Aptamer-Coated PLGA Nanoparticles Selectively Internalize into Epithelial Ovarian Cancer Cells In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Fluorescein Diacetate-Loaded Nanoparticles
2.3. ICG, Paclitaxel, and Paclitaxel-Loaded Nanoparticles
2.4. Aptamer Labeling of PLGA Nanoparticles
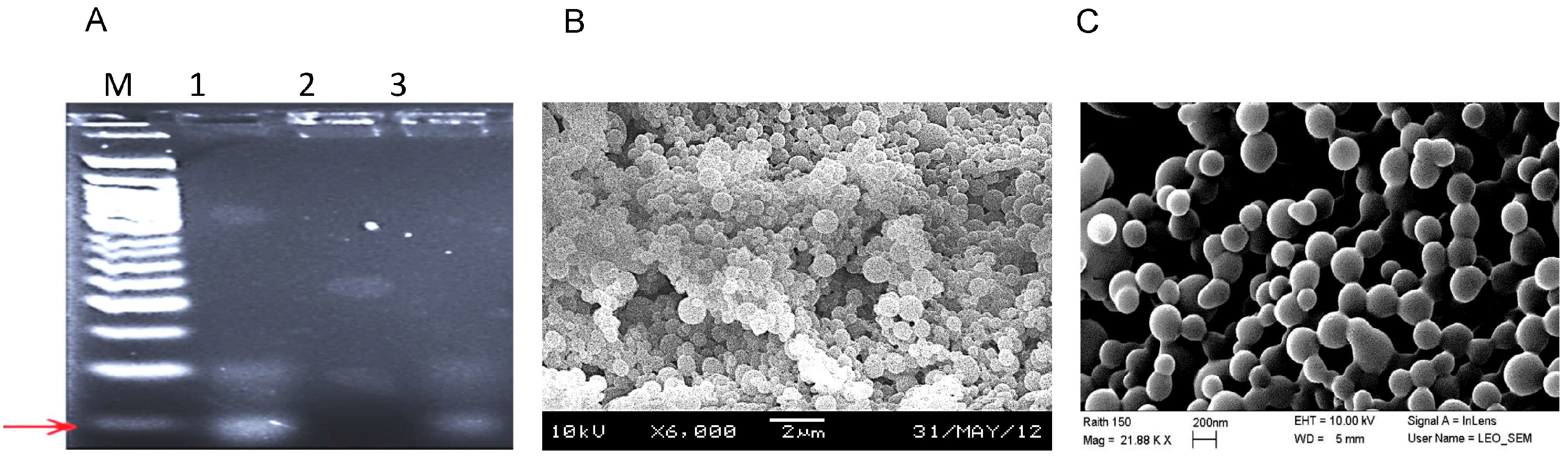
2.5. Nanoparticle Aptamer Characterization
2.6. SEM Imaging
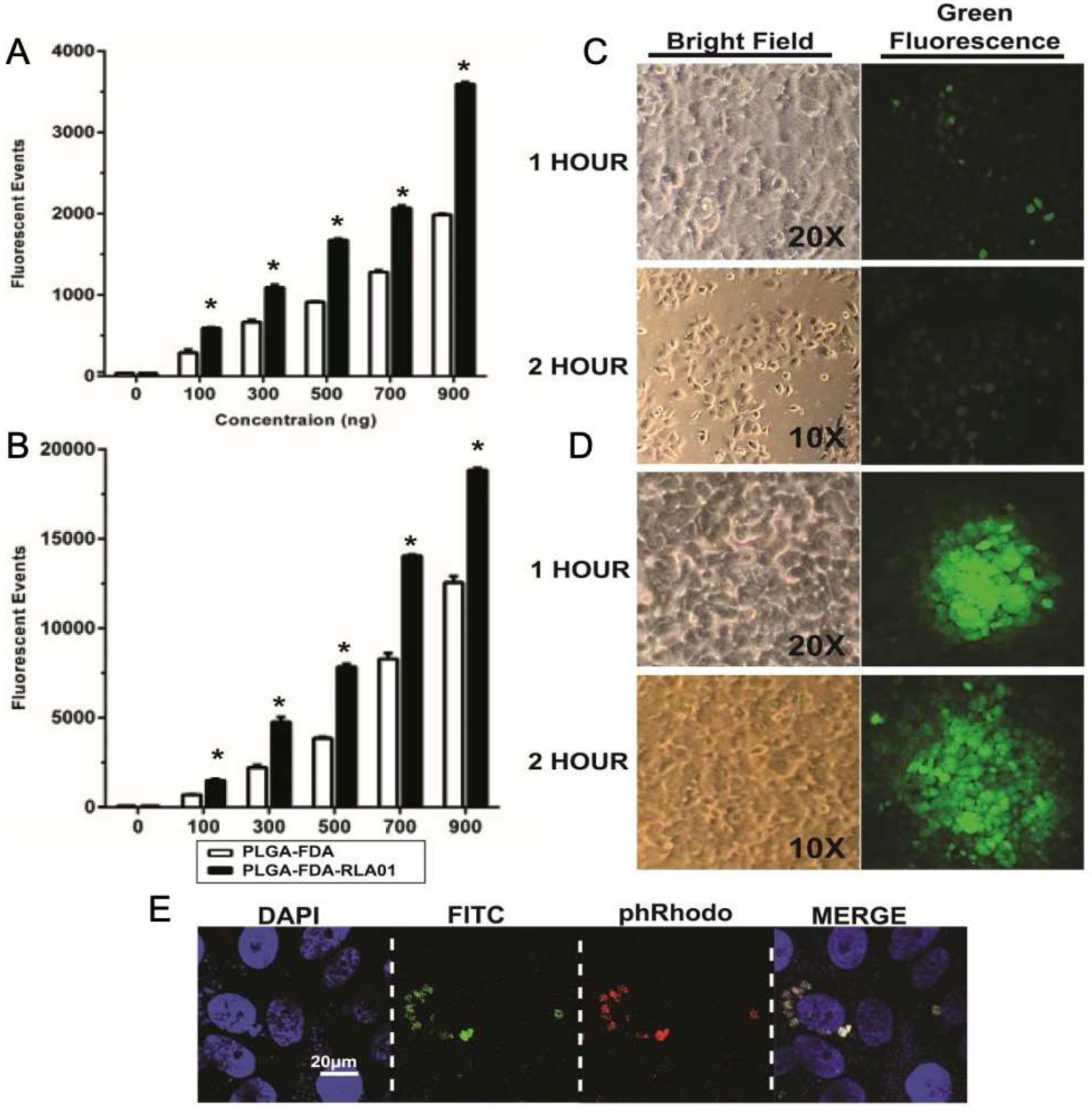
2.7. Confocal and Light Microscopy
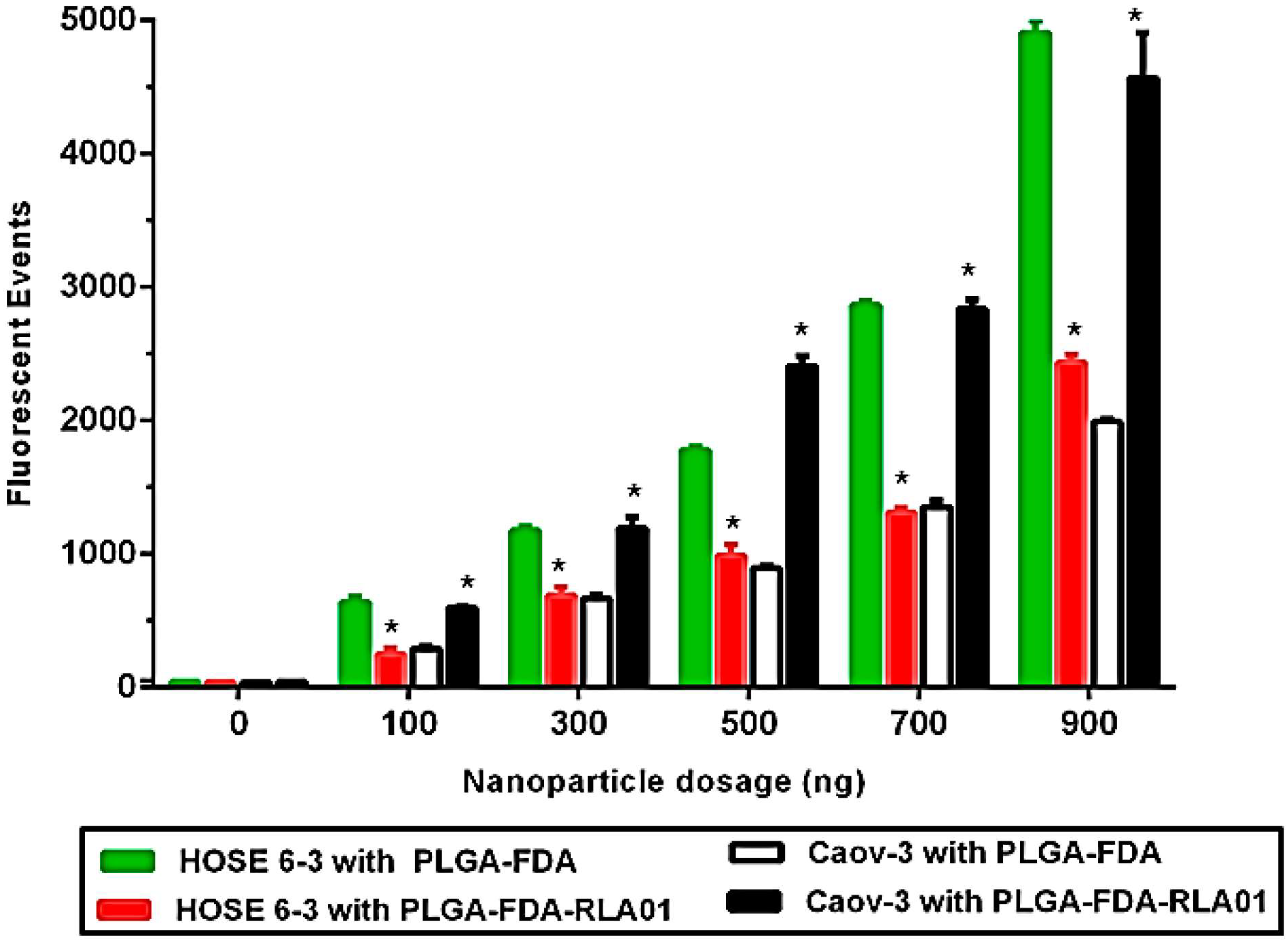
2.8. Flow Cytometry
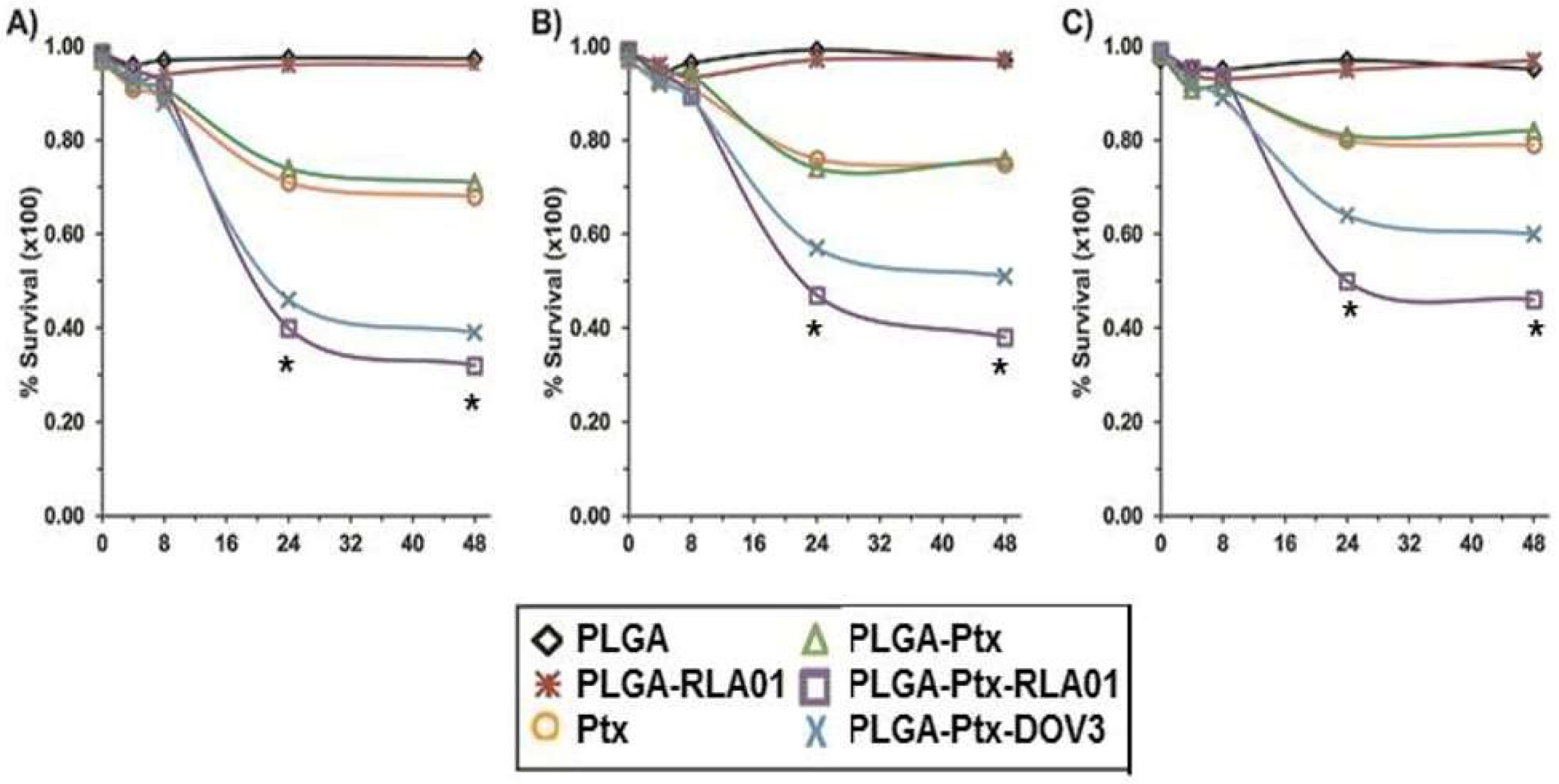
2.9. Cell Proliferation and Apoptosis Assays
2.10. In Vivo Xenograft Studies
3. Results
3.1. Labeling the Surface of Nanoparticles with Aptamer RLA01
3.2. Cellular Uptake of Aptamer-Labeled Nanoparticles
3.3. Targeted Efficacy of Cell Killing with RLA01-Labeled Paclitaxel-Loaded Nanoparticles
3.4. Xenograft Model to Assess In Vivo Accumulation and Honing of RLA01-Labeled Nanoparticles to EOC Tumors
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- SEER Cancer Statistics Factsheets: Ovary Cancer; American Cancer Society: Atlanta, GA, USA, 2025.
- Conic, I.; Dimov, I.; Tasic-Dimov, D.; Djordjevic, B.; Stefanovic, V. Ovarian epithelial cancer stem cells. Sci. World J. 2011, 11, 1243–1269. [Google Scholar] [CrossRef][Green Version]
- Saad, A.F.; Hu, W.; Sood, A.K. Microenvironment and pathogenesis of epithelial ovarian cancer. Horm. Cancer 2010, 1, 277–290. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Coleman, R.L.; Markman, M. Ovarian cancer. Lancet 2009, 374, 1371–1382. [Google Scholar] [CrossRef]
- McGuire, W.P., 3rd; Markman, M. Primary ovarian cancer chemotherapy: Current standards of care. Br. J. Cancer 2003, 89 (Suppl. S3), S3–S8. [Google Scholar] [CrossRef]
- Kroep, J.R. Advances in epithelial ovarian cancer therapy. Curr. Pharm. Des. 2012, 18, 3735–3740. [Google Scholar] [CrossRef]
- Bicaku, E.; Xiong, Y.; Marchion, D.C.; Chon, H.S.; Stickles, X.B.; Chen, N.; Lancaster, J.M. In vitro analysis of ovarian cancer response to cisplatin, carboplatin, and paclitaxel identifies common pathways that are also associated with overall patient survival. Br. J. Cancer 2012, 106, 1967–1975. [Google Scholar] [CrossRef]
- Mantia-Smaldone, G.M.; Edwards, R.P.; Vlad, A.M. Targeted treatment of recurrent platinum-resistant ovarian cancer: Current and emerging therapies. Cancer Manag. Res. 2011, 3, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Markman, M. Current standards of care for chemotherapy of optimally cytoreduced advanced epithelial ovarian cancer. Gynecol. Oncol. 2013, 131, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Kroep, J.R.; Nortier, J.W. The role of bevacizumab in advanced epithelial ovarian cancer. Curr. Pharm. Des. 2012, 18, 3775–3783. [Google Scholar] [CrossRef] [PubMed]
- Markman, M. Current status of intraperitoneal antineoplastic drug delivery. Cancer Treat. Res. 2007, 134, 153–169. [Google Scholar]
- Markman, M. Intraperitoneal antineoplastic drug delivery: Rationale and results. Lancet Oncol. 2003, 4, 277–283. [Google Scholar] [CrossRef]
- Cannistra, S.A.; Matulonis, U.A.; Penson, R.T.; Hambleton, J.; Dupont, J.; Mackey, H.; McGuire, W. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J. Clin. Oncol. 2007, 25, 5180–5186. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Dalton, H.; Benjamin, I.; Tanovic, A. Trabectedin as a new chemotherapy option in the treatment of relapsed platinum sensitive ovarian cancer. Curr. Pharm. Des. 2012, 18, 3754–3769. [Google Scholar] [CrossRef]
- Audeh, M.W.; Carmichael, J.; Penson, R.T.; Friedlander, M.; Powell, B.; Bell-McGuinn, K.M.; Tutt, A. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: A proof-of-concept trial. Lancet 2010, 376, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Gu, F.X.; Langer, R.; Farokhzad, O.C.; Lippard, S.J. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc. Natl. Acad. Sci. USA 2008, 105, 17356–17361. [Google Scholar] [CrossRef]
- Shiao, Y.S.; Chiu, H.H.; Wu, P.H.; Huang, Y.F. Aptamer-Functionalized Gold Nanoparticles As Photoresponsive Nanoplatform for Co-Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 21832–21841. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kwon, S.; Singh, R.K.; Perez, R.A.; Abou Neel, E.A.; Kim, H.W.; Chrzanowski, W. Silica-based mesoporous nanoparticles for controlled drug delivery. J. Tissue Eng. 2013, 4, 2041731413503357. [Google Scholar] [CrossRef]
- Yin, J.; He, X.; Wang, K.; Qing, Z.; Wu, X.; Shi, H.; Yang, X. One-step engineering of silver nanoclusters-aptamer assemblies as luminescent labels to target tumor cells. Nanoscale 2012, 4, 110–112. [Google Scholar] [CrossRef]
- Dhar, S.; Kolishetti, N.; Lippard, S.J.; Farokhzad, O.C. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 1850–1855. [Google Scholar] [CrossRef]
- Guo, J.; Gao, X.; Su, L.; Xia, H.; Gu, G.; Pang, Z.; Chen, H. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 2011, 32, 8010–8020. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Markman, J.L.; Rekechenetskiy, A.; Holler, E.; Ljubimova, J.Y. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1866–1879. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Vasir, J.K.; Labhasetwar, V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv. Drug Deliv. Rev. 2007, 59, 718–728. [Google Scholar] [CrossRef]
- Fang, X.; Tan, W. Aptamers generated from cell-SELEX for molecular medicine: A chemical biology approach. Acc. Chem. Res. 2010, 43, 48–57. [Google Scholar] [CrossRef]
- McKeague, M.; Derosa, M.C. Challenges and opportunities for small molecule aptamer development. J. Nucleic Acids 2012, 2012, 748913. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef]
- Lee, J.H.; Yigit, M.V.; Mazumdar, D.; Lu, Y. Molecular diagnostic and drug delivery agents based on aptamer-nanomaterial conjugates. Adv. Drug Deliv. Rev. 2010, 62, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Wang, H.; Chen, Y.; Zhang, X.; Zhu, H.; Yang, C.; Liu, C. Molecular aptamers for drug delivery. Trends Biotechnol. 2011, 29, 634–640. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, H.; Cai, W. Tumor-targeted drug delivery with aptamers. Curr. Med. Chem. 2011, 18, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Ye, M.; Donovan, M.J.; Song, E.; Zhao, Z.; Tan, W. Nucleic acid aptamers: An emerging frontier in cancer therapy. Chem. Commun. (Camb.) 2012, 48, 10472–10480. [Google Scholar] [CrossRef]
- Talekar, M.; Kendall, J.; Denny, W.; Garg, S. Targeting of nanoparticles in cancer: Drug delivery and diagnostics. Anticancer. Drugs 2011, 22, 949–962. [Google Scholar] [CrossRef]
- Fu, Z.; Xiang, J. Aptamer-Functionalized Nanoparticles in Targeted Delivery and Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9123. [Google Scholar] [CrossRef]
- Group, T.I.C.O.N.I. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: The ICON3 randomised trial. Lancet 2002, 360, 505–515. [Google Scholar] [CrossRef]
- Benedetto, G.; Vestal, C.G.; Richardson, C. Aptamer-Functionalized Nanoparticles as Smart Bombs: The Unrealized Potential for Personalized Medicine and Targeted Cancer Treatment. Target. Oncol. 2015, 10, 467–485. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Xue, Z.; Tsang, C.; Qiao, X.; Dong, L.; Gao, Y. Aptamer nucleotide analog drug conjugates in the targeting therapy of cancers. Front. Cell Dev. Biol. 2022, 10, 1053984. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jin, C.; Wang, Y.; Fang, X.; Zhang, X.; Chen, Z.; Tan, W. Aptamer-conjugated nanomaterials for specific cancer cell recognition and targeted cancer therapy. NPG Asia Mater. 2014, 6, e95. [Google Scholar] [CrossRef]
- Narwade, M.; Shaikh, A.; Gajbhiye, K.R.; Kesharwani, P.; Gajbhiye, V. Advanced cancer targeting using aptamer functionalized nanocarriers for site-specific cargo delivery. Biomater. Res. 2023, 27, 42. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Md, S.; Kesharwani, P. Aptamer grafted nanoparticle as targeted therapeutic tool for the treatment of breast cancer. Biomed. Pharmacother. 2022, 146, 112530. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, S.; Chanda, K.; Balamurali, M.M. Recent Advancements of Aptamers in Cancer Therapy. ACS Omega 2023, 8, 32231–32243. [Google Scholar] [CrossRef]
- Wong, K.Y.; Wong, M.S.; Liu, J. Aptamer-functionalized liposomes for drug delivery. Biomed. J. 2024, 47, 100685. [Google Scholar] [CrossRef]
- Guarneri, V.; Dieci, M.V.; Conte, P. Enhancing intracellular taxane delivery: Current role and perspectives of nanoparticle albumin-bound paclitaxel in the treatment of advanced breast cancer. Expert. Opin. Pharmacother. 2012, 13, 395–406. [Google Scholar] [CrossRef]
- Gordon, E.M.; Hall, F.L. Rexin-G, a targeted genetic medicine for cancer. Expert. Opin. Biol. Ther. 2010, 10, 819–832. [Google Scholar] [CrossRef]
- Deng, W.G.; Kawashima, H.; Wu, G.; Jayachandran, G.; Xu, K.; Minna, J.D.; Ji, L. Synergistic tumor suppression by coexpression of FUS1 and p53 is associated with down-regulation of murine double minute-2 and activation of the apoptotic protease-activating factor 1-dependent apoptotic pathway in human non-small cell lung cancer cells. Cancer Res. 2007, 67, 709–717. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stewart, M.; McFarlane, R.; Cameron, E.; Lang, K.; Campbell, M.; Toth, S.; Neil, J.C. Conditional expression and oncogenicity of c-myc linked to a CD2 gene dominant control region. Int. J. Cancer 1993, 53, 1023–1030. [Google Scholar] [CrossRef]
- Zheng, G.X.; Do, B.T.; Webster, D.E.; Khavari, P.A.; Chang, H.Y. Dicer-microRNA-Myc circuit promotes transcription of hundreds of long noncoding RNAs. Nat. Struct. Mol. Biol. 2014, 21, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.; Hamburg, S.I.; Borad, M.; Seetharam, M.; Kundranda, M.; Lee, P.; Frelund, P.; Gibert, M.; Mast, C.; Semple, S.; et al. A Phasi I Dose Escalation Study of TKM-080301, a RNA Therapeutic Directed Aptamer Against PLK1, in Patients with Advanced Solid Tumors. In Proceedings of the 104th Annual Meeting of the American Association of Cancer Research AACR, Washington, DC, USA, 6–10 March 2013. [Google Scholar]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Dausse, E.; Da Rocha Gomes, S.; Toulme, J.J. Aptamers: A new class of oligonucleotides in the drug discovery pipeline? Curr. Opin. Pharmacol. 2009, 9, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Bayrac, A.T.; Sefah, K.; Parekh, P.; Bayrac, C.; Gulbakan, B.; Oktem, H.A.; Tan, W. In vitro Selection of DNA Aptamers to Glioblastoma Multiforme. ACS Chem. Neurosci. 2011, 2, 175–181. [Google Scholar] [CrossRef]
- Benedetto, G.; Hamp, T.J.; Wesselman, P.J.; Richards, C. Identification of epithelial ovarian tumor-specific aptamers. Nucleic Acid. Ther. 2015, 25, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Berezovski, M.V.; Lechmann, M.; Musheev, M.U.; Mak, T.W.; Krylov, S.N. Aptamer-facilitated biomarker discovery (AptaBiD). J. Am. Chem. Soc. 2008, 130, 9137–9143. [Google Scholar] [CrossRef]
- Van Simaeys, D.; Lopez-Colon, D.; Sefah, K.; Sutphen, R.; Jimenez, E.; Tan, W. Study of the molecular recognition of aptamers selected through ovarian cancer cell-SELEX. PLoS ONE 2010, 5, e13770. [Google Scholar] [CrossRef]
- Ghavami, S.; Hashemi, M.; Ande, S.R.; Yeganeh, B.; Xiao, W.; Eshraghi, M.; Los, M. Apoptosis and cancer: Mutations within caspase genes. J. Med. Genet. 2009, 46, 497–510. [Google Scholar] [CrossRef]
- Yu, Z.; Kuncewicz, T.; Dubinsky, W.P.; Kone, B.C. Nitric oxide-dependent negative feedback of PARP-1 trans-activation of the inducible nitric-oxide synthase gene. J. Biol. Chem. 2006, 281, 9101–9109. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetto, G.; Fowler, A.; Langdon, D.; Raine, M.; White, M.L.; Ogle, J.; Garmon, C.; Ogle, C.; Richardson, C. Aptamer-Coated PLGA Nanoparticles Selectively Internalize into Epithelial Ovarian Cancer Cells In Vitro and In Vivo. Biomolecules 2025, 15, 1123. https://doi.org/10.3390/biom15081123
Benedetto G, Fowler A, Langdon D, Raine M, White ML, Ogle J, Garmon C, Ogle C, Richardson C. Aptamer-Coated PLGA Nanoparticles Selectively Internalize into Epithelial Ovarian Cancer Cells In Vitro and In Vivo. Biomolecules. 2025; 15(8):1123. https://doi.org/10.3390/biom15081123
Chicago/Turabian StyleBenedetto, Gregory, Anthony Fowler, Dan Langdon, Maya Raine, Molly Lynne White, Joshua Ogle, Corey Garmon, Craig Ogle, and Christine Richardson. 2025. "Aptamer-Coated PLGA Nanoparticles Selectively Internalize into Epithelial Ovarian Cancer Cells In Vitro and In Vivo" Biomolecules 15, no. 8: 1123. https://doi.org/10.3390/biom15081123
APA StyleBenedetto, G., Fowler, A., Langdon, D., Raine, M., White, M. L., Ogle, J., Garmon, C., Ogle, C., & Richardson, C. (2025). Aptamer-Coated PLGA Nanoparticles Selectively Internalize into Epithelial Ovarian Cancer Cells In Vitro and In Vivo. Biomolecules, 15(8), 1123. https://doi.org/10.3390/biom15081123



