Dual Oxytocin Signals in Striatal Astrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Purified Striatal Astrocytic Processes
2.3. Endogenous Glutamate Release
2.4. Intracellular [Ca2+] Assay
2.5. Calculations and Statistical Analysis
2.6. Materials
3. Results
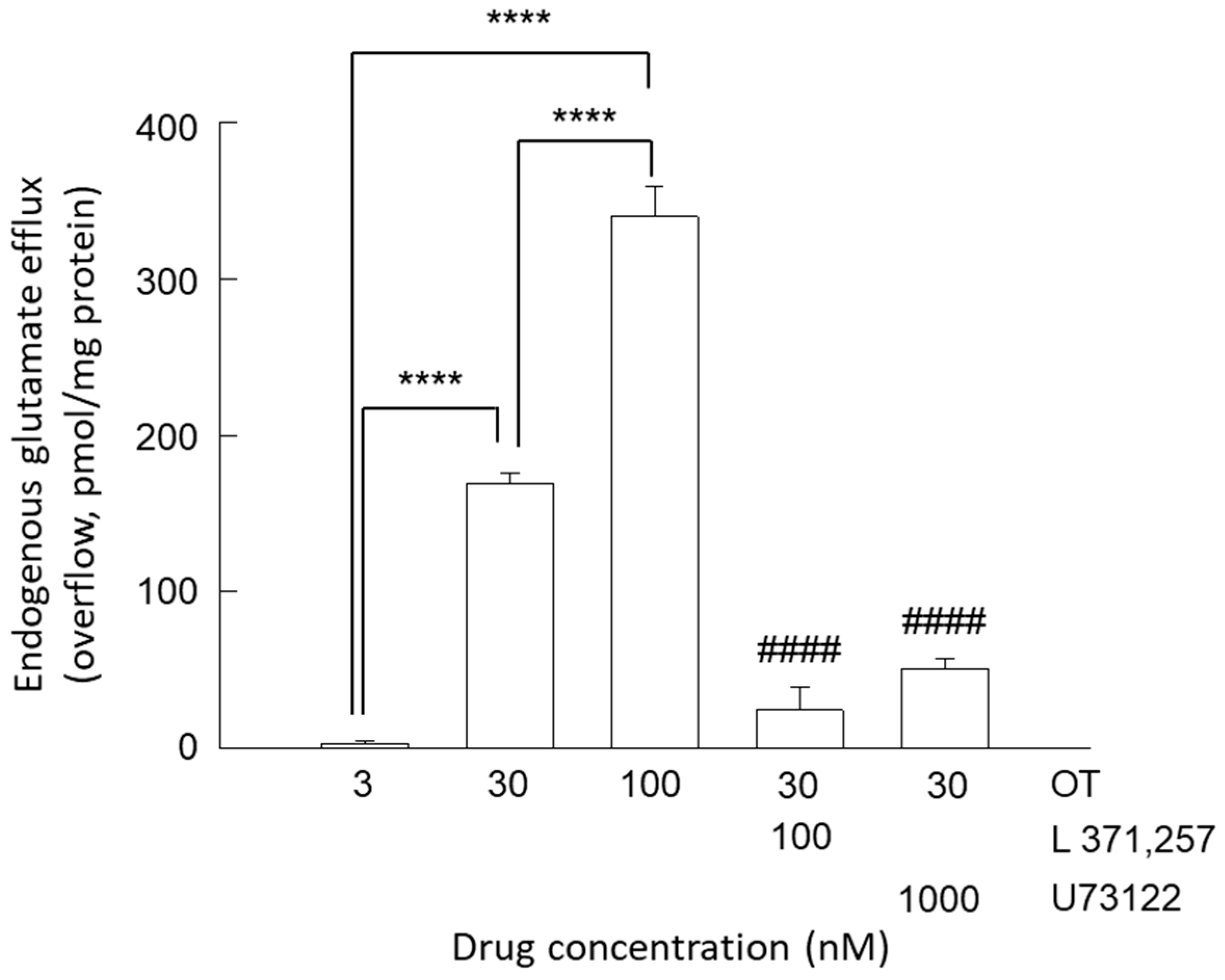
3.1. Release of Glutamate from Astrocyte Processes: Concentration-Dependent Responses to OT
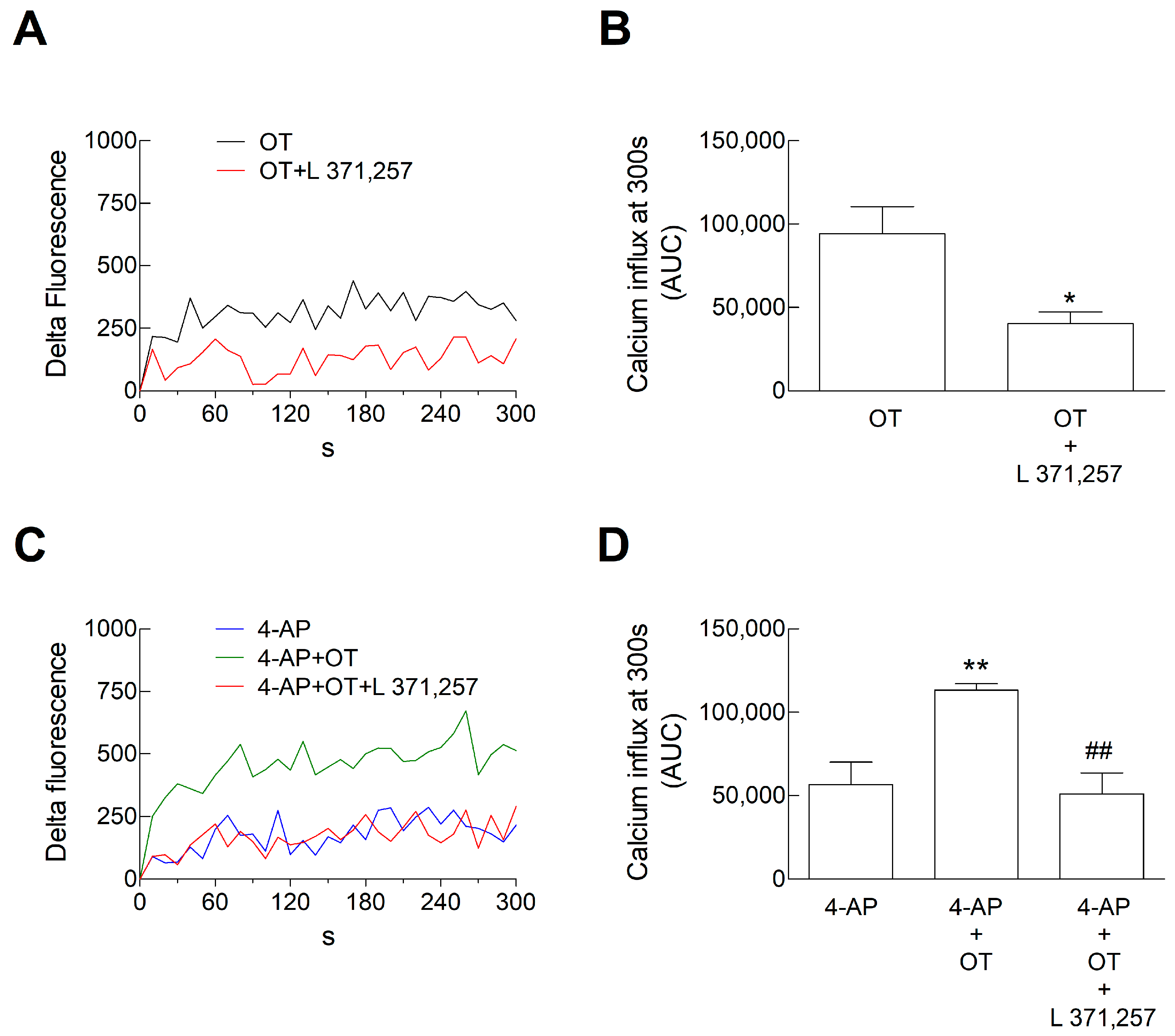
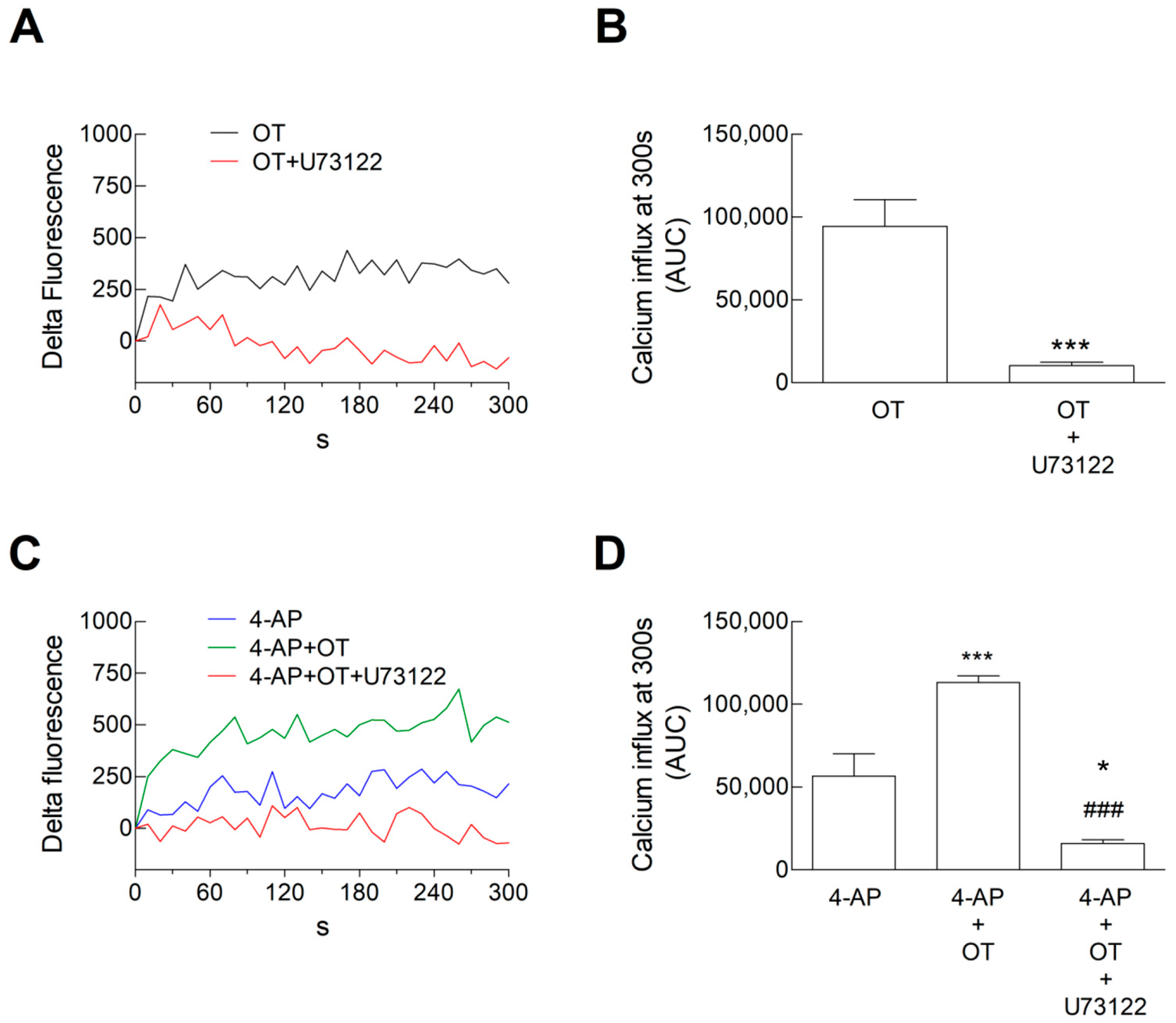
3.2. Ca2+ Signals in Astrocyte Processes: Responses to OT
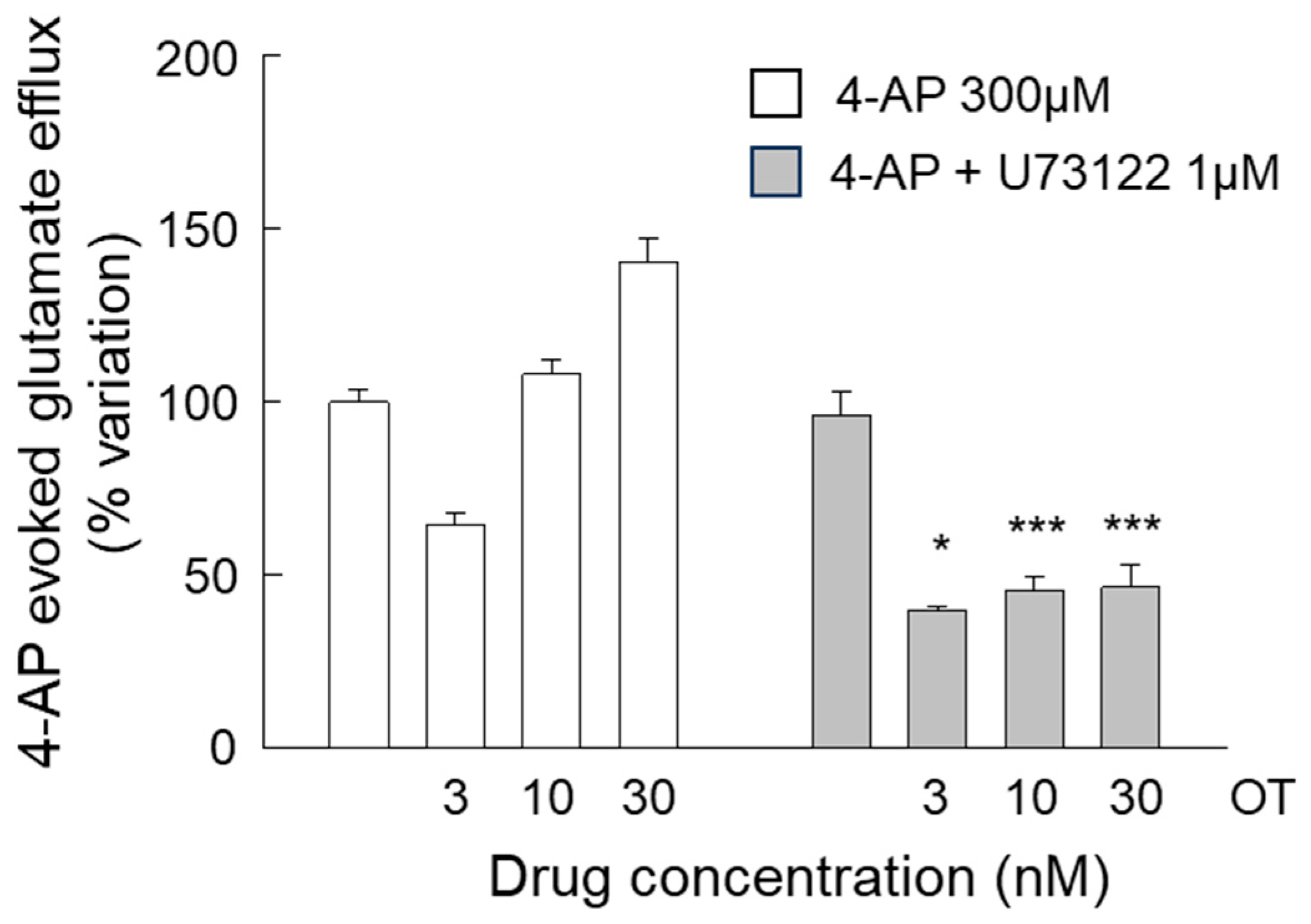
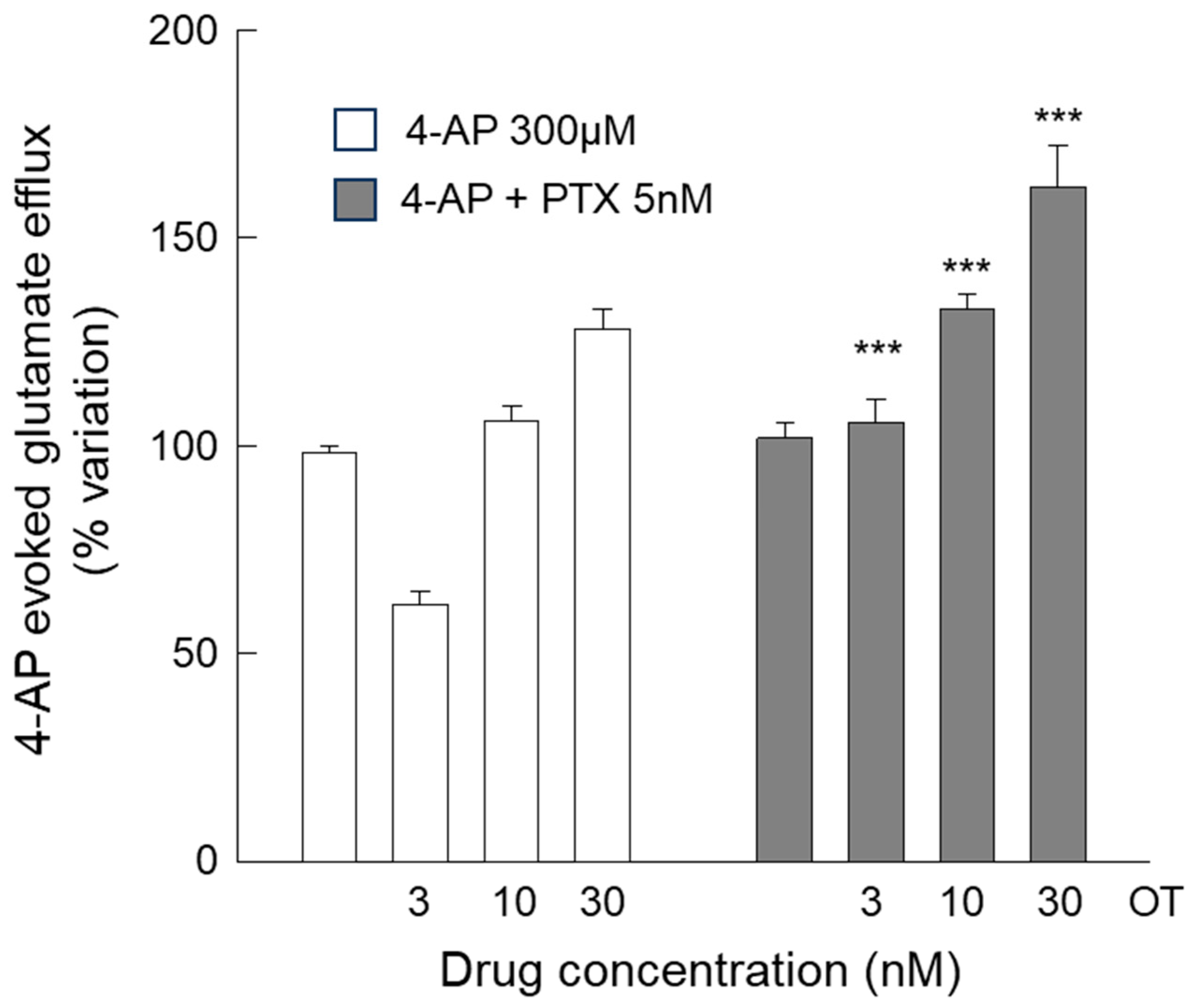
3.3. Release of Glutamate from Astrocyte Processes: Insight into the Different Concentration-Dependent Responses to OT
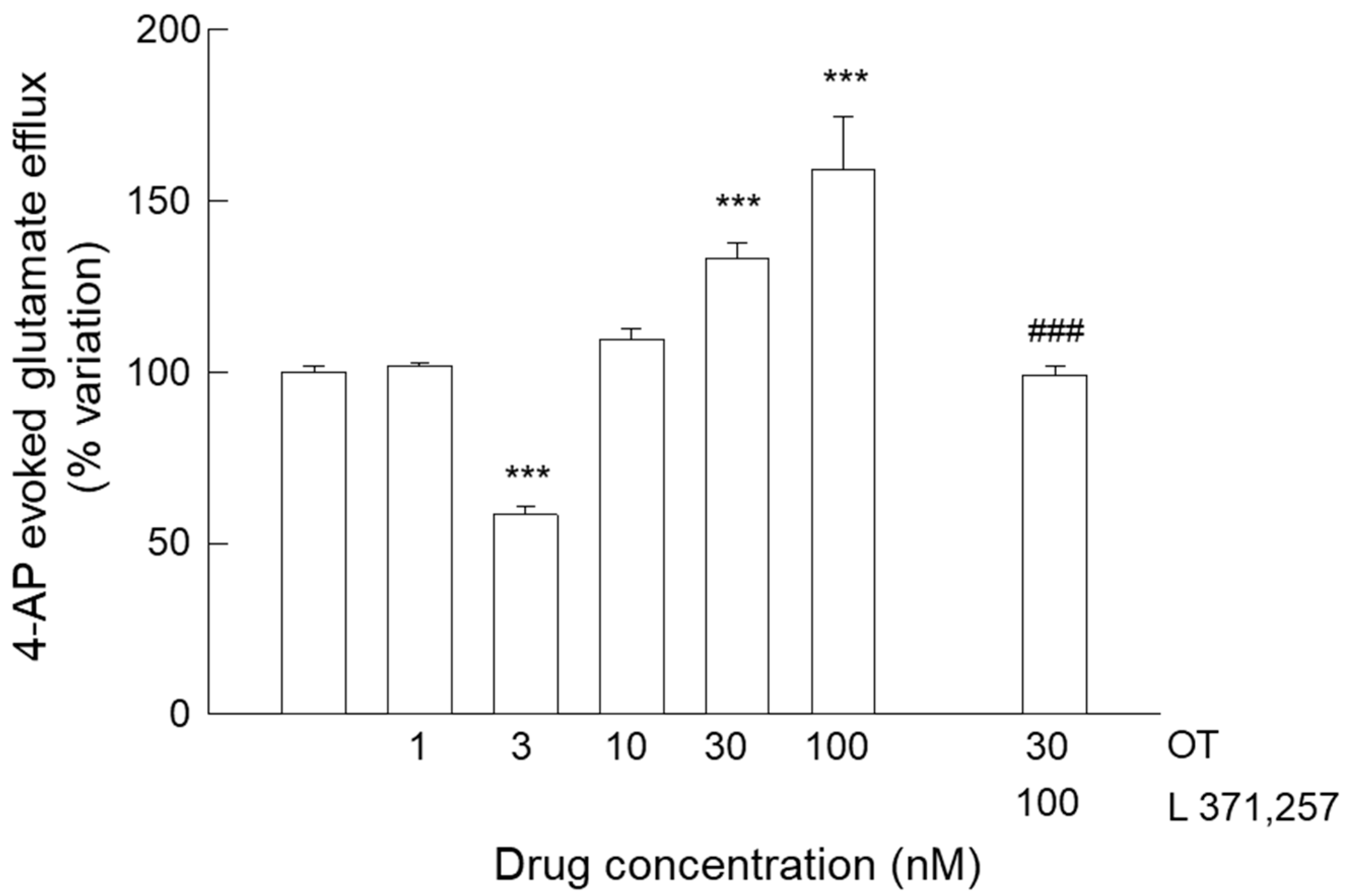
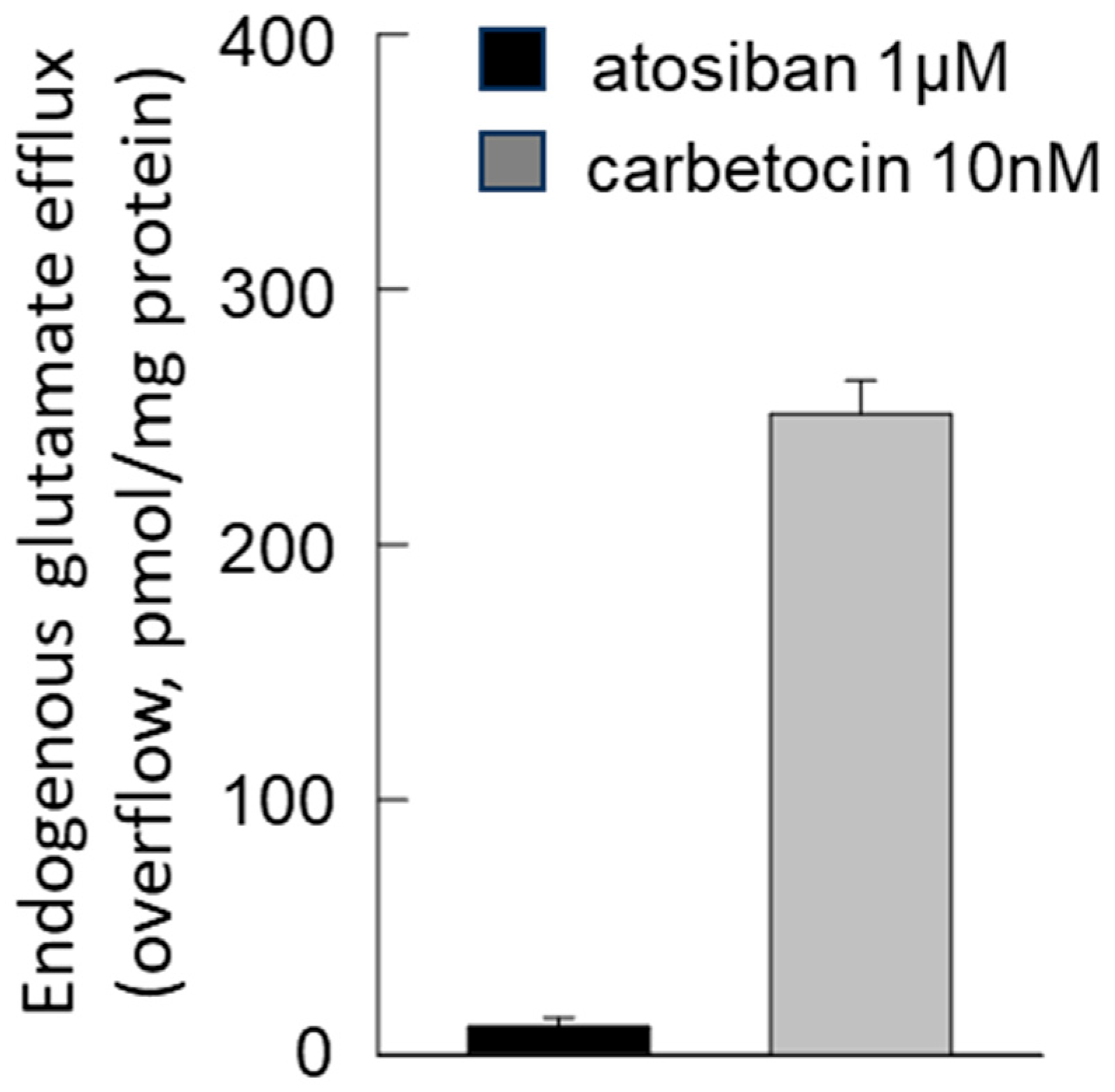
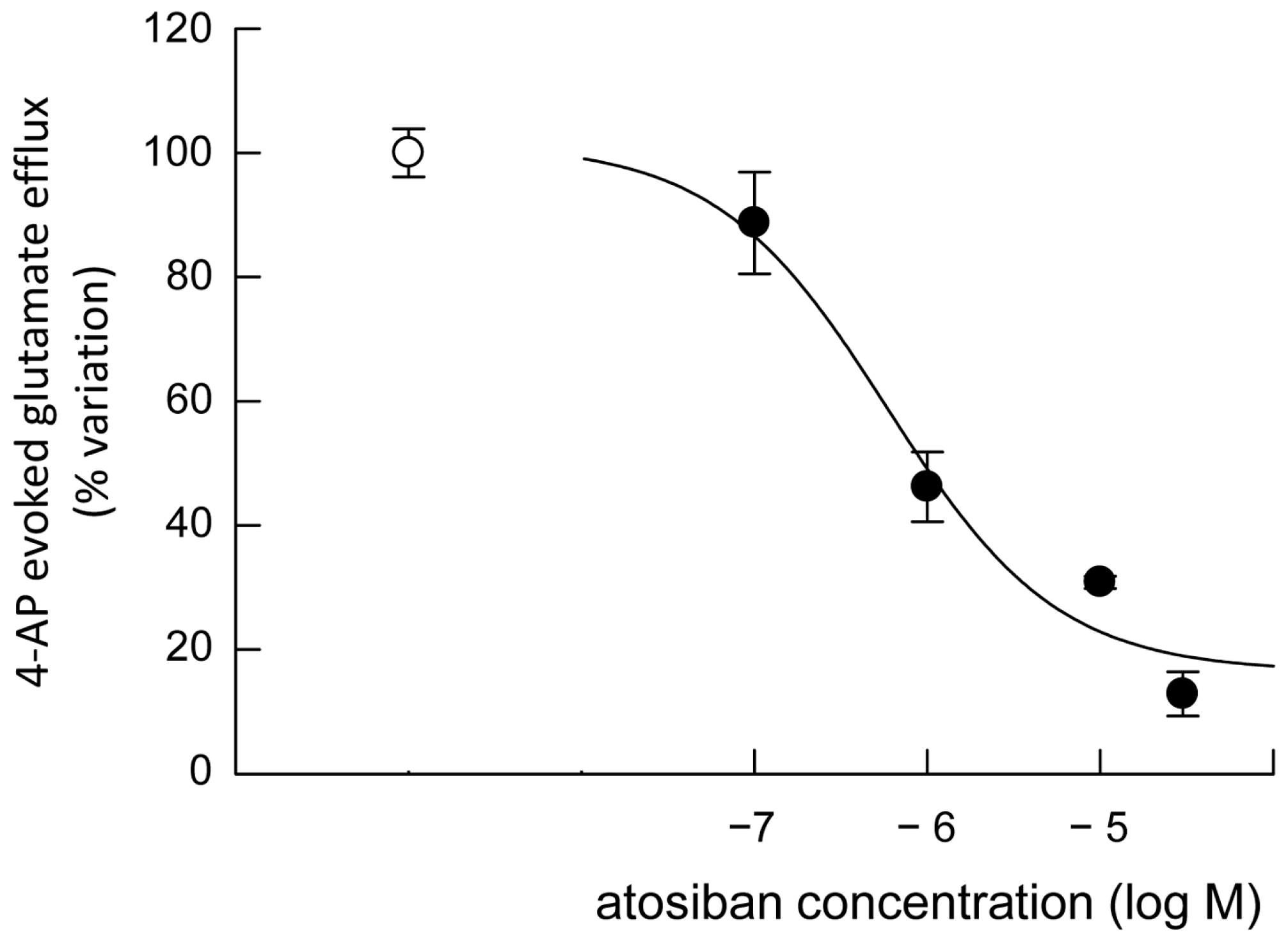
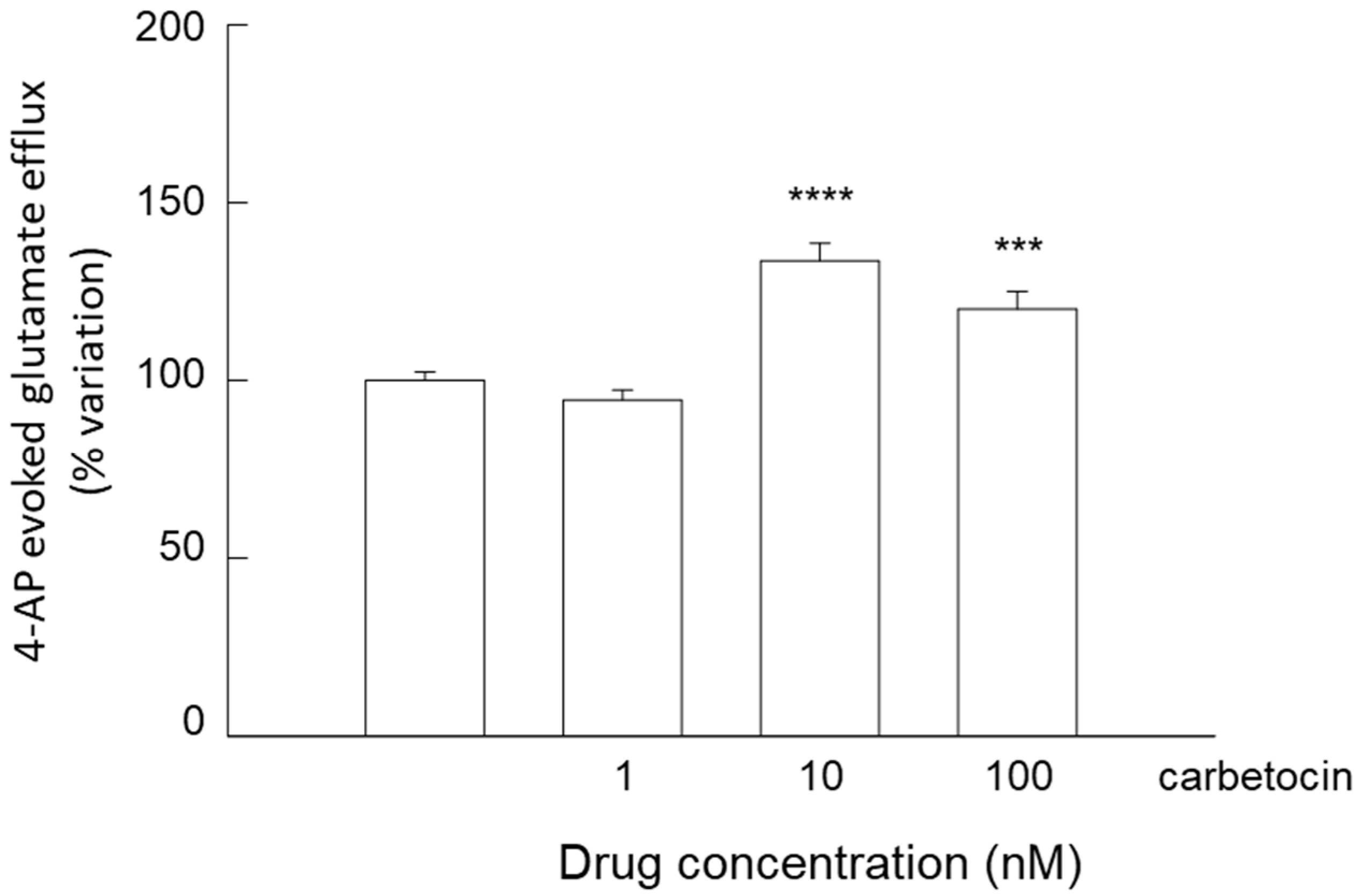
3.4. Release of Glutamate from Astrocyte Processes: Responses to OTR Biased Agonists
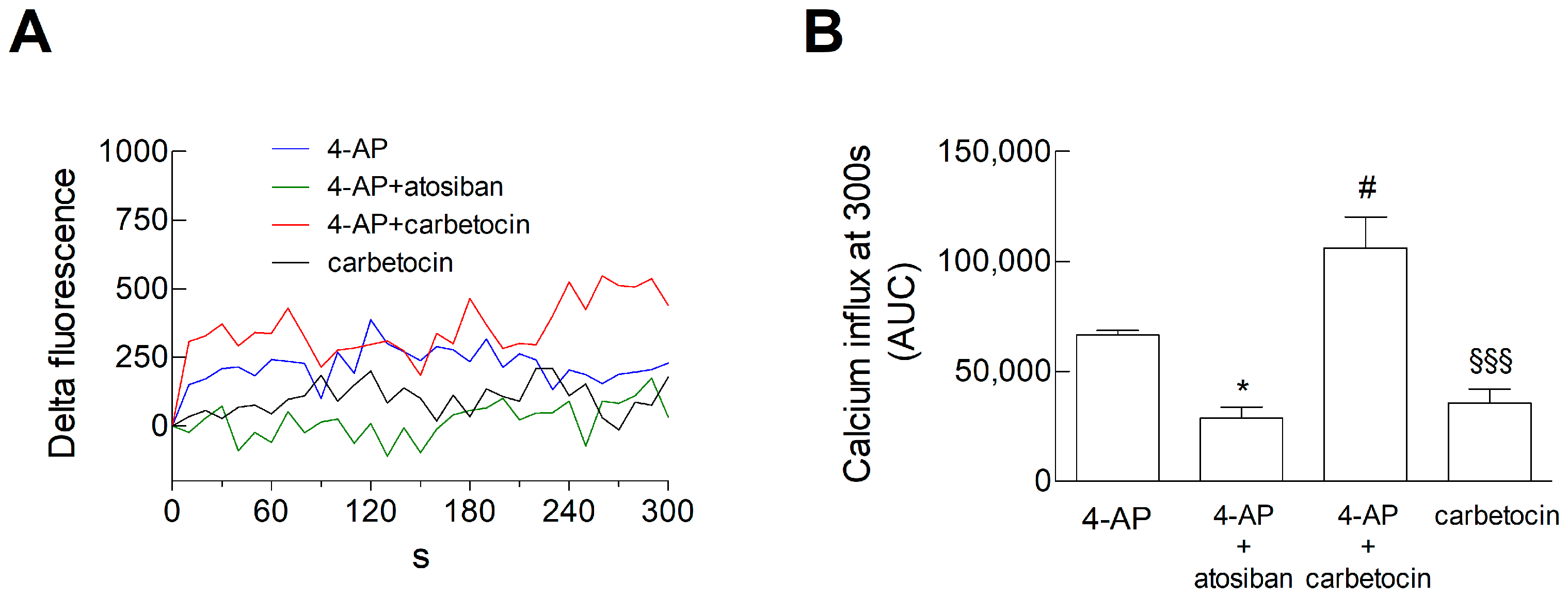
3.5. Ca2+ Signals in Astrocyte Processes: Responses to OTR Biased Agonists
4. Discussion
4.1. OT Can Induce Dual Responses in Astrocyte Processes, Namely the Inhibition and Facilitation of Both Ca2+ Signals and Glutamate Release
4.2. The Inhibitory Response Was Dependent on the Activation of Gi Coupled to the Inhibition of Adenyl Cyclase, and the Facilitatory Response Was Dependent on the Activation of the PLC Intracellular Pathway
4.3. Both the Inhibitory and the Facilitatory Responses Were Evoked by OT in the Same Nanomolar Concentration Range
4.4. The Biased Agonists Atosiban and Carbetocin Duplicate OT’s Inhibitory and Facilitatory Effects, Respectively, on Both Glutamate Release and Ca2+ Signals
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-AP | 4-aminopyridine |
| cAMP | cyclic adenosine monophosphate |
| CG | calcium green |
| D2 | dopamine D2 receptor |
| IP3 | inositol-1,4,5-triphosphate |
| OT | oxytocin |
| OTR | oxytocin receptor |
| PAPs | perisynaptic astrocyte processes |
| PD | Parkinson’s disease |
| PKA | protein kinase A |
| PLC | phospholipase C |
| PTX | pertussis toxin |
| V1aR | vasopressin receptor 1a |
| V1bR | vasopressin receptor 1b |
| VGLUT | vesicular glutamate transporter |
References
- Porter, J.T.; McCarthy, K.D. Astrocytic neurotransmitter receptors in situ and in vivo. Prog. Neurobiol. 1997, 51, 439–455. [Google Scholar] [CrossRef]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef]
- Nedergaard, M.; Verkhratsky, A. Artifact versus reality-how astrocytes contribute to synaptic events. Glia 2012, 60, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, L.; Nie, H.; Deng, D.Y.B. Calcium Signaling in Astrocytes and Its Role in the Central Nervous System Injury. Mol. Neurobiol. 2025; epub ahead of print. [Google Scholar] [CrossRef]
- Haydon, P.G.; Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006, 86, 1009–1031. [Google Scholar] [CrossRef]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Chounlamountry, K.; Kessler, J.P. The ultrastructure of perisynaptic glia in the nucleus tractus solitarii of the adult rat: Comparison between single synapses and multisynaptic arrangements. Glia 2011, 59, 655–663. [Google Scholar] [CrossRef]
- Lachamp, P.; Crest, M.; Kessler, J.P. Vesicular glutamate transporters type 1 and 2 expression in axon terminals of the rat nucleus of the solitary tract. Neuroscience 2006, 137, 73–81. [Google Scholar] [CrossRef]
- Newman, E.A.; Zahs, K.R. Modulation of neuronal activity by glial cells in the retina. J. Neurosci. 1998, 18, 4022–4028. [Google Scholar] [CrossRef] [PubMed]
- Papouin, T.; Dunphy, J.; Tolman, M.; Foley, J.C.; Haydon, P.G. Astrocytic control of synaptic function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160154. [Google Scholar] [CrossRef] [PubMed]
- Montana, V.; Malarkey, E.B.; Verderio, C.; Matteoli, M.; Parpura, V. Vesicular transmitter release from astrocytes. Glia 2006, 54, 700–715. [Google Scholar] [CrossRef]
- Bergersen, L.H.; Gundersen, V. Morphological evidence for vesicular glutamate release from astrocytes. Neuroscience 2009, 158, 260–265. [Google Scholar] [CrossRef]
- Santello, M.; Volterra, A. Synaptic modulation by astrocytes Ca2+—Dependent glutamate release. Neuroscience 2009, 158, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Parpura, V.; Verkhratsky, A. The astrocyte excitability brief: From receptors to gliotransmission. Neurochem. Int. 2012, 61, 610–621. [Google Scholar] [CrossRef]
- Martın, R.; Bajo-Grañeras, R.; Moratalla, R.; Perea, G.; Araque, A. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 2015, 349, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Sardinha, V.M.; Guerra-Gomes, S.; Araque, A.; Sousa, N. Do stars govern our actions? Astrocyte involvement in rodent behavior. Trends Neurosci. 2015, 38, 535–549. [Google Scholar] [CrossRef]
- Kruyer, A.; Kalivas, P.W. Astrocytes as cellular mediators of cue reactivity in addiction. Curr. Opin. Pharmacol. 2021, 56, 1–6. [Google Scholar] [CrossRef]
- Favetta, G.; Bubacco, L. Beyond neurons: How does dopamine signaling impact astrocytic functions and pathophysiology? Prog. Neurobiol. 2025, 251, 102798. [Google Scholar] [CrossRef] [PubMed]
- Corkrum, M.; Covelo, A.; Lines, J.; Bellocchio, L.; Pisansky, M.; Loke, K.; Quintana, R.; Rothwell, P.E.; Lujan, R.; Marsicano, G.; et al. Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity. Neuron 2020, 105, 1036–1047.e5. [Google Scholar] [CrossRef]
- Corkrum, M.; Araque, A. Astrocyte-neuron signaling in the mesolimbic dopamine system: The hidden stars of dopamine signaling. Neuropsychopharmacology 2021, 46, 1864–1872. [Google Scholar] [CrossRef]
- Adermark, L.; Lagström, O.; Loftén, A.; Licheri, V.; Havenäng, A.; Loi, E.A.; Stomberg, R.; Söderpalm, B.; Domi, A.; Ericson, M. Astrocytes modulate extracellular neurotransmitter levels and excitatory neurotransmission in dorsolateral striatum via dopamine D2 receptor signaling. Neuropsychopharmacology 2022, 47, 1493–1502. [Google Scholar] [CrossRef]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron. 2010, 68, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Taylor, A.M.W.; Nagai, J.; Golshani, P.; Evans, C.J.; Coppola, G.; Khakh, B.S. Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron 2018, 99, 1170–1187.e9. [Google Scholar] [CrossRef]
- Mitterauer, B.J. Pathophysiology of schizophrenia based on impaired glial-neuronal interactions. Open J. Med. Psychol. 2014, 3, 42110. [Google Scholar] [CrossRef]
- Xia, M.; Abazyan, S.; Jouroukhin, Y.; Pletnikov, M. Behavioral sequelae of astrocyte dysfunction: Focus on animal models of schizophrenia. Schizophr. Res. 2014, 176, 72–82. [Google Scholar] [CrossRef]
- Bernstein, H.G.; Steiner, J.; Guest, P.C.; Dobrowolny, H.; Bogerts, B. Glial cells as key players in schizophrenia pathology: Recent insights and concepts of therapy. Schizophr. Res. 2015, 161, 4–18. [Google Scholar] [CrossRef]
- Booth, H.D.E.; Hirst, W.D.; Wade-Martins, R. The role of astrocyte dysfunction in Parkinson’s disease pathogenesis. Trends Neurosci. 2017, 40, 358–370. [Google Scholar] [CrossRef]
- Kincaid, A.E.; Zheng, T.; Wilson, C.J. Connectivity and convergence of single corticostriatal axons. J. Neurosci. 1998, 18, 4722–4731. [Google Scholar] [CrossRef] [PubMed]
- Doig, N.M.; Moss, J.; Bolam, J.P. Cortical and thalamic innervation of direct and indirect pathway medium-sized spiny neurons in mouse striatum. J. Neurosci. 2010, 30, 14610–14618. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, N.; Wagner Valladolid, M.; Quesada-Ramírez, L.; Farrer, M.J.; Milnerwood, A.J. Chronic and Acute Manipulation of Cortical Glutamate Transmission Induces Structural and Synaptic Changes in Co-cultured Striatal Neurons. Front. Cell Neurosci. 2021, 15, 569031. [Google Scholar] [CrossRef]
- Amato, S.; Averna, M.; Guidolin, D.; Pedrazzi, M.; Pelassa, S.; Capraro, M.; Passalacqua, M.; Bozzo, M.; Gatta, E.; Anderlini, D.; et al. Heterodimer of A2A and Oxytocin Receptors Regulating Glutamate Release in Adult Striatal Astrocytes. Int. J. Mol. Sci. 2022, 23, 2326. [Google Scholar] [CrossRef]
- Amato, S.; Averna, M.; Guidolin, D.; Ceccoli, C.; Gatta, E.; Candiani, S.; Pedrazzi, M.; Capraro, M.; Maura, G.; Agnati, L.F.; et al. Heteromerization of Dopamine D2 and Oxytocin Receptor in Adult Striatal Astrocytes. Int. J. Mol. Sci. 2023, 24, 4677. [Google Scholar] [CrossRef]
- Amato, S.; Averna, M.; Farsetti, E.; Guidolin, D.; Pedrazzi, M.; Gatta, E.; Candiani, S.; Maura, G.; Agnati, L.F.; Cervetto, C.; et al. Control of Dopamine Signal in High-Order Receptor Complex on Striatal Astrocytes. Int. J. Mol. Sci. 2024, 25, 8610. [Google Scholar] [CrossRef]
- Manjila, S.B.; Betty, R.; Kim, Y. Missing pieces in decoding the brain oxytocin puzzle: Functional insights from mouse brain wiring diagrams. Front. Neurosci. 2022, 16, 1044736. [Google Scholar] [CrossRef]
- Leonzino, M.; Ponzoni, L.; Braida, D.; Gigliucci, V.; Busnelli, M.; Ceresini, I.; Duque-Wilckens, N.; Nishimori, K.; Trainor, B.C.; Sala, M.; et al. Impaired approach to novelty and striatal alterations in the oxytocin receptor deficient mouse model of autism. Horm. Behav. 2019, 114, 104543. [Google Scholar] [CrossRef]
- Báez-Mendoza, R.; Schultz, W. The role of the striatum in social behavior. Front. Neurosci. 2013, 7, 233. [Google Scholar] [CrossRef]
- Russell, J.A.; Brunton, P.J. Oxytocin: Control of Secretion by the Brain and Central Roles. In Reference Module in Neuroscience and Biobehavioral Psychology; Elsevier: Oxford, UK, 2017; pp. 1–14. [Google Scholar]
- Nebl, P.J.; Gordon, A.K. The Effect of Female Orgasm Frequency on Female Mate Selection: A Test of Two Hypotheses. Evol. Psychol. 2022, 20, 14747049221083536. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nakano, Y.; Iwata, N.; Soejima, Y.; Suyama, A.; Hasegawa, T.; Otsuka, F. Oxytocin enhances progesterone production with upregulation of BMP-15 activity by granulosa cells. Biochem. Biophys. Res. Commun. 2023, 646, 103–109. [Google Scholar] [CrossRef]
- Saller, S.; Kunz, L.; Dissen, G.; Stouffer, R.; Ojeda, S.; Berg, D.; Berg, U.; Mayerhofer, A. Oxytocin receptors in the primate ovary: Molecular identity and link to apoptosis in human granulosa cells. Hum. Reprod. 2010, 25, 969–976. [Google Scholar] [CrossRef]
- Ghazy, A.A.; Soliman, O.A.; Elbahnasi, A.I.; Alawy, A.Y.; Mansour, A.M.; Gowayed, M.A. Role of Oxytocin in Different Neuropsychiatric, Neurodegenerative, and Neurodevelopmental Disorders. In Reviews of Physiology, Bio-Chemistry and Pharmacology; Pedersen, S.H.F., Ed.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Son, S.; Manjila, S.B.; Newmaster, K.T.; Wu, Y.T.; Vanselow, D.J.; Ciarletta, M.; Anthony, T.E.; Cheng, K.C.; Kim, Y. Whole-Brain Wiring Diagram of Oxytocin System in Adult Mice. J. Neurosci. 2022, 42, 5021–5033. [Google Scholar] [CrossRef]
- Agnati, L.F.; Fuxe, K.; Zoli, M.; Ozini, I.; Toffano, G.; Ferraguti, F. A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: The volume transmission and the wiring transmission. Acta Physiol. Scand. 1986, 128, 201–207. [Google Scholar]
- Wei, J.; Zheng, H.; Li, G.; Chen, Z.; Fang, G.; Yan, J. Involvement of oxytocin receptor deficiency in psychiatric disorders and behavioral abnormalities. Front. Cell. Neurosci. 2023, 17, 1164796. [Google Scholar] [CrossRef]
- Mitre, M.; Minder, J.; Morina, E.X.; Chao, M.V.; Froemke, R.C. Oxytocin Modulation of Neural Circuits. Curr. Top. Behav. Neurosci. 2018, 35, 31–53. [Google Scholar]
- Talpo, F.; Spaiardi, P.; Castagno, A.N.; Maniezzi, C.; Raffin, F.; Terribile, G.; Sancini, G.; Pisani, A.; Biella, G.R. Neuromodulatory functions exerted by oxytocin on different populations of hippocampal neurons in rodents. Front. Cell. Neurosci. 2023, 17, 1082010. [Google Scholar] [CrossRef]
- Castagno, A.N.; Spaiardi, P.; Trucco, A.; Maniezzi, C.; Raffin, F.; Mancini, M.; Nicois, A.; Cazzola, J.; Pedrinazzi, M.; Del Papa, P.; et al. Oxytocin Modifies the Excitability and the Action Potential Shape of the Hippocampal CA1 GABAergic Interneurons. Int. J. Mol. Sci. 2024, 25, 2613. [Google Scholar] [CrossRef]
- Di Scala-Guenot, D.; Mouginot, D.; Strosser, M.T. Increase of Intracellular Calcium Induced by Oxytocin in Hypothalamic Cultured Astrocytes. Glia 1994, 11, 269–276. [Google Scholar] [CrossRef]
- Kuo, J.; Hariri, O.R.; Micevych, P. An interaction of oxytocin receptors with metabotropic glutamate receptors in hypothalamic astrocytes. J. Neuroendocr. 2009, 21, 1001–1006. [Google Scholar] [CrossRef]
- Zatkova, M.; Bacova, Z.; Puerta, F.; Lestanova, Z.; Alanazi, M.; Kiss, A.; Reichova, A.; Castejon, A.M.; Ostatnikova, D.; Bakos, J. Projection length stimulated by oxytocin is modulated by the inhibition of calcium signaling in U-87MG cells. J. Neural. Transm. 2018, 125, 1847–1856. [Google Scholar] [CrossRef]
- Wahis, J.; Baudon, A.; Althammer, F.; Kerspern, D.; Goyon, S.; Hagiwara, D.; Lefevre, A.; Barteczko, L.; Boury-Jamot, B.; Bellanger, B.; et al. Astrocytes mediate the effect of oxytocin in the central amygdala on neuronal activity and affective states in rodents. Nat. Neurosci. 2021, 24, 529–541. [Google Scholar] [CrossRef]
- Langle, S.L.; Poulain, D.A.; Theodosis, D.T. Induction of rapid, activity-dependent neuronal-glial remodelling in the adult rat hypothalamus in vitro. Eur. J. Neurosci. 2003, 18, 206–214. [Google Scholar] [CrossRef]
- Meinung, C.P.; Boi, L.; Pandamooz, S.; Mazaud, D.; Ghézali, G.; Rouach, N.; Neumann, I.D. OXTR-mediated signaling in astrocytes contributes to anxiolysis. Mol. Psychiatry 2025, 30, 2620–2634. [Google Scholar] [CrossRef]
- Romero-Fernandez, W.; Borroto-Escuela, D.O.; Agnati, L.F.; Fuxe, K. Evidence for the existence of dopamine D2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor-receptor interactions. Mol. Psychiatry 2013, 18, 849–850. [Google Scholar] [CrossRef]
- Stoop, R. Neuromodulation by Oxytocin and Vasopressin. Neuron 2012, 76, 142–159. [Google Scholar] [CrossRef]
- Ayar, A.; Ozcan, M.; Alcin, E.; Serhatlioglu, I.; Ozcan, S.; Kutlu, S.; Kelestimur, H. Oxytocin activates calcium signaling in rat sensory neurons through a protein kinase C-dependent mechanism. J. Physiol. Biochem. 2014, 70, 43–48. [Google Scholar] [CrossRef]
- Eliava, M.; Melchior, M.; Knobloch-Bollmann, H.S.; Wahis, J.; da Silva Gouveia, M.; Tang, Y.; Ciobanu, A.C.; Triana Del Rio, R.; Roth, L.C.; Althammer, F.; et al. A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron 2016, 89, 1291–1304. [Google Scholar] [CrossRef]
- Gravati, M.; Busnelli, M.; Bulgheroni, E.; Reversi, A.; Spaiardi, P.; Parenti, M.; Toselli, M.; Chini, B. Dual modulation of inward rectifier potassium currents in olfactory neuronal cells by promiscuous G protein coupling of the oxytocin receptor. J. Neuro-chem. 2010, 114, 1424–1435. [Google Scholar] [CrossRef]
- Tirko, N.N.; Eyring, K.W.; Carcea, I.; Mitre, M.; Chao, M.V.; Froemke, R.C.; Tsien, R.W. Oxytocin transforms firing mode of CA2 hippocampal neurons. Neuron 2018, 100, 593608.e3. [Google Scholar] [CrossRef]
- Wiegand, V.; Gimpl, G. Specification of the cholesterol interaction with the oxytocin receptor using a chimeric receptor approach. Eur. J. Pharmacol. 2012, 676, 12–19. [Google Scholar] [CrossRef]
- Kombian, S.B.; Hirasawa, M.; Mouginot, D.; Pittman, Q.J. Modulation of synaptic transmission by oxytocin and vasopressin in the supraoptic nucleus. Prog. Brain Res. 2002, 139, 235–246. [Google Scholar]
- Osako, Y.; Otsuka, T.; Taniguchi, M.; Oka, T.; Kaba, H. Oxytocin Depresses Spontaneous γ-Aminobutyric Acid-Ergic Inhibitory Postsynaptic Currents in Cultured Mitral Cells of the Rat Olfactory Bulb by a Presynaptic Mechanism. Neurosci. Lett. 2000, 289, 25–28. [Google Scholar] [CrossRef]
- Hirasawa, M.; Kombian, S.B.; Pittman, Q.J. Oxytocin Retrogradely Inhibits Evoked, but Not Miniature, EPSCs in the Rat Supraoptic Nucleus: Role of N- and P/Q-Type Calcium Channels. J. Physiol. 2001, 532, 595–607. [Google Scholar] [CrossRef]
- Busnelli, M.; Saulière, A.; Manning, M.; Bouvier, M.; Galés, C.; Chini, B. Functional Selective Oxyto-cin-derived Agonists Discriminate between Individual G Protein Family Subtypes. J. Biol. Chem. 2012, 287, 3617–3629. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Aboghazleh, R.; Boyajian, S.D.; Atiyat, A.; Udwan, M.; Al-Helalat, M.; Al-Rashaideh, R. Rodent brain extraction and dissection: A comprehensive approach. MethodsX 2023, 12, 102516. [Google Scholar] [CrossRef]
- Chiu, K.; Lau, W.M.; Lau, H.T.; So, K.; Chang, R.C. Micro-dissection of Rat Brain for RNA or Protein Extraction from Specific Brain Region. J. Vis. Exp. 2007, 7, 269. [Google Scholar]
- Nakamura, Y.; Iga, K.; Shibata, T.; Shudo, M.; Kataoka, K. Glial plasmalemmal vesicles: A subcellular fraction from rat hippo-campal homogenate distinct from synaptosomes. Glia 1993, 9, 48–56. [Google Scholar] [CrossRef]
- Raiteri, M.; Sala, R.; Fassio, A.; Rossetto, O.; Bonanno, G. Entrapping of impermeant probes of different size into nonpermea-bilized synaptosomes as a method to study presynaptic mechanisms. J. Neurochem. 2000, 74, 423–431. [Google Scholar] [CrossRef]
- Cervetto, C.; Venturini, A.; Passalacqua, M.; Guidolin, D.; Genedani, S.; Fuxe, K.; Borroto-Esquela, D.O.; Cortelli, P.; Woods, A.S.; Maura, G.; et al. A2A-D2 receptor–receptor interaction modulates gliotransmitter release from striatal astrocyte processes. J. Neurochem. 2017, 140, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Venturini, A.; Guidolin, D.; Maura, G.; Passalacqua, M.; Tacchetti, C.; Cortelli, P.; Genedani, S.; Candiani, S.; Ramoino, P.; et al. Homocysteine and A2A-D2 receptor-receptor interaction at striatal astrocyte processes. J. Mol. Neurosci. 2018, 65, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Vergani, L.; Passalacqua, M.; Ragazzoni, M.; Venturini, A.; Cecconi, F.; Berretta, N.; Mercuri, N.; D’Amelio, M.; Maura, G.; et al. Astrocyte-Dependent Vulnerability to Excitotoxicity in Spermine Oxi-dase-Overexpressing Mouse. Neuromol. Med. 2016, 18, 50–68. [Google Scholar] [CrossRef]
- Venturini, A.; Passalacqua, M.; Pelassa, S.; Pastorino, F.; Tedesco, M.; Cortese, K.; Gagliani, M.C.; Leo, G.; Maura, G.; Guidolin, D.; et al. Exosomes From Astrocyte Processes: Signaling to Neurons. Front. Pharmacol. 2019, 10, 1452. [Google Scholar] [CrossRef]
- Derouiche, A.; Geiger, K.D. Perspectives for ezrin and radixin in astrocytes: Kinases, functions and pathology. Int. J. Mol. Sci. 2019, 20, 3776. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Micheli, L.; Cervetto, C.; Toti, A.; Lucarini, E.; Parisio, C.; Marcoli, M.; Ghelardini, C. Neuronal alarmin IL-1α evokes astrocyte-mediated protective signals: Effectiveness in chemotherapy-induced neuropathic pain. Neurobiol. Dis. 2022, 168, 105716. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Arena, C.; Gado, F.; Di Cesare Mannelli, L.; Cervetto, C.; Carpi, S.; Reynoso-Moreno, I.; Polini, B.; Vallini, E.; Chicca, S.; Lucarini, E.; et al. The endocannabinoid system dual-target ligand N-cycloheptyl-1,2-dihydro-5-bromo-1-(4-fluorobenzyl)-6-methyl-2-oxo-pyridine-3-carboxamide improves disease severity in a mouse model of multiple sclerosis. Eur. J. Med. Chem. 2020, 208, 112858. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Averna, M.; Vergani, L.; Pedrazzi, M.; Amato, S.; Pelassa, S.; Giuliani, S.; Baldini, F.; Maura, G.; Mariottini, P.; et al. Reactive Astrocytosis in a Mouse Model of Chronic Polyamine Catabolism Activation. Biomolecules 2021, 11, 1274. [Google Scholar] [CrossRef]
- Alberi, S.; Dreifuss, J.J.; Raggenbass, M. The oxytocin-induced inward current in vagal neurons of the rat is mediated by G protein activation but not by an increase in the intracellular calcium concentration. Eur. J. Neurosci. 1997, 9, 2605–2612. [Google Scholar] [CrossRef]
- Cassoni, P.; Sapino, A.; Stella, A.; Fortunati, N.; Bussolati, G. Presence and significance of oxytocin receptors in human neuroblastomas and glial tumors. Int. J. Cancer 1998, 77, 695–700. [Google Scholar] [CrossRef]
- Burns, D.L. Subunit structure and enzymic activity of pertussis toxin. Microbiol. Sci. 1988, 5, 285–287. [Google Scholar]
- Jin, W.; Lo, T.M.; Loh, H.H.; Thayer, S.A. U73122 inhibits phospholipase C-dependent calcium mobilization in neuronal cells. Brain Res. 1994, 642, 237–243. [Google Scholar] [CrossRef]
- Reversi, A.; Rimoldi, V.; Marrocco, T.; Cassoni, P.; Bussolati, G.; Parenti, M.; Chini, B. The oxytocin receptor antagonist atosiban inhibits cell growth via a “biased agonist” mechanism. J. Biol. Chem. 2005, 280, 16311–16318. [Google Scholar] [CrossRef]
- Passoni, I.; Leonzino, M.; Gigliucci, V.; Chini, B.; Busnelli, M. Carbetocin is a Functional Selective Gq Agonist That Does Not Promote Oxytocin Receptor Recycling After Inducing β-Arrestin-Independent Internalisation. J. Neuroendocrinol. 2016, 28, jne.12363. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Frattaroli, D.; Venturini, A.; Passalacqua, M.; Nobile, M.; Alloisio, S.; Tacchetti, C.; Maura, G.; Agnati, L.F.; Marcoli, M. Calcium-permeable AMPA receptors trigger vesicular glutamate re-lease from Bergmann gliosomes. Neuropharmacology 2015, 99, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Ormel, L.; Stensrud, M.J.; Bergersen, L.H.; Gundersen, V. VGLUT1 is localized in astrocytic processes in several brain regions. Glia 2012, 60, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, N.; Kantroo, M.; Stobart, J.L. Extracellular Calcium Influx Pathways in Astrocyte Calcium Microdomain Physiology. Biomolecules 2021, 11, 1467. [Google Scholar] [CrossRef]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.R.; Robitaille, R.; Volterra, A. Gliotransmitters travel in time and space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef]
- Bindocci, E.; Savtchouk, I.; Liaudet, N.; Becker, D.; Carriero, G.; Volterra, A. Three-dimensional calcium imaging advances understanding of astrocyte biology. Science 2017, 356, eaai8185. [Google Scholar] [CrossRef]
- Panatier, A.; Vallée, J.; Haber, M.; Murai, K.K.; Lacaille, J.C.; Robitaille, R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 2011, 146, 785–798. [Google Scholar] [CrossRef]
- Matos, M.; Bosson, A.; Riebe, I.; Reynell, C.; Vallée, J.; Laplante, I.; Panatier, A.; Robitaille, R.; Lacaille, C. Astrocytes detect and upregulate transmission at inhibitory synapses of somatostatin interneurons onto pyramidal cells. Nat. Commun. 2018, 9, 4254. [Google Scholar] [CrossRef]
- Di Castro, M.A.; Chuquet, J.; Liaudet, N.; Bhaukaurally, K.; Santello, M.; Bouvier, D.; Tiret, P.; Volterra, A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 2011, 14, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Boddum, K.; Jensen, T.P.; Magloire, V.; Kristiansen, U.; Rusakov, D.A.; Pavlov, I.; Walker, M.C. Astrocytic GABA transporter activity modulates excitatory neurotransmission. Nat. Commun. 2016, 7, 13572. [Google Scholar] [CrossRef]
- De Pittà, M.; Brunel, N.; Volterra, A. Astrocytes: Orchestrating synaptic plasticity? Neuroscience 2016, 323, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Kastanenka, K.V.; Moreno-Bote, R.; De Pittà, M.; Perea, G.; Eraso-Pichot, A.; Masgrau, R.; Poskanzer, K.E.; Galea, E. A roadmap to integrate astrocytes into Systems Neuroscience. Glia 2020, 68, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Letellier, M.; Park, Y.K.; Chater, T.E.; Chipman, P.H.; Gautam, S.G.; Oshima-Takago, T.; Goda, Y. Astrocytes regulate hetero-geneity of presynaptic strengths in hippocampal networks. Proc. Natl. Acad. Sci. USA 2016, 113, E2685–E2694. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.; Perea, G.; de Sevilla, D.F.; Gómez-Gonzalo, M.; Núñez, A.; Martín, E.D.; Araque, A. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 2012, 10, e1001259. [Google Scholar] [CrossRef]
- Bernardinelli, Y.; Randall, J.; Janett, E.; Nikonenko, I.; König, S.; Jones, E.V.; Flores, C.E.; Murai, K.K.; Bochet, C.G.; Holtmaat, A.; et al. Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr. Biol. 2014, 24, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Molotkov, D.; Zobova, S.; Arcas, J.M.; Khiroug, L. Calcium-induced outgrowth of astrocytic peripheral processes requires actin binding by Profilin-1. Cell Calcium. 2013, 53, 338–348. [Google Scholar] [CrossRef]
- Tanaka, M.; Shih, P.-Y.; Gomi, H.; Yoshida, T.; Nakai, J.; Ando, R.; Furuichi, T.; Mikoshiba, K.; Semyanov, A.; Itohara, S. Astrocytic Ca2+ signals are required for the functional integrity of tripartite synapses. Mol. Brain 2013, 6, 6. [Google Scholar] [CrossRef]
- Bernardinelli, Y.; Muller, D.; Nikonenko, I. Astrocyte-synapse structural plasticity. Neural Plast. 2014, 2014, 232105. [Google Scholar] [CrossRef]
- Theodosis, D.T.; Poulain, D.A. Activity-dependent neuronal-glial and synaptic plasticity in the adult mammalian hypothala-mus. Neuroscience 1993, 57, 501–535. [Google Scholar] [CrossRef]
- Hatton, G.I. Function-related plasticity in hypothalamus. Annu. Rev. Neurosci. 1997, 20, 375–397. [Google Scholar] [CrossRef]
- Meinung, C.P. Oxytocin Receptor-Mediated Signaling in Astrocytes. Ph.D Thesis, Universität Regensburg, Regensburg, Germany, 2020. [Google Scholar]
- Panatier, A.; Theodosis, D.T.; Mothet, J.-P.; Touquet, B.; Pollegioni, L.; Poulain, D.A.; Oliet, S.H.R. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 2006, 125, 775–784. [Google Scholar] [CrossRef]
- Hirrlinger, J.; Hülsmann, S.; Kirchhoff, F. Astroglial processes show spontaneous motility at active synaptic terminals In Situ. Eur. J. Neurosci. 2004, 20, 2235–2239. [Google Scholar] [CrossRef]
- Lavialle, M.; Aumann, G.; Anlauf, E.; Pröls, F.; Arpin, M.; Derouiche, A. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 12915–12919. [Google Scholar] [CrossRef]
- Oliet, S.H.; Piet, R.; Poulain, D.A. Control of glutamate clearance and synaptic efficacy by glial coverage of neu-rons. Science 2001, 292, 923–926. [Google Scholar] [CrossRef]
- Piet, R.; Vargová, L.; Syková, E.; Poulain, D.A.; Oliet, S.H.R. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc. Natl. Acad. Sci. USA 2004, 101, 2151–2155. [Google Scholar] [CrossRef]
- Agnati, L.F.; Leo, G.; Zanardi, A.; Genedani, S.; Rivera, A.; Fuxe, K.; Guidolin, D. Volume transmission and wiring transmission from cellular to molecular networks: History and perspectives. Acta Physiol. 2006, 187, 329–344. [Google Scholar] [CrossRef]
- Marcoli, M.; Agnati, L.F.; Benedetti, F.; Genedani, S.; Guidolin, D.; Ferraro, L.; Maura, G.; Fuxe, K. On the role of the extracellular space on the holistic behavior of the brain. Rev. Neurosci. 2015, 26, 489–506. [Google Scholar] [CrossRef]
- Amzica, F. In vivo electrophysiological evidences for cortical neuron–glia interactions during slow (<1 Hz) and paroxysmal sleep oscillations. J. Physiol. Paris 2002, 96, 209–219. [Google Scholar]
- Amzica, F.; Massimini, M. Glial and neuronal interactions during slow wave and paroxysmal activities in the neocortex. Cereb. Cortex 2002, 12, 1101–1113. [Google Scholar] [CrossRef]
- Dallérac, G.; Chever, O.; Rouach, N. How do astrocytes shape synaptic transmission? Insights from electrophysiology. Front. Cell. Neurosci. 2013, 7, 159. [Google Scholar]
- Choi, H.B.; Gordon, G.R.; Zhou, N.; Tai, C.; Rungta, R.L.; Martinez, J.; Milner, T.A.; Ryu, J.K.; McLarnon, J.G.; Tresguerres, M.; et al. Metabolic communication between astrocytes and neurons via bicarbonate-responsive soluble adenylyl cyclase. Neuron 2012, 75, 1094–1104. [Google Scholar] [CrossRef]
- Lasič, E.; Lisjak, M.; Horvat, A.; Božić, M.; Šakanović, A.; Anderluh, G.; Verkhratsky, A.; Vardjan, N.; Jorgačevski, J.; Stenovec, M.; et al. Astrocyte Specific Remodeling of Plasmalemmal Cholesterol Composition by Ketamine Indicates a New Mechanism of Antidepressant Action. Sci. Rep. 2019, 9, 10957. [Google Scholar] [CrossRef]
- Chini, B.; Verhage, M.; Grinevich, V. The Action Radius of Oxytocin Release in the Mammalian CNS: From Single Vesicles to Be-havior. Trends Pharmacol. Sci. 2017, 38, 982–991. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Zhang, M.; Lam, S.; Sham, P.C.; Bu, B.; Chua, S.E.; Wang, W.; McAlonan, G.M. The effect of oxytocin on social and non-social behaviour and striatal protein expression in C57BL/6N mice. PLoS ONE 2015, 10, e0145638. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Geng, Y.; Zhao, W.; Zhou, F.; Wang, J.; Markett, S.; Biswal, B.B.; Ma, Y.; Kendrick, K.M.; et al. Oxytocin differentially modulates specific dorsal and ventral striatal functional connec-tions with frontal and cerebellar regions. Neuroimage 2019, 184, 781–789. [Google Scholar] [CrossRef]
- Barnett, D.; Bohmbach, K.; Grelot, V.; Charlet, A.; Dallérac, G.; Ju, Y.H.; Nagai, J.; Orr, A.G. Astrocytes as Drivers and Disruptors of Behavior: New Advances in Basic Mechanisms and Therapeutic Targeting. J. Neurosci. 2023, 43, 7463–7471. [Google Scholar] [CrossRef]
- Agnati, L.F.; Marcoli, M.; Maura, G.; Woods, A.; Guidolin, D. The brain as a “hyper-network”: The key role of neural networks as main producers of the integrated brain actions especially via the "broadcasted" neuroconnectomics. J. Neural Transm. 2018, 125, 883–897. [Google Scholar] [CrossRef]
- Erbaş, O.; Altuntaş, I. Oxytocin and Neuroprotective Effects. In Oxytocin and Health; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Wang, Y.; Xu, H.; Chen, S.; Chen, J.; Zheng, Q.; Ma, Y.; Zhao, X.; Shi, Y.; Xiao, L. Oxytocin Protects Nigrostriatal Dopamine Signal via Activating GABAergic Circuit in the MPTP-Induced Parkinson’s Disease Model. Adv. Sci. 2024, 11, e2310244. [Google Scholar] [CrossRef]
- Erbaş, O.; Oltulu, F.; Taşkiran, D. Amelioration of rotenone-induced dopaminergic cell death in the striatum by oxytocin treatment. Peptides 2012, 38, 312–317. [Google Scholar] [CrossRef]
- Erbaş, O.; Oltulu, F.; Taşkiran, D. Suppression of exaggerated neuronal oscillations by oxytocin in a rat model of Parkinson’s disease. Gen. Physiol. Biophys. 2013, 32, 517–525. [Google Scholar] [CrossRef][Green Version]
- Almansoub, H.A.M.M.; Tang, H.; Wu, Y.; Wang, D.Q.; Mahaman, Y.A.R.; Salissou, M.T.M.; Lu, Y.; Hu, F.; Zhou, L.T.; Al-mansob, Y.A.M.; et al. Oxytocin Alleviates MPTP-Induced Neurotoxicity in Mice by Targeting MicroRNA-26a/Death-Associated Protein Kinase 1 Pathway. J. Alzheimer’s Dis. 2020, 74, 883–901. [Google Scholar] [CrossRef]
- Xu, X.; Mei, B.; Yang, Y.; Li, J.; Weng, J.; Yang, Y.; Zhu, Q.; Zhang, H.; Liu, X. Astrocytes Lingering at a Crossroads: Neuroprotection and Neurodegeneration in Neurocognitive Dysfunction. Int. J. Biol. Sci. 2025, 21, 3122–3143. [Google Scholar] [CrossRef]
- Koyama, Y. Functional alterations of astrocytes in mental disorders: Pharmacological significance as a drug target. Front. Cell. Neurosci. 2015, 9, 261. [Google Scholar] [CrossRef]
- Derevyanko, A.; Tao, T.; Allen, N.J. Common alterations to astrocytes across neurodegenerative disorders. Curr. Opin. Neurobiol. 2025, 90, 102970. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Y.; Chen, G. Cognitive disorders: Potential astrocyte-based mechanism. Brain Res. Bull. 2025, 20, 111181. [Google Scholar] [CrossRef]
- Endo, F.; Kasai, A.; Soto, J.S.; Yu, X.; Qu, Z.; Hashimoto, H.; Gradinaru, V.; Kawaguchi, R.; Khakh, B.S. Molecular basis of astrocyte diversity and morphology across the CNS in health and disease. Science 2022, 378, eadc9020. [Google Scholar] [CrossRef]
- Kim, S.H.; MacIntyre, D.A.; Hanyaloglu, A.C.; Blanks, A.M.; Thornton, S.; Bennett, P.R.; Terzidou, V. The oxytocin receptor antagonist, Atosiban, activates pro-inflammatory pathways in human amnion via G(αi) signalling. Mol. Cell. Endocrinol. 2016, 420, 11–23. [Google Scholar] [CrossRef]
- Mak, P.; Broussard, C.; Vacy, K.; Broadbear, J.H. Modulation of anxiety behavior in the elevated plus maze using peptidic oxytocin and vasopressin receptor ligands in the rat. J. Psychopharmacol. 2012, 26, 532–542. [Google Scholar] [CrossRef]
- Chaviaras, S.; Mak, P.; Ralph, D.; Krishnan, L.; Broadbear, J.H. Assessing the antidepressant-like effects of carbetocin, an oxytocin agonist, using a modification of the forced swimming test. Psychopharmacology 2010, 210, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zanos, P.; Georgiou, P.; Wright, S.R.; Hourani, S.M.; Kitchen, I.; Winsky-Sommerer, R.; Bailey, A. The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology 2014, 39, 855–865. [Google Scholar] [CrossRef]
- Georgiou, P.; Zanos, P.; Garcia-Carmona, J.A.; Hourani, S.; Kitchen, I.; Kieffer, B.L.; Laorden, M.L.; Bailey, A. The oxytocin analogue carbetocin prevents priming-induced reinstatement of morphine-seeking: Involvement of dopaminergic, noradrenergic and MOPr systems. Eur. Neuropsychopharmacol. 2015, 25, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Feifel, D.; Shilling, P.D.; Belcher, A.M. The effects of oxytocin and its analog, carbetocin, on genetic deficits in sensorimotor gat-ing. Eur. Neuropsychopharmacol. 2012, 22, 374–378. [Google Scholar] [CrossRef][Green Version]
- Klenerova, V.; Krejci, I.; Sida, P.; Hlinak, Z.; Hynie, S. Oxytocin and carbetocin effects on spontaneous behavior of male rats: Mod-ulation by oxytocin receptor antagonists. Neuro Endocrinol. Lett. 2009, 30, 335–342. [Google Scholar]
- Klenerova, V.; Krejci, I.; Sida, P.; Hlinak, Z.; Hynie, S. Modulary effects of oxytocin and carbetocin on stress-induced changes in rat behavior in the open-field. J. Physiol. Pharmacol. 2009, 60, 57–62. [Google Scholar]
- Klenerova, V.; Krejci, I.; Sida, P.; Hlinak, Z.; Hynie, S. Oxytocin and carbetocin ameliorating effects on restraint stress-induced short- and long-term behavioral changes in rats. Neuro Endocrinol. Lett. 2010, 31, 622–630. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farsetti, E.; Amato, S.; Averna, M.; Guidolin, D.; Pedrazzi, M.; Maura, G.; Agnati, L.F.; Cervetto, C.; Marcoli, M. Dual Oxytocin Signals in Striatal Astrocytes. Biomolecules 2025, 15, 1122. https://doi.org/10.3390/biom15081122
Farsetti E, Amato S, Averna M, Guidolin D, Pedrazzi M, Maura G, Agnati LF, Cervetto C, Marcoli M. Dual Oxytocin Signals in Striatal Astrocytes. Biomolecules. 2025; 15(8):1122. https://doi.org/10.3390/biom15081122
Chicago/Turabian StyleFarsetti, Elisa, Sarah Amato, Monica Averna, Diego Guidolin, Marco Pedrazzi, Guido Maura, Luigi Francesco Agnati, Chiara Cervetto, and Manuela Marcoli. 2025. "Dual Oxytocin Signals in Striatal Astrocytes" Biomolecules 15, no. 8: 1122. https://doi.org/10.3390/biom15081122
APA StyleFarsetti, E., Amato, S., Averna, M., Guidolin, D., Pedrazzi, M., Maura, G., Agnati, L. F., Cervetto, C., & Marcoli, M. (2025). Dual Oxytocin Signals in Striatal Astrocytes. Biomolecules, 15(8), 1122. https://doi.org/10.3390/biom15081122









