Regulatory Effect of PGE2-EP2/EP4 Receptor Pathway on Staphylococcus aureus-Induced Inflammatory Factors in Dairy Cow Neutrophils
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Antibodies and Molecular Reagents
2.1.2. Chemical Inhibitors
2.1.3. Equipment
2.2. Isolation, Culture, and Identification of Neutrophils in Dairy Cows
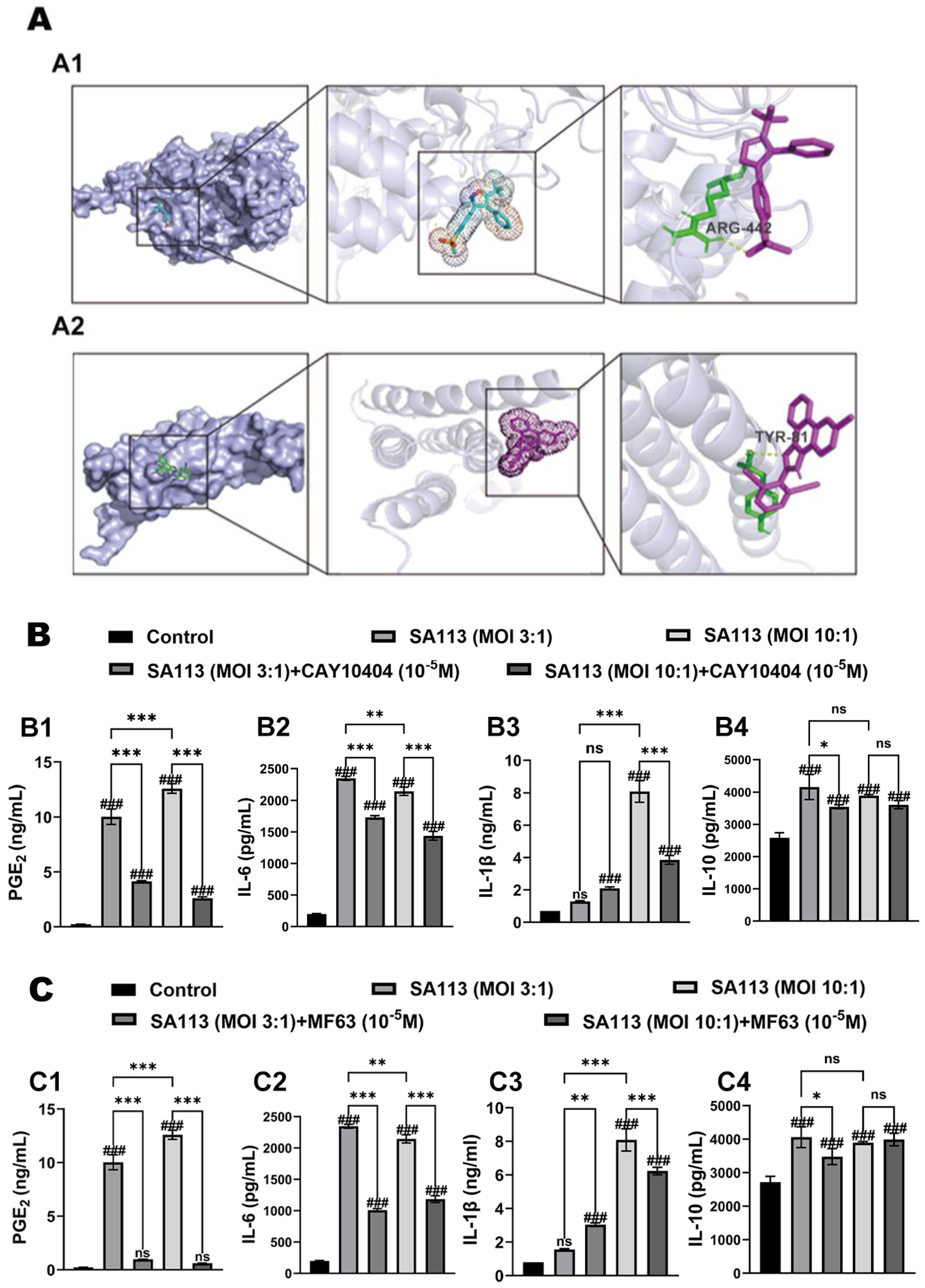
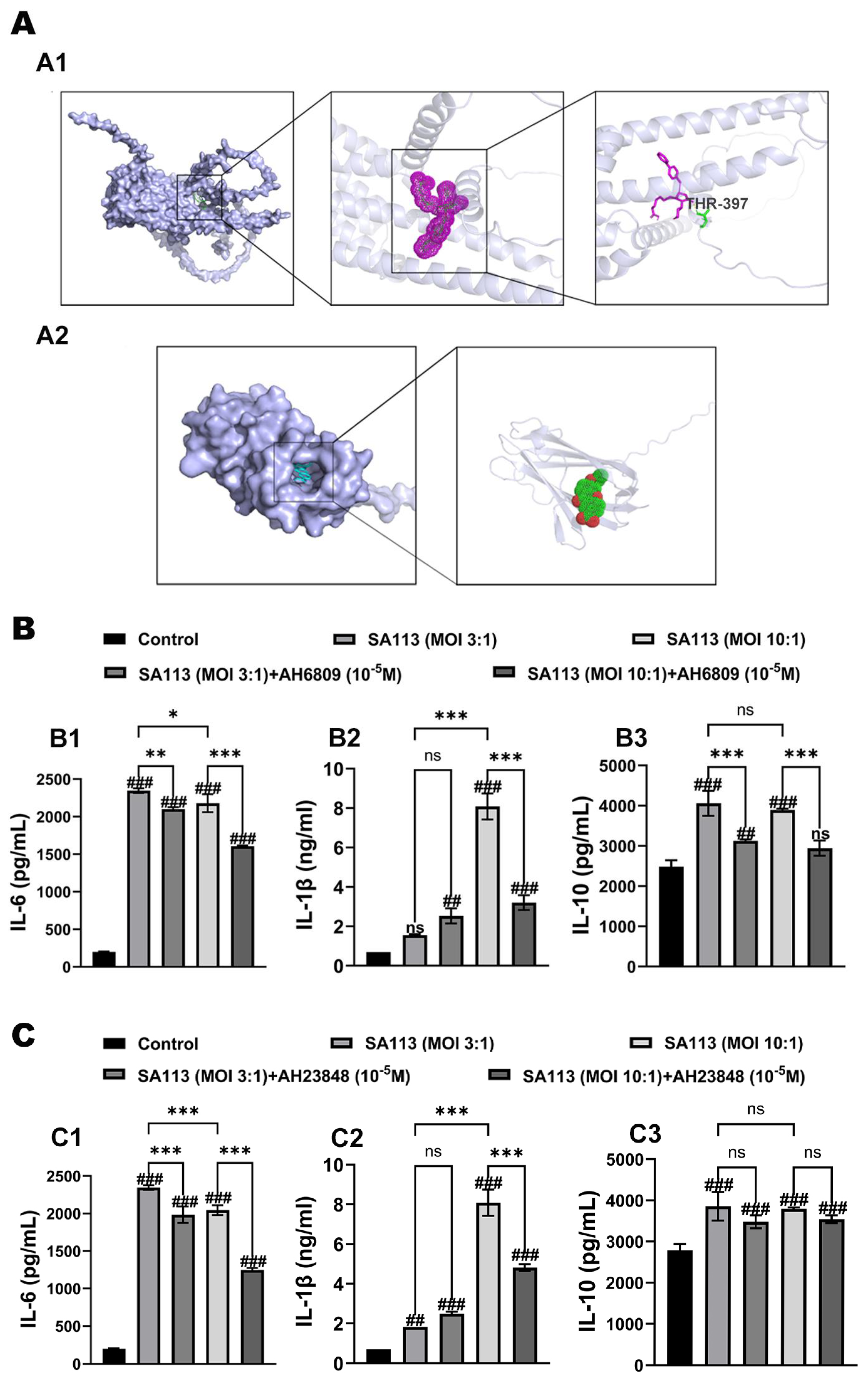
2.3. Molecular Docking
2.4. Effects of Various Inhibitors on Cytokine Production in Bovine Neutrophils Induced by S. aureus
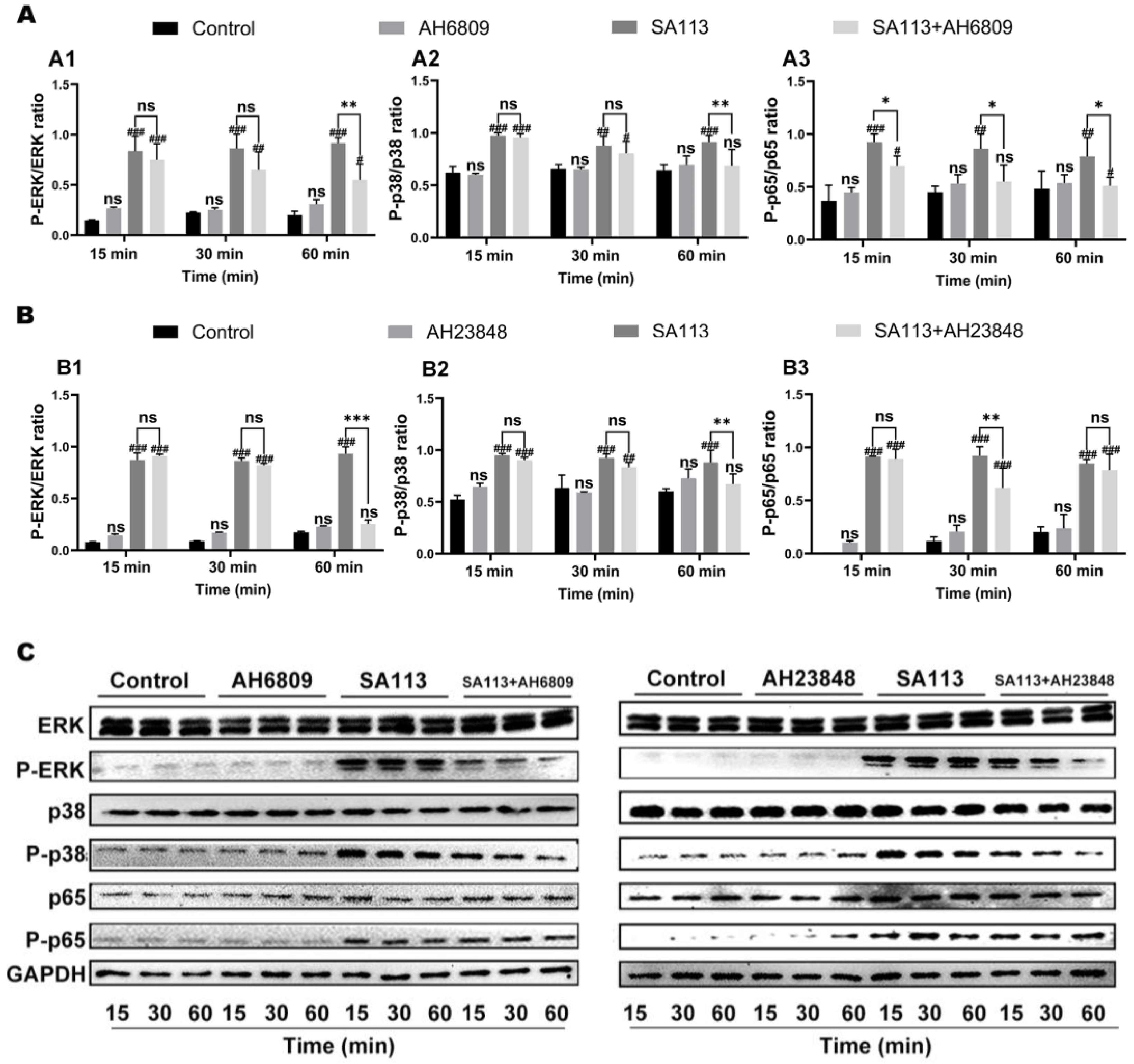
2.5. Effects of Various Inhibitors on the Activation of MAPK and NF-κB Signaling Pathways in Bovine Neutrophils Induced by S. aureus
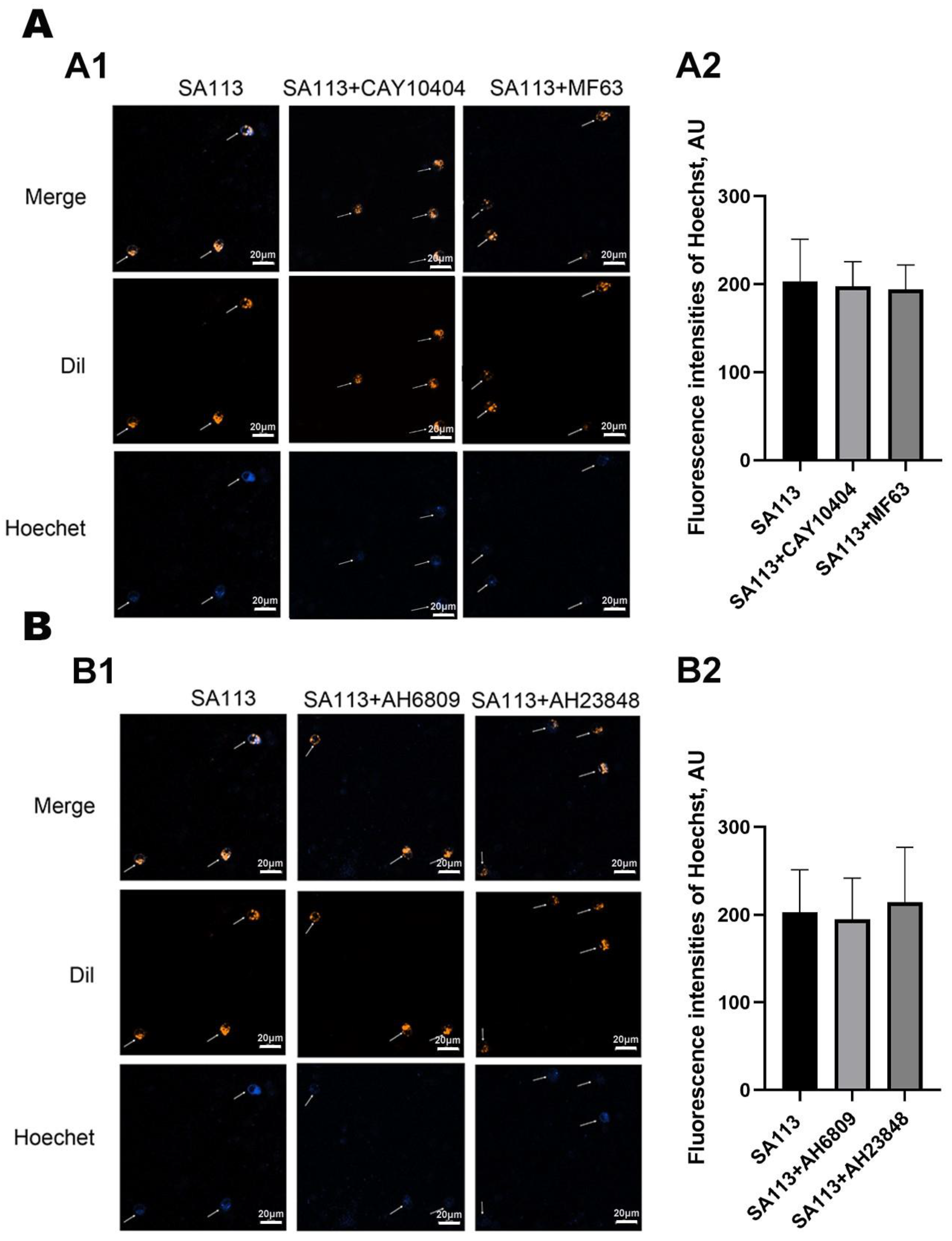
2.6. Effect of Various Inhibitors on Phagocytic Activity of Dairy Cow Neutrophils
2.7. Statistical Analysis
3. Results
3.1. Effects of COX-2 and mPGES-1 Inhibitors on Expression of Inflammatory Factors in Dairy Cow Neutrophils Induced by S. aureus
3.1.1. Verification of Molecular Docking for Inhibitors
3.1.2. Effect of COX-2 and mPGES-1 Inhibitors on Expression Levels of PGE2 and Inflammatory Factors in Dairy Cow Neutrophils Induced by S. aureus Infection
3.1.3. Effects of COX-2 and mPGES-1 Inhibitors on Activation of MAPK and NF-κB Signaling Pathways in Dairy Cow Neutrophils Induced by S. aureus Infection
3.2. Effects of EP2 and EP4 Receptor Inhibitors on the Post-Inflammatory Response of Dairy Cow Neutrophils Induced by S. aureus Infection
3.2.1. Verification of Molecular Docking for Inhibitors
3.2.2. Effects of EP2 and EP4 Receptor Inhibitors on Cytokine Expression in Dairy Cow Neutrophils Induced by S. aureus Infection
3.2.3. Effects of EP2 and EP4 Receptor Inhibition on Activation of MAPK and NF-κB Pathways in Dairy Cow Neutrophils Induced by S. aureus Infection
3.3. Effect of COX-2 and mPGES-1 Inhibitors, as Well as EP2 and EP4 Receptor Antagonists, on the Phagocytic Activity of Neutrophils in Dairy Cows Against S. aureus Infection
3.3.1. Effect of COX-2 and mPGES-1 Inhibitors on Phagocytic Activity of Neutrophils in Dairy Cows Against S. aureus Infection
3.3.2. Effect of EP2 and EP4 Receptor Antagonists on Phagocytic Activity of Neutrophils in Dairy Cows Against S. aureus Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| AH23848 | EP4 receptor blocker |
| ANOVA | Analysis of variance |
| CAY10404 | Cyclooxygenase-2 suppressor |
| COX-2 | Cyclooxygenase-2 |
| ELISA | Enzyme-linked immunosorbent assay |
| ERK | Extracellular signal-regulated kinase |
| mPGES-1 | Microsomal prostaglandin E synthase-1 |
| MF63 | Microsomal prostaglandin E synthase-1 inhibitor |
| NSAIDs | Non-steroidal anti-inflammatory medications |
| PGE2 | Prostaglandin E2 |
References
- Kitaya, K.; Takeuchi, T.; Mizuta, S.; Matsubayashi, H.; Ishikawa, T. Endometritis: New time, new concepts. Fertil. Steril. 2018, 110, 344–350. [Google Scholar] [CrossRef]
- Wang, M.L.; Liu, M.C.; Xu, J.; An, L.G.; Wang, J.F.; Zhu, Y.H. Uterine Microbiota of Dairy Cows With Clinical and Subclinical Endometritis. Front. Microbiol. 2018, 9, 2691. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Berends, E.T.M.; Zheng, X.H.; Hill, P.J.; Chan, R.; Torres, V.J.; Wozniak, D.J. Leukocidins and the nuclease nuc prevent neutrophil-mediated killing of Staphylococcus aureus biofilms. Infect. Immun. 2020, 88, e00372-00320. [Google Scholar] [CrossRef]
- Kienle, K.; Lammermann, T. Neutrophil swarming: An essential process of the neutrophil tissue response. Immunol. Rev. 2016, 273, 76–93. [Google Scholar] [CrossRef]
- McGuinness, W.A.; Kobayashi, S.D.; DeLeo, F.R. Evasion of Neutrophil Killing by Staphylococcus aureus. Pathogens 2016, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Skendros, P.; Mitroulis, I.; Ritis, K. Autophagy in neutrophils: From granulopoiesis to neutrophil extracellular traps. Front. Cell Dev. Biol. 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zhang, T.; Liu, M.; Zhou, L.Y.; Qi, M.Z.; Xie, X.; Shi, X.Y.; Gu, X.P.; Ma, Z.L. Exosomal PGE2 from M2 macrophages inhibits neutrophil recruitment and NET formation through lipid mediator class switching in sepsis. J. Biomed. Sci. 2023, 30, 62. [Google Scholar] [CrossRef]
- Loynes, C.A.; Lee, J.A.; Robertson, A.L.; Steel, M.J.; Ellett, F.; Feng, Y.; Levy, B.D.; Whyte, M.K.B.; Renshaw, S.A. PGE2 production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci. Adv. 2018, 4, eaar8320. [Google Scholar] [CrossRef]
- Wang, Z.X.; Wei, X.Y.; Ji, C.L.; Yu, W.H.; Song, C.W.; Wang, C.Z. PGE2 inhibits neutrophil phagocytosis through the EP2R-cAMP-PTEN pathway. Immun. Inflamm. Dis. 2022, 10, e662. [Google Scholar] [CrossRef]
- Mahesh, G.; Anil Kumar, K.; Reddanna, P. Overview on the discovery and development of anti-inflammatory drugs: Should the focus be on synthesis or degradation of PGE2? J. Inflamm. Res. 2021, 14, 253–263. [Google Scholar] [CrossRef]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Hu, S.; Wan, Q.; Xu, C.; Chen, H.; Zhu, L.; Xu, Z.; Meng, J.; Breyer, R.M.; Li, N.; et al. Protective role of mPGES-1 (Microsomal Prostaglandin E Synthase-1)-Derived PGE2 (Prostaglandin E2) and the Endothelial EP4 (Prostaglandin E Receptor) in vascular responses to injury. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1115–1124. [Google Scholar] [CrossRef]
- McLane, R.D.; Le Cozannet-Laidin, L.; Boyle, M.S.; Lanzillotta, L.; Taylor, Z.L.; Anthony, S.R.; Tranter, M.; Onorato, A.J. Synthesis and PGE2 inhibitory activity of novel diarylheptanoids. Bioorg. Med. Chem. Lett. 2018, 28, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Kehm, R.D.; Hopper, J.L.; John, E.M.; Phillips, K.A.; MacInnis, R.J.; Dite, G.S.; Milne, R.L.; Liao, Y.Y.; Zeinomar, N.; Knight, J.A.; et al. Regular use of aspirin and other non-steroidal anti-inflammatory drugs and breast cancer risk for women at familial or genetic risk: A cohort study. Breast Cancer Res. 2019, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.O.; Hjemdahl, P. Lessons from 20 years with COX-2 inhibitors: Importance of dose-response considerations and fair play in comparative trials. J. Intern. Med. 2022, 292, 557–574. [Google Scholar] [CrossRef]
- Ng, P.Y.; Ng, A.K.; Ip, A.; Sin, W.C.; Yiu, K.H. Atherothrombotic Outcomes After Sodium-Glucose Cotransporter 2 Inhibitors Versus Dipeptidyl Peptidase-4 Inhibitors in Patients With Type 2 Diabetes: A Territory-Wide Retrospective Cohort Study. J. Am. Heart Assoc. 2025, 14, e037207. [Google Scholar] [CrossRef]
- Weigert, A.; Strack, E.; Snodgrass, R.G.; Brune, B. mPGES-1 and ALOX5/-15 in tumor-associated macrophages. Cancer Metastasis Rev. 2018, 37, 317–334. [Google Scholar] [CrossRef]
- Bergqvist, F.; Morgenstern, R.; Jakobsson, P.J. A review on mPGES-1 inhibitors: From preclinical studies to clinical applications. Prostaglandins Other Lipid Mediat. 2020, 147, 106383. [Google Scholar] [CrossRef]
- Kim, S.H.; Hashimoto, Y.; Cho, S.N.; Roszik, J.; Milton, D.R.; Dal, F.; Kim, S.F.; Menter, D.G.; Yang, P.; Ekmekcioglu, S.; et al. Microsomal PGE2 synthase-1 regulates melanoma cell survival and associates with melanoma disease progression. Pigment Cell Melanoma Res. 2016, 29, 297–308. [Google Scholar] [CrossRef]
- Salazar, F.; Vazquez, M.L.; Masferrer, J.L.; Mbalaviele, G.; Llinas, M.T.; Saez, F.; Arhancet, G.; Salazar, F.J. Renal effects induced by prolonged mPGES1 inhibition. Am. J. Physiol. Ren. Physiol. 2014, 306, F68–F74. [Google Scholar] [CrossRef][Green Version]
- Li, T.T.; Liu, B.; Guan, H.; Mao, W.; Wang, L.R.; Zhang, C.; Hai, L.L.; Liu, K.; Cao, J.S. PGE2 increases inflammatory damage in Escherichia coli-infected bovine endometrial tissue in vitro via the EP4-PKA signaling pathway. Biol. Reprod. 2019, 100, 175–186. [Google Scholar] [CrossRef]
- Arora, M.; Choudhary, S.; Singh, P.K.; Sapra, B.; Silakari, O. Structural investigation on the selective COX-2 inhibitors mediated cardiotoxicity: A review. Life Sci. 2020, 251, 117631. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.H.; Jia, J.P. Natural COX-2 inhibitors as promising anti-inflammatory agents: An update. Curr. Med. Chem. 2021, 28, 3622–3646. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Smith, C.L.; Hu, L.; Campanale, K.M.; Stoltz, R.; Huffman, L.G., Jr.; McNearney, T.A.; Yang, X.Y.; Ackermann, B.L.; Dean, R.; et al. Pharmacodynamic comparison of LY3023703, a novel microsomal prostaglandin e synthase 1 inhibitor, with celecoxib. Clin. Pharmacol. Ther. 2016, 99, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, M.; Doble, M. Transcriptional regulation of mPGES1 in cancer: An alternative approach to drug discovery? Curr. Drug Targets 2017, 18, 119–131. [Google Scholar] [CrossRef]
- Tuure, L.; Pemmari, A.; Hamalainen, M.; Moilanen, T.; Moilanen, E. Regulation of gene expression by MF63, a selective inhibitor of microsomal PGE synthase 1 (mPGES1) in human osteoarthritic chondrocytes. Br. J. Pharmacol. 2020, 177, 4134–4146. [Google Scholar] [CrossRef]
- Thimmappa, P.Y.; Vasishta, S.; Ganesh, K.; Nair, A.S.; Joshi, M.B. Neutrophil (dys)function due to altered immuno-metabolic axis in type 2 diabetes: Implications in combating infections. Hum. Cell 2023, 36, 1265–1282. [Google Scholar] [CrossRef]
- Ueta, M.; Sotozono, C.; Yamada, K.; Yokoi, N.; Inatomi, T.; Kinoshita, S. Expression of prostaglandin E receptor subtype EP4 in conjunctival epithelium of patients with ocular surface disorders: Case-control study. BMJ Open 2012, 2, e001330. [Google Scholar] [CrossRef][Green Version]
- Zhang, M.J.; Feigenson, M.; Sheu, T.J.; Awad, H.A.; Schwarz, E.M.; Jonason, J.H.; Loiselle, A.E.; O’Keefe, R.J. Loss of the PGE2 receptor EP1 enhances bone acquisition, which protects against age and ovariectomy-induced impairments in bone strength. Bone 2015, 72, 92–100. [Google Scholar] [CrossRef]
- Qiu, J.; Li, Q.; Li, J.; Zhou, F.; Sang, P.; Xia, Z.; Wang, W.; Wang, L.; Yu, Y.; Jiang, J. Complementary roles of EP2 and EP4 receptors in malignant glioma. Br. J. Pharmacol. 2023, 180, 2623–2640. [Google Scholar] [CrossRef]
- Steinmetz-Späh, J.; Jakobsson, P.J. The anti-inflammatory and vasoprotective properties of mPGES-1 inhibition offer promising therapeutic potential. Expert Opin. Ther. Targets 2023, 27, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Neuschäfer-Rube, F.; Schön, T.; Kahnt, I.; Püschel, G.P. LDL-dependent regulation of TNFα/PGE2 induced COX-2/mPGES-1 expression in human macrophage cell lines. Inflammation 2023, 46, 893–911. [Google Scholar] [CrossRef] [PubMed]
- Rausch-Derra, L.; Huebner, M.; Wofford, J.; Rhodes, L. A prospective, randomized, masked, placebo-controlled multisite clinical study of grapiprant, an EP4 prostaglandin receptor antagonist (PRA), in dogs with osteoarthritis. J. Vet. Intern. Med. 2016, 30, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, W.; Wu, N.N.; Rong, N.; Kang, C.; Jian, S.J.; Chen, C.L.; Chen, C.; Zhang, X.Y. Synthesis of Escherichia coli OmpA oral nanoparticles and evaluation of immune function against the main pathogenic bacteria of cow mastitis. Vaccines 2021, 9, 304. [Google Scholar] [CrossRef]
- Yimin; Kohanawa, M.; Zhao, S.J.; Ozaki, M.; Haga, S.; Nan, G.X.; Kuge, Y.; Tamaki, N. Contribution of toll-like receptor 2 to the innate response against Staphylococcus aureus infection in mice. PLoS ONE 2013, 8, e74287. [Google Scholar] [CrossRef]
- Bogado Pascottini, O.; LeBlanc, S.J.; Gnemi, G.; Leroy, J.; Opsomer, G. Genesis of clinical and subclinical endometritis in dairy cows. Reproduction 2023, 166, R15–R24. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Jia, Y.; Cheng, M.L.; Wu, D.J.; Tohti, K.; Zhu, J.G. Single-chain fragment variables targeting leukocidin ED can alleviate the inflammation of Staphylococcus aureus-induced mastitis in mice. Int. J. Mol. Sci. 2021, 23, 334. [Google Scholar] [CrossRef]
- Zhang, K.; Jia, Y.; Qian, Y.H.; Jiang, X.Y.; Zhang, S.Y.; Liu, B.; Cao, J.S.; Song, Y.L.; Mao, W. Staphylococcus aureus increases Prostaglandin E2 secretion in cow neutrophils by activating TLR2, TLR4, and NLRP3 inflammasome signaling pathways. Front. Microbiol. 2023, 14, 1163261. [Google Scholar] [CrossRef]
- Wu, J.D.; Liu, B.; Mao, W.; Feng, S.; Yao, Y.; Bai, F.; Shen, Y.; Guleng, A.; Jirigala, B.; Cao, J.S. Prostaglandin E2 regulates activation of mouse peritoneal macrophages by Staphylococcus aureus through Toll-Like Receptor 2, Toll-Like Receptor 4, and NLRP3 inflammasome signaling. J. Innate Immun. 2020, 12, 154–169. [Google Scholar] [CrossRef]
- Ohkura, N.; Yoshiba, K.; Yoshiba, N.; Oda, Y.; Edanami, N.; Ohshima, H.; Takenaka, S.; Okiji, T.; Noiri, Y. Prostaglandin E2-transporting pathway and its roles via EP2/EP4 in cultured human dental pulp. J. Endod. 2023, 49, 410–418. [Google Scholar] [CrossRef]
- Gao, Y.H.; Shang, B.B.; He, Y.Y.; Deng, W.; Wang, L.; Sui, S.G. The mechanism of Gejie Zhilao Pill in treating tuberculosis based on network pharmacology and molecular docking verification. Front. Cell. Infect. Microbiol. 2024, 14, 1405627. [Google Scholar] [CrossRef]
- Gill, S.K.; Yao, Y.W.; Kay, L.J.; Bewley, M.A.; Marriott, H.M.; Peachell, P.T. The anti-inflammatory effects of PGE2 on human lung macrophages are mediated by the EP4 receptor. Br. J. Pharmacol. 2016, 173, 3099–3109. [Google Scholar] [CrossRef]
- Jayasinghe, A.M.K.; Kirindage, K.; Fernando, I.P.S.; Kim, K.N.; Oh, J.Y.; Ahn, G. The anti-inflammatory effect of low molecular weight fucoidan from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophages via inhibiting NF-kappaB/MAPK signaling pathways. Mar. Drugs 2023, 21, 601. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.Y.; Hou, Y.N.; Li, J.; Ma, K.; Yao, G.D.; Liu, W.W.; Hayashi, T.; Itoh, K.; Tashiro, S.I.; Onodera, S.; et al. Prostaglandin E2 attenuates synergistic bactericidal effects between COX inhibitors and antibiotics on Staphylococcus aureus. Prostaglandins Leukot. Essent. Fat. Acids 2018, 133, 16–22. [Google Scholar] [CrossRef]
- Krause, J.; Geginat, G.; Tammer, I. Prostaglandin E2 from Candida albicans Stimulates the Growth of Staphylococcus aureus in Mixed Biofilms. PLoS ONE 2015, 10, e0135404. [Google Scholar] [CrossRef]
- Laudy, A.E.; Mrowka, A.; Krajewska, J.; Tyski, S. The influence of efflux pump inhibitors on the activity of non-antibiotic NSAIDS against gram-negative rods. PLoS ONE 2016, 11, e0147131. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, C.; Liu, B.; Zhang, S.; Wang, Y.; Qian, Y.; Gong, Z.; Zhao, J.; Yang, X.; Bai, Y.; et al. Regulatory Effect of PGE2-EP2/EP4 Receptor Pathway on Staphylococcus aureus-Induced Inflammatory Factors in Dairy Cow Neutrophils. Biomolecules 2025, 15, 1062. https://doi.org/10.3390/biom15081062
Zhao Y, Wang C, Liu B, Zhang S, Wang Y, Qian Y, Gong Z, Zhao J, Yang X, Bai Y, et al. Regulatory Effect of PGE2-EP2/EP4 Receptor Pathway on Staphylococcus aureus-Induced Inflammatory Factors in Dairy Cow Neutrophils. Biomolecules. 2025; 15(8):1062. https://doi.org/10.3390/biom15081062
Chicago/Turabian StyleZhao, Yi, Chao Wang, Bo Liu, Shuangyi Zhang, Yongfei Wang, Yinghong Qian, Zhiguo Gong, Jiamin Zhao, Xiaolin Yang, Yuting Bai, and et al. 2025. "Regulatory Effect of PGE2-EP2/EP4 Receptor Pathway on Staphylococcus aureus-Induced Inflammatory Factors in Dairy Cow Neutrophils" Biomolecules 15, no. 8: 1062. https://doi.org/10.3390/biom15081062
APA StyleZhao, Y., Wang, C., Liu, B., Zhang, S., Wang, Y., Qian, Y., Gong, Z., Zhao, J., Yang, X., Bai, Y., & Mao, W. (2025). Regulatory Effect of PGE2-EP2/EP4 Receptor Pathway on Staphylococcus aureus-Induced Inflammatory Factors in Dairy Cow Neutrophils. Biomolecules, 15(8), 1062. https://doi.org/10.3390/biom15081062




