A Distinct miRNA Profile in Intimal Hyperplasia of Failed Arteriovenous Fistulas Reveals Key Pathogenic Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. microRNA Expression Profiling in Human AVF Tissues
2.3. Validation of miRNA Expression by Real-Time PCR
2.4. Cell Models of AVF Pathogenesis and Treatments
2.5. Cell Transfection
2.6. miRNA Expression Analysis in Vascular Cell Models
2.7. Analysis of Cell Proliferation
2.8. Scratch Wound Assay
2.9. Inflammatory Cytokine Detection
2.10. Statistical Analysis
3. Results
3.1. Study Population and Clinical Data
3.2. miRNA Profiling Highlights a Subset of miRNAs Differently Expressed Between Normal and IH Veins from CKD Patients
3.3. Analysis of the AVF IH miRNA Subset in Human Vascular Cell Models
3.4. Pioglitazone, a PPAR-γ Agonist, Modulates miRNA Expression Levels In Vitro
3.5. miR-449a-5p Promotes Cell Proliferation, Migration and the Inflammatory Process
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AVF | arteriovenous fistula |
| AVFCs | arteriovenous fistula cells |
| CKD | chronic kidney disease |
| FDR | false discovery rate |
| HAOSMCs | human aortic smooth muscle cells |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| IH | intimal hyperplasia |
| miRNA | microRNA |
| NV | normal veins |
| NVCs | normal vein cells |
References
- Ma, S.; Duan, S.; Liu, Y.; Wang, H. Intimal Hyperplasia of Arteriovenous Fistula. Ann. Vasc. Surg. 2022, 85, 444–453. [Google Scholar] [CrossRef]
- Déglise, S.; Bechelli, C.; Allagnat, F. Vascular Smooth Muscle Cells in Intimal Hyperplasia, an Update. Front. Physiol. 2023, 13, 1081881. [Google Scholar] [CrossRef]
- Roy-Chaudhury, P.; Arend, L.; Zhang, J.; Krishnamoorthy, M.; Wang, Y.; Banerjee, R.; Samaha, A.; Munda, R. Neointimal Hyperplasia in Early Arteriovenous Fistula Failure. Am. J. Kidney Dis. 2007, 50, 782–790. [Google Scholar] [CrossRef]
- Martinez, L.; Tabbara, M.; Duque, J.C.; Selman, G.; Falcon, N.S.; Paez, A.; Griswold, A.J.; Ramos-Echazabal, G.; Hernandez, D.R.; Velazquez, O.C.; et al. Transcriptomics of Human Arteriovenous Fistula Failure: Genes Associated With Nonmaturation. Am. J. Kidney Dis. 2019, 74, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Chatterjee, P.; Dave, J.M.; Ostriker, A.C.; Greif, D.M.; Rzucidlo, E.M.; Martin, K.A. Targeting Smooth Muscle Cell Phenotypic Switching in Vascular Disease. JVS-Vasc. Sci. 2021, 2, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Mauro, R.; Rocchi, C.; Vasuri, F.; Pini, A.; Croci Chiocchini, A.L.; Ciavarella, C.; La Manna, G.; Pasquinelli, G.; Faggioli, G.; Gargiulo, M. Tissue Ki67 Proliferative Index Expression and Pathological Changes in Hemodialysis Arteriovenous Fistulae: Preliminary Single-Center Results. J. Vasc. Access 2021, 24, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Wei, S.; Xie, B.; Wang, Z.; Li, M.; Qiao, Z.; Sun, P.; Wang, W. Endothelial Nitric Oxide Synthase (eNOS) Mediates Neointimal Thickness in Arteriovenous Fistulae with Different Anastomotic Angles in Rats. J. Vasc. Access 2022, 23, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Moonen, J.-R.A.J.; Lee, E.S.; Schmidt, M.; Maleszewska, M.; Koerts, J.A.; Brouwer, L.A.; van Kooten, T.G.; van Luyn, M.J.A.; Zeebregts, C.J.; Krenning, G.; et al. Endothelial-to-Mesenchymal Transition Contributes to Fibro-Proliferative Vascular Disease and Is Modulated by Fluid Shear Stress. Cardiovasc. Res. 2015, 108, 377–386. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lai, Y.-J.; Tung, Y.-C.; Wu, L.-S.; Hsu, L.-A.; Tseng, C.-N.; Chang, G.-J.; Yang, K.-C.; Yeh, Y.-H. Osteopontin Mediation of Disturbed Flow–Induced Endothelial Mesenchymal Transition through CD44 Is a Novel Mechanism of Neointimal Hyperplasia in Arteriovenous Fistulae for Hemodialysis Access. Kidney Int. 2023, 103, 702–718. [Google Scholar] [CrossRef]
- Xu, Y.; Korayem, A.; Cruz-Solbes, A.S.; Chandel, N.; Sakata, T.; Mazurek, R.; Mavropoulos, S.A.; Kariya, T.; Aikawa, T.; Yamada, K.P.; et al. Inhibition of Endothelial-to-Mesenchymal Transition in a Large Animal Preclinical Arteriovenous Fistula Model Leads to Improved Remodelling and Reduced Stenosis. Cardiovasc. Res. 2024, 120, 1768–1779. [Google Scholar] [CrossRef]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a Big Role in Gene Regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Wang, K.; Deng, P.; Sun, Y.; Ye, P.; Zhang, A.; Wu, C.; Yue, Z.; Chen, Z.; Xia, J. MicroRNA-155 Promotes Neointimal Hyperplasia through Smooth Muscle-like Cell-Derived RANTES in Arteriovenous Fistulas. J. Vasc. Surg. 2018, 67, 933–944.e3. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, C.; Chen, J.; Yang, M.; Afzal, T.A.; An, W.; Maguire, E.M.; He, S.; Luo, J.; Wang, X.; et al. miRNA-200c-3p Promotes Endothelial to Mesenchymal Transition and Neointimal Hyperplasia in Artery Bypass Grafts. J. Pathol. 2021, 253, 209–224. [Google Scholar] [CrossRef]
- Kilari, S.; Cai, C.; Zhao, C.; Sharma, A.; Chernogubova, E.; Simeon, M.; Wu, C.-C.; Song, H.-L.; Maegdefessel, L.; Misra, S. The Role of MicroRNA-21 in Venous Neointimal Hyperplasia: Implications for Targeting miR-21 for VNH Treatment. Mol. Ther. 2019, 27, 1681–1693. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Huang, W.; Zhang, J.; Shi, Y.; Zhang, L. Altered microRNA Expression in Stenoses of Native Arteriovenous Fistulas in Hemodialysis Patients. J. Vasc. Surg. 2016, 63, 1034–1043.e3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ciavarella, C.; Motta, I.; Vasuri, F.; Palumbo, T.; Lisi, A.P.; Costa, A.; Astolfi, A.; Valente, S.; Versura, P.; Fornasiero, E.F.; et al. The PPAR-γ Agonist Pioglitazone Modulates Proliferation and Migration in HUVEC, HAOSMC and Human Arteriovenous Fistula-Derived Cells. Int. J. Mol. Sci. 2023, 24, 4424. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−∆∆Ct Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA Function with Experimental Support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0: Network-Based Visual Analytics for miRNA Functional Analysis and Systems Biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef]
- Shahri, J.J.; Saberianpour, S.; Kazemzadeh, G. Arteriovenous Fistula Aneurysm: Bench to Bedside. Indian J. Surg. 2023, 85, 219–227. [Google Scholar] [CrossRef]
- Mestres, G.; Barahona, F.; Yugueros, X.; Gamé, V.; Gil-Sala, D.; Blanco, C.; Fontseré, N.; Riambau, V. Inflow Artery Aneurysmal Degeneration After Long Term Native Arteriovenous Fistula for Haemodialysis. Eur. J. Vasc. Endovasc. Surg. 2023, 66, 849–854. [Google Scholar] [CrossRef]
- González-López, P.; Ares-Carral, C.; López-Pastor, A.R.; Infante-Menéndez, J.; González Illaness, T.; Vega de Ceniga, M.; Esparza, L.; Beneit, N.; Martín-Ventura, J.L.; Escribano, Ó.; et al. Implication of miR-155-5p and miR-143-3p in the Vascular Insulin Resistance and Instability of Human and Experimental Atherosclerotic Plaque. Int. J. Mol. Sci. 2022, 23, 10253. [Google Scholar] [CrossRef]
- Lopes-Ramos, C.M.; Paulson, J.N.; Chen, C.-Y.; Kuijjer, M.L.; Fagny, M.; Platig, J.; Sonawane, A.R.; DeMeo, D.L.; Quackenbush, J.; Glass, K. Regulatory Network Changes between Cell Lines and Their Tissues of Origin. BMC Genom. 2017, 18, 723. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Q.; Li, J.; Huang, C.; Feng, Y.-Q. Plasma MicroRNA-29c Levels Are Associated with Carotid Intima-Media Thickness and Is a Potential Biomarker for the Early Detection of Atherosclerosis. Cell. Physiol. Biochem. 2018, 50, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, A.J.; Liu, Y.; Fang, Y.; Ding, X.; Liang, M. The miR-29 Family: Genomics, Cell Biology, and Relevance to Renal and Cardiovascular Injury. Physiol. Genom. 2012, 44, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Torella, D.; Iaconetti, C.; Tarallo, R.; Marino, F.; Giurato, G.; Veneziano, C.; Aquila, I.; Scalise, M.; Mancuso, T.; Cianflone, E.; et al. miRNA Regulation of the Hyperproliferative Phenotype of Vascular Smooth Muscle Cells in Diabetes. Diabetes 2018, 67, 2554–2568. [Google Scholar] [CrossRef]
- Sharma, S.; Eghbali, M. Influence of Sex Differences on microRNA Gene Regulation in Disease. Biol. Sex Differ. 2014, 5, 3. [Google Scholar] [CrossRef]
- Islam, R.; Hong, Z. YAP/TAZ as Mechanobiological Signaling Pathway in Cardiovascular Physiological Regulation and Pathogenesis. Mechanobiol. Med. 2024, 2, 100085. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, X.; Wang, S.; Shi, B. MiR-449a Suppresses Cell Migration and Invasion by Targeting PLAGL2 in Breast Cancer. Pathol. Res. Pract. 2018, 214, 790–795. [Google Scholar] [CrossRef]
- Barati, T.; Mirzaei, Z.; Ebrahimi, A.; Shekari Khaniani, M.; Mansoori Derakhshan, S. miR-449a: A Promising Biomarker and Therapeutic Target in Cancer and Other Diseases. Cell Biochem. Biophys. 2024, 82, 1629–1650. [Google Scholar] [CrossRef]
- Zhu, R.; Qi, W.; Liu, T.; Liu, F. MicroRNA 449a Can Attenuate Protective Effect of Urokinase Against Pulmonary Embolism. Front. Pharmacol. 2022, 13, 713848. [Google Scholar] [CrossRef]
- Yu, J.; Liu, H.; Chen, Y.; Wang, L.; Chen, P.; Zhao, Y.; Ou, C.; Chen, W.; Hu, J.; Wang, Y.; et al. miR-449a Disturbs Atherosclerotic Plaque Stability in Streptozotocin and High-Fat Diet-Induced Diabetic Mice by Targeting CEACAM1. Diabetol. Metab. Syndr. 2024, 16, 98. [Google Scholar] [CrossRef]
- Jiang, L.; Hao, C.; Li, Z.; Zhang, P.; Wang, S.; Yang, S.; Wei, F.; Zhang, J. miR-449a Induces EndMT, Promotes the Development of Atherosclerosis by Targeting the Interaction between AdipoR2 and E-Cadherin in Lipid Rafts. Biomed. Pharmacother. 2019, 109, 2293–2304. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, H.; Zhong, W.; Zhang, Q.; Yu, Z. Expression Profiling and Bioinformatics Analysis of Circulating microRNAs in Patients with Acute Myocardial Infarction. J. Clin. Lab. Anal. 2020, 34, e23099. [Google Scholar] [CrossRef] [PubMed]
- Nesti, L.; Tricò, D.; Mengozzi, A.; Natali, A. Rethinking Pioglitazone as a Cardioprotective Agent: A New Perspective on an Overlooked Drug. Cardiovasc. Diabetol. 2021, 20, 109. [Google Scholar] [CrossRef] [PubMed]
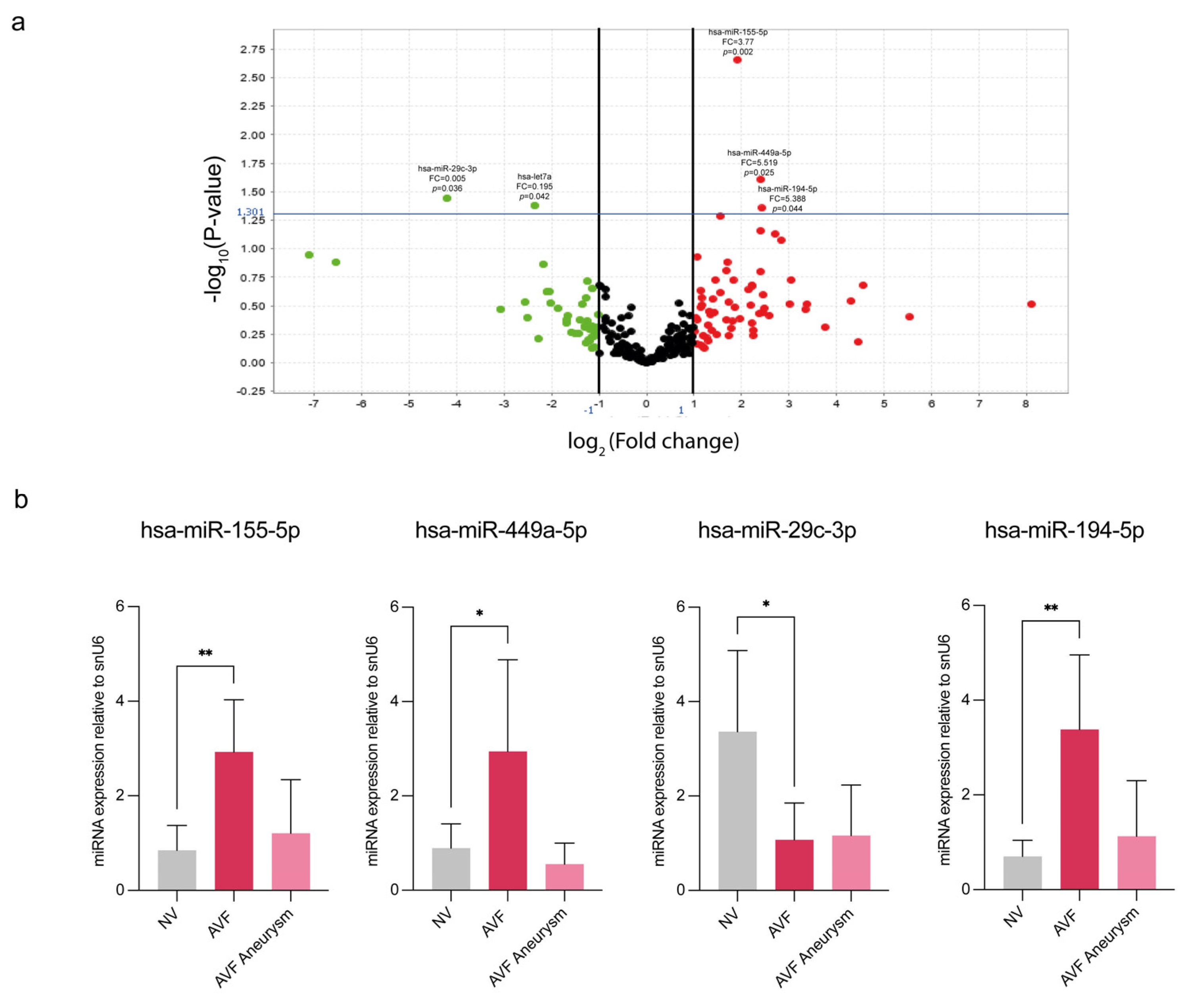
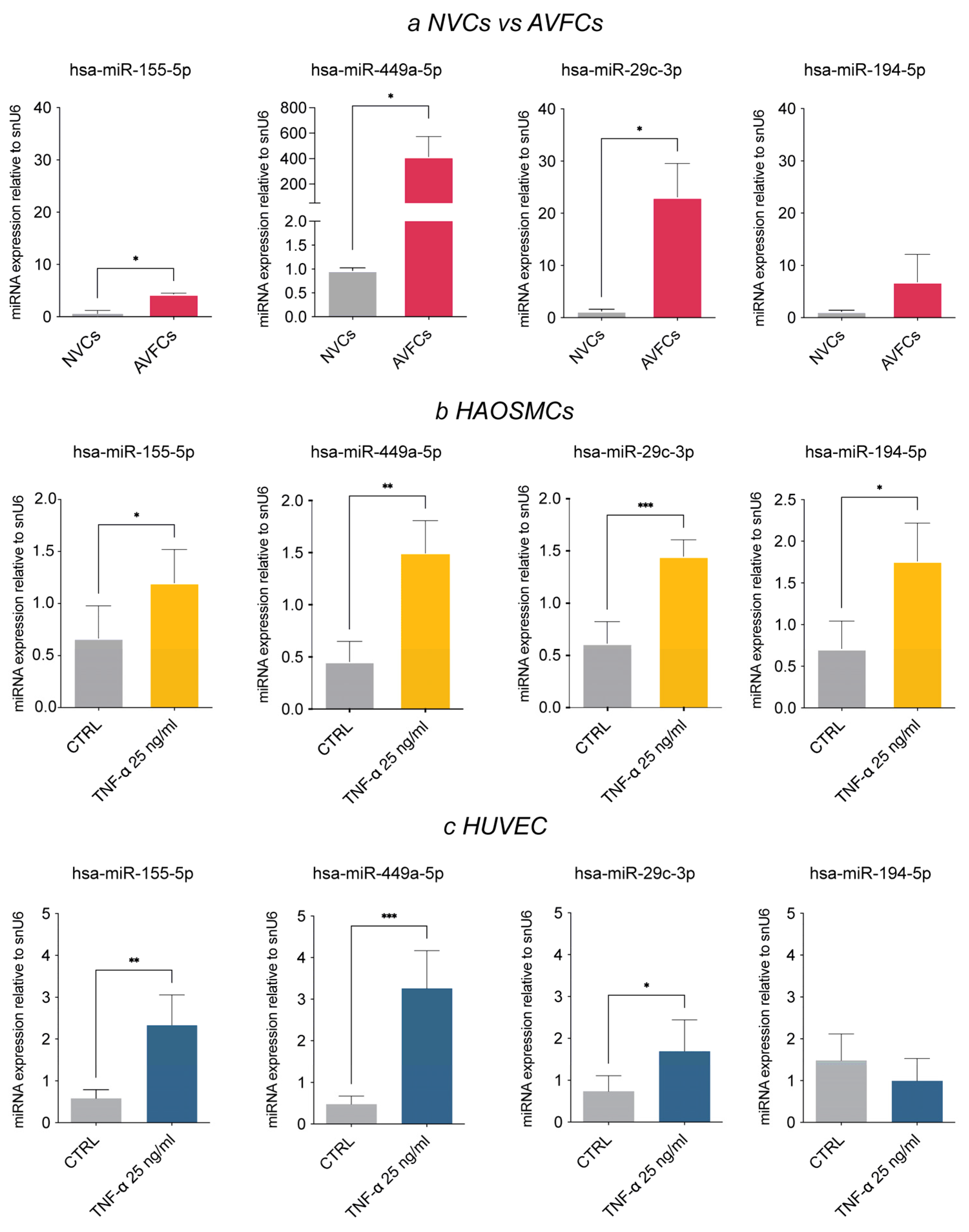
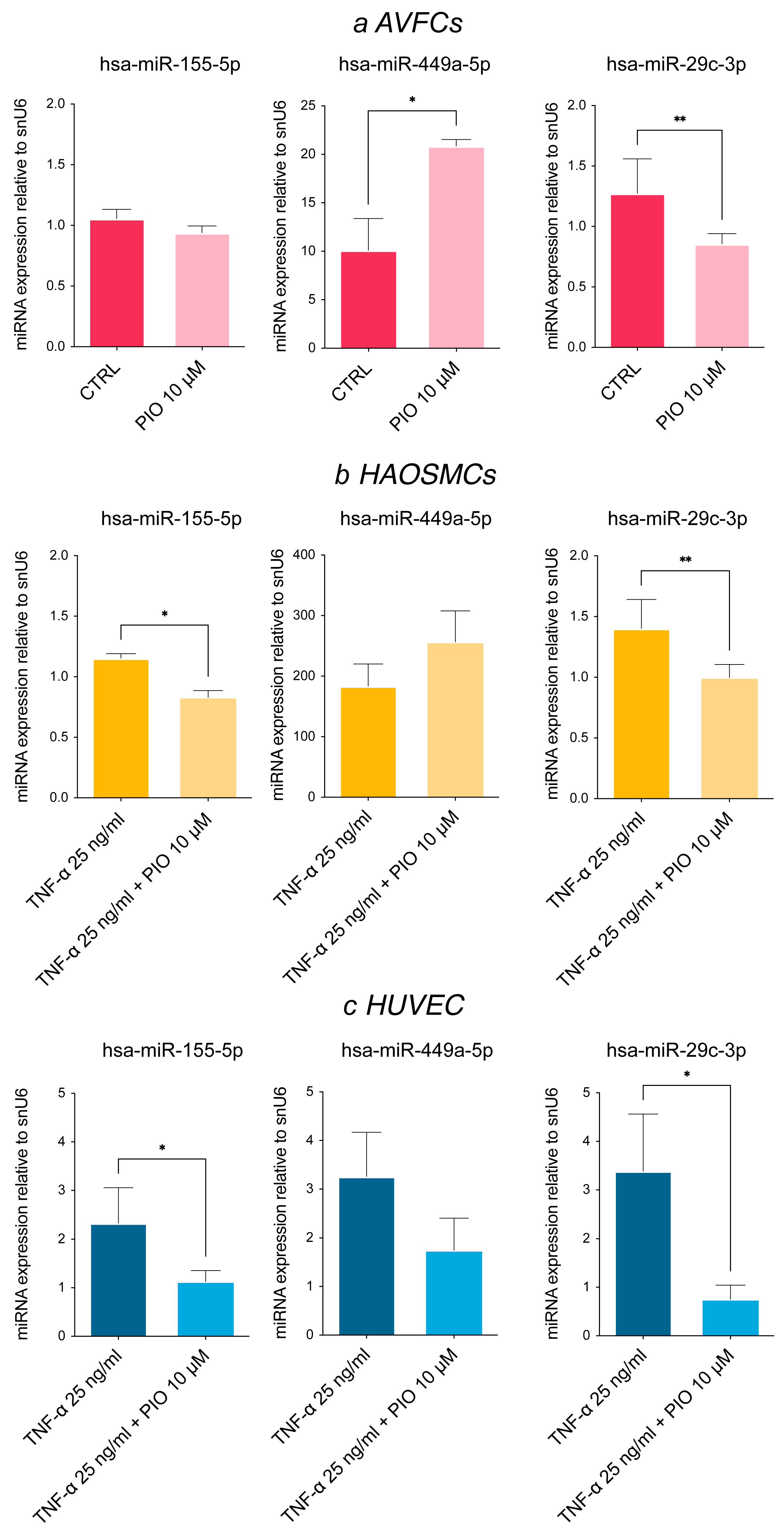
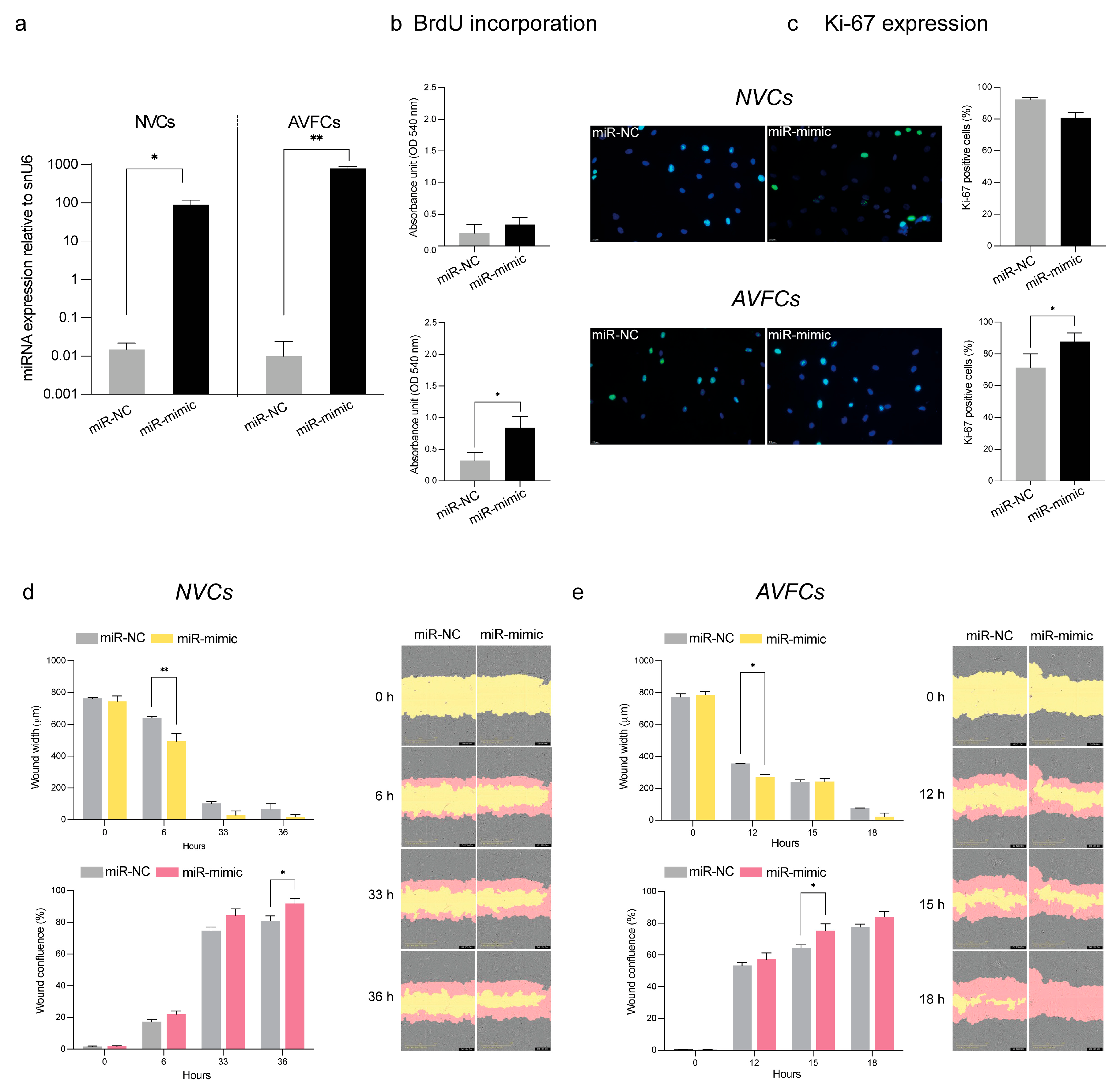
| Demographic and Clinical Data | Patient Group | |
|---|---|---|
| NV (n = 8) | AVF (n = 8) | |
| Mean age | 69 ± 16 | 60 ± 12 |
| Males | 7 | 4 |
| Females | 1 | 7 |
| Diabetes | 0 | 1 |
| Hypertension | 7 | 10 |
| Vein fibrosis | 1 | 1 |
| Vein stenosis | 0 | 1 |
| Array Card | Single Assay qPCR | ||||
|---|---|---|---|---|---|
| miRNA Assay Name | miRBase Accession Number | Fold Change AVF vs. NV | p Value | Fold Change AVF vs. NV | p Value |
| hsa-miR-155-5p | MIMAT0000646 | 3.77 | 0.002 | 2.930 | 0.002 |
| hsa-miR-449a-5p | MIMAT0001541 | 5.319 | 0.025 | 2.945 | 0.03 |
| hsa-miR-29c-3p | MIMAT0000681 | 0.055 | 0.036 | 0.870 | 0.01 |
| hsa-miR-194-5p | MIMAT0000460 | 5.388 | 0.044 | 3.38 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciavarella, C.; Vasuri, F.; Degiovanni, A.; Christ, L.; Mauro, R.; Gargiulo, M.; Pasquinelli, G. A Distinct miRNA Profile in Intimal Hyperplasia of Failed Arteriovenous Fistulas Reveals Key Pathogenic Pathways. Biomolecules 2025, 15, 1064. https://doi.org/10.3390/biom15081064
Ciavarella C, Vasuri F, Degiovanni A, Christ L, Mauro R, Gargiulo M, Pasquinelli G. A Distinct miRNA Profile in Intimal Hyperplasia of Failed Arteriovenous Fistulas Reveals Key Pathogenic Pathways. Biomolecules. 2025; 15(8):1064. https://doi.org/10.3390/biom15081064
Chicago/Turabian StyleCiavarella, Carmen, Francesco Vasuri, Alessio Degiovanni, Lena Christ, Raffaella Mauro, Mauro Gargiulo, and Gianandrea Pasquinelli. 2025. "A Distinct miRNA Profile in Intimal Hyperplasia of Failed Arteriovenous Fistulas Reveals Key Pathogenic Pathways" Biomolecules 15, no. 8: 1064. https://doi.org/10.3390/biom15081064
APA StyleCiavarella, C., Vasuri, F., Degiovanni, A., Christ, L., Mauro, R., Gargiulo, M., & Pasquinelli, G. (2025). A Distinct miRNA Profile in Intimal Hyperplasia of Failed Arteriovenous Fistulas Reveals Key Pathogenic Pathways. Biomolecules, 15(8), 1064. https://doi.org/10.3390/biom15081064








