Valence-Driven Cognitive Flexibility: Neurochemical and Circuit-Level Insights from Animal Models and Their Relevance to Schizophrenia
Abstract
1. Introduction
2. Assessing Cognitive Flexibility Deficits in SZ
3. Assessing Cognitive Flexibility Deficits in Rodents: Methodology, Neural Circuits and Neurotransmitters
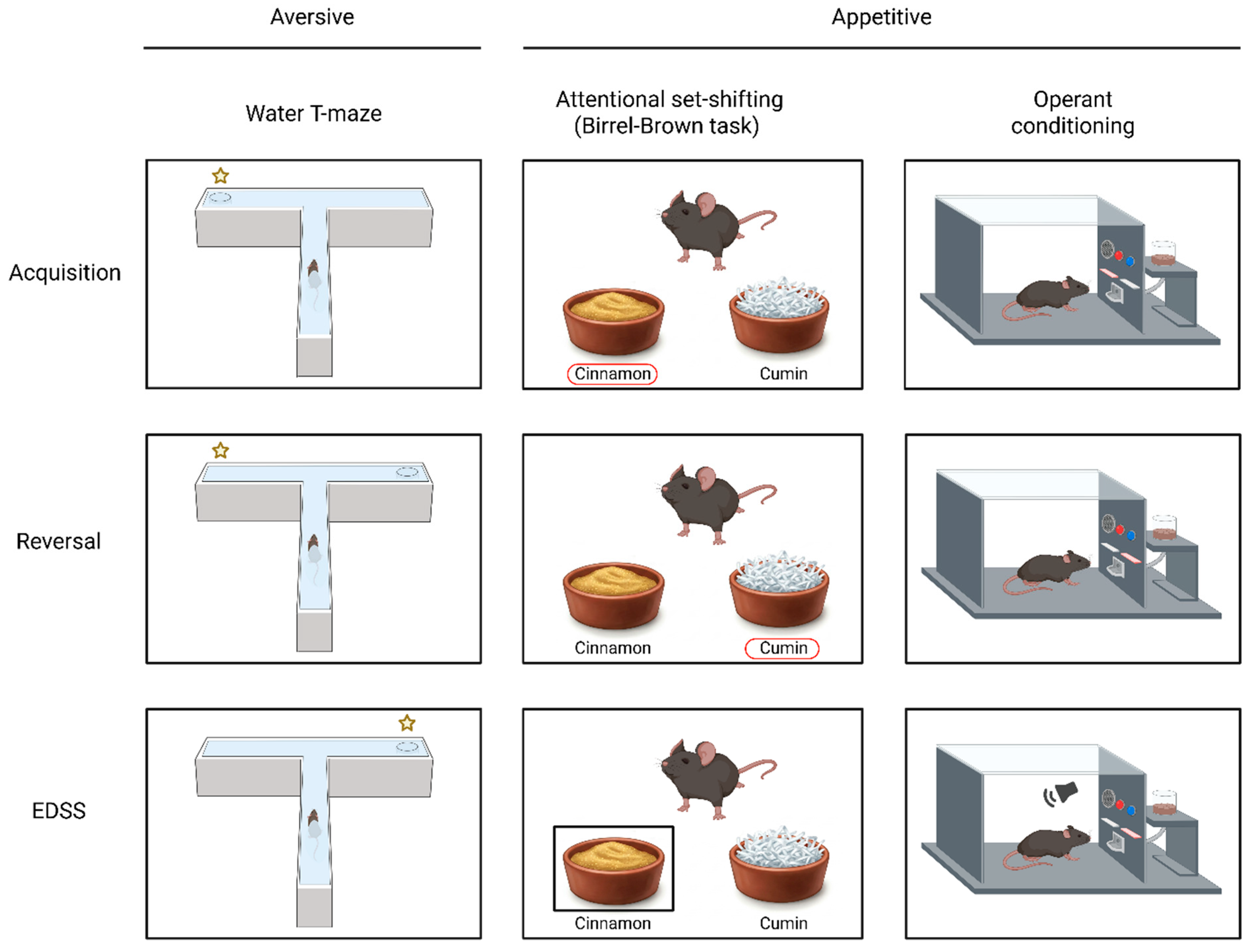
3.1. Aversive Learning Tasks
3.2. Appetitive Learning Tasks
3.3. The Neural Substrates of Cognitive Flexibility
3.3.1. Glutamate
3.3.2. GABA
3.3.3. Dopamine
3.3.4. Serotonin
3.3.5. Additional Neural Substrates
3.3.6. Environmental Manipulations
| References | Mice/Rats | Aversive/Appetitive | Manipulation | Methods | Task | Reversal | EDSS | Brain Region | Neurotransmitters |
|---|---|---|---|---|---|---|---|---|---|
| Bardgett et al., 2003 [59] | Mice | Aversive | Pharmacological | Acute injection of MK-801 | water T-maze | ↓ | Glutamate | ||
| Chadman et al., 2006 [60] | Rats | Appetitive | Pharmacological | Acute injection of MK-801 | water T-maze | ↓ | Glutamate | ||
| McLean et al., 2010 [61] | Rats | Appetitive | Pharmacological | Acute injection of PCP | Operant conditioning | ↓ | Glutamate | ||
| Abdul-Monim et al., 2006 [62] | Rats | Appetitive | Pharmacological | Acute injection of PCP | Operant conditioning | ↓ | Glutamate | ||
| Patrono et al., 2023 [63] | Rats | Appetitive | Optogenetic stimulations | Injection of MK-801 | ASST | ↓, ↑ | ↓, ↑ | PFC, ventral hippocampus | GABA |
| Li et al., 2016 [64] | Rats | Aversive | Pharmacological | Chromic injection of MK-801 | Morris water maze | ↓ | Glutamate | ||
| Thonnard et al., 2019 [65] | Rats | Aversive | Pharmacological | Chromic injection of MK-801 | Morris water maze | ↓ | Glutamate | ||
| Watson & Stanton, 2009 [66] | Rats | Appetitive | Pharmacological | bilateral intrahippocampal administration of MK-801 | T-maze | ↓ | Glutamate | ||
| Dong et al., 2013 [67] | Rats | Aversive | Pharmacological | systemic or intra-hippocampal blockade of NMDA receptor Grin2b subunit | MWM | ↓ | Hippocampus | Glutamate | |
| Duffy et al., 2008 [68] | Mice | Aversive | Pharmacological | blockade of NMDA receptor Grin2b subunit, D-Serine administration | MWM | ↓, ↑ | Glutamate | ||
| Darvas & Palmiter, 2015 [72] | Mice | Aversive | Genetic | Grin1 knockout | Water U-maze | - | ↑ | Dorsal striatum | Dopamine |
| Marquardt et al., 2014 [73] | Mice | Appetitive | Genetic | Grin2a knockout | ASST | - | ↓ | Glutamate | |
| Brigman et al., 2013 [74] | Mice | Appetitive | Genetic and Pharmacological | Cortical Grin2b knockout and OFC-specific Grin2b blocking | Operant conditioning | ↓ | mPFC and OFC | Glutamate | |
| Labrie et al., 2009 [75] | Mice | Aversive | Genetic | DAO1G181R mice with inactivation of DAO enzyme | MWM | ↑ | Glutamate | ||
| Zeleznikow-Johnston et al., 2018 [78] | Mice | Appetitive | Genetic | mGluR5 knockout | Operant conditioning | ↓ | Glutamate | ||
| Lim et al., 2019 [79] | Mice | Appetitive | Genetic | mGluR5 knockout | Operant conditioning | ↓ | Glutamate | ||
| Xu et al., 2013 [80] | Mice | Aversive | Pharmacological | increasing mGluR5 activity using positive allosteric modulators | MWM | ↑ | Glutamate | ||
| Gastambide et al., 2012 [81] | Rats | Appetitive | Pharmacological | increasing mGluR5 activity using positive allosteric modulators | ASST | ↓, ↑ | ↓ | Glutamate | |
| Joffe et al., 2019 [82] | Mice | Appetitive | Pharmacological | increasing mGluR5 activity using positive allosteric modulators | Operant conditioning | ↑ | Glutamate | ||
| Balschun et al., 2010 [85] | Mice | Aversive | Genetic | Vglut1 knockout | MWM | ↓ | Glutamate | ||
| Granseth et al., 2015 [86] | Mice | Appetitive | Genetic | Vglut1 knockout | Operant conditioning | ↓ | Glutamate | ||
| Lander et al., 2019 [32] | Mice | Aversive | Genetic | Knockdown and knockout of Glud1 | Water T-maze | ↓ | ↓ | Glutamate | |
| Asraf et al., 2023 [25] | Mice | Aversive | Genetic | Knockdown of Glud1 | Water T-maze | ↓ | mPFC | Glutamate | |
| Morellini et al., 2010 [88] | Mice | Aversive | Genetic | Knockout of Tnr | MWM | ↑ | Hippocampus | Glutamate and GABA | |
| Barnes et al., 2023 [89] | Rats | Aversive | Optogenetic stimulations | Optogenetic activation orinhibition of glutamatergic neurons in vmOFC | Operant conditioning | ↓, ↑ | OFC | Glutamate | |
| Rajagopal et al., 2018 [93] | Mice | Appetitive | Pharmacological | Administration of TPA-023, a GABAA partial agonist | Operant conditioning | ↓, ↑ | Glutamate and GABA | ||
| Brigman et al., 2006 [96] | Mice | Appetitive | Genetic | Knockdown of reelin | Operant conditioning | ↓ | - | PFC | GABA |
| Hausrat et al., 2015 [97] | Mice | Aversive | Genetic | Knockout of Rdx | MWM | ↓ | GABA | ||
| Bissonette et al., 2010 [94] | Mice | Appetitive | Genetic | Knockout of Plaur | Foraging reversal | ↓ | OFC | GABA | |
| Bissonette et al., 2015 [95] | Mice | Appetitive | Genetic | Knockout of Plaur | Foraging reversal | ↓ | OFC | GABA | |
| Boulougouris et al., 2009 [105] | Rats | Appetitive | Pharmacological | Administration of D2/D3 receptor agonist | Operant conditioning | ↓ | Dopamine | ||
| Izquierdo et al., 2006 [106] | Mice | Appetitive | Pharmacological | Administration of D1-like agonist | Operant conditioning | ↓ | Dopamine | ||
| Connolly et al., 2014 [107] | Rats | Appetitive | Pharmacological | Administration of D4 receptor antagonist | ASST | ↓, ↑ | - | Glutamate and Dopamine | |
| DeSteno & Schmauss, 2009 [108] | Mice | Appetitive | Pharmacological | Administration of typical antipsychotic D2 receptor blocker haloperidol | ASST | ↓ | ↓ | Dopamine | |
| Kruzich & Grandy, 2004 [109] | Mice | Appetitive | Genetic | Knockout of D2 receptor | Odor discrimination | ↓ | Dopamine | ||
| Kruzich et al., 2006 [110] | Mice | Appetitive | Genetic | Knockout of D2 receptor | Odor discrimination | ↓ | Dopamine | ||
| Morita et al., 2016 [111] | Mice | Appetitive | Genetic | Knockout of D2 long receptor | Operant conditioning | ↓ | Dopamine | ||
| Kellendonk et al., 2006 [112] | Mice | Appetitive | Genetic | Transient overexpression of D2 receptors in striatum | Odor discrimination | ↓ | Dopamine | ||
| Brigman et al., 2010 [119] | Mice | Appetitive | Genetic and Pharmacological | Pharmacological blockade or genetic deletion (either partial or complete) of serotonin transporter 5-HTT | Operant conditioning | ↑ | Serotonin | ||
| Odland et al., 2021 [120] | Mice | Appetitive | Pharmacological | Administration of selective 5-HTT inhibitor fluoxetine | Operant conditioning | ↑ | Serotonin | ||
| Amodeo et al., 2020 [121] | Mice | Appetitive | Pharmacological | Administration of 5-HT2A agonist and/or 5-HT2C antagonist | T-maze | ↓ | Serotonin | ||
| Boulougouris et al., 2008 [122] | Rats | Appetitive | Pharmacological | Administration of 5-HT2A or 5-HT2C antagonists | Operant conditioning | ↓, ↑ | Serotonin | ||
| Boulougouris et al., 2010 [123] | Rats | Appetitive | Pharmacological | Administration of Intra-OFC 5-HT2C receptor antagonism | Operant conditioning | ↑ | OFC | Serotonin | |
| Han et al., 2011 [136] | Rats | Aversive | Environmental | Social isolation | MWM | ↓ | |||
| Lander et al., 2017 [137] | Mice | Aversive | Environmental | Social isolation | Water T-maze | ↓ | ↓ | PFC | |
| Butts et al., 2013 [138] | Rats | Appetitive | Environmental | Stress | Operant conditioning | - | ↓ | ||
| Thai et al., 2013 [139] | Rats | Appetitive | Environmental | Stress | Operant conditioning | ↓ | - | ||
| Goodwill et al., 2018 [140] | Mice | Appetitive | Environmental | Stress | ASST | ↓ | ↓ | OFC and mPFC | GABA |
| Zeleznikow-Johnston et al., 2017 [146] | Mice | Appetitive | Environmental | Environmental enrichment | Operant conditioning | ↑ | |||
| Kikuchi et al., 2022 [147] | Mice | Appetitive | Environmental | Environmental enrichment | Operant conditioning | ↑ | |||
| Sampedro-Piquero et al., 2015 [148] | Mice | Aversive | Environmental | Environmental enrichment | 4-radial arm water maze | ↑ | OFC and mPFC | ||
| Aarde et al., 2021 [151] | Mice | Appetitive | Environmental | Sex differences | Operant conditioning | ↓ | OFC and mPFC |
4. Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lange, F.; Seer, C.; Loens, S.; Wegner, F.; Schrader, C.; Dressler, D.; Dengler, R.; Kopp, B. Neural Mechanisms Underlying Cognitive Inflexibility in Parkinson’s Disease. Neuropsychologia 2016, 93, 142–150. [Google Scholar] [CrossRef]
- Gruner, P.; Pittenger, C. Cognitive Inflexibility in Obsessive-Compulsive Disorder. Neuroscience 2017, 345, 243–255. [Google Scholar] [CrossRef]
- Aumer, P.; Brandt, G.A.; Hirjak, D.; Bähner, F. Impaired Cognitive Flexibility in Schizophrenia: A Systematic Review of Behavioral and Neurobiological Findings. Biomark. Neuropsychiatry 2024, 11, 100111. [Google Scholar] [CrossRef]
- Barch, D.M.; Braver, T.S.; Carter, C.S.; Poldrack, R.A.; Robbins, T.W. CNTRICS Final Task Selection: Executive Control. Schizophr. Bull. 2009, 35, 115–135. [Google Scholar] [CrossRef]
- Lencz, T.; Smith, C.W.; McLaughlin, D.; Auther, A.; Nakayama, E.; Hovey, L.; Cornblatt, B.A. Generalized and Specific Neurocognitive Deficits in Prodromal Schizophrenia. Biol. Psychiatry 2006, 59, 863–871. [Google Scholar] [CrossRef]
- Deary, I.J.; Corley, J.; Gow, A.J.; Harris, S.E.; Houlihan, L.M.; Marioni, R.E.; Penke, L.; Rafnsson, S.B.; Starr, J.M. Age-Associated Cognitive Decline. Br. Med. Bull. 2009, 92, 135–152. [Google Scholar] [CrossRef]
- Jeste, D.V.; Wolkowitz, O.M.; Palmer, B.W. Divergent Trajectories of Physical, Cognitive, and Psychosocial Aging in Schizophrenia. Schizophr. Bull. 2011, 37, 451–455. [Google Scholar] [CrossRef]
- Herold, C.J.; Schmid, L.A.; Lässer, M.M.; Seidl, U.; Schröder, J. Cognitive Performance in Patients with Chronic Schizophrenia Across the Lifespan. GeroPsych 2017, 30, 35–44. [Google Scholar] [CrossRef]
- Finger, E.C.; Marsh, A.A.; Mitchell, D.G.; Reid, M.E.; Sims, C.; Budhani, S.; Kosson, D.S.; Chen, G.; Towbin, K.E.; Leibenluft, E.; et al. Abnormal Ventromedial Prefrontal Cortex Function in Children with Psychopathic Traits During Reversal Learning. Arch. Gen. Psychiatry 2008, 65, 586–594. [Google Scholar] [CrossRef]
- Gilmour, G.; Arguello, A.; Bari, A.; Brown, V.J.; Carter, C.; Floresco, S.B.; Jentsch, D.J.; Tait, D.S.; Young, J.W.; Robbins, T.W. Measuring the Construct of Executive Control in Schizophrenia: Defining and Validating Translational Animal Paradigms for Discovery Research. Neurosci. Biobehav. Rev. 2013, 37, 2125–2140. [Google Scholar] [CrossRef]
- Crider, A. Perseveration in Schizophrenia. Schizophr. Bull. 1997, 23, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.O.; Kruppa, J.A.; Fink, G.R.; Herpertz-Dahlmann, B.; Konrad, K.; Schulte-Rüther, M. Developmental Differences in Probabilistic Reversal Learning: A Computational Modeling Approach. Front. Neurosci. 2021, 14, 536596. [Google Scholar] [CrossRef]
- Miles, S.; Howlett, C.A.; Berryman, C.; Nedeljkovic, M.; Moseley, G.L.; Phillipou, A. Considerations for Using the Wisconsin Card Sorting Test to Assess Cognitive Flexibility. Behav. Res. Methods 2021, 53, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, L.; Molnar, C.; Horner, M.D.; Anderson, B.; Forster, L.; George, M.S.; Nahas, Z. Neurocognitive Deficits and Prefrontal Cortical Atrophy in Patients with Schizophrenia. Schizophr. Res. 2008, 101, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Dempster, K.; Norman, R.; Théberge, J.; Densmore, M.; Schaefer, B.; Williamson, P. Glutamatergic Metabolite Correlations with Neuropsychological Tests in First Episode Schizophrenia. Psychiatry Res. Neuroimaging 2015, 233, 180–185. [Google Scholar] [CrossRef]
- Xu, F.; Xian, Z. Study Investigating Executive Function in Schizophrenia Patients and Their Unaffected Siblings. PLoS ONE 2023, 18, e0285034. [Google Scholar] [CrossRef]
- Waltz, J.A.; Gold, J.M. Probabilistic Reversal Learning Impairments in Schizophrenia: Further Evidence of Orbitofrontal Dysfunction. Schizophr. Res. 2007, 93, 296–303. [Google Scholar] [CrossRef]
- Greenzang, C.; Manoach, D.S.; Goff, D.C.; Barton, J.J.S. Task-Switching in Schizophrenia: Active Switching Costs and Passive Carry-over Effects in an Antisaccade Paradigm. Exp. Brain Res. 2007, 181, 493–502. [Google Scholar] [CrossRef]
- Kieffaber, P.D.; Kappenman, E.S.; Bodkins, M.; Shekhar, A.; O’Donnell, B.F.; Hetrick, W.P. Switch and Maintenance of Task Set in Schizophrenia. Schizophr. Res. 2006, 84, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.F.; Goldberg, T.E.; Kolachana, B.S.; Callicott, J.H.; Mazzanti, C.M.; Straub, R.E.; Goldman, D.; Weinberger, D.R. Effect of COMT Val 108/158 Met Genotype on Frontal Lobe Function and Risk for Schizophrenia. Proc. Natl. Acad. Sci. USA 2001, 98, 6917–6922. [Google Scholar] [CrossRef]
- Hampshire, A.; Owen, A.M. Fractionating Attentional Control Using Event-Related FMRI. Cereb. Cortex 2005, 16, 1679–1689. [Google Scholar] [CrossRef]
- Kim, C.; Johnson, N.F.; Cilles, S.E.; Gold, B.T. Common and Distinct Mechanisms of Cognitive Flexibility in Prefrontal Cortex. J. Neurosci. 2011, 31, 4771–4779. [Google Scholar] [CrossRef]
- Lobellova, V.; Entlerova, M.; Svojanovska, B.; Hatalova, H.; Prokopova, I.; Petrasek, T.; Vales, K.; Kubik, S.; Fajnerova, I.; Stuchlik, A. Two Learning Tasks Provide Evidence for Disrupted Behavioural Flexibility in an Animal Model of Schizophrenia-like Behaviour Induced by Acute MK-801: A Dose–Response Study. Behav. Brain Res. 2013, 246, 55–62. [Google Scholar] [CrossRef]
- Badowska, D.M.; Brzózka, M.M.; Kannaiyan, N.; Thomas, C.; Dibaj, P.; Chowdhury, A.; Steffens, H.; Turck, C.W.; Falkai, P.; Schmitt, A.; et al. Modulation of Cognition and Neuronal Plasticity in Gain- and Loss-of-Function Mouse Models of the Schizophrenia Risk Gene Tcf4. Transl. Psychiatry 2020, 10, 343. [Google Scholar] [CrossRef]
- Asraf, K.; Zaidan, H.; Natoor, B.; Gaisler-Salomon, I. Synergistic, Long-Term Effects of Glutamate Dehydrogenase 1 Deficiency and Mild Stress on Cognitive Function and MPFC Gene and MiRNA Expression. Transl. Psychiatry 2023, 13, 248. [Google Scholar] [CrossRef]
- Karvat, G.; Kimchi, T. Acetylcholine Elevation Relieves Cognitive Rigidity and Social Deficiency in a Mouse Model of Autism. Neuropsychopharmacology 2014, 39, 831–840. [Google Scholar] [CrossRef]
- Ortega, L.A.; Tracy, B.A.; Gould, T.J.; Parikh, V. Effects of Chronic Low- and High-Dose Nicotine on Cognitive Flexibility in C57BL/6J Mice. Behav. Brain Res. 2013, 238, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, L.A.; Frajmund, L.A.; van Nuijs, D.; van der Heijden, D.C.N.; Middeldorp, J.; Hol, E.M. Both Male and Female APPswe/PSEN1dE9 Mice Are Impaired in Spatial Memory and Cognitive Flexibility at 9 Months of Age. Neurobiol. Aging 2022, 113, 28–38. [Google Scholar] [CrossRef] [PubMed]
- van den Boom, B.J.G.; Mooij, A.H.; Misevičiūtė, I.; Denys, D.; Willuhn, I. Behavioral Flexibility in a Mouse Model for Obsessive-compulsive Disorder: Impaired Pavlovian Reversal Learning in SAPAP3 Mutants. Genes Brain Behav. 2019, 18, e12557. [Google Scholar] [CrossRef]
- Morris, R. Developments of a Water-Maze Procedure for Studying Spatial Learning in the Rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Ayalon, L.; Doron, R.; Weiner, I.; Joel, D. Amelioration of Behavioral Deficits in a Rat Model of Huntington’s Disease by an Excitotoxic Lesion to the Globus Pallidus. Exp. Neurol. 2004, 186, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Lander, S.S.; Khan, U.; Lewandowski, N.; Chakraborty, D.; Provenzano, F.A.; Mingote, S.; Chornyy, S.; Frigerio, F.; Maechler, P.; Kaphzan, H.; et al. Glutamate Dehydrogenase–Deficient Mice Display Schizophrenia-Like Behavioral Abnormalities and CA1-Specific Hippocampal Dysfunction. Schizophr. Bull. 2019, 45, 127–137. [Google Scholar] [CrossRef]
- Martínez-Canabal, A.; López-Oropeza, G.; Sotres-Bayón, F. Hippocampal Neurogenesis Facilitates Cognitive Flexibility in a Fear Discrimination Task. Front. Behav. Neurosci. 2024, 17, 1331928. [Google Scholar] [CrossRef]
- Yun, M.; Kim, E.; Jung, M.W. Enhanced Fear Limits Behavioral Flexibility in Shank2-Deficient Mice. Mol. Autism 2022, 13, 40. [Google Scholar] [CrossRef]
- Bali, A.; Jaggi, A.S. Electric Foot Shock Stress: A Useful Tool in Neuropsychiatric Studies. Rev. Neurosci. 2015, 26, 655–677. [Google Scholar] [CrossRef]
- Chambers, K.C. Conditioned Taste Aversions. World J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Bouton, M.E.; Maren, S.; McNally, G.P. Behavioral and Neurobiological Mechanisms of Pavlovian and Instrumental Extinction Learning. Physiol. Rev. 2021, 101, 611–681. [Google Scholar] [CrossRef] [PubMed]
- Dickson, P.E.; Calton, M.A.; Mittleman, G. Performance of C57BL/6J and DBA/2J Mice on a Touchscreen-Based Attentional Set-Shifting Task. Behav. Brain Res. 2014, 261, 158–170. [Google Scholar] [CrossRef]
- Ragozzino, M.E.; Detrick, S.; Kesner, R.P. Involvement of the Prelimbic–Infralimbic Areas of the Rodent Prefrontal Cortex in Behavioral Flexibility for Place and Response Learning. J. Neurosci. 1999, 19, 4585–4594. [Google Scholar] [CrossRef]
- Ragozzino, M.E. The Effects of Dopamine D(1) Receptor Blockade in the Prelimbic-Infralimbic Areas on Behavioral Flexibility. Learn. Mem. 2002, 9, 18–28. [Google Scholar] [CrossRef]
- Cinalli, D.A.; Cohen, S.J.; Calubag, M.; Oz, G.; Zhou, L.; Stackman, R.W. DREADD-Inactivation of Dorsal CA1 Pyramidal Neurons in Mice Impairs Retrieval of Object and Spatial Memories. Hippocampus 2023, 33, 6–17. [Google Scholar] [CrossRef]
- Moser, E.; Moser, M.; Andersen, P. Spatial Learning Impairment Parallels the Magnitude of Dorsal Hippocampal Lesions, but Is Hardly Present Following Ventral Lesions. J. Neurosci. 1993, 13, 3916–3925. [Google Scholar] [CrossRef]
- Moser, M.B.; Moser, E.I.; Forrest, E.; Andersen, P.; Morris, R.G. Spatial Learning with a Minislab in the Dorsal Hippocampus. Proc. Natl. Acad. Sci. USA 1995, 92, 9697–9701. [Google Scholar] [CrossRef] [PubMed]
- D’Hooge, R.; De Deyn, P.P. Applications of the Morris Water Maze in the Study of Learning and Memory. Brain Res. Rev. 2001, 36, 60–90. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, J.P.C.; Sánchez-Santed, F.; Heinsbroek, R.P.W.; Donker, A.; Postmes, P. A Behavioural Analysis of Rats with Damage to the Medial Prefrontal Cortex Using the Morris Water Maze: Evidence for Behavioural Flexibility, but Not for Impaired Spatial Navigation. Brain Res. 1994, 652, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Bissonette, G.B.; Powell, E.M. Reversal Learning and Attentional Set-Shifting in Mice. Neuropharmacology 2012, 62, 1168–1174. [Google Scholar] [CrossRef]
- Marquis, J.-P.; Goulet, S.; Doré, F.Y. Neonatal Ventral Hippocampus Lesions Disrupt Extra-Dimensional Shift and Alter Dendritic Spine Density in the Medial Prefrontal Cortex of Juvenile Rats. Neurobiol. Learn. Mem. 2008, 90, 339–346. [Google Scholar] [CrossRef]
- Newman, L.A.; McGaughy, J. Attentional Effects of Lesions to the Anterior Cingulate Cortex: How Prior Reinforcement Influences Distractibility. Behav. Neurosci. 2011, 125, 360–371. [Google Scholar] [CrossRef]
- Bissonette, G.B.; Martins, G.J.; Franz, T.M.; Harper, E.S.; Schoenbaum, G.; Powell, E.M. Double Dissociation of the Effects of Medial and Orbital Prefrontal Cortical Lesions on Attentional and Affective Shifts in Mice. J. Neurosci. 2008, 28, 11124–11130. [Google Scholar] [CrossRef]
- Boulougouris, V.; Dalley, J.W.; Robbins, T.W. Effects of Orbitofrontal, Infralimbic and Prelimbic Cortical Lesions on Serial Spatial Reversal Learning in the Rat. Behav. Brain Res. 2007, 179, 219–228. [Google Scholar] [CrossRef]
- Ghods-Sharifi, S.; Haluk, D.M.; Floresco, S.B. Differential Effects of Inactivation of the Orbitofrontal Cortex on Strategy Set-Shifting and Reversal Learning. Neurobiol. Learn. Mem. 2008, 89, 567–573. [Google Scholar] [CrossRef]
- Ragozzino, M.E. The Contribution of the Medial Prefrontal Cortex, Orbitofrontal Cortex, and Dorsomedial Striatum to Behavioral Flexibility. Ann. N. Y. Acad. Sci. 2007, 1121, 355–375. [Google Scholar] [CrossRef]
- Chandler, D.J. Evidence for a Specialized Role of the Locus Coeruleus Noradrenergic System in Cortical Circuitries and Behavioral Operations. Brain Res. 2016, 1641, 197–206. [Google Scholar] [CrossRef]
- Janitzky, K.; Lippert, M.T.; Engelhorn, A.; Tegtmeier, J.; Goldschmidt, J.; Heinze, H.-J.; Ohl, F.W. Optogenetic Silencing of Locus Coeruleus Activity in Mice Impairs Cognitive Flexibility in an Attentional Set-Shifting Task. Front. Behav. Neurosci. 2015, 9, 286. [Google Scholar] [CrossRef]
- McGaughy, J.; Ross, R.S.; Eichenbaum, H. Noradrenergic, but Not Cholinergic, Deafferentation of Prefrontal Cortex Impairs Attentional Set-Shifting. Neuroscience 2008, 153, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Luscher, C.; Malenka, R.C. NMDA Receptor-Dependent Long-Term Potentiation and Long-Term Depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4, a005710. [Google Scholar] [CrossRef] [PubMed]
- Uno, Y.; Coyle, J.T. Glutamate Hypothesis in Schizophrenia. Psychiatry Clin. Neurosci. 2019, 73, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Bhat, A.A.; Masoodi, T.; Hashem, S.; Akhtar, S.; Ali, T.A.; Amjad, S.; Chawla, S.; Bagga, P.; Frenneaux, M.P.; et al. Genetics of Glutamate and Its Receptors in Autism Spectrum Disorder. Mol. Psychiatry 2022, 27, 2380–2392. [Google Scholar] [CrossRef]
- Bardgett, M.E.; Boeckman, R.; Krochmal, D.; Fernando, H.; Ahrens, R.; Csernansky, J.G. NMDA Receptor Blockade and Hippocampal Neuronal Loss Impair Fear Conditioning and Position Habit Reversal in C57Bl/6 Mice. Brain Res. Bull. 2003, 60, 131–142. [Google Scholar] [CrossRef]
- Chadman, K.K.; Watson, D.J.; Stanton, M.E. NMDA Receptor Antagonism Impairs Reversal Learning in Developing Rats. Behav. Neurosci. 2006, 120, 1071–1083. [Google Scholar] [CrossRef]
- McLean, S.L.; Neill, J.C.; Idris, N.F.; Marston, H.M.; Wong, E.H.F.; Shahid, M. Effects of Asenapine, Olanzapine, and Risperidone on Psychotomimetic-Induced Reversal-Learning Deficits in the Rat. Behav. Brain Res. 2010, 214, 240–247. [Google Scholar] [CrossRef]
- Abdul-Monim, Z.; Reynolds, G.P.; Neill, J.C. The Effect of Atypical and Classical Antipsychotics on Sub-Chronic PCP-Induced Cognitive Deficits in a Reversal-Learning Paradigm. Behav. Brain Res. 2006, 169, 263–273. [Google Scholar] [CrossRef]
- Patrono, E.; Hrůzova, K.; Svoboda, J.; Stuchlík, A. The Role of Optogenetic Stimulations of Parvalbumin-Positive Interneurons in the Prefrontal Cortex and the Ventral Hippocampus on an Acute MK-801 Model of Schizophrenia-like Cognitive Inflexibility. Schizophr. Res. 2023, 252, 198–205. [Google Scholar] [CrossRef]
- Li, J.-T.; Su, Y.-A.; Wang, H.-L.; Zhao, Y.-Y.; Liao, X.-M.; Wang, X.-D.; Si, T.-M. Repeated Blockade of NMDA Receptors During Adolescence Impairs Reversal Learning and Disrupts GABAergic Interneurons in Rat Medial Prefrontal Cortex. Front. Mol. Neurosci. 2016, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Thonnard, D.; Dreesen, E.; Callaerts-Vegh, Z.; D’Hooge, R. NMDA Receptor Dependence of Reversal Learning and the Flexible Use of Cognitively Demanding Search Strategies in Mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 90, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.J.; Stanton, M.E. Medial Prefrontal Administration of MK-801 Impairs T-Maze Discrimination Reversal Learning in Weanling Rats. Behav. Brain Res. 2009, 205, 57–66. [Google Scholar] [CrossRef]
- Dong, Z.; Bai, Y.; Wu, X.; Li, H.; Gong, B.; Howland, J.G.; Huang, Y.; He, W.; Li, T.; Wang, Y.T. Hippocampal Long-Term Depression Mediates Spatial Reversal Learning in the Morris Water Maze. Neuropharmacology 2013, 64, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.; Labrie, V.; Roder, J.C. D-Serine Augments NMDA-NR2B Receptor-Dependent Hippocampal Long-Term Depression and Spatial Reversal Learning. Neuropsychopharmacology 2008, 33, 1004–1018. [Google Scholar] [CrossRef]
- Stefani, M.R.; Groth, K.; Moghaddam, B. Glutamate Receptors in the Rat Medial Prefrontal Cortex Regulate Set-Shifting Ability. Behav. Neurosci. 2003, 117, 728–737. [Google Scholar] [CrossRef]
- Lipina, T.; Men, X.; Blundell, M.; Salahpour, A.; Ramsey, A.J. Abnormal Sensory Perception Masks Behavioral Performance of Grin1 Knockdown Mice. Genes Brain Behav. 2022, 21, e12825. [Google Scholar] [CrossRef]
- Sanderson, D.J.; Bannerman, D.M. The Role of Habituation in Hippocampus-dependent Spatial Working Memory Tasks: Evidence from GluA1 AMPA Receptor Subunit Knockout Mice. Hippocampus 2012, 22, 981–994. [Google Scholar] [CrossRef]
- Darvas, M.; Palmiter, R.D. Specific Contributions of N-Methyl-d-Aspartate Receptors in the Dorsal Striatum to Cognitive Flexibility. Neuroscience 2015, 284, 934–942. [Google Scholar] [CrossRef]
- Marquardt, K.; Saha, M.; Mishina, M.; Young, J.W.; Brigman, J.L. Loss of GluN2A-containing NMDA Receptors Impairs Extra-dimensional Set-shifting. Genes Brain Behav. 2014, 13, 611–617. [Google Scholar] [CrossRef]
- Brigman, J.L.; Daut, R.A.; Wright, T.; Gunduz-Cinar, O.; Graybeal, C.; Davis, M.I.; Jiang, Z.; Saksida, L.M.; Jinde, S.; Pease, M.; et al. GluN2B in Corticostriatal Circuits Governs Choice Learning and Choice Shifting. Nat. Neurosci. 2013, 16, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Labrie, V.; Duffy, S.; Wang, W.; Barger, S.W.; Baker, G.B.; Roder, J.C. Genetic Inactivation of D-Amino Acid Oxidase Enhances Extinction and Reversal Learning in Mice. Learn. Mem. 2009, 16, 28–37. [Google Scholar] [CrossRef]
- Esterlis, I.; Holmes, S.E.; Sharma, P.; Krystal, J.H.; DeLorenzo, C. Metabotropic Glutamatergic Receptor 5 and Stress Disorders: Knowledge Gained From Receptor Imaging Studies. Biol. Psychiatry 2018, 84, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Jong, Y.-J.I.; O’Malley, K.L. Mechanisms Associated with Activation of Intracellular Metabotropic Glutamate Receptor, MGluR5. Neurochem. Res. 2017, 42, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Zeleznikow-Johnston, A.M.; Renoir, T.; Churilov, L.; Li, S.; Burrows, E.L.; Hannan, A.J. Touchscreen Testing Reveals Clinically Relevant Cognitive Abnormalities in a Mouse Model of Schizophrenia Lacking Metabotropic Glutamate Receptor 5. Sci. Rep. 2018, 8, 16412. [Google Scholar] [CrossRef]
- Lim, J.; Kim, E.; Noh, H.J.; Kang, S.; Phillips, B.U.; Kim, D.G.; Bussey, T.J.; Saksida, L.; Heath, C.J.; Kim, C.H. Assessment of MGluR5 KO Mice under Conditions of Low Stress Using a Rodent Touchscreen Apparatus Reveals Impaired Behavioural Flexibility Driven by Perseverative Responses. Mol. Brain 2019, 12, 37. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.; Kraniotis, S.; He, Q.; Marshall, J.J.; Nomura, T.; Stauffer, S.R.; Lindsley, C.W.; Conn, P.J.; Contractor, A. Potentiating MGluR5 Function with a Positive Allosteric Modulator Enhances Adaptive Learning. Learn. Mem. 2013, 20, 438–445. [Google Scholar] [CrossRef]
- Gastambide, F.; Cotel, M.-C.; Gilmour, G.; O’Neill, M.J.; Robbins, T.W.; Tricklebank, M.D. Selective Remediation of Reversal Learning Deficits in the Neurodevelopmental MAM Model of Schizophrenia by a Novel MGlu5 Positive Allosteric Modulator. Neuropsychopharmacology 2012, 37, 1057–1066. [Google Scholar] [CrossRef]
- Joffe, M.E.; Santiago, C.I.; Stansley, B.J.; Maksymetz, J.; Gogliotti, R.G.; Engers, J.L.; Nicoletti, F.; Lindsley, C.W.; Conn, P.J. Mechanisms Underlying Prelimbic Prefrontal Cortex MGlu3/MGlu5-Dependent Plasticity and Reversal Learning Deficits Following Acute Stress. Neuropharmacology 2019, 144, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Sherry, D.M.; Liu, X.; Fremeau, R.T.; Seal, R.P.; Edwards, R.H.; Copenhagen, D.R. Vesicular Glutamate Transporter 3 Expression Identifies Glutamatergic Amacrine Cells in the Rodent Retina. J. Comp. Neurol. 2004, 477, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Vigneault, É.; Poirel, O.; Riad, M.; Prud’homme, J.; Dumas, S.; Turecki, G.; Fasano, C.; Mechawar, N.; El Mestikawy, S. Distribution of Vesicular Glutamate Transporters in the Human Brain. Front. Neuroanat. 2015, 9, 23. [Google Scholar] [CrossRef]
- Balschun, D.; Moechars, D.; Callaerts-Vegh, Z.; Vermaercke, B.; Van Acker, N.; Andries, L.; D’Hooge, R. Vesicular Glutamate Transporter VGLUT1 Has a Role in Hippocampal Long-Term Potentiation and Spatial Reversal Learning. Cereb. Cortex 2010, 20, 684–693. [Google Scholar] [CrossRef]
- Granseth, B.; Andersson, F.K.; Lindström, S.H. The Initial Stage of Reversal Learning Is Impaired in Mice Hemizygous for the Vesicular Glutamate Transporter (VGluT1). Genes Brain Behav. 2015, 14, 477–485. [Google Scholar] [CrossRef]
- Lander, S.S.; Chornyy, S.; Safory, H.; Gross, A.; Wolosker, H.; Gaisler-Salomon, I. Glutamate Dehydrogenase Deficiency Disrupts Glutamate Homeostasis in Hippocampus and Prefrontal Cortex and Impairs Recognition Memory. Genes Brain Behav. 2020, 19, e12636. [Google Scholar] [CrossRef]
- Morellini, F.; Sivukhina, E.; Stoenica, L.; Oulianova, E.; Bukalo, O.; Jakovcevski, I.; Dityatev, A.; Irintchev, A.; Schachner, M. Improved Reversal Learning and Working Memory and Enhanced Reactivity to Novelty in Mice with Enhanced GABAergic Innervation in the Dentate Gyrus. Cereb. Cortex 2010, 20, 2712–2727. [Google Scholar] [CrossRef]
- Barnes, S.A.; Dillon, D.G.; Young, J.W.; Thomas, M.L.; Faget, L.; Yoo, J.H.; Der-Avakian, A.; Hnasko, T.S.; Geyer, M.A.; Ramanathan, D.S. Modulation of Ventromedial Orbitofrontal Cortical Glutamatergic Activity Affects the Explore-Exploit Balance and Influences Value-Based Decision-Making. Cereb. Cortex 2023, 33, 5783–5796. [Google Scholar] [CrossRef] [PubMed]
- Rüsch, N.; Tebartz van Elst, L.; Valerius, G.; Büchert, M.; Thiel, T.; Ebert, D.; Hennig, J.; Olbrich, H.-M. Neurochemical and Structural Correlates of Executive Dysfunction in Schizophrenia. Schizophr. Res. 2008, 99, 155–163. [Google Scholar] [CrossRef]
- Shirayama, Y.; Obata, T.; Matsuzawa, D.; Nonaka, H.; Kanazawa, Y.; Yoshitome, E.; Ikehira, H.; Hashimoto, K.; Iyo, M. Specific Metabolites in the Medial Prefrontal Cortex Are Associated with the Neurocognitive Deficits in Schizophrenia: A Preliminary Study. Neuroimage 2010, 49, 2783–2790. [Google Scholar] [CrossRef]
- Stock, A.-K.; Werner, A.; Kuntke, P.; Petasch, M.-S.; Bensmann, W.; Zink, N.; Koyun, A.H.; Quednow, B.B.; Beste, C. Gamma-Aminobutyric Acid and Glutamate Concentrations in the Striatum and Anterior Cingulate Cortex Not Found to Be Associated with Cognitive Flexibility. Brain Sci. 2023, 13, 1192. [Google Scholar] [CrossRef]
- Rajagopal, L.; Huang, M.; Michael, E.; Kwon, S.; Meltzer, H.Y. TPA-023 Attenuates Subchronic Phencyclidine-Induced Declarative and Reversal Learning Deficits via GABAA Receptor Agonist Mechanism: Possible Therapeutic Target for Cognitive Deficit in Schizophrenia. Neuropsychopharmacology 2018, 43, 2468–2477. [Google Scholar] [CrossRef]
- Bissonette, G.B.; Bae, M.H.; Suresh, T.; Jaffe, D.E.; Powell, E.M. Astrocyte-Mediated Hepatocyte Growth Factor/Scatter Factor Supplementation Restores GABAergic Interneurons and Corrects Reversal Learning Deficits in Mice. J. Neurosci. 2010, 30, 2918–2923. [Google Scholar] [CrossRef] [PubMed]
- Bissonette, G.B.; Schoenbaum, G.; Roesch, M.R.; Powell, E.M. Interneurons Are Necessary for Coordinated Activity During Reversal Learning in Orbitofrontal Cortex. Biol. Psychiatry 2015, 77, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Brigman, J.L.; Padukiewicz, K.E.; Sutherland, M.L.; Rothblat, L.A. Executive Functions in the Heterozygous Reeler Mouse Model of Schizophrenia. Behav. Neurosci. 2006, 120, 984–988. [Google Scholar] [CrossRef]
- Hausrat, T.J.; Muhia, M.; Gerrow, K.; Thomas, P.; Hirdes, W.; Tsukita, S.; Heisler, F.F.; Herich, L.; Dubroqua, S.; Breiden, P.; et al. Radixin Regulates Synaptic GABAA Receptor Density and Is Essential for Reversal Learning and Short-Term Memory. Nat. Commun. 2015, 6, 6872. [Google Scholar] [CrossRef]
- Tse, M.T.; Piantadosi, P.T.; Floresco, S.B. Prefrontal Cortical Gamma-Aminobutyric Acid Transmission and Cognitive Function: Drawing Links to Schizophrenia from Preclinical Research. Biol. Psychiatry 2015, 77, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Yoshimura, R.; Kakeda, S.; Moriya, J.; Hayashi, K.; Ikenouchi-Sugita, A.; Umene-Nakano, W.; Hori, H.; Ueda, N.; Korogi, Y.; et al. Associations between Plasma Levels of 3-methoxy-4-hydroxyphenylglycol (MHPG) and Negative Symptoms or Cognitive Impairments in Early-stage Schizophrenia. Hum. Psychopharmacol. Clin. Exp. 2009, 24, 639–645. [Google Scholar] [CrossRef]
- Ayano, G. Dopamine: Receptors, Functions, Synthesis, Pathways, Locations and Mental Disorders: Review of Literatures. J. Ment. Disord. Treat. 2016, 2, 2. [Google Scholar] [CrossRef]
- Uhl, G.R. Dopamine Transporter: Basic Science and Human Variation of a Key Molecule for Dopaminergic Function, Locomotion, and Parkinsonism. Mov. Disord. 2003, 18, S71–S80. [Google Scholar] [CrossRef] [PubMed]
- Curtin, D.; Taylor, E.M.; Bellgrove, M.A.; Chong, T.T.-J.; Coxon, J.P. Dopamine D2 Receptor Modulates Exercise Related Effect on Cortical Excitation/Inhibition and Motor Skill Acquisition. J. Neurosci. 2024, 44, e2028232024. [Google Scholar] [CrossRef]
- Di Domenico, D.; Mapelli, L. Dopaminergic Modulation of Prefrontal Cortex Inhibition. Biomedicines 2023, 11, 1276. [Google Scholar] [CrossRef]
- Curtin, D.; Taylor, E.M.; Bellgrove, M.A.; Chong, T.T.-J.; Coxon, J.P. D2 Receptor Blockade Eliminates Exercise-Induced Changes in Cortical Inhibition and Excitation. Brain Stimul. 2023, 16, 727–733. [Google Scholar] [CrossRef]
- Boulougouris, V.; Castañé, A.; Robbins, T.W. Dopamine D2/D3 Receptor Agonist Quinpirole Impairs Spatial Reversal Learning in Rats: Investigation of D3 Receptor Involvement in Persistent Behavior. Psychopharmacology 2009, 202, 611–620. [Google Scholar] [CrossRef]
- Izquierdo, A.; Wiedholz, L.; Millstein, R.; Yang, R.; Bussey, T.; Saksida, L.; Holmes, A. Genetic and Dopaminergic Modulation of Reversal Learning in a Touchscreen-Based Operant Procedure for Mice. Behav. Brain Res. 2006, 171, 181–188. [Google Scholar] [CrossRef]
- Connolly, N.P.; Gomez-Serrano, M. D4 Dopamine Receptor-Specific Antagonist Improves Reversal Learning Impairment in Amphetamine-Treated Male Rats. Exp. Clin. Psychopharmacol. 2014, 22, 557–564. [Google Scholar] [CrossRef] [PubMed]
- DeSteno, D.A.; Schmauss, C. A Role for Dopamine D2 Receptors in Reversal Learning. Neuroscience 2009, 162, 118–127. [Google Scholar] [CrossRef]
- Kruzich, P.J.; Grandy, D.K. Dopamine D2receptors Mediate Two-Odor Discrimination and Reversal Learning in C57BL/6 Mice. BMC Neurosci. 2004, 5, 12. [Google Scholar] [CrossRef][Green Version]
- Kruzich, P.J.; Mitchell, S.H.; Younkin, A.; Grandy, D.K. Dopamine D2 Receptors Mediate Reversal Learning in Male C57BL/6J Mice. Cogn. Affect. Behav. Neurosci. 2006, 6, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Wang, Y.; Sasaoka, T.; Okada, K.; Niwa, M.; Sawa, A.; Hikida, T. Dopamine D2L Receptor Is Required for Visual Discrimination and Reversal Learning. Mol. Neuropsychiatry 2016, 2, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Kellendonk, C.; Simpson, E.H.; Polan, H.J.; Malleret, G.; Vronskaya, S.; Winiger, V.; Moore, H.; Kandel, E.R. Transient and Selective Overexpression of Dopamine D2 Receptors in the Striatum Causes Persistent Abnormalities in Prefrontal Cortex Functioning. Neuron 2006, 49, 603–615. [Google Scholar] [CrossRef]
- Veselinović, T.; Vernaleken, I.; Janouschek, H.; Cumming, P.; Paulzen, M.; Mottaghy, F.M.; Gründer, G. The Role of Striatal Dopamine D2/3 Receptors in Cognitive Performance in Drug-Free Patients with Schizophrenia. Psychopharmacology 2018, 235, 2221–2232. [Google Scholar] [CrossRef]
- Nelson, C.L.M.; Amsbaugh, H.M.; Reilly, J.L.; Rosen, C.; Marvin, R.W.; Ragozzino, M.E.; Bishop, J.R.; Sweeney, J.A.; Hill, S.K. Beneficial and Adverse Effects of Antipsychotic Medication on Cognitive Flexibility Are Related to COMT Genotype in First Episode Psychosis. Schizophr. Res. 2018, 202, 212–216. [Google Scholar] [CrossRef]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A Review. J. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Spies, M.; Knudsen, G.M.; Lanzenberger, R.; Kasper, S. The Serotonin Transporter in Psychiatric Disorders: Insights from PET Imaging. Lancet Psychiatry 2015, 2, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.-J.; Xue, Y.-R.; Shao, H.; Wei, C.; Liu, T.; He, J.; Yang, Y.-H.; Wang, H.-M.; Li, N.; Ren, S.-Q.; et al. Hippocampal Excitation-Inhibition Balance Underlies the 5-HT2C Receptor in Modulating Depressive Behaviours. Brain 2024, 147, 3764–3779. [Google Scholar] [CrossRef]
- Carlos-Lima, E.; Higa, G.S.V.; Viana, F.J.C.; Tamais, A.M.; Cruvinel, E.; Borges, F.d.S.; Francis-Oliveira, J.; Ulrich, H.; De Pasquale, R. Serotonergic Modulation of the Excitation/Inhibition Balance in the Visual Cortex. Int. J. Mol. Sci. 2023, 25, 519. [Google Scholar] [CrossRef]
- Brigman, J.L.; Mathur, P.; Harvey-White, J.; Izquierdo, A.; Saksida, L.M.; Bussey, T.J.; Fox, S.; Deneris, E.; Murphy, D.L.; Holmes, A. Pharmacological or Genetic Inactivation of the Serotonin Transporter Improves Reversal Learning in Mice. Cereb. Cortex 2010, 20, 1955–1963. [Google Scholar] [CrossRef]
- Odland, A.U.; Sandahl, R.; Andreasen, J.T. Sequential Reversal Learning: A New Touchscreen Schedule for Assessing Cognitive Flexibility in Mice. Psychopharmacology 2021, 238, 383–397. [Google Scholar] [CrossRef]
- Amodeo, D.A.; Hassan, O.; Klein, L.; Halberstadt, A.L.; Powell, S.B. Acute Serotonin 2A Receptor Activation Impairs Behavioral Flexibility in Mice. Behav. Brain Res. 2020, 395, 112861. [Google Scholar] [CrossRef]
- Boulougouris, V.; Glennon, J.C.; Robbins, T.W. Dissociable Effects of Selective 5-HT2A and 5-HT2C Receptor Antagonists on Serial Spatial Reversal Learning in Rats. Neuropsychopharmacology 2008, 33, 2007–2019. [Google Scholar] [CrossRef]
- Boulougouris, V.; Robbins, T.W. Enhancement of Spatial Reversal Learning by 5-HT2C Receptor Antagonism Is Neuroanatomically Specific. J. Neurosci. 2010, 30, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Üçok, A.; Alpsan, H.; Çakır, S.; Saruhan-Direskeneli, G. Association of a Serotonin Receptor 2A Gene Polymorphism with Cognitive Functions in Patients with Schizophrenia. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2007, 144B, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Bosia, M.; Anselmetti, S.; Pirovano, A.; Ermoli, E.; Marino, E.; Bramanti, P.; Smeraldi, E.; Cavallaro, R. HTTLPR Functional Polymorphism in Schizophrenia: Executive Functions vs. Sustained Attention Dissociation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 81–85. [Google Scholar] [CrossRef]
- Gajardo, I.; Salazar, C.S.; Lopez-Espíndola, D.; Estay, C.; Flores-Muñoz, C.; Elgueta, C.; Gonzalez-Jamett, A.M.; Martínez, A.D.; Muñoz, P.; Ardiles, Á.O. Lack of Pannexin 1 Alters Synaptic GluN2 Subunit Composition and Spatial Reversal Learning in Mice. Front. Mol. Neurosci. 2018, 11, 114. [Google Scholar] [CrossRef]
- Privitera, L.; Hogg, E.L.; Gaestel, M.; Wall, M.J.; Corrêa, S.A.L. The MK2 Cascade Regulates MGluR-Dependent Synaptic Plasticity and Reversal Learning. Neuropharmacology 2019, 155, 121–130. [Google Scholar] [CrossRef]
- D’Adamo, P.; Wolfer, D.P.; Kopp, C.; Tobler, I.; Toniolo, D.; Lipp, H.-P. Mice Deficient for the Synaptic Vesicle Protein Rab3a Show Impaired Spatial Reversal Learning and Increased Explorative Activity but None of the Behavioral Changes Shown by Mice Deficient for the Rab3a Regulator Gdi1. Eur. J. Neurosci. 2004, 19, 1895–1905. [Google Scholar] [CrossRef]
- Tsetsenis, T.; Younts, T.J.; Chiu, C.Q.; Kaeser, P.S.; Castillo, P.E.; Südhof, T.C. Rab3B Protein Is Required for Long-Term Depression of Hippocampal Inhibitory Synapses and for Normal Reversal Learning. Proc. Natl. Acad. Sci. USA 2011, 108, 14300–14305. [Google Scholar] [CrossRef]
- Egerton, A.; Brett, R.R.; Pratt, J.A. Acute Δ9-Tetrahydrocannabinol-Induced Deficits in Reversal Learning: Neural Correlates of Affective Inflexibility. Neuropsychopharmacology 2005, 30, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Sokolic, L.; Long, L.E.; Hunt, G.E.; Arnold, J.C.; McGregor, I.S. Disruptive Effects of the Prototypical Cannabinoid Δ9-Tetrahydrocannabinol and the Fatty Acid Amide Inhibitor URB-597 on Go/No-Go Auditory Discrimination Performance and Olfactory Reversal Learning in Rats. Behav. Pharmacol. 2011, 22, 191–202. [Google Scholar] [CrossRef]
- Seeger, T.; Fedorova, I.; Zheng, F.; Miyakawa, T.; Koustova, E.; Gomeza, J.; Basile, A.S.; Alzheimer, C.; Wess, J. M2 Muscarinic Acetylcholine Receptor Knock-Out Mice Show Deficits in Behavioral Flexibility, Working Memory, and Hippocampal Plasticity. J. Neurosci. 2004, 24, 10117–10127. [Google Scholar] [CrossRef] [PubMed]
- De Castro, B.M.; Pereira, G.S.; Magalhães, V.; Rossato, J.I.; De Jaeger, X.; Martins-Silva, C.; Leles, B.; Lima, P.; Gomez, M.V.; Gainetdinov, R.R.; et al. Reduced Expression of the Vesicular Acetylcholine Transporter Causes Learning Deficits in Mice. Genes Brain Behav. 2009, 8, 23–35. [Google Scholar] [CrossRef]
- Rissman, E.F.; Heck, A.L.; Leonard, J.E.; Shupnik, M.A.; Gustafsson, J.-. Å. Disruption of Estrogen Receptor β Gene Impairs Spatial Learning in Female Mice. Proc. Natl. Acad. Sci. USA 2002, 99, 3996–4001. [Google Scholar] [CrossRef]
- Frye, C.A.; Rhodes, M.E.; Dudek, B. Estradiol to Aged Female or Male Mice Improves Learning in Inhibitory Avoidance and Water Maze Tasks. Brain Res. 2005, 1036, 101–108. [Google Scholar] [CrossRef]
- Han, X.; Wang, W.; Xue, X.; Shao, F.; Li, N. Brief Social Isolation in Early Adolescence Affects Reversal Learning and Forebrain BDNF Expression in Adult Rats. Brain Res. Bull. 2011, 86, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Lander, S.S.; Linder-Shacham, D.; Gaisler-Salomon, I. Differential Effects of Social Isolation in Adolescent and Adult Mice on Behavior and Cortical Gene Expression. Behav. Brain Res. 2017, 316, 245–254. [Google Scholar] [CrossRef]
- Butts, K.A.; Floresco, S.B.; Phillips, A.G. Acute Stress Impairs Set-Shifting but Not Reversal Learning. Behav. Brain Res. 2013, 252, 222–229. [Google Scholar] [CrossRef]
- Thai, C.A.; Zhang, Y.; Howland, J.G. Effects of Acute Restraint Stress on Set-Shifting and Reversal Learning in Male Rats. Cogn. Affect. Behav. Neurosci. 2013, 13, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Goodwill, H.L.; Manzano-Nieves, G.; LaChance, P.; Teramoto, S.; Lin, S.; Lopez, C.; Stevenson, R.J.; Theyel, B.B.; Moore, C.I.; Connors, B.W.; et al. Early Life Stress Drives Sex-Selective Impairment in Reversal Learning by Affecting Parvalbumin Interneurons in Orbitofrontal Cortex of Mice. Cell Rep. 2018, 25, 2299–2307.e4. [Google Scholar] [CrossRef]
- Munarriz-Cuezva, E.; Meana, J.J. Poly (I:C)-induced Maternal Immune Activation Generates Impairment of Reversal Learning Performance in Offspring. J. Neurochem. 2025, 169, e16212. [Google Scholar] [CrossRef]
- Amodeo, D.A.; Lai, C.-Y.; Hassan, O.; Mukamel, E.A.; Behrens, M.M.; Powell, S.B. Maternal Immune Activation Impairs Cognitive Flexibility and Alters Transcription in Frontal Cortex. Neurobiol. Dis. 2019, 125, 211–218. [Google Scholar] [CrossRef]
- Nakagawa, K.; Yoshino, H.; Ogawa, Y.; Yamamuro, K.; Kimoto, S.; Noriyama, Y.; Makinodan, M.; Yamashita, M.; Saito, Y.; Kishimoto, T. Maternal Immune Activation Affects Hippocampal Excitatory and Inhibitory Synaptic Transmission in Offspring from an Early Developmental Period to Adulthood. Front. Cell. Neurosci. 2020, 14, 241. [Google Scholar] [CrossRef]
- Su, Y.; Lian, J.; Hodgson, J.; Zhang, W.; Deng, C. Prenatal Poly I:C Challenge Affects Behaviors and Neurotransmission via Elevated Neuroinflammation Responses in Female Juvenile Rats. Int. J. Neuropsychopharmacol. 2022, 25, 160–171. [Google Scholar] [CrossRef]
- Santoni, M.; Frau, R.; Pistis, M. Transgenerational Sex-Dependent Disruption of Dopamine Function Induced by Maternal Immune Activation. Front. Pharmacol. 2022, 13, 821498. [Google Scholar] [CrossRef]
- Zeleznikow-Johnston, A.; Burrows, E.L.; Renoir, T.; Hannan, A.J. Environmental Enrichment Enhances Cognitive Flexibility in C57BL/6 Mice on a Touchscreen Reversal Learning Task. Neuropharmacology 2017, 117, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Yoneda, M.; Nishikawa, K.; Noda, T.; Hasegawa, H.; Fujisaku, T.; Ohno-Shosaku, T. Effects of Environmental Enrichment on Exploratory Behavior, Win-Stay and Lose-Shift Performance, Motor Sequence Learning, and Reversal Learning during the Three-Lever Operant Task in Mice. Behav. Brain Res. 2022, 429, 113904. [Google Scholar] [CrossRef] [PubMed]
- Sampedro-Piquero, P.; Zancada-Menendez, C.; Begega, A. Housing Condition-Related Changes Involved in Reversal Learning and Its c-Fos Associated Activity in the Prefrontal Cortex. Neuroscience 2015, 307, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Meyer, U. Maternal Immune Activation and Neuropsychiatric Illness: A Translational Research Perspective. Am. J. Psychiatry 2018, 175, 1073–1083. [Google Scholar] [CrossRef]
- Socrates, A.J.; Mullins, N.; Gur, R.C.; Gur, R.E.; Stahl, E.; O’Reilly, P.F.; Reichenberg, A.; Jones, H.; Zammit, S.; Velthorst, E. Polygenic Risk of Social Isolation Behavior and Its Influence on Psychopathology and Personality. Mol. Psychiatry 2024, 29, 3599–3606. [Google Scholar] [CrossRef]
- Aarde, S.M.; Genner, R.M.; Hrncir, H.; Arnold, A.P.; Jentsch, J.D. Sex Chromosome Complement Affects Multiple Aspects of Reversal-learning Task Performance in Mice. Genes Brain Behav. 2021, 20, e12685. [Google Scholar] [CrossRef] [PubMed]
- Shields, G.S.; Trainor, B.C.; Lam, J.C.W.; Yonelinas, A.P. Acute Stress Impairs Cognitive Flexibility in Men, Not Women. Stress 2016, 19, 542–546. [Google Scholar] [CrossRef]
- DeCasien, A.R.; Guma, E.; Liu, S.; Raznahan, A. Sex Differences in the Human Brain: A Roadmap for More Careful Analysis and Interpretation of a Biological Reality. Biol. Sex Differ. 2022, 13, 43. [Google Scholar] [CrossRef]
- Premachandran, H.; Zhao, M.; Arruda-Carvalho, M. Sex Differences in the Development of the Rodent Corticolimbic System. Front. Neurosci. 2020, 14, 583477. [Google Scholar] [CrossRef]
- McEwen, B.S.; Milner, T.A. Understanding the Broad Influence of Sex Hormones and Sex Differences in the Brain. J. Neurosci. Res. 2017, 95, 24–39. [Google Scholar] [CrossRef]
- Becker, J.B.; Chartoff, E. Sex Differences in Neural Mechanisms Mediating Reward and Addiction. Neuropsychopharmacology 2019, 44, 166–183. [Google Scholar] [CrossRef]
- Ortona, E.; Pierdominici, M.; Maselli, A.; Veroni, C.; Aloisi, F.; Shoenfeld, Y. Sex-Based Differences in Autoimmune Diseases. Ann. Dell’istituto Super. Di Sanita 2016, 52, 205–212. [Google Scholar] [CrossRef]
- Bangasser, D.A.; Eck, S.R.; Ordoñes Sanchez, E. Sex Differences in Stress Reactivity in Arousal and Attention Systems. Neuropsychopharmacology 2019, 44, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, E.V.; Seo, D.; Sinha, R. Sex Differences in Neural Stress Responses and Correlation with Subjective Stress and Stress Regulation. Neurobiol. Stress 2019, 11, 100177. [Google Scholar] [CrossRef] [PubMed]
- Shansky, R.M. Sex Differences in Behavioral Strategies: Avoiding Interpretational Pitfalls. Curr. Opin. Neurobiol. 2018, 49, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Brandner, C. Strategy Selection during Exploratory Behavior: Sex Differences. Judgm. Decis. Mak. 2007, 2, 326–332. [Google Scholar] [CrossRef]
- Abel, K.M.; Drake, R.; Goldstein, J.M. Sex Differences in Schizophrenia. Int. Rev. Psychiatry 2010, 22, 417–428. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asraf, K.; Gaisler-Salomon, I. Valence-Driven Cognitive Flexibility: Neurochemical and Circuit-Level Insights from Animal Models and Their Relevance to Schizophrenia. Biomolecules 2025, 15, 1154. https://doi.org/10.3390/biom15081154
Asraf K, Gaisler-Salomon I. Valence-Driven Cognitive Flexibility: Neurochemical and Circuit-Level Insights from Animal Models and Their Relevance to Schizophrenia. Biomolecules. 2025; 15(8):1154. https://doi.org/10.3390/biom15081154
Chicago/Turabian StyleAsraf, Kfir, and Inna Gaisler-Salomon. 2025. "Valence-Driven Cognitive Flexibility: Neurochemical and Circuit-Level Insights from Animal Models and Their Relevance to Schizophrenia" Biomolecules 15, no. 8: 1154. https://doi.org/10.3390/biom15081154
APA StyleAsraf, K., & Gaisler-Salomon, I. (2025). Valence-Driven Cognitive Flexibility: Neurochemical and Circuit-Level Insights from Animal Models and Their Relevance to Schizophrenia. Biomolecules, 15(8), 1154. https://doi.org/10.3390/biom15081154







