alpha particle priming doses (LET ~140 keV/μm): 0.5, 2, or 2.5 Gy; X-rays (250 kV, 3 Gy/min): multiple doses up to ~12 Gy; irradiations separated by ≤3–4 min | V79 Chinese hamster lung fibroblast cells | Clonogenic survival | • Sequential exposure with ≤4 min delay showed strong synergistic effect at 2.5 Gy alpha • Survival curve shoulder (Dq) nearly eliminated • X-ray survival curve slope (Do) unchanged • Synergy attributed to alpha-induced damage interfering with sublethal damage repair from X-rays | [52] |
| Alpha particles (5.50 MeV, 0.18 Gy/min): 0–3 Gy; X-rays (280 kVp, 0.75 Gy/min): 0–15 Gy; mixed exposures: 0.06 or 1 Gy alpha + graded X-ray doses | Rat lung epithelial cells (F344, LEC) | Cell survival (clonogenic), micronuclei induction (FISH), mitotic delay | • Simultaneous exposures caused greater-than-additive effects on cell killing and micronuclei frequency • High-dose alpha (1 Gy) removed shoulder from survival curve • Synergistic slopes observed in micronuclei assays even at low alpha dose (0.06 Gy) | [53] |
| Alpha particles (Columbia University charged-particle microbeam): 1 or 20 particles per nucleus; X-rays: 0.02–0.5 Gy pretreatment, 4h before alpha exposure; 3 Gy challenge, 4 h after alpha exposure | Human–hamster hybrid AL cells (CHO–human chromosome 11) | Mutation at CD59 locus (analysis via multiplex PCR) | • Low-dose X-ray priming (0.02–0.1 Gy) suppressed bystander mutagenesis (~58–62% reduction) • 0.5 Gy priming had minimal effect • Bystander cells showed elevated mutant yield after 3 Gy X-ray challenge (supra-additive) • Priming + alpha exposure increased complex CD59− mutation spectrum | [54] |
| Alpha particles (5.50 MeV, 0.223 Gy/min): 0.83 Gy; gamma rays (662 keV, 0.372 Gy/min): 1.02 Gy; total dose of 1.85 Gy; 5 min transfer time between irradiations | U2OS human osteosarcoma cells stably expressing NBS1-GFP | DNA repair foci frequency, size, intensity, and mobility (NBS1-GFP live-cell imaging) | • Stronger synergistic effect observed for α→γ sequence • Slower repair kinetics, larger and more persistent foci • ↑ Intensity and ↓ mobility • γ→α induced faster decay and lower focus intensity • Results suggest order-dependent DDR engagement and impaired repair after alpha priming | [55] |
| Alpha particles (LET ~91 keV/μm): 2.5 Gy; gamma rays (0.73 Gy/min): 2.5 Gy; fractionated regimens used as well | Breast cancer (MDA-MB-231), Osteosarcoma (U2OS) | γH2AX foci (TEM, immunofluorescence), colony formation, viability | • Mixed beam causes more γH2AX foci • Stronger reduction in viability/colony formation • Delayed chromatin recompaction enhances cell kill | [56] |
| Alpha (LET ~126 keV/μm, 50 rad/min): ~25% of total dose; gamma (LET ~0.31 keV/μm, 154 rad/min): ~75% of total dose | Diploid yeast (S. cerevisiae, strain BZ34) | Mutation frequency (reversion to arginine independence) | • Statistically significant synergistic effect observed • Reversion frequency 1.34× higher than additive prediction • Enhanced mutagenic effect attributed to interaction between low- and high-LET damage pathways | [57] |
| Alpha, beta, and gamma radiation (mixed radionuclides from Chernobyl fallout including 134Cs, 137Cs, 144Ce, 154Eu, etc.): total dose 1–515 mSv (chronic); gamma rays (60Co source): 0.1–29, 600 mSv (chronic) | Barley (Hordeum vulgare, waxy mutant line); field-grown in contaminated plots and gamma-field controls | Waxy-reversion frequency in haploid pollen; mutation frequency in generative cells | • Combined radionuclide IR caused higher mutation rates per mSv than external gamma • Mutagenicity not explained by dose alone • Enhanced genotoxicity linked to multi-type exposure, chemical synergies, and heterogeneous contamination | [58] |
| Beta (90Sr-90Y, low LET): 1.2–4.8 krad; gamma rays (60Co, low LET): 1.2–4.8 krad; beta and gamma combined (varied proportions): total 1.2–4.8 krad at 8.4 or 17.8 rad/min | Soybean plants (Glycine max cv. Hill) at unifoliolate leaf stage; grown to maturity in field | Survival, plant height, lateral growth frequency and length, vegetative yield, seed yield | • Combined exposure affected lateral growth and yield depending on beta/gamma dose component • Gamma slightly more damaging overall • Interaction effects seen in vegetative vs. reproductive response • Dose-rate and composition sensitivity evident | [59] |
| Alpha particles (5.50 MeV): ~0.9–4.9 kBq/g lung; beta particles (0.062 MeV): ~0.4–2.2 MBq/g lung | F344/Crl rats (inhalation, in vivo) | Radiation pneumonitis mortality rate; respiratory function (vital capacity, compliance, CO diffusion) | • Combined exposure produced additive effects • Validated hazard models for lethality and morbidity • Alpha radiation ~7× more biologically effective than beta • Respiratory dysfunction and fibrosis observed | [60] |
| X-rays (250 kVp): 3 Gy; neutrons (IND-spectrum, high-LET): 0.15–0.75 Gy (5–25% of total 3 Gy dose); neutrons (83%) + gamma (17%): total 0.75 Gy | Human peripheral blood (ex vivo from 5 donors) | Gene expression profiling (microarray, RT-qPCR); TP53 signaling; immune suppression | • Neutron–photon mixtures caused increasing transcriptomic alterations with higher neutron % • 25% neutron had strongest TP53 and immune effects • 22 genes uniquely responded to neutron-containing exposures, suggesting gene-level synergism even without simultaneous irradiation | [61] |
| X-rays (250 kVp): 3 Gy; neutrons (IND-spectrum, broad energy range simulating Hiroshima 1–1.5 km epicenter): 0.75 Gy or contributing 5% (0.15 Gy), 15% (0.45 Gy), or 25% (0.75 Gy) of 3 Gy total mixed dose | C57BL/6 mice (peripheral blood, 7 days post-exposure) | Gene expression (microarray, RT-qPCR); repression of translation and ribosomal gene sets | • Strong synergistic effect at the gene expression level • Mixed exposures→unique suppression of mRNA translation, tRNA processing, EIF2/mTOR signaling, and ribosomal protein genes linked to bone marrow failure • Effects not seen in pure X-ray group • Synergy evident even at 5% neutron | [62] |
| X-rays (250 kVp): 1 or 3 Gy; monoenergetic neutrons (0.35, 0.45, 5.9, 13.7 MeV): 0.1 or 0.3 Gy; sequential exposures with <2 min delay (neutrons always first) | C3H 10T½ mouse fibroblast cells | Oncogenic transformation (type II/III foci); clonogenic survival | • No significant synergistic effect • Transformation frequencies matched additive prediction across all neutron energies • Low-dose combined exposures showed additive behavior even with high-LET neutrons | [63] |
| X-rays (140 kVp): variable dose per fraction (up to ~30 Gy total); neutrons (3 MeV): variable dose per fraction (up to ~25 Gy total); mixed fields included combinations with photon contamination levels of 11%, 32%, 53%, and 72% | Mouse foot skin (WHT/Gy fBSVS mice) | Acute skin reaction scoring | • Strong synergistic interaction during simultaneous exposure • Full quadratic-term interaction confirmed • Synergy declined but persisted up to 6 h delay • Dose-response curves shifted with increasing photon content, supporting sublethal damage interaction model | [64] |
| X-rays (180 kVp): up to ~635 rad; neutrons (14 MeV): up to ~430 rad; various single and sequential doses used; exposures spaced by 5–10 min | L5 mouse fibroblast cell line (subclone of L cells); suspension culture in Eagle MEM | Cell reproductive capacity (colony formation > 50 cells) | • Survival lower in sequentially irradiated cells vs. single modality • Combined exposures showed interaction • RBE values for neutrons 2.5–1.5 vs. X-rays • Evidence of partial overlap but mechanistic differences between neutron and X-ray cell killing | [65] |
| X-rays (250 kVp): 0.2 Gy priming and 1 Gy challenge; neutrons (LET 60–70 keV/μm): 0.2 Gy priming and 1 Gy X-ray challenge; Bragg peak negative pi mesons (LET 35–55 keV/μm): 0.2 Gy priming and 1 Gy X-ray challenge | V79-379A Chinese hamster fibroblasts | Colony-forming ability (>50 cells); plating efficiency; survival after challenge; induction of adaptive response | • Sequential high-LET priming (neutrons/pions) ↑ resistance to subsequent X-rays if 4–6 h elapsed • Adaptation required minimum 0.2 Gy priming • Effect was transient, protein-synthesis-dependent, and indicative of inducible DNA repair mechanisms | [66] |
| X-rays (250 kVp, 3 Gy/min): up to 8.5 Gy; fast neutrons (3.15 MeV, 0.47 Gy/min; 11.3% gamma contamination): up to 3.75 Gy; sequential exposures with 6 min delay (room temp), or 3 h delay (with recovery at 37 °C) | V79 Chinese hamster fibroblasts (in suspension) | Clonogenic survival | • Synergistic interaction observed with 6 min delay • ↓ X-ray survival curve shoulder (Dq), especially after neutron priming • No interaction with 3 h delay • Effects consistent with partial inhibition of repair between exposures | [67] |
| X-rays (250 kVp, 150 rad/min): doses combined with neutrons in split exposures; fast neutrons (25 MeV, Fermilab): 280 or 420 rad; fast neutrons (0.86 MeV, JANUS): up to ~1.6 krad | V79 Chinese hamster cells | Colony-forming ability | • Increased survival after neutron + X-ray fractionation • Εvidence of repair of sublethal damage • Sequential exposure (neutron followed by X-ray) enhanced sublethal damage repair vs. neutron alone | [68] |
| X-rays (250 kVp): 1250 rad; fast neutrons (produced by 16 MeV deuterons on Be target): 540 rad; sequential irradiation with 15 min to 4 h interval | Mouse jejunum (crypt cells) | Crypt survival | • Combined X-rays and neutrons caused additive or interacting effects depending on time between doses • Interaction seen if <4 hrs between • Sublethal damage from neutrons and X-rays showed similar repair kinetics • Full repair→additive effects • Short interval→partial repair and enhanced damage | [69] |
| Neutron beam (HB11, mixed field of protons from neutron capture and induced/incident gamma): 0.25–1.7 Gy; gamma rays (60Co source, used for calibration): 0.25–3 Gy; exposures in water phantom at 37 °C | Human peripheral blood lymphocytes (PBLs) from 6 donors | Chromosomal aberrations (dicentric chromosomes) | • No synergistic effect observed • Mixed beam-induced dicentric yields matched additive predictions from gamma + calculated proton components • RBE values: 3.0 (mixed beam), 7.2 (protons) • Results consistent across varying neutron/gamma ratios | [70] |
| Neutrons (0.1–8 MeV): 0.02 Gy/min; gamma rays: 0.1 Gy/min; total dose: 0.5, 0.75, or 1.0 Gy; mixed beams 1:1 neutron/gamma; sequential exposure (within 8 min) in both orders (γ→n and n→γ) | Human peripheral blood mononuclear cells (PBMCs) from 3 donors | Gene expression (RT-qPCR of FDXR, BBC3, etc.); chromosomal aberrations (dicentric chromosome assay, DCA) | • No synergistic effect detected for either endpoint • Mixed-beam responses were additive regardless of sequence • RBEs for neutrons ranged from 1.39 to 3.91 (gene expression) and 7.30 (dicentrics) • Gamma–neutron order had no significant influence | [71] |
| Νeutrons (2.5 MeV): 1.42 Gy; gamma rays (137Cs source): 1.42 Gy; combined neutron + gamma: 0.71 Gy each; 252Cf neutrons: 0–0.71 Gy | I: Human peripheral blood from 3 healthy adult males; II: AHH-1 human lymphocytes | Gene expression profiling (RNA-seq, qPCR); dose–response of BAX, DDB2, FDXR (qPCR) | • Neutrons induced more differentially expressed genes and pathways than gamma rays • Combined exposure activated genes overlapping with both mono-exposures • BAX, DDB2, FDXR showed neutron-specific dose-responses, suggesting their role as molecular targets of neutron damage | [72] |
| Fast neutrons (~6 MeV, 2–3% photon contamination): up to 7.5 Gy; gamma rays (60Co source): up to 7.5 Gy; mixed exposures with 60% gamma + 40% neutrons and vice versa, delivered sequentially with <3 min interval | Chinese hamster V79 cells | Clonogenic survival (colony formation assay) | • Survival after mixed exposure was independent of sequence • Synergistic effects reflected in altered survival curve slopes • Zaider–Rossi model accurately described both sequential and simultaneous outcomes | [73] |
| Neutrons (0.5 eV–10 MeV); gamma rays (0.1–4 MeV); IR-8 nuclear reactor mixed field; simultaneous exposure at total doses from 25 mGy to 2 Gy; compared to gamma-only irradiation in the same dose range. | Neonatal mouse neural stem/progenitor cells (NSCs/NPCs) cultured in vitro | DNA double-strand breaks (γH2AX foci), focus size and repair kinetics, neurosphere formation, cell survival | • Low doses (25–50 mGy) stimulated proliferation • >100 mGy reduced survival • Gamma–neutron IR had higher RBE (max 9.7) vs. gamma alone • Induced large γH2AX foci with slow repair • Sensitivity of NSCs/NPCs to mixed radiation highlighted | [74] |
| Mixed field of fission neutrons and gamma rays (MUTR reactor); neutron dose rate: 0.32 Gy/min; gamma dose rate: 0.42 Gy/min; gamma rays (60Co control): 0.15–1.3 Gy/min; total doses up to ~7 Gy | V79-4 Chinese hamster lung fibroblast cells (monolayer culture) | Clonogenic survival | • Dose-dependent loss of clonogenic survival • Mixed neutron–gamma field caused increased cell killing via interaction effects • Nanodosimetry model suggested localized DNA damage at nanoscales • Steeper survival decline than with gamma rays alone • Clonogenic assay confirmed greater loss of reproductive capacity | [75] |
| Neutrons (14.7 MeV): 10–15 rad/min (5 ± 2% gamma contamination); gamma rays (60Co): 150 rad/min; combined doses with 50% or 75% gamma ray contribution; sequential exposures with immediate succession or extended up to 45 min | Chinese hamster ovary cells (in flasks, room temperature) | Clonogenic survival | • Significant synergistic effect observed at both 50% and 75% gamma ray mix • Survival lower than additive prediction • Minimal impact of sequence or delay up to 45 min • Katz model predictions matched experimental interaction magnitude | [76] |
| Neutrons (14.8 MeV, 4.8 rad/min, 40% of total dose); gamma rays (60Co, 8 rad/min, 60% of total dose); total dose varied across experiments; exposures conducted both simultaneously and sequentially (with ~5 min interval between modalities for sequential exposure) | V79 Chinese hamster lung fibroblast cells (monolayers at 37 °C) | Clonogenic survival | • Simultaneous exposure produced a supra-additive effect • 29% lower survival at 10% level vs. additive prediction • Sequential exposures (either order) matched additive model • Results indicate dose-rate sensitive synergism when beams are mixed | [77] |
| Neutrons (14.8-MeV, 2 cGy/min, 40% of the beam); gamma rays (60Co, 3 cGy/min, 60% of the beam); delivered as pulsed beams in 3 min intervals; total dose varied; exposures given either sequentially (alternating pulses) or simultaneously (combined pulses) | V79 Chinese hamster lung fibroblasts (monolayers at 37 °C) | Clonogenic survival | • Simultaneous exposures caused significantly more cell killing than sequential • Dose ratio at 1% survival = 1.08 • Survival curves fitted better with quadratic models • Confirms supra-additive effect under simultaneous delivery • Supports interaction between damage types | [78] |
| Mixed gamma–neutron radiation (TRIGA Mark-F reactor; ~60% gamma, ~40% neutron): 570–30,000 rads; delivered in steady-state (1000 rad/min) or pulsed mode (88% of dose in <40 ms) | Male Sprague–Dawley rats; serum and urine sampled 6–72 h post-exposure | Fluorescence intensity in serum (360, 465 nm) and urine (400, 425 nm) | • Serum fluorescence at 465 nm ↓ dose-dependently at 24 h (570–9300 rads) • 360 nm peak ↑ at 72 h (non-dose-dependent) • Urine fluorescence at 425 nm ↑ with dose (1000–30,000 rads) • Limited utility as biological dosimeter | [79] |
| Mixed neutron–gamma radiation (light water-moderated research reactor; neutron peak energy 0.4–0.6 MeV; ~30% gamma component)l total dose of 0.5, 2, or 4.5 Gy; whole-body exposure in rotating cage | Male F1 (C57BL × DBA2) mice; erythrocytes, lymphocytes, platelets isolated from whole blood | Changes in membrane lectin-binding capacity; alterations in cell surface morphology; intracellular membrane structural changes | • ↑ Lectin-binding observed as early as 30 min • Lymphocytes most sensitive (up to 2.5× increase) • Dose- and time-dependent oscillatory membrane responses • Membrane ultrastructure altered (filopodia, vacuolization, ER dilation) • Effects not dose-proportional above 0.5 Gy | [80] |
| Gamma rays (60Co, ~2.0–2.5 MeV): 0.1 Gy; mixed neutron (0.5–10 MeV) and gamma radiation (2.0–2.5 MeV): 1 Gy | C57BL/6J male mice; hippocampus and brain immune cells analyzed | Behavioral performance (open-field test, Morris water maze, MWM); microglial and astrocyte populations (flow cytometry); cytokine and neurotrophin levels (qPCR, ELISA) | • Combined irradiation impaired hippocampus-dependent memory (unlike gamma, neutron alone) • ↑ M2 microglia, astrocytes, BDNF and NT-4 expression • ↓ TNF-α and IL-1β vs. gamma, neutron • Suggests anti-inflammatory neuroadaptation post gamma-priming | [81] |
| Mixed neutron–gamma radiation (neutrons: 0.5–3.0 MeV, ~85% of dose; gamma rays: <10% of dose): 375 rads or 1000 rads | ICR female mice (germfree and conventional); small intestine | Mucosal atrophy, crypt regeneration, occurrence of diarrhea, survival time | • At 375 rads, mucosal recovery occurred in both groups before death • At 1000 rads, lesion progression and death occurred earlier in conventional mice • Villus atrophy, lipid-filled cells, and delayed regeneration observed in germfree mice • Epithelial denudation not seen | [82] |
| Neutrons (43 MeV): 4–8.5 Gy; gamma rays (60Co): up to 20 Gy; delivered sequentially with short intervals (0–3 h) between modalities | V79 Chinese hamster cells in plateau phase (G1 arrest) | Clonogenic survival (colony formation assay) | • Interaction effect shown as significant reduction in or elimination of survival curve shoulder • Damage from both modalities appears to involve similar PLD • Response mimics that seen with β-araA treatment | [83] |
| Epithermal neutron source (Studsvik: includes fast neutrons > 1 MeV and γ-rays): total dose 8.2–16.2 Gy/h, depth-dependent; epithermal neutron source (Birmingham: neutrons ≤ 1 MeV and γ-rays): total dose 0.58–1.04 Gy/h, depth-dependent | Chinese hamster V79 fibroblast cells | Clonogenic survival | • ↓ Clonogenic survival with depth • ↑ RBE values (Studsvik > Birmingham) • Evidence of high-LET and low-LET interaction enhancing biological damage | [84] |
| Neutrons (0.2–9 MeV, 0.96 Gy/h, ~17% gamma ray component): 0.15–0.75 Gy; gamma rays (0.17 Gy/h): 0.03–0.15 Gy; X-rays (1.23 Gy/min): 2.1–2.82 Gy; total dose of 3 Gy; equitoxic 0.9 Gy (0.75 Gy neutrons + 0.15 Gy gamma rays) | Mouse (C57BL/6J) serum | Changes in serum lipid classes (LPC, PC, TG, DG, PS, CE, SM, LPE) | • Synergistic pro-inflammatory and hyperlipidemic lipidomic changes • ↑ LPC/PC ratio (inflammatory biomarker) • ↑ TG and PS | [85] |
| Fast neutrons (14.5 ± 1.04 MeV, high LET): 0.7 Gy (2 Gy EQD); protons (67–83 MeV, spread-out Bragg peak): 2 Gy (2 Gy EQD); combined sequential exposure (neutron→proton, 2 h interval): 4 Gy EQD | Human breast cancer cell lines (MCF-7, MDA-MB-231) | Cancer stem cell fraction (ALDH+/CD44+/CD24−); stemness gene expression (OCT4, NANOG, SOX2) | • Combined exposure decreased CSC fraction additively in MCF-7 and antagonistically in MDA-MB-231 • No significant changes in stemness gene expression • Response depended on cell line and radiation sequence | [86] |
| Neon ions (425 MeV/amu, LET ~234 keV/μm, 500–600 rad/min): up to 6 Gy; X-rays (225 kVp, 270 rad/min): up to 8 Gy; sequential exposures with 0–24 h interval | V79 Chinese hamster lung fibroblasts (asynchronous monolayer) | Clonogenic survival | • Synergistic reduction in survival with both sequences • Strongest effect with minimal delay • High-LET priming eliminated shoulder of X-ray curve • Low-LET priming steepened neon ion curve • Synergy diminished with 3–24 h interval, indicating repairable, interacting damage types | [87] |
| Neon ions (425 MeV/u, LET ~234 keV/μm): 3.3 Gy; X-rays (225 kVp): 5.5 Gy; Argon ions (570 MeV/u, LET ~117 keV/μm): 2.04 or 3.57 Gy | Chinese hamster V79 fibroblasts (synchronized at GI/S, mid-S, and late-S phases) | Clonogenic survival | • Strong synergistic effect, greatest in late S-phase • Interaction diminished with ≥3 h delay • Survival response depended on cell cycle stage and priming radiation type • Supports repairable, phase-specific sublethal damage interaction | [88] |
| Deuterons (50 keV/μm): 2 or 5.6 Gy; 3He ions (96 keV/μm): 2.5 or 4 Gy; 3He ions (160 keV/μm): 4 Gy; X-rays (50 kVp): graded doses following each high-LET dose | Chinese hamster V79 cells (synchronized in late S-phase) | Clonogenic survival | • Strong synergistic effects observed across all LETs and doses •↑ ER with LET and priming dose • Mixed irradiation decreased survival more than additive prediction • Results consistent with interaction of sublethal damage from high- and low-LET radiation | [89] |
| Priming dose: 7 Gy neon ions (557 MeV/u, LET 115–240 keV/μm) or 20 Gy X-rays (225 kVp, 6 Gy/min); top-off X-ray doses: 7.5, 15, or 25 Gy given at 0.5, 4, or 24 h later | Rat rhabdomyosarcoma tumors (R2C5 subline in WAG/Rij rats) | Tumor growth delay (doubling time to 2× volume) | • No significant synergistic effect • Growth delays similar regardless of whether X-rays followed neon ions or X-rays • Top-off doses produced near-additive outcomes even at 0.5 h interval • Rapid sublethal damage repair likely prevented interaction | [90] |
| Type and Dose | Biological Systems | Biological Endpoints | Effects | Ref. |
|---|---|---|---|---|
| X-rays (6–10 MV): 50.4 Gy in 28 fractions; proton beams (250 MeV): 46.2 GyE in 28 fractions, delivered as concomitant boost > 6 h after X-rays | Supratentorial glioblastoma multiforme patients (n = 20) [Clinical study] | Overall survival, progression-free survival, toxicity | • Median survival 21.6 months; 1- and 2-year survival rates 71.1% and 45.3% • Manageable acute hematologic toxicity • Occasional late leukoencephalopathy | [91] |
| Photons (60Co gamma rays or 6 MV X-rays): 50.4 Gy in 28 fractions; proton beams (250 MeV): 25.5 GyE in 17 fractions as concomitant boost (minimum 6 h interval); total 75.9 GyE in 45 fractions over 5.5 weeks | Stage II–IV oropharyngeal squamous cell carcinoma patients (n = 29) [Clinical study] | Locoregional control, disease-free survival, acute and late toxicity | • 5-year locoregional control 84% • Pronounced acute mucosal toxicity (mucositis, dysphagia) • Grade 3 late effects in 11% (fibrosis, trismus, vocal cord paralysis) | [92] |
| X-rays (4–10 MV): ~50–55 Gy; proton beams (160 MeV): 16–28 GyE, combined sequentially in daily fractions; total prescribed dose 66–83 CGE | Patients with skull base and cervical spine chordomas and chondrosarcomas (n = 621) [Clinical study] | Local control, overall survival, normal tissue toxicity | • 10-year local control: chondrosarcoma 94%, chordoma 54% • Male chordoma patients had better outcomes • Main late toxicities included temporal lobe injury (13%), optic neuropathy (4.4%), endocrinopathy (40%) | [93] |
| Protons (95–105 MeV); carbon ions (6C, 400 MeV/n); total RBE-weighted dose ≅ 8.4 ± 0.2 Gy; four sequential exposure schemes with 30–45% 12C contribution; intervals: 0–4 h; sequence: p→C or C→p | Chinese hamster fibrosarcoma cells (B14-150) [In vitro study] | Clonogenic survival | • Significant synergistic effect observed only in C→p sequence with 45% 12C contribution (K = 0.65) • Effect diminished with 30% 12C • Antagonism seen in p→C sequence (K > 1) • ↑* Survival with longer interval in p→C, but ↓* in C→p | [94] |
| Proton beam (OPTIS2; Bragg peak; LET ≅ 0.5–2 keV/μm): 7.5 Gy or 5 Gy; Targeted radionuclide therapy with 177Lu-Folate or 177Lu-PSMA-617 (β−, Eₘₐₓ ≅ 0.5 MeV; LET ≅ 0.2 keV/μm): ~7.5 Gy (8.5 MBq) or ~5 Gy (1.25 MBq) | CD1 nude mice with KB xenografts; BALB/c nude mice with PC-3 PIP xenografts [Preclinical study] | Tumor growth delay (TGDI2/5), median survival, relative tumor volume | • Combination therapy showed additive or synergistic effect depending on tumor model • KB model showed significant synergy (↑ TGDI2/5, no endpoints) • PC-3 PIP model showed additive trend only • Combination well tolerated in both cases | [95] |
| X-rays (160 kV): 2 Gy; 177Lu-PSMA-617: ~40 MBq (400 pmol), administered 4 h after EBRT (preclinical part of the study) | LNCaP xenografts in BALB/c nu/nu mice [Preclinical study] | Tumor growth delay, median survival (in mice, preclinical) | • Combined external beam RT + RLT in mice prolonged tumor doubling time 2.7-fold vs. RT alone • Median survival extended from 22.5 days (untreated) to 44 days | [96] |
| Protons (67–83 MeV, LET ~ 3 keV/µm); heavy recoils (HR, induced by 14.5 MeV neutrons, LET ~ 290 keV/µm); total dose: 6.6–6.8 Gy (RBE); varying p/HR ratios (60/40%, 80/20%); intervals: 0–8 h | B14-150 Chinese hamster fibrosarcoma cells (confluent monolayers) [In vitro study] | Cell survival (clonogenic assay) | • HR→p sequence with 40% HR most effective for reducing survival • p→HR sequence showed partial recovery (T½ ~1.1–1.3 h) • Combinations showed mostly antagonistic interaction (K > 1) • Survival inversely related to HR dose contribution | [97] |
| Proton pencil beam scanning (96–104 MeV; LET ≅ 0.5–1 keV/μm): 40 Gy × 2 (total 80 Gy); neutron radiation (14.1 MeV; high-LET ≅ 100 keV/μm): 5 Gy; sequential exposure in mice with 3 h interval: neutrons before or after protons; neutron dose ~15% of total; CT-guided tumor targeting | SHK mice with solid Ehrlich ascites carcinoma [Preclinical study] | Tumor growth suppression, skin radiation reactions (RTOG/EORTC), relapse frequency, remission duration, survival | • All groups showed tumor suppression • Neutron-after-proton group showed milder skin toxicity and better tolerance • Neutron-before-proton group showed severe toxicity and shortest survival • Combined exposures ↑ relapse rate and ↓ lifespan vs. protons alone | [98] |
| Protons (88–109 MeV); neutrons (14.5 MeV, D-T generator); total dose ~8.6 Gy; neutron/proton dose ratios of 30:70 or 40:60; sequential exposures with 0–8 h delay; survival modeled vs. independent action | Chinese hamster fibrosarcoma cells (B14-150) [In vitro study] | Clonogenic survival (colony assay) | • All neutron–proton schemes showed synergistic effects (K < 1) • Strongest synergy when neutrons delivered first and comprised 40% of dose • Survival significantly below additive prediction • No recovery observed in neutron-first sequence | [99] |
| Priming dose: 0.075 Gy X-rays; challenging dose: 1.75 Gy 137Cs gamma rays; 6 h interval between doses; exposures in mice and ex vivo human thymocytes | Mouse thymocytes (C57BL/6J); human pediatric thymocytes (1 mo–3 yrs) [In vitro study] | Cell cycle (sub-G1), DNA damage (γH2AX), apoptosis (Caspase-3, PARP1), ferroptosis (xCT, GPX4), epigenetic markers (DNMTs, TDG, MBD4) | • Strong synergistic response: earlier and enhanced apoptosis, cell cycle arrest, ferroptosis, and DNA damage response in combined vs. single dose • Priming dose→sensitized “radiation awareness” state • Consistent mouse–human similarity in response | [100] |
| X-rays (320 kVp, whole thorax): 12.5 or 13 Gy; soft X-rays (10 kVp, dorsal skin, 10% surface): 30 Gy | WAG/RijCmcr rats; lung and skin monitored for 210 days post-irradiation [Preclinical study] | Survival (IACUC criteria), breathing interval, lung collagen (fibrosis), mast cell count, skin wound area | • Combined lung + skin irradiation delayed pneumonitis onset and improved survival vs. thorax alone (13 Gy) • Lung collagen ↓ by skin co-irradiation • Captopril enhanced skin healing and delayed lung injury further | [101] |
| X-rays (150 kV, orthovoltage): 9 Gy; photodynamic therapy (THPTS, 760 nm): 20 J/cm2 | Bladder cancer organoids (T-24, RT-112) [In vitro study] | Organoid viability, multimodal cell death (apoptosis, ferroptosis, pyroptosis), quantified immune infiltration (e.g., Jurkat migration assay) | • IR + PDT showed additive/synergistic cytotoxicity • Multimodal cell death • Non-malignant tissue unaffected • ↑ T-cell infiltration | [102] |
| PDT (non-coherent light, 370–680 nm): 30 mW/cm2, 90–180 s; RT (gamma rays, 60Co source, 1.0–1.62 Gy/min): 2 Gy (range tested: 0–15 Gy) | Ehrlich ascites carcinoma cells in BALB/c mice [Preclinical study] | Tumor growth inhibition, plasma membrane damage (Trypan blue assay), DNA damage (chromosomal aberrations) | • PDT damaged membranes • RT caused DNA breaks • Combo had additive tumor inhibition (~33–38%) • HPde acted as dual sensitizer | [103] |
| X-rays: 2, 10, 20 Gy; PDT: 2.5 J/cm2 at 690 nm; PDT given ~10 min before RT | Heterocellular pancreatic cancer spheroids (MIA PaCa-2, Capan2, AsPC-1) co-cultured with patient-derived fibroblasts [In vitro study] | Viability (live/dead staining), necrosis, apoptosis (flow cytometry), DNA damage (γ-H2AX), proliferation (PCNA) | • Low-dose PDT and RT showed synergistic effects • PDT→necrosis and apoptosis • RT decreased spheroid growth • Combination→smaller, less viable spheroids than expected additively • Effects varied by cell line | [104] |
| X-rays (50 kV): 0–10 Gy; nanoparticles: MC540-SAO:Eu@mSiO2 at 50 µg/mL; X-PDT combines RT and nanoparticle-mediated PDT; applied 5 min after injection | Radioresistant human NSCLC cells (H1299); subcutaneous and intrathoracic mouse tumor models [In vitro and preclinical study] | Cell viability (MTT), clonogenic survival, apoptosis/necrosis, DNA damage (comet, γ-H2AX), lipid peroxidation (ROS), tumor growth delay | • Strong synergistic effect: X-PDT significantly more effective than RT alone in vitro and in vivo • Enhanced apoptosis, necrosis, DNA, and lipid damage • Tumor suppression in deep tissues • No systemic toxicity observed | [105] |
| PDT (non-coherent light, 730 nm): 45 mW/cm2, 30–108 J/cm2; indocyanine green (ICG) 50 μM; RT (X-rays, 100 kVp): 2–8 Gy; combination: 4 Gy X-rays + 60 J/cm2 light + 50 μM ICG | MCF-7 breast cancer cells [In vitro study] | Cell viability (MTT assay) | • ICG alone non-toxic but effective photosensitizer • Combo of ICG + light + X-ray killed 96.6% cells • Low-dose X-ray enhances PDT efficacy | [106] |
| RT (320 kV X-rays): 4 or 8 Gy; UV-C (200–280 nm)-emitting nanoscintillators (LuPO4: Pr3⁺, Nd3⁺): 2.5 mg/mL | A549 lung cancer 3D spheroid model [In vitro study] | Tumor spheroid growth; cell death pathways (apoptosis, necrosis); cell cycle arrest (G2/M) | • ↑ Tumor growth inhibition (up to 30% size reduction with uniform NP distribution) • ↑ Apoptosis and necrosis vs. radiation alone • ↑ Permanent G2/M cell cycle arrest • Effect present under hypoxia but reduced vs. normoxia • No nonspecific toxicity from nanoparticles alone | [107] |
| RT (X-rays, 320 kVp): 2–4 Gy; UVC (220–285 nm, via LuPO4:Pr3⁺ NPs, generated in situ by X-rays; NP concentration: 0.5–7.5 mg/mL (optimal: 2.5 mg/mL) | HFF1 normal human fibroblasts, XP17BE UV-sensitive fibroblasts [In vitro study] | Clonogenic survival, CPD formation (ELISA) | • ↑ Cell killing with combined treatment (↓ survival to ~2% at 7.5 mg/mL NPs + 2 Gy) • ↑ CPD formation (~50% equivalent to 15 J/m2 UV-C) • ↑ Effect in XP17BE vs. HFF1 cells • NPs alone cause mild dose-dependent toxicity at ≥5 mg/mL | [108] |
| RT (X-rays (6 MV): 2 Gy; NIR laser (808 nm): 2 W/cm2 for 3–20 min at 43 °C | Human glioblastoma U87MG cells [In vitro study] | Colony formation; cell viability | • ↓ Plating efficiency with IUdR-PLGA-NGO + X-ray + NIR vs. all other groups • ↑ Nanoparticle uptake • Enhanced radio- and thermo-sensitization • No cytotoxicity from laser or nanoparticles alone | [109] |
| Pelvic external beam RT (6 or 15 MV photons): 45 Gy in 25 fractions; HDR brachytherapy (192Ir): 4 fractions of 7–7.5 Gy each during RT (some fractions after RT) | Cervical cancer patients (n = 13, FIGO stages IB–IIIB) [Clinical study] | Tumor volume kinetics (MRI), gross tumor volume (GTV) and high-risk clinical target volume (HR-CTV) reduction | • Tumor volume reduction ~70–75% by first brachytherapy fraction • Further modest shrinkage thereafter • Early initiation of brachytherapy feasible during EBRT | [110] |
| Modulated electrons (9–15 MeV); IMRT (6 MV photons); total dose 38.5 Gy in 10 fractions over 5 days; combined delivery per fraction | Cohort of 7 breast cancer patients with early-stage disease treated post-lumpectomy [Clinical study] | Acute skin toxicity (CTCAE) | • Combined MERT + IMRT achieved comparable target coverage to IMRT alone but reduced dose to ipsilateral lung and heart • No grade ≥ 2 toxicity • Cosmetic results rated as excellent/good in most cases | [111] |
| RT (X-rays): 6 Gy; NIR light (730 nm): 0.4–0.8 W/cm2 for 5–8 min; UCNP@NBOF-FePc-PFA at 80–100 μg/mL | Murine breast cancer cells (4T1.2), U251 glioma cells; BALB/c mice (tumor-bearing) [In vitro and preclinical study] | Tumor cell apoptosis, ROS generation | • Highly synergistic tri-modal effect (radiotherapy + photothermal + photodynamic therapy) • ~96% tumor inhibition in vivo • Massive cell apoptosis • ↑ ROS and temperature under dual irradiation | [112] |
| X-rays (6 MV): 2–6 Gy; diode laser (808 nm): 1.0–1.5 W/cm2 for 10 min; applied sequentially ± Pt nanoparticles (100 μg/mL) | B16/F10 melanoma cells [In vitro study] | Cell viability (MTT assay), intracellular ROS production | • Combined X-ray + laser irradiation with PtNPs significantly decreased viability (~80–90% reduction) vs. either modality alone • Synergistic ROS generation observed | [113] |
| X-rays (6 MV): 4 Gy; NIR laser (808 nm): 1 W/cm2 for 3 min at ~ 42 °C; RT applied 4–6 h after the first laser irradiation | Mouse 4T1 TNBC tumor model (BALB/c nude mice); 4T1 cells in vitro [In vitro and preclinical study] | ROS generation; tumor growth delay; colony formation; apoptosis (TUNEL) | • Strong synergistic effect: 60% complete tumor eradication in vivo • Highest ROS production in combined group • Lowest colony survival and strongest apoptosis in vitro • INS NPs showed excellent tumor targeting and magnetic guidance | [114] |
| NIR-PIT (690 nm laser): external exposure (50 + 100 J/cm2); interstitial exposure (50 + 100 J/cm); combined exposure (25 + 50 J/cm2 external + 25 + 50 J/cm interstitial) | EGFR-positive A431-luc tumor xenografts in nude mice [Preclinical study] | Tumor volume, bioluminescence (viability), survival | • Combined external/interstitial light led to greatest tumor volume reduction and survival vs. either alone • Improved light delivery and tumor coverage enhanced treatment efficacy | [115] |
| Carbon ions (320 MeV/n, LET 46.6 keV/μm): 0.8–4.4 Gy; X-rays (4 MV): 2–8 Gy; carbon 0.4–2.2 Gy + X-ray 1–4 Gy; exposures within 15 min or 72 h apart | Human salivary gland cancer cells (HSG) [In vitro study] | Clonogenic survival (colony formation assay) | • No synergistic effect observed • Combined exposures followed additive prediction model based on GyE • Survival curves and parameters aligned with additive model • Effect independent of irradiation sequence | [116] |
| Carbon ions (290 MeV/u, LET 13–100 keV/μm): 2.0–6.8 Gy; silicon ions (490 MeV/u, LET 55 keV/μm): 3.0 Gy; argon ions (500 MeV/u, LET 85 keV/μm): 2.5–3.0 Gy; iron ions (500 MeV/u and 200 MeV/u, LET 200–860 keV/μm): 1.75–3.5 Gy; X-rays (150 or 200 kVp): 8.0 Gy (priming or test dose) | V79 Chinese hamster cells [In vitro study] | Clonogenic survival | • ↑ Cell killing with sequential ion + X-ray exposure vs. single beams • ↓ SLDR with increasing LET; high-LET ions (≥80 keV/μm) cause largely irreparable damage • LET-dependent reduction in repairable fraction • Evidence of combinatorial suppression of SLDR | [117] |
| Whole-brain photons (60Co source): 45 Gy (1.5 Gy/fraction); neutrons (deuteron–deuterium source, RBE~4.5): 5.2 Gy boost; neutrons given 5–20 min before photons, over 6 weeks | Patients with anaplastic astrocytoma and glioblastoma multiforme (n = 44) [Clinical study] | Tumor control, median survival, histologic response | • Anaplastic astrocytoma (AA) median survival 40.3 months vs. 11 months for glioblastoma multiforme (GBM) • Neutron–photon sequencing within minutes associated with improved survival in AA • High rates of tumor necrosis observed | [118] |
| Whole-brain photons (1.5 Gy/fraction, total 45 Gy); neutron boosts (3.6–6.0 Gy total, 12 fractions twice weekly, given within 3 h of photons | Supratentorial glioblastoma multiforme and anaplastic astrocytoma patients (n = 190) [Clinical study] | Overall survival, radiation injury (autopsy pathology) | • Median survival 9.9 months (glioblastoma) and 22 months (anaplastic astrocytoma) • Higher neutron doses showed trend to worse survival in astrocytoma • Autopsies revealed frequent tumor sterilization but extensive radiation injury | [119] |
| Fast neutrons (30 MeV d-Be, <5% gamma contribution): up to 35.78 Gy (fractionated, 5 n); gamma rays (60Co, 1.00 Gy/min): up to 104.4 Gy (fractionated, 5 gamma); mixed beam (2n + 3 gamma over 5 days): neutrons ~6.78–7.66 Gy, gamma ~66 Gy total | C3H mice bearing syngeneic NFSa fibrosarcoma [Preclinical study] | Cell survival (lung colony assay) | • Mixed-beam (N-γ-γ-γ-N) yielded survival and TCD50 curves indistinguishable from calculated additive effects • No interaction observed • Neutron RBE ~3 vs. gamma rays • Fractionation increased Do and extrapolation number for gamma rays but not neutrons | [120] |
| Proton beams (130–165 MeV spread-out Bragg peak): 2–8 Gy; in situ thermal neutrons and boron neutron capture (α,7Li): 20–80 ppm 10B, 2 h pre-irradiation | Human tumor cell lines (HSG, MG63, SAS, G-361) [In vitro study] | Clonogenic survival | • Proton beams with 80 ppm boron increased RBE up to 1.63 and SER up to 1.57 vs. protons alone • Higher intracellular boron correlated with greater sensitization | [121] |
| BNCT; mixed neutron (thermal: 22.4%, epithermal: 2.4%, fast: 16.7%) + gamma (58.5% of total dose) beam: 1.25 Gy per fraction (neutron)/2 Gy per fraction (gamma) | CHO-K1 Chinese hamster ovary cells [In vitro study] | Clonogenic survival; DNA double-strand breaks (53BP1 foci count and size) | • Fractionated neutron IR led to fewer foci but larger size • Higher D0 vs. single dose • Suggests persistent clustered DSBs due to high LET • Gamma IR showed less foci size difference • Importance of damage complexity in fractionated high-LET fields highlighted | [122] |
| BNCT; alpha particles (3.2 MeV, LET ~120 keV/μm): 0.5–2.0 Gy; gamma rays (60Co): 3.4–8.6 Gy; mixed doses matched for equivalent biological effect (e.g., 0.5 Gy alpha + 3.4 Gy gamma); simultaneous exposure using dual-source setup at 10 °C | V79-4 Chinese hamster cells [In vitro study] | Clonogenic survival (colony formation assay) | • No significant synergistic effect observed • Mixed-beam survival closely matched additive prediction • Results suggest lack of sublethal damage interaction with alpha particles under these conditions | [123] |
| BNCT; alpha particles: 2 or 2.5 Gy; X-rays: variable doses; simultaneous vs. non-simultaneous delivery assessed | V79 Chinese hamster lung fibroblast cells [In vitro study] | Cell survival (clonogenic assay) | • Strong synergistic effect on cell killing observed only at 2.5 Gy alpha when combined simultaneously with X-rays • Survival curve steepened, suggesting alpha exposure impairs DNA repair from low-LET X-rays | [124] |
| BNCT; neutron mixed beam (thermal <0.5 eV: 25%, epithermal 0.5–10 keV: ~2.6%, fast >10 keV: 18–19%, LET range not specified): 0.9–1.0 Gy; gamma rays (60Co, 40 mGy/min): 0.9–1.0 Gy (controls) | CHO-K1 (wild-type) and xrs5 (Ku80-deficient) Chinese hamster ovary cells [In vitro study] | Clonogenic survival; DNA double-strand breaks (53BP1 foci count, size, spatial distribution) | • RBE at 10% survival: 3.3 (CHO-K1) and 1.2 (xrs5) • Focus number and size similar to gamma rays, but neutron-induced foci were spatially clustered • Indicates potential for more complex DNA damage from neutron components | [125] |
| Gadolinium neutron capture therapy (GdNCT): ~56.8 μg 157Gd/g tumor; boron neutron capture therapy (BNCT): ~158 μg 10B/g tumor; thermal neutron irradiation: 1 × 109 neutrons cm−2·s−1 for 60 min | Mice bearing recurrent head-and-neck tumors (HTB-43 xenografts) [Preclinical study] | Tumor regression, cancer stem cell depletion, survival prolongation | • Nearly complete tumor eradication • Suppression of recurrence biomarkers (TGF-α, p53, CD44, PGE2, HIF-α) • Enhanced apoptosis and necrosis | [126] |
| 252Cf neutrons (0.2–0.3 Gy/hr), 137Cs gamma rays (0.7–0.85 Gy/hr) and 60Co gamma rays (1.25–2 Gy/min); total body irradiation with doses up to ~13 Gy, depending on endpoint and mix ratio | Balb/c mice, whole-body irradiation model; gastrointestinal and bone marrow systems assessed [Preclinical study] | GI-50 (6–10 day survival) and BM-50 (30 day survival) syndromes | • Dose required to cause syndromes ↓ sharply up to 35% neutron contribution, then plateaued • Minimal repair in neutron-rich exposures • Mixed beams ≥35% neutrons behaved like high-LET radiation • Fractionation and dose-rate effects negligible for 252Cf but substantial for photons | [127] |
| Type and Dose | Biological Systems | Biological Endpoints | Effects | Ref. |
|---|---|---|---|---|
| Two-ion exposure; protons (1 GeV/n, 0.5 Gy/min): 2Gy; Fe ions (1 GeV/n, 1 Gy/min): 0.75 Gy; sequential exposure with intervals of 2, 30, or 60 min; cells kept at 37 °C between exposures | Human mammary epithelial cells (CH184B5F5/M10) | Chromosome aberrations (mBAND on chromosome 3); intra- and inter-chromosomal exchanges; inversion, deletion, translocation frequency | • Highest aberration frequency observed at 30 min interval • Dual exposure yielded more damage than predicted sum • Supports enhanced susceptibility to Fe damage during early repair phase after proton exposure • Synergy likely driven by interaction of partially repaired lesions | [128] |
| Two-ion exposure; protons (1 GeV/amu): 1 cGy; Fe ions (1 GeV/amu): 1cGy; applied sequentially with intervals from 3 min to 24 h | AG01522 normal human skin fibroblasts | Micronucleus formation and 53BP1 foci induction in irradiated and bystander cells | • Direct exposure→similar DNA damage levels regardless of single or combined exposure • Bystander response unchanged in signaling cells • Prior proton exposure suppressed bystander response in recipient cells | [129] |
| Two-ion exposure; protons (1 GeV); titanium ions (1 GeV/n, LET 108.1 keV/μm) or iron ions (1 GeV/n, LET 151.3 keV/μm); protons: 0–20 cGy; HZE dose: 20 cGy; sequential exposure with 15 min delay | Primary human fibroblasts | Neoplastic transformation (anchorage-independent growth in soft agar); clonogenic survival | • Marked synergistic ↑* in transformation when protons preceded HZE by 15 min • Effect evident even at 1 cGy proton + 20 cGy HZE • Split doses of same ion species did not replicate synergy • Results emphasize protons’ role in sensitizing to subsequent HZE exposure | [130] |
| Two-ion exposure; protons (1 GeV/n); iron or titanium ions (both 1 GeV/n; Fe LET 151.3 keV/μm, Ti LET 108.1 keV/μm; protons: 20 cGy; Fe or Ti ions: 20 cGy; 2.5 min to 48 h in-between irradiations; reverse order also tested | Primary human neonatal fibroblasts | Anchorage-independent growth (soft agar assay); clonogenic survival | • Marked synergistic effect when protons preceded Fe or Ti by 2.5 min–1 h (Fe) or up to ~6 h (Ti) • Transformants per survivor ~3× additive prediction • No synergy when HZE delivered first or at longer intervals • Survival unaffected, suggesting transformation-specific interaction | [131] |
| Two-ion exposure; 1H (150 MeV/n): 0.5 Gy; oxygen ions (16O, 600 MeV/n): 0.1 Gy; whole-body irradiation with a 1 h interval | Male C57BL/6 mice; hippocampus (dentate gyrus, CA1) | Short-term spatial memory (Y-maze); dendritic complexity (Sholl); spine density (mushroom, stubby); synaptic marker expression (Nr2a, Nr2b, GluR1, synapsin-1, drebrin, SAP97) | • Impaired memory and ↓ novel arm recognition • ↓ mushroom spines, ↑ stubby spines in DG and CA1 • Altered dendritic arborization and complexity • ↑ GluR1, Nr2a, synapsin-1, drebrin • Hippocampal remodeling consistent with cognitive dysfunction | [132] |
| Two-ion exposure; 1H (150 MeV/n): 0.5 Gy; 16O (600 MeV/n): 0.1 Gy | Male C57Bl/6J mice, hippocampal neurons | Short-term memory (Y-maze), recognition memory (novel object recognition, NOR), dendritic morphology, spine density, SNP analysis | • Memory deficits • Reduced novel object recognition • ↓ mushroom spine density • Altered dendritic morphology (↑ in dentate gyrus, ↓ in CA1) • ↑ SNPs in Txnrd2/3 | [133] |
| Two-ion exposure; 1H (1 GeV, LET 0.223 keV/µm): 3 × 17 cGy every other day; 56Fe (1 GeV/nucleon, LET 151.4 keV/µm): 15 cGy, 2 days after last 1H dose (see related commentary) | Cardiovascular system (murine heart) | Left ventricular ejection fraction, posterior wall thickness, LV end-systolic and end-diastolic pressure, dP/dtmax, dP/dtmin; cardiac fibrosis (Masson’s trichrome); infarct size; VEGF-A, p-Akt, p-Erk1/2 expression | • Sequence-dependent cardiac effects: increased fibrosis and LV hypertrophy (56Fe + 1H) • Impaired post-MI recovery and increased infarct size (1H + 56Fe) | [134] |
| Three-ion exposure; protons (1 GeV, 60%); 16O ions (250 MeV/n, 20%); 28Si ions (263 MeV/n, 20%); total doses: 0, 25, 50, or 200 cGy; sequentially delivered in rapid succession to mimic cosmic radiation exposure | B6D2F1 (C57BL/6J × DBA2/J F1) male and female mice | Behavioral (home-cage activity, depressive behavior), cognitive (object recognition, fear conditioning), molecular (BDNF, CD68, MAP-2 levels), microbiome diversity | • 50–200 cGy impaired object recognition • 50 cGy ↑ depressive behavior and activity (males) • Radiation altered BDNF (↓ in males), CD68 (↑ in females) • Gut microbiome diversity ↑ in dose-dependent fashion | [135] |
| Three-ion exposure; protons (120 MeV/n): 20 cGy; helium (250 MeV/n): 5 cGy; silicon (300 MeV/n): 5 cGy; whole-body irradiation in 3 orders (H→He→Si, Si→He→H, and H→He +24h→Si); total dose: 30 cGy; CDDO-EA given 3 days pre-IR to 1 day post-IR | K-rasLA1 lung cancer-susceptible mice; lung tissue and plasma | Lesion number (hyperplasia, adenoma, atypia, carcinoma); plasma MDA levels (oxidative stress marker); tumor incidence | • H→He→Si sequence ↑ premalignant lesions, MDA, and adenocarcinomas • Delaying Si by 24 h or using Si→H→He sequence ↓ effects • CDDO-EA countermeasure normalized lesion count and MDA • Sequence- and timing-dependent cancer risk highlighted | [136] |
| Three-ion exposure; protons (1000 MeV, LET 0.24 keV/μm): 1.2 Gy; Si ions (500 MeV/n, LET 54 keV/μm): 0.15 Gy; Fe ions (600 MeV/n, LET 190 keV/μm): 0.15 Gy; total dose: 1.5 Gy (whole-body, sequential rapid switching) | WAG/RijCmcr male rats (6 mo); heart, kidney, blood; 270-day follow-up | Perivascular cardiac fibrosis, blood pressure, serum cholesterol, renal histology, cytokine levels (IL-5, IL-18, IL-17A), macrophage (CD68+) infiltration | • 1.5 Gy→perivascular fibrosis and ↑ systolic BP • ↑ CD68+ cells in heart/kidney • Cytokine shifts at 30–60 d • Single-ion beams did not induce pathology • Threshold for fibrosis likely between 0.75–1.5 Gy | [137] |
| Four-ion simplified mixed field GCR (Smf-GCR); protons 1000 MeV/n, He 250 MeV/n, O 325 MeV/n, Si 300 MeV/n; LET 0.22–69 keV/μm); total dose of 0.5 Gy (0.3 Gy H, 0.1 Gy He, 0.05 Gy O, 0.05 Gy Si) delivered in sequence over ~15 min | Male and female Apc1638N/+ mice (intestinal tumor model) | Intestinal tumor count and classification (adenoma vs. carcinoma) | • Smf-GCR induced more GI tumors and carcinomas than gamma rays • Heavy-ion fraction (O + Si) accounted for >95% of tumorigenic effect • Males had higher tumor burden • Data highlight heavy-ion dominance in GCR-associated GI cancer risk | [138] |
| Five-ion simplified GCRsim (H 1000 MeV/n, Si 600 MeV/n, He 250 MeV/n, O 350 MeV/n, Fe 600 MeV/n, plus H 250 MeV/n); total dose of 150 cGy; single whole-body exposure | C57BL/6 male mice (6 months old) | Cardiac function (echocardiography, MRI, pressure-volume loops), aortic histology | • ↑ Arterial elastance • ↓ Preload-recruitable stroke work • Elastin fiber disruption in aorta • Modest ↓ in cardiac output and stroke volume | [139] |
| Five-ion simplified GCRsim (protons 250/1000 MeV, He 250 MeV/n, O 350 MeV/n, Si 600 MeV/n, Fe 600 MeV/n); total dose of 15 or 50 cGy | Male and female C57Bl/6J mice, immune and endocrine systems (blood, adrenal glands) | Organ weights (thymus, spleen, adrenals), plasma hormone levels (aldosterone, corticosterone), immune cell profiles (phagocytosis, NLR), transcriptomics | • ↓ Thymus/spleen/adrenal weights in males • ↓ Aldosterone in males • ↑ NLR in females (3 days) • ↑ Phagocytosis in males • Sex-specific gene expression changes at 14 days | [140] |
| Five-ion simplified GCRsim [protons (1 GeV, 250 MeV), He (250 MeV/n); O (350 MeV/n), Si (600 MeV/n), Fe (600 MeV/n)]; total dose of 50 cGy; whole-body irradiation | Male BALB/c mice (n = 12, irradiated vs. sham); hippocampus and bone marrow; tissues analyzed 3 months post-IR | Short-term and spatial memory (Y-maze and Morris water maze); neural cell population changes (astrocytes, NPCs, microglia, oligodendrocytes, assessed via flow cytometry; cytogenetics (G-banding, SKY); proteomics (TMT-based differential protein expression analysis) | • GCR exposure impaired short-term and spatial memory • No glial cell changes • Chromosome aberrations ↑ 4× vs. sham • 113 proteins differentially expressed (fold change > 1.5) • Network analysis linked protein shifts to cognition and neurodegeneration | [141] |
| Five-ion simplified GCRsim; protons (1000 MeV, LET 0.20 keV/μm): 17.5 cGy; Si (600 MeV/n, LET 50.4 keV/μm): 0.5 cGy; He (250 MeV/n, LET 1.60 keV/μm): 9 cGy; O (350 MeV/n, LET 20.9 keV/μm): 3 cGy; protons (250 MeV, LET 0.40 keV/μm): 19.5 cGy; Fe (1000 MeV/n): 0.5 cGy; total dose: 50 cGy or 100 cGy depending on group. | Male and female mice; hippocampus, blood, cortex | Spatial learning (RAWM), sociability, social memory, recognition memory; microglia phenotype (CD68, CD107a), synaptic density (PSD-95, Synapsin-1), blood monocyte levels | • GCRsim caused sex-specific spatial learning deficits (males only) • Linked to microglia activation and ↑ synapses • Microglia depletion reversed deficits • Early blood monocyte levels predicted late cognitive decline in males | [142] |
| Five-ion simplified GCRsim (H, He, O, Si, Fe): 0.5 or 0.75 Gy; gamma rays: 0.75 or 2 Gy; whole-body irradiation | Male and female C57BL/6J wild-type and APPswe/PS1dE9 transgenic mice; brain, heart, kidney, plasma | Spatial memory (Y-maze), anxiety (EPM, OFT), sensorimotor gating (PPI), rotarod, MRI volumes, gene expression (VLCAD, Casp3, GLUT4), cytokine levels | • No effect on Aβ • GCRsim and gamma caused sex-specific MRI and behavior changes • GCRsim ↓ VLCAD, Casp3, GLUT4 • Male Tg mice more affected neurologically • GCRsim ↑ hippocampal volumes and ventricular enlargement • Minor kidney/heart fibrosis and altered cytokines | [143] |
| Five-ion simplified GCRsim (H, He, O, Si, Fe); total dose of 500 mGy delivered over ~4.5 h in 6 sequential beams; TGF-βRI inhibitor (IPW-5371) administered in diet pre- and post-IR | BALB/c (male) and CD1 (male/female) mice; cardiac tissue and plasma collected 12–20 weeks post-IR | Cardiac structure/function, collagen deposition, capillary density, immune markers (CD2, CD4, CD45, TLR4), TGF-β1 expression | • GCRsim caused minor cardiac changes • ↑ Collagen and ↓ TLR4 in CD1 males mitigated by TGF-β inhibition • CD1 females showed ↑ capillaries and ↓ ventricular mass • Combined GCRsim + inhibitor altered immune cell marker profiles in both sexes | [144] |
| Five-ion simplified GCRsim (H 1000 MeV/n, He 250 MeV/n, O 350 MeV/n, Si 600 MeV/n, Fe 600 MeV/n); total dose of 5, 15, or 50 cG; whole-body irradiation | Male and female C57BL/6J mice; undisturbed home-cage behavior | Species-typical behaviors: burrowing, grooming, rearing, nest building (Deacon score); Neuroscore test battery (7 tasks) | • No sensorimotor deficits detected • Sex- and dose-specific changes in burrowing (↑ at 15 cGy in females) and grooming (↑ at 50 cGy in females) • Nestlet construction differed by sex and dose • More robust female performance • Delayed effects subtle and behavior-dependent | [145] |
| Five-ion simplified GCRsim beam; total dose of 15 cGy (0.5 cGy/min over ~20 min) | Male Wistar rats (8–9 months old) | Sleep architecture (NREM, REM, TST), EEG spectral power (delta, theta, alpha, sigma, beta bands), core body temperature (CBT) | • ↓ Dark period TST • ↓ NREM • ↓ REM • ↓ NREM delta power • ↓ REM theta power • ↑ NREM/REM alpha and sigma power • ↓ CBT during light period | [146] |
| Five-ion simplified GCRsim beam; total dose of 0.5 Gy delivered over ~20 min | Female C57Bl/6 mice (liver, heart, plasma, soleus muscle); human 3D microvessel cultures (HUVEC-derived) | Microvessel integrity (angiogenesis, collapse), DNA double-strand breaks (53BP1 foci), mitochondrial function, inflammatory pathways (cytokines, ISGs) | • ↓ Microvessel collapse • ↓ DNA DSBs • ↓ Inflammatory signaling (TNF-α, IL-6) • ↑ Mitochondrial function rescue after miRNA antagomir treatment | [147] |
| Five-ion simplified GCRsim beam; total dose of 0.75 Gy; gamma rays: 2 Gy; whole-body irradiation | Male and female transgenic mice (APP;E3F, APP;E4F); hippocampus, plasma, feces | Locomotion/anxiety (open field), motor coordination/learning (rotarod), recognition memory (NOR), spatial memory (Y-maze); hippocampal Aβ pathology (S97, ThioS), brain ApoE levels, plasma cytokine and lipids levels; gut microbiome composition (16S rRNA) | • Modest cognitive and neuropathological effects of GCRsim vs. sham • Radiation interacted with sex, genotype, travel • GCRsim ↓ plasma IL-6, TNF-α, HDL • Long-term microbiome shifts correlated with plaque burden and memory • ApoE genotype shaped responses | [148] |
| Five-ion simplified GCRsim (1 GeV/n protons 35%, 250 MeV/n protons 39%, 250 MeV/n helium 18%, 350 MeV/n oxygen 6%, 600 MeV/n silicon 1%, 600 MeV/n iron 1%; total dose of 10 cGy | Male and female Wistar rats | Risk-taking propensity (RTP), decision-making performance, processing speed | • ↑ Risk-taking in females (↓ profitable choices at 30–60 days) • ↑ Decision latency in males (~2× slower at 30 days) • Performance recovery by 90 days | [149] |
| Five- or six-beam simplified GCRsim; total dose of 5 or 30 cGy; whole-body irradiation | Male C57BL/6J mice; hippocampus, cortex | Hippocampal inhibitory synaptic activity, LFP oscillations, spatial and recognition memory, anxiety behavior | • Mixed-ion exposure disrupted hippocampal GABAergic signaling • Slowed sharp-wave ripple frequency • Impaired memory and ↑ anxiety-like behavior at 30 cGy | [150] |
| Five-ion simplified GCRsim (1 GeV/n protons 35%, 250 MeV/n protons 39%, He 18%, O 6%, Si 1%, Fe 1%); total dose of 10 cGy | Female Wistar rats | Task switching performance, stimulus-response training success, switch cost errors | • ↓ Switch task accuracy (−20%) • ↑ Perseverative errors (anterograde interference) • ↑ Failure to complete training stages • No change in response times | [151] |
| Five-ion simplified GCRsim (1 GeV/n protons 17.5 cGy, 250 MeV protons 19.5 cGy, He 9 cGy, O 3 cGy, Si 0.5 cGy, Fe low dose); total dose of 50 cGy | Male and female CD1 mice, retina tissue | BRB integrity (AQP-4, PECAM-1, ZO-1 expression), oxidative stress (4-HNE), apoptosis (TUNEL assay) | • ↑ AQP-4 expression (female > male) • ↑ PECAM-1 expression (male > female) • ↓ ZO-1 expression • ↑ Oxidative stress (4-HNE) • ↑ Retinal apoptosis | [152] |
| Five-ion simplified GCRsim (1 GeV/n protons 35%, 250 MeV/n protons 39%, He 18%, O 6%, Si 1%, Fe 1%); total dose of 75 cGy | H9c2 myoblasts (rat), ES-D3 pluripotent cells (murine), Hy926 endothelial cells (human) | Mitochondrial function (MTT, TMRE), oxidative stress (DHE), cell senescence (ONPG), neoplastic transformation (Afp-tdTomato expression) | • ↓ Mitochondrial function • ↑ Oxidative stress • ↑ Senescence markers • ↑ Neoplastic transformation markers (Afp-tdTomato) | [153] |
| X-rays (160 kVp): 0.1–1.0 Gy pretreatment, 8 Gy challenge; five-ion simplified GCRsim [1000 MeV/n proton: 26.25 cGy; 250 MeV/n proton: 29.25 cGy, helium (250 MeV/n): 13.50 cGy, oxygen (350 MeV/n): 4.50 cGy, silicon (600 MeV/n): 0.75 cGy, iron (600 MeV/n): 0.75 cGy]: 75 cGy challenge | H9c2 rat cardiomyoblast cells | Cell doubling time, MTT viability, mitochondrial ROS (DHE/MitoSox), membrane potential (TMRE) | • X-ray pretreatment restored replicative capacity and ↓ cytosolic superoxide after GCRsim • Strongest adaptive effect at 0.5–1.0 Gy • Mitochondrial metrics unaffected • Supports low-dose X-ray hormesis as potential countermeasure | [154] |
| Five-ion simplified GCRsim: 10 cGy; 4He (250 MeV/n, LET 1.6 keV/μm): 10 cGy; whole-body irradiation of male Wistar rats; comparisons between single-ion (He) and complex-ion (GCRsim) groups | Male Wistar rats | Attentional set-shifting performance (ATSET): attempts to reach criterion (ATRC, error rate), mean correct latency (processing speed), stage-specific set shifting (SD, IDR, EDS, etc.), practice effects (practice savings ratio). | • GCRsim and He exposure both impaired SD stage of ATSET • GCRsim-exposed rats required more time/iterations to solve tasks • PE ↓ (50% slower than pretest vs. 30% improvement in sham) • UCFlex deficits also observed • Findings suggest diminished cognitive flexibility and ↓ learning from repetition after space radiation exposure | [155] |
| Five-ion simplified GCRsim; protons (1000 MeV/n, 35%), Si (600 MeV/n, 1%), He (250 MeV/n, 18%), O (350 MeV/n, 6%), Fe (600 MeV/n, 1%), final proton fraction (250 MeV/n, 39%); total dose 500 mGy delivered sequentially; amifostine pretreatment (107 or 214 mg/kg i.p. 1 h prior) | C57Bl/6J male and female mice | Behavioral testing (novel object recognition), locomotion/habituation and anxiety-like behavior (open field), home-cage activity (light/dark cycle), whole-brain cFos immunoreactivity and connectivity | • Combined heavy-ion irradiation impaired novel object recognition in males but not females • Amifostine pretreatment mitigated deficits in males and modulated brain regional connectivity • Sex-specific behavioral effects observed | [156] |
| Five-ion simplified GCRsim (mixed field; total dose 0.1–2.0 Gy); also separately evaluated same dose of: proton (150 MeV, LET 0.54 keV/μm), carbon (600 MeV/n, LET 9.18 keV/μm), iron (600 MeV/n, LET 172.4 keV/μm), and gamma rays (137Cs) | Latently CMV-infected Kasumi-3 human myeloblast cells | CMV reactivation (viral DNA load, qPCR), cell viability (% live cells), cell size (μm), viral genomic variation (sequencing), viral transcriptomics (UL49) | • All IR types induced CMV reactivation in dose- and time-dependent fashion • GCRsim triggered UL49 upregulation (logFC 1.48) • Carbon/iron led to strongest CMV load • No genomic variation • Cell viability and size affected by LET | [157] |
| Six-beam simplified GCRsim (74% protons, 18% helium, 6% oxygen, 1% silicon, 1% iron); total dose of 10 cGy | Male Wistar rats | Approach time, pull duration, movement accuracy (misses/contacts), reach endpoint concentration | • ↑ Pull duration • Mild ↑ approach time (some individuals) • No significant change in movement accuracy or reach concentration vss Sham at 72 h | [158] |
| Six-ion simplified GCRsim; protons (1 GeV, LET 0.24 keV/μm); He (250 MeV/n, LET 1.6 keV/μm); O (250 MeV/n, LET 25 keV/μm); Si (263 MeV/n, LET 78 keV/μm); Ti (1 GeV/n, LET 107 keV/μm); Fe (1 GeV/n, LET 151 keV/μm); total dose of 25, 50, or 200 cGy; rapid sequential whole-body irradiation | B6D2F1 male and female mice (n = 99); cortical and hippocampal brain tissue, feces (microbiome) | Locomotion and anxiety-like behavior (open field), recognition memory (object recognition), depression-like behaviour (forced swim), associative learning and memory (contextual/cued fear conditioning, passive avoidance); cortical and hippocampal BDNF, CD68, MAP-2 levels (ELISA); gut microbiome composition (16S rRNA sequencing) | • 50 and 200 cGy impaired object recognition and passive avoidance memory in females • Altered BDNF/CD68 patterns in males • Microbiome composition changed in sex- and dose-dependent ways • Strong link between microbiota and behavioral metrics | [159] |
| Six-beam simplified GCRsim [H (1 GeV); Si (600 MeV/n); He (250 MeV/n); O (350 MeV/n); Fe (600 MeV/n); H (250 MeV)]; total dose: 5 cGy or 30 cGy; whole-body irradiation | Middle-aged male mice; hippocampus (CA1) | Memory updating, discrimination index (DI), long-term potentiation (LTP), p-cofilin expression | • 30 cGy GCR impaired memory updating and LTP • 5 cGy had mild or no effect • Systemic HDAC3 inhibition reversed LTP impairment • ↓ p-Cofilin suggests cytoskeletal mechanism • First evidence of epigenetic rescue of GCR-induced synaptic dysfunction | [160] |
| Seven-ion simplified GCRsim (H, He, C, O, Si, Ti, Fe; 20–1000 MeV/n); chronic exposure: 2.08 cGy/day × 6 days/week × 4 weeks (total 50 cGy); whole-body irradiation | Female ApcMin/+ mice; mammary tissues and serum | Mammary tumor incidence, ductal morphology, serum estradiol and SPP1, ERα/ERRα expression (IHC/qPCR) | • ↑ Ductal overgrowth, ↑ mammary tumors (24% vs. 5%) • ↑ Serum estradiol, ERα, ERRα, SPP1 expression • Estrogen and inflammatory signaling linked to tumorigenesis • Conserved expression patterns in human breast cancer tissues | [161] |
| Thirty-three-beam GCRsim (33 separate beams involving H, He, C, O, Si, Ti, Fe, and other ions; various LETs and energies); acute dose: ~40 cGy in 2 h; chronic dose: ~50 cGy over 24 sessions; whole-body irradiation | C57BL/6J mice (n = 178 male, 91 female); hippocampus, mPFC, corpus callosum | Memory updating (object in updated location, OUL), recognition memory (NOR), anxiety-like behavior (light–dark box, LDB), social interaction (SIT), aggression/dominance (tube dominance); hippocampal synaptic plasticity (LTP), excitatory/inhibitory synaptic currents (sEPSC/sIPSC), synapse morphology (PSD length, spine size) | • Chronic GCR impaired memory updating in both sexes • Acute exposure disrupted excitatory synaptic signaling • LTP ↓ in both sexes • Chronic GCR thinned PSD in large spines, altered myelination in small/large axons • Sex-specific deficits in behavior and synaptic function | [162] |
| Thirty-three-beam GCRsim (H, He, C, O, Si, Ti, Fe, etc.; 40–49.9 cGy total); acute dose: 40 cGy in 2 h; chronic dose: ~50 Gy over 4 weeks; whole-body irradiation | Male C57BL/6 mice; prefrontal cortex (PFC) | Reward sensitivity/motivation (touchscreen-based economic demand), sustained attention and reaction time (psychomotor vigilance tasks); PFC neurotransmitter responsiveness (DA, 5-HT, NE, Glu, GABA levels) | • No change in motivation, but attentional deficits and slowed reaction times in GCR groups • DA signaling blunted in PFC • Chronic GCR ↑ all neurotransmitters under stimulation • Acute and chronic GCR reorganized neurotransmitter networks (DA, 5-HT, NE) • DA-GABA-Glu connectivity disrupted • Suggests persistent PFC network dysfunction | [163] |
| Thirty-three-beam GCRsim (H, He, C, O, Si, Ti, Fe, etc.); acute dose: 40 cGy in 1 day; chronic: 50 cGy over 24 days (2.08 cGy/day); whole-body irradiation | Male and female C57BL/6J mice | Locomotion (distance traveled, stop time), stop clustering, home base stability, edge preference (% stops at periphery), navigation accuracy (path circuity), progression speed (peak speed), heading changes (directional control). | • Acute exposure caused slower, more circuitous return paths under light conditions • Chronic exposure had less disruption • Light-dependent deficits in spatial navigation emerged only with acute irradiation | [164] |
| Thirty-three-beam GCRsim (H, He, O, Si, Ti, Fe, etc.) ± neutrons (10 cGy); acute (1.5–2 h) or chronic (4–6 week) exposure to 50, 75, or 100 cGy; 10 cGy neutron added 6 mo after acute 75 cGy GCRsim | K-rasLA1 lung cancer-susceptible mice (male/female); lung tissue and plasma; 1-year follow-up | Adenocarcinoma incidence, premalignant lesion number/size, survival, lipid peroxidation (MDA assay) | • GCRsim dose- and schedule-dependent ↑ in lung adenocarcinoma • Chronic exposure > acute • 10 cGy neutrons post-GCRsim ↑ malignancy • No survival impact except with neutrons • Lesion size ↑ at 100 cGy • Implications for Mars mission cancer risk | [165] |
| Thirty-three-beam GCRsim; acute dose: 0.75 Gy over ~1.5 h, whole-body irradiation ± antioxidant CDDO-EA pre/post | Female C57BL/6J mice (6-month-old); dentate gyrus and cortex; 14.25-month follow-up | Pattern separation and cognitive flexibility (location discrimination reversal, LDR), stimulus–response acquisition/extinction, social interaction, recognition memory (NOR), locomotion and anxiety-like behaviour (open field), hippocampal neurogenesis (DCX+ immature neurons) | • GCRsim caused deficits in cognitive flexibility and ↓ DCX+ neurons • CDDO-EA mitigated LDR impairments • Anxiety, sociability, and locomotion unaffected • Female resilience differed from male studies | [166] |
| Thirty-three-beam GCRsim; acute dose: 0.75 Gy over 1.5 h; whole-body irradiation | Male C57BL/6J mice (n = 22–24/group) | Sociability, social novelty preference, anxiety-like behavior, object recognition memory (NOR) | • 33-GCR did not alter NOR or anxiety but blunted preference for social novelty • CDDO-EA did not prevent this effect • CDDO-EA + GCR also impaired sociability • Findings highlight CNS vulnerability to complex mixed-field space radiation and need for targeted neuroprotective countermeasures | [167] |
| Thirty-three-beam GCRsim (seven ion species across 20–1000 MeV/n); total dose of 50 cGy | Female ApcMin/+ mice, mammary gland tissues | Ductal proliferation, ductal overgrowth, preneoplasia markers (Spp1, Rrm2) | • ↑ Ductal branching and hyperplasia • ↑ Cyclin D1+ cell proliferation • ↑ Spp1 expression (gene and protein) • ↑ Rrm2 expression (mRNA and protein) | [168] |
| X-rays (190 kVp): 0–2 Gy; alpha particles (LET 90.9 keV/μm): 0–2 Gy; mixed beam (X-rays + alpha, 1:1 dose ratio): 0–2 Gy total (0–1 Gy each component) | Human peripheral blood lymphocytes (2 male donors, in vitro) | Chromosomal aberrations, mRNA expression (FDXR, CDKN1A, MDM2), alternative transcription | • Synergistic increase in chromosomal aberrations and gene expression across seasons • Alpha > X-ray effectiveness • No synergism in alternative transcription • Inter/intra-donor variability observed | [169] |
| UV-B: 25–100 J/m2; protons (LET ~4.7 keV/μm): 0.25–0.5 Gy; gamma rays: 0.5 Gy; sequential exposure (≤20 min apart) | Human non-malignant cells: HaCaT keratinocytes, Hs27 fibroblasts, CRL 9855 monocytes, PBMCs | DNA damage (γH2AX, dicentrics), gene expression, viability (MTS, LDH), genomic instability | • Marked synergistic effects in co-exposed fibroblasts and keratinocytes • ↑ γH2AX foci, pan-nuclear staining, stress gene upregulation, and ↓ viability • Synergy less evident in UV-B-sensitive monocytes • Gamma + UV-B mimicked proton + UV-B responses | [170] |
| Neutrons (5 MeV p/d on Be target, LET 10–200 keV/μm): 0.33 Gy; photons (concomitant): 0.07 Gy (acute); neutrons/photons (252Cf, LET ~100 keV/μm): 0.4 Gy total at ≤1 mGy/day (chronic); 7-ion simplified GCRsim (H, He, C, O, Si, Ti, Fe ions, various energies): 0.4 Gy (acute ~2 h or 19 fractions over 1 month) | C3H male and BALB/c female mice | Locomotion and anxiety-like behavior (open field), recognition memory (NOR), contextual/cued fear conditioning | • Chronic mixed-field exposure impaired novel object recognition • Acute GCRsim ↓ exploratory behavior • Fractionated GCRsim→trend toward lower fear learning • Aspirin failed to mitigate radiation effects and worsened object recognition in sham controls | [171] |
| 40Ar (550 MeV/n, 86 keV/μm), 28Si (100 MeV/n, 150 keV/μm), 56Fe (115 MeV/n, 442 keV/μm); X-rays (150 kVp); dose combinations ranged from 1–11 Gy total; simultaneous exposure | Hamster V79 fibroblasts; human lymphocytes | Clonogenic survival (V79); chromosome 2 aberrations (FISH-PCC, lymphocytes) | • Additive effects for Ar and Si ions with X-rays • Slight non-additive deviation (mild synergy) observed for Fe ions, especially at 1:1 dose ratio • Fe + X-ray survival and chromosomal damage slightly exceeded predicted additive response | [172] |
| Gamma rays (661.7 keV): 0.4 Gy; 12C ions (450 MeV/n, 10.3 keV/μm): 0.14 Gy; whole-body gamma ray exposure followed 24 h later by head-only 12C ions | Adult male Wistar rats; nucleus accumbens (NAc) and dorsal striatum (dST) | Locomotor activity, grip strength, monoamine and choline metabolism (HPLC), gene/protein expression of STX1A and SNCA (qPCR, immunoblotting) | • IR caused hyperlocomotion and enhanced intrasession habituation • ↑ Choline and α-synuclein, ↓ STX1A in NAc • ↓ 5-HIAA • STX1A protein ↓ in dST • Suggests link to vesicle trafficking and neurotransmission modulation | [173] |
| Gamma rays (661.7 keV): 0.4 Gy; 12C ions (450 MeV/n, 10.3 keV/μm): 0.14 Gy; whole-body gamma irradiation daily for 3 days, followed by acute 12C head-only exposure on day 4 | Wistar rats (n = 14), pituitary gland analyzed post-mortem | C/EBP-β protein isoform levels (LAP*, LAP, LIP, Western blot); mRNA expression of C/EBP-β isoforms (qPCR) | • No change in mRNA levels, but 1.76× increase in C/EBP-β LIP isoform protein in irradiated rats • Suggests translation-stage regulation • Indicates ER stress and potential apoptosis signaling via HPA axis modulation | [174] |
| Galactic cosmic rays (GCRs); microgravity, circadian disruption, and other spaceflight stressors; estimated GCR dose: ~76 mGy (physical), ~146 mSv (effective) over 340 days; complex exposure mix including HZE particles; environmental stressors act in parallel | One monozygotic twin in space (TW) vs. Earth-based twin (HR) | Multi-system effects: transcriptomic, epigenetic, proteomic, metabolomic, immune, cardiovascular, ocular, microbiome, cognitive | • Multiple synergistic effects inferred • Persistent chromosomal inversions (DNA damage), gene dysregulation, telomere elongation→rapid shortening • Altered immune networks, inflammation, cognitive decline • Consistent with complex biological interaction of radiation with other spaceflight stressors | [175] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parousis-Paraskevas, O.; Gkikoudi, A.; Al-Qaaod, A.; Vasilopoulos, S.N.; Manda, G.; Beinke, C.; Haghdoost, S.; Terzoudi, G.I.; Krasniqi, F.; Georgakilas, A.G. Combined Radiations: Biological Effects of Mixed Exposures Across the Radiation Spectrum. Biomolecules 2025, 15, 1282. https://doi.org/10.3390/biom15091282
Parousis-Paraskevas O, Gkikoudi A, Al-Qaaod A, Vasilopoulos SN, Manda G, Beinke C, Haghdoost S, Terzoudi GI, Krasniqi F, Georgakilas AG. Combined Radiations: Biological Effects of Mixed Exposures Across the Radiation Spectrum. Biomolecules. 2025; 15(9):1282. https://doi.org/10.3390/biom15091282
Chicago/Turabian StyleParousis-Paraskevas, Orfeas, Angeliki Gkikoudi, Amer Al-Qaaod, Spyridon N. Vasilopoulos, Gina Manda, Christina Beinke, Siamak Haghdoost, Georgia I. Terzoudi, Faton Krasniqi, and Alexandros G. Georgakilas. 2025. "Combined Radiations: Biological Effects of Mixed Exposures Across the Radiation Spectrum" Biomolecules 15, no. 9: 1282. https://doi.org/10.3390/biom15091282
APA StyleParousis-Paraskevas, O., Gkikoudi, A., Al-Qaaod, A., Vasilopoulos, S. N., Manda, G., Beinke, C., Haghdoost, S., Terzoudi, G. I., Krasniqi, F., & Georgakilas, A. G. (2025). Combined Radiations: Biological Effects of Mixed Exposures Across the Radiation Spectrum. Biomolecules, 15(9), 1282. https://doi.org/10.3390/biom15091282









