Retinal Gatekeepers: Molecular Mechanism and Therapeutic Role of Cysteine and Selenocysteine
Abstract
1. Introduction
2. The Retinal Gatekeepers
3. Retina and Oxidative Stress: A Delicate Balance
4. Oxidative Stress and Retinal Degeneration: A Link Worth Exploring
4.1. Age-Related Macular Degeneration
4.2. Retinitis Pigmentosa
4.3. Diabetic Retinopathy
5. Counteracting Oxidative Stress: A Strategy for Treating Retinal Diseases
5.1. Small Antioxidants
5.2. KEAP1–NRF2 Pathway
5.3. Gene Therapy
5.4. N-Acetyl-L-Cysteine
5.5. Selenium
5.6. Endogenous Redox Defenses
5.7. Advantages and Current Limitations
6. Discussion
7. Conclusions
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaffey, N.; Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell. 4th Edn. Ann. Bot. 2003, 91, 401. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of Oxidative Stress in Neurodegenerative Disorders: A Review of Reactive Oxygen Species and Prevention by Antioxidants. Brain Commun. 2023, 6, fcad356. [Google Scholar] [CrossRef]
- Rusciano, D.; Bagnoli, P. Oxygen, the Paradox of Life and the Eye. Front. Biosci.-Landmark 2024, 29, 319. [Google Scholar] [CrossRef]
- Zhang, Y.; Roh, Y.J.; Han, S.-J.; Park, I.; Lee, H.M.; Ok, Y.S.; Lee, B.C.; Lee, S.-R. Role of Selenoproteins in Redox Regulation of Signaling and the Antioxidant System: A Review. Antioxidants 2020, 9, 383. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W.; Shah, A.; Yu, M.; Liu, Y.; He, L.; Dang, J.; Yang, L.; Yan, M.; Ying, Y.; et al. Sodium Iodate Induces Ferroptosis in Human Retinal Pigment Epithelium ARPE-19 Cells. Cell Death Dis. 2021, 12, 230. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the Transsulfuration Pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Zou, C. Redox Regulation and Reaction Mechanism of Human Cystathionine-β-Synthase: A PLP-Dependent Hemesensor Protein. Arch. Biochem. Biophys. 2005, 433, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Sbodio, J.I.; Snyder, S.H. Cysteine Metabolism in Neuronal Redox Homeostasis. Trends Pharmacol. Sci. 2018, 39, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Yadav, V.; Banerjee, R. Heme-Dependent Metabolite Switching Regulates H2S Synthesis in Response to Endoplasmic Reticulum (ER) Stress. J. Biol. Chem. 2016, 291, 16418–16423. [Google Scholar] [CrossRef]
- Banerjee, R. Catalytic Promiscuity and Heme-Dependent Redox Regulation of H 2 S Synthesis. Curr. Opin. Chem. Biol. 2017, 37, 115–121. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Tang, G.; Wu, L.; Wang, R. Rat Pancreatic Level of Cystathionine γ-Lyase Is Regulated by Glucose Level via Specificity Protein 1 (SP1) Phosphorylation. Diabetologia 2011, 54, 2615–2625. [Google Scholar] [CrossRef]
- Zhang, H.; Forman, H.J. Glutathione Synthesis and Its Role in Redox Signaling. Semin. Cell Dev. Biol. 2012, 23, 722–728. [Google Scholar] [CrossRef]
- Murphy, R.C.; Zarini, S. Glutathione Adducts of Oxyeicosanoids. Prostaglandins Other Lipid Mediat. 2002, 68–69, 471–482. [Google Scholar] [CrossRef]
- Łukaszewicz-Hussain, A. The role of glutathione and glutathione-related enzymes in antioxidative process. Med. Pr. 2003, 54, 473–479. [Google Scholar]
- Lu, S.C. Regulation of Glutathione Synthesis. Mol. Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Giustarini, D.; Milzani, A.; Dalle-Donne, I.; Rossi, R. How to Increase Cellular Glutathione. Antioxidants 2023, 12, 1094. [Google Scholar] [CrossRef]
- Ayalasomayajula, S.P.; Kompella, U.B. Induction of Vascular Endothelial Growth Factor by 4-Hydroxynonenal and Its Prevention by Glutathione Precursors in Retinal Pigment Epithelial Cells. Eur. J. Pharmacol. 2002, 449, 213–220. [Google Scholar] [CrossRef]
- Bulteau, A.-L.; Chavatte, L. Update on Selenoprotein Biosynthesis. Antioxid. Redox Signal. 2015, 23, 775–794. [Google Scholar] [CrossRef]
- Mariotti, M.; Ridge, P.G.; Zhang, Y.; Lobanov, A.V.; Pringle, T.H.; Guigo, R.; Hatfield, D.L.; Gladyshev, V.N. Composition and Evolution of the Vertebrate and Mammalian Selenoproteomes. PLoS ONE 2012, 7, e33066. [Google Scholar] [CrossRef] [PubMed]
- Fagegaltier, D. Structural Analysis of New Local Features in SECIS RNA Hairpins. Nucleic Acids Res. 2000, 28, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Rebsch, C.M.; Kinzy, S.A.; Fletcher, J.E.; Copeland, P.R. Efficiency of Mammalian Selenocysteine Incorporation. J. Biol. Chem. 2004, 279, 37852–37859. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Carlson, B.A.; Lee, B.J.; Tsuji, P.A.; Copeland, P.R.; Schweizer, U.; Gladyshev, V.N.; Hatfield, D.L. Selenocysteine tRNA[Ser]Sec, the Central Component of Selenoprotein Biosynthesis: Isolation, Identification, Modification, and Sequencing. In Methods in Molecular Biology (Clifton, N.J.); Chavatte, L., Ed.; Springer New York: New York, NY, USA, 2018; Volume 1661, pp. 43–60. ISBN 978-1-4939-7257-9. [Google Scholar]
- Carlson, B.A.; Yoo, M.-H.; Tsuji, P.A.; Gladyshev, V.N.; Hatfield, D.L. Mouse Models Targeting Selenocysteine tRNA Expression for Elucidating the Role of Selenoproteins in Health and Development. Molecules 2009, 14, 3509–3527. [Google Scholar] [CrossRef]
- Vindry, C.; Ohlmann, T.; Chavatte, L. Translation Regulation of Mammalian Selenoproteins. Biochim. Biophys. Acta BBA-Gen. Subj. 2018, 1862, 2480–2492. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Sies, H. Protection against Reactive Oxygen Species by Selenoproteins. Biochim. Biophys. Acta BBA-Gen. Subj. 2009, 1790, 1478–1485. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Glutathione Peroxidases and Redox-Regulated Transcription Factors. Biol. Chem. 2006, 387, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From Selenium to Selenoproteins: Synthesis, Identity, and Their Role in Human Health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef]
- Cheng, W.-H.; Ho, Y.-S.; Valentine, B.A.; Ross, D.A.; Combs, G.F.; Lei, X.G. Cellular Glutathione Peroxidase Is the Mediator of Body Selenium to Protect against Paraquat Lethality in Transgenic Mice. J. Nutr. 1998, 128, 1070–1076. [Google Scholar] [CrossRef]
- Björnstedt, M.; Hamberg, M.; Kumar, S.; Xue, J.; Holmgren, A. Human Thioredoxin Reductase Directly Reduces Lipid Hydroperoxides by NADPH and Selenocystine Strongly Stimulates the Reaction via Catalytically Generated Selenols. J. Biol. Chem. 1995, 270, 11761–11764. [Google Scholar] [CrossRef]
- Moskovitz, J.; Singh, V.K.; Requena, J.; Wilkinson, B.J.; Jayaswal, R.K.; Stadtman, E.R. Purification and Characterization of Methionine Sulfoxide Reductases from Mouse and Staphylococcus Aureus and Their Substrate Stereospecificity. Biochem. Biophys. Res. Commun. 2002, 290, 62–65. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Selenoprotein P—Expression, Functions, and Roles in Mammals. Biochim. Biophys. Acta BBA-Gen. Subj. 2009, 1790, 1441–1447. [Google Scholar] [CrossRef]
- Tan, S.M.; Stefanovic, N.; Tan, G.; Wilkinson-Berka, J.L.; De Haan, J.B. Lack of the Antioxidant Glutathione Peroxidase-1 (GPx1) Exacerbates Retinopathy of Prematurity in Mice. Investig. Opthalmology Vis. Sci. 2013, 54, 555. [Google Scholar] [CrossRef]
- Roggia, M.F.; Imai, H.; Shiraya, T.; Noda, Y.; Ueta, T. Protective Role of Glutathione Peroxidase 4 in Laser-Induced Choroidal Neovascularization in Mice. PLoS ONE 2014, 9, e98864. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Szaflik, J.P.; Szaflik, M.; Ulinska, M.; Szaflik, J.; Majsterek, I. Analysis of Antioxidative Factors Related to AMD Risk Development in the Polish Patients. Acta Ophthalmol. 2017, 95, 530–536. [Google Scholar] [CrossRef]
- Chang, C.; Worley, B.L.; Phaëton, R.; Hempel, N. Extracellular Glutathione Peroxidase GPx3 and Its Role in Cancer. Cancers 2020, 12, 2197. [Google Scholar] [CrossRef]
- Maiorino, M.; Scapin, M.; Ursini, F.; Biasolo, M.; Bosello, V.; Flohé, L. Distinct Promoters Determine Alternative Transcription of Gpx-4 into Phospholipid-Hydroperoxide Glutathione Peroxidase Variants. J. Biol. Chem. 2003, 278, 34286–34290. [Google Scholar] [CrossRef]
- Ueta, T.; Inoue, T.; Furukawa, T.; Tamaki, Y.; Nakagawa, Y.; Imai, H.; Yanagi, Y. Glutathione Peroxidase 4 Is Required for Maturation of Photoreceptor Cells. J. Biol. Chem. 2012, 287, 7675–7682. [Google Scholar] [CrossRef]
- Hansson, H.-A.; Holmgren, A.; Norstedt, G.; Rozell, B. Changes in the Distribution of Insulin-like Growth Factor I, Thioredoxin, Thioredoxin Reductase and Ribonucleotide Reductase during the Development of the Retina. Exp. Eye Res. 1989, 48, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Dyer, M.A.; Cepko, C.L. Regulating Proliferation during Retinal Development. Nat. Rev. Neurosci. 2001, 2, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Tanito, M.; Huang, Z.; Li, F.; Zhou, X.; Zaharia, A.; Yodoi, J.; McGinnis, J.F.; Cao, W. Delay of Photoreceptor Degeneration in Tubby Mouse by Sulforaphane. J. Neurochem. 2007, 101, 1041–1052. [Google Scholar] [CrossRef]
- Ren, X.; Léveillard, T. Modulating Antioxidant Systems as a Therapeutic Approach to Retinal Degeneration. Redox Biol. 2022, 57, 102510. [Google Scholar] [CrossRef]
- Shibuki, H.; Katai, N.; Yodoi, J.; Uchida, K.; Yoshimura, N. Lipid Peroxidation and Peroxynitrite in Retinal Ischemia-Reperfusion Injury. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3607–3614. [Google Scholar] [PubMed]
- Shibuki, H.; Katai, N.; Kuroiwa, S.; Kurokawa, T.; Yodoi, J.; Yoshimura, N. Protective Effect of Adult T-Cell Leukemia-Derived Factor on Retinal Ischemia-Reperfusion Injury in the Rat. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1470–1477. [Google Scholar] [PubMed]
- Yamamoto, M.; Ohira, A.; Honda, O.; Sato, N.; Furuke, K.; Yodoi, J.; Honda, Y. Analysis of Localization of Adult T-Cell Leukemia-Derived Factor in the Transient Ischemic Rat Retina After Treatment with OP-1206 α-CD, a Prostaglandin E1 Analogue. J. Histochem. Cytochem. 1997, 45, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Masutani, H.; Nakamura, H.; Ohira, A.; Yodoi, J. Cytoprotective Effect of Thioredoxin against Retinal Photic Injury in Mice. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1162–1167. [Google Scholar]
- Tanito, M.; Masutani, H.; Nakamura, H.; Oka, S.; Ohira, A.; Yodoi, J. Attenuation of Retinal Photooxidative Damage in Thioredoxin Transgenic Mice. Neurosci. Lett. 2002, 326, 142–146. [Google Scholar] [CrossRef]
- Kong, L.; Zhou, X.; Li, F.; Yodoi, J.; McGinnis, J.; Cao, W. Neuroprotective Effect of Overexpression of Thioredoxin on Photoreceptor Degeneration in Tubby Mice. Neurobiol. Dis. 2010, 38, 446–455. [Google Scholar] [CrossRef]
- Ahuja-Jensen, P.; Johnsen-Soriano, S.; Ahuja, S.; Bosch-Morell, F.; Sancho-Tello, M.; Romero, F.J.; Abrahamson, M.; Van Veen, T. Low Glutathione Peroxidase in Rd1 Mouse Retina Increases Oxidative Stress and Proteases. NeuroReport 2007, 18, 797–801. [Google Scholar] [CrossRef]
- Nishitoh, H.; Matsuzawa, A.; Tobiume, K.; Saegusa, K.; Takeda, K.; Inoue, K.; Hori, S.; Kakizuka, A.; Ichijo, H. ASK1 Is Essential for Endoplasmic Reticulum Stress-Induced Neuronal Cell Death Triggered by Expanded Polyglutamine Repeats. Genes Dev. 2002, 16, 1345–1355. [Google Scholar] [CrossRef]
- Guo, X.; Namekata, K.; Kimura, A.; Harada, C.; Harada, T. ASK1 in Neurodegeneration. Adv. Biol. Regul. 2017, 66, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, S.; Hu, D.; Xing, Y.; Shen, Y. N-Methyl-N-Nitrosourea-Induced Retinal Degeneration in Mice. Exp. Eye Res. 2014, 121, 102–113. [Google Scholar] [CrossRef]
- Tooker, R.E.; Vigh, J. Light-evoked S-nitrosylation in the Retina. J. Comp. Neurol. 2015, 523, 2082–2110. [Google Scholar] [CrossRef]
- Benhar, M.; Forrester, M.T.; Stamler, J.S. Protein Denitrosylation: Enzymatic Mechanisms and Cellular Functions. Nat. Rev. Mol. Cell Biol. 2009, 10, 721–732. [Google Scholar] [CrossRef]
- Shelton, M.D.; Distler, A.M.; Kern, T.S.; Mieyal, J.J. Glutaredoxin Regulates Autocrine and Paracrine Proinflammatory Responses in Retinal Glial (Müller) Cells. J. Biol. Chem. 2009, 284, 4760–4766. [Google Scholar] [CrossRef]
- Hurley, J.B.; Lindsay, K.J.; Du, J. Glucose, Lactate, and Shuttling of Metabolites in Vertebrate Retinas. J. Neurosci. Res. 2015, 93, 1079–1092. [Google Scholar] [CrossRef]
- Kennedy, C.J.; Rakoczy, P.E.; Constable, I.J. Lipofuscin of the Retinal Pigment Epithelium: A Review. Eye 1995, 9, 763–771. [Google Scholar] [CrossRef]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic Energy Use and Supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef] [PubMed]
- Domènech, E.B.; Marfany, G. The Relevance of Oxidative Stress in the Pathogenesis and Therapy of Retinal Dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.P.; Chihuailaf, R.H. Antioxidants and the Integrity of Ocular Tissues. Vet. Med. Int. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Barrios, A.; Álvarez, L.; García, M.; Artime, E.; Pereiro, R.; González-Iglesias, H. Antioxidant Defenses in the Human Eye: A Focus on Metallothioneins. Antioxidants 2021, 10, 89. [Google Scholar] [CrossRef]
- Ban, N.; Ozawa, Y.; Osada, H.; Lin, J.B.; Toda, E.; Watanabe, M.; Yuki, K.; Kubota, S.; Apte, R.S.; Tsubota, K. Neuroprotective Role of Retinal SIRT3 against Acute Photo-Stress. Npj Aging Mech. Dis. 2017, 3, 19. [Google Scholar] [CrossRef]
- Sasaki, M.; Yuki, K.; Kurihara, T.; Miyake, S.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K.; Ozawa, Y. Biological Role of Lutein in the Light-Induced Retinal Degeneration. J. Nutr. Biochem. 2012, 23, 423–429. [Google Scholar] [CrossRef]
- Nakazawa, M.; Maeda, S.; Yokoyama, N.; Nakagawa, T.; Yonezawa, T.; Ohno, K.; Matsuki, N. Sphingosine-1-Phosphate (S1P) Signaling Regulates the Production of Intestinal IgA and Its Potential Role in the Pathogenesis of Canine Inflammatory Bowel Disease. J. Vet. Med. Sci. 2019, 81, 1249–1258. [Google Scholar] [CrossRef]
- Narimatsu, T.; Ozawa, Y.; Miyake, S.; Nagai, N.; Tsubota, K. Angiotensin II Type 1 Receptor Blockade Suppresses Light-Induced Neural Damage in the Mouse Retina. Free Radic. Biol. Med. 2014, 71, 176–185. [Google Scholar] [CrossRef]
- Osada, H.; Okamoto, T.; Kawashima, H.; Toda, E.; Miyake, S.; Nagai, N.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Neuroprotective Effect of Bilberry Extract in a Murine Model of Photo-Stressed Retina. PLoS ONE 2017, 12, e0178627. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, T.; Ozawa, Y.; Miyake, S.; Kubota, S.; Hirasawa, M.; Nagai, N.; Shimmura, S.; Tsubota, K. Disruption of Cell-Cell Junctions and Induction of Pathological Cytokines in the Retinal Pigment Epithelium of Light-Exposed Mice. Investig. Opthalmology Vis. Sci. 2013, 54, 4555. [Google Scholar] [CrossRef]
- Chen, Y.; Sawada, O.; Kohno, H.; Le, Y.-Z.; Subauste, C.; Maeda, T.; Maeda, A. Autophagy Protects the Retina from Light-Induced Degeneration. J. Biol. Chem. 2013, 288, 7506–7518. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione Depletion Induces Ferroptosis, Autophagy, and Premature Cell Senescence in Retinal Pigment Epithelial Cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; Han, Y.; Zhou, F.; Zhang, D.; Ji, B.; Hu, J.; Lv, Y.; Cai, S.; Wei, Y.; et al. Identification of Anthocyanin Components of Wild Chinese Blueberries and Amelioration of Light-Induced Retinal Damage in Pigmented Rabbit Using Whole Berries. J. Agric. Food Chem. 2011, 59, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.C.; Roszkowska, A.M.; Izzotti, A. Environmental Light and Endogenous Antioxidants as the Main Determinants of Non-Cancer Ocular Diseases. Mutat. Res. Mutat. Res. 2013, 752, 153–171. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Wu, Y.; Ji, B. Docosahexaenoic Acid Aggravates Photooxidative Damage in Retinal Pigment Epithelial Cells via Lipid Peroxidation. J. Photochem. Photobiol. B 2014, 140, 85–93. [Google Scholar] [CrossRef]
- Roehlecke, C.; Schaller, A.; Knels, L.; Funk, R.H.W. The Influence of Sublethal Blue Light Exposure on Human RPE Cells. Mol. Vis. 2009, 15, 1929–1938. [Google Scholar] [PubMed]
- Hui, S.; Yi, L.; Fengling, Q.L. Effects of Light Exposure and Use of Intraocular Lens on Retinal Pigment Epithelial Cells In Vitro. Photochem. Photobiol. 2009, 85, 966–969. [Google Scholar] [CrossRef]
- Lu, L.; Hackett, S.F.; Mincey, A.; Lai, H.; Campochiaro, P.A. Effects of Different Types of Oxidative Stress in RPE Cells. J. Cell. Physiol. 2006, 206, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, P.; Davidson, P.C.; Jones, D.P.; Hagen, T.M.; Reed, R.L.; Drews-Botsch, C. Protection of Retinal Pigment Epithelium from Oxidative Injury by Glutathione and Precursors. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3661–3668. [Google Scholar]
- Cao, J.Y.; Dixon, S.J. Mechanisms of Ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef]
- Wood, J.P.; Pergande, G.; Osborne, N.N. Prevention of Glutathione Depletion-Induced Apoptosis in Cultured Human RPE Cells by Flupirtine. Restor. Neurol. Neurosci. 1998, 12, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Yaung, J.; Kannan, R.; He, S.; Ryan, S.J.; Hinton, D.R. Hepatocyte Growth Factor Protects RPE Cells from Apoptosis Induced by Glutathione Depletion. Investig. Opthalmology Vis. Sci. 2005, 46, 4311. [Google Scholar] [CrossRef]
- Li, S.-Y.; Zhao, N.; Wei, D.; Pu, N.; Hao, X.-N.; Huang, J.-M.; Peng, G.-H.; Tao, Y. Ferroptosis in the Ageing Retina: A Malevolent Fire of Diabetic Retinopathy. Ageing Res. Rev. 2024, 93, 102142. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The Chemistry and Biological Activities of N-Acetylcysteine. Biochim. Biophys. Acta BBA-Gen. Subj. 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Nakamura, M.; Kuse, Y.; Tsuruma, K.; Shimazawa, M.; Hara, H. The Involvement of the Oxidative Stress in Murine Blue LED Light-Induced Retinal Damage Model. Biol. Pharm. Bull. 2017, 40, 1219–1225. [Google Scholar] [CrossRef]
- The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration: The Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial. JAMA 2013, 309, 2005. [Google Scholar] [CrossRef] [PubMed]
- Ganea, E.; Harding, J.J. Glutathione-Related Enzymes and the Eye. Curr. Eye Res. 2006, 31, 1–11. [Google Scholar] [CrossRef]
- The Eye Diseases Prevalence Research Group. Causes and Prevalence of Visual Impairment Among Adults in the UnitedStates. Arch. Ophthalmol. 2004, 122, 477. [Google Scholar] [CrossRef]
- World Health Organization World Report on Vision; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151657-0.
- Blasiak, J.; Petrovski, G.; Veréb, Z.; Facskó, A.; Kaarniranta, K. Oxidative Stress, Hypoxia, and Autophagy in the Neovascular Processes of Age-Related Macular Degeneration. BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Plestina-Borjan, I.; Katusic, D.; Medvidovic-Grubisic, M.; Supe-Domic, D.; Bucan, K.; Tandara, L.; Rogosic, V. Association of Age-Related Macular Degeneration with Erythrocyte Antioxidant Enzymes Activity and Serum Total Antioxidant Status. Oxid. Med. Cell. Longev. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.S.; Symons, R.C.A.; Komeima, K.; Shen, J.; Xiao, W.; Swaim, M.E.; Gong, Y.Y.; Kachi, S.; Campochiaro, P.A. Differential Sensitivity of Cones to Iron-Mediated Oxidative Damage. Investig. Opthalmology Vis. Sci. 2007, 48, 438. [Google Scholar] [CrossRef]
- Zarbin, M.A. Current Concepts in the Pathogenesis of Age-Related Macular Degeneration. Arch. Ophthalmol. 2004, 122, 598. [Google Scholar] [CrossRef]
- Shen, J.K.; Dong, A.; Hackett, S.F.; Bell, W.R.; Green, W.R.; Campochiaro, P.A. Oxidative Damage in Age-Related Macular Degeneration. Histol. Histopathol. 2007, 22, 1301–1308. [Google Scholar] [CrossRef]
- Hollyfield, J.G.; Bonilha, V.L.; Rayborn, M.E.; Yang, X.; Shadrach, K.G.; Lu, L.; Ufret, R.L.; Salomon, R.G.; Perez, V.L. Oxidative Damage–Induced Inflammation Initiates Age-Related Macular Degeneration. Nat. Med. 2008, 14, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Thurman, J.M.; Renner, B.; Kunchithapautham, K.; Ferreira, V.P.; Pangburn, M.K.; Ablonczy, Z.; Tomlinson, S.; Holers, V.M.; Rohrer, B. Oxidative Stress Renders Retinal Pigment Epithelial Cells Susceptible to Complement-Mediated Injury. J. Biol. Chem. 2009, 284, 16939–16947. [Google Scholar] [CrossRef]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated Autophagy in the RPE Is Associated with Increased Susceptibility to Oxidative Stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of Antioxidant Enzymes and Small Molecular Weight Antioxidants in the Pathogenesis of Age-Related Macular Degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef]
- Nguyen, T.; Sherratt, P.J.; Pickett, C.B. Regulatory Mechanisms Controlling Gene Expression Mediated by the Antioxidant Response Element. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 233–260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, F.; Yan, A.; Xia, X. Madecassoside Protects Retinal Pigment Epithelial Cells against Hydrogen Peroxide-Induced Oxidative Stress and Apoptosis through the Activation of Nrf2/HO-1 Pathway. Biosci. Rep. 2020, 40, BSR20194347. [Google Scholar] [CrossRef]
- Brantley, M.A.; Osborn, M.P.; Sanders, B.J.; Rezaei, K.A.; Lu, P.; Li, C.; Milne, G.L.; Cai, J.; Sternberg, P. Plasma Biomarkers of Oxidative Stress and Genetic Variants in Age-Related Macular Degeneration. Am. J. Ophthalmol. 2012, 153, 460–467.e1. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, P.G.; Ferrington, D.A.; Kannan, R. Glutathione Metabolism and the Novel Role of Mitochondrial GSH in Retinal Degeneration. Antioxidants 2021, 10, 661. [Google Scholar] [CrossRef]
- Samiec, P.S.; Drews-Botsch, C.; Flagg, E.W.; Kurtz, J.C.; Sternberg, P.; Reed, R.L.; Jones, D.P. Glutathione in Human Plasma: Decline in Association with Aging, Age-Related Macular Degeneration, and Diabetes. Free Radic. Biol. Med. 1998, 24, 699–704. [Google Scholar] [CrossRef]
- Bharathselvi, M.; Biswas, S.; Raman, R.; Selvi, R.; Coral, K.; Narayanansamy, A.; Ramakrishnan, S.; Sulochana, K.N. Homocysteine & Its Metabolite Homocysteine-Thiolactone & Deficiency of Copper in Patients with Age Related Macular Degeneration—A Pilot Study. Indian J. Med. Res. 2016, 143, 756–762. [Google Scholar] [CrossRef]
- Nowak, M.; Świȩtochowska, E.; Wielkoszyński, T.; Marek, B.; Karpe, J.; Górski, J.; Głogowska-szelαg, J.; Kos-kudła, B.; Ostrowska, Z. Changes in Blood Antioxidants and Several Lipid Peroxidation Products in Women with Age-Related Macular Degeneration. Eur. J. Ophthalmol. 2003, 13, 281–286. [Google Scholar] [CrossRef]
- Kularatne, R.N.; Bulumulla, C.; Catchpole, T.; Takacs, A.; Christie, A.; Stefan, M.C.; Csaky, K.G. Protection of Human Retinal Pigment Epithelial Cells from Oxidative Damage Using Cysteine Prodrugs. Free Radic. Biol. Med. 2020, 152, 386–394. [Google Scholar] [CrossRef]
- Tosi, G.M.; Giustarini, D.; Franci, L.; Minetti, A.; Imperatore, F.; Caldi, E.; Fiorenzani, P.; Aloisi, A.M.; Sparatore, A.; Rossi, R.; et al. Superior Properties of N-Acetylcysteine Ethyl Ester over N-Acetyl Cysteine to Prevent Retinal Pigment Epithelial Cells Oxidative Damage. Int. J. Mol. Sci. 2021, 22, 600. [Google Scholar] [CrossRef]
- Cohen, S.M.; Olin, K.L.; Feuer, W.J.; Hjelmeland, L.; Keen, C.L.; Morse, L.S. Low Glutathione Reductase and Peroxidase Activity in Age-Related Macular Degeneration. Br. J. Ophthalmol. 1994, 78, 791–794. [Google Scholar] [CrossRef]
- Zafrilla, P.; Losada, M.; Perez, A.; Caravaca, G.; Mulero, J. Biomarkers of Oxidative Stress in Patients with Wet Age Related Macular Degeneration. J. Nutr. Health Aging 2013, 17, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Ulańczyk, Z.; Grabowicz, A.; Cecerska-Heryć, E.; Śleboda-Taront, D.; Krytkowska, E.; Mozolewska-Piotrowska, K.; Safranow, K.; Kawa, M.P.; Dołęgowska, B.; Machalińska, A. Dietary and Lifestyle Factors Modulate the Activity of the Endogenous Antioxidant System in Patients with Age-Related Macular Degeneration: Correlations with Disease Severity. Antioxidants 2020, 9, 954. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Małecka, K.; Kabiesz, A.; Nowak, M.; Śpiewak, D. Age Related Macular Degeneration—Challenge for Future: Pathogenesis and New Perspectives for the Treatment. Eur. Geriatr. Med. 2015, 6, 69–75. [Google Scholar] [CrossRef]
- Brodzka, S.; Baszyński, J.; Rektor, K.; Hołderna-Bona, K.; Stanek, E.; Kurhaluk, N.; Tkaczenko, H.; Malukiewicz, G.; Woźniak, A.; Kamiński, P. The Role of Glutathione in Age-Related Macular Degeneration (AMD). Int. J. Mol. Sci. 2024, 25, 4158. [Google Scholar] [CrossRef]
- Athanasiou, D.; Aguila, M.; Bellingham, J.; Li, W.; McCulley, C.; Reeves, P.J.; Cheetham, M.E. The Molecular and Cellular Basis of Rhodopsin Retinitis Pigmentosa Reveals Potential Strategies for Therapy. Prog. Retin. Eye Res. 2018, 62, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Narayan, D.S.; Wood, J.P.M.; Chidlow, G.; Casson, R.J. A Review of the Mechanisms of Cone Degeneration in Retinitis Pigmentosa. Acta Ophthalmol. 2016, 94, 748–754. [Google Scholar] [CrossRef]
- Verbakel, S.K.; Van Huet, R.A.C.; Boon, C.J.F.; Den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-Syndromic Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Balem, F.; Tirupula, K.; Man, D.; Dhiman, H.K.; Yanamala, N.; Ollesch, J.; Planas-Iglesias, J.; Jennings, B.J.; Gerwert, K.; et al. Comparison of the Molecular Properties of Retinitis Pigmentosa P23H and N15S Amino Acid Replacements in Rhodopsin. PLoS ONE 2019, 14, e0214639. [Google Scholar] [CrossRef]
- Chan, P.; Stolz, J.; Kohl, S.; Chiang, W.-C.; Lin, J.H. Endoplasmic Reticulum Stress in Human Photoreceptor Diseases. Brain Res. 2016, 1648, 538–541. [Google Scholar] [CrossRef]
- Duricka, D.L.; Brown, R.L.; Varnum, M.D. Defective Trafficking of Cone Photoreceptor CNG Channels Induces the Unfolded Protein Response and ER-Stress-Associated Cell Death. Biochem. J. 2012, 441, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Sizova, O.S.; Shinde, V.M.; Lenox, A.R.; Gorbatyuk, M.S. Modulation of Cellular Signaling Pathways in P23H Rhodopsin Photoreceptors. Cell. Signal. 2014, 26, 665–672. [Google Scholar] [CrossRef]
- Tsubura, A.; Yoshizawa, K.; Kuwata, M.; Uehara, N. Animal Models for Retinitis Pigmentosa Induced by MNU.; Disease Progression, Mechanisms and Therapeutic Trials. Histol. Histopathol. 2010, 25, 933–944. [Google Scholar] [CrossRef]
- Carmody, R.J.; Cotter, T.G. Oxidative Stress Induces Caspase-Independent Retinal Apoptosis in Vitro. Cell Death Differ. 2000, 7, 282–291. [Google Scholar] [CrossRef]
- Luft, R. The Development of Mitochondrial Medicine. Proc. Natl. Acad. Sci. USA 1994, 91, 8731–8738. [Google Scholar] [CrossRef]
- Rodriguez, M.C.; MacDonald, J.R.; Mahoney, D.J.; Parise, G.; Beal, M.F.; Tarnopolsky, M.A. Beneficial Effects of Creatine, CoQ10, and Lipoic Acid in Mitochondrial Disorders. Muscle Nerve 2007, 35, 235–242. [Google Scholar] [CrossRef]
- Liu, J.; Ames, B.N. Reducing Mitochondrial Decay with Mitochondrial Nutrients to Delay and Treat Cognitive Dysfunction, Alzheimer’s Disease, and Parkinson’s Disease. Nutr. Neurosci. 2005, 8, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Komeima, K.; Rogers, B.S.; Lu, L.; Campochiaro, P.A. Antioxidants Reduce Cone Cell Death in a Model of Retinitis Pigmentosa. Proc. Natl. Acad. Sci. USA 2006, 103, 11300–11305. [Google Scholar] [CrossRef]
- Usui, S.; Komeima, K.; Lee, S.Y.; Jo, Y.-J.; Ueno, S.; Rogers, B.S.; Wu, Z.; Shen, J.; Lu, L.; Oveson, B.C.; et al. Increased Expression of Catalase and Superoxide Dismutase 2 Reduces Cone Cell Death in Retinitis Pigmentosa. Mol. Ther. 2009, 17, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Song, J.; Wang, C.; Li, Y.; Dunaief, J.L. Berberine Protects against Light-Induced Photoreceptor Degeneration in the Mouse Retina. Exp. Eye Res. 2016, 145, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Piano, I.; D’Antongiovanni, V.; Testai, L.; Calderone, V.; Gargini, C. A Nutraceutical Strategy to Slowing Down the Progression of Cone Death in an Animal Model of Retinitis Pigmentosa. Front. Neurosci. 2019, 13, 461. [Google Scholar] [CrossRef]
- Berson, E.L. A Randomized Trial of Vitamin A and Vitamin E Supplementation for Retinitis Pigmentosa. Arch. Ophthalmol. 1993, 111, 761. [Google Scholar] [CrossRef]
- Espinós, C.; Galindo, M.I.; García-Gimeno, M.A.; Ibáñez-Cabellos, J.S.; Martínez-Rubio, D.; Millán, J.M.; Rodrigo, R.; Sanz, P.; Seco-Cervera, M.; Sevilla, T.; et al. Oxidative Stress, a Crossroad Between Rare Diseases and Neurodegeneration. Antioxidants 2020, 9, 313. [Google Scholar] [CrossRef]
- Léveillard, T.; Mohand-Saïd, S.; Lorentz, O.; Hicks, D.; Fintz, A.-C.; Clérin, E.; Simonutti, M.; Forster, V.; Cavusoglu, N.; Chalmel, F.; et al. Identification and Characterization of Rod-Derived Cone Viability Factor. Nat. Genet. 2004, 36, 755–759. [Google Scholar] [CrossRef]
- Punzo, C.; Xiong, W.; Cepko, C.L. Loss of Daylight Vision in Retinal Degeneration: Are Oxidative Stress and Metabolic Dysregulation to Blame? J. Biol. Chem. 2012, 287, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Roska, B. Rods Feed Cones to Keep them Alive. Cell 2015, 161, 706–708. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dong, A.; Shen, J.; Krause, M.; Hackett, S.F.; Campochiaro, P.A. Increased Expression of Glial Cell Line-derived Neurotrophic Factor Protects against Oxidative Damage-induced Retinal Degeneration. J. Neurochem. 2007, 103, 1041–1052. [Google Scholar] [CrossRef]
- Usui, S.; Oveson, B.C.; Lee, S.Y.; Jo, Y.; Yoshida, T.; Miki, A.; Miki, K.; Iwase, T.; Lu, L.; Campochiaro, P.A. NADPH Oxidase Plays a Central Role in Cone Cell Death in Retinitis Pigmentosa. J. Neurochem. 2009, 110, 1028–1037. [Google Scholar] [CrossRef]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a Therapeutic Target for Common Pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Pallardó, F.V.; Lyakhovich, A.; Tiano, L.; Trifuoggi, M. Mitigating the Pro-Oxidant State and Melanogenesis of Retinitis Pigmentosa: By Counteracting Mitochondrial Dysfunction. Cell. Mol. Life Sci. 2021, 78, 7491–7503. [Google Scholar] [CrossRef] [PubMed]
- Gallenga, C.E.; Lonardi, M.; Pacetti, S.; Violanti, S.S.; Tassinari, P.; Di Virgilio, F.; Tognon, M.; Perri, P. Molecular Mechanisms Related to Oxidative Stress in Retinitis Pigmentosa. Antioxidants 2021, 10, 848. [Google Scholar] [CrossRef] [PubMed]
- Usui, S.; Oveson, B.C.; Iwase, T.; Lu, L.; Lee, S.Y.; Jo, Y.J.; Wu, Z.; Choi, E.Y.; Samulski, R.J.; Campochiaro, P.A. Overexpression of SOD in retina: Need for increase in H2O2-detoxifying enzyme in same cellular compartment. Free Radic Biol Med. 2011, 51, 1347–1354. [Google Scholar] [CrossRef]
- Wong, T.Y.; Cheung, C.M.G.; Larsen, M.; Sharma, S.; Simó, R. Diabetic Retinopathy. Nat. Rev. Dis. Primer 2016, 2, 16012. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Bourne, R.R.A.; Briant, P.S.; Flaxman, S.R.; Taylor, H.R.B.; Jonas, J.B.; Abdoli, A.A.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.G.; et al. Causes of Blindness and Vision Impairment in 2020 and Trends over 30 Years, and Prevalence of Avoidable Blindness in Relation to VISION 2020: The Right to Sight: An Analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Du, Y.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor Cells Are Major Contributors to Diabetes-Induced Oxidative Stress and Local Inflammation in the Retina. Proc. Natl. Acad. Sci. USA 2013, 110, 16586–16591. [Google Scholar] [CrossRef]
- Kanwar, M.; Chan, P.-S.; Kern, T.S.; Kowluru, R.A. Oxidative Damage in the Retinal Mitochondria of Diabetic Mice: Possible Protection by Superoxide Dismutase. Investig. Opthalmology Vis. Sci. 2007, 48, 3805. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Miller, C.M.; Kern, T.S. Hyperglycemia Increases Mitochondrial Superoxide in Retina and Retinal Cells. Free Radic. Biol. Med. 2003, 35, 1491–1499. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.-P.; et al. Normalizing Mitochondrial Superoxide Production Blocks Three Pathways of Hyperglycaemic Damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Du, X.-L.; Edelstein, D.; Rossetti, L.; Fantus, I.G.; Goldberg, H.; Ziyadeh, F.; Wu, J.; Brownlee, M. Hyperglycemia-Induced Mitochondrial Superoxide Overproduction Activates the Hexosamine Pathway and Induces Plasminogen Activator Inhibitor-1 Expression by Increasing Sp1 Glycosylation. Proc. Natl. Acad. Sci. USA 2000, 97, 12222–12226. [Google Scholar] [CrossRef]
- Hammes, H.P.; Martin, S.; Federlin, K.; Geisen, K.; Brownlee, M. Aminoguanidine Treatment Inhibits the Development of Experimental Diabetic Retinopathy. Proc. Natl. Acad. Sci. USA 1991, 88, 11555–11558. [Google Scholar] [CrossRef]
- Wells-Knecht, K.J.; Zyzak, D.V.; Litchfield, J.E.; Thorpe, S.R.; Baynes, J.W. Identification of Glyoxal and Arabinose as Intermediates in the Autoxidative Modification of Proteins by Glucose. Biochemistry 1995, 34, 3702–3709. [Google Scholar] [CrossRef]
- Ishii, H.; Jirousek, M.R.; Koya, D.; Takagi, C.; Xia, P.; Clermont, A.; Bursell, S.-E.; Kern, T.S.; Ballas, L.M.; Heath, W.F.; et al. Amelioration of Vascular Dysfunctions in Diabetic Rats by an Oral PKC β Inhibitor. Science 1996, 272, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, K.H.; Merola, L.O.; Field, R.A. Sorbitol Pathway: Presence in Nerve and Cord with Substrate Accumulation in Diabetes. Science 1966, 151, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Matsumura, T.; Edelstein, D.; Rossetti, L.; Zsengellér, Z.; Szabó, C.; Brownlee, M. Inhibition of GAPDH Activity by Poly(ADP-Ribose) Polymerase Activates Three Major Pathways of Hyperglycemic Damage in Endothelial Cells. J. Clin. Investig. 2003, 112, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Peralta, D.; Bronowska, A.K.; Morgan, B.; Dóka, É.; Van Laer, K.; Nagy, P.; Gräter, F.; Dick, T.P. A Proton Relay Enhances H2O2 Sensitivity of GAPDH to Facilitate Metabolic Adaptation. Nat. Chem. Biol. 2015, 11, 156–163. [Google Scholar] [CrossRef]
- Gardiner, T.; Anderson, H.; Stitt, A. Inhibition of Advanced Glycation End-products Protects against Retinal Capillary Basement Membrane Expansion during Long-term Diabetes. J. Pathol. 2003, 201, 328–333. [Google Scholar] [CrossRef]
- Zong, H.; Ward, M.; Stitt, A.W. AGEs, RAGE, and Diabetic Retinopathy. Curr. Diab. Rep. 2011, 11, 244–252. [Google Scholar] [CrossRef]
- Zhang, M.; Kho, A.L.; Anilkumar, N.; Chibber, R.; Pagano, P.J.; Shah, A.M.; Cave, A.C. Glycated Proteins Stimulate Reactive Oxygen Species Production in Cardiac Myocytes: Involvement of Nox2 (Gp91phox)-Containing NADPH Oxidase. Circulation 2006, 113, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, E.L.M.; Empsen, C.; Geerts, A.; Van Grunsven, L.A. Advanced Glycation End Products Induce Production of Reactive Oxygen Species via the Activation of NADPH Oxidase in Murine Hepatic Stellate Cells. J. Hepatol. 2010, 52, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Ward, M.; Madden, A.; Yong, P.H.; Limb, G.A.; Curtis, T.M.; Stitt, A.W. Hyperglycaemia-Induced pro-Inflammatory Responses by Retinal Müller Glia Are Regulated by the Receptor for Advanced Glycation End-Products (RAGE). Diabetologia 2010, 53, 2656–2666. [Google Scholar] [CrossRef]
- Yao, D.; Brownlee, M. Hyperglycemia-Induced Reactive Oxygen Species Increase Expression of the Receptor for Advanced Glycation End Products (RAGE) and RAGE Ligands. Diabetes 2010, 59, 249–255. [Google Scholar] [CrossRef]
- Curtis, T.M.; Hamilton, R.; Yong, P.-H.; McVicar, C.M.; Berner, A.; Pringle, R.; Uchida, K.; Nagai, R.; Brockbank, S.; Stitt, A.W. Müller Glial Dysfunction during Diabetic Retinopathy in Rats Is Linked to Accumulation of Advanced Glycation End-Products and Advanced Lipoxidation End-Products. Diabetologia 2011, 54, 690–698. [Google Scholar] [CrossRef]
- Stitt, A.W.; Hughes, S.-J.; Canning, P.; Lynch, O.; Cox, O.; Frizzell, N.; Thorpe, S.R.; Cotter, T.G.; Curtis, T.M.; Gardiner, T.A. Substrates Modified by Advanced Glycation End-Products Cause Dysfunction and Death in Retinal Pericytes by Reducing Survival Signals Mediated by Platelet-Derived Growth Factor. Diabetologia 2004, 47, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Jiang, D.; Tang, L. Advanced Glycation End-Products Induce Apoptosis Involving the Signaling Pathways of Oxidative Stress in Bovine Retinal Pericytes. Life Sci. 2006, 79, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.M.; Kim, C.-S.; Sohn, E.; Lee, Y.M.; Jo, K.; Kim, J.S. Puerarin Inhibits the Retinal Pericyte Apoptosis Induced by Advanced Glycation End Products in Vitro and in Vivo by Inhibiting NADPH Oxidase-Related Oxidative Stress. Free Radic. Biol. Med. 2012, 53, 357–365. [Google Scholar] [CrossRef]
- Yong, P.H.; Zong, H.; Medina, R.J.; Limb, G.A.; Uchida, K.; Stitt, A.W.; Curtis, T.M. Evidence Supporting a Role for N-(3-Formyl-3,4-Dehydropiperidino)Lysine Accumulation in Müller Glia Dysfunction and Death in Diabetic Retinopathy. Mol. Vis. 2010, 16, 2524–2538. [Google Scholar]
- Lee, A.Y.W.; Chung, S.S.M. Contributions of Polyol Pathway to Oxidative Stress in Diabetic Cataract. FASEB J. 1999, 13, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced Glycoxidation and Lipoxidation End Products (AGEs and ALEs): An Overview of Their Mechanisms of Formation. Free Radic. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Nakamura, J.; Hamada, Y.; Naruse, K.; Nakashima, E.; Kato, K.; Kasuya, Y.; Yasuda, Y.; Kamiya, H.; Hotta, N. The Role of Polyol Pathway in Glucose-Induced Apoptosis of Cultured Retinal Pericytes. Diabetes Res. Clin. Pract. 2003, 60, 1–9. [Google Scholar] [CrossRef]
- Lorenzi, M. The Polyol Pathway as a Mechanism for Diabetic Retinopathy: Attractive, Elusive, and Resilient. J. Diabetes Res. 2007, 2007, 061038. [Google Scholar] [CrossRef]
- Robinson, W.G.; Tillis, T.N.; Laver, N.; Kinoshita, J.H. Diabetes-Related Histopathologies of the Rat Retina Prevented with an Aldose Reductase Inhibitor. Exp. Eye Res. 1990, 50, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Robison, W.G.; Nagata, M.; Laver, N.; Hohman, T.C.; Kinoshita, J.H. Diabetic-like Retinopathy in Rats Prevented with an Aldose Reductase Inhibitor. Investig. Ophthalmol. Vis. Sci. 1989, 30, 2285–2292. [Google Scholar] [PubMed]
- Xia, P.; Inoguchi, T.; Kern, T.S.; Engerman, R.L.; Oates, P.J.; King, G.L. Characterization of the Mechanism for the Chronic Activation of Diacylglycerol-Protein Kinase C Pathway in Diabetes and Hypergalactosemia. Diabetes 1994, 43, 1122–1129. [Google Scholar] [CrossRef]
- Pan, D.; Xu, L.; Guo, M. The Role of Protein Kinase C in Diabetic Microvascular Complications. Front. Endocrinol. 2022, 13, 973058. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Hayes, R.G.; Stitt, A.W.; McAuley, E.; Archer, D.B. Constitutive Nitric Oxide Synthase Expression in Retinal Vascular Endothelial Cells Is Suppressed by High Glucose and Advanced Glycation End Products. Diabetes 1998, 47, 945–952. [Google Scholar] [CrossRef]
- Nonaka, A.; Kiryu, J.; Tsujikawa, A.; Yamashiro, K.; Miyamoto, K.; Nishiwaki, H.; Honda, Y.; Ogura, Y. PKC-Beta Inhibitor (LY333531) Attenuates Leukocyte Entrapment in Retinal Microcirculation of Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2702–2706. [Google Scholar]
- Aiello, L.P.; Bursell, S.-E.; Clermont, A.; Duh, E.; Ishii, H.; Takagi, C.; Mori, F.; Ciulla, T.A.; Ways, K.; Jirousek, M.; et al. Vascular Endothelial Growth Factor–Induced Retinal Permeability Is Mediated by Protein Kinase C In Vivo and Suppressed by an Orally Effective β-Isoform–Selective Inhibitor. Diabetes 1997, 46, 1473–1480. [Google Scholar] [CrossRef]
- Murakami, T.; Frey, T.; Lin, C.; Antonetti, D.A. Protein Kinase Cβ Phosphorylates Occludin Regulating Tight Junction Trafficking in Vascular Endothelial Growth Factor–Induced Permeability In Vivo. Diabetes 2012, 61, 1573–1583. [Google Scholar] [CrossRef]
- Pieper, G.M. Riaz-ul-Haq Activation of Nuclear Factor-κB in Cultured Endothelial Cells by Increased Glucose Concentration: Prevention by Calphostin C. J. Cardiovasc. Pharmacol. 1997, 30, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Yerneni, K.K.; Bai, W.; Khan, B.V.; Medford, R.M.; Natarajan, R. Hyperglycemia-Induced Activation of Nuclear Transcription Factor kappaB in Vascular Smooth Muscle Cells. Diabetes 1999, 48, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z. Crosstalk of Reactive Oxygen Species and NF-κB Signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Gauss, K.A.; Nelson-Overton, L.K.; Siemsen, D.W.; Gao, Y.; DeLeo, F.R.; Quinn, M.T. Role of NF-κB in Transcriptional Regulation of the Phagocyte NADPH Oxidase by Tumor Necrosis Factor-α. J. Leukoc. Biol. 2007, 82, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xu, M.; Ren, Q.; Fan, Y.; Liu, B.; Chen, J.; Wang, Z.; Sun, X. Downregulation of Fatty Acid Binding Protein 4 Alleviates Lipid Peroxidation and Oxidative Stress in Diabetic Retinopathy by Regulating Peroxisome Proliferator-Activated Receptor γ-Mediated Ferroptosis. Bioengineered 2022, 13, 10540–10551. [Google Scholar] [CrossRef]
- Zheng, L.; Du, Y.; Miller, C.; Gubitosi-Klug, R.A.; Kern, T.S.; Ball, S.; Berkowitz, B.A. Critical Role of Inducible Nitric Oxide Synthase in Degeneration of Retinal Capillaries in Mice with Streptozotocin-Induced Diabetes. Diabetologia 2007, 50, 1987–1996. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kowluru, A.; Mishra, M.; Kumar, B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 2015, 48, 40–61. [Google Scholar] [CrossRef]
- González De Vega, R.; García, M.; Fernández-Sánchez, M.L.; González-Iglesias, H.; Sanz-Medel, A. Protective Effect of Selenium Supplementation Following Oxidative Stress Mediated by Glucose on Retinal Pigment Epithelium. Metallomics 2018, 10, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Al-Bassam, L.; Shearman, G.C.; Brocchini, S.; Alany, R.G.; Williams, G.R. The Potential of Selenium-Based Therapies for Ocular Oxidative Stress. Pharmaceutics 2024, 16, 631. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Shi, X.; Liu, X.; Wang, H.; Liu, K.; Xu, Y. Porous Se@SiO2 nanospheres alleviate diabetic retinopathy by inhibiting excess lipid peroxidation and inflammation. Mol. Med. 2024, 30, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Feng, K.; Liu, R.; Pan, J.; Zhang, L.; Lu, X. Vitamins and Mineral Supplements for Retinitis Pigmentosa. J. Ophthalmol. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Hoffman, D.R.; Hughbanks-Wheaton, D.K.; Pearson, N.S.; Fish, G.E.; Spencer, R.; Takacs, A.; Klein, M.; Locke, K.G.; Birch, D.G. Four-Year Placebo-Controlled Trial of Docosahexaenoic Acid in X-Linked Retinitis Pigmentosa (DHAX Trial): A Randomized Clinical Trial. JAMA Ophthalmol. 2014, 132, 866. [Google Scholar] [CrossRef]
- Sanz, M.M.; Johnson, L.E.; Ahuja, S.; Ekström, P.A.R.; Romero, J.; Van Veen, T. Significant Photoreceptor Rescue by Treatment with a Combination of Antioxidants in an Animal Model for Retinal Degeneration. Neuroscience 2007, 145, 1120–1129. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Sperduto, R.D.; SanGiovanni, J.P.; Davis, M.D.; Ferris, F.L. Ten-Year Follow-up of Age-Related Macular Degeneration in the Age-Related Eye Disease Study: AREDS Report No. 36. JAMA Ophthalmol. 2014, 132, 272. [Google Scholar] [CrossRef]
- The Age-Related Eye Disease Study (AREDS). Control. Clin. Trials 1999, 20, 573–600. [CrossRef]
- Batliwala, S.; Xavier, C.; Liu, Y.; Wu, H.; Pang, I.-H. Involvement of Nrf2 in Ocular Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 1703810. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, Y.; Wang, J.; Sternberg, P.; Freeman, M.L.; Grossniklaus, H.E.; Cai, J. Age-Related Retinopathy in NRF2-Deficient Mice. PLoS ONE 2011, 6, e19456. [Google Scholar] [CrossRef]
- Xu, Z.; Wei, Y.; Gong, J.; Cho, H.; Park, J.K.; Sung, E.-R.; Huang, H.; Wu, L.; Eberhart, C.; Handa, J.T.; et al. NRF2 Plays a Protective Role in Diabetic Retinopathy in Mice. Diabetologia 2014, 57, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Deliyanti, D.; Alrashdi, S.F.; Tan, S.M.; Meyer, C.; Ward, K.W.; De Haan, J.B.; Wilkinson-Berka, J.L. Nrf2 Activation Is a Potential Therapeutic Approach to Attenuate Diabetic Retinopathy. Investig. Opthalmology Vis. Sci. 2018, 59, 815. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Ji, X.; Ivanchenko, M.V.; Chung, M.; Piper, M.; Rana, P.; Wang, S.K.; Xue, Y.; West, E.; Zhao, S.R.; et al. Nrf2 Overexpression Rescues the RPE in Mouse Models of Retinitis Pigmentosa. JCI Insight 2021, 6, e145029. [Google Scholar] [CrossRef]
- Pan, H.; He, M.; Liu, R.; Brecha, N.C.; Yu, A.C.H.; Pu, M. Sulforaphane Protects Rodent Retinas against Ischemia-Reperfusion Injury through the Activation of the Nrf2/HO-1 Antioxidant Pathway. PLoS ONE 2014, 9, e114186. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, Y.; Hatano, E.; Inoue, T.; Yoshida, K.; Kondo, M.; Terasaki, H. Cytoprotective Effects of a Novel Nrf2 Activator, RS9, in Rhodopsin Pro347Leu Rabbits. Curr. Eye Res. 2016, 41, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hu, L. Nrf2 Activation through the Inhibition of Keap1–Nrf2 Protein–Protein Interaction. Med. Chem. Res. 2020, 29, 846–867. [Google Scholar] [CrossRef]
- Sugano, E.; Murayama, N.; Takahashi, M.; Tabata, K.; Tamai, M.; Tomita, H. Essential Role of Thioredoxin 2 in Mitigating Oxidative Stress in Retinal Epithelial Cells. J. Ophthalmol. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Gimeno-Hernández, R.; Cantó, A.; Fernández-Carbonell, A.; Olivar, T.; Hernández-Rabaza, V.; Almansa, I.; Miranda, M. Thioredoxin Delays Photoreceptor Degeneration, Oxidative and Inflammation Alterations in Retinitis Pigmentosa. Front. Pharmacol. 2020, 11, 590572. [Google Scholar] [CrossRef]
- Tulsawani, R.; Kelly, L.S.; Fatma, N.; Chhunchha, B.; Kubo, E.; Kumar, A.; Singh, D.P. Neuroprotective Effect of Peroxiredoxin 6 against Hypoxia-Induced Retinal Ganglion Cell Damage. BMC Neurosci. 2010, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Pascual, I.; Larrayoz, I.M.; Campos, M.M.; Rodriguez, I.R. Methionine Sulfoxide Reductase B2 Is Highly Expressed in the Retina and Protects Retinal Pigmented Epithelium Cells from Oxidative Damage. Exp. Eye Res. 2010, 90, 420–428. [Google Scholar] [CrossRef]
- Dun, Y.; Vargas, J.; Brot, N.; Finnemann, S.C. Independent Roles of Methionine Sulfoxide Reductase A in Mitochondrial ATP Synthesis and as Antioxidant in Retinal Pigment Epithelial Cells. Free Radic. Biol. Med. 2013, 65, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Aït-Ali, N.; Fridlich, R.; Millet-Puel, G.; Clérin, E.; Delalande, F.; Jaillard, C.; Blond, F.; Perrocheau, L.; Reichman, S.; Byrne, L.C.; et al. Rod-Derived Cone Viability Factor Promotes Cone Survival by Stimulating Aerobic Glycolysis. Cell 2015, 161, 817–832. [Google Scholar] [CrossRef]
- Elachouri, G.; Lee-Rivera, I.; Clérin, E.; Argentini, M.; Fridlich, R.; Blond, F.; Ferracane, V.; Yang, Y.; Raffelsberger, W.; Wan, J.; et al. Thioredoxin Rod-Derived Cone Viability Factor Protects against Photooxidative Retinal Damage. Free Radic. Biol. Med. 2015, 81, 22–29. [Google Scholar] [CrossRef]
- Punzo, C.; Kornacker, K.; Cepko, C.L. Stimulation of the Insulin/mTOR Pathway Delays Cone Death in a Mouse Model of Retinitis Pigmentosa. Nat. Neurosci. 2009, 12, 44–52. [Google Scholar] [CrossRef] [PubMed]
- DeBalsi, K.L.; Wong, K.E.; Koves, T.R.; Slentz, D.H.; Seiler, S.E.; Wittmann, A.H.; Ilkayeva, O.R.; Stevens, R.D.; Perry, C.G.R.; Lark, D.S.; et al. Targeted Metabolomics Connects Thioredoxin-Interacting Protein (TXNIP) to Mitochondrial Fuel Selection and Regulation of Specific Oxidoreductase Enzymes in Skeletal Muscle. J. Biol. Chem. 2014, 289, 8106–8120. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, S.K.; Rana, P.; West, E.R.; Hong, C.M.; Feng, H.; Wu, D.M.; Cepko, C.L. AAV-Txnip Prolongs Cone Survival and Vision in Mouse Models of Retinitis Pigmentosa. eLife 2021, 10, e66240. [Google Scholar] [CrossRef]
- Terluk, M.R.; Ebeling, M.C.; Fisher, C.R.; Kapphahn, R.J.; Yuan, C.; Kartha, R.V.; Montezuma, S.R.; Ferrington, D.A. N-Acetyl-L-Cysteine Protects Human Retinal Pigment Epithelial Cells from Oxidative Damage: Implications for Age-Related Macular Degeneration. Oxid. Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-Selenium and Its Nanomedicine Applications: A Critical Review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef]
- Xia, Y.; Hill, K.E.; Li, P.; Xu, J.; Zhou, D.; Motley, A.K.; Wang, L.; Byrne, D.W.; Burk, R.F. Optimization of Selenoprotein P and Other Plasma Selenium Biomarkers for the Assessment of the Selenium Nutritional Requirement: A Placebo-Controlled, Double-Blind Study of Selenomethionine Supplementation in Selenium-Deficient Chinese Subjects. Am. J. Clin. Nutr. 2010, 92, 525–531. [Google Scholar] [CrossRef]
- Higuchi, A.; Takahashi, K.; Hirashima, M.; Kawakita, T.; Tsubota, K. Selenoprotein P Controls Oxidative Stress in Cornea. PLoS ONE 2010, 5, e9911. [Google Scholar] [CrossRef]
- Ou, L.; Wu, Z.; Hu, X.; Huang, J.; Yi, Z.; Gong, Z.; Li, H.; Peng, K.; Shu, C.; Koole, L.H. A Tissue-Adhesive F127 Hydrogel Delivers Antioxidative Copper-Selenide Nanoparticles for the Treatment of Dry Eye Disease. Acta Biomater. 2024, 175, 353–368. [Google Scholar] [CrossRef]
- Higuchi, A.; Inoue, H.; Kawakita, T.; Ogishima, T.; Tsubota, K. Selenium Compound Protects Corneal Epithelium against Oxidative Stress. PLoS ONE 2012, 7, e45612. [Google Scholar] [CrossRef]
- She, C.; Shang, F.; Cui, M.; Yang, X.; Liu, N. Association between Dietary Antioxidants and Risk for Diabetic Retinopathy in a Chinese Population. Eye 2021, 35, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, D.; Nazıroğlu, M.; Vanyorek, L.; Muhamad, S. Involvement of TRPM2 Channel on Hypoxia-Induced Oxidative Injury, Inflammation, and Cell Death in Retinal Pigment Epithelial Cells: Modulator Action of Selenium Nanoparticles. Biol. Trace Elem. Res. 2021, 199, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Ananth, S.; Miyauchi, S.; Thangaraju, M.; Jadeja, R.N.; Bartoli, M.; Ganapathy, V.; Martin, P.M. Selenomethionine (Se-Met) Induces the Cystine/Glutamate Exchanger SLC7A11 in Cultured Human Retinal Pigment Epithelial (RPE) Cells: Implications for Antioxidant Therapy in Aging Retina. Antioxidants 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Atasi, L.; Ho, Y.-S. Role of Mitochondrial Superoxide Dismutase in the Development of Diabetic Retinopathy. Investig. Opthalmology Vis. Sci. 2006, 47, 1594. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kowluru, V.; Xiong, Y.; Ho, Y.-S. Overexpression of Mitochondrial Superoxide Dismutase in Mice Protects the Retina from Diabetes-Induced Oxidative Stress. Free Radic. Biol. Med. 2006, 41, 1191–1196. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide Activates Mitochondrial Uncoupling Proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, X.; Bi, H.; Zhu, Q.; Wu, J.; Xia, X.; Ren, Q.; Ho, P.C.P. Expression Modification of Uncoupling Proteins and MnSOD in Retinal Endothelial Cells and Pericytes Induced by High Glucose: The Role of Reactive Oxygen Species in Diabetic Retinopathy. Exp. Eye Res. 2006, 83, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.C.; Rosales, M.A.B.; Biswas, S.K.; Lopes De Faria, J.B.; Lopes De Faria, J.M. Diabetic Retinal Neurodegeneration Is Associated with Mitochondrial Oxidative Stress and Is Improved by an Angiotensin Receptor Blocker in a Model Combining Hypertension and Diabetes. Diabetes 2009, 58, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A. Effect of Reinstitution of Good Glycemic Control on Retinal Oxidative Stress and Nitrative Stress in Diabetic Rats. Diabetes 2003, 52, 818–823. [Google Scholar] [CrossRef]
- Mishra, M.; Zhong, Q.; Kowluru, R.A. Epigenetic Modifications of Nrf2-Mediated Glutamate–Cysteine Ligase: Implications for the Development of Diabetic Retinopathy and the Metabolic Memory Phenomenon Associated with Its Continued Progression. Free Radic. Biol. Med. 2014, 75, 129–139. [Google Scholar] [CrossRef]
- Harvey, C.J.; Thimmulappa, R.K.; Singh, A.; Blake, D.J.; Ling, G.; Wakabayashi, N.; Fujii, J.; Myers, A.; Biswal, S. Nrf2-Regulated Glutathione Recycling Independent of Biosynthesis Is Critical for Cell Survival during Oxidative Stress. Free Radic. Biol. Med. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- Dreger, H.; Westphal, K.; Weller, A.; Baumann, G.; Stangl, V.; Meiners, S.; Stangl, K. Nrf2-Dependent Upregulation of Antioxidative Enzymes: A Novel Pathway for Proteasome Inhibitor-Mediated Cardioprotection. Cardiovasc. Res. 2009, 83, 354–361. [Google Scholar] [CrossRef]
- Alam, J.; Stewart, D.; Touchard, C.; Boinapally, S.; Choi, A.M.K.; Cook, J.L. Nrf2, a Cap’n’Collar Transcription Factor, Regulates Induction of the Heme Oxygenase-1 Gene. J. Biol. Chem. 1999, 274, 26071–26078. [Google Scholar] [CrossRef]
- Albert-Garay, J.S.; Riesgo-Escovar, J.R.; Sánchez-Chávez, G.; Salceda, R. Retinal Nrf2 Expression in Normal and Early Streptozotocin-Diabetic Rats. Neurochem. Int. 2021, 145, 105007. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Chen, X. Protective Effects of Sulforaphane on Diabetic Retinopathy: Activation of the Nrf2 Pathway and Inhibition of NLRP3 Inflammasome Formation. Exp. Anim. 2019, 68, 221–231. [Google Scholar] [CrossRef]
- Mishra, M.; Duraisamy, A.J.; Kowluru, R.A. Sirt1: A Guardian of the Development of Diabetic Retinopathy. Diabetes 2018, 67, 745–754. [Google Scholar] [CrossRef]
- Karbasforooshan, H.; Karimi, G. The Role of SIRT1 in Diabetic Retinopathy. Biomed. Pharmacother. 2018, 97, 190–194. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, H.-Z.; Wan, Y.; Zhang, Q.-J.; Wei, Y.-S.; Huang, S.; Liu, J.-J.; Lu, Y.-B.; Zhang, Z.-Q.; Yang, R.-F.; et al. Repression of P66Shc Expression by SIRT1 Contributes to the Prevention of Hyperglycemia-Induced Endothelial Dysfunction. Circ. Res. 2011, 109, 639–648. [Google Scholar] [CrossRef]
- Haydinger, C.D.; Oliver, G.F.; Ashander, L.M.; Smith, J.R. Oxidative Stress and Its Regulation in Diabetic Retinopathy. Antioxidants 2023, 12, 1649. [Google Scholar] [CrossRef]
- Krajewska, J.B.; Waszczykowska, A. Gene therapy strategies in ophthalmology—An overview of current developments and future prospects. J. Appl. Genet. 2025. [Google Scholar] [CrossRef]
- Castelli, V.; Paladini, A.; d’Angelo, M.; Allegretti, M.; Mantelli, F.; Brandolini, L.; Cocchiaro, P.; Cimini, A.; Varrassi, G. Taurine and oxidative stress in retinal health and disease. CNS Neurosci. Ther. 2021, 27, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Alfonsetti, M.; Castelli, V.; d’Angelo, M.; Benedetti, E.; Allegretti, M.; Barboni, B.; Cimini, A. Looking for In Vitro Models for Retinal Diseases. Int. J. Mol. Sci. 2021, 22, 10334. [Google Scholar] [CrossRef]
- Bertram, K.M.; Baglole, C.J.; Phipps, R.P.; Libby, R.T. Molecular Regulation of Cigarette Smoke Induced-Oxidative Stress in Human Retinal Pigment Epithelial Cells: Implications for Age-Related Macular Degeneration. Am. J. Physiol.-Cell Physiol. 2009, 297, C1200–C1210. [Google Scholar] [CrossRef]
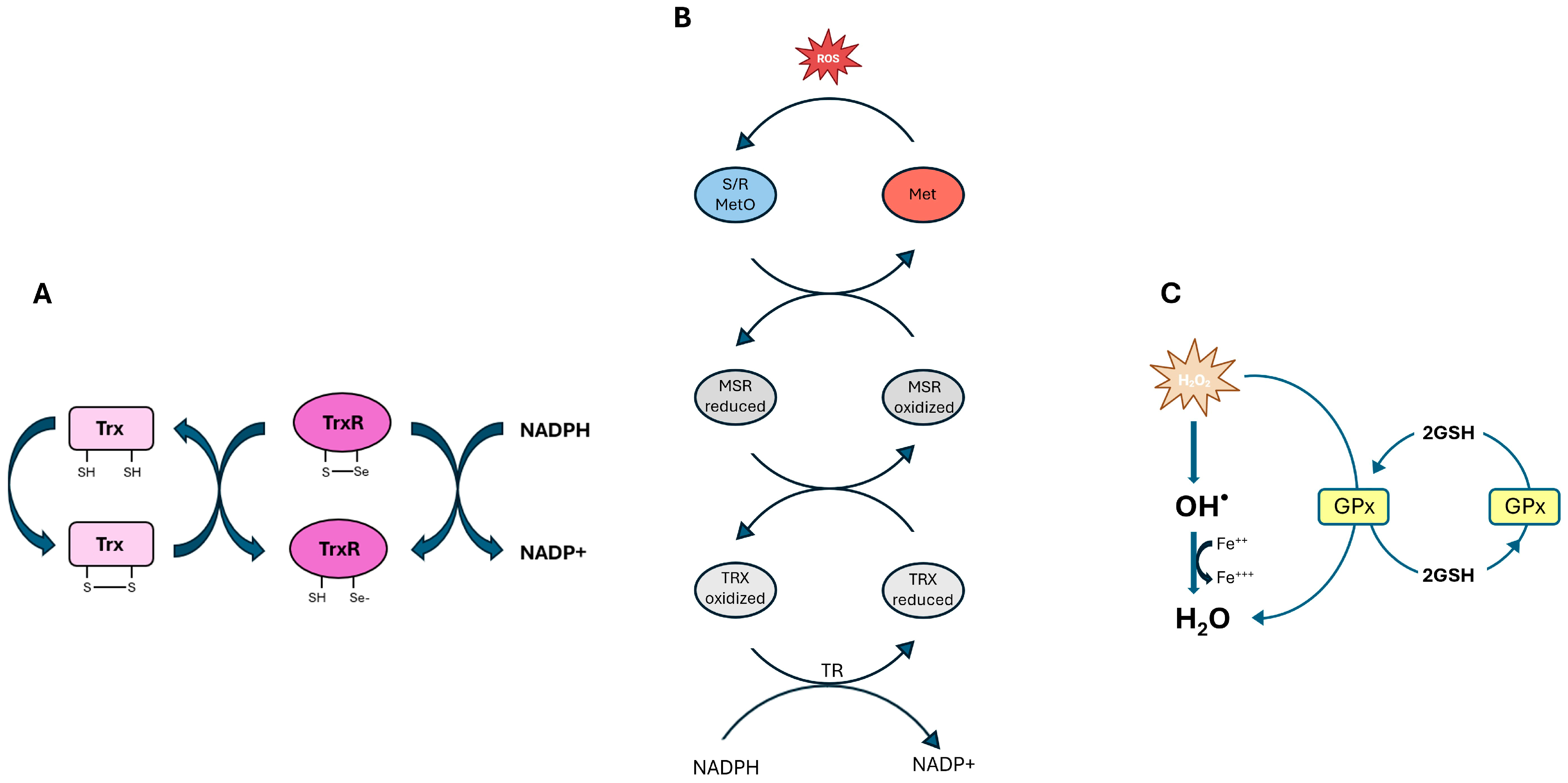
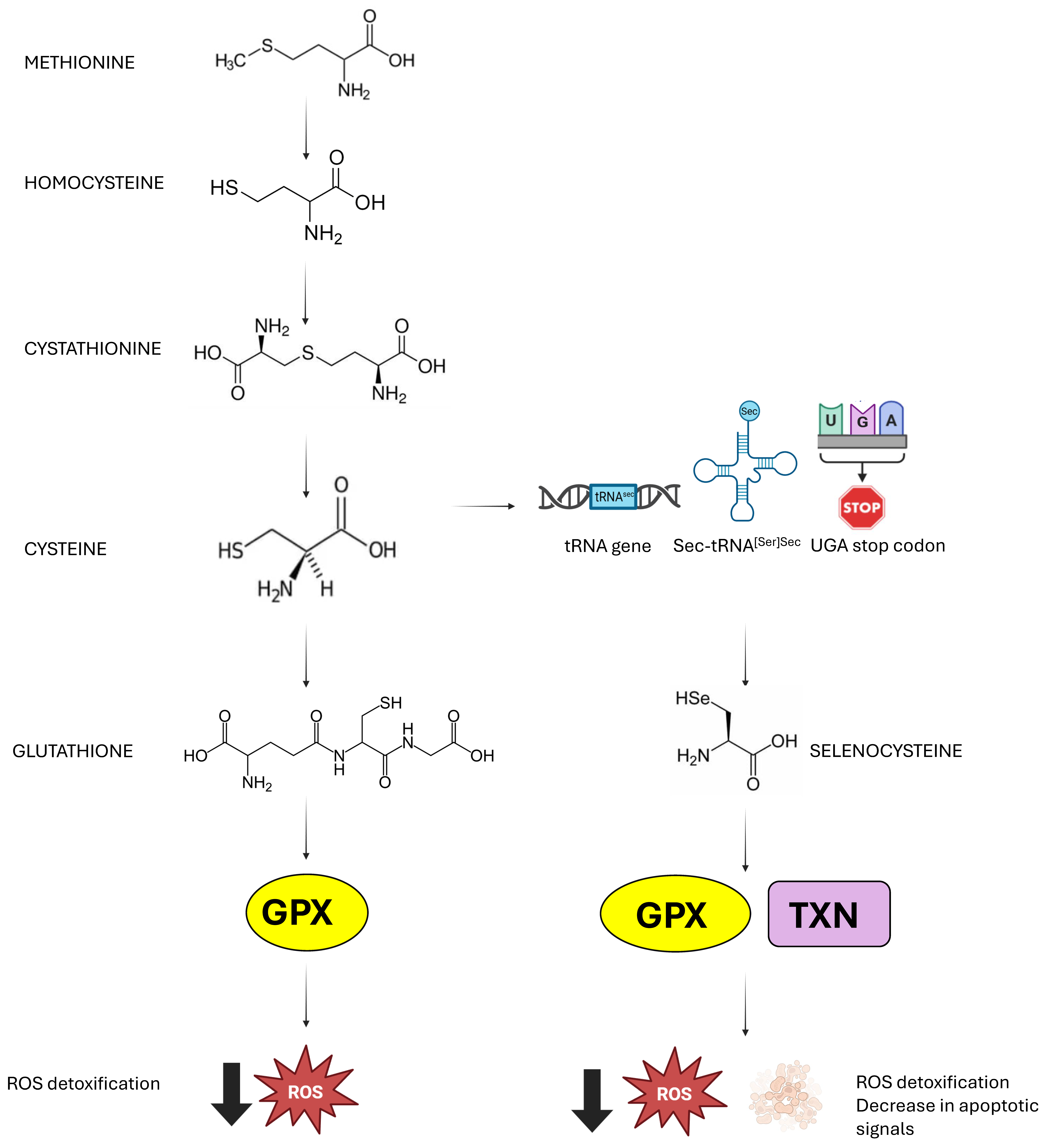
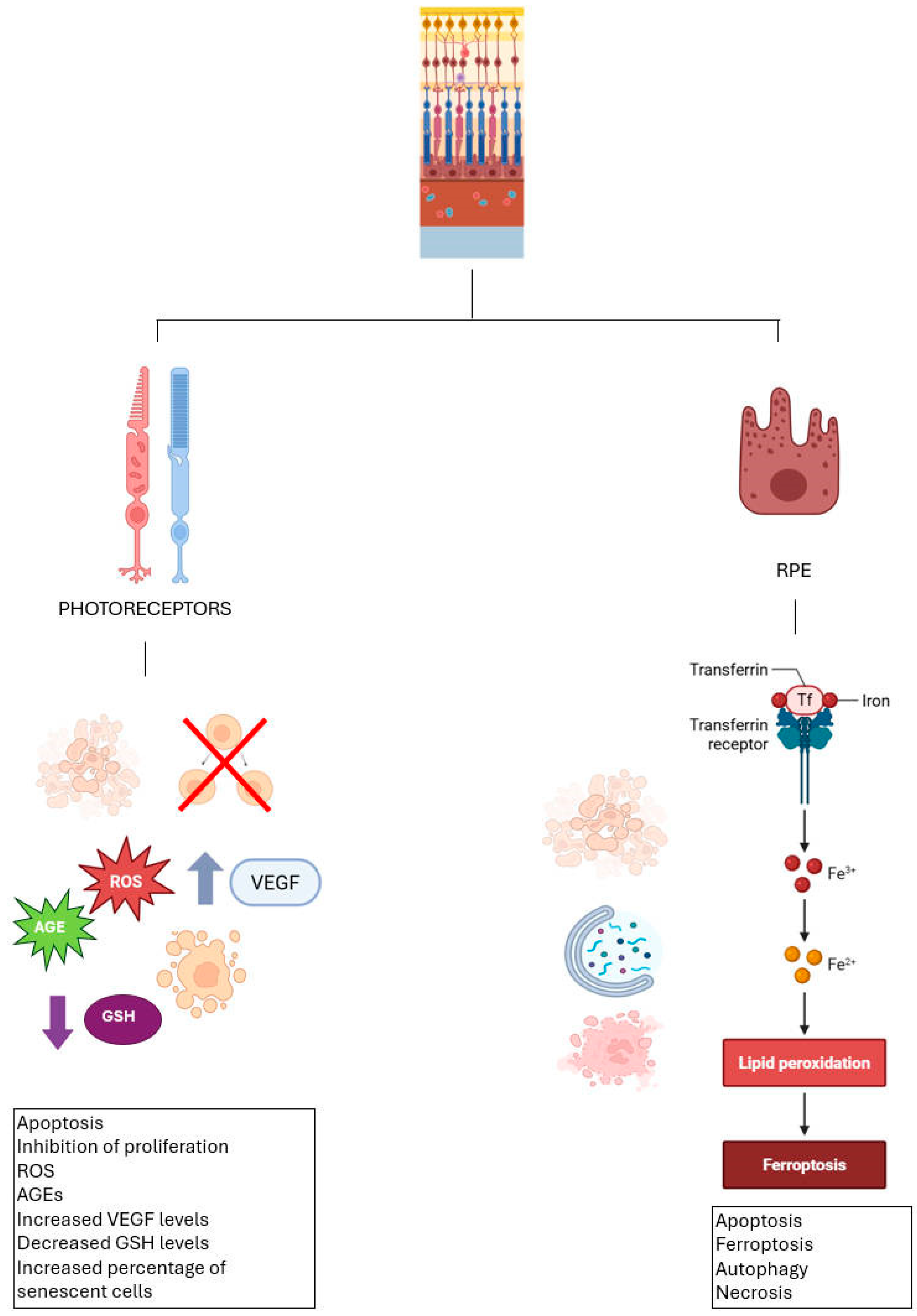
| Selenoprotein | Function | Distribution | Characteristic | Ref |
|---|---|---|---|---|
| GPX1 | Reduces H2O2 and organic peroxides using GSH | High expression during early retinal development | Major defense against oxidative stress; supports early retinal protection | [30,34,35,36] |
| GPX3 | Extracellular detoxification of peroxides | The most abundant GPX isoform in the retina; extracellular space | Protects cell surfaces and basement membranes | [37] |
| GPX4 | Reduces lipid hydroperoxides and inhibits ferroptosis | Inner segments of photoreceptors, RPE, and choroid | Essential for photoreceptor survival; regulates lipid peroxidation | [35,38,39] |
| TXNRD | Regenerates reduced thioredoxin using NADPH | Retinal ganglion cell, inner nuclear layer, photoreceptors | Declines in mature retina are essential for redox signaling | [40,41,42] |
| MSRB | Reduces oxidized methionine residues in proteins | Ubiquitous; systemic antioxidant role | Less specific to the retina, but it contributes to redox balance | [27,31] |
| Selenoprotein P | Selenium transport in plasma: minor antioxidant function | Systemic; marginal retinal expression | Mainly involved in selenium homeostasis | [33] |
| Therapeutic Strategy | Therapeutic Target | Methods | Treatment Outcomes | Models | Ref |
|---|---|---|---|---|---|
| Small Antioxidants | rod, cones, macula, photoreceptor outer segment membranes and retina. | Supplementation with vitamins A, E, beta-carotene, lutein, DHA | Slowing of visual decline in RP and AMD. | RP patients,10–50 years old, 29 men/33 woman; 4757 participants aged 55 to 80 with or without AMD. | [188,189,190,191,192] |
| KEAP1–NRF2 Pathway | BRB, retina, Müller cells, RPE, I/R retina, retinas of diabetic Long Evans rats. | Activation of NRF2 pathway | Protection against oxidative damage in AMD and DR; involves REDD1-mediated degradation, activation of HO-1, GSH biosynthesis, and TXN expression. | NRF2-deficient mice; DR mice, Sprague Dawley rats; I/R mice; Streptozotocin-Diabetic Rats. | [193,194,195,196,197,198,230,231] |
| Gene Therapy | rod, cones, vitreous, RPE cells, retinal ganglion cell. | AAV-mediated delivery of TXN2, MSR, PRDX, RdCVF/L genes | Improved retinal survival; modulates redox balance, ER stress, and glucose metabolism. | Neural retina of C57BL/6J mice; Pro347Leu rabbits; NRF2 KO mice; retinal epithelial cells; rd1 mouse; hypoxia-induced retinal ganglion cell; retinal pigmented epithelium cells; cone-enriched cultures from chicken embryos | [129,199,200,201,202,203,204,205,206,207,208] |
| N-acetyl-L-cysteine (NAC) | RPE, photoreceptors. | NAC treatment | Increase in GSH, improvement of mitochondrial function, reduction in ROS and protection of RPE cells, particularly in AMD patient-derived cultures. | Human retinal pigment epithelial cells | [211] |
| Selenium | oxidative metabolism, respiratory kinetics, cone, plasma, cornea, corneal epithelium, RPE. | Selenium compounds (e.g., Se-lactoferrin, SeNPs, SeMet) enhan | Enhancement of GPx and SOD2 activity; reduce oxidative stress, inflammation, and protection against dry eye and DR-related damage. | TKO mice; TXNIPfl/fl mice; TXNIPSKM−/− mice; RP mice; 98 healthy Chinese subjects; human retinal pigment epithelial cells; Sprague Dawley rats; human corneal epithelial cell; C57BL/6J mice; 415 Chinese diabetic patients with or without DR. | [29,185,209,210,212,213,214,215,216,217,218] |
| Endogenous Redox Defense | Retinal mitochondria, retinal pericytes, RPE, vascular and neuronal retina, retinal microvessels. | Enhancing SOD2, UCPs, GSH system, NRF2, and SIRT1 activity | Protection of retinal cells from hyperglycemia-induced oxidative and vascular damage, especially in DR. | Diabetic mice; retinal pericytes; human retinal pigment epithelial cells; bovine retinal endothelial cells; retinal capillary endothelial cells and pericytes; spontaneously hypertensive rats; normotensive Wistar–Kyoto rats; human eye globes; NRF2−/− CD-1 mice; neonatal rat cardiac myocytes; mouse macrophage; mouse fibroblast; SIRT1-overexpressin mice | [143,167,186,219,220,221,222,223,224,225,226,227,228,229,232] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maceroni, E.; Cimini, A.; Quintiliani, M.; d’Angelo, M.; Castelli, V. Retinal Gatekeepers: Molecular Mechanism and Therapeutic Role of Cysteine and Selenocysteine. Biomolecules 2025, 15, 1203. https://doi.org/10.3390/biom15081203
Maceroni E, Cimini A, Quintiliani M, d’Angelo M, Castelli V. Retinal Gatekeepers: Molecular Mechanism and Therapeutic Role of Cysteine and Selenocysteine. Biomolecules. 2025; 15(8):1203. https://doi.org/10.3390/biom15081203
Chicago/Turabian StyleMaceroni, Eleonora, Annamaria Cimini, Massimiliano Quintiliani, Michele d’Angelo, and Vanessa Castelli. 2025. "Retinal Gatekeepers: Molecular Mechanism and Therapeutic Role of Cysteine and Selenocysteine" Biomolecules 15, no. 8: 1203. https://doi.org/10.3390/biom15081203
APA StyleMaceroni, E., Cimini, A., Quintiliani, M., d’Angelo, M., & Castelli, V. (2025). Retinal Gatekeepers: Molecular Mechanism and Therapeutic Role of Cysteine and Selenocysteine. Biomolecules, 15(8), 1203. https://doi.org/10.3390/biom15081203







