Complement Cascades and Brain Disorders
Abstract
1. Introduction
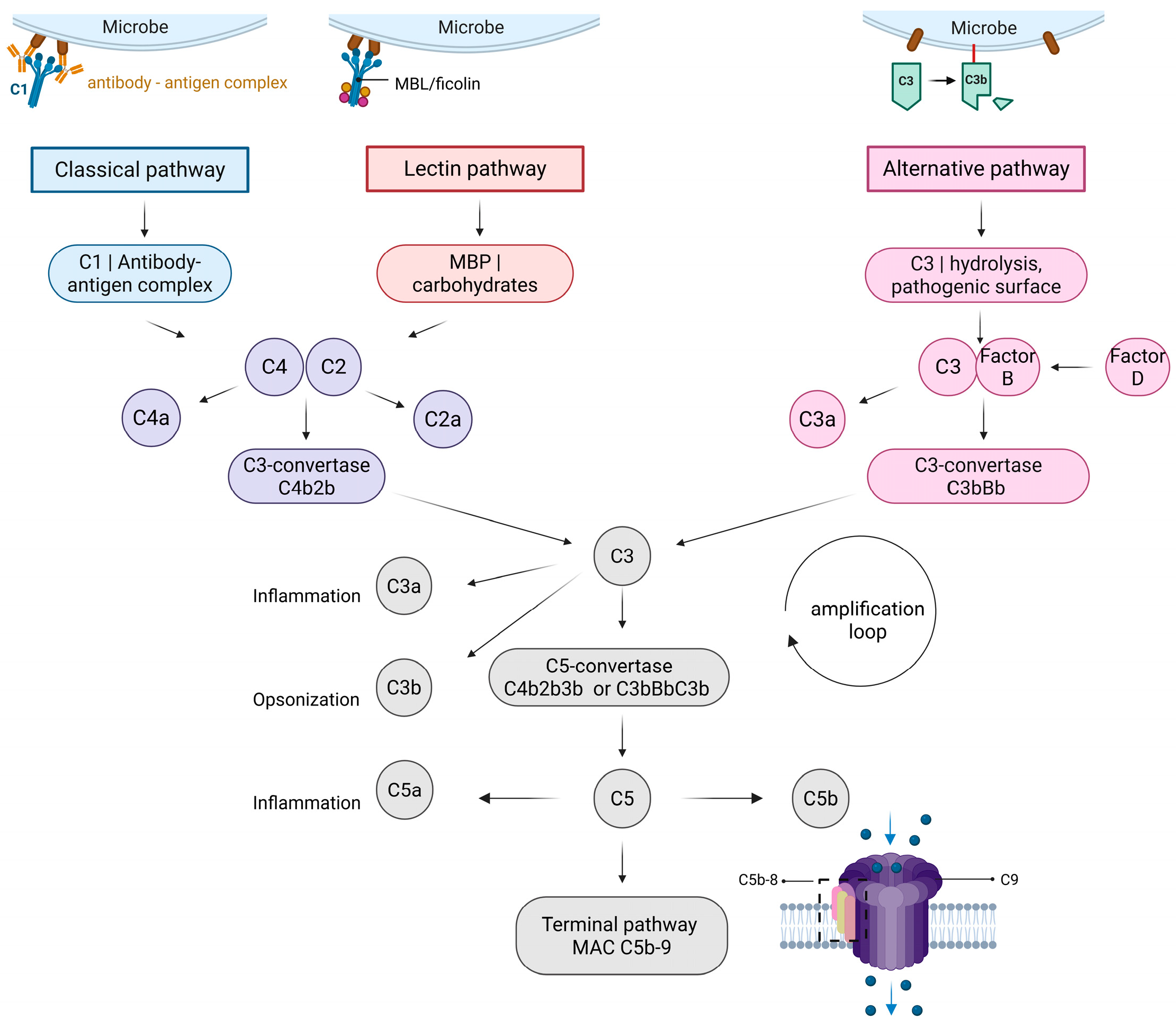
1.1. Classical Pathway
1.2. Lectin Pathway
1.3. Alternative Pathway
1.4. Terminal Pathway
1.5. Regulation of the Complement System
2. Complement and the CNS
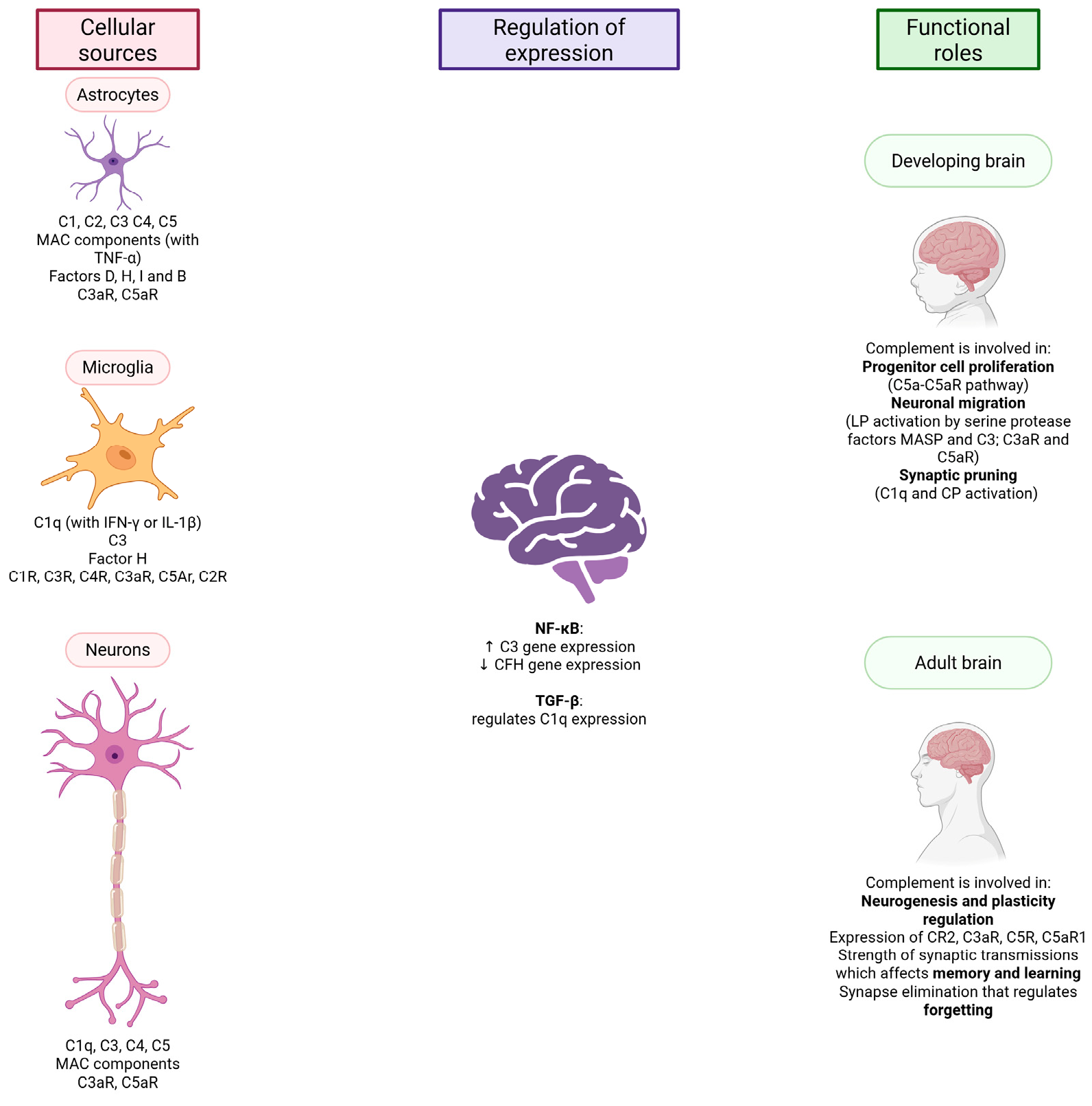
2.1. Production of Complement Components in the CNS
2.2. The Complement System and the Developing Brain
2.3. The Complement System and the Adult Brain
3. The Complement System and CNS Pathologies
3.1. Alzheimer’s Disease
3.2. Schizophrenia
3.3. Glioblastoma
4. Potential Clinical Use
4.1. Complement and Laboratory Diagnostics
4.2. Complement and Therapeutics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-beta |
| AD | Alzheimer’s disease |
| aHUS | Atypical hemolytic uremic syndrome |
| ANCA | Anti-neutrophil cytoplasmic autoantibody |
| APP | Amyloid precursor protein |
| APTT | Activated partial thromboplastin time |
| AQP4 | Aquaporin 4 |
| BBB | Blood–brain barrier |
| BM-MSCs | Bone marrow–derived MSCs |
| C1-IA | Complement 1 inactivator |
| C4BP | C4b binding protein |
| C5aR1 | C5a receptor 1 |
| CFH | Complement Factor H |
| CNS | Central nervous system |
| CR1 | Complement receptor 1 |
| CSF | Cerebrospinal fluid |
| DAF | Decay-accelerating factor |
| DALYs | Disability-adjusted life years |
| EDTA | Ethylenediaminetetraacetic acid |
| EMA | European Medicines Agency |
| EIA | Enzyme immuno-assay |
| EVs | Extracellular vesicles |
| FDA | Food and Drug Administration |
| FB | Factor B |
| FD | Factor D |
| FH | Soluble factor H |
| FI | Factor I |
| FHR5 | Complement factor H–related protein 5 |
| GFP | Green fluorescent protein |
| gMG | Generalized myasthenia gravis |
| GWAS | Genome-wide association study |
| IDHwt | Isocitrate dehydrogenase wild type |
| IFN-γ | Interferon-γ |
| IHC | Immunohistochemistry |
| MAC | Membrane attack complex |
| MASP | MBL-associated serine proteases |
| MBL | Mannose-binding lectin |
| MCP | Membrane cofactor protein |
| MHC | Major histocompatibility complex |
| MSCs | Mesenchymal stem cells |
| MSLCs | Mesenchymal stem-like cells |
| NF-κB | Nuclear factor κ B |
| NGS | Next generation sequencing |
| NMOSD | Neuromyelitis optica spectrum disorder |
| PNH | Paroxysmal nocturnal hemoglobinuria |
| PRMs | Pattern recognition molecules |
| SLE | Systemic lupus erythematosus |
| TAMs | Tumor-associated macrophages |
| TGF-β | Transforming growth factor beta |
| TNF-α | Tumor necrosis factor |
| VEGF | Vascular endothelial growth factor |
References
- Mastellos, D.C.; Hajishengallis, G.; Lambris, J.D. A guide to complement biology, pathology and therapeutic opportunity. Nat. Rev. Immunol. 2024, 24, 118–141. [Google Scholar] [CrossRef]
- Ehrlich, P.; Morgenroth, J. Ueber haemolysine: Zweite mittheilung. In Berliner Klinische Wochenschrift; Schumacher: Berlin, Germany, 1899. [Google Scholar]
- Chen, M.; Daha, M.R.; Kallenberg, C.G. The complement system in systemic autoimmune disease. J. Autoimmun. 2010, 34, J276–J286. [Google Scholar] [CrossRef]
- Petr, V.; Thurman, J.M. The role of complement in kidney disease. Nat. Rev. Nephrol. 2023, 19, 771–787. [Google Scholar] [CrossRef]
- Afshar-Kharghan, V. The role of the complement system in cancer. J. Clin. Investig. 2017, 127, 780–789. [Google Scholar] [CrossRef]
- Dalakas, M.C.; Alexopoulos, H.; Spaeth, P.J. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat. Rev. Neurol. 2020, 16, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Shapira, Y.; Agmon-Levin, N.; Shoenfeld, Y. Defining and analyzing geoepidemiology and human autoimmunity. J. Autoimmun. 2010, 34, J168–J177. [Google Scholar] [CrossRef]
- Bello, A.K.; Okpechi, I.G.; Levin, A.; Ye, F.; Damster, S.; Arruebo, S.; Donner, J.-A.; Caskey, F.J.; Cho, Y.; Davids, M.R.; et al. An update on the global disparities in kidney disease burden and care across world countries and regions. Lancet Glob. Health 2024, 12, e382–e395. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, J.D.; Seeher, K.M.; Schiess, N.; Nichols, E.; Cao, B.; Servili, C.; Cavallera, V.; Cousin, E.; Hagins, H.; Moberg, M.E.; et al. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Gillespie, K. Projected Global Burden of Brain Disorders Through 2050 (P7-15.001). Neurology 2024, 102, 3234. [Google Scholar] [CrossRef]
- Gomez-Arboledas, A.; Acharya, M.M.; Tenner, A.J. The Role of Complement in Synaptic Pruning and Neurodegeneration. Immunotargets Ther. 2021, 10, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Bohlson, S.S.; Garred, P.; Kemper, C.; Tenner, A.J. Complement Nomenclature-Deconvoluted. Front. Immunol. 2019, 10, 1308. [Google Scholar] [CrossRef] [PubMed]
- Seifert, L.; Zahner, G.; Meyer-Schwesinger, C.; Hickstein, N.; Dehde, S.; Wulf, S.; Köllner, S.M.S.; Lucas, R.; Kylies, D.; Froembling, S.; et al. The classical pathway triggers pathogenic complement activation in membranous nephropathy. Nat. Commun. 2023, 14, 473. [Google Scholar] [CrossRef] [PubMed]
- Dobó, J.; Kocsis, A.; Farkas, B.; Demeter, F.; Cervenak, L.; Gál, P. The Lectin Pathway of the Complement System-Activation, Regulation, Disease Connections and Interplay with Other (Proteolytic) Systems. Int. J. Mol. Sci. 2024, 25, 1566. [Google Scholar] [CrossRef]
- Fujita, T. Evolution of the lectin–complement pathway and its role in innate immunity. Nat. Rev. Immunol. 2002, 2, 346–353. [Google Scholar] [CrossRef]
- Heitzeneder, S.; Seidel, M.; Förster-Waldl, E.; Heitger, A. Mannan-binding lectin deficiency—Good news, bad news, doesn’t matter? Clin. Immunol. 2012, 143, 22–38. [Google Scholar] [CrossRef]
- Pangburn, M.K. Initiation of the alternative pathway of complement and the history of “tickover”. Immunol. Rev. 2023, 313, 64–70. [Google Scholar] [CrossRef]
- Sekine, H.; Machida, T.; Fujita, T. Factor D. Immunol. Rev. 2023, 313, 15–24. [Google Scholar] [CrossRef]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Shah, A.; Kishore, U.; Shastri, A. Complement System in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 13647. [Google Scholar] [CrossRef]
- Abbott, J.D.; Ball, G.; Boumpas, D.; Bridges, S.L.; Chatham, W.; Curtis, J.; Daniel, C.; Hughes, L.B.; Kao, A.H.; Langford, C.; et al. (Eds.) Terminal complement complex. In Rheumatology and Immunology Therapy; Springer: Berlin/Heidelberg, Germany, 2004; pp. 841–842. [Google Scholar]
- Xie, C.B.; Jane-Wit, D.; Pober, J.S. Complement Membrane Attack Complex: New Roles, Mechanisms of Action, and Therapeutic Targets. Am. J. Pathol. 2020, 190, 1138–1150. [Google Scholar] [CrossRef]
- Koelman, D.L.H.; Brouwer, M.C.; van de Beek, D. Targeting the complement system in bacterial meningitis. Brain 2019, 142, 3325–3337. [Google Scholar] [CrossRef]
- Ricklin, D.; Mastellos, D.C.; Reis, E.S.; Lambris, J.D. The renaissance of complement therapeutics. Nat. Rev. Nephrol. 2018, 14, 26–47. [Google Scholar] [CrossRef] [PubMed]
- Monach, P.A. Complement. Arthritis Rheumatol. 2024, 76, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Remuzzi, G. Overview of Complement Activation and Regulation. Semin. Nephrol. 2013, 33, 479–492. [Google Scholar] [CrossRef]
- Xue, X.; Wu, J.; Ricklin, D.; Forneris, F.; Di Crescenzio, P.; Schmidt, C.Q.; Granneman, J.; Sharp, T.H.; Lambris, J.D.; Gros, P. Regulator-dependent mechanisms of C3b processing by factor I allow differentiation of immune responses. Nat. Struct. Mol. Biol. 2017, 24, 643–651. [Google Scholar] [CrossRef]
- Werner, L.M.; Criss, A.K. Diverse Functions of C4b-Binding Protein in Health and Disease. J. Immunol. 2023, 211, 1443–1449. [Google Scholar] [CrossRef]
- Lubbers, R.; van Essen, M.F.; van Kooten, C.; Trouw, L.A. Production of complement components by cells of the immune system. Clin. Exp. Immunol. 2017, 188, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Fatoba, O.; Itokazu, T.; Yamashita, T. Complement cascade functions during brain development and neurodegeneration. FEBS J. 2022, 289, 2085–2109. [Google Scholar] [CrossRef]
- Chen, Y.; Chu, J.M.T.; Chang, R.C.C.; Wong, G.T.C. The Complement System in the Central Nervous System: From Neurodevelopment to Neurodegeneration. Biomolecules 2022, 12, 337. [Google Scholar] [CrossRef]
- Magdalon, J.; Mansur, F.; Teles e Silva, A.L.; de Goes, V.A.; Reiner, O.; Sertié, A.L. Complement System in Brain Architecture and Neurodevelopmental Disorders. Front. Neurosci. 2020, 14, 23. [Google Scholar] [CrossRef]
- Coulthard, L.G.; Hawksworth, O.A.; Woodruff, T.M. Complement: The Emerging Architect of the Developing Brain. Trends Neurosci. 2018, 41, 373–384. [Google Scholar] [CrossRef]
- Györffy, B.A.; Kun, J.; Török, G.; Bulyáki, É.; Borhegyi, Z.; Gulyássy, P.; Kis, V.; Szocsics, P.; Micsonai, A.; Matkó, J.; et al. Local apoptotic-like mechanisms underlie complement-mediated synaptic pruning. Proc. Natl. Acad. Sci. USA 2018, 115, 6303–6308. [Google Scholar] [CrossRef]
- Bohlson, S.S.; Tenner, A.J. Complement in the Brain: Contributions to Neuroprotection, Neuronal Plasticity, and Neuroinflammation. Annu. Rev. Immunol. 2023, 41, 431–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yue, H.; Hu, Z.; Shen, Y.; Ma, J.; Li, J.; Wang, X.-D.; Wang, L.; Sun, B.; Shi, P.; et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science 2020, 367, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Yarmoska, S.K.; Alawieh, A.M.; Tomlinson, S.; Hoang, K.B. Modulation of the Complement System by Neoplastic Disease of the Central Nervous System. Front. Immunol. 2021, 12, 689435. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Ziabska, K.; Ziemka-Nalecz, M.; Pawelec, P.; Sypecka, J.; Zalewska, T. Aberrant Complement System Activation in Neurological Disorders. Int. J. Mol. Sci. 2021, 22, 4675. [Google Scholar] [CrossRef]
- Bouwens, T.A.; Trouw, L.A.; Veerhuis, R.; Dirven, C.M.; Lamfers, M.L.; Al-Khawaja, H. Complement activation in Glioblastoma multiforme pathophysiology: Evidence from serum levels and presence of complement activation products in tumor tissue. J. Neuroimmunol. 2015, 278, 271–276. [Google Scholar] [CrossRef]
- DeCordova, S.; Abdelgany, A.; Murugaiah, V.; Pathan, A.A.; Nayak, A.; Walker, T.; Shastri, A.; Alrokayan, S.H.; Khan, H.A.; Singh, S.K.; et al. Secretion of functionally active complement factor H related protein 5 (FHR5) by primary tumour cells derived from Glioblastoma Multiforme patients. Immunobiology 2019, 224, 625–631. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Schwartz, J.B.; Abner, E.L.; Jicha, G.A.; Kapogiannis, D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann. Neurol. 2018, 83, 544–552. [Google Scholar] [CrossRef]
- Walker, D.G.; McGeer, P.L. Complement gene expression in human brain: Comparison between normal and Alzheimer disease cases. Mol. Brain Res. 1992, 14, 109–116. [Google Scholar] [CrossRef]
- Daborg, J.; Andreasson, U.; Pekna, M.; Lautner, R.; Hanse, E.; Minthon, L.; Blennow, K.; Hansson, O.; Zetterberg, H. Cerebrospinal fluid levels of complement proteins C3, C4 and CR1 in Alzheimer’s disease. J. Neural Transm. 2012, 119, 789–797. [Google Scholar] [CrossRef]
- Zhu, X.-C.; Yu, J.-T.; Jiang, T.; Wang, P.; Cao, L.; Tan, L. CR1 in Alzheimer’s Disease. Mol. Neurobiol. 2015, 51, 753–765. [Google Scholar] [CrossRef]
- Lu, L.; Yao, Q.-y.; Ruan, S.-S.; Hu, J.-W.; Long, W.-j.; Dai, W.-Z.; Ma, T.; Zhu, X.-C. Explore the role of CR1 genetic variants in late-onset Alzheimer’s disease susceptibility. Psychiatr. Genet. 2021, 31, 216–229. [Google Scholar] [CrossRef]
- Yuan, H.; Du, L.; Ge, P. Complement receptor 1 genetic polymorphism contributes to sporadic Alzheimer’s disease susceptibility in Caucasians: A meta-analysis. Biosci. Rep. 2020, 40, BSR20200321. [Google Scholar] [CrossRef] [PubMed]
- Sekar, A.; Bialas, A.R.; de Rivera, H.; Davis, A.; Hammond, T.R.; Kamitaki, N.; Tooley, K.; Presumey, J.; Baum, M.; Van Doren, V.; et al. Schizophrenia risk from complex variation of complement component 4. Nature 2016, 530, 177–183. [Google Scholar] [CrossRef]
- Severance, E.G.; Gressitt, K.L.; Buka, S.L.; Cannon, T.D.; Yolken, R.H. Maternal complement C1q and increased odds for psychosis in adult offspring. Schizophr. Res. 2014, 159, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ni, P.; Tian, Y.; Zhao, L.; Li, M.; Li, X.; Wei, W.; Wei, J.; Wang, Q.; Guo, W.; et al. Association of elevated levels of peripheral complement components with cortical thinning and impaired logical memory in drug-naïve patients with first-episode schizophrenia. Schizophrenia 2023, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Ah-Pine, F.; Malaterre-Septembre, A.; Bedoui, Y.; Khettab, M.; Neal, J.W.; Freppel, S.; Gasque, P. Complement Activation and Up-Regulated Expression of Anaphylatoxin C3a/C3aR in Glioblastoma: Deciphering the Links with TGF-beta and VEGF. Cancers 2023, 15, 2647. [Google Scholar] [CrossRef]
- Jonsson, K.F.; Liljedahl, E.; Osther, K.; Bengzon, J.; Melander Skattum, L.; Redebrandt, H.N. Complement Components in Peripheral Blood from Adult Patients with IDH Wild-Type Glioblastoma. World Neurosurg. 2023, 177, e742–e747. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.D.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754. [Google Scholar] [CrossRef]
- Nikolac Perkovic, M.; Videtic Paska, A.; Konjevod, M.; Kouter, K.; Svob Strac, D.; Nedic Erjavec, G.; Pivac, N. Epigenetics of Alzheimer’s Disease. Biomolecules 2021, 11, 195. [Google Scholar] [CrossRef]
- Carapeto, A.P.; Marcuello, C.; Faísca, P.F.N.; Rodrigues, M.S. Morphological and Biophysical Study of S100A9 Protein Fibrils by Atomic Force Microscopy Imaging and Nanomechanical Analysis. Biomolecules 2024, 14, 1091. [Google Scholar] [CrossRef]
- Ziaunys, M.; Sakalauskas, A.; Mikalauskaite, K.; Smirnovas, V. Polymorphism of Alpha-Synuclein Amyloid Fibrils Depends on Ionic Strength and Protein Concentration. Int. J. Mol. Sci. 2021, 22, 12382. [Google Scholar] [CrossRef]
- Rogers, J.; Cooper, N.R.; Webster, S.; Schultz, J.; McGeer, P.L.; Styren, S.D.; Civin, W.H.; Brachova, L.; Bradt, B.; Ward, P.; et al. Complement activation by beta-amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1992, 89, 10016–10020. [Google Scholar] [CrossRef] [PubMed]
- Dzamba, D.; Harantova, L.; Butenko, O.; Anderova, M. Glial Cells—The Key Elements of Alzheimer´s Disease. Curr. Alzheimer Res. 2016, 13, 894–911. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Publishing. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, TX, USA, 2013. [Google Scholar]
- Cannon, T.D.; Chung, Y.; He, G.; Sun, D.; Jacobson, A.; van Erp, T.G.; McEwen, S.; Addington, J.; Bearden, C.E.; Cadenhead, K.; et al. Progressive reduction in cortical thickness as psychosis develops: A multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol. Psychiatry 2015, 77, 147–157. [Google Scholar] [CrossRef]
- Glantz, L.A.; Lewis, D.A. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry 2000, 57, 65–73. [Google Scholar] [CrossRef]
- Carpenter, W.T., Jr.; Buchanan, R.W. Schizophrenia. N. Engl. J. Med. 1994, 330, 681–690. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Kendler, K.S.; Neale, M.C. Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry 2003, 60, 1187–1192. [Google Scholar] [CrossRef]
- Ingraham, L.J.; Kety, S.S. Adoption studies of schizophrenia. Am. J. Med. Genet. 2000, 97, 18–22. [Google Scholar] [CrossRef]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef]
- Purcell, S.M.; Wray, N.R.; Stone, J.L.; Visscher, P.M.; O’Donovan, M.C.; Sullivan, P.F.; Sklar, P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009, 460, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Melbourne, J.K.; Rosen, C.; Feiner, B.; Sharma, R.P. C4A mRNA expression in PBMCs predicts the presence and severity of delusions in schizophrenia and bipolar disorder with psychosis. Schizophr. Res. 2018, 197, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Asyraf, A.J.M.; El Huda, A.R.N.; Hanisah, M.N.; Norsidah, K.Z.; Norlelawati, A.T. Relationship of selective complement markers with schizophrenia. J. Neuroimmunol. 2022, 363, 577793. [Google Scholar] [CrossRef]
- Mayilyan, K.R.; Krarup, A.; Soghoyan, A.F.; Jensenius, J.C.; Sim, R.B. l-ficolin-MASP arm of the complement system in schizophrenia. Immunobiology 2023, 228, 152349. [Google Scholar] [CrossRef]
- Weathers, S.P.; Gilbert, M.R. Advances in treating glioblastoma. F1000Prime Rep. 2014, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Fornvik, K.; Maddahi, A.; Persson, O.; Osther, K.; Salford, L.G.; Nittby Redebrandt, H. C1-inactivator is upregulated in glioblastoma. PLoS ONE 2017, 12, e0183086. [Google Scholar] [CrossRef]
- Sun, L.; Hui, A.M.; Su, Q.; Vortmeyer, A.; Kotliarov, Y.; Pastorino, S.; Passaniti, A.; Menon, J.; Walling, J.; Bailey, R.; et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 2006, 9, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.J.; Kim, S.; Oh, Y.; Suh, Y.; Kaushik, N.; Lee, J.H.; Lee, H.J.; Kim, M.J.; Park, M.J.; Kim, R.K.; et al. Crosstalk between GBM cells and mesenchymal stemlike cells promotes the invasiveness of GBM through the C5a/p38/ZEB1 axis. Neuro-Oncology 2020, 22, 1452–1462. [Google Scholar] [CrossRef]
- Zhu, H.; Yu, X.; Zhang, S.; Shu, K. Targeting the Complement Pathway in Malignant Glioma Microenvironments. Front. Cell Dev. Biol. 2021, 9, 657472. [Google Scholar] [CrossRef]
- Willrich, M.A.V.; Braun, K.M.P.; Moyer, A.M.; Jeffrey, D.H.; Frazer-Abel, A. Complement testing in the clinical laboratory. Crit. Rev. Clin. Lab. Sci. 2021, 58, 447–478. [Google Scholar] [CrossRef]
- Yang, S.; McGookey, M.; Wang, Y.; Cataland, S.R.; Wu, H.M. Effect of Blood Sampling, Processing, and Storage on the Measurement of Complement Activation Biomarkers. Am. J. Clin. Pathol. 2015, 143, 558–565. [Google Scholar] [CrossRef]
- Wong, R.S.M.; Pullon, H.W.H.; Amine, I.; Bogdanovic, A.; Deschatelets, P.; Francois, C.G.; Ignatova, K.; Issaragrisil, S.; Niparuck, P.; Numbenjapon, T.; et al. Inhibition of C3 with pegcetacoplan results in normalization of hemolysis markers in paroxysmal nocturnal hemoglobinuria. Ann. Hematol. 2022, 101, 1971–1986. [Google Scholar] [CrossRef]
- Pouw, R.B.; Ricklin, D. Tipping the balance: Intricate roles of the complement system in disease and therapy. Semin. Immunopathol. 2021, 43, 757–771. [Google Scholar] [CrossRef]
- Crew, P.E.; McNamara, L.; Waldron, P.E.; McCulley, L.; Christopher Jones, S.; Bersoff-Matcha, S.J. Antibiotic prophylaxis in vaccinated eculizumab recipients who developed meningococcal disease. J. Infect. 2020, 80, 350–371. [Google Scholar] [CrossRef] [PubMed]
- Uzawa, A.; Oertel, F.C.; Mori, M.; Paul, F.; Kuwabara, S. NMOSD and MOGAD: An evolving disease spectrum. Nat. Rev. Neurol. 2024, 20, 602–619. [Google Scholar] [CrossRef]
- Zelek, W.M.; Morgan, B.P. Targeting complement in neurodegeneration: Challenges, risks, and strategies. Trends Pharmacol. Sci. 2022, 43, 615–628. [Google Scholar] [CrossRef]
- Mughal, Z.U.N.; Ahmed, B.; Amin, F.; Sadiq, A.; Rangwala, B.S. Lecanemab: A Hopeful Alzheimer’s Disease Treatment. Ann. Neurosci. 2024, 31, 83–85. [Google Scholar] [CrossRef]
- Gomez-Arboledas, A.; Carvalho, K.; Balderrama-Gutierrez, G.; Chu, S.H.; Liang, H.Y.; Schartz, N.D.; Selvan, P.; Petrisko, T.J.; Pan, M.A.; Mortazavi, A.; et al. C5aR1 antagonism alters microglial polarization and mitigates disease progression in a mouse model of Alzheimer’s disease. Acta Neuropathol. Commun. 2022, 10, 116. [Google Scholar] [CrossRef]
- van Leeuwen, J.R.; Popov, T.; Obergfell, A.; Rabelink, T.J.; Teng, Y.K.O. Preliminary Assessment of Safety and Tolerability of Avacopan During the Early Access Program for ANCA-Associated Vasculitis. Biol. Targets Ther. 2023, 17, 11–14. [Google Scholar] [CrossRef]
- Liljedahl, E.; Konradsson, E.; Gustafsson, E.; Jonsson, K.F.; Olofsson, J.K.; Osther, K.; Ceberg, C.; Redebrandt, H.N. Combined anti-C1-INH and radiotherapy against glioblastoma. BMC Cancer 2023, 23, 106. [Google Scholar] [CrossRef]
- Hettmann, T.; Gillies, S.D.; Kleinschmidt, M.; Piechotta, A.; Makioka, K.; Lemere, C.A.; Schilling, S.; Rahfeld, J.U.; Lues, I. Development of the clinical candidate PBD-C06, a humanized pGlu3-Aβ-specific antibody against Alzheimer’s disease with reduced complement activation. Sci. Rep. 2020, 10, 3294. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Liu, W.; Wang, L.; Shi, Y.; Hu, Y.; Yang, J.; Li, G.; Huang, H.; Cui, D. Extracellular vesicle biomarkers for complement dysfunction in schizophrenia. Brain 2023, 147, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Upthegrove, R.; Manzanares-Teson, N.; Barnes, N.M. Cytokine function in medication-naive first episode psychosis: A systematic review and meta-analysis. Schizophr. Res. 2014, 155, 101–108. [Google Scholar] [CrossRef] [PubMed]
| Disease | Complement-Related Biomarker | Potential Diagnostic or Prognostic Value | Sample | |
|---|---|---|---|---|
| Alzheimer’s disease | C1q, C3, C4 | Increased levels linked to disease progression | Blood, CFS, brain | [43,44,45] |
| CR1 | Genetic variations associated with increased risk for late-onset AD | Blood | [46,47,48] | |
| Schizophrenia | C4 | Genetic variations associated with increased risk | Blood | [49] |
| C1q, C2, C3, C4 | Increased levels linked to disease progression | Blood | [50,51] | |
| Glioblastoma | C3a/C3aR | Increased expression linked to poor prognosis | Tumor | [52] |
| C3 | Increased levels linked to poor prognosis | Tumor | [41] | |
| C1b, MBL | Increased levels associated with increased risk | Blood | [41] | |
| FB | Decreased levels associated with increased risk | Blood | [41] | |
| C1r, C1q | Decreased levels linked to poor patient survival | Blood | [53] |
| Testing Type | Sample | Component | Consideration | Methodology |
|---|---|---|---|---|
| Functional testing | Serum | Classical pathway Alternative pathway Lectin pathway | To look for complement deficiency and assess activation state of the pathways | Hemolytic assays, EIA |
| Individual component quantification and functional assessment | EDTA-plasma/serum | C1, MBL, ficolins 1–3, properdin, C2–C9, FB, FD | Component may be present but in a dysfunctional state Monitoring of anti-complement therapy | Radial immunodiffusion, nephelometry, EIA |
| Control protein | EDTA-plasma/serum | C1-inhibitor, C4BP, FI, FH, properdin | To investigate suspected imbalance of complement | Immunodiffusion, nephelometry, EIA |
| Activation products | EDTA-plasma | C3a, C3dg, C4a, C4d, Bb, C5a, sC5b9 | To distinguish primary from secondary complement deficiency To assess the involvement of an individual pathway To investigate suspected imbalance of complement | Rocket immunoelectrophoresis, EIA |
| Autoantibodies | EDTA-plasma/serum | anti-C1q, anti-C1-INH, anti-MBL, anti-C3b, anti-FH, anti-FI, anti-FB, C3-Nefs | Detected in autoimmune diseases | EIA, hemolytic assays |
| Molecular analysis of complement genes | EDTA whole blood | Genes of interest | To confirm complement disorder | NGS. hydrolysis probes |
| Therapeutic Agent | Target | Disease | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Eculizumab | C5 | PNH aHUS gMG NMOSD | Approved by the FDA and EMA Safe Well-tolerated | 2000-fold increased risk of meningococcal infections Focused on rare diseases Burden on healthcare system Limited accessibility | [82,83,84,85] |
| Ravalizumab (Ultomiris)) | C5 | PNH | Approved by FDA Longer half-life than eculizumab Improved dosing frequency | Target diversity results in not reaching clinical endpoints | [82,84,85] |
| Pegcetacoplan (Empaveli, Apellis) | C3 | PNH | Approved by the FDA and EMA Superior to eculizumab Long-term treatment is well tolerated | Greater risk of Neisseria meningitidis, Streptococcus pneumoniae, and Hemophilus influenzae type B infections | [82,85] |
| Lecanemab (Leqembi) | Aβ | AD | Approved by the FDA Able to slow patients’ cognitive decline in early disease stages Highly selective for Aβ protofibrils Lowers Aβ plaques Minimizes Aβ deposition Improves clinical degradation | Amyloid-associated imaging defects (edema or microhemorrhages) | [86] |
| PMX205 | C5aR1 | AD | Reduces total number of Aβ plaques and total Aβ load, number of ThioS + plaques, synaptic loss, and dystrophic neurites Promotes neuroprotective microglial phenotype | Might have an effect in the initial plaque deposition stages, but not once the amyloid plaques are formed | [87] |
| CCX168 (Avacopan, Chemocentryx/Vifor) | C5aR1 | ANCA-associated vasculitis | Already in use Oral administration | 10% of patients developed renal and urinary disorders | [88] |
| Anti-C1 inhibitor antibody | C1 | Glioma (rat model) | Potential radiosensitizing effect, i.e., increased survival of animals with subcutaneous glioblastoma | Drug delivery issues Difficult to achieve and/or sustain therapeutic concentration | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovčevska, I.; Videtič Paska, A.; Kouter, K. Complement Cascades and Brain Disorders. Biomolecules 2025, 15, 1179. https://doi.org/10.3390/biom15081179
Jovčevska I, Videtič Paska A, Kouter K. Complement Cascades and Brain Disorders. Biomolecules. 2025; 15(8):1179. https://doi.org/10.3390/biom15081179
Chicago/Turabian StyleJovčevska, Ivana, Alja Videtič Paska, and Katarina Kouter. 2025. "Complement Cascades and Brain Disorders" Biomolecules 15, no. 8: 1179. https://doi.org/10.3390/biom15081179
APA StyleJovčevska, I., Videtič Paska, A., & Kouter, K. (2025). Complement Cascades and Brain Disorders. Biomolecules, 15(8), 1179. https://doi.org/10.3390/biom15081179







