Bio-Fabricated Aluminum Oxide Nanoparticles Derived from Waste Pharmaceutical Packages: Insight into Characterization and Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Biogenic P/Al2O3-NPs
2.1.1. pH Zero Point Charge (pHzpc)
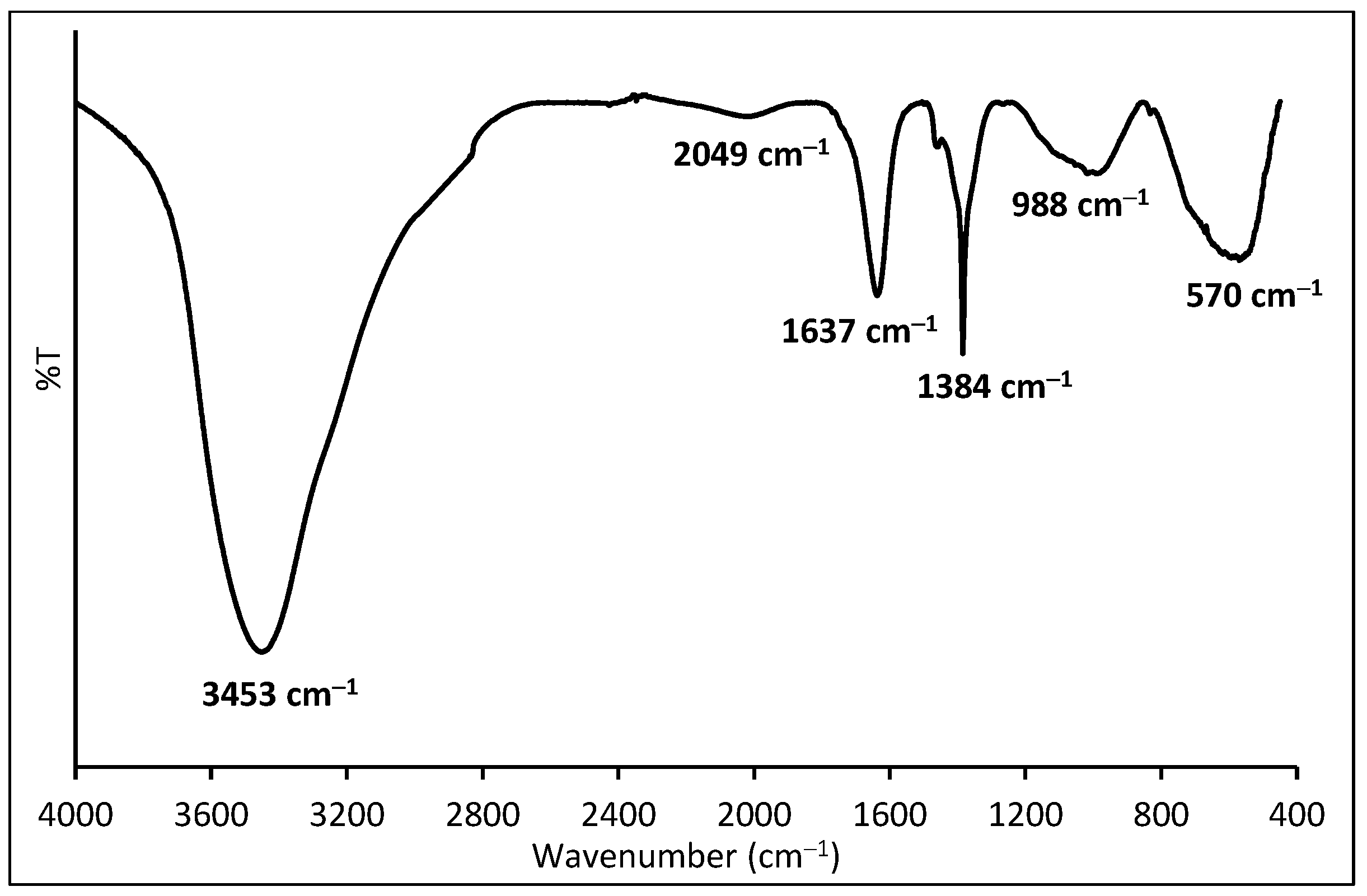
2.1.2. FTIR Spectra
2.1.3. Energy-Dispersive X-Ray Spectroscopy
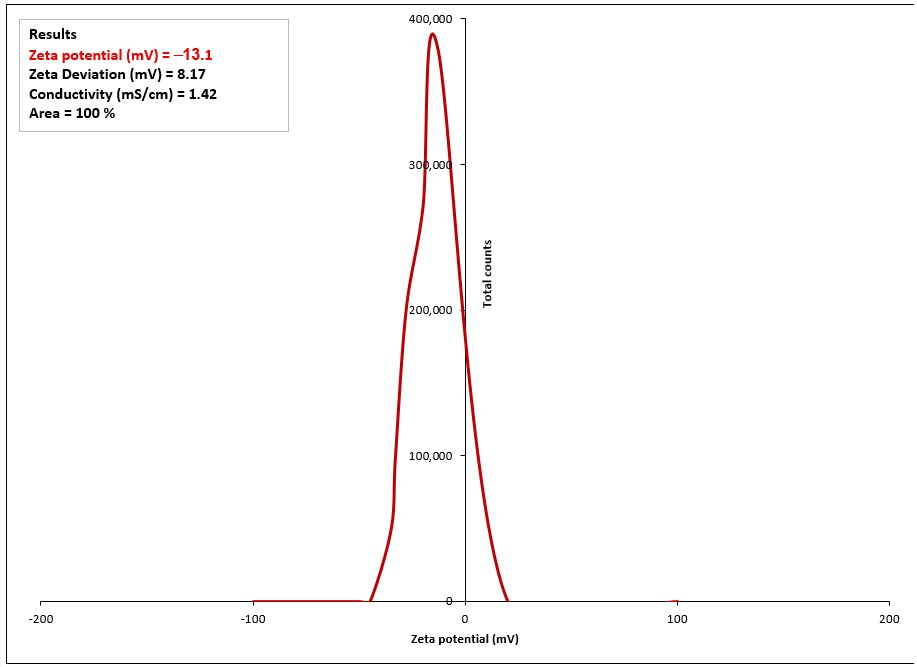
2.1.4. Zeta Potential Analysis
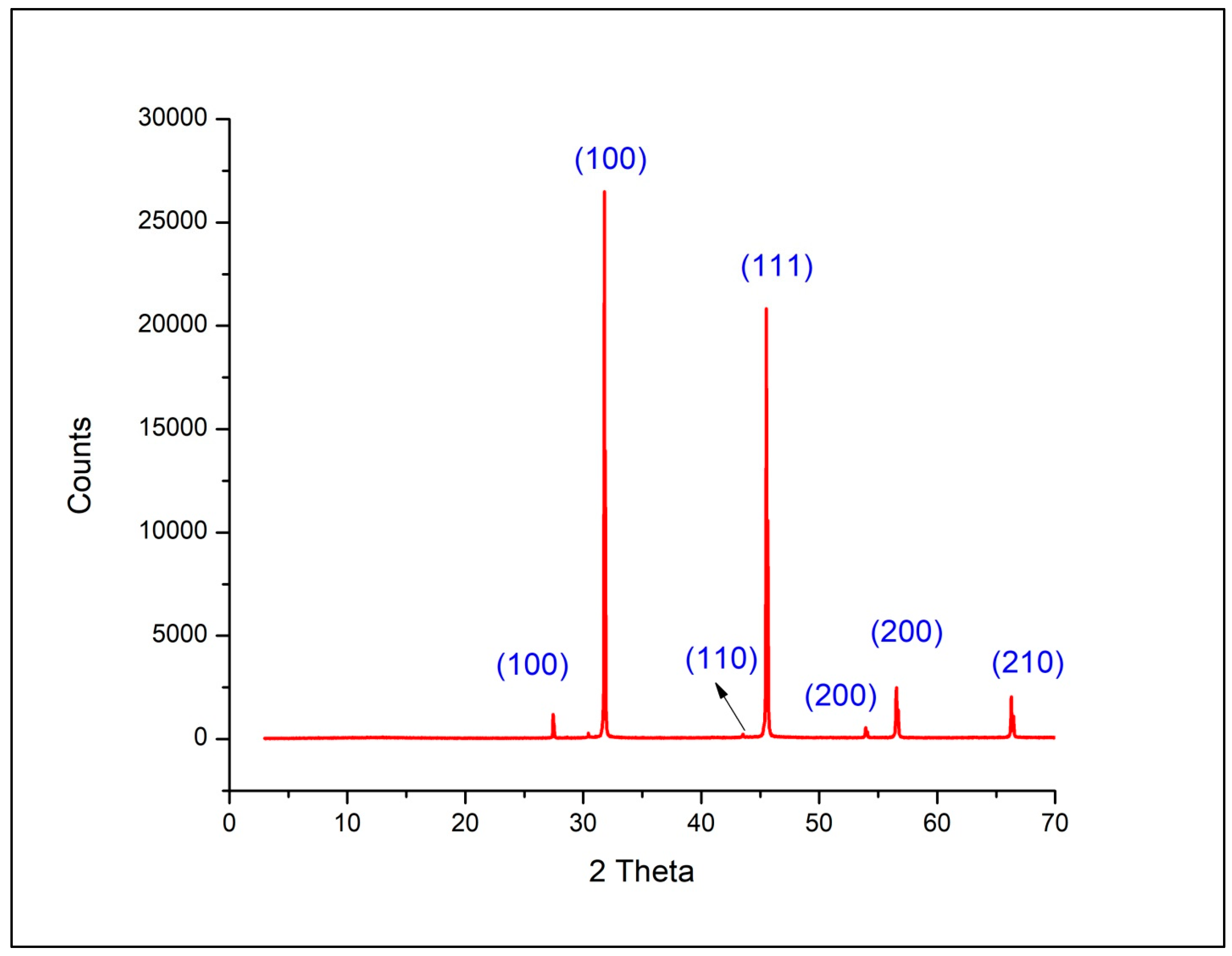
2.1.5. X-Ray Diffraction Analysis
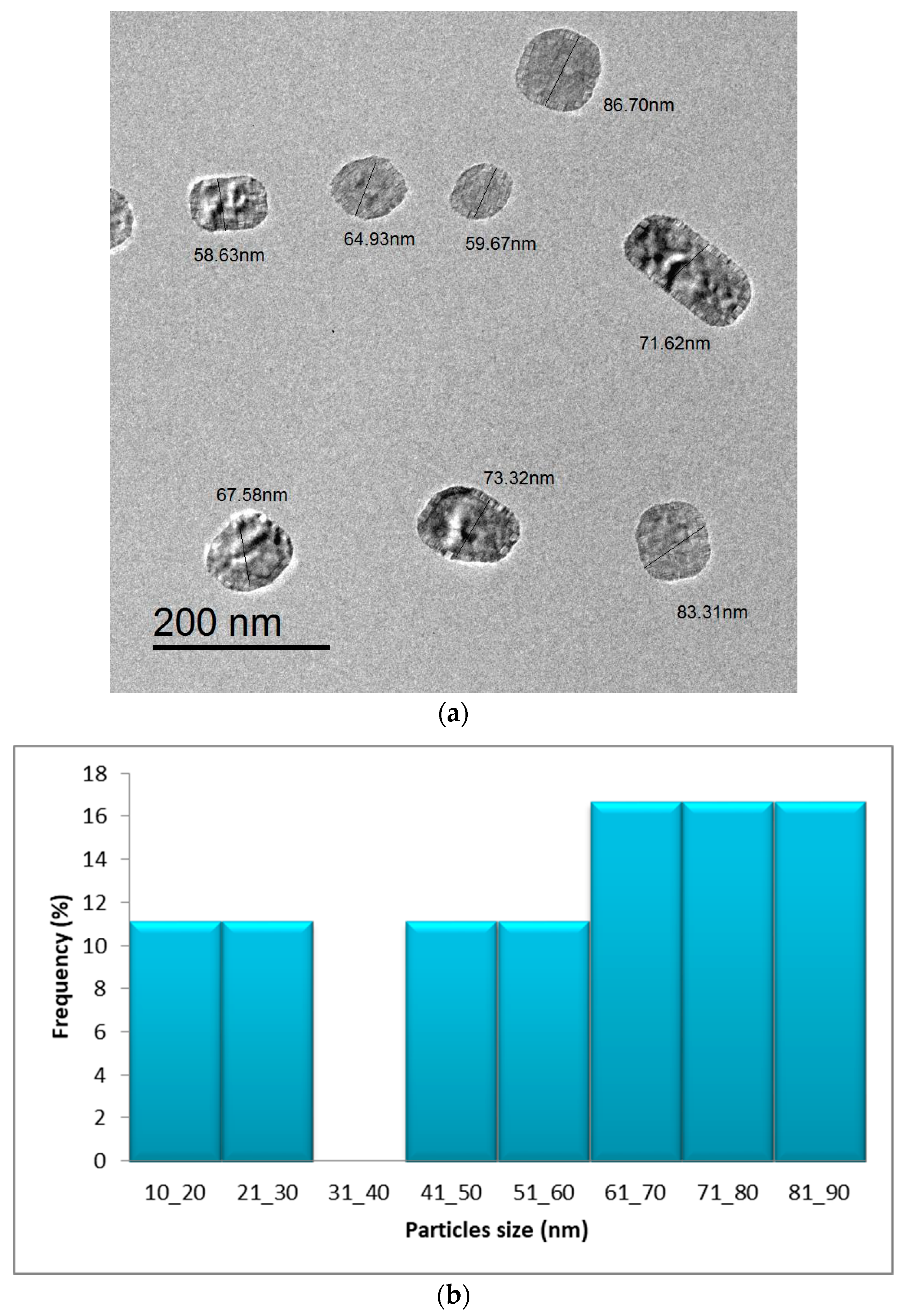
2.1.6. TEM Analysis
2.2. Adsorption Study
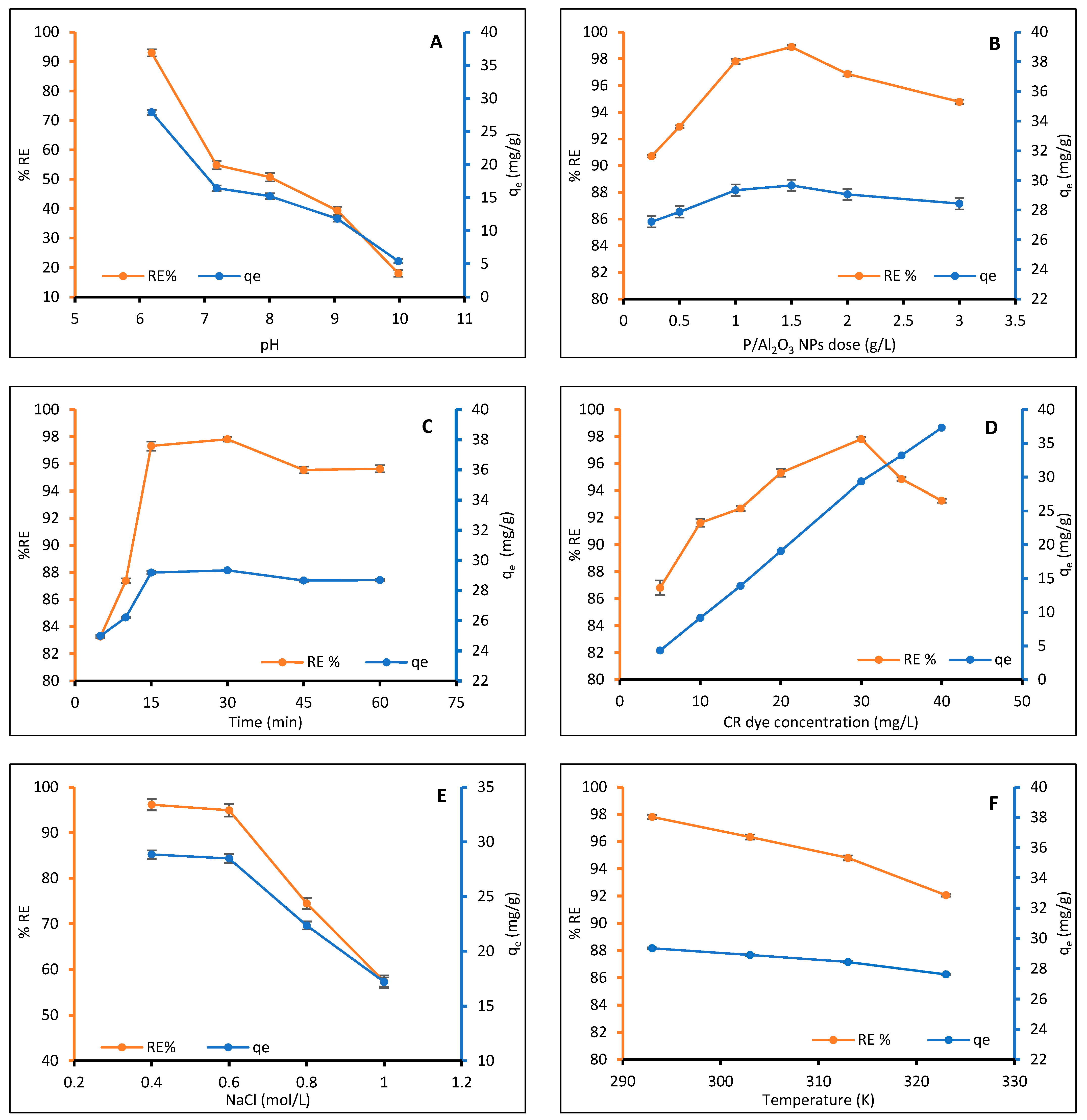
2.2.1. Effect of pH
2.2.2. Effect of P/Al2O3-NPs Dosage
2.2.3. Effect of Contact Time
2.2.4. Effect of Initial Concentration of CR Dye
2.2.5. Effect of Ionic Strength
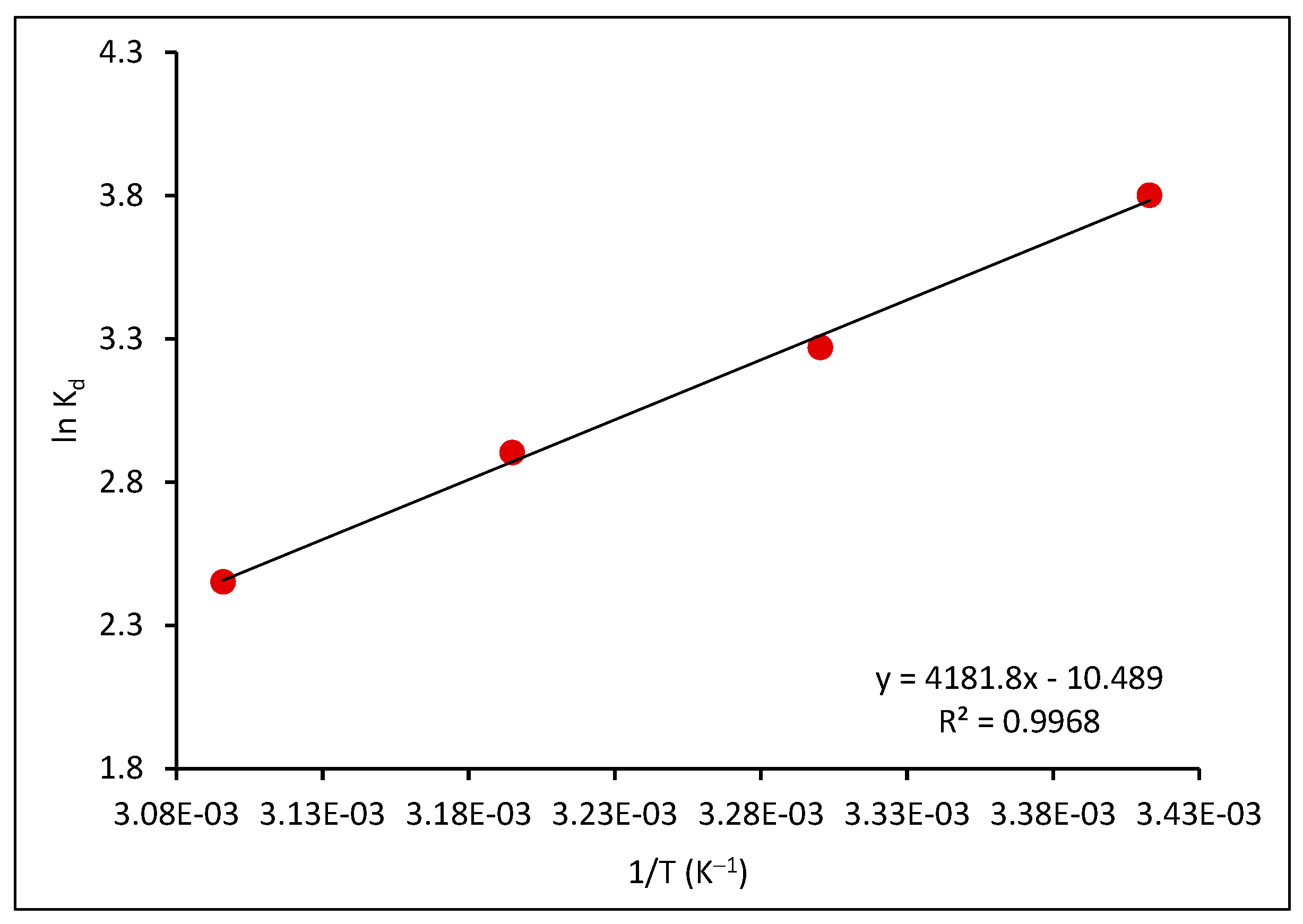
2.2.6. Effect of Temperature and Thermodynamic Functions
2.2.7. Kinetic Studies
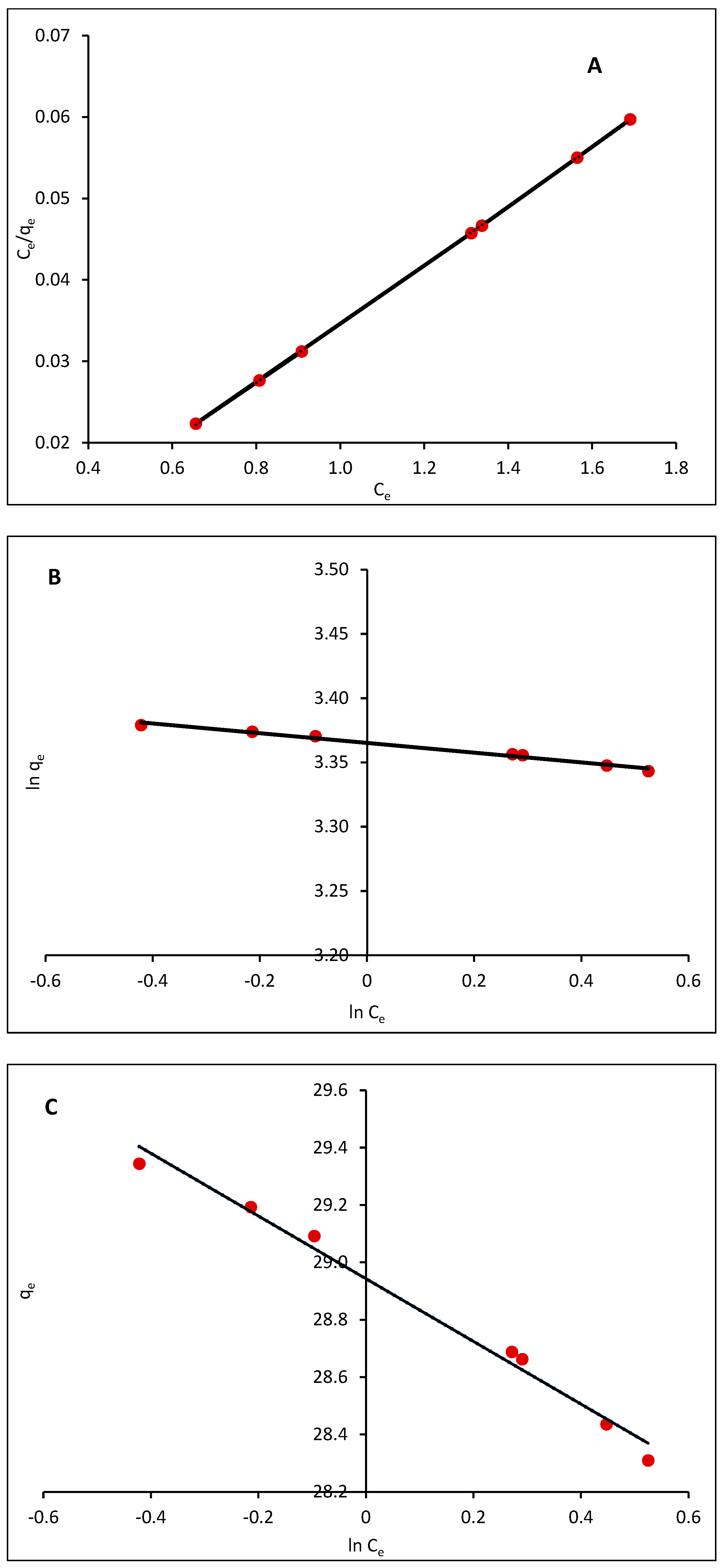
2.2.8. Adsorption Isotherms
2.3. Adsorption Mechanism of CR Dye onto Biogenic P/Al2O3-NPs
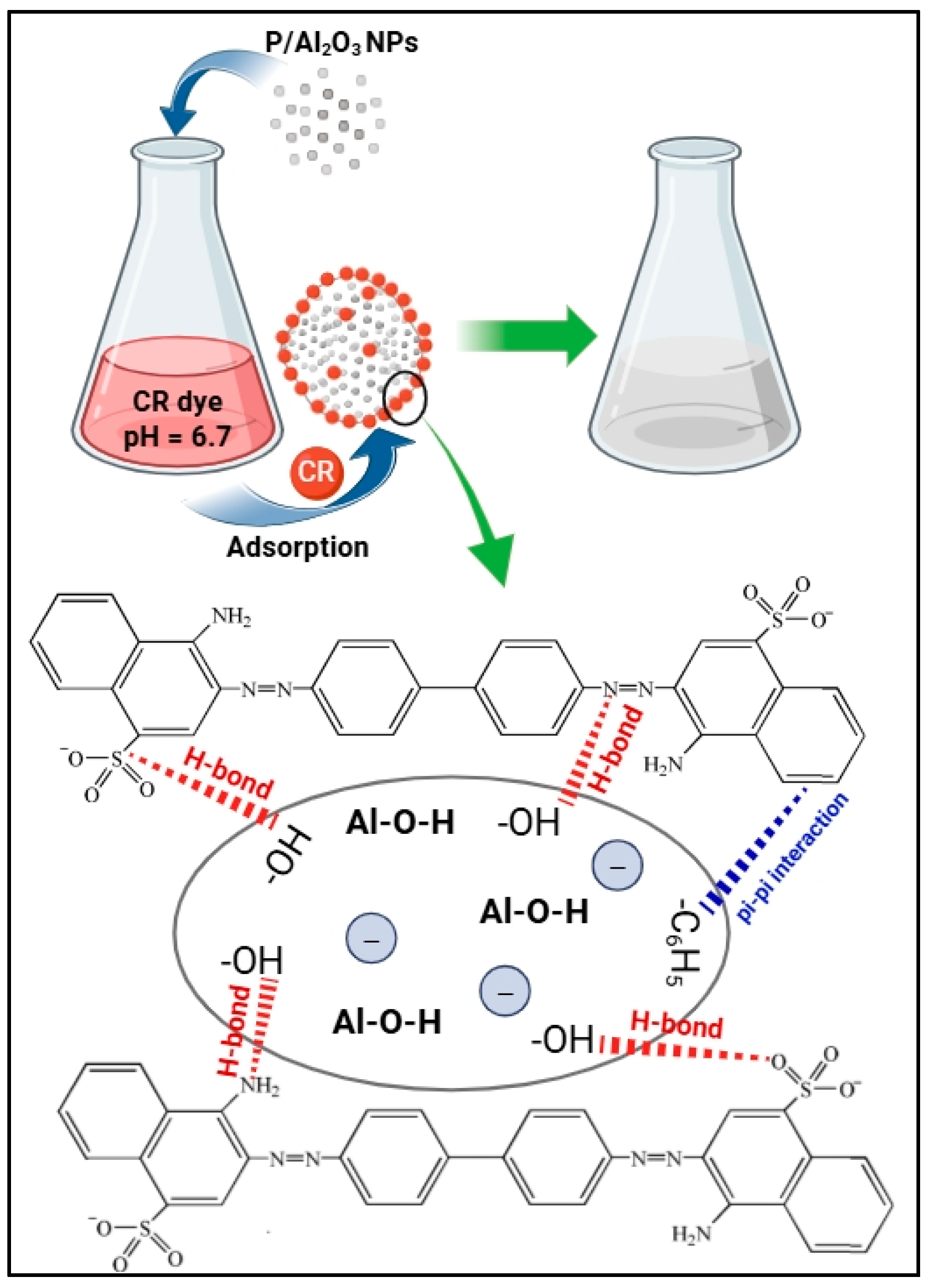
2.4. Comparative Study
3. Materials and Methods
3.1. Materials
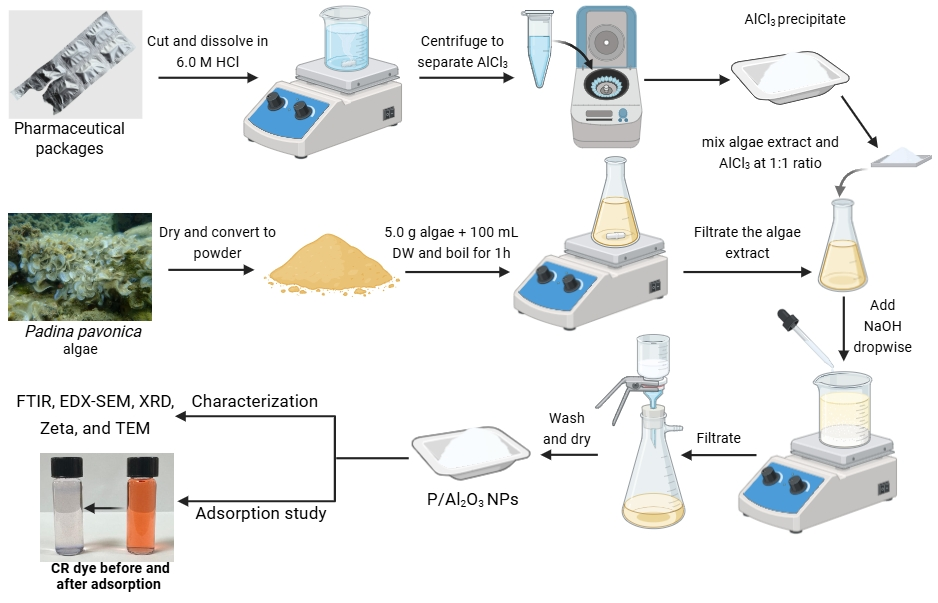
3.2. Fabrication of P/Al2O3-NPs
3.3. Adsorption Study
3.3.1. Effect of pH
3.3.2. Effect of P/Al2O3-NPs Dosages
3.3.3. Effect of Contact Time
3.3.4. Effect of Initial Concentration of CR Dye
3.3.5. Effect of Ionic Strength
3.3.6. Effect of Temperature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cecchin, I.; Reddy, K.R.; Thomé, A.; Tessaro, E.F.; Schnaid, F. Nanobioremediation: Integration of Nanoparticles and Bioremediation for Sustainable Remediation of Chlorinated Organic Contaminants in Soils. Int. Biodeterior. Biodegrad. 2017, 119, 419–428. [Google Scholar] [CrossRef]
- Pedrosa de Oliveira, D.; Costa, J.S.R.; Oliveira-Nascimento, L. Sustainability of Blisters for Medicines in Tablet Form. Sustain. Chem. Pharm. 2021, 21, 100423. [Google Scholar] [CrossRef]
- Rimšaitė, A.; Mumladze, T.; Denafas, G. Feasibilities of Aluminium Recovery from Combined Packaging Waste. SSRG Int. J. Agric. Environ. Sci. (SSRG-IJAES) 2019, 6, 103–111. [Google Scholar] [CrossRef]
- Agarwal, V.; Halli, P.; Helin, S.; Tesfaye, F.; Lundström, M. Electrohydraulic Fragmentation of Aluminum and Polymer Fractions from Waste Pharmaceutical Blisters. ACS Sustain. Chem. Eng. 2020, 8, 4137–4145. [Google Scholar] [CrossRef]
- Shukla, S.; Chernyaev, A.; Halli, P.; Aromaa, J.; Lundström, M. Leaching of Waste Pharmaceutical Blister Package Aluminium in Sulphuric Acid Media. Metals 2023, 13, 1118. [Google Scholar] [CrossRef]
- Yusuf, N.K.; Lajis, M.A.; Ahmad, A. Multiresponse Optimization and Environmental Analysis in Direct Recycling Hot Press Forging of Aluminum AA6061. Materials 2019, 12, 1918. [Google Scholar] [CrossRef]
- Capuzzi, S.; Timelli, G. Preparation and Melting of Scrap in Aluminum Recycling: A Review. Metals 2018, 8, 249. [Google Scholar] [CrossRef]
- Magalhães-Ghiotto, G.A.V.; de Oliveira, A.M.; Natal, J.P.S.; Bergamasco, R.; Gomes, R.G. Green Nanoparticles in Water Treatment: A Review of Research Trends, Applications, Environmental Aspects and Large-Scale Production. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100526. [Google Scholar] [CrossRef]
- Goodluck, N.; Okechukwu, H.; Chijioke, G. Biogenic Nanoparticles and Their Environmental Applications in Bioremediation and Pollution Control. Int. J. Innov. Sci. Res. Technol. 2021, 6, 113–121. [Google Scholar]
- Alprol, A.E.; Eleryan, A.; Abouelwafa, A.; Gad, A.M.; Hamad, T.M. Green Synthesis of Zinc Oxide Nanoparticles Using Padina Pavonica Extract for Efficient Photocatalytic Removal of Methylene Blue. Sci. Rep. 2024, 14, 32160. [Google Scholar] [CrossRef]
- El-Kassas, H.Y.; Aly-Eldeen, M.A.; Gharib, S.M. Green Synthesis of Iron Oxide (Fe3O4) Nanoparticles Using Two Selected Brown Seaweeds: Characterization and Application for Lead Bioremediation. Acta Oceanol. Sin. 2016, 35, 89–98. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.A.; Sundararaju, S.; Ramaraj, R.; Maniam, G.P.; Govindan, N. Synthesis of Silver Nanoparticles Using Marine Macroalgae Padina Sp. and Its Antibacterial Activity towards Pathogenic Bacteria. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 1–15. [Google Scholar] [CrossRef]
- Saleh, A.K.; Shaban, A.S.; Diab, M.A.; Debarnot, D.; Elzaref, A.S. Green Synthesis and Characterization of Aluminum Oxide Nanoparticles Using Phoenix Dactylifera Seed Extract along with Antimicrobial Activity, Phytotoxicity, and Cytological Effects on Vicia Faba Seeds. Biomass Convers. Biorefin. 2024, 14, 31859–31875. [Google Scholar] [CrossRef]
- Sumesh, K.R.; Kanthavel, K. Green Synthesis of Aluminium Oxide Nanoparticles and Its Applications in Mechanical and Thermal Stability of Hybrid Natural Composites. J. Polym. Environ. 2019, 27, 2189–2200. [Google Scholar] [CrossRef]
- Nagarajan, P.; Subramaniyan, V.; Elavarasan, V.; Mohandoss, N.; Subramaniyan, P.; Vijayakumar, S. Biofabricated Aluminium Oxide Nanoparticles Derived from Citrus aurantium L.: Antimicrobial, Anti-Proliferation, and Photocatalytic Efficiencies. Sustainability 2023, 15, 1743. [Google Scholar] [CrossRef]
- Goutam, S.P.; Avinashi, S.K.; Yadav, M.; Roy, D.; Shastri, R. Green Synthesis and Characterization of Aluminium Oxide Nanoparticles Using Leaf Extract of Rosa. Adv. Sci. Eng. Med. 2018, 10, 719–722. [Google Scholar] [CrossRef]
- Selvaraj, P.; Neethu, E.; Rathika, P.; Jayaseeli, J.P.R.; Jermy, B.R.; AbdulAzeez, S.; Borgio, J.F.; Dhas, T.S. Antibacterial Potentials of Methanolic Extract and Silver Nanoparticles from Marine Algae. Biocatal. Agric. Biotechnol. 2020, 28, 101719. [Google Scholar] [CrossRef]
- Koopi, H.; Buazar, F. A Novel One-Pot Biosynthesis of Pure Alpha Aluminum Oxide Nanoparticles Using the Macroalgae Sargassum Ilicifolium: A Green Marine Approach. Ceram. Int. 2018, 44, 8940–8945. [Google Scholar] [CrossRef]
- Chu, T.P.M.; Nguyen, N.T.; Vu, T.L.; Dao, T.H.; Dinh, L.C.; Nguyen, H.L.; Hoang, T.H.; Le, T.S.; Pham, T.D. Synthesis, Characterization, and Modification of Alumina Nanoparticles for Cationic Dye Removal. Materials 2019, 12, 450. [Google Scholar] [CrossRef]
- Banerjee, S.; Dubey, S.; Gautam, R.K.; Chattopadhyaya, M.C.; Sharma, Y.C. Adsorption Characteristics of Alumina Nanoparticles for the Removal of Hazardous Dye, Orange G from Aqueous Solutions. Arab. J. Chem. 2019, 12, 5339–5354. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of Synthetic Dyes from Wastewaters: A Review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Langhals, H. Color Chemistry. Synthesis, Properties and Applications of Organic Dyes and Pigments, 3rd revised ed.; Zollinger, H., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; Volume 43. [Google Scholar]
- Chequer, F.M.D.; Dorta, D.J.; de Oliveira, D.P.; Chequer, F.M.D.; Dorta, D.J.; de Oliveira, D.P. Azo Dyes and Their Metabolites: Does the Discharge of the Azo Dye into Water Bodies Represent Human and Ecological Risks? IntechOpen: London, UK, 2011; ISBN 978-953-307-704-8. [Google Scholar]
- Miandad, R.; Kumar, R.; Barakat, M.A.; Basheer, C.; Aburiazaiza, A.S.; Nizami, A.S.; Rehan, M. Untapped Conversion of Plastic Waste Char into Carbon-Metal LDOs for the Adsorption of Congo Red. J. Colloid Interface Sci. 2018, 511, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Mall, I.D.; Srivastava, V.C.; Kumar, G.V.A.; Mishra, I.M. Characterization and Utilization of Mesoporous Fertilizer Plant Waste Carbon for Adsorptive Removal of Dyes from Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2006, 278, 175–187. [Google Scholar] [CrossRef]
- Kam, O.R.; Garikoe, I.; Bakouan, C.; Guel, B.; Khamis, M.; Wait, I.; Nenov, V.; Zerbo, J.K. Low-Cost Synthesis of Alumina Nanoparticles and Their Usage for Bisphenol-A Removal from Aqueous Solutions. Processes 2021, 9, 1709. [Google Scholar] [CrossRef]
- Fawcett, D.; Verduin, J.J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A Review of Current Research into the Biogenic Synthesis of Metal and Metal Oxide Nanoparticles via Marine Algae and Seagrasses. J. Nanosci. 2017, 2017, 8013850. [Google Scholar] [CrossRef]
- Asemani, M.; Rabbani, A.R. Detailed FTIR Spectroscopy Characterization of Crude Oil Extracted Asphaltenes: Curve Resolve of Overlapping Bands. J. Pet. Sci. Eng. 2020, 185, 106618. [Google Scholar] [CrossRef]
- Myronyuk, I.F.; Mandzyuk, V.I.; Sachko, V.M.; Gun’ko, V.M. Structural and Morphological Features of Disperse Alumina Synthesized Using Aluminum Nitrate Nonahydrate. Nanoscale Res. Lett. 2016, 11, 153. [Google Scholar] [CrossRef]
- Yang, D.; Paul, B.; Xu, W.; Yuan, Y.; Liu, E.; Ke, X.; Wellard, R.M.; Guo, C.; Xu, Y.; Sun, Y.; et al. Alumina Nanofibers Grafted with Functional Groups: A New Design in Efficient Sorbents for Removal of Toxic Contaminants from Water. Water Res. 2010, 44, 741–750. [Google Scholar] [CrossRef]
- Bukar, A.M.; Abdullah Jesse, F.F.; Che Abdullah, C.A.; M Noordin, M.; Z Kyari, M.; Norman, A.; Mohd-Lila, M.A. In Vitro Cytotoxicity Evaluation of Green Synthesized Alumina Nanoscales on Different Mammalian Cell Lines. Sci. Rep. 2024, 14, 22826. [Google Scholar] [CrossRef]
- Gharbi, A.H.; Laouini, S.E.; Hemmami, H.; Bouafia, A.; Gherbi, M.T.; Ben Amor, I.; Hasan, G.G.; Abdullah, M.M.S.; Trzepieciński, T.; Abdullah, J.A.A. Eco-Friendly Synthesis of Al2O3 Nanoparticles: Comprehensive Characterization Properties, Mechanics, and Photocatalytic Dye Adsorption Study. Coatings 2024, 14, 848. [Google Scholar] [CrossRef]
- Manyasree, D.; Kiranmayi, P.; Kumar, R. Synthesis, characterization and antibacterial activity of aluminium oxide nanoparticles. Int. J. Pharm. Pharm. Sci. 2018, 10, 32–35. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Alhumairi, A.M.; Saddiq, A.A. Simultaneous Bioremediation of Petroleum Hydrocarbons and Production of Biofuels by the Micro-Green Alga, Cyanobacteria, and Its Consortium. Heliyon 2023, 9, e16656. [Google Scholar] [CrossRef]
- Berg, J.M.; Romoser, A.; Banerjee, N.; Zebda, R.; Sayes, C.M. The Relationship between PH and Zeta Potential of ~30 Nm Metal Oxide Nanoparticle Suspensions Relevant to in Vitro Toxicological Evaluations. Nanotoxicology 2009, 3, 276–283. [Google Scholar] [CrossRef]
- Manogar, P.; Esther Morvinyabesh, J.; Ramesh, P.; Dayana Jeyaleela, G.; Amalan, V.; Ajarem, J.S.; Allam, A.A.; Seong Khim, J.; Vijayakumar, N. Biosynthesis and Antimicrobial Activity of Aluminium Oxide Nanoparticles Using Lyngbya Majuscula Extract. Mater. Lett. 2022, 311, 131569. [Google Scholar] [CrossRef]
- Man, J.; Zhang, S.; Li, J.; Zhao, B.; Chen, Y. Effects of Electrolyte PH on Morphologies and Mechanical Properties of α-Al2O3/Ni Composite Coatings and Role of Zeta Potentials in Co-Deposition Process. Surf. Coat. Technol. 2014, 249, 118–124. [Google Scholar] [CrossRef]
- Das, T.; Kaur, M.; Kaur, N.; Bhowmik, P.K.; Han, H.; Sohal, H.S.; Husain, F.M. Greener and Magnetic Fe3O4 Nanoparticles as a Recyclable Catalyst for Knoevenagel Condensation and Degradation of Industrial Congo Red Dye. Green. Process. Synth. 2025, 14, 20240257. [Google Scholar] [CrossRef]
- Zhang, L.; Han, X.; Mu, J.; Zhang, L.; Han, X.; Mu, J. A Novel Adsorbent Prepared by Aluminum-Containing Waste Residue and Its Application for Congo Red Wastewater Decolorization. Adv. Mater. Phys. Chem. 2023, 13, 161–176. [Google Scholar] [CrossRef]
- Alarifi, I.M.; Al-Ghamdi, Y.O.; Darwesh, R.; Ansari, M.O.; Uddin, M.K. Properties and Application of MoS2 Nanopowder: Characterization, Congo Red Dye Adsorption, and Optimization. J. Mater. Res. Technol. 2021, 13, 1169–1180. [Google Scholar] [CrossRef]
- Sachin; Pramanik, B.K.; Singh, N.; Zizhou, R.; Houshyar, S.; Cole, I.; Yin, H. Fast and Effective Removal of Congo Red by Doped ZnO Nanoparticles. Nanomaterials 2023, 13, 566. [Google Scholar] [CrossRef]
- Patel, S.; Desai, R.; Patel, B.; Ali, D.; Dawane, V.; Gadhvi, K.; Yadav, V.K.; Choudhary, N.; Sahoo, D.K.; Patel, A. Phytonanofabrication of Iron Oxide Particles from the Acacia jacquemontii Plant and Their Potential Application for the Removal of Brilliant Green and Congo Red Dye from Wastewater. Front. Bioeng. Biotechnol. 2023, 11, 1319927. [Google Scholar] [CrossRef]
- El-Shafey, S.; Salama, A.; Abouzeid, R. Nanocomposite of Cellulose Nanofibers/Alumina for Effective Adsorption of Brilliant Blue and Ethyl Orange Dyes: Equilibrium, Kinetic and Mechanism Studies. Desalination Water Treat. 2025, 321, 100875. [Google Scholar] [CrossRef]
- Ergović Ravančić, M.; Habuda-Stanić, M. Fluoride Adsorption from Water Using Activated Carbon Modified with Nitric Acid and Hydrogen Peroxide. Water 2024, 16, 3439. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Mousavi, S.A.; Atashkar, S. Kinetic and Isotherm Studies on the Removal of Reactive Red 2 from Aqueous Solutions Using Phosphoric Acid Activated Carbon. AQUA—Water Infrastruct. Ecosyst. Soc. 2023, 72, 123–138. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mostafa, M.; El-Sherbeeny, A.M.; El-Meligy, M.A.; Nadeem, A. Instantaneous Adsorption of Synthetic Dyes from an Aqueous Environment Using Kaolinite Nanotubes: Equilibrium and Thermodynamic Studies. ACS Omega 2021, 6, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kaur, R. Removal of Rhodamine-B Dye from Aqueous Solution onto Pigeon Dropping: Adsorption, Kinetic, Equilibrium and Thermodynamic Studies. J. Mater. Environ. Sci. 2014, 5, 1830–1838. [Google Scholar]
- Eletta, O.A.A.; Mustapha, S.I.; Ajayi, O.A.; Ahmed, A.T. Optimization of Dye Removal from Textile Wastewater Using Activated Carbon from Sawdust. Niger. J. Technol. Dev. 2018, 15, 26–32. [Google Scholar] [CrossRef]
- Pal, S.; Patra, A.S.; Ghorai, S.; Sarkar, A.K.; Mahato, V.; Sarkar, S.; Singh, R.P. Efficient and Rapid Adsorption Characteristics of Templating Modified Guar Gum and Silica Nanocomposite toward Removal of Toxic Reactive Blue and Congo Red Dyes. Bioresour. Technol. 2015, 191, 291–299. [Google Scholar] [CrossRef]
- Firmino, H.C.T.; Nascimento, E.P.; Arzuza, L.C.C.; Araujo, R.N.; Sousa, B.V.; Neves, G.A.; Morales, M.A.; Menezes, R.R. High-Efficiency Adsorption Removal of Congo Red Dye from Water Using Magnetic NiFe2O4 Nanofibers: An Efficient Adsorbent. Materials 2025, 18, 754. [Google Scholar] [CrossRef]
- Kato, S.; Kansha, Y. Comprehensive Review of Industrial Wastewater Treatment Techniques. Environ. Sci. Pollut. Res. 2024, 31, 51064–51097. [Google Scholar] [CrossRef]
- Molavi, H.; Pourghaderi, A.; Shojaei, A. Experimental Study on the Influence of Initial PH, Ionic Strength, and Temperature on the Selective Adsorption of Dyes onto Nanodiamonds. J. Chem. Eng. Data 2019, 64, 1508–1514. [Google Scholar] [CrossRef]
- Constantino, R.E.; Hamdan-Mansour, A.M.; Henderson, A.; Noll-Nelson, B.; Doswell, W.; Braxter, B. Assessing the Readability and Usability of Online H-E-L-P Intervention for IPV Survivors. Open J. Nurs. 2014, 04, 150–157. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, T.; Ye, X.; Li, Q.; Guo, M.; Liu, H.; Wu, Z. Dye Adsorption by Resins: Effect of Ionic Strength on Hydrophobic and Electrostatic Interactions. Chem. Eng. J. 2013, 228, 392–397. [Google Scholar] [CrossRef]
- Namasivayam, C.; Kavitha, D. Removal of Congo Red from Water by Adsorption onto Activated Carbon Prepared from Coir Pith, an Agricultural Solid Waste. Dye. Pigment. 2002, 54, 47–58. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Suhas; Mohan, D. Equilibrium Uptake and Sorption Dynamics for the Removal of a Basic Dye (Basic Red) Using Low-Cost Adsorbents. J. Colloid. Interface Sci. 2003, 265, 257–264. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Ahmad Puad, N.A.; Bello, O.S. Kinetic, Equilibrium and Thermodynamic Studies of Synthetic Dye Removal Using Pomegranate Peel Activated Carbon Prepared by Microwave-Induced KOH Activation. Water Resour. Ind. 2014, 6, 18–35. [Google Scholar] [CrossRef]
- Ojedokun, A.T.; Bello, O.S. Kinetic Modeling of Liquid-Phase Adsorption of Congo Red Dye Using Guava Leaf-Based Activated Carbon. Appl. Water Sci. 2017, 7, 1965–1977. [Google Scholar] [CrossRef]
- Roy, T.K.; Mondal, N.K. Potentiality of Eichhornia Shoots Ash towards Removal of Congo Red from Aqueous Solution: Isotherms, Kinetics, Thermodynamics and Optimization Studies. Groundw. Sustain. Dev. 2019, 9, 100269. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Makete, E. Removal of Congo Red Dye from Aqueous Media Using Litchi Seeds Powder: Equilibrium, Kinetics and Thermodynamics. Phys. Chem. Earth Parts A/B/C 2021, 123, 103007. [Google Scholar] [CrossRef]
- Ahmad Aftab, R.; Zaidi, S.; Aslam Parwaz Khan, A.; Arish Usman, M.; Khan, A.Y.; Tariq Saeed Chani, M.; Asiri, A.M. Removal of Congo Red from Water by Adsorption onto Activated Carbon Derived from Waste Black Cardamom Peels and Machine Learning Modeling. Alex. Eng. J. 2023, 71, 355–369. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E.; Gryglewicz, G. Adsorption Characteristics of Congo Red on Coal-Based Mesoporous Activated Carbon. Dye. Pigment. 2007, 74, 34–40. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über Die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Jahanbakhshi, M.; Khalafvandi, S.A.; Alian, M.; Razeghi Jahromi, D. Decontamination of Congo Red Dye from Aqueous Solution Using Nanoclay/Chitosan-Graft-Gelatin Nanocomposite Hydrogel. J. Mol. Liq. 2024, 395, 123839. [Google Scholar] [CrossRef]
- Wang, H.; Luo, W.; Guo, R.; Li, D.; Xue, B. Effective Adsorption of Congo Red Dye by Magnetic Chitosan Prepared by Solvent-Free Ball Milling. Mater. Chem. Phys. 2022, 292, 126857. [Google Scholar] [CrossRef]
- Reza Masoodi, H.; Sadat Pourhosseini, R.; Bagheri, S. The Role of Nature of Aromatic Ring on Cooperativity between π–π Stacking and Ion–π Interactions: A Computational Study. Comput. Theor. Chem. 2023, 1220, 114022. [Google Scholar] [CrossRef]
- Castellanos, N.J.; Martinez Rojas, Z.; Camargo, H.A.; Biswas, S.; Granados-Oliveros, G. Congo Red Decomposition by Photocatalytic Formation of Hydroxyl Radicals (·OH) Using Titanium Metal–Organic Frameworks. Transit. Met. Chem. 2019, 44, 77–87. [Google Scholar] [CrossRef]
- Chatterjee, S.; Guha, N.; Krishnan, S.; Singh, A.K.; Mathur, P.; Rai, D.K. Selective and Recyclable Congo Red Dye Adsorption by Spherical Fe3O4 Nanoparticles Functionalized with 1,2,4,5-Benzenetetracarboxylic Acid. Sci. Rep. 2020, 10, 111. [Google Scholar] [CrossRef]
- Mezgebe, K.; Mulugeta, E. Synthesis and Pharmacological Activities of Azo Dye Derivatives Incorporating Heterocyclic Scaffolds: A Review. RSC Adv. 2022, 12, 25932–25946. [Google Scholar] [CrossRef]
- Nascimento Pinheiro, H.; Freitas Veloso, F.; Oliveira Monteiro da Silva Abreu, F.; Lucas Isidio de Oliveira Almeida, J.; Rocha Lima Cardial, M. Polysaccharides-Based Adsorbents for Removal of Congo Red Dyes from Wastewater. MOJ Ecol. Environ. Sci. 2023, 8, 138–141. [Google Scholar] [CrossRef]
- Quintanilla-Villanueva, G.E.; Sicardi-Segade, A.; Luna-Moreno, D.; Núñez-Salas, R.E.; Villarreal-Chiu, J.F.; Rodríguez-Delgado, M.M. Recent Advances in Congo Red Degradation by TiO2-Based Photocatalysts Under Visible Light. Catalysts 2025, 15, 84. [Google Scholar] [CrossRef]
- Alhasan, H.S.; Omran, A.R.; Al Mahmud, A.; Mady, A.H.; Thalji, M.R. Toxic Congo Red Dye Photodegradation Employing Green Synthesis of Zinc Oxide Nanoparticles Using Gum Arabic. Water 2024, 16, 2202. [Google Scholar] [CrossRef]
- Omotunde, O.I.; Gurgur, E.; Ojo, A.M.; Abiodun, O.M.; Okoronkwo, A.E. Oxidant-Assisted Catalytic Degradation of Congo Red by Pd/NiO Nanoparticles under Visible Light. Acad. Eng. 2025, 2. [Google Scholar] [CrossRef]
- Fardood, S.T.; Moradnia, F.; Moradi, S.; Forootan, R.; Zare, F.Y.; Heidari, M. Eco-Friendly Synthesis and Characterization of α-Fe2O3 Nanoparticles and Study of Their Photocatalytic Activity for Degradation of Congo Red Dye. Nanochem. Res. 2019, 4, 140–147. [Google Scholar] [CrossRef]
- Kaur, H.; Bouzid, G.; Kumar, S.; Kaur, R.; Ayachi, S.; Alam, M.W. Optimized Green Synthesis of Dual-Phase Metal Oxide Nanoparticles for Environmental Remediation. Curr. Appl. Phys. 2025, 78, 29–47. [Google Scholar] [CrossRef]
- Patil, Y.; Attarde, S.; Dhake, R.; Fegade, U.; Alaghaz, A.N.M.A. Adsorption of Congo Red Dye Using Metal Oxide Nano-Adsorbents: Past, Present, and Future Perspective. Int. J. Chem. Kinet. 2023, 55, 579–605. [Google Scholar] [CrossRef]
- Adebayo, M.A.; Jabar, J.M.; Amoko, J.S.; Openiyi, E.O.; Shodiya, O.O. Coconut Husk-Raw Clay-Fe Composite: Preparation, Characteristics and Mechanisms of Congo Red Adsorption. Sci. Rep. 2022, 12, 14370. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an Anionic Dye (Congo Red) from Aqueous Solutions by Pine Bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef]
- Olusegun, S.J.; Mohallem, N.D.S. Comparative Adsorption Mechanism of Doxycycline and Congo Red Using Synthesized Kaolinite Supported CoFe2O4 Nanoparticles. Environ. Pollut. 2020, 260, 114019. [Google Scholar] [CrossRef]
- Khezami, L.; Ben Aissa, M.A.; Modwi, A.; Guesmi, A.; Algethami, F.K.; Bououdina, M. Efficient Removal of Organic Dyes by Cr-Doped ZnO Nanoparticles. Biomass Convers. Biorefin. 2024, 14, 4177–4190. [Google Scholar] [CrossRef]
- Adesina, A.O.; Elvis, O.A.; Mohallem, N.D.S.; Olusegun, S.J. Adsorption of Methylene Blue and Congo Red from Aqueous Solution Using Synthesized Alumina–Zirconia Composite. Environ. Technol. 2021, 42, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Radwan, A.; Mohamed, S.O.; Khalil, M.M.H.; El-Sewify, I.M. Effective Adsorption of Fluorescent Congo Red Azo Dye from Aqueous Solution by Green Synthesized Nanosphere ZnO/CuO Composite Using Propolis as Bee Byproduct Extract. Sci. Rep. 2024, 14, 9061. [Google Scholar] [CrossRef]
- Ejeta, B.A.; Aaga, G.F.; Fereja, W.M.; Mengesha, B. Biofabrication of Highly Effective and Easily Regenerated CuO Nanoparticles as Adsorbents for Congo Red and Malachite Green Removal. Sci. Rep. 2024, 14, 24116. [Google Scholar] [CrossRef]
- Al-Salihi, S.; Jasim, A.M.; Fidalgo, M.M.; Xing, Y. Removal of Congo Red Dyes from Aqueous Solutions by Porous γ-Alumina Nanoshells. Chemosphere 2022, 286, 131769. [Google Scholar] [CrossRef]
- Somasekhara Reddy, M.C.; Sivaramakrishna, L.; Varada Reddy, A. The Use of an Agricultural Waste Material, Jujuba Seeds for the Removal of Anionic Dye (Congo Red) from Aqueous Medium. J. Hazard. Mater. 2012, 203–204, 118–127. [Google Scholar] [CrossRef]

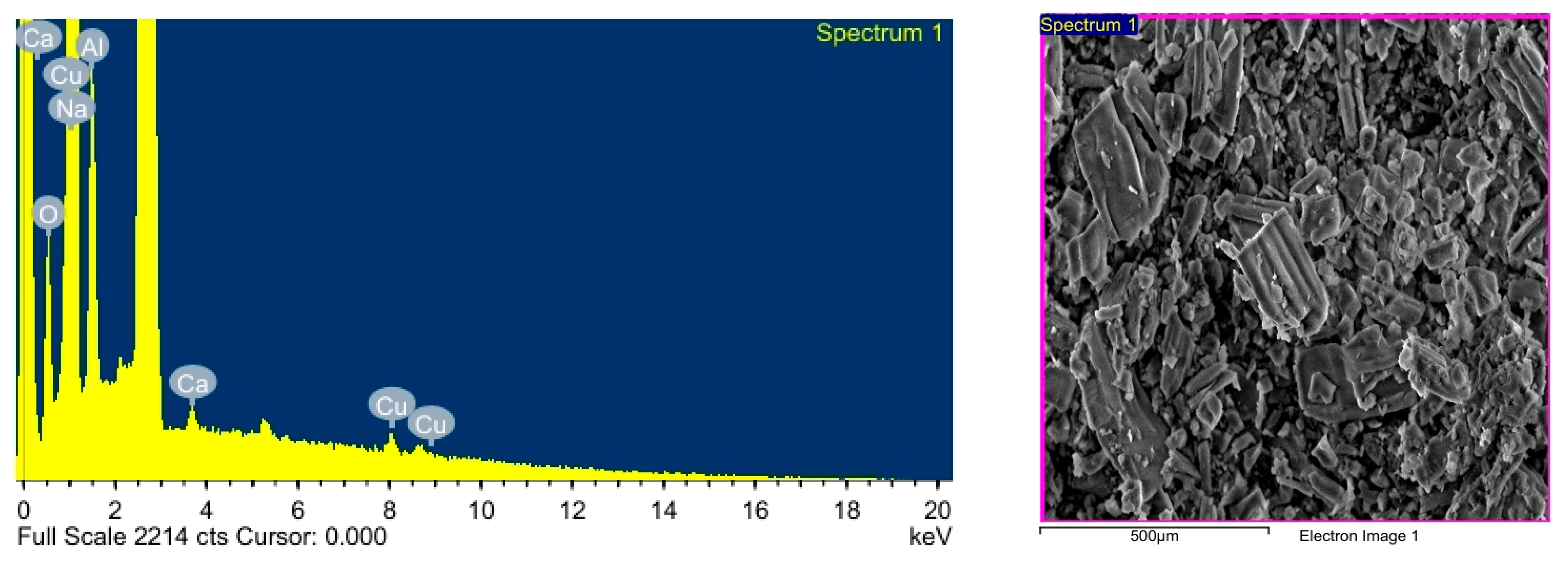

| Element | Weight (%) | Atomic (%) |
|---|---|---|
| O | 13.83 | 18.76 |
| Na | 72.69 | 70.92 |
| Al | 11.00 | 9.140 |
| Ca | 0.680 | 0.380 |
| Cu | 2.250 | 0.790 |
| Total | 100.0 |
| 2 Theta | D Value (A) | Intensity (%) | Size (nm) | hkl |
|---|---|---|---|---|
| 27.453 | 2.246 | 4.8 | 82.06 | 100 |
| 30.433 | 2.935 | 0.9 | 82.61 | 100 |
| 31.787 | 2.819 | 100 | 82.89 | 100 |
| 43.527 | 2.077 | 0.8 | 85.83 | 110 |
| 45.519 | 1.991 | 87.4 | 86.45 | 111 |
| 53.932 | 1.699 | 2.2 | 89.44 | 200 |
| 56.537 | 1.626 | 11.8 | 90.51 | 200 |
| 66.280 | 1.409 | 9.4 | 95.20 | 210 |
| Temperature (K) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (kJ mol−1) |
|---|---|---|---|
| 293 | −9.258 | −34.767 | −0.0872 |
| 303 | −8.236 | ||
| 313 | −7.554 | ||
| 323 | −6.581 |
| Pseudo-First Order | Pseudo-Second Order | Intraparticle Diffusion | |||
|---|---|---|---|---|---|
| qe | 36.6972 | qe | 29.0710 | Ci | 24.9062 |
| K1 | −0.3361 | K2 | 0.0684 | Kip | 0.6043 |
| R2 | 0.8234 | R2 | 0.9994 | R2 | 0.5124 |
| qe (exp.) | 29.3440 mg/g | ||||
| Isotherm | Equations | Parameter Values | |
|---|---|---|---|
| Langmuir | (7) | qmax (mg/g) = 27.778 KL (L/mg) = 24.000 RL = 0.0014 R2 = 0.9999 | |
| (8) | |||
| where —equilibrium adsorbent capacity, —maximum adsorbent capacity, —equilibrium constant of Langmuir, —dimensionless separation factor. | |||
| Freundlich | (9) | KF (mg/g) = 28.943 n = −26.385 R2 = 0.985 | |
| where —Freundlich adsorption constant, —heterogeneity factor. | |||
| Temkin | (10) | KT (L/g) = 3.099 × 10−12 BT (KJ/mol) = −1.093 bT (mg/g) = −2.229 R2 = 0.9864 | |
| (11) | |||
| where —Temkin isotherm equilibrium binding constant, —maximum binding heat of sorption, —binding energy | |||
| Adsorbent | qmax (mg/g) | %RE | pH | Equilibrium Time | Isotherm Model | Ref. |
|---|---|---|---|---|---|---|
| Doped ZnO | 230.0 | ~99.0 | 4 | 10 min | Langmuir | [41] |
| NiFe2O4 nanofibers (Ni500) | 18.6 | 97.0 | - | 30 min | Langmuir | [50] |
| Coconut husk–raw clay–Fe | 1032.0 | - | 2 | 480 min | Langmuir | [78] |
| Pine bark | 0.5 | 94.5 | 2 | 7 days | Freundlich | [79] |
| Kaolinite-supported CoFe2O4 NPs | 547.0 | - | - | 12 h | Langmuir | [80] |
| Sol–gel-Cr-doped ZnO NPs | 155.5 | - | 7 | 110 min | Langmuir | [81] |
| Al2O3-ZrO2 | 57.5 | 80.0 | 4 | 60 min | Langmuir | [82] |
| Biosynthesized ZnO/CuO (BZC) | 90.1 | - | 5 | 120 min | Langmuir | [83] |
| Bio-CuO NPs | 0.13 | 99.5 | 4 | 80 min | Langmuir | [84] |
| g-Al2O3 nanoshells | 370.4 | 7 | 30 min | Langmuir | [85] | |
| Biogenic P/Al2O3-NPs | 29.3 | 97.8 | 6 | 30 min | Langmuir | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ahmari, J.M.; Alghanmi, R.M.; Hamouda, R.A. Bio-Fabricated Aluminum Oxide Nanoparticles Derived from Waste Pharmaceutical Packages: Insight into Characterization and Applications. Biomolecules 2025, 15, 984. https://doi.org/10.3390/biom15070984
Al-Ahmari JM, Alghanmi RM, Hamouda RA. Bio-Fabricated Aluminum Oxide Nanoparticles Derived from Waste Pharmaceutical Packages: Insight into Characterization and Applications. Biomolecules. 2025; 15(7):984. https://doi.org/10.3390/biom15070984
Chicago/Turabian StyleAl-Ahmari, Jamilah M., Reem M. Alghanmi, and Ragaa A. Hamouda. 2025. "Bio-Fabricated Aluminum Oxide Nanoparticles Derived from Waste Pharmaceutical Packages: Insight into Characterization and Applications" Biomolecules 15, no. 7: 984. https://doi.org/10.3390/biom15070984
APA StyleAl-Ahmari, J. M., Alghanmi, R. M., & Hamouda, R. A. (2025). Bio-Fabricated Aluminum Oxide Nanoparticles Derived from Waste Pharmaceutical Packages: Insight into Characterization and Applications. Biomolecules, 15(7), 984. https://doi.org/10.3390/biom15070984





