1. Introduction
Mitochondrial dysfunction contributes to heart failure, which remains one of the leading causes of heart disease [
1,
2,
3]. Because mitochondria supply approximately 95% of ATP through oxidative phosphorylation (OXPHOS), even subtle mitochondrial dysfunction can disrupt the balance from adaptive to maladaptive remodeling [
4]. Beyond ATP production, mitochondria also regulate calcium handling, redox signaling, apoptosis, and innate immunity, all of which demand functional mitochondria [
5,
6,
7]. Such functional mitochondria are governed by various quality control processes, including proteostasis, fusion–fission dynamics, mitophagy, and apoptosis, which act as first responders to cardiac stress [
7,
8,
9,
10]. Hence, disturbances in mitochondrial function can lead to chronic compromised cardiac function over time, affecting ATP production.
Cardiomyocytes are uniquely vulnerable to intrinsic mitochondrial stress due to their terminally differentiated nature and limited regenerative capacity. At the same time, targeting these cells therapeutically is particularly challenging. In contrast, many extra-cardiac tissues possess robust regenerative and adaptive capabilities and may serve as potential reservoirs for initiating systemic protective responses [
11,
12]. Mounting evidence from metabolic and aging research suggests that mitochondrial perturbations in peripheral organs, such as skeletal muscle, liver, or adipose tissue, can trigger systemic stress responses, most notably mitochondrial stress that affects whole-body homeostasis and impacts distant organs [
13,
14,
15]. This raises the intriguing therapeutic possibility of targeting other organs as an alternative to correcting mitochondrial defects within the diseased heart.
Mitochondrial matrix protein quality control (mPQC) is crucial for maintaining cardiac bioenergetics and cellular integrity, particularly in the post-mitotic myocardium. The heart, being a high-demand organ, relies heavily on well-orchestrated mitochondrial proteostasis mechanisms, which are primarily regulated by matrix proteases such as LONP1, along with other counterparts, including CLPXP, m-AAA, and iAAA proteases, to prevent the accumulation of damaged proteins and maintain oxidative phosphorylation [
8]. Loss of mPQC function in cardiomyocytes could compromise mitochondrial efficiency, elevate oxidative stress, and lead to maladaptive remodeling and heart failure. However, emerging studies suggest that mitochondrial stress responses are not strictly cell-autonomous and may involve compensatory signaling across tissues [
14]. While cardiac mPQC defects could impair heart function, it remains unclear whether such defects can be mitigated through inter-organ crosstalk, a process by which distant organs communicate via circulating metabolites, cytokines, or hormones to trigger adaptive or maladaptive responses. For example, mitochondrial stress in skeletal muscle or liver can release signaling molecules that influence cardiac mitochondrial function, offering a potentially valuable strategy given the challenges of directly targeting the heart. This raises the question: can the heart, when mPQC is impaired, benefit from mitochondrial stress responses initiated in distant tissues?
In this context, LONP1 which is a highly conserved ATP-dependent mitochondrial matrix protease, emerges as a critical regulator of systemic mitochondrial health. Beyond its role in degrading misfolded proteins, LONP1 modulates mitochondrial transcription, redox homeostasis, and stress signaling [
8]. A growing body of evidence has implicated
Lonp1 haploinsufficiency as a critical contributor to mitochondrial and cardiac dysfunction. For instance, Venkatesh et al. (2019) demonstrated that reduced
Lonp1 expression sensitizes cardiomyocytes to ischemia–reperfusion injury, suggesting a key protective role for LONP1 in cardiac stress adaptation [
16]. Similarly, De Gaetano et al. (2020) reported that
Lonp1 deficiency compromises mitochondrial ultrastructure and bioenergetics in embryonic fibroblasts, while Zhao et al. (2022) demonstrated that deletion of
Lonp1 in embryonic cardiac tissues leads to embryonic lethality due to arrested cardiac development [
17,
18]. Despite these significant advances, the consequences of
Lonp1 haploinsufficiency, specifically partial reductions in LONP1 activity, remain poorly understood, especially in young adult cardiomyocytes. Most studies to date have focused on either complete gene deletion or overexpression models, leaving a critical gap in understanding the physiological and pathophysiological relevance of more subtle, chronic deficits in
Lonp1 expression, which may better reflect disease or aging contexts. We hypothesized that moderate impairment of LONP1 in peripheral tissues might prime the organism to better tolerate mitochondrial dysfunction elsewhere, including in the heart. Such an adaptive preconditioning or “mitochondrial hormesis” model has been proposed in the context of aging and neurodegeneration, but its relevance to cardiac disease remains poorly defined [
19,
20].
To test this hypothesis, we employed two mouse models of Lonp1 haploinsufficiency, a cardiomyocyte-specific heterozygous knockout (Lonp1CKO-HET) and a global heterozygous knockout (Lonp1GKO-HET), as homozygous global Lonp1 deletion is embryonic lethal. Our study aimed to determine whether systemic modulation of mitochondrial proteostasis by partial deletion of Lonp1 could indirectly protect the heart in settings of cardiac mPQC dysfunction. By comparing the mitochondrial stress signatures, cardiac function, and compensatory responses in these models, we reveal that systemic mPQC deficiency, but not cardiac-specific deficiency, shows adaptive mechanisms that safeguard the heart. These findings suggest a novel therapeutic paradigm in which targeting mitochondrial proteostasis in extra-cardiac tissues may offer cardioprotection in conditions of heart disease.
2. Materials and Methods
2.1. Reagents and Chemicals
All TaqMan assay probes were purchased from Bio-Rad, CA, USA, except for the 18S probe (Cat. # 4333760F, Applied Biosystems, Life Technologies, CA, USA) and the Tert probe (Cat. # 4458368, Applied Biosystems, Thermo Fisher, CA, USA) (
Table S1). Chemicals were purchased from BioRad, Sigma, Applied Biosystem, Southern Biotech, Electron Microscopy Sciences, and GenScript unless otherwise mentioned.
2.2. Animal Models
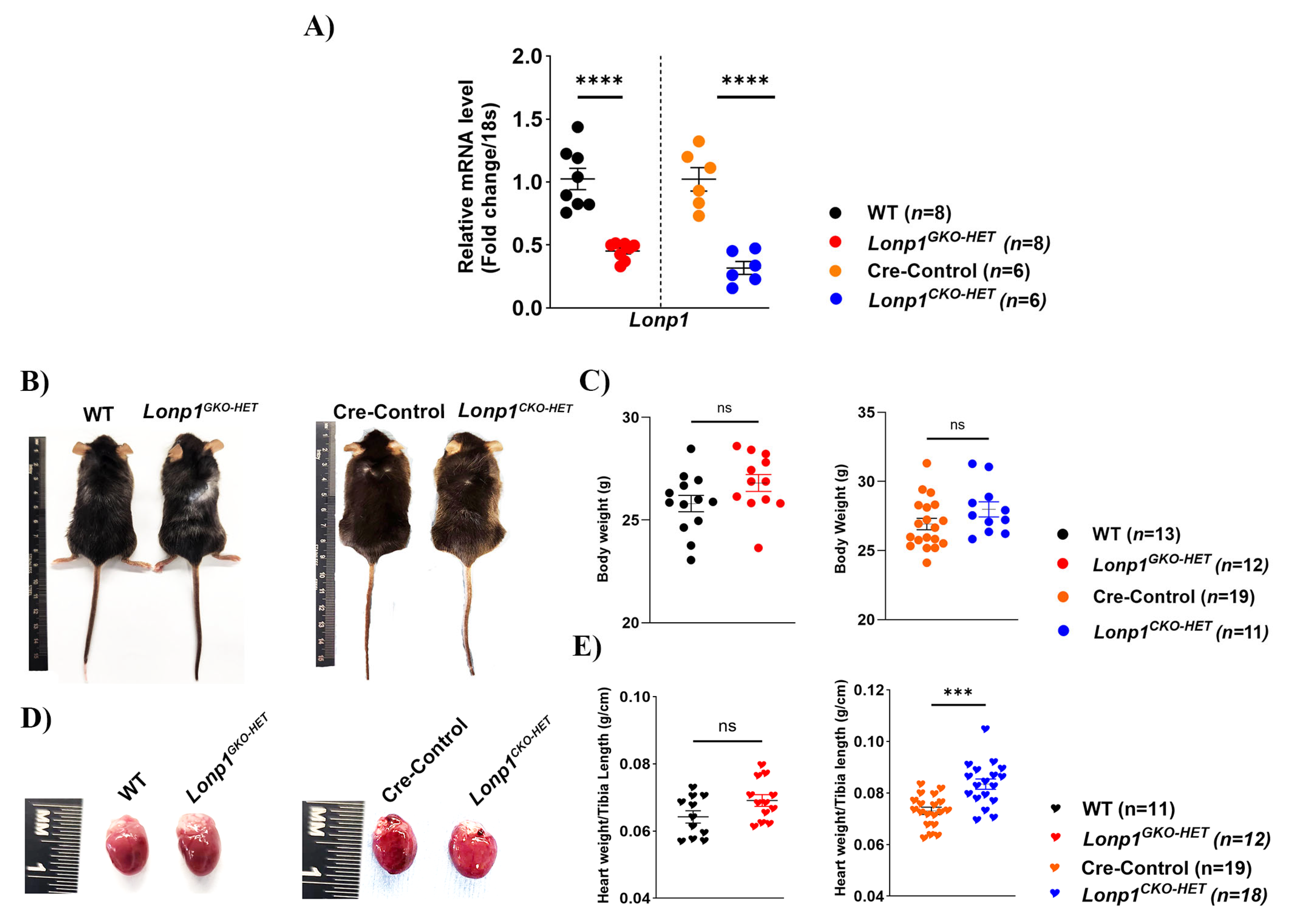
All animal procedures were approved in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of West Virginia University, USA (approval code 2203051893 on 6 April 2022). The C57BL/6 background whole-body heterozygous knockout
Lonp1GKO-HET mice were obtained from Dr. Carlos López-Otín (Universidad de Oviedo, Spain), and described previously in Quiros et al., 2014 [
21]. Cardiac-specific
Lonp1 heterozygous knock out (
Lonp1CKO-HET) mice were generated using homozygous
Lonp1-floxed mice (
Lonp1flfl) (Obtained from Dr. Bin Lu, University of South China) crossed with mice expressing Cre recombinase under the endogenous α myosin heavy chain (α-MHC) promoter procured from Jackson Laboratory (Strain: 005657). Littermates of WT and Cre-WT mice (referred to Cre-Control) were used as control mice in all experiments, respectively. The young adult mice (~12-week-old) from both sexes were included in the study. Twelve-week-old mice were chosen to evaluate early mitochondrial and cardiac phenotypes before the onset of age-related changes, allowing for the detection of primary effects due to
Lonp1 haploinsufficiency without secondary influences. All the mice were housed (minimum 3 to maximum 5 per cage) and were fed with standard laboratory chow in a temperature- and light/dark cycle-controlled animal facility room with free access to food and water.
2.3. Genotype
Tail and Ear biopsies were collected from the litter to determine the genotype of the mice. DNA was extracted from the samples by lysing them at 75 °C for 10 min, followed by 5 min of incubation at 95 °C for enzyme activation using the kappa hot start mouse genotyping kit (Cat. # 07961804001, Roche Diagnostics, IN, USA). End-point polymerase chain reaction (PCR) was performed using the following primers synthesized by Eton Bioscience Inc., CA, USA. For Lonp1GKO-HET, we employed Forward (5′CCCTGACTGCAGAGATTGTGAA3′), (5′CAGGACATAGCGTTGGCTACC3′), and a common reverse primer (5′TTCAGTGCCAGTGCCTTAGAGT3′), whereas for the Lonp1CKO-HET we employed both flox; forward (5′GGATCACCCTGAGTTCCCAGTT3′) and reverse (5′CACCACCTATAGCAGGTGCGAA3′), and Cre; forward (5′GCCTGCATTACCGGTCGATGC3′), and reverse (5′CAGGGTGTTATAAGCAATCCC3′) primers. PCR products were amplified using a recommended protocol, and 2% agarose gel electrophoresis was used to separate the PCR products and determine the genotype. The WT genotype is indicated by the presence of a band at 200 bp, Lonp1GKO-HET bands at 200 bp plus 300 bp, αMHC Cre band at 501 bp, and Lonp1flfl bands at 473 bp plus 573 bp.
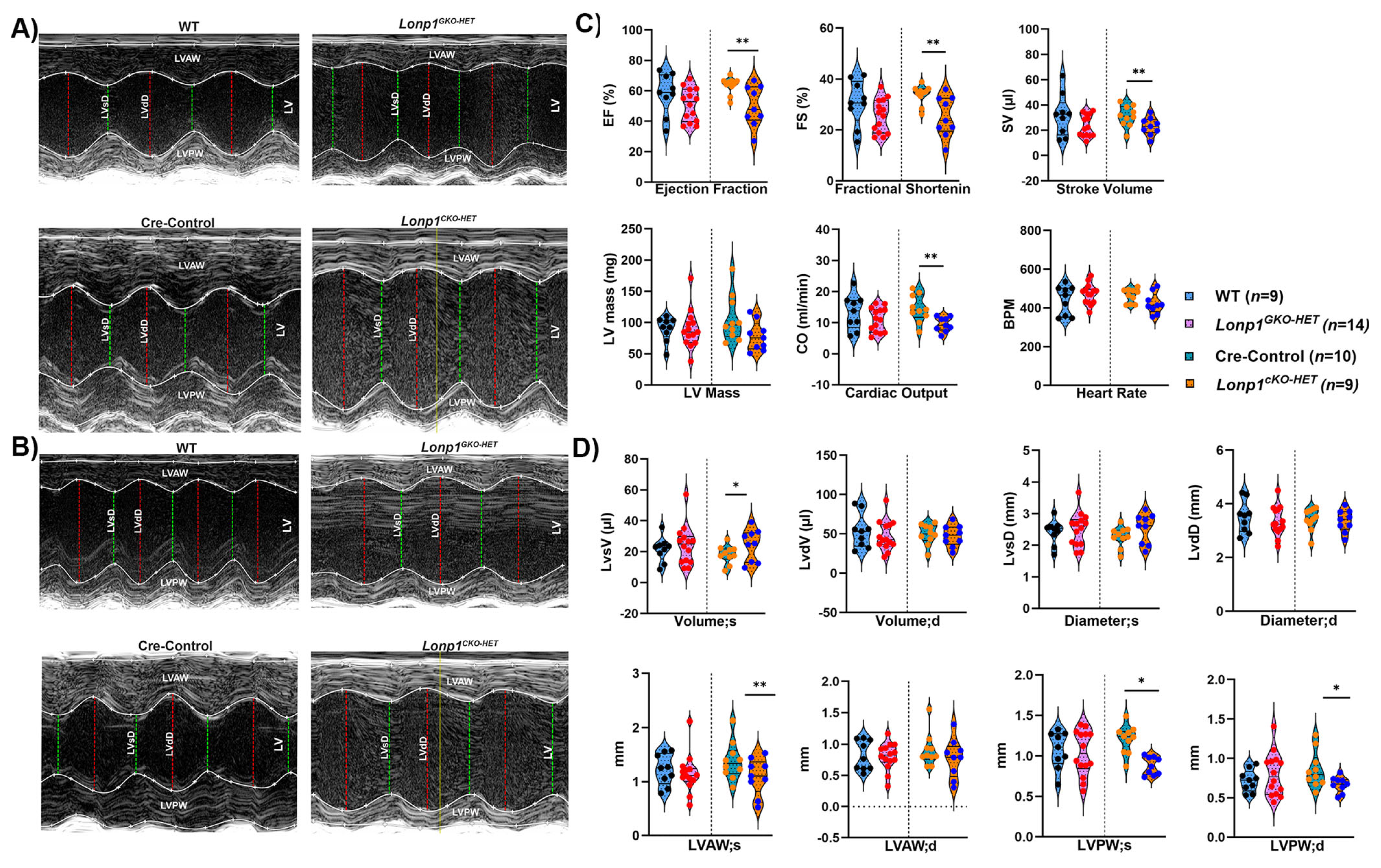
2.4. Transthoracic Echocardiographic Functional Assessment
Echocardiography on mice was performed by a single trained individual in a blindfolded approach in the WVU Animal Models and Imaging Facility. The mice were sedated with 1.5–2% isoflurane anesthesia via nose cone to ensure consistent and precise measurements of heart rate and function of left ventricle (LV). The echocardiogram images were acquired in parasternal long (PSLAX) and short axis (SAX) using a linear array transducer UHF57x (57 MHz) on the VevoF2 Micro-Ultrasound Imaging System (Visual Sonics, Toronto, ON, Canada). Both Brightness mode (B-mode) and Motion mode (M-mode) images of the heart were acquired at the level of papillary muscles to measure the ejection fraction (EF), fractional shortening (FS), stroke volume (SV), cardiac output (CO), left ventricular systolic, diastolic volume and diameter, anterior and posterior wall thickness. The echocardiographic analysis was performed utilizing a speckle tracking algorithm in VisualSonics Vevo Strain software 2.0 (Vevo F2, Visual Sonics, Toronto, ON, Canada) by an individual blindfolded to the mouse identifier. The endocardium and epicardium were tracked and marked up via speckle tracking and these segments were analyzed for at least three to five consecutive cardiac cycles to define the highest value achieved per parameter and averaged to give a comprehensive perspective of the heart. Raw values are provided in
Table S2.
2.5. Histological Analysis of Cardiac Morphology
Mice were weighed and euthanized by cervical dislocation. The hearts were excised, weighed, rinsed in 1× ice-cold PBS, and dry blotted. The excised heart tissue was sliced horizontally using a Zivic Mouse Heart Slicer Matrix (Cat. # HSMS001-1, Zivic Instruments, PA, USA), enabling precise tissue region, and then fixed in a 10% Formalin solution (Sigma Aldrich). Subsequently, the tissues were hydrated and dehydrated in graded alcohol solutions, cleared with xylene, and embedded in paraffin wax. The paraffin blocks of the hearts were cut at 5 µm, deparaffinized in descending graded alcohol, and stained with hematoxylin and eosin (HE), and Masson’s Trichrome. Collagen volume fraction (CVF) was quantified by analyzing Masson’s Trichrome-stained heart sections using FIJI (ImageJ 1.54f, Fiji distribution version 2.15.0, National Institute of Health, USA) software. For each sample, multiple fields were randomly selected across the left ventricular myocardium. The collagen-stained area (blue) and total tissue area were quantified by applying a standardized color thresholding algorithm that selectively isolates blue pixels corresponding to collagen matrix, as distinct from the red-stained muscle fibers. CVF was then calculated as the ratio of collagen-stained area to total tissue area, and the mean CVF value from multiple fields was averaged per sample. These per-sample averages were used for statistical comparison across groups and expressed as a percentage of total tissue area. This method enables objective and reproducible quantification of myocardial fibrosis. Images were acquired at 10× and 40× magnification using the MIF Olympus slide scanner (Both Brightfield and fluorescence modes). Raw values are provided in
Table S2.
2.6. Gene Expression Assay by RT-PCR
The experimental mice heart tissues were snap frozen in liquid nitrogen, ground to a fine powder employing pre-chilled mortar, followed by homogenization, and total RNA was extracted using the Qiagen RNeasy mini kit for fibrous tissue (Cat# 74704, Qiagen Inc.). The concentration of RNA (ng/µL) in each sample was measured using a Denovix 11 series Spectrophotometer. The RNA (100–500 ng) was utilized to synthesize cDNA by employing an iScriptTM cDNA synthesis kit from BioRad (Cat #170 8891), followed by cDNA quantification. qRT-PCR was performed employing TaqMan-based gene expression assays for each gene target as listed in
Table S1. The relative fold expression was normalized against 18S and quantified using the ΔΔCt method. The list of TaqMan gene expression assays used in this study is provided in
Table S1, and the corresponding raw Ct and ΔΔCt values used for calculating relative expression are provided in
Table S2.
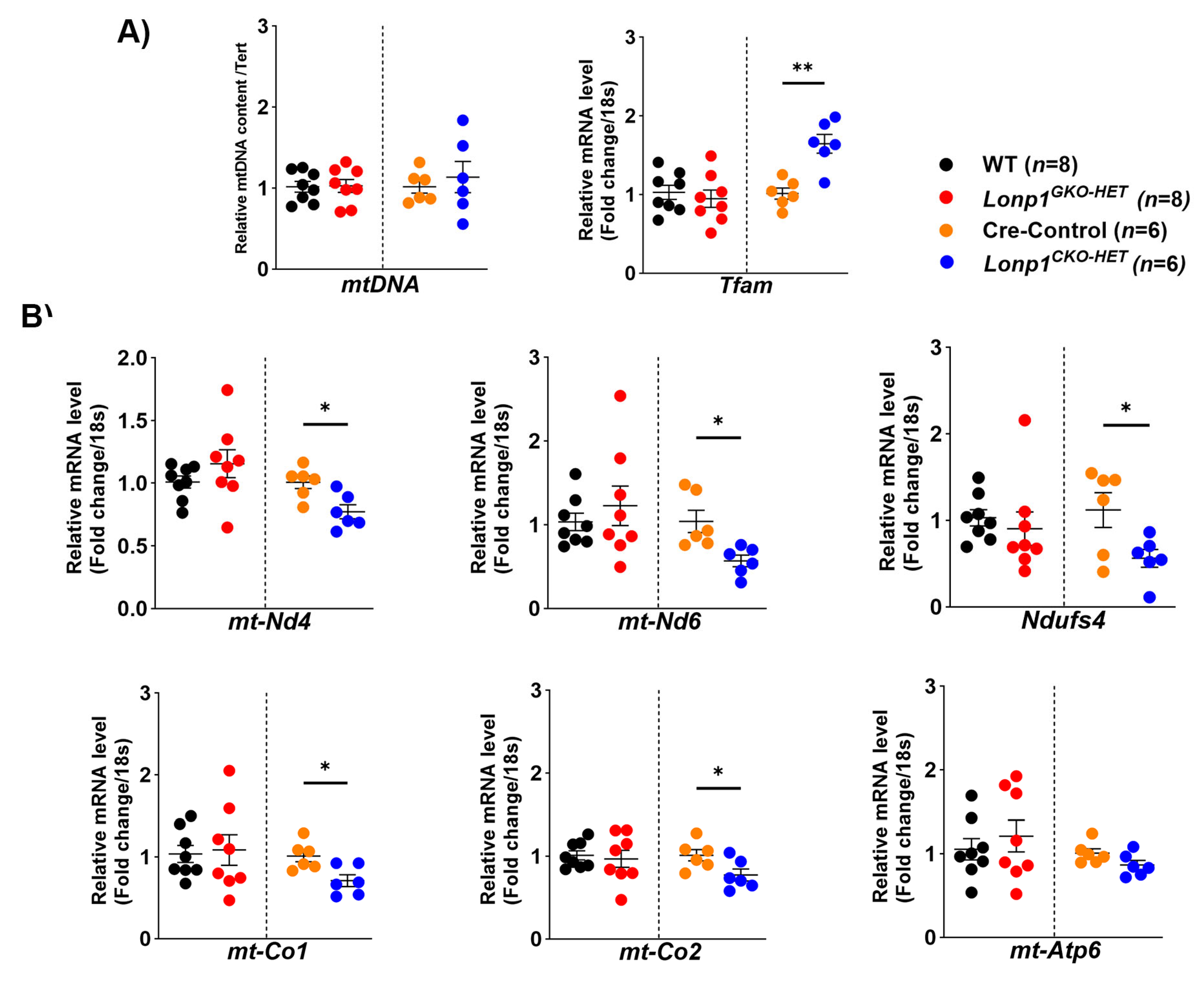
2.7. Determination of Mitochondrial DNA Content
Relative levels of mitochondrial DNA copy number (mtDNA-CN) from the heart tissues were measured using a real-time qPCR assay. Genomic DNA was isolated from mouse heart tissue following the modified simple DNA isolation method described in Muthu et al., 2025 [
22]. 100 ng of the isolated genomic DNA was employed to quantify relative mtDNA content by amplifying both the
mt-Co1 gene and the nuclear
Tert gene (
Table S1). Amplification was performed using BioRad Universal PCR Master Mix (Cat#1725134, BioRad, CA, USA). The qPCR conditions included an initial denaturation at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. All reactions were carried out in triplicate. The relative fold quantitation of the mtDNA copy number was calculated using the ΔΔCt method. Raw values are provided in
Table S2.
2.8. Mitochondria Isolation from the Whole Heart
Hearts were harvested, squeezed to remove blood, and rinsed in ice-cold PBS. Then, mitochondrial isolation was performed at 4 °C using buffer 1 [100 KCL mmol/L, 50 MOPS mmol/L, 5 MgSO4·7H2O mmol/L, 1 EGTA mmol/L, and 1 ATP mmol/L (PH 7.4)] at a 1:10 (weight: volume) ratio to homogenize the heart tissue. The samples were centrifuged at 700× g for 10 min, followed by the collection of the supernatant and subsequent centrifugation at 10,000× g. The pellet was washed in buffer 1 and centrifuged twice at 10,000× g. The precipitated pellet from 700 g was further processed in buffer 2 [100 KCL mmol/L, 50 MOPS mmol/L, and 0.5 EGTA mmol/L (PH 7.4)], and digested in trypsin (5 mg/g) for 10 min, then the digestion was stopped by adding protease inhibitor cocktail and centrifuged at 700× g for 10 min, the supernatant was collected and recentrifuged at 10,000× g for 10 min. Mitochondria from the initial supernatant and digested pellets were combined in a sucrose buffer that contains 220 sucrose mmol/L, 70 mannitol mmol/L, 10 Tris-HCL mmol/L, and 1 EDTA mmol/L (PH 7.4). The further purification of total mitochondria was performed using a sucrose gradient of 23%, 15%, 10%, and 3% Percoll solution and centrifuging in a Beckman Optima MAX-XP Ultracentrifuge (Beckman Coulter, Fullerton, CA, USA) at 32,000× g for 8 min and then the final mitochondrial isolation was stored in KME buffer [KCL 100 mM, MOPS 50 mM, and EGTA 0.5 mM (PH 7.4)]. The final pellet of mitochondria was used for mitochondrial ETC complex activity analysis.
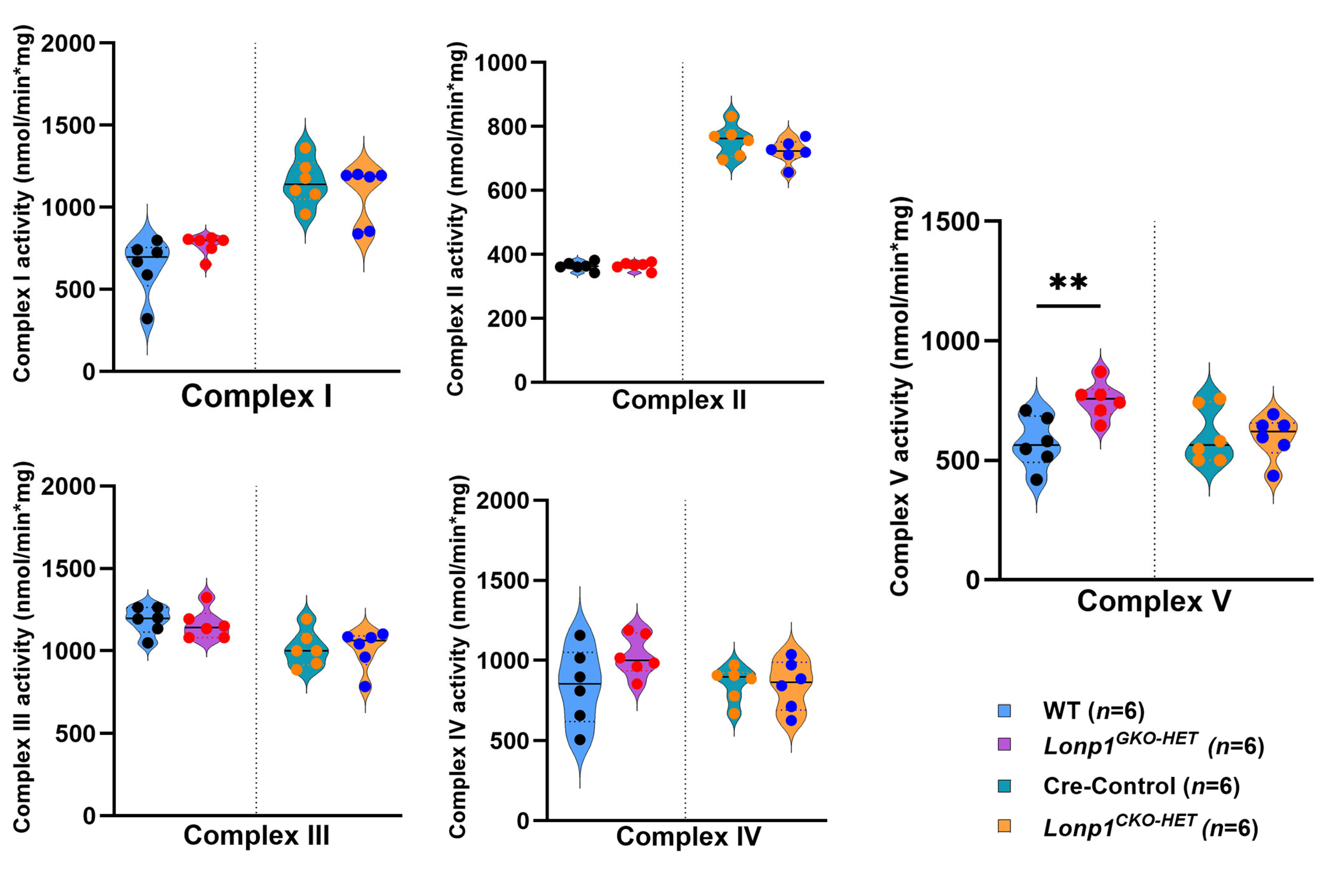
2.9. Enzyme Activity of Electron Transport Complexes I–V
The total protein concentration was determined by the BCA method from isolated mitochondria. The ETC complexes I, II, III, and IV, activities were measured using protein homogenates as described in Kunovac et al. 2021 [
23]. Briefly, the activities of ETC complexes I to IV were determined by measuring the oxidation of NADH at 340 nm in the presence of decyl ubiquinone and rotenone (I), 2,6-dichlorophenolindophenol (DCPIP) reduction at 600 nm (II), cytochrome C reduction at 550 nm (III), and oxidation of reduced cytochrome c at 550 nm (IV), respectively. The complex V activity was determined by measuring oligomycin-sensitive ATPase activity through the pyruvate kinase and phosphoenolpyruvate pathway. The final values were represented as unit/nanogram or milligram of protein (I–V), where unit = nanomoles of oxidized substrate (minute
−1). Raw values are provided in
Table S2.
2.10. Statistical Analysis
The significant differences between control and Lonp1 haploinsufficiency groups of both Lonp1GKO-HET and Lonp1CKO-HET models were individually compared using the unpaired Student’s t-test using GraphPad Prism software version 10.4.2, and p < 0.05 was considered statistically significant. Data are expressed as mean ± standard error of the mean.
4. Discussion
Our findings demonstrate that cardiomyocyte-specific haploinsufficiency of
Lonp1 leads to early signs of cardiac and mitochondrial stress, whereas global haploinsufficiency does not elicit comparable changes. A multifunctional mitochondrial LONP1 is crucial for cellular proteostasis, whereas homozygous deletion of
Lonp1 in mice results in embryonic lethality [
8,
21]. Therefore, we have employed global heterozygous mice, which are born normal and live similarly to wild-type mice, and compared them with cardiac-specific heterozygous mice [
16]. Our results highlight the unique vulnerability of post-mitotic cardiomyocytes to impaired mitochondrial proteostasis and suggest that systemic, extra-cardiac mitochondrial stress resulting from
Lonp1 haploinsufficiency may buffer against such localized stress, possibly through compensatory inter-organ signaling.
The differential mitochondrial stress response between the two models further underscores this tissue-specific sensitivity.
Lonp1CKO-HET hearts exhibited robust transcriptional upregulation of mitochondrial stress markers, including
Clpx,
Spg7,
Hspa9, and
Hspd1, without alterations in integrated stress response factors like
Atf4 and
Atf3. A similar stress response, serving as an adaptive mechanism, has been observed in other models of
Lonp1 inhibition [
25]. The activation of the mitochondrial unfolded protein response (UPR
mt) mitigates mitochondrial dysfunction and preserves cellular viability in the absence of a broader integrated stress response, suggesting a conserved protective role for LONP1-regulated proteostasis across tissues [
25]. Conversely,
Lonp1GKO-HET hearts did not show similar transcriptional alterations, suggesting that systemic
Lonp1 reduction may activate compensatory mechanisms in extra-cardiac tissues that mitigate mitochondrial stress specifically in the heart. This lack of stress response in the global model implies a potential inter-organ communication axis that buffers cardiac mitochondria against proteostasis imbalance, possibly through circulating factors or metabolic adaptations [
26]. These findings underscore the concept that localized mitochondrial stress, such as in post-mitotic cardiomyocytes, cannot always be recapitulated by global gene dosage reduction, highlighting the heart’s unique susceptibility and limited capacity to compensate for intrinsic mitochondrial dysfunction.
Mitochondrial biogenesis and OXPHOS gene expression were also differentially affected between the two models of
Lonp1 haploinsufficiency. Interestingly, although mtDNA content remained unchanged, the significant upregulation of
Tfam in
Lonp1CKO-HET hearts may reflect a pre-emptive compensatory mechanism aimed at enhancing mitochondrial biogenesis and transcriptional capacity in response to early mitochondrial stress, consistent with an adaptive attempt to preserve mitochondrial function. This was accompanied by a notable downregulation of mitochondrial-encoded subunits of Complex I (
mt-Nd4,
mt-Nd6) and Complex IV (
mt-Co1,
mt-Co2), suggesting impaired mitochondrial respiratory capacity in cardiomyocytes under proteostatic stress. These findings are consistent with previous reports indicating that LONP1 is required for maintaining mtDNA integrity and mitochondrial transcription, and its loss can lead to defective respiratory chain complex assembly [
21]. It has been demonstrated that LONP1 reduction in different cell types, such as fibroblasts and neonatal rat ventricular myocytes, led to decreased levels of respiratory chain function and mitochondrial membrane potential, implicating LONP1 as a key regulator of mitochondrial protein homeostasis and energetic function, consistent with the observation in
Lonp1CKO-HET hearts [
27].
In contrast,
Lonp1GKO-HET hearts did not exhibit similar transcriptional changes in OXPHOS genes. Surprisingly, they showed an increase in Complex V activity, suggesting a systemic adaptation that enhances mitochondrial ATP production. This finding aligns with evidence that moderate mitochondrial stress can trigger adaptive responses via mitochondrial hormesis (mitohormesis), where mild impairment leads to compensatory upregulation of mitochondrial function in distant tissues [
19,
20]. Although we did not directly assess the origin of systemic compensation in this study, our findings support the concept that
Lonp1 haploinsufficiency in extra-cardiac tissues can trigger adaptive responses that mitigate cardiac mitochondrial dysfunction. Previous studies have shown that mitochondrial perturbations in metabolically active organs such as skeletal muscle, liver, and adipose tissue can initiate the release of mitokines, a hormone-like signals such as FGF21 and GDF15, which exert systemic effects on mitochondrial biogenesis, oxidative stress, and cellular metabolism in distant organs, including the heart [
13,
14,
15,
26,
28]. In particular, FGF21, which is secreted by the liver and skeletal muscle in response to mitochondrial stress, has been shown to improve mitochondrial function and reduce inflammation in cardiac tissues [
28]. Likewise, GDF15 is another stress-responsive cytokine that mediates metabolic adaptation in response to mitochondrial dysfunction and has been implicated in protective cardiac signaling [
26]. Therefore, it is possible that
Lonp1GKO-HET mice, which exhibit moderate mitochondrial proteostatic imbalance in multiple organs, activate such mitokine signaling pathways that confer endocrine-mediated cardio protection.
Moreover, it has been demonstrated that mitochondrial stress in skeletal muscle can upregulate antioxidant defense and reduce cardiac oxidative stress, potentially through altered nutrient flux or humoral signaling [
26]. The lack of mitochondrial stress response activation in
Lonp1GKO-HET hearts, combined with increased Complex V activity, supports the concept of a systemic metabolic preconditioning effect, a phenomenon consistent with mitohormesis, where mild stress induces adaptive resilience [
19,
29,
30]. While the specific organ responsible for initiating the compensatory response remains to be determined, skeletal muscle and liver are strong candidates due to their high mitochondrial density, secretory profile, and established roles in mitokine production. Notably, the lack of such a compensatory response in
Lonp1CKO-HET hearts underscores the tissue-autonomous vulnerability of cardiomyocytes to mitochondrial proteostasis imbalance and further supports the notion that systemic mitochondrial stress may paradoxically buffer local dysfunction through endocrine or metabolic crosstalk.
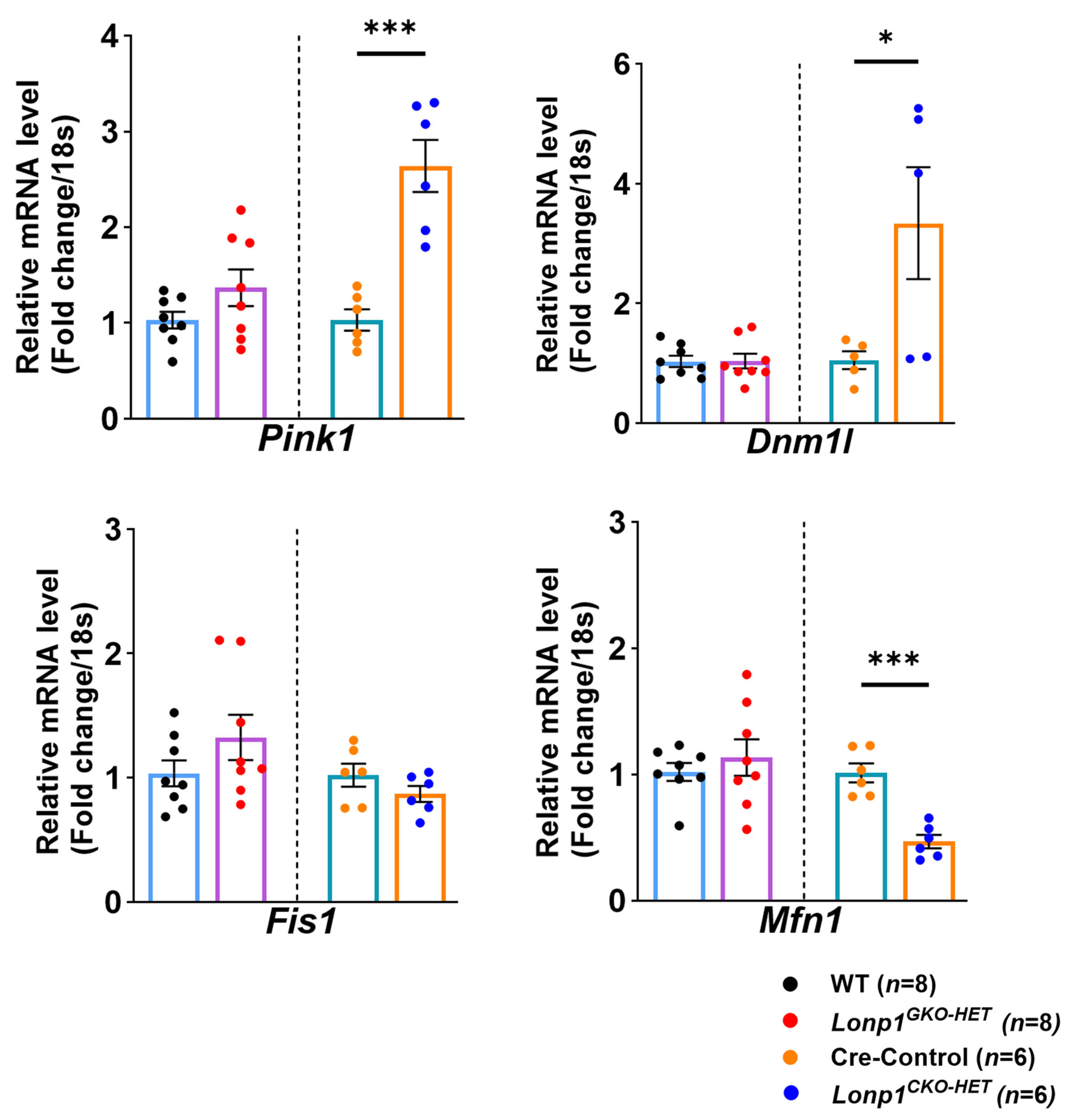
Furthermore, alterations in mitochondrial dynamics were evident only in
Lonp1CKO-HET hearts, which displayed significant upregulation of
Pink1 and
Dnm1l, markers of mitophagy and mitochondrial fission, respectively, alongside downregulation of the fusion regulator
Mfn1. These changes reflect an adaptive mitochondrial quality control (QC) mechanism that promotes organelle turnover and remodeling in response to proteostatic stress. This pattern is consistent with prior reports that
Lonp1 deficiency leads to the activation of mitophagy via the Pink1/Parkin signaling pathway [
28,
31]. The downregulation of
Mfn1, which is the major driver of mitochondrial fusion, is also in line with the observed suppression of fusion machinery, which facilitates mitochondrial fragmentation in its absence; however, whether that helps in the removal of damaged mitochondria via mitophagy is unclear [
32,
33]. In contrast, these mitochondrial dynamic regulators remained unchanged in
Lonp1GKO-HET hearts, suggesting that systemic reduction of
Lonp1 does not elicit the same local mitochondrial stress activation in the heart. This reinforces the concept that systemic mitochondrial proteostasis disruption is buffered by extra-cardiac tissues, preventing maladaptive mitochondrial remodeling within the myocardium. Such buffering may be mediated through systemic metabolic adaptations or mitokine signaling, as proposed in models of mild mitochondrial dysfunction, where whole-body adaptations preserve organ-specific mitochondrial integrity [
28,
34].
In summary, our study reveals that the heart’s reliance on efficient mitochondrial proteostasis makes it uniquely sensitive to Lonp1 loss when not supported by systemic compensatory mechanisms. While systemic Lonp1 haploinsufficiency appears to maintain cardiac function through unidentified protective inter-organ mechanisms, as targeted cardiac loss results in early functional decline and mitochondrial stress. These findings underscore the importance of tissue context in mitochondrial matrix protein quality control, suggesting that enhancing systemic mitochondrial proteostasis could be a novel therapeutic strategy for protecting the heart in mitochondrial disorders. Our study offers important translational insights into mitochondrial-targeted therapies for cardiac disease. We show that systemic Lonp1 haploinsufficiency triggers mild mitochondrial stress and adaptive responses (mitohormesis), potentially preconditioning the heart against dysfunction. In contrast, cardiomyocyte-specific Lonp1 reduction leads to a trend towards cardiac dysfunction without systemic compensation, highlighting the heart’s reliance on intrinsic LONP1 activity. These findings suggest that systemic modulation of LONP1 may be a safer and more flexible therapeutic approach than direct cardiac targeting, offering a novel strategy to enhance cardiac resilience through inter-organ mitochondrial signaling.
Our study focused on 12-week-old mice to assess early mitochondrial stress responses and cardiac function before potential age-associated compensatory changes. While this time point provides insights into early pathophysiological events, it remains unknown whether
Lonp1 haploinsufficiency leads to progressive deterioration over time. Previous studies suggest that mitochondrial proteostasis deficits can accumulate with age [
8,
22,
25], raising the possibility that older
Lonp1CKO-HET mice may exhibit more severe cardiac remodeling or dysfunction. However, longitudinal or age-cohort studies are underway in these specific models.
4.1. Limitations
Our study has several limitations. Firstly, while our findings suggest protective inter-organ communication in systemic Lonp1 haploinsufficiency, we did not directly identify the source organs or molecular mediators (e.g., mitokines or metabolic hormones) responsible for this compensation. However, based on existing literature, we predict that skeletal muscle and liver could be the primary contributors through the secretion of FGF21 and GDF15 in response to mitochondrial stress. Secondly, the analyses were limited to young adult mice under baseline conditions, and it remains unclear how these models respond to aging or cardiac stress. Lastly, potential sex-specific effects were noted but not fully explored due to limited sample size, warranting further investigation in future studies.
4.2. Future Direction
Future studies should focus on identifying the specific extra-cardiac tissues and signaling pathways involved in this systemic compensation. Additionally, exploring whether enhancing mitochondrial proteostasis in peripheral organs can confer cardioprotection under pathological conditions may open new avenues for treating mitochondrial cardiomyopathies. Furthermore, evaluating the plasma levels of mitokines such as FGF21 and GDF15 and determining their expression profiles in skeletal muscle and liver in Lonp1GKO-HET mice could offer insights into the inter-organ crosstalk mechanism. Incorporating ultrastructural analyses using transmission electron microscopy (TEM) in the future will be valuable for directly visualizing mitochondrial morphology (e.g., swelling, fragmentation, cristae structure) and validating the transcriptional and functional findings observed in Lonp1 haploinsufficient hearts. Future studies incorporating omics-based approaches, including proteomics, metabolomics, and transcriptomics, will be essential to identify circulating factors and molecular networks that mediate inter-organ crosstalk in response to Lonp1 haploinsufficiency.