m6A Methylation Modification: Perspectives on the Early Reproduction of Females
Abstract
1. Introduction
2. The Basic Theory of m6A Methylation Modification
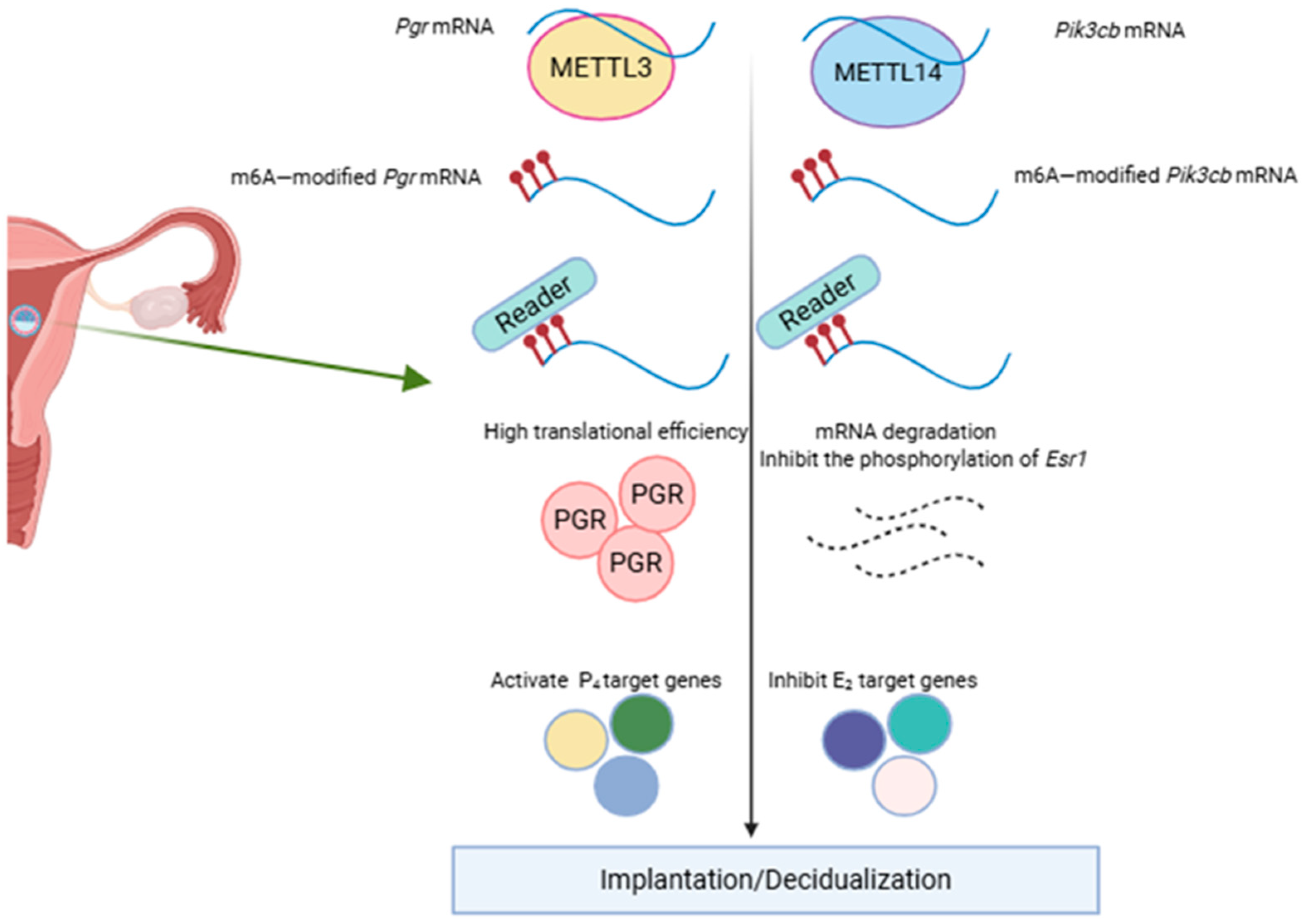
3. The Theoretical Basis of Uterine Receptivity and Decidualization
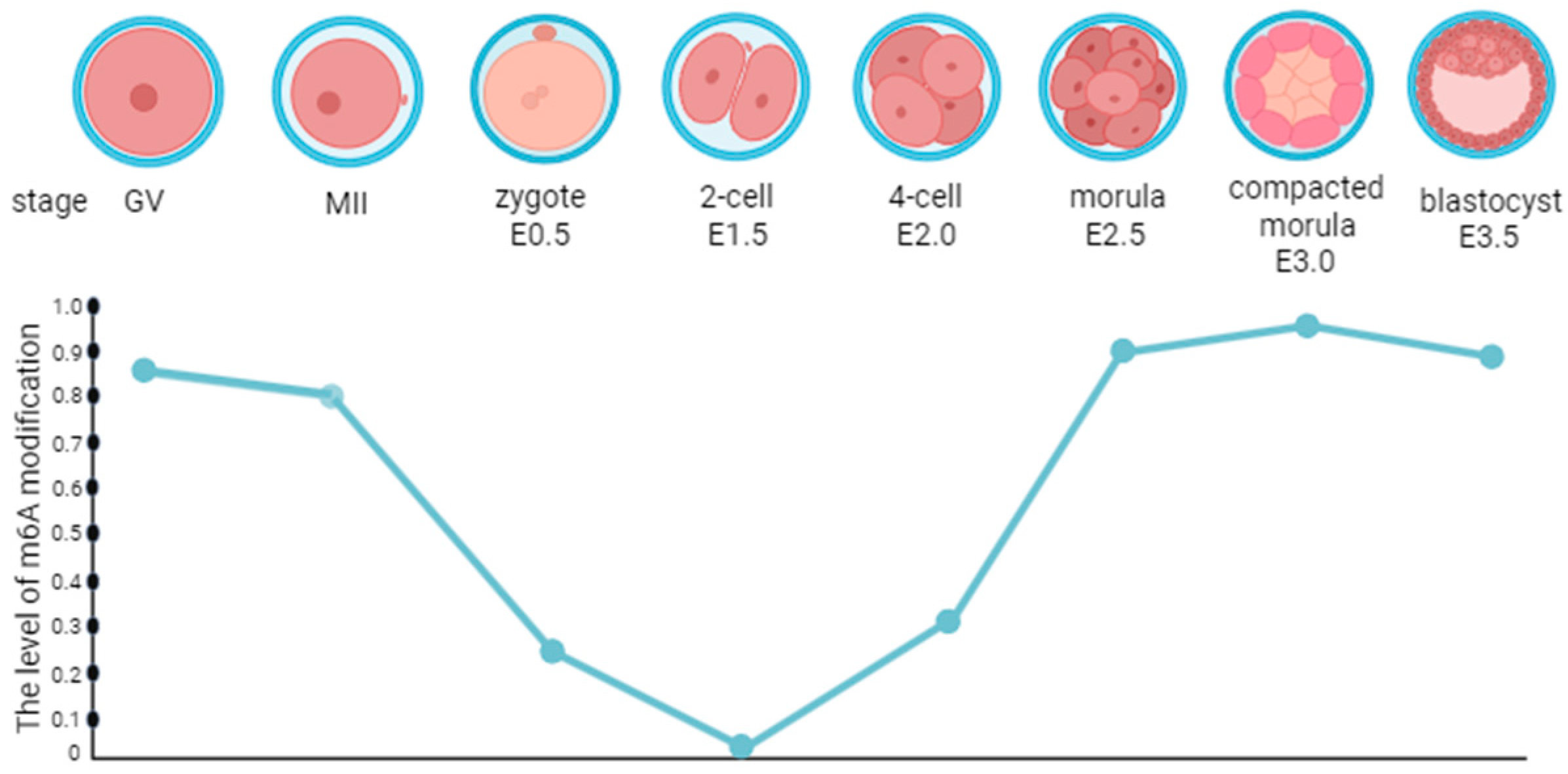
4. The Regulation of m6A Methylation on Preimplantation Embryo Development
5. m6A Modification in the Early Stages of Female Reproduction
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Zhu, Y.; Wang, T.; Xu, X.; Tang, Q.; Li, J.; Wang, Y.; Hu, W.; Wu, W. Feasibility analysis of incorporating infertility into medical insurance in China. Front. Endocrinol. 2022, 13, 967739. [Google Scholar] [CrossRef]
- Stentz, N.C.; Koelper, N.; Barnhart, K.T.; Sammel, M.D.; Senapati, S. Infertility and mortality. Am. J. Obstet. Gynecol. 2020, 222, 251.e1–251.e10. [Google Scholar] [CrossRef]
- Geula, S.; Moshitch-Moshkovitz, S.; Dominissini, D.; Mansour, A.A.; Kol, N.; Salmon-Divon, M.; Hershkovitz, V.; Peer, E.; Mor, N.; Manor, Y.S.; et al. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 2015, 347, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.G.; Lu, X.; Guo, L.; Hou, G.M.; Ma, X.S.; Li, Q.N.; Huang, L.; Fan, L.H.; Zhao, Z.H.; Ou, X.H.; et al. Mettl14 is required for mouse postimplantation development by facilitating epiblast maturation. FASEB J. 2019, 33, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-H.; Zhang, G.-L.; Jiang, R.-F.; Hong, Y.-Q.; Zhang, Q.-Y.; He, J.-P.; Liu, X.-R.; Yang, Z.-S.; Yang, L.; Jiang, X.; et al. METTL3 is essential for normal progesterone signaling during embryo implantation via m6A-mediated translation control of progesterone receptor. Proc. Natl. Acad. Sci. USA 2023, 120, e2214684120. [Google Scholar] [CrossRef]
- Kobayashi, R.; Kawabata-Iwakawa, R.; Terakawa, J.; Sugiyama, M.; Morita, S.; Horii, T.; Hatada, I. Aberrant activation of estrogen receptor-α signaling in Mettl14-deficient uteri impairs embryo implantation. FASEB J. 2023, 37, e23093. [Google Scholar] [CrossRef]
- Chen, K.; Liang, J.; Qin, T.; Zhang, Y.; Chen, X.; Wang, Z. The Role of Extracellular Vesicles in Embryo Implantation. Front. Endocrinol. 2022, 13, 809596. [Google Scholar] [CrossRef]
- Ma, Y.; Gu, M.; Chen, L.; Shen, H.; Pan, Y.; Pang, Y.; Miao, S.; Tong, R.; Huang, H.; Zhu, Y.; et al. Recent advances in critical nodes of embryo engineering technology. Theranostics 2021, 11, 7391–7424. [Google Scholar] [CrossRef]
- Fazleabas, A.; Kim, J.; Srinivasan, S.; Donnelly, K.; Brudney, A.; Jaffe, R. Implantation in the Baboon: Endometrial Responses. Semin. Reprod. Med. 2008, 17, 257–265. [Google Scholar] [CrossRef]
- Nimbkar-Joshi, S.; Rosario, G.; Katkam, R.R.; Manjramkar, D.D.; Metkari, S.M.; Puri, C.P.; Sachdeva, G. Embryo-induced alterations in the molecular phenotype of primate endometrium. J. Reprod. Immunol. 2009, 83, 65–71. [Google Scholar] [CrossRef]
- Simón, C.; Martín, J.C.; Pellicer, A. Paracrine regulators of implantation. Best Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 815–826. [Google Scholar] [CrossRef]
- Wang, H.; Dey, S.K. Roadmap to embryo implantation: Clues from mouse models. Nat. Rev. Genet. 2006, 7, 185–199. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, H.; Kong, S.; Wang, S.; Wang, H.; Wang, H.; Armant, D.R. Physiological and molecular determinants of embryo implantation. Mol. Asp. Med. 2013, 34, 939–980. [Google Scholar] [CrossRef]
- Delaunay, S.; Frye, M. RNA modifications regulating cell fate in cancer. Nat. Cell Biol. 2019, 21, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.D.; Jaffrey, S.R. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol. 2014, 15, 313–326. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, J.; Liao, A. The regulation and potential roles of m6A modifications in early embryonic development and immune tolerance at the maternal-fetal interface. Front. Immunol. 2022, 13, 988130. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yue, Y.; Han, D.; Wang, X.; Fu, Y.; Zhang, L.; Jia, G.; Yu, M.; Lu, Z.; Deng, X.; et al. A METTL3–METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2013, 10, 93–95. [Google Scholar] [CrossRef]
- Knuckles, P.; Lence, T.; Haussmann, I.U.; Jacob, D.; Kreim, N.; Carl, S.H.; Masiello, I.; Hares, T.; Villasenor, R.; Hess, D.; et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m6A machinery component Wtap/Fl(2)d. Genes Dev. 2018, 32, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, K.E.; Chen, B.; Liu, K.; Hunter, O.V.; Xie, Y.; Tu, B.P.; Conrad, N.K. The U6 snRNA m 6 A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell 2017, 169, 824–835.e14. [Google Scholar] [CrossRef]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.-M.; Li, C.J.; Vågbø, C.B.; Shi, Y.; Wang, W.-L.; Song, S.-H.; et al. ALKBH5 Is a Mammalian RNA Demethylase that Impacts RNA Metabolism and Mouse Fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.-G.; et al. N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef]
- Xu, C.; Liu, K.; Tempel, W.; Demetriades, M.; Aik, W.; Schofield, C.J.; Min, J. Structures of Human ALKBH5 Demethylase Reveal a Unique Binding Mode for Specific Single-stranded N6-Methyladenosine RNA Demethylation. J. Biol. Chem. 2014, 289, 17299–17311. [Google Scholar] [CrossRef]
- Zaccara, S.; Ries, R.J.; Jaffrey, S.R. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.C.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.; et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2013, 505, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Weng, H.; Sun, W.; Qin, X.; Shi, H.; Wu, H.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L.; et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018, 20, 285–295. [Google Scholar] [CrossRef]
- Meyer, K.D.; Patil, D.P.; Zhou, J.; Zinoviev, A.; Skabkin, M.A.; Elemento, O.; Pestova, T.V.; Qian, S.-B.; Jaffrey, S.R. 5′ UTR m6A Promotes Cap-Independent Translation. Cell 2015, 163, 999–1010. [Google Scholar] [CrossRef]
- Fang, F.; Wang, X.; Li, Z.; Ni, K.; Xiong, C. Epigenetic regulation of mRNA N6-methyladenosine modifications in mammalian gametogenesis. Mol. Hum. Reprod. 2021, 27, gaab025. [Google Scholar] [CrossRef]
- Batista, P.J.; Molinie, B.; Wang, J.; Qu, K.; Zhang, J.; Li, L.; Bouley, D.M.; Lujan, E.; Haddad, B.; Daneshvar, K.; et al. m6A RNA Modification Controls Cell Fate Transition in Mammalian Embryonic Stem Cells. Cell Stem Cell 2014, 15, 707–719. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, H.; Zhang, X.; Liu, W.; Ding, Y.; Huang, D.; Zhai, J.; Wei, W.; Wen, J.; Chen, D.; et al. METTL3 drives NAFLD-related hepatocellular carcinoma and is a therapeutic target for boosting immunotherapy. Cell Rep. Med. 2023, 4, 101144. [Google Scholar] [CrossRef]
- De Jesus, D.F.; Zhang, Z.; Kahraman, S.; Brown, N.K.; Chen, M.; Hu, J.; Gupta, M.K.; He, C.; Kulkarni, R.N. m6A mRNA methylation regulates human β-cell biology in physiological states and in type 2 diabetes. Nat. Metab. 2019, 1, 765–774. [Google Scholar] [CrossRef]
- Bao, Y.; Zhai, J.; Chen, H.; Wong, C.C.; Liang, C.; Ding, Y.; Huang, D.; Gou, H.; Chen, D.; Pan, Y.; et al. Targeting m6A reader YTHDF1 augments antitumour immunity and boosts anti-PD-1 efficacy in colorectal cancer. Gut 2023, 72, 1497–1509. [Google Scholar] [CrossRef]
- Huang, C.S.; Zhu, Y.Q.; Xu, Q.C.; Chen, S.; Huang, Y.; Zhao, G.; Ni, X.; Liu, B.; Zhao, W.; Yin, X.Y. YTHDF2 promotes intrahepatic cholangiocarcinoma progression and desensitises cisplatin treatment by increasing CDKN1B mRNA degradation. Clin. Transl. Med. 2022, 12, e848. [Google Scholar] [CrossRef]
- Zhou, C.; She, X.; Gu, C.; Hu, Y.; Ma, M.; Qiu, Q.; Sun, T.; Xu, X.; Chen, H.; Zheng, Z. FTO fuels diabetes-induced vascular endothelial dysfunction associated with inflammation by erasing m6A methylation of TNIP1. J. Clin. Investig. 2023, 133, e160517. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, T.; Chen, M.-H.; Wu, R.-X.; Wang, J.; Hu, X.-D.; Meng, T.; Wu, A.-H.; Li, Y.; Yang, Y.-F.; Lei, Y.; et al. Author Correction: ALKBH5-mediated m6A modification of IL-11 drives macrophage-to-myofibroblast transition and pathological cardiac fibrosis in mice. Nat. Commun. 2024, 15, 5595. [Google Scholar] [CrossRef]
- Vasquez, Y.M.; DeMayo, F.J. Role of nuclear receptors in blastocyst implantation. Semin. Cell Dev. Biol. 2013, 24, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.R. Uterine receptivity and the plasma membrane transformation. Cell Res. 2004, 14, 259–267. [Google Scholar] [CrossRef]
- Ye, X. Uterine Luminal Epithelium as the Transient Gateway for Embryo Implantation. Trends Endocrinol. Metab. 2020, 31, 165–180. [Google Scholar] [CrossRef]
- Fukui, Y.; Hirota, Y.; Matsuo, M.; Gebril, M.; Akaeda, S.; Hiraoka, T.; Osuga, Y. Uterine receptivity, embryo attachment, and embryo invasion: Multistep processes in embryo implantation. Reprod. Med. Biol. 2019, 18, 234–240. [Google Scholar] [CrossRef]
- Liu, H.; Huang, X.; Mor, G.; Liao, A. Epigenetic modifications working in the decidualization and endometrial receptivity. Cell. Mol. Life Sci. 2019, 77, 2091–2101. [Google Scholar] [CrossRef]
- Li, Q.; Kannan, A.; DeMayo, F.J.; Lydon, J.P.; Cooke, P.S.; Yamagishi, H.; Srivastava, D.; Bagchi, M.K.; Bagchi, I.C. The Antiproliferative Action of Progesterone in Uterine Epithelium Is Mediated by Hand2. Science 2011, 331, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Levens, E.D.; Stefansson, L.; Nieman, L.K. Indian Hedgehog and Its Targets in Human Endometrium: Menstrual Cycle Expression and Response to CDB-2914. J. Clin. Endocrinol. Metab. 2010, 95, 5330–5337. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Zhao, X.; Das, S.K.; Hogan, B.L.M.; Dey, S.K. Indian Hedgehog as a Progesterone-Responsive Factor Mediating Epithelial–Mesenchymal Interactions in the Mouse Uterus. Dev. Biol. 2002, 245, 280–290. [Google Scholar] [CrossRef]
- Barsh, G.; Kurihara, I.; Lee, D.-K.; Petit, F.G.; Jeong, J.; Lee, K.; Lydon, J.P.; DeMayo, F.J.; Tsai, M.-J.; Tsai, S.Y. COUP-TFII Mediates Progesterone Regulation of Uterine Implantation by Controlling ER Activity. PLoS Genet. 2007, 3, e102. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Sun, H.; Jiang, H.; Zhang, P.; Huang, Y.; Liu, Z.; Yu, Y.; Xu, Z.; Xiang, H.; et al. The Role of N6-methyladenosine Modification in Gametogenesis and Embryogenesis: Impact on Fertility. Genom. Proteom. Bioinform. 2024, 22, qzae050. [Google Scholar] [CrossRef]
- Faulds, K.J.; Egelston, J.N.; Sedivy, L.J.; Mitchell, M.K.; Garimella, S.; Kozlowski, H.; D’Alessandro, A.; Hansen, K.C.; Balsbaugh, J.L.; Phiel, C.J. Glycogen synthase kinase-3 (GSK-3) activity regulates mRNA methylation in mouse embryonic stem cells. J. Biol. Chem. 2018, 293, 10731–10743. [Google Scholar] [CrossRef]
- Hao, J.; Xianfeng, Y.; Gao, W.; Wei, J.; Qi, M.; Han, L.; Shi, S.; Lin, C.; Wang, D. The perturbed expression of m6A in parthenogenetic mouse embryos. Genet. Mol. Biol. 2019, 42, 666–670. [Google Scholar] [CrossRef]
- Lence, T.; Akhtar, J.; Bayer, M.; Schmid, K.; Spindler, L.; Ho, C.H.; Kreim, N.; Andrade-Navarro, M.A.; Poeck, B.; Helm, M.; et al. m6A modulates neuronal functions and sex determination in Drosophila. Nature 2016, 540, 242–247. [Google Scholar] [CrossRef]
- Yu, T.; Qi, X.; Zhang, L.; Ning, W.; Gao, D.; Xu, T.; Ma, Y.; Knott, J.G.; Sathanawongs, A.; Cao, Z.; et al. Dynamic reprogramming and function of RNA N6-methyladenosine modification during porcine early embryonic development. Zygote 2021, 29, 417–426. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Skuland, T.; Zhou, C.; Li, A.; Hashim, A.; Jermstad, I.; Khan, S.; Dalen, K.T.; Greggains, G.D.; et al. The RNA m6A landscape of mouse oocytes and preimplantation embryos. Nat. Struct. Mol. Biol. 2023, 30, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Hu, Y.; Ren, C.; Cao, Q.; Zhou, S.; Cao, Y.; Li, M.; Shu, W.; Huo, R. METTL3-mediated m6A is required for murine oocyte maturation and maternal-to-zygotic transition. Cell Cycle 2020, 19, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Kasowitz, S.D.; Ma, J.; Anderson, S.J.; Leu, N.A.; Xu, Y.; Gregory, B.D.; Schultz, R.M.; Wang, P.J. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genet. 2018, 14, e1007412. [Google Scholar] [CrossRef]
- Lee, K.; Majumdar, M.K.; Buyaner, D.; Hendricks, J.K.; Pittenger, M.F.; Mosca, J.D. Human Mesenchymal Stem Cells Maintain Transgene Expression during Expansion and Differentiation. Mol. Ther. 2001, 3, 857–866. [Google Scholar] [CrossRef]
- Wei, J.; Yu, X.; Yang, L.; Liu, X.; Gao, B.; Huang, B.; Dou, X.; Liu, J.; Zou, Z.; Cui, X.-L.; et al. FTO mediates LINE1 m6A demethylation and chromatin regulation in mESCs and mouse development. Science 2022, 376, 968–973. [Google Scholar] [CrossRef]
- Li, X.-C.; Jin, F.; Wang, B.-Y.; Yin, X.-J.; Hong, W.; Tian, F.-J. The m6A demethylase ALKBH5 controls trophoblast invasion at the maternal-fetal interface by regulating the stability of CYR61 mRNA. Theranostics 2019, 9, 3853–3865. [Google Scholar] [CrossRef]
- Lessey, B.A.; Young, S.L. What exactly is endometrial receptivity? Fertil. Steril. 2019, 111, 611–617. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kim, J.-S. A Review of Mechanisms of Implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef]
- Han, M.; Cao, Y.; Zhou, W.; Zhou, M.; Zhou, X.; Zhang, D.; Xu, B.; Zhang, A. Increased expression of HMGB1 in the implantation phase endometrium is related to recurrent implantation failure. Mol. Biol. Rep. 2022, 49, 1701–1710. [Google Scholar] [CrossRef]
- Macklon, N. Recurrent implantation failure is a pathology with a specific transcriptomic signature. Fertil. Steril. 2017, 108, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Basatvat, S.; Russell, J.M.; Saare, M.; Thurston, L.M.; Salumets, A.; Fazeli, A. Potential innate immunity-related markers of endometrial receptivity and recurrent implantation failure (RIF). Reprod. Biol. 2021, 21, 100569. [Google Scholar] [CrossRef]
- Du, L.; Deng, W.; Zeng, S.; Xu, P.; Huang, L.; Liang, Y.; Wang, Y.; Xu, H.; Tang, J.; Bi, S.; et al. Single-cell transcriptome analysis reveals defective decidua stromal niche attributes to recurrent spontaneous abortion. Cell Prolif. 2021, 54, e13125. [Google Scholar] [CrossRef]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone Action in Endometrial Cancer, Endometriosis, Uterine Fibroids, and Breast Cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yildiz, S.; Adli, M.; Wei, J.-J. Adenomyosis pathogenesis: Insights from next-generation sequencing. Hum. Reprod. Update 2021, 27, 1086–1097. [Google Scholar] [CrossRef]
- Patel, B.; Elguero, S.; Thakore, S.; Dahoud, W.; Bedaiwy, M.; Mesiano, S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Update 2014, 21, 155–173. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, M.; Wu, J.; Wang, S.; Yang, X.; Yi, M.; Zhang, X.; Fang, X. Exploring diagnostic m6A regulators in endometriosis. Aging 2020, 12, 25916–25938. [Google Scholar] [CrossRef]
- Li, X.; Xiong, W.; Long, X.; Dai, X.; Peng, Y.; Xu, Y.; Zhang, Z.; Zhang, L.; Liu, Y. Inhibition of METTL3/m6A/miR126 promotes the migration and invasion of endometrial stromal cells in endometriosis. Biol. Reprod. 2021, 105, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Zhao, X.; Wu, H.; Li, J.; Cheng, Y.; Guo, Q.; Cao, X.; Liang, T.; Sun, L.; et al. METTL3-mediated m6A modification of SIRT1 mRNA inhibits progression of endometriosis by cellular senescence enhancing. J. Transl. Med. 2023, 21, 407. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, M.; Morin, M.; Corriveau, H.; Hamel, M.; Nadeau, S.; Filiatrault, J.; Dumoulin, C. Characteristics of Lower Limb Muscle Strength, Balance, Mobility, and Function in Older Women with Urge and Mixed Urinary Incontinence: An Observational Pilot Study. Physiother. Can. 2019, 71, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Eckert, M.A.; Harada, B.T.; Liu, S.M.; Lu, Z.; Yu, K.; Tienda, S.M.; Chryplewicz, A.; Zhu, A.C.; Yang, Y.; et al. m6A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat. Cell Biol. 2018, 20, 1074–1083. [Google Scholar] [CrossRef]
- Ruan, P.; Wang, S.; Yang, C.; Huang, X.; Sun, P.; Tan, A. m6A mRNA methylation regulates the ERK/NF-kappaB/AKT signaling pathway through the PAPPA/IGFBP4 axis to promote proliferation and tumor formation in endometrial cancer. Cell Biol. Toxicol. 2023, 39, 1611–1626. [Google Scholar] [CrossRef]
- Li, X.; Jin, J.; Long, X.; Weng, R.; Xiong, W.; Liang, J.; Liu, J.; Sun, J.; Cai, X.; Zhang, L.; et al. METTL3-regulated m6A modification impairs the decidualization of endometrial stromal cells by regulating YTHDF2-mediated degradation of FOXO1 mRNA in endometriosis-related infertility. Reprod. Biol. Endocrinol. 2023, 21, 99. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Q.Y.; Liu, J.L. Deciphering mouse uterine receptivity for embryo implantation at single-cell resolution. Cell Prolif. 2021, 54, e13128. [Google Scholar] [CrossRef]
- Xin, Q.; Kong, S.; Yan, J.; Qiu, J.; He, B.; Zhou, C.; Ni, Z.; Bao, H.; Huang, L.; Lu, J.; et al. Polycomb subunit BMI1 determines uterine progesterone responsiveness essential for normal embryo implantation. J. Clin. Investig. 2017, 128, 175–189. [Google Scholar] [CrossRef]
- Dou, X.; Huang, L.; Xiao, Y.; Liu, C.; Li, Y.; Zhang, X.; Yu, L.; Zhao, R.; Yang, L.; Chen, C.; et al. METTL14 is a chromatin regulator independent of its RNA N6-methyladenosine methyltransferase activity. Protein Cell 2023, 14, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Sarkar, R.; Li, Y.; He, L.; Kang, W.; Liao, W.; Liu, W.; Nguyen, T.; Zhang, L.; Deng, Z.; et al. N6-adenomethylation of GsdmC is essential for Lgr5+ stem cell survival to maintain normal colonic epithelial morphogenesis. Dev. Cell 2022, 57, 1976–1994.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ding, C.; Chen, H.; Zhao, J.; Chen, Z.; Chen, B.; Mao, K.; Hao, Y.; Roulis, M.; Xu, H.; et al. m6A mRNA modification maintains colonic epithelial cell homeostasis via NF-κB–mediated antiapoptotic pathway. Sci. Adv. 2022, 8, eabl5723. [Google Scholar] [CrossRef]
- Danan, C.H.; Naughton, K.E.; Hayer, K.E.; Vellappan, S.; McMillan, E.A.; Zhou, Y.; Matsuda, R.; Nettleford, S.K.; Katada, K.; Parham, L.R.; et al. Intestinal transit-amplifying cells require METTL3 for growth factor signaling and cell survival. JCI Insight 2023, 8, e171657. [Google Scholar] [CrossRef]
- Lin, Z.; Hsu, P.J.; Xing, X.; Fang, J.; Lu, Z.; Zou, Q.; Zhang, K.-J.; Zhang, X.; Zhou, Y.; Zhang, T.; et al. Mettl3-/Mettl14-mediated mRNA N6-methyladenosine modulates murine spermatogenesis. Cell Res. 2017, 27, 1216–1230. [Google Scholar] [CrossRef]
- Norwitz, E.R.; Schust, D.J.; Fisher, S.J. Implantation and the Survival of Early Pregnancy. N. Engl. J. Med. 2001, 345, 1400–1408. [Google Scholar] [CrossRef]
- Wu, D.; Spencer, C.B.; Ortoga, L.; Zhang, H.; Miao, C. Histone lactylation-regulated METTL3 promotes ferroptosis via m6A-modification on ACSL4 in sepsis-associated lung injury. Redox Biol. 2024, 74, 103194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, T.; Zhang, Z.; Piao, C.; Kong, C.; Zhang, X. METTL14-mediated m6A modification of ZFP14 inhibits clear cell renal cell carcinoma progression via promoting STAT3 ubiquitination. Clin. Transl. Med. 2025, 15, e70232. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zheng, Z. m6A Methylation Modification: Perspectives on the Early Reproduction of Females. Biomolecules 2025, 15, 1102. https://doi.org/10.3390/biom15081102
Yang Y, Zheng Z. m6A Methylation Modification: Perspectives on the Early Reproduction of Females. Biomolecules. 2025; 15(8):1102. https://doi.org/10.3390/biom15081102
Chicago/Turabian StyleYang, Yan, and Zhanhong Zheng. 2025. "m6A Methylation Modification: Perspectives on the Early Reproduction of Females" Biomolecules 15, no. 8: 1102. https://doi.org/10.3390/biom15081102
APA StyleYang, Y., & Zheng, Z. (2025). m6A Methylation Modification: Perspectives on the Early Reproduction of Females. Biomolecules, 15(8), 1102. https://doi.org/10.3390/biom15081102




