Consequences of Adhesion Molecule Close Homolog of L1 Deficiency for Neurons and Glial Cells in the Mouse Spinal Cord After Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Procedures
2.3. Antibodies
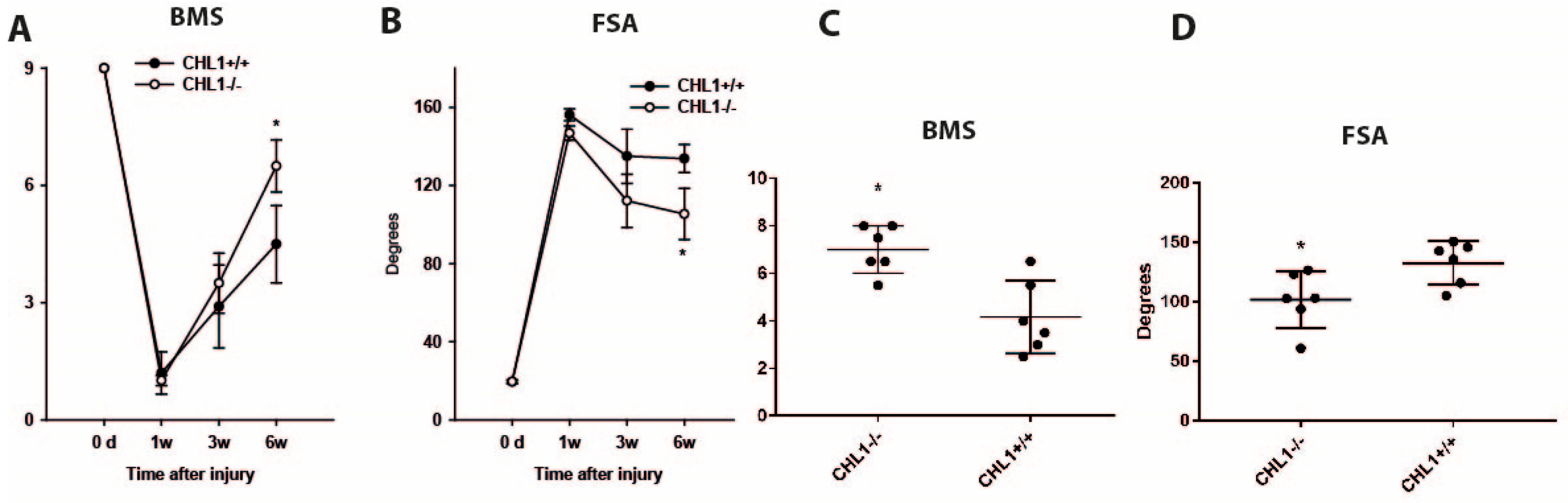
2.4. Motor Function Analysis
2.5. Tissue Fixation and Sectioning
2.6. Immunohistochemistry
2.7. Stereological Analysis
2.8. Estimation of Spinal Cord Volumes
2.9. Photographic Documentation
2.10. Statistical Analysis
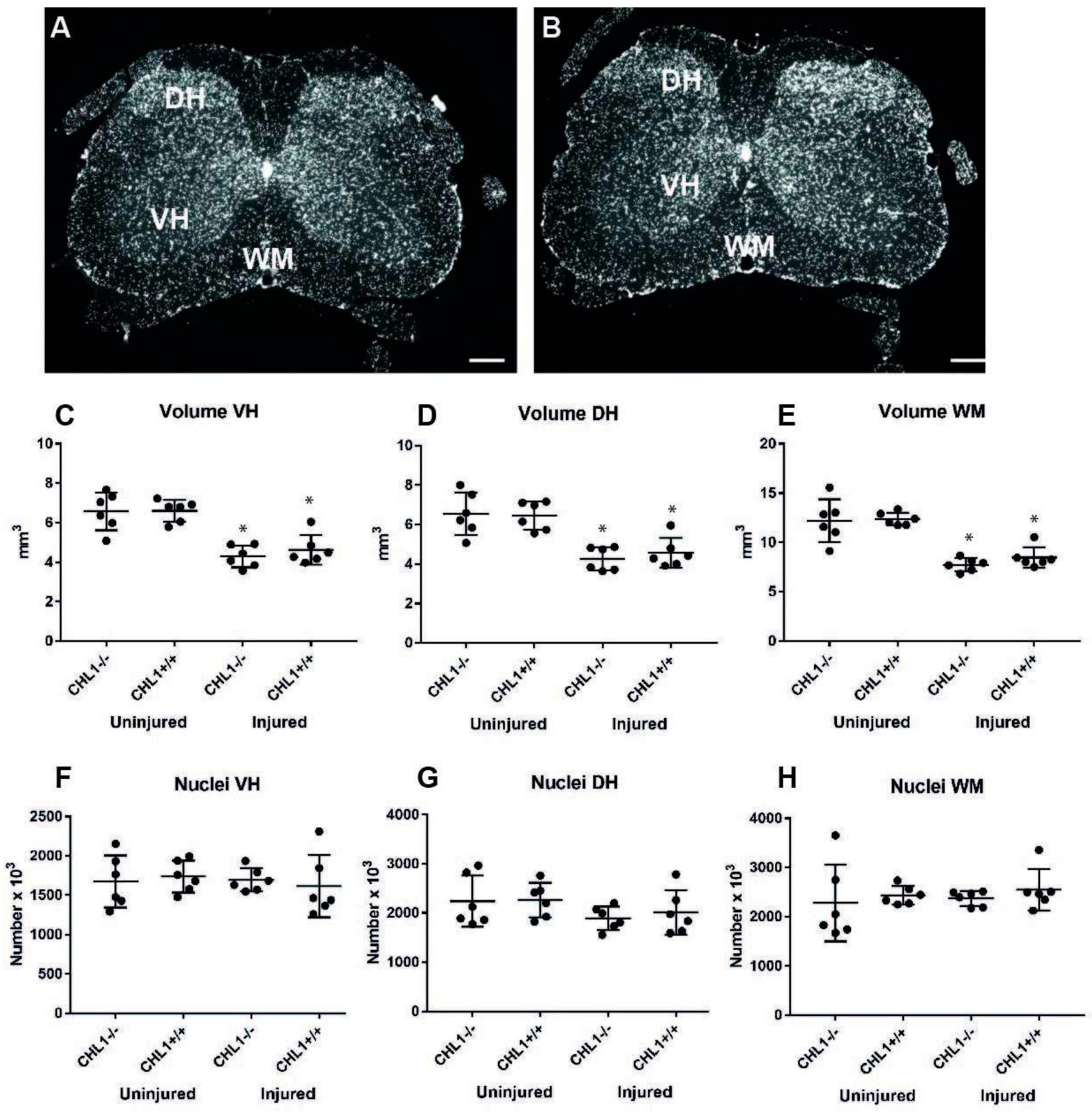
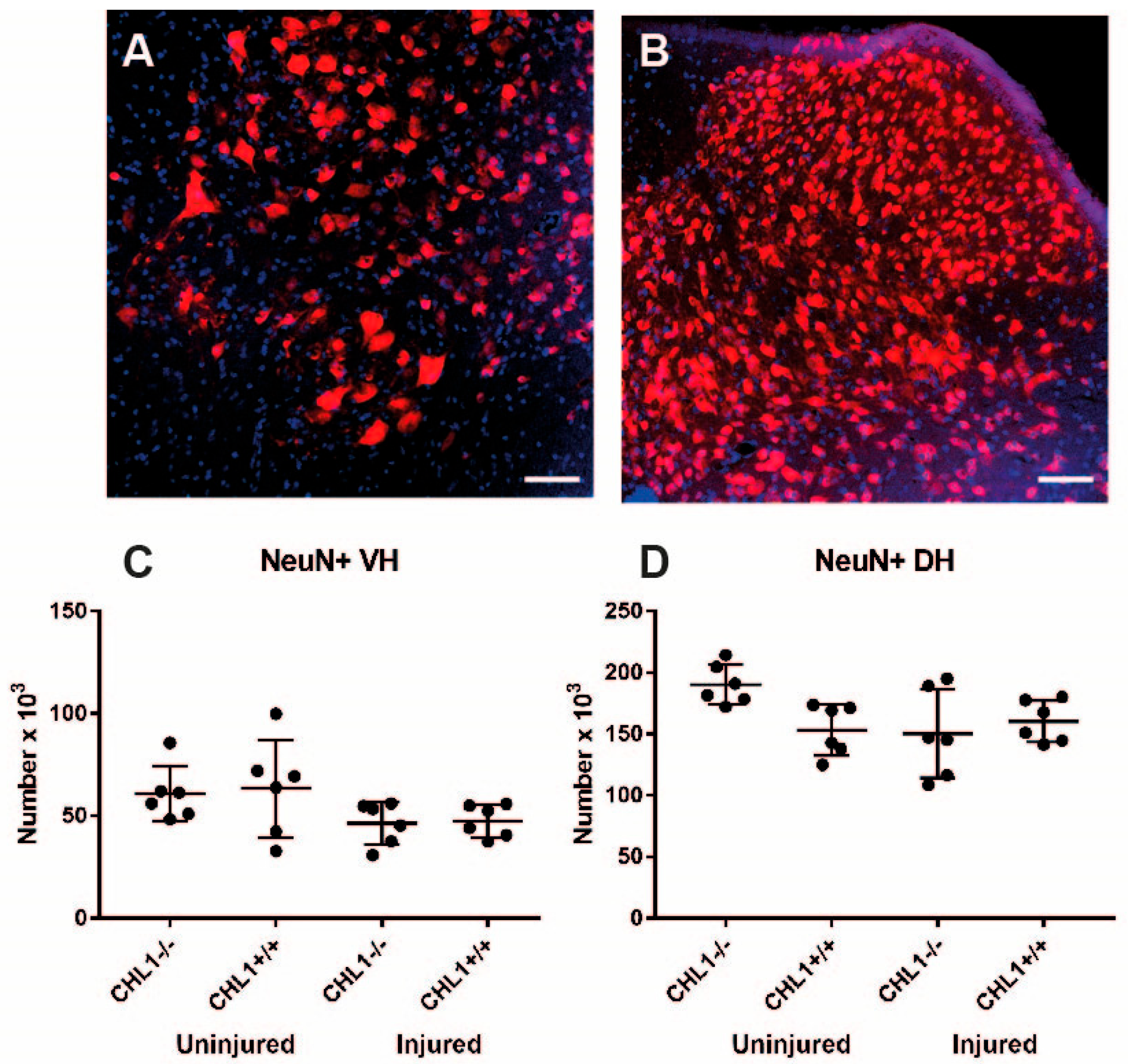
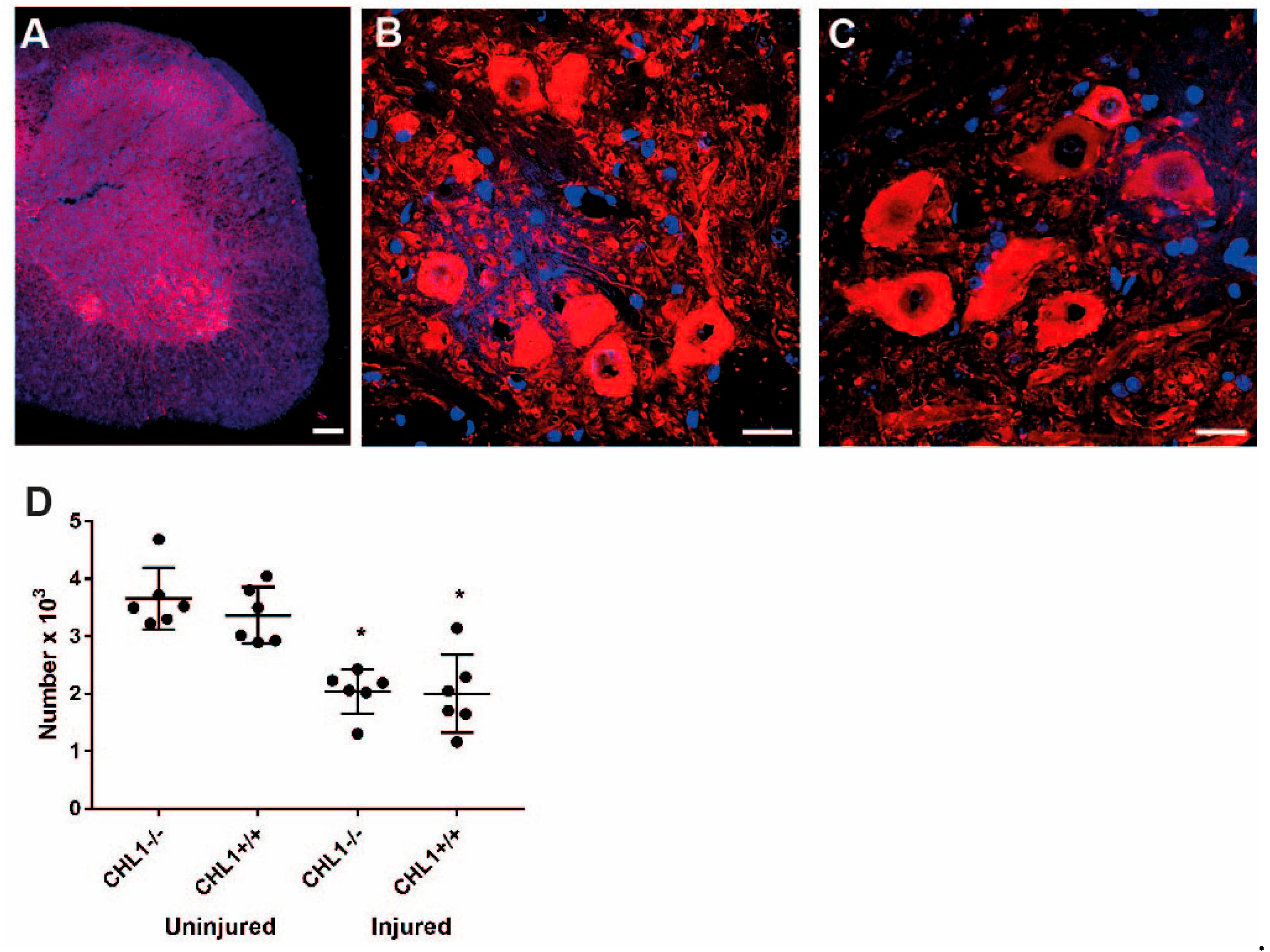
3. Results
3.1. Reduction of the Lumbar Spinal Cord Volume Without Cell Loss After Injury
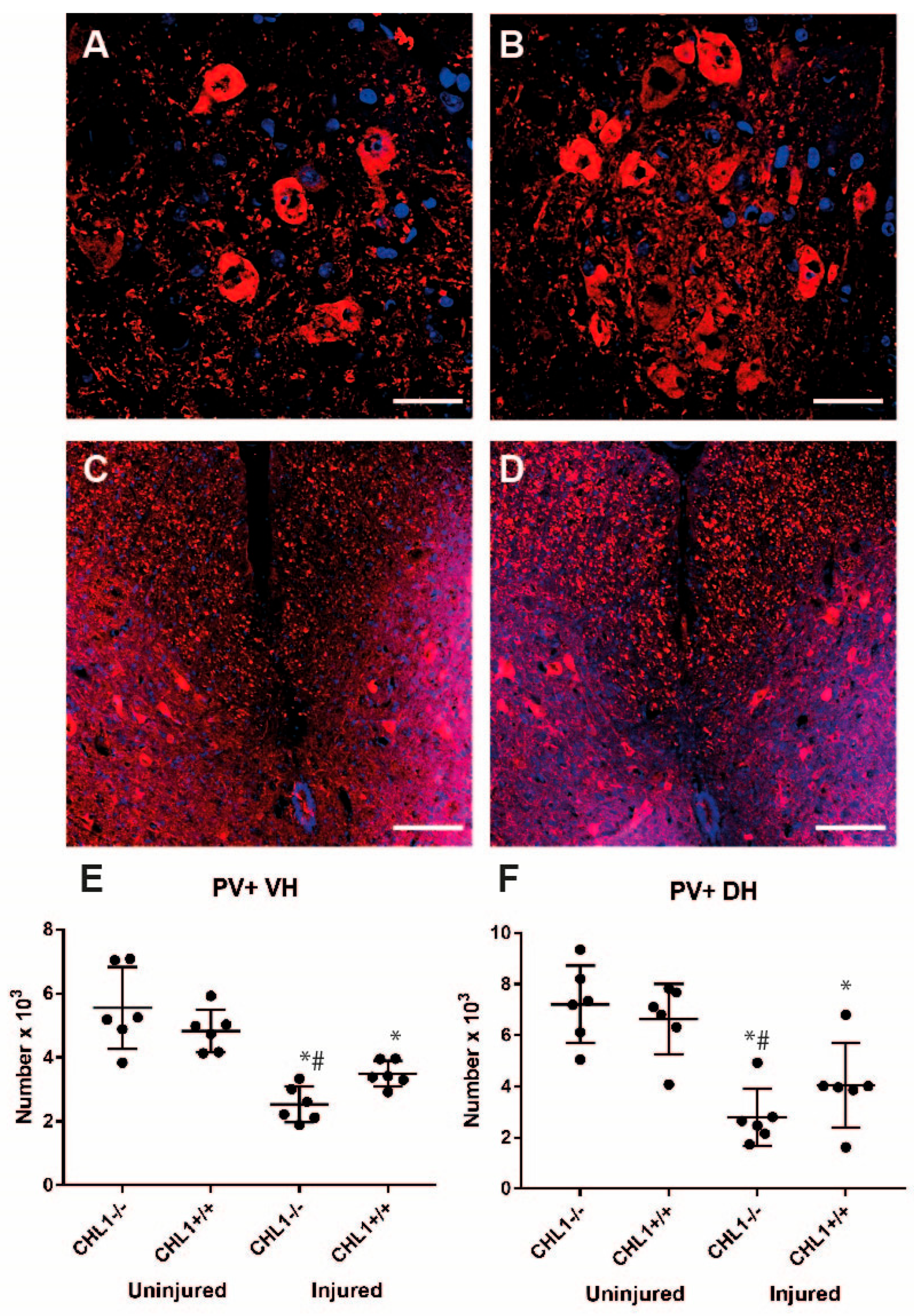
3.2. Loss of Motoneurons and Parvalbumin-Positive Interneurons After Injury
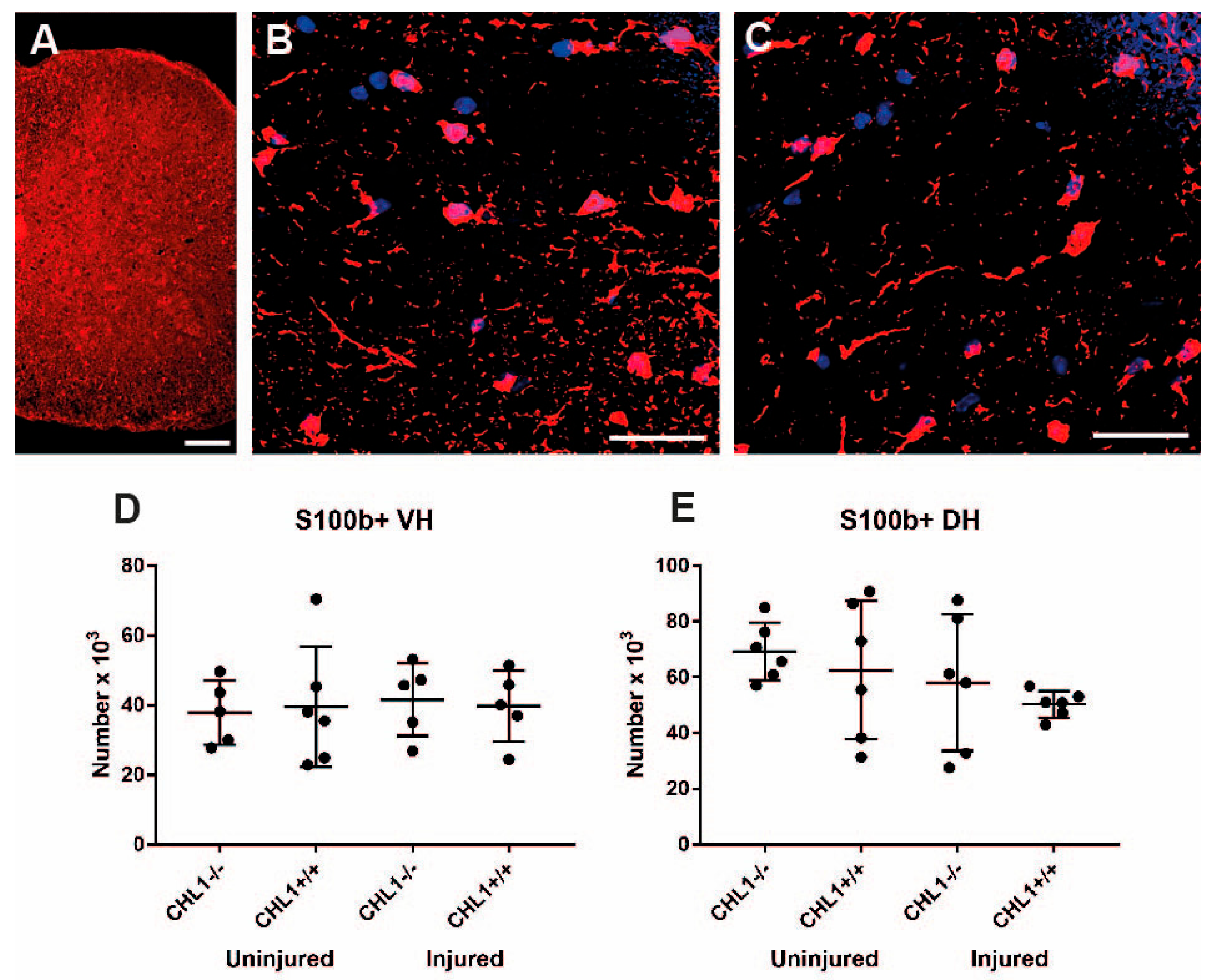
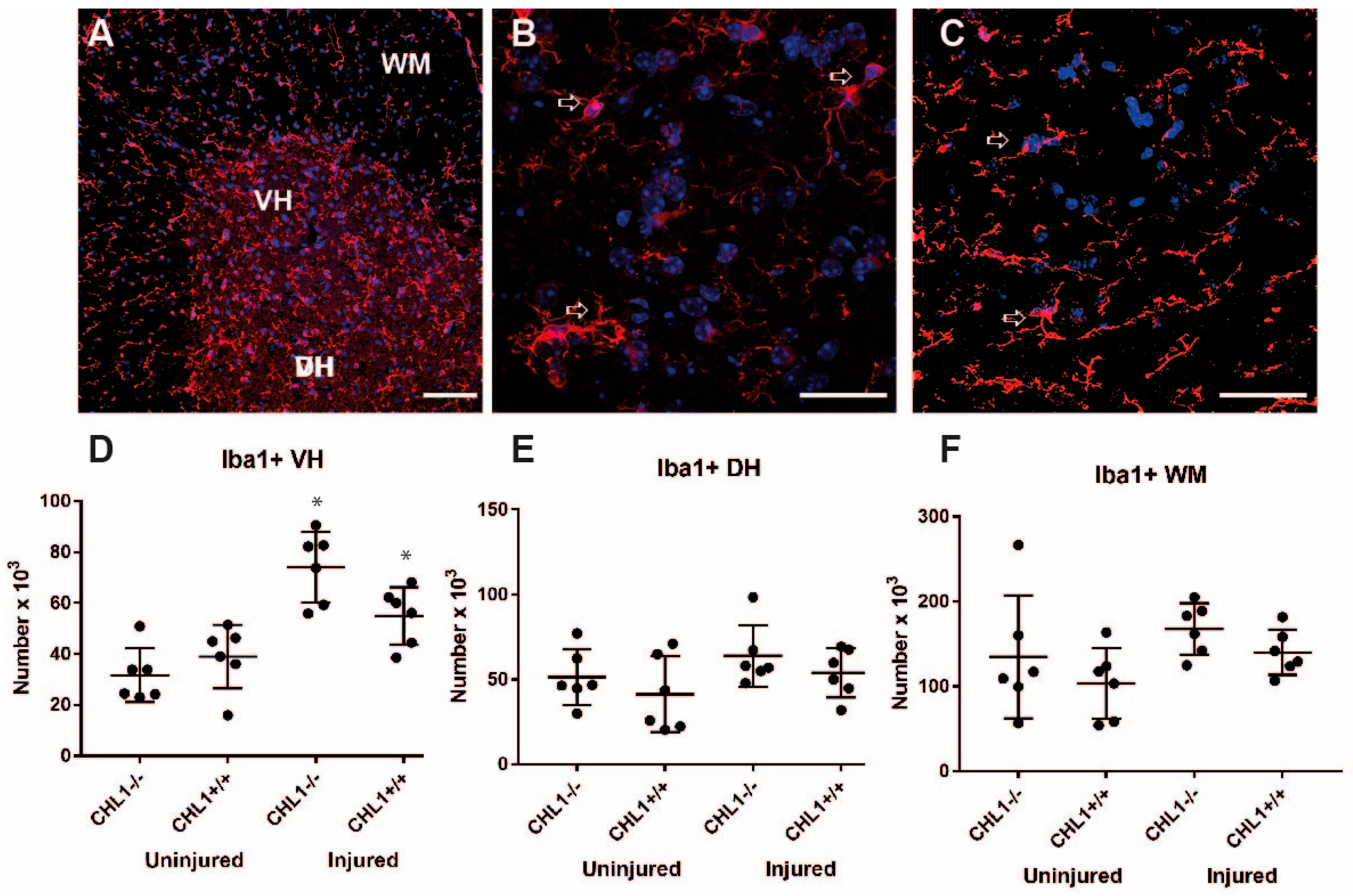
3.3. Glial Cell Populations in the Lumbar Spinal Cord After Injury
4. Discussion
4.1. The Effect of Injury in CHL1+/+ Mice
4.2. The Effect of Injury in CHL1−/− Mice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHL1 | Close homolog of L1 |
| ChAT | Choline-acetyl transferase |
| PV | Parvalbumin |
| Iba1 | Ionized calcium binding adaptor molecule 1 |
| CNP | 2′,3′-cyclic nucleotide 3′phosphodiesterase |
| PBS | Phosphate buffered saline |
| RT | Room temperature |
| GFAP | Glial fibrillary acidic protein |
| SD | Standard deviation |
| ANOVA | Analysis of variance |
| GM | Gray matter |
| WM | White matter |
| VH | Ventral horn |
| DH | Dorsal horn |
References
- Griffin, J.M.; Bradke, F. Therapeutic repair for spinal cord injury: Combinatory approaches to address a multifaceted problem. EMBO Mol. Med. 2020, 12, e11505. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The biology of regeneration failure and Success after spinal cord injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef] [PubMed]
- Alfadil, E.; Bradke, F. Moving through the crowd. Where are we at understanding physiological axon growth? Semin. Cell Dev. Biol. 2023, 140, 63–71. [Google Scholar] [CrossRef]
- Clifford, T.; Finkel, Z.; Rodriguez, B.; Joseph, A.; Cai, L. Current advancements in spinal cord injury research-glial scar formation and neural regeneration. Cells 2023, 12, 853. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhai, X.; Yu, C. Spatial distribution-based progression of spinal cord injury pathology: A key role for neuroimmune cells. Front. Immunol. 2025, 15, 1505755. [Google Scholar] [CrossRef]
- Jakovcevski, I.; Förster, E.; Reiss, G.; Schachner, M. Impact of depletion of microglia/macrophages on regeneration after spinal cord injury. Neuroscience 2021, 459, 129–141. [Google Scholar] [CrossRef]
- Tomé, D.; Almeida, R.D. The injured axon: Intrinsic mechanisms driving axonal regeneration. Trends Neurosci. 2024, 47, 875–891. [Google Scholar] [CrossRef]
- Jakovcevski, I.; Wu, J.; Karl, N.; Leshchyns’ka, I.; Sytnyk, V.; Chen, J.; Irintchev, A.; Schachner, M. Glial scar expression of CHL1, the close homolog of the adhesion molecule L1, limits recovery after spinal cord injury. J. Neurosci. 2007, 27, 7222–7233. [Google Scholar] [CrossRef]
- Theis, T.; Ayala, C.; Tschang, M.; Philip, M.; Nagaraj, V.; Shrirao, A.; Voronin, G.; Young, W.; Schachner, M. CHL1-deficient and wild-type male mice do not differ in locomotor recovery from spinal cord injury. J. Spine Res. Surg. 2022, 4, 96–103. [Google Scholar]
- Bjugn, R.; Nyengaard, J.R.; Rosland, J.H. Spinal cord transection—No loss of distal ventral horn neurons. Exp. Neurol. 1997, 148, 179–186. [Google Scholar] [CrossRef]
- Young, I.J. Morphological and histochemical studies of partially and totally deafferented spinal cord segments. Exp. Neurol. 1966, 14, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Nakagawa, T.; Kubota, T. Neuronal loss and expression of neurotrophic factors in a model of rat chronic compressive spinal cord injury. Spine 2006, 31, 2059–2066. [Google Scholar] [CrossRef]
- McBride, R.L.; Feringa, E.R. Ventral horn motoneurons 10, 20 and 52 weeks after T-9 spinal cord transection. Brain Res. Bull. 1992, 28, 57–60. [Google Scholar] [CrossRef]
- Jiang, Y.; Cai, Y.; Yang, N.; Gao, S.; Li, Q.; Pang, Y.; Su, P. Molecular mechanisms of spinal cord injury repair across vertebrates: A comparative review. Eur. J. Neurosci. 2024, 60, 4552–4568. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.G.; Becker, T.; Hugnot, J.P. The spinal ependymal zone as a source of endogenous repair cells across vertebrates. Prog. Neurobiol. 2018, 170, 67–80. [Google Scholar] [CrossRef]
- Schmalbach, B.; Lepsveridze, E.; Djogo, N.; Papashvili, G.; Kuang, F.; Leshchyns’ka, I.; Sytnyk, V.; Nikonenko, A.G.; Dityatev, A.; Jakovcevski, I.; et al. Age-dependent loss of parvalbumin-expressing hippocampal interneurons in mice deficient in CHL1, a mental retardation and schizophrenia susceptibility gene. J. Neurochem. 2015, 135, 830–844. [Google Scholar] [CrossRef]
- Howard, C.V.; Reed, M.G. Surface-weighted star volume: Concept and estimation. J. Microsc. 1998, 190, 350–356. [Google Scholar]
- Antal, M. Molecular anatomy of synaptic and extrasynaptic neurotransmission between nociceptive primary afferents and spinal dorsal horn neurons. Int. J. Mol. Sci. 2025, 26, 2356. [Google Scholar] [CrossRef]
- Alvarez, F.J.; Jonas, P.C.; Sapir, T.; Hartley, R.; Berrocal, M.C.; Geiman, E.J.; Todd, A.J.; Goulding, M. Postnatal phenotype and localization of spinal cord V1 derived interneurons. J. Comp. Neurol. 2005, 493, 177–192. [Google Scholar] [CrossRef]
- Dougherty, K.J.; Sawchuk, M.A.; Hochman, S. Phenotypic diversity and expression of GABAergic inhibitory interneurons during postnatal development in lumbar spinal cord of glutamic acid decarboxylase 67-green fluorescent protein mice. Neuroscience 2009, 163, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Gradwell, M.A.; Boyle, K.A.; Browne, T.J.; Bell, A.M.; Leonardo, J.; Peralta Reyes, F.S.; Dickie, A.C.; Smith, K.M.; Callister, R.J.; Dayas, C.V.; et al. Diversity of inhibitory and excitatory parvalbumin interneuron circuits in the dorsal horn. Pain 2022, 163, e432–e452. [Google Scholar] [CrossRef]
- Du, J.; Yi, M.; Zhou, F.; He, W.; Yang, A.; Qiu, M.; Huang, H. S100B is selectively expressed by gray matter protoplasmic astrocytes and myelinating oligodendrocytes in the developing CNS. Mol. Brain 2021, 14, 154. [Google Scholar] [CrossRef]
- Marangon, D.; Castro ESilva, J.H.; Cerrato, V.; Boda, E.; Lecca, D. Oligodendrocyte progenitors in glial scar: A bet on remyelination. Cells 2024, 13, 1024. [Google Scholar] [CrossRef] [PubMed]
- Filippatou, A.G.; Calabresi, P.A.; Saidha, S.; Murphy, O.C. Spotlight on trans-synaptic degeneration in the visual pathway in multiple sclerosis. Eye Brain 2023, 15, 153–160. [Google Scholar] [CrossRef]
- Wall, R.V.; Basavarajappa, D.; Klistoner, A.; Graham, S.; You, Y. Mechanisms of transsynaptic degeneration in the aging brain. Aging Dis. 2024, 15, 2149–2167. [Google Scholar] [CrossRef] [PubMed]
- Hama, A.; Pappas, T.G.D.; Sagen, J. Adrenal medullary implants reduce transsynaptic degeneration in the spinal cord of rats following chronic constriction nerve injury. Exp. Neurol. 1996, 137, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Dong Teng, Y.; Mocchetti, I.; Taveira-DaSilva, A.; Gillis, R.; Wrathall, J. Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after spinal cord injury. J. Neurosci. 1999, 19, 7037–7047. [Google Scholar] [CrossRef]
- Eidelberg, E.; Nguyen, L.H.; Polich, R.; Walden, J.G. Transsynaptic degeneration of motoneurones caudal to spinal cord lesions. Brain Res. Bull. 1989, 22, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Huber, E.; David, G.; Thompson, A.J.; Weiskopf, N.; Mohammadi, S.; Freund, P. Dorsal and ventral horn atrophy is associated with clinical outcome after spinal cord injury. Neurology 2018, 90, e1510–e1522. [Google Scholar] [CrossRef]
- Keller-Peck, C.R.; Mullen, R.J. Patterns of neuronal differentiation in neural tube mutant mice: Curly tail and Pax3 splotch-delayed. J. Comp. Neurol. 1996, 368, 516–526. [Google Scholar] [CrossRef]
- Bocker, H.T.; Heinrich, T.; Liebmann, L.; Hennings, J.C.; Seemann, E.; Gerth, M.; Preobraschenski, J.; Kessels, M.M.; Westermann, M.; Isbrandt, D.; et al. The Na+/H+ Exchanger Nhe1 Modulates Network Excitability via GABA Release. Cereb. Cortex 2019, 29, 4263–4276. [Google Scholar] [CrossRef]
- Verret, L.; Mann, E.O.; Hang, G.B.; Barth, A.M.; Cobos, I.; Ho, K.; Devidze, N.; Masliah, E.; Kreitzer, A.C.; Mody, I.; et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 2012, 149, 708–721. [Google Scholar] [CrossRef]
- Behrens, M.M.; Ali, S.S.; Dugan, L.L. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J. Neurosci. 2008, 28, 13957–13966. [Google Scholar] [CrossRef]
- Dekkers, J.; Bayley, P.; Dick, J.R.T.; Schwaller, B.; Berchtold, M.W.; Greensmith, L. Overexpression of parvalbumin in transgenic mice rescues motoneurons from injury-induced cell death. J. Neurosci. 2004, 123, 459–466. [Google Scholar] [CrossRef][Green Version]
- Deumens, R.; Joosten, E.A.; Waxman, S.G.; Hains, B.C. Locomotor dysfunction and pain: The scylla and charybdis of fiber sprouting after spinal cord injury. Mol. Neurobiol. 2008, 37, 52–63. [Google Scholar] [CrossRef]
- Jang, K.; Garraway, S.M. A review of dorsal root ganglia and primary sensory neuron plasticity mediating inflammatory and chronic neuropathic pain. Neurobiol. Pain 2024, 15, 100151. [Google Scholar] [CrossRef]
- Clerc, N.; Moqrich, A. Diverse roles and modulations of IA in spinal cord pain circuits. Cell Rep. 2022, 38, 110588. [Google Scholar] [CrossRef]
- Yamanaka, H.; Kobayashi, K.; Okubo, M.; Fukuoka, T.; Noguchi, K. Increase of close homolog of cell adhesion molecule L1 in primary afferent by nerve injury and the contribution to neuropathic pain. J. Comp. Neurol. 2011, 519, 1597–1615. [Google Scholar] [CrossRef]
- Yang, H.; Lu, P.; McKay, H.M.; Bernot, T.; Keirstead, H.; Steward, O.; Gage, F.H.; Edgerton, V.R.; Tuszynski, M.H. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J. Neurosci. 2006, 26, 2157–2166. [Google Scholar] [CrossRef]
- Lee, H.J.; Jakovcevski, I.; Radonjic, N.; Hoelters, L.; Schachner, M.; Irintchev, A. Better functional outcome of compression spinal cord injury in mice is associated with enhanced H-reflex responses. Exp. Neurol. 2009, 216, 365–374. [Google Scholar] [CrossRef]
- Ladeby, R.; Wirenfeldt, M.; Garcia-Ovejero, D.; Fenger, C.; Dissing-Olesen, L.; Dalmau, I.; Finsen, B. Microglial cell population dynamics in the injured adult central nervous system. Brain Res. Rev. 2005, 48, 196–206. [Google Scholar] [CrossRef]
- Katic, J.; Loers, G.; Kleene, R.; Karl, N.; Schmidt, C.; Buck, F.; Zmijewski, J.W.; Jakovcevski, I.; Preissner, K.T.; Schachner, M. Interaction of the cell adhesion molecule CHL1 with vitronectin, integrins, and the plasminogen activator inhibitor-2 promotes CHL1-induced neurite outgrowth and neuronal migration. J. Neurosci. 2014, 34, 14606–14623. [Google Scholar] [CrossRef]
- Acar, A. Stereological Analyses of Neurons and Glial Cells in the Lesioned and Unlesioned Spinal Cord of Wild-Type and CHL1-Deficient Mice. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 2009. Unpublished Dissertation. [Google Scholar]

| Change | Injured vs. Uninjured CHL1+/+ | Injured CHL1−/− vs. Injured CHL1+/+ |
|---|---|---|
| Volume | ↓ | = |
| All cells/DAPI | = | = |
| All neurons/NeuN | = | = |
| Motoneurons/ChAT | ↓ | = |
| Interneurons/PV | ↓ | ↓ |
| Astrocytes/S100b | = | = |
| Oligodendrocytes/CNP | WM ↓ GM = | = |
| Microglia/Iba 1 | VH ↑ DH = WM = | = |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakovcevski, I.; Acar, A.; Schwindenhammer, B.; Hamad, M.I.K.; Reiss, G.; Förster, E.; Schachner, M. Consequences of Adhesion Molecule Close Homolog of L1 Deficiency for Neurons and Glial Cells in the Mouse Spinal Cord After Injury. Biomolecules 2025, 15, 1247. https://doi.org/10.3390/biom15091247
Jakovcevski I, Acar A, Schwindenhammer B, Hamad MIK, Reiss G, Förster E, Schachner M. Consequences of Adhesion Molecule Close Homolog of L1 Deficiency for Neurons and Glial Cells in the Mouse Spinal Cord After Injury. Biomolecules. 2025; 15(9):1247. https://doi.org/10.3390/biom15091247
Chicago/Turabian StyleJakovcevski, Igor, Ayse Acar, Benjamin Schwindenhammer, Mohammad I. K. Hamad, Gebhard Reiss, Eckart Förster, and Melitta Schachner. 2025. "Consequences of Adhesion Molecule Close Homolog of L1 Deficiency for Neurons and Glial Cells in the Mouse Spinal Cord After Injury" Biomolecules 15, no. 9: 1247. https://doi.org/10.3390/biom15091247
APA StyleJakovcevski, I., Acar, A., Schwindenhammer, B., Hamad, M. I. K., Reiss, G., Förster, E., & Schachner, M. (2025). Consequences of Adhesion Molecule Close Homolog of L1 Deficiency for Neurons and Glial Cells in the Mouse Spinal Cord After Injury. Biomolecules, 15(9), 1247. https://doi.org/10.3390/biom15091247









