Icatibant Acts as a Balanced Ligand of MRGPRX2 in Human Skin Mast Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Treatments
2.2. β-Hexosaminidase Release Assay
2.3. Immunoblot Analysis
2.4. Flow Cytometry
2.5. Statistical Analysis
3. Results
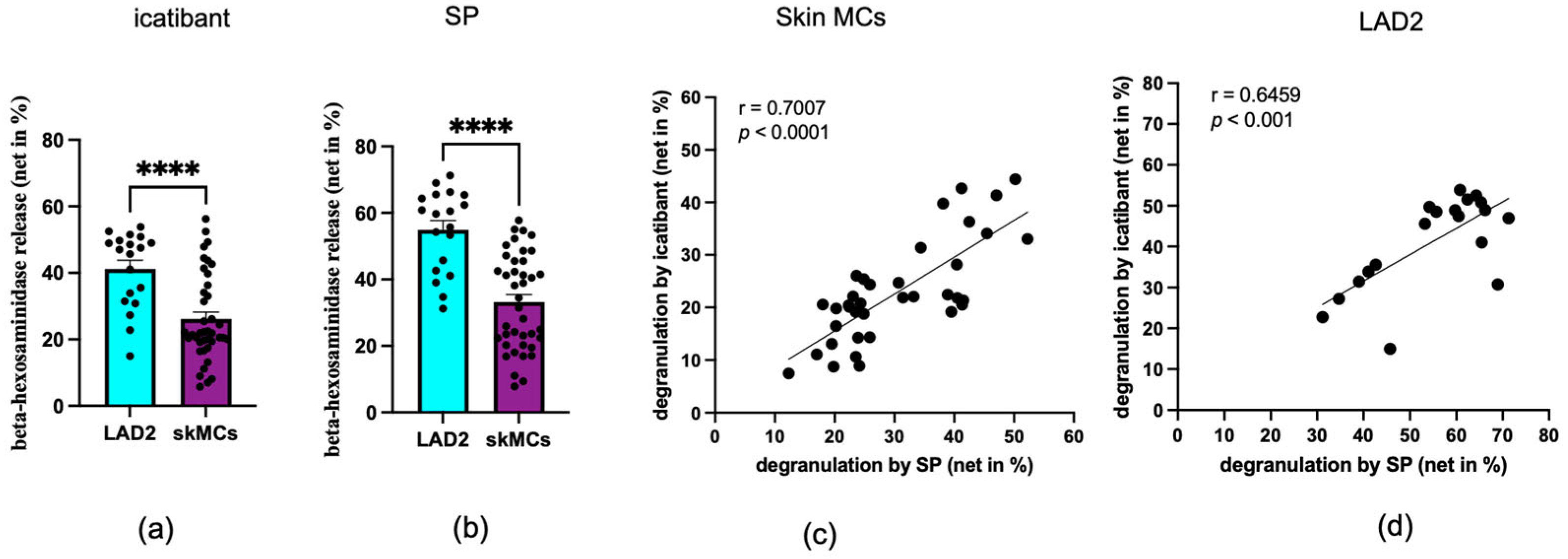
3.1. Skin MCs and LAD2 Cells Degranulate with Icatibant
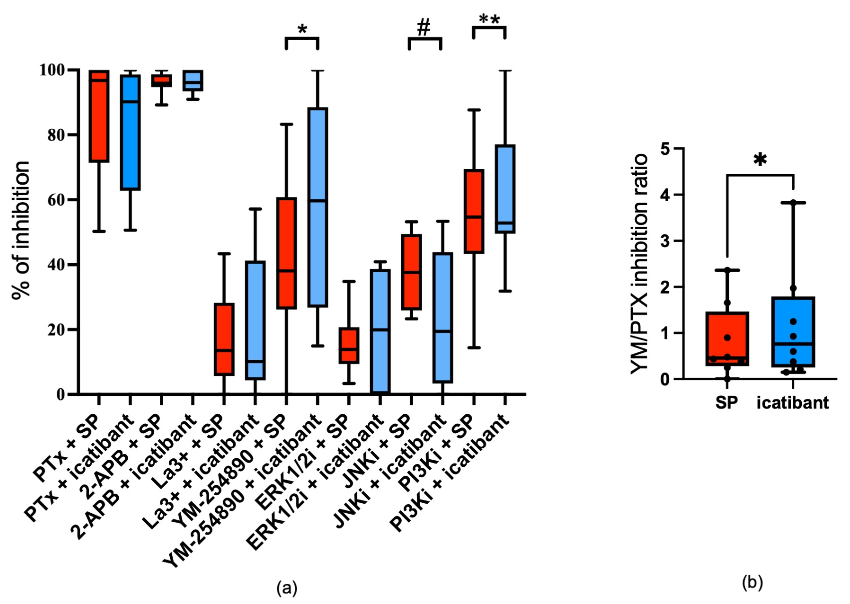
3.2. Comparative Signal Transduction for SP and Icatibant
3.3. Shared and Distinct Signaling Prerequisites Underlie the Degranulation Responses Elicited by Icatibant and SP
3.4. Icatibant Triggers MRGPRX2 Internalization in Skin MCs
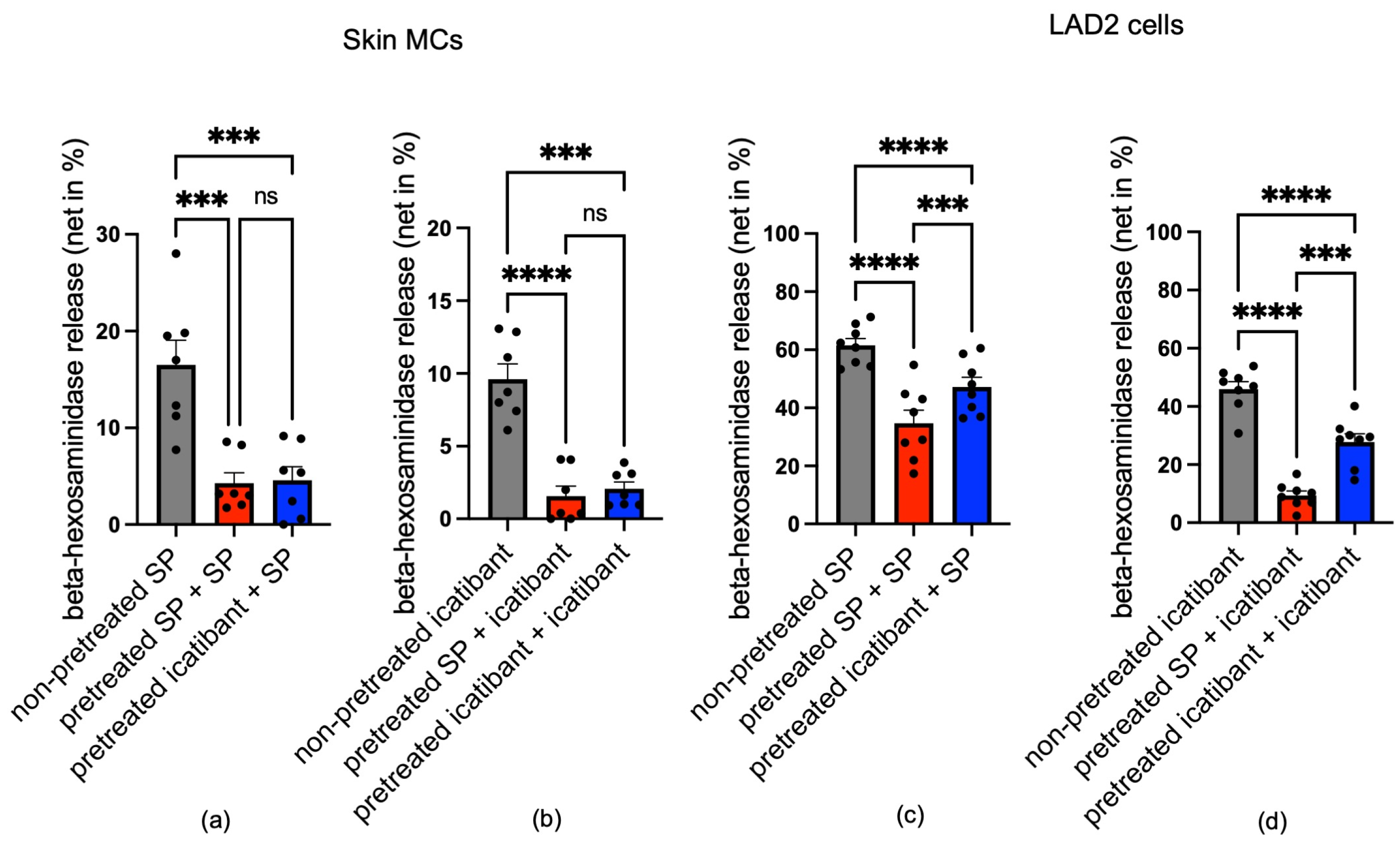
3.5. Icatibant Potently Desensitizes MRGPRX2 in Skin MCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRGPRX2 | Mas-related G-protein-coupled receptor member X2 |
| LAD2 | Laboratory of Allergic Diseases 2 mast cell line |
| MCs | Mast cells |
| GPCR | G-protein-coupled receptor |
| GDP | Guanosine diphosphate |
| GTP | Guanosine triphosphate |
| GRK | G-protein-coupled receptor kinase |
| Akt | Protein kinase B |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| P | Phosphorylated |
| I | Inhibitor |
| Min | Minute |
| TANGO | Transcriptional activation following arrestin translocation |
| HTLA | HEK293-derived TANGO Luciferase Assay cell line |
| PI3K | Phosphoinositide 3-kinase |
| JNK | c-Jun N-terminal kinase |
| SP | Substance P |
| PTX | Pertussis toxin |
| 2-APB | 2-Aminoethoxydiphenyl borate |
| La3+ | Lanthanum ion |
| TSLPR | Thymic stromal lymphopoietin receptor |
| IL-33 | Interleukin-33 |
References
- Quan, P.L.; Sabaté-Brescó, M.; Guo, Y.; Martín, M.; Gastaminza, G. The Multifaceted Mas-Related G Protein-Coupled Receptor Member X2 in Allergic Diseases and Beyond. Int. J. Mol. Sci. 2021, 22, 4421. [Google Scholar] [CrossRef]
- Roy, S.; Chompunud Na Ayudhya, C.; Thapaliya, M.; Deepak, V.; Ali, H. Multifaceted MRGPRX2: New insight into the role of mast cells in health and disease. J. Allergy Clin. Immunol. 2021, 148, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Metz, M.; Starkl, P.; Marichal, T.; Tsai, M. Mast cells and IgE in defense against lethality of venoms: Possible “benefit” of allergy. Allergo J. Int. 2020, 29, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Beaven, M.A.; Metcalfe, D.D. Mast cells signal their importance in health and disease. J. Allergy Clin. Immunol. 2018, 142, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Kühn, H.; Kolkhir, P.; Babina, M.; Düll, M.; Frischbutter, S.; Fok, J.S.; Jiao, Q.; Metz, M.; Scheffel, J.; Wolf, K.; et al. Mas-related G protein-coupled receptor X2 and its activators in dermatologic allergies. J. Allergy Clin. Immunol. 2021, 147, 456–469. [Google Scholar] [CrossRef]
- Al Hamwi, G.; Riedel, Y.K.; Clemens, S.; Namasivayam, V.; Thimm, D.; Müller, C.E. MAS-related G protein-coupled receptors X (MRGPRX): Orphan GPCRs with potential as targets for future drugs. Pharmacol. Ther. 2022, 238, 108259. [Google Scholar] [CrossRef]
- Foer, D.; Wien, M.; Karlson, E.W.; Song, W.; Boyce, J.A.; Brennan, P.J. Patient Characteristics Associated With Reactions to Mrgprx2-Activating Drugs in an Electronic Health Record-Linked Biobank. J. Allergy Clin. Immunol. Pract. 2023, 11, 492–499.e492. [Google Scholar] [CrossRef]
- Corbière, A.; Loste, A.; Gaudenzio, N. MRGPRX2 sensing of cationic compounds-A bridge between nociception and skin diseases? Exp. Dermatol. 2021, 30, 193–200. [Google Scholar] [CrossRef]
- Kumar, M.; Duraisamy, K.; Chow, B.K. Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2). Cells 2021, 10, 1033. [Google Scholar] [CrossRef]
- Maier, P.; Macht, M.; Beck, S.; Kolkhir, P.; Babina, M.; Kremer, A.E.; Zahn, D.; Wolf, K. MRGPRX2 ligandome: Molecular simulations reveal three categories of ligand-receptor interactions. J. Struct. Biol. 2025, 217, 108193. [Google Scholar] [CrossRef]
- Shiraishi, A.; Wada, A.; Satake, H. Evolutionary scenarios for the specific recognition of nonhomologous endogenous peptides by G protein–coupled receptor paralogs. J. Biol. Chem. 2025, 301, 108125. [Google Scholar] [CrossRef]
- Lansu, K.; Karpiak, J.; Liu, J.; Huang, X.P.; McCorvy, J.D.; Kroeze, W.K.; Che, T.; Nagase, H.; Carroll, F.I.; Jin, J.; et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat. Chem. Biol. 2017, 13, 529–536. [Google Scholar] [CrossRef]
- Cao, C.; Kang, H.J.; Singh, I.; Chen, H.; Zhang, C.; Ye, W.; Hayes, B.W.; Liu, J.; Gumpper, R.H.; Bender, B.J.; et al. Structure, function and pharmacology of human itch GPCRs. Nature 2021, 600, 170–175. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Bal, G.; Franke, K.; Zuberbier, T.; Babina, M. β-arrestin-1 and β-arrestin-2 Restrain MRGPRX2-Triggered Degranulation and ERK1/2 Activation in Human Skin Mast Cells. Front. Allergy 2022, 3, 930233. [Google Scholar] [CrossRef]
- Babina, M.; Wang, Z.; Roy, S.; Guhl, S.; Franke, K.; Artuc, M.; Ali, H.; Zuberbier, T. MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through β-Arrestin and Lack of Correlation with the FcεRI Pathway. J. Investig. Dermatol. 2021, 141, 1286–1296.e1284. [Google Scholar] [CrossRef]
- Chompunud Na Ayudhya, C.; Amponnawarat, A.; Ali, H. Substance P Serves as a Balanced Agonist for MRGPRX2 and a Single Tyrosine Residue Is Required for β-Arrestin Recruitment and Receptor Internalization. Int. J. Mol. Sci. 2021, 22, 5318. [Google Scholar] [CrossRef] [PubMed]
- Busse, P.; Kaplan, A. Specific Targeting of Plasma Kallikrein for Treatment of Hereditary Angioedema: A Revolutionary Decade. J. Allergy Clin. Immunol. Pract. 2022, 10, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Porebski, G.; Kwiecien, K.; Pawica, M.; Kwitniewski, M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front. Immunol. 2018, 9, 3027. [Google Scholar] [CrossRef] [PubMed]
- Shtessel, M.; Limjunyawong, N.; Oliver, E.T.; Chichester, K.; Gao, L.; Dong, X.; Saini, S.S. MRGPRX2 Activation Causes Increased Skin Reactivity in Patients with Chronic Spontaneous Urticaria. J. Investig. Dermatol. 2021, 141, 678–681.e672. [Google Scholar] [CrossRef]
- Syed, M.; Kammala, A.K.; Callahan, B.; Oskeritzian, C.A.; Subramanian, H. Lactic acid suppresses MRGPRX2 mediated mast cell responses. Cell. Immunol. 2021, 368, 104422. [Google Scholar] [CrossRef]
- Dondalska, A.; Rönnberg, E.; Ma, H.; Pålsson, S.A.; Magnusdottir, E.; Gao, T.; Adam, L.; Lerner, E.A.; Nilsson, G.; Lagerström, M.; et al. Amelioration of Compound 48/80-Mediated Itch and LL-37-Induced Inflammation by a Single-Stranded Oligonucleotide. Front. Immunol. 2020, 11, 559589. [Google Scholar] [CrossRef]
- Murakami, T.; Suzuki, K.; Niyonsaba, F.; Tada, H.; Reich, J.; Tamura, H.; Nagaoka, I. MrgX2-mediated internalization of LL-37 and degranulation of human LAD2 mast cells. Mol. Med. Rep. 2018, 18, 4951–4959. [Google Scholar] [CrossRef]
- Raj, S.; Lu, L.; Unsworth, L.D. Screening Peptides that Activate MRGPRX2 using Engineered HEK Cells. J. Vis. Exp. 2021, 177, e62448. [Google Scholar] [CrossRef]
- Roy, S.; Ganguly, A.; Haque, M.; Ali, H. Angiogenic Host Defense Peptide AG-30/5C and Bradykinin B(2) Receptor Antagonist Icatibant Are G Protein Biased Agonists for MRGPRX2 in Mast Cells. J. Immunol. 2019, 202, 1229–1238. [Google Scholar] [CrossRef]
- Alkanfari, I.; Gupta, K.; Jahan, T.; Ali, H. Naturally Occurring Missense MRGPRX2 Variants Display Loss of Function Phenotype for Mast Cell Degranulation in Response to Substance P, Hemokinin-1, Human β-Defensin-3, and Icatibant. J. Immunol. 2018, 201, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, H.; Furuno, M.; Edamura, K.; Noguchi, M. Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells. J. Leukoc. Biol. 2019, 106, 1069–1077. [Google Scholar] [CrossRef]
- Lowman, M.A.; Benyon, R.C.; Church, M.K. Characterization of neuropeptide-induced histamine release from human dispersed skin mast cells. Br. J. Pharmacol. 1988, 95, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Motakis, E.; Guhl, S.; Ishizu, Y.; Itoh, M.; Kawaji, H.; de Hoon, M.; Lassmann, T.; Carninci, P.; Hayashizaki, Y.; Zuberbier, T.; et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 2014, 123, e58–e67. [Google Scholar] [CrossRef]
- Varricchi, G.; Pecoraro, A.; Loffredo, S.; Poto, R.; Rivellese, F.; Genovese, A.; Marone, G.; Spadaro, G. Heterogeneity of Human Mast Cells With Respect to MRGPRX2 Receptor Expression and Function. Front. Cell. Neurosci. 2019, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Plum, T.; Wang, X.; Rettel, M.; Krijgsveld, J.; Feyerabend, T.B.; Rodewald, H.R. Human Mast Cell Proteome Reveals Unique Lineage, Putative Functions, and Structural Basis for Cell Ablation. Immunity 2020, 52, 404–416.e405. [Google Scholar] [CrossRef]
- Babina, M.; Guhl, S.; Artuc, M.; Zuberbier, T. Allergic FcεRI- and pseudo-allergic MRGPRX2-triggered mast cell activation routes are independent and inversely regulated by SCF. Allergy 2018, 73, 256–260. [Google Scholar] [CrossRef]
- Wang, Z.; Guhl, S.; Franke, K.; Artuc, M.; Zuberbier, T.; Babina, M. IL-33 and MRGPRX2-Triggered Activation of Human Skin Mast Cells-Elimination of Receptor Expression on Chronic Exposure, but Reinforced Degranulation on Acute Priming. Cells 2019, 8, 341. [Google Scholar] [CrossRef]
- Wang, Z.; Franke, K.; Zuberbier, T.; Babina, M. Cytokine Stimulation by MRGPRX2 Occurs with Lower Potency than by FcεRI Aggregation but with Similar Dependence on the Extracellular Signal-Regulated Kinase 1/2 Module in Human Skin Mast Cells. J. Investig. Dermatol. 2022, 142, 414–424.e8. [Google Scholar] [CrossRef]
- Babina, M.; Guhl, S.; Artuc, M.; Trivedi, N.N.; Zuberbier, T. Phenotypic variability in human skin mast cells. Exp. Dermatol. 2016, 25, 434–439. [Google Scholar] [CrossRef]
- Babina, M.; Wang, Z.; Franke, K.; Guhl, S.; Artuc, M.; Zuberbier, T. Yin-Yang of IL-33 in Human Skin Mast Cells: Reduced Degranulation, but Augmented Histamine Synthesis through p38 Activation. J. Investig. Dermatol. 2019, 139, 1516–1525.e3. [Google Scholar] [CrossRef]
- Babina, M.; Wang, Z.; Artuc, M.; Guhl, S.; Zuberbier, T. MRGPRX2 is negatively targeted by SCF and IL-4 to diminish pseudo-allergic stimulation of skin mast cells in culture. Exp. Dermatol. 2018, 27, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Akula, S.; Tripathi, S.R.; Franke, K.; Wernersson, S.; Babina, M.; Hellman, L. Cultures of Human Skin Mast Cells, an Attractive In Vitro Model for Studies of Human Mast Cell Biology. Cells 2024, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Guhl, S.; Artuc, M.; Neou, A.; Babina, M.; Zuberbier, T. Long-term cultured human skin mast cells are suitable for pharmacological studies of anti-allergic drugs due to high responsiveness to FcεRI cross-linking. Biosci. Biotechnol. Biochem. 2011, 75, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Kirshenbaum, A.S.; Akin, C.; Wu, Y.; Rottem, M.; Goff, J.P.; Beaven, M.A.; Rao, V.K.; Metcalfe, D.D. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk. Res. 2003, 27, 677–682. [Google Scholar] [CrossRef]
- Li, Z.; Schneikert, J.; Bal, G.; Jin, M.; Franke, K.; Zuberbier, T.; Babina, M. Intrinsic Regulatory Mechanisms Protect Human Skin Mast Cells from Excessive MRGPRX2 Activation: Paucity in LAD2 (Laboratory of Allergic Diseases 2) Cells Contributes to Hyperresponsiveness of the Mast Cell Line. J. Investig. Dermatol. 2024, 145, 1215–1219. [Google Scholar] [CrossRef]
- Franke, K.; Wang, Z.; Zuberbier, T.; Babina, M. Cytokines Stimulated by IL-33 in Human Skin Mast Cells: Involvement of NF-κB and p38 at Distinct Levels and Potent Co-Operation with FcεRI and MRGPRX2. Int. J. Mol. Sci. 2021, 22, 3580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Franke, K.; Bal, G.; Li, Z.; Zuberbier, T.; Babina, M. MRGPRX2-Mediated Degranulation of Human Skin Mast Cells Requires the Operation of G(αi), G(αq), Ca++ Channels, ERK1/2 and PI3K-Interconnection between Early and Late Signaling. Cells 2022, 11, 953. [Google Scholar] [CrossRef]
- Babina, M. The pseudo-allergic/neurogenic route of mast cell activation via MRGPRX2: Discovery, functional programs, regulation, relevance to disease, and relation with allergic stimulation. Itch 2020, 5, e32. [Google Scholar] [CrossRef]
- Baldo, B.A. MRGPRX2, drug pseudoallergies, inflammatory diseases, mechanisms and distinguishing MRGPRX2- and IgE/FcεRI-mediated events. Br. J. Clin. Pharmacol. 2023, 89, 3232–3246. [Google Scholar] [CrossRef]
- Grimes, J.; Desai, S.; Charter, N.W.; Lodge, J.; Moita Santos, R.; Isidro-Llobet, A.; Mason, A.M.; Wu, Z.; Wolfe, L.A., 3rd; Anantharaman, L.; et al. MrgX2 is a promiscuous receptor for basic peptides causing mast cell pseudo-allergic and anaphylactoid reactions. Pharmacol. Res. Perspect. 2019, 7, e00547. [Google Scholar] [CrossRef]
- Franke, K.; Bal, G.; Li, Z.; Zuberbier, T.; Babina, M. Clorfl86/RHEX Is a Negative Regulator of SCF/KIT Signaling in Human Skin Mast Cells. Cells 2023, 12, 1306. [Google Scholar] [CrossRef]
- Redhu, D.; Franke, K.; Aparicio-Soto, M.; Kumari, V.; Pazur, K.; Illerhaus, A.; Hartmann, K.; Worm, M.; Babina, M. Mast cells instruct keratinocytes to produce thymic stromal lymphopoietin: Relevance of the tryptase/protease-activated receptor 2 axis. J. Allergy Clin. Immunol. 2022, 149, 2053–2061.e2056. [Google Scholar] [CrossRef]
- Babina, M.; Wang, Z.; Franke, K.; Zuberbier, T. Thymic Stromal Lymphopoietin Promotes MRGPRX2-Triggered Degranulation of Skin Mast Cells in a STAT5-Dependent Manner with Further Support from JNK. Cells 2021, 10, 102. [Google Scholar] [CrossRef]
- Franke, K.; Bal, G.; Li, Z.; Zuberbier, T.; Babina, M. CREB Is Activated by the SCF/KIT Axis in a Partially ERK-Dependent Manner and Orchestrates Survival and the Induction of Immediate Early Genes in Human Skin Mast Cells. Int. J. Mol. Sci. 2023, 24, 4135. [Google Scholar] [CrossRef]
- Babina, M.; Wang, Z.; Li, Z.; Franke, K.; Guhl, S.; Artuc, M.; Zuberbier, T. FcεRI- and MRGPRX2-evoked acute degranulation responses are fully additive in human skin mast cells. Allergy 2022, 77, 1906–1909. [Google Scholar] [CrossRef]
- Peng, Q.; McEuen, A.R.; Benyon, R.C.; Walls, A.F. The heterogeneity of mast cell tryptase from human lung and skin. Eur. J. Biochem. 2003, 270, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Worm, M.; Edenharter, G.; Ruëff, F.; Scherer, K.; Pföhler, C.; Mahler, V.; Treudler, R.; Lang, R.; Nemat, K.; Koehli, A.; et al. Symptom profile and risk factors of anaphylaxis in Central Europe. Allergy 2012, 67, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Robas, N.; Mead, E.; Fidock, M. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J. Biol. Chem. 2003, 278, 44400–44404. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Gupta, K.; Lee, D.; Bayir, A.K.; Ahn, H.; Ali, H. β-Defensins activate human mast cells via Mas-related gene X2. J. Immunol. 2013, 191, 345–352. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Zhang, Y.; Lai, Y.; Chen, W.; Xiao, Z.; Zhang, W.; Jin, M.; Yu, B. LL-37-induced human mast cell activation through G protein-coupled receptor MrgX2. Int. Immunopharmacol. 2017, 49, 6–12. [Google Scholar] [CrossRef]
- Chompunud Na Ayudhya, C.; Roy, S.; Alkanfari, I.; Ganguly, A.; Ali, H. Identification of Gain and Loss of Function Missense Variants in MRGPRX2’s Transmembrane and Intracellular Domains for Mast Cell Activation by Substance P. Int. J. Mol. Sci. 2019, 20, 5247. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Mobley, Y.R.; Choi, H.W.; Bist, P.; Salinas, C.A.; Brown, Z.D.; Chen, S.L.; Staats, H.F.; Abraham, S.N. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv. 2019, 5, eaav0216. [Google Scholar] [CrossRef]
- Yang, F.; Guo, L.; Li, Y.; Wang, G.; Wang, J.; Zhang, C.; Fang, G.X.; Chen, X.; Liu, L.; Yan, X.; et al. Structure, function and pharmacology of human itch receptor complexes. Nature 2021, 600, 164–169. [Google Scholar] [CrossRef]
- Reddy, V.B.; Graham, T.A.; Azimi, E.; Lerner, E.A. A single amino acid in MRGPRX2 necessary for binding and activation by pruritogens. J. Allergy Clin. Immunol. 2017, 140, 1726–1728. [Google Scholar] [CrossRef]
- Joshi, S.R.; Khan, D.A. Non-IgE-Mediated Drug Hypersensitivity Reactions. Curr. Allergy Asthma Rep. 2021, 21, 41. [Google Scholar] [CrossRef]
- Giannetti, M.P.; Nicoloro-SantaBarbara, J.; Godwin, G.; Middlesworth, J.; Espeland, A.; Castells, M.C. Drug and Venom Allergy in Mastocytosis. Immunol. Allergy Clin. N. Am. 2023, 43, 699–710. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Schneikert, J.; Bal, G.; Zuberbier, T.; Babina, M. Icatibant Acts as a Balanced Ligand of MRGPRX2 in Human Skin Mast Cells. Biomolecules 2025, 15, 1224. https://doi.org/10.3390/biom15091224
Li Z, Schneikert J, Bal G, Zuberbier T, Babina M. Icatibant Acts as a Balanced Ligand of MRGPRX2 in Human Skin Mast Cells. Biomolecules. 2025; 15(9):1224. https://doi.org/10.3390/biom15091224
Chicago/Turabian StyleLi, Zhuoran, Jean Schneikert, Gürkan Bal, Torsten Zuberbier, and Magda Babina. 2025. "Icatibant Acts as a Balanced Ligand of MRGPRX2 in Human Skin Mast Cells" Biomolecules 15, no. 9: 1224. https://doi.org/10.3390/biom15091224
APA StyleLi, Z., Schneikert, J., Bal, G., Zuberbier, T., & Babina, M. (2025). Icatibant Acts as a Balanced Ligand of MRGPRX2 in Human Skin Mast Cells. Biomolecules, 15(9), 1224. https://doi.org/10.3390/biom15091224








