Serum Growth Differentiation Factor 15 (GDF15) Levels Reflect Ischemic Etiology in Heart Failure Patients with Iron Deficiency: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Clinical Data and Standard Biochemical Determinations
2.3. Serum GDF15 Determination
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
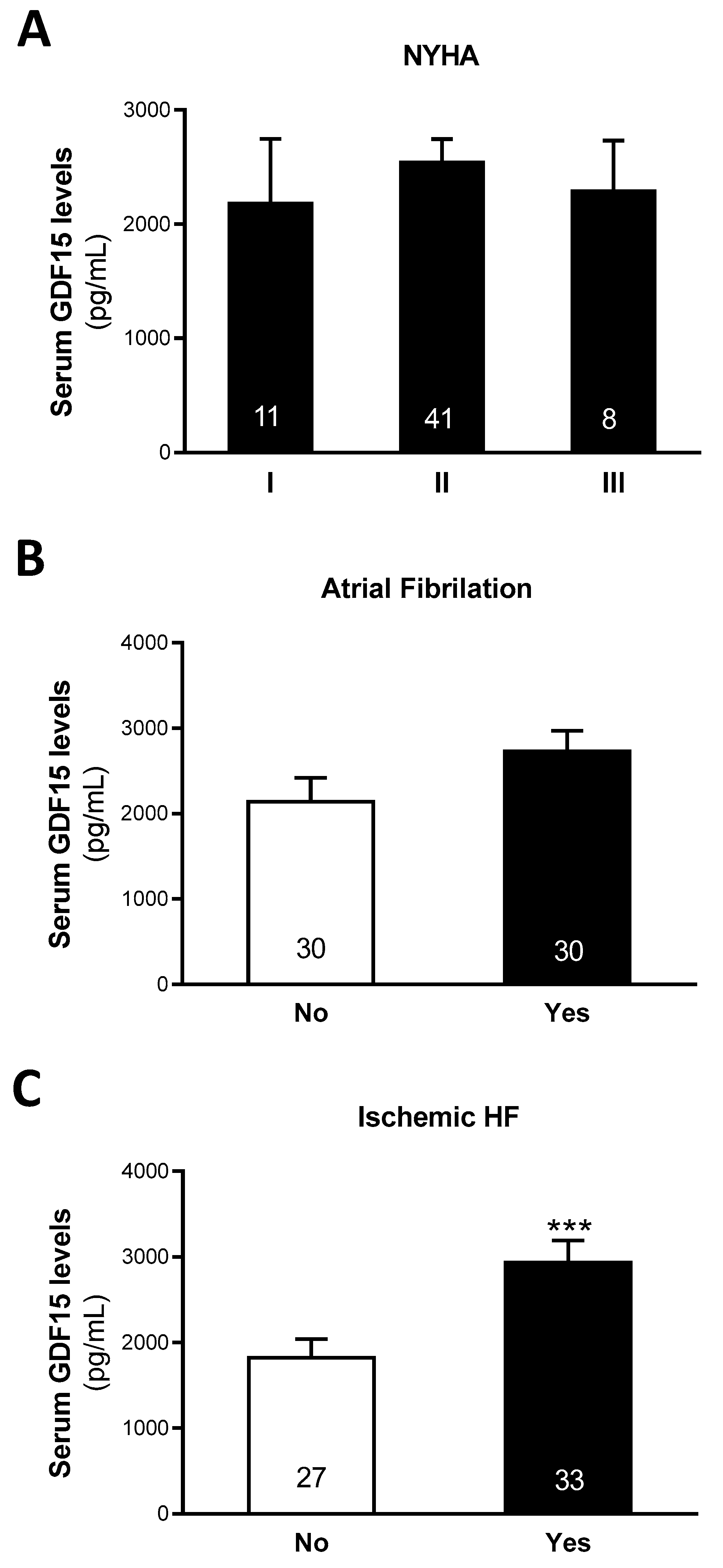
3.2. Relationship Between Serum GDF15, Cardiac Function and Iron-Related Hallmarks
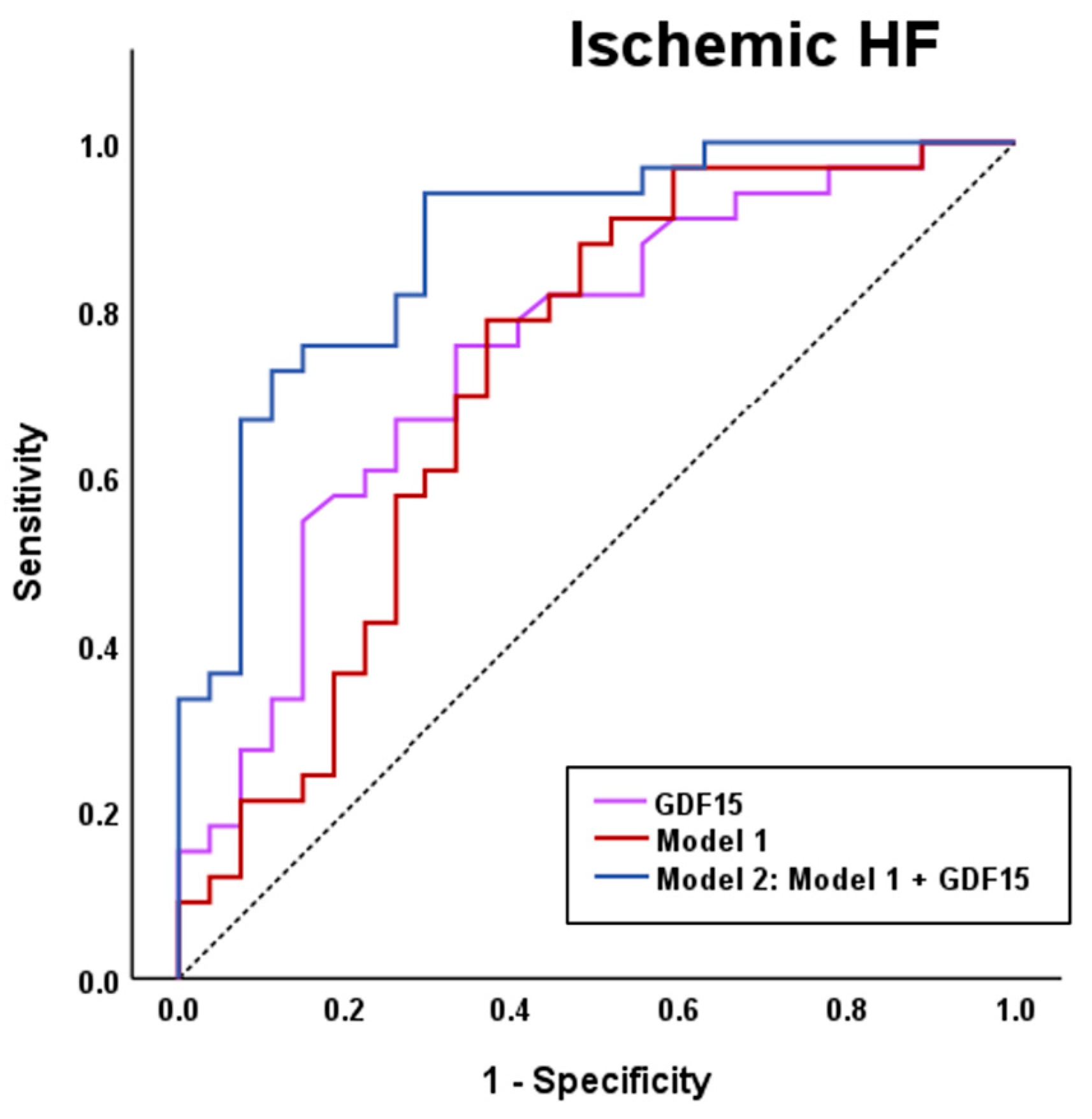
3.3. Serum GDF15 Levels Classify Patients with HF of Ischemic Etiology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GDF15 | Growth differentiation factor 15 |
| HF | Heart failure |
| ID | Iron deficiency |
| CV | Cardiovascular |
| TAPSE | Tricuspid annular plane systolic excursion |
| 6MWT | 6-minute walking test |
| NT-proBNP | N-terminal prohormone of brain natriuretic peptide |
| LVEF | Left ventricle ejection fraction |
| TSAT | Transferrin saturation |
| BMI | Body mass index |
| NYHA | New York Heart Association |
| ASE | American Society of Echocardiography |
| EACVI | European Association of Cardiovascular Imaging |
| STEMI | ST-Elevation Myocardial Infarction |
| ID-MS | Isotopic dilution-mass spectrometry |
| HRP | Horseradish peroxidase |
| TMB | Tetra-methylbenzidine |
| ANCOVA | Analysis of covariance |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
References
- Clephas, P.R.D.; de Boer, R.A.; Brugts, J.J. Benefits of Remote Hemodynamic Monitoring in Heart Failure. Trends Cardiovasc. Med. 2024, 34, 468–476. [Google Scholar] [CrossRef]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of Cardiovascular Disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Klip, I.T.; Comin-Colet, J.; Voors, A.A.; Ponikowski, P.; Enjuanes, C.; Banasiak, W.; Lok, D.J.; Rosentryt, P.; Torrens, A.; Polonski, L.; et al. Iron Deficiency in Chronic Heart Failure: An International Pooled Analysis. Am. Heart J. 2013, 165, 575–582.e3. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Ebner, N.; Evertz, R.; Ponikowski, P.; Anker, S.D. Iron Deficiency in Heart Failure: An Overview. JACC Heart Fail. 2019, 7, 36–46. [Google Scholar] [CrossRef] [PubMed]
- González-Costello, J.; Comín-Colet, J.; Lupón, J.; Enjuanes, C.; de Antonio, M.; Fuentes, L.; Moliner-Borja, P.; Farré, N.; Zamora, E.; Manito, N.; et al. Importance of Iron Deficiency in Patients with Chronic Heart Failure as a Predictor of Mortality and Hospitalizations: Insights from an Observational Cohort Study. BMC Cardiovasc. Disord. 2018, 18, 206. [Google Scholar] [CrossRef]
- Enjuanes, C.; Bruguera, J.; Grau, M.; Cladellas, M.; Gonzalez, G.; Meroño, O.; Moliner-Borja, P.; Verdú, J.M.; Farré, N.; Comín-Colet, J. Iron Status in Chronic Heart Failure: Impact on Symptoms, Functional Class and Submaximal Exercise Capacity. Rev. Española Cardiol. (Engl. Ed.) 2016, 69, 247–255. [Google Scholar] [CrossRef]
- Enjuanes, C.; Klip, I.T.; Bruguera, J.; Cladellas, M.; Ponikowski, P.; Banasiak, W.; van Veldhuisen, D.J.; van der Meer, P.; Jankowska, E.A.; Comín-Colet, J. Iron Deficiency and Health-Related Quality of Life in Chronic Heart Failure: Results from a Multicenter European Study. Int. J. Cardiol. 2014, 174, 268–275. [Google Scholar] [CrossRef]
- Moliner, P.; Enjuanes, C.; Tajes, M.; Cainzos-Achirica, M.; Lupón, J.; Garay, A.; Jimenez-Marrero, S.; Yun, S.; Farré, N.; Cladellas, M.; et al. Association Between Norepinephrine Levels and Abnormal Iron Status in Patients with Chronic Heart Failure: Is Iron Deficiency More Than a Comorbidity? J. Am. Heart Assoc. 2019, 8, e010887. [Google Scholar] [CrossRef]
- Maeder, M.T.; Khammy, O.; dos Remedios, C.; Kaye, D.M. Myocardial and Systemic Iron Depletion in Heart Failure. J. Am. Coll. Cardiol. 2011, 58, 474–480. [Google Scholar] [CrossRef]
- van Veldhuisen, D.J.; Anker, S.D.; Ponikowski, P.; Macdougall, I.C. Anemia and Iron Deficiency in Heart Failure: Mechanisms and Therapeutic Approaches. Nat. Rev. Cardiol. 2011, 8, 485–493. [Google Scholar] [CrossRef]
- Hoes, M.F.; Grote Beverborg, N.; Kijlstra, J.D.; Kuipers, J.; Swinkels, D.W.; Giepmans, B.N.G.; Rodenburg, R.J.; van Veldhuisen, D.J.; de Boer, R.A.; van der Meer, P. Iron Deficiency Impairs Contractility of Human Cardiomyocytes through Decreased Mitochondrial Function. Eur. J. Heart Fail. 2018, 20, 910–919. [Google Scholar] [CrossRef]
- Haddad, S.; Wang, Y.; Galy, B.; Korf-Klingebiel, M.; Hirsch, V.; Baru, A.M.; Rostami, F.; Reboll, M.R.; Heineke, J.; Flögel, U.; et al. Iron-Regulatory Proteins Secure Iron Availability in Cardiomyocytes to Prevent Heart Failure. Eur. Heart J. 2016, 38, 362–372. [Google Scholar] [CrossRef]
- Melenovsky, V.; Petrak, J.; Mracek, T.; Benes, J.; Borlaug, B.A.; Nuskova, H.; Pluhacek, T.; Spatenka, J.; Kovalcikova, J.; Drahota, Z.; et al. Myocardial Iron Content and Mitochondrial Function in Human Heart Failure: A Direct Tissue Analysis. Eur. J. Heart Fail. 2017, 19, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Sutil-Vega, M.; Rizzo, M.; Martínez-Rubio, A. Anemia and Iron Deficiency in Heart Failure: A Review of Echocardiographic Features. Echocardiography 2019, 36, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jamieson, K.L.; Grenier, J.; Nikhanj, A.; Tang, Z.; Wang, F.; Wang, S.; Seidman, J.G.; Seidman, C.E.; Thompson, R.; et al. Myocardial Iron Deficiency and Mitochondrial Dysfunction in Advanced Heart Failure in Humans. J. Am. Heart Assoc. 2022, 11, e022853. [Google Scholar] [CrossRef] [PubMed]
- Abu-Assi, E.; López-López, A.; González-Salvado, V.; Redondo-Diéguez, A.; Peña-Gil, C.; Bouzas-Cruz, N.; Raposeiras-Roubín, S.; Riziq-Yousef Abumuaileq, R.; García-Acuña, J.M.; González-Juanatey, J.R. The Risk of Cardiovascular Events After an Acute Coronary Event Remains High, Especially During the First Year, Despite Revascularization. Rev. Esp. Cardiol. (Engl. Ed). 2016, 69, 11–18. [Google Scholar] [CrossRef]
- Cahill, T.J.; Kharbanda, R.K. Heart Failure after Myocardial Infarction in the Era of Primary Percutaneous Coronary Intervention: Mechanisms, Incidence and Identification of Patients at Risk. World J. Cardiol. 2017, 9, 407–415. [Google Scholar] [CrossRef]
- Kelly, D.J.; Gershlick, T.; Witzenbichler, B.; Guagliumi, G.; Fahy, M.; Dangas, G.; Mehran, R.; Stone, G.W. Incidence and Predictors of Heart Failure Following Percutaneous Coronary Intervention in ST-Segment Elevation Myocardial Infarction: The HORIZONS-AMI Trial. Am. Heart J. 2011, 162, 663–670. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Zeller, M.; Cottin, Y.; Vergely, C. GDF15 and Cardiac Cells: Current Concepts and New Insights. Int. J. Mol. Sci. 2021, 22, 8889. [Google Scholar] [CrossRef]
- Luo, J.-W.; Duan, W.-H.; Song, L.; Yu, Y.-Q.; Shi, D.-Z. A Meta-Analysis of Growth Differentiation Factor-15 and Prognosis in Chronic Heart Failure. Front. Cardiovasc. Med. 2021, 8, 630818. [Google Scholar] [CrossRef]
- di Candia, A.M.; de Avila, D.X.; Moreira, G.R.; Villacorta, H.; Maisel, A.S. Growth Differentiation Factor-15, a Novel Systemic Biomarker of Oxidative Stress, Inflammation, and Cellular Aging: Potential Role in Cardiovascular Diseases. Am. Heart J. Plus: Cardiol. Res. Pract. 2021, 9, 100046. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Kempf, T.; Rector, T.S.; Tapken, H.; Allhoff, T.; Jantzen, F.; Kuskowski, M.; Cohn, J.N.; Drexler, H.; Wollert, K.C. Serial Measurement of Growth-Differentiation Factor-15 in Heart Failure. Circulation 2010, 122, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A.; Patel, P.; Das, S.R.; Ayers, C.R.; Khera, A.; Martinez-Rumayor, A.; Berry, J.D.; McGuire, D.K.; de Lemos, J.A. Association of Growth Differentiation Factor-15 with Coronary Atherosclerosis and Mortality in a Young, Multiethnic Population: Observations from the Dallas Heart Study. Clin. Chem. 2012, 58, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Izumiya, Y.; Hanatani, S.; Kimura, Y.; Takashio, S.; Yamamoto, E.; Kusaka, H.; Tokitsu, T.; Rokutanda, T.; Araki, S.; Tsujita, K.; et al. Growth Differentiation Factor-15 Is a Useful Prognostic Marker in Patients with Heart Failure with Preserved Ejection Fraction. Can. J. Cardiol. 2014, 30, 338–344. [Google Scholar] [CrossRef]
- Sharma, A.; Stevens, S.R.; Lucas, J.; Fiuzat, M.; Adams, K.F.; Whellan, D.J.; Donahue, M.P.; Kitzman, D.W.; Piña, I.L.; Zannad, F.; et al. Utility of Growth Differentiation Factor-15, A Marker of Oxidative Stress and Inflammation, in Chronic Heart Failure. JACC Heart Fail. 2017, 5, 724–734. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Li, Y.; Jing, X.; Yang, J. Prognostic Value of Growth Differentiation Factor-15 in Chinese Patients with Heart Failure: A Prospective Observational Study. Cardiol. J. 2018, 25, 245–253. [Google Scholar] [CrossRef]
- Girona, J.; Guardiola, M.; Barroso, E.; García-Altares, M.; Ibarretxe, D.; Plana, N.; Ribalta, J.; Amigó, N.; Correig, X.; Vázquez-Carrera, M.; et al. GDF15 Circulating Levels Are Associated with Metabolic-Associated Liver Injury and Atherosclerotic Cardiovascular Disease. Int. J. Mol. Sci. 2025, 26, 2039. [Google Scholar] [CrossRef]
- Liuizė, A.; Mongirdienė, A. TGF-β Isoforms and GDF-15 in the Development and Progression of Atherosclerosis. Int. J. Mol. Sci. 2024, 25, 2104. [Google Scholar] [CrossRef]
- Guardiola, M.; Girona, J.; Barroso, E.; García-Altares, M.; Ibarretxe, D.; Plana, N.; Ribalta, J.; Correig, X.; Vázquez-Carrera, M.; Masana, L.; et al. The GDF15 3′ UTR Polymorphism Rs1054564 Is Associated with Diabetes and Subclinical Atherosclerosis. Int. J. Mol. Sci. 2024, 25, 11985. [Google Scholar] [CrossRef]
- Ferrannini, G.; Manca, M.L.; Magnoni, M.; Andreotti, F.; Andreini, D.; Latini, R.; Maseri, A.; Maggioni, A.P.; Ostroff, R.M.; Williams, S.A.; et al. Coronary Artery Disease and Type 2 Diabetes: A Proteomic Study. Diabetes Care 2020, 43, 843–851. [Google Scholar] [CrossRef]
- Hagström, E.; Held, C.; Stewart, R.A.H.; Aylward, P.E.; Budaj, A.; Cannon, C.P.; Koenig, W.; Krug-Gourley, S.; Mohler, E.R.; Steg, P.G.; et al. Growth Differentiation Factor 15 Predicts All-Cause Morbidity and Mortality in Stable Coronary Heart Disease. Clin. Chem. 2017, 63, 325–333. [Google Scholar] [CrossRef]
- Khan, S.Q.; Ng, K.; Dhillon, O.; Kelly, D.; Quinn, P.; Squire, I.B.; Davies, J.E.; Ng, L.L. Growth Differentiation Factor-15 as a Prognostic Marker in Patients with Acute Myocardial Infarction. Eur. Heart J. 2009, 30, 1057–1065. [Google Scholar] [CrossRef]
- Lakhal, S.; Talbot, N.P.; Crosby, A.; Stoepker, C.; Townsend, A.R.M.; Robbins, P.A.; Pugh, C.W.; Ratcliffe, P.J.; Mole, D.R. Regulation of Growth Differentiation Factor 15 Expression by Intracellular Iron. Blood 2009, 113, 1555–1563. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, R.; Delanghe, J.R.; Speeckaert, M.M. Growth Differentiation Factor 15 (GDF-15) in Kidney Diseases. Adv. Clin. Chem. 2023, 114, 1–46. [Google Scholar] [CrossRef]
- Przybyłowski, P.; Wasilewski, G.; Bachorzewska-Gajewska, H.; Golabek, K.; Dobrzycki, S.; Małyszko, J. Growth Differentiation Factor 15 Is Related to Anemia and Iron Metabolism in Heart Allograft Recipients and Patients with Chronic Heart Failure. Transplant. Proc. 2014, 46, 2852–2855. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Gullestad, L.; Kou, L.; Young, J.B.; Pfeffer, M.A.; van Veldhuisen, D.J.; Swedberg, K.; Mcmurray, J.J.V.; Desai, A.S.; Anand, I.S.; et al. Growth Differentiation Factor 15 Predicts Poor Prognosis in Patients with Heart Failure and Reduced Ejection Fraction and Anemia: Results from RED-HF. Clin. Res. Cardiol. 2022, 111, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Polo, R.; Ras-Jiménez, M.D.M.; Basalo Carbajales, M.D.C.; Jovells-Vaqué, S.; Garcia-Pinilla, J.M.; Cobo-Marcos, M.; de Juan-Bagudá, J.; Fonseca, C.; Francesch Manzano, J.; Cosa, A.E.; et al. Myocardial Performance Improvement After Iron Replacement in Heart Failure Patients: The IRON-PATH II Echo-Substudy. J. Clin. Med. 2025, 14, 4048. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a Novel Macrophage Inhibitory Cytokine, Is a Divergent Member of the TGF-β Superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef]
- Assadi, A.; Zahabi, A.; Hart, R.A. GDF15, an Update of the Physiological and Pathological Roles It Plays: A Review. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1535–1546. [Google Scholar] [CrossRef]
- Tsai, V.W.W.; Lin, S.; Brown, D.A.; Salis, A.; Breit, S.N. Anorexia–Cachexia and Obesity Treatment May Be Two Sides of the Same Coin: Role of the TGF-b Superfamily Cytokine MIC-1/GDF15. Int. J. Obes. 2016, 40, 193–197. [Google Scholar] [CrossRef]
- Breit, S.N.; Johnen, H.; Cook, A.D.; Tsai, V.W.W.; Mohammad, M.G.; Kuffner, T.; Zhang, H.P.; Marquis, C.P.; Jiang, L.; Lockwood, G.; et al. The TGF-β Superfamily Cytokine, MIC-1/GDF15: A Pleotrophic Cytokine with Roles in Inflammation, Cancer and Metabolism. Growth Factors 2011, 29, 187–195. [Google Scholar] [CrossRef]
- Planavila, A.; Fernández-Solà, J.; Villarroya, F. Cardiokines as Modulators of Stress-Induced Cardiac Disorders. Adv. Protein Chem. Struct. Biol. 2017, 108, 227–256. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Kempf, T.; Wallentin, L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clin. Chem. 2017, 63, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Insights Into Mechanisms of GDF15 and Receptor GFRAL: Therapeutic Targets. Trends Endocrinol. Metab. 2020, 31, 939–951. [Google Scholar] [CrossRef]
- Al-Naseem, A.; Sallam, A.; Choudhury, S.; Thachil, J. Iron Deficiency without Anaemia: A Diagnosis That Matters. Clin. Med. 2021, 21, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Martens, P. The Effect of Iron Deficiency on Cardiac Function and Structure in Heart Failure with Reduced Ejection Fraction. Card. Fail. Rev. 2022, 8, e06. [Google Scholar] [CrossRef]
- Yin, D.; Yan, X.; Bai, X.; Tian, A.; Gao, Y.; Li, J. Prognostic Value of Growth Differentiation Factors 15 in Acute Heart Failure Patients with Preserved Ejection Fraction. ESC Heart Fail. 2023, 10, 1025–1034. [Google Scholar] [CrossRef]
- Li, J.; Cui, Y.; Huang, A.; Li, Q.; Jia, W.; Liu, K.; Qi, X. Additional Diagnostic Value of Growth Differentiation Factor-15 (GDF-15) to N-Terminal B-Type Natriuretic Peptide (NT-ProBNP) in Patients with Different Stages of Heart Failure. Med. Sci. Monit. 2018, 24, 4992–4999. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, L.; Zhang, H.; Zhang, X.-L.; Shao, H.-Y. Correlation between Growth Differentiation Factor-15 and the Severity of Chronic Heart Failure in Patients with Coronary Atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12844–12848. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Liu, H.; Ng, C.Y.; Xu, G.; Liu, E.; Li, G.; Liu, T. Circulating Serum Levels of Growth Differentiation Factor-15 and Neuregulin-1 in Patients with Paroxysmal Non-Valvular Atrial Fibrillation. Int. J. Cardiol. 2014, 172, E311–E313. [Google Scholar] [CrossRef]
- Tan, Z.; Song, T.; Huang, S.; Liu, M.; Ma, J.; Zhang, J.; Yu, P.; Liu, X. Relationship between Serum Growth Differentiation Factor 15, Fibroblast Growth Factor-23 and Risk of Atrial Fibrillation: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 899667. [Google Scholar] [CrossRef] [PubMed]
| (N = 60) | |
|---|---|
| Demographic Data and Comorbidities | |
| Age (years) | 72.5 (62.3–79.0) |
| Gender (F) | 16.7% |
| Hypertension | 80.0% |
| Diabetes | 60.0% |
| Obesity | 33.9% |
| Dyslipidaemia | 66.1% |
| Acute myocardial infarction | 50.8% |
| Peripheral artery disease | 21.7% |
| Stroke | 15.0% |
| Atrial fibrillation | 50.0% |
| Ischemic etiology of HF | 55.0% |
| NYHA Functional Class | |
| I | 18.3% |
| II | 68.3% |
| III | 13.3% |
| IV | 0% |
| Clinical and Analytical Data | |
| Systolic BP (mmHg) | 116.0 (102.3–133.0) |
| Diastolic BP (mmHg) | 65.0 (58.3–73.8) |
| Weight (Kg) | 76.5 (67.3–83.8) |
| BMI (Kg/m2) | 27.0 (24.0–32.0) |
| Heart rate (bpm) | 69.5 (60.0–80.8) |
| LVEF (%) | 36.2 (31.3–43.0) |
| TAPSE (mm) | 18.7 (16.3–20.7) |
| 6MWT (m) | 355.5 (292.5–404.8) |
| NT-proBNP (pg/dL) | 1692.0 (639.0–3106.0) |
| Creatinine (μmol/L) | 108.4 (84.5–141.3) |
| Glomerular filtration rate (mL/min) | 55.3 (40.7–76.0) |
| Iron (μmol/L) | 7.6 (5.8–10.0) |
| Hemoglobin (g/dL) | 13.8 (12.5–14.6) |
| Ferritin (ng/mL) | 81.2 (47.3–176.5) |
| Transferrin (μmol/L) | 31.0 (27.5–35.4) |
| TSAT (%) | 16.0 (14.0–20.0) |
| GDF15 (pg/mL) | 2273.8 (1497.9–3322.4) |
| Variables | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| ρ | p-Value | ρ | p-Value | |
| Systolic BP | 0.128 | 0.332 | 0.311 | 0.020 |
| Diastolic BP | −0.201 | 0.124 | 0.036 | 0.795 |
| Weight | −0.546 | <0.001 | −0.402 | 0.002 |
| BMI | −0.481 | <0.001 | −0.283 | 0.033 |
| Heart rate | 0.053 | 0.690 | 0.180 | 0.185 |
| LVEF | −0.251 | 0.053 | −0.106 | 0.437 |
| TAPSE | −0.327 | 0.017 | −0.288 | 0.045 |
| 6MWT | −0.654 | <0.001 | −0.445 | <0.001 |
| NT-proBNP | 0.597 | <0.001 | 0.427 | 0.001 |
| Creatinine | 0.577 | <0.001 | 0.394 | 0.003 |
| Glomerular filtration | −0.608 | <0.001 | −0.424 | 0.001 |
| Iron | 0.283 | 0.028 | 0.080 | 0.560 |
| Hemoglobin | −0.490 | <0.001 | −0.424 | 0.001 |
| Ferritin | 0.342 | 0.007 | 0.328 | 0.014 |
| Transferrin | −0.231 | 0.078 | −0.117 | 0.396 |
| TSAT | 0.123 | 0.389 | −0.050 | 0.735 |
| Variables | Crude | Adjusted | ||||
|---|---|---|---|---|---|---|
| β | p-Value | R2 | β | p-Value | R2 | |
| Systolic BP | 0.233 | 0.073 | 0.054 | 0.301 | 0.013 | 0.280 |
| Diastolic BP | −0.100 | 0.449 | 0.010 | 0.039 | 0.772 | 0.192 |
| Weight | −0.460 | <0.001 | 0.212 | −0.429 | 0.002 | 0.263 |
| BMI | −0.390 | 0.002 | 0.152 | −0.295 | 0.033 | 0.191 |
| Heart rate | 0.130 | 0.321 | 0.017 | 0.161 | 0.192 | 0.216 |
| LVEF | −0.229 | 0.079 | 0.052 | −0.101 | 0.442 | 0.199 |
| TAPSE | −0.364 | 0.007 | 0.132 | −0.275 | 0.045 | 0.243 |
| 6MWT | −0.539 | <0.001 | 0.291 | −0.509 | <0.001 | 0.370 |
| NT-proBNP | 0.565 | <0.001 | 0.320 | 0.479 | 0.001 | 0.337 |
| Creatinine | 0.522 | <0.001 | 0.273 | 0.428 | 0.003 | 0.317 |
| Glomerular filtration | −0.557 | <0.001 | 0.310 | −0.474 | 0.001 | 0.337 |
| Iron | 0.191 | 0.144 | 0.036 | 0.084 | 0.516 | 0.197 |
| Hemoglobin | −0.476 | <0.001 | 0.226 | −0.451 | <0.001 | 0.340 |
| Ferritin | 0.327 | 0.011 | 0.107 | 0.303 | 0.014 | 0.277 |
| Transferrin | −0.249 | 0.057 | 0.062 | −0.122 | 0.395 | 0.205 |
| TSAT | 0.004 | 0.978 | 0.000 | −0.273 | 0.756 | 0.162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajes, M.; Ras-Jiménez, M.d.M.; Girona, J.; Ramos-Polo, R.; Guardiola, M.; García-Pinilla, J.M.; Ribalta, J.; Cobo-Marcos, M.; Masana, L.; de Juan-Bagudá, J.; et al. Serum Growth Differentiation Factor 15 (GDF15) Levels Reflect Ischemic Etiology in Heart Failure Patients with Iron Deficiency: A Cross-Sectional Study. Biomolecules 2025, 15, 1234. https://doi.org/10.3390/biom15091234
Tajes M, Ras-Jiménez MdM, Girona J, Ramos-Polo R, Guardiola M, García-Pinilla JM, Ribalta J, Cobo-Marcos M, Masana L, de Juan-Bagudá J, et al. Serum Growth Differentiation Factor 15 (GDF15) Levels Reflect Ischemic Etiology in Heart Failure Patients with Iron Deficiency: A Cross-Sectional Study. Biomolecules. 2025; 15(9):1234. https://doi.org/10.3390/biom15091234
Chicago/Turabian StyleTajes, Marta, Maria del Mar Ras-Jiménez, Josefa Girona, Raúl Ramos-Polo, Montse Guardiola, José Manuel García-Pinilla, Josep Ribalta, Marta Cobo-Marcos, Lluís Masana, Javier de Juan-Bagudá, and et al. 2025. "Serum Growth Differentiation Factor 15 (GDF15) Levels Reflect Ischemic Etiology in Heart Failure Patients with Iron Deficiency: A Cross-Sectional Study" Biomolecules 15, no. 9: 1234. https://doi.org/10.3390/biom15091234
APA StyleTajes, M., Ras-Jiménez, M. d. M., Girona, J., Ramos-Polo, R., Guardiola, M., García-Pinilla, J. M., Ribalta, J., Cobo-Marcos, M., Masana, L., de Juan-Bagudá, J., Fonseca, C., Enjuanes, C., Vázquez-Carrera, M., Comin-Colet, J., & Rodríguez-Calvo, R. (2025). Serum Growth Differentiation Factor 15 (GDF15) Levels Reflect Ischemic Etiology in Heart Failure Patients with Iron Deficiency: A Cross-Sectional Study. Biomolecules, 15(9), 1234. https://doi.org/10.3390/biom15091234







