Nematode Pheromones as Key Mediators of Behavior, Development, and Ecological Interactions
Abstract
1. Introduction
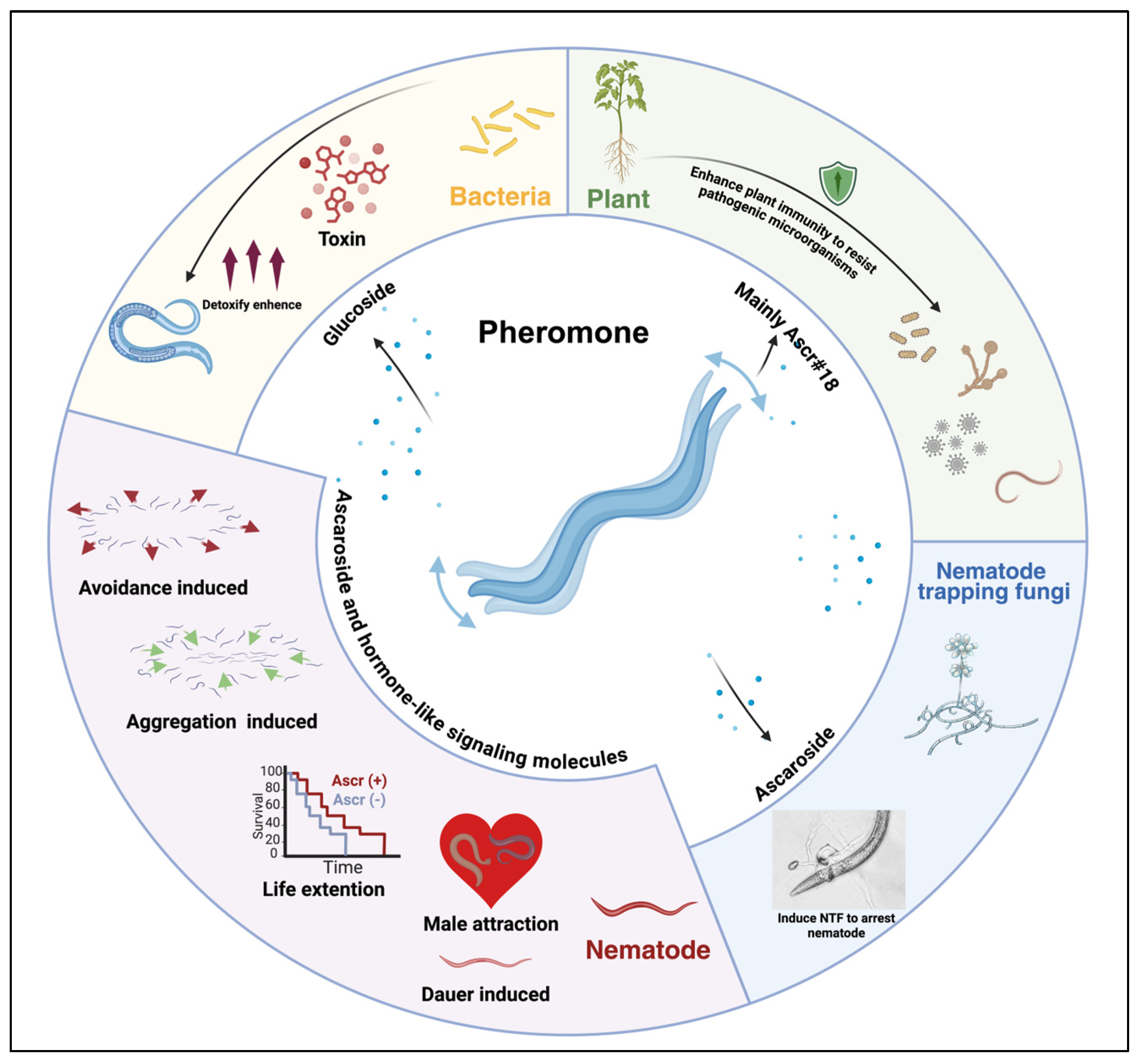
2. Nematode Pheromone-Mediated Communication in Ecosystems
3. Classification and Function of Nematode Pheromones
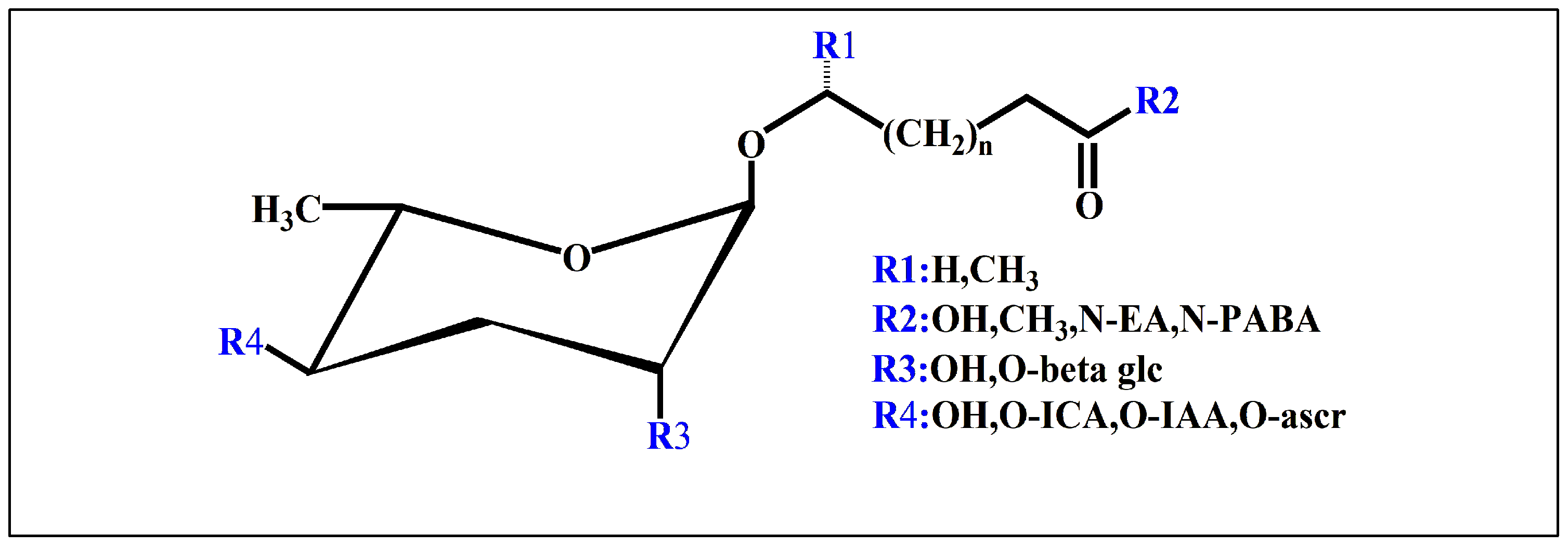
3.1. Nematode Ascaroside Pheromones
3.2. Nematode Glucoside Pheromones
3.3. Other Nematode Pheromones
4. Regulatory Functions and Mechanisms of Nematode Pheromones
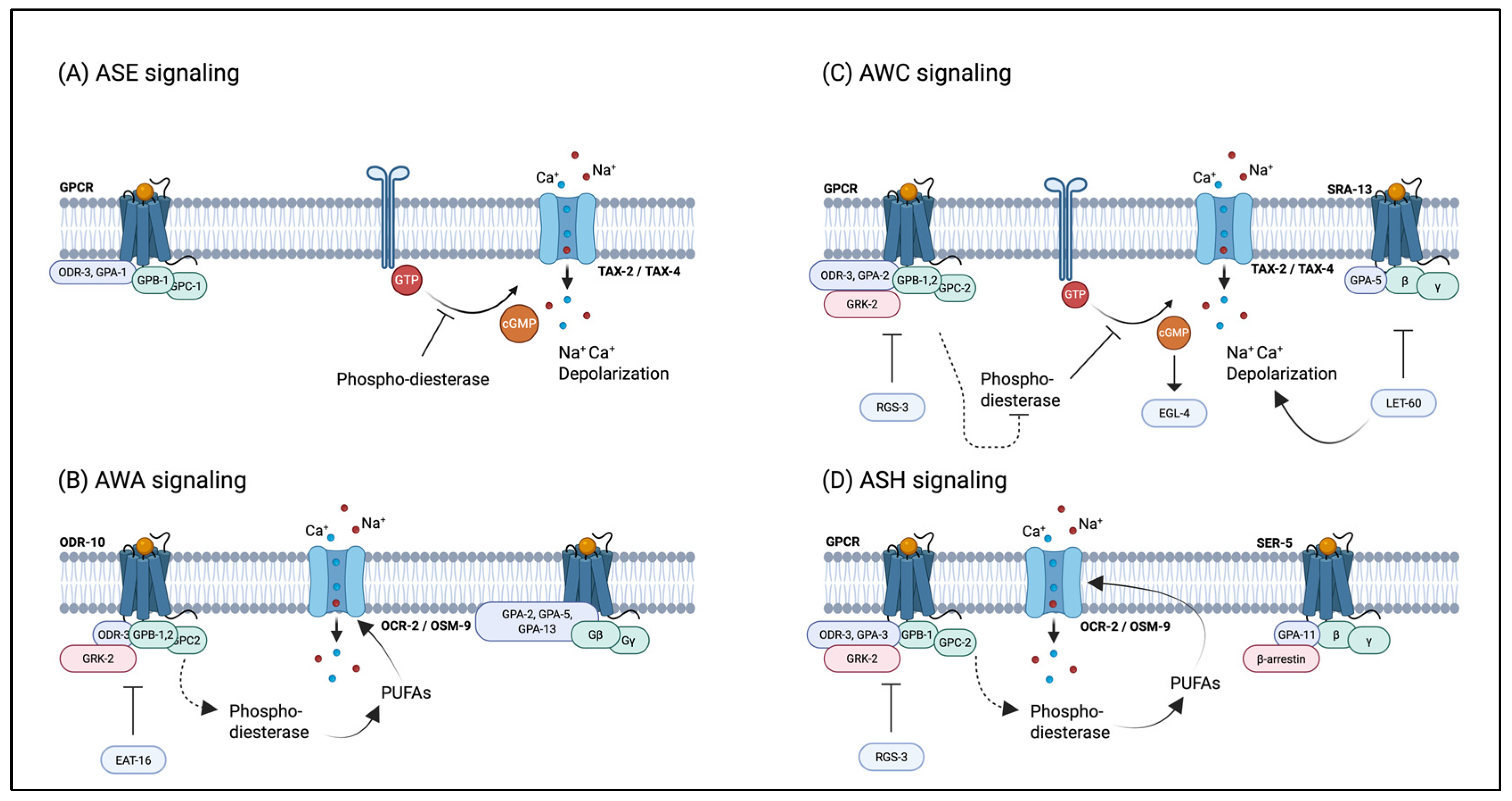
4.1. Regulation of Nematode Behavior by Pheromones
4.2. Dauer Larva Induction and Regulatory Mechanisms
4.3. Pheromonal Regulation of Nematode Mating Behavior
4.4. Pheromone-Mediated Modulation of Nematode Lifespan
5. Nematic Pheromones Mediate Interspecies Interactions
5.1. Nematode Pheromones Modulate the Interaction Between Nematodes and Fungi
5.2. Regulation of Nematode–Pathogen Interactions by Nematode Pheromones
5.3. Nematode Pheromones Modulate Nematode-Plant Interactions
5.4. Other Aspects of Communication Through Nematode Pheromones
6. Nematode Pheromone Biosynthesis
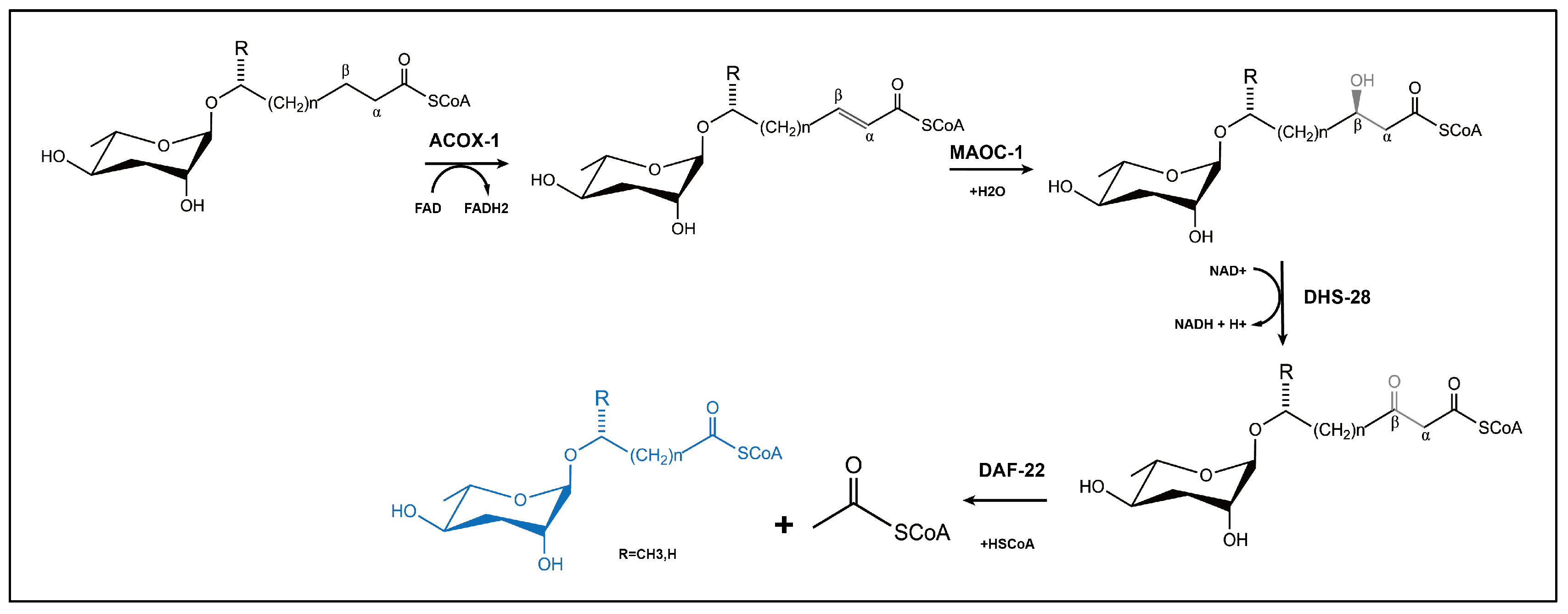
6.1. Biosynthesis of Nematode Ascaroside
6.2. Biosynthesis of Nematode Glucosides
7. Application of Pheromones in Biological Control
7.1. Pheromone-Based Pest Control
7.2. Pheromone-Based Nematode Control
8. Challenges and Unresolved Issues in Nematode Pheromone Research
9. Bridging the Lab-to-Field Gap: Practical Challenges and Emerging Frontiers in Ascaroside-Based Nematode Management
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ullah, A.; Bano, A.; Khan, N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Khan, M.R. Nematode pests of agricultural crops, a global overview. Nov. Biol. Biotechnol. Appl. Plant Nematode Manag. 2023. [Google Scholar] [CrossRef]
- Basu, S.; Clark, R.E.; Fu, Z.; Lee, B.W.; Crowder, D.W. Insect alarm pheromones in response to predators: Ecological trade-offs and molecular mechanisms. Insect Biochem. Mol. Biol. 2021, 128, 103514. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.H.; George, J.; Reddy, G.V.; Zeng, X.; Guerrero, A. Latest developments in insect sex pheromone research and its application in agricultural pest management. Insects 2021, 12, 484. [Google Scholar] [CrossRef]
- Kannan, K.; Galizia, C.G.; Nouvian, M. Olfactory strategies in the defensive behaviour of insects. Insects 2022, 13, 470. [Google Scholar] [CrossRef]
- Zhou, S.; Jander, G. Molecular ecology of plant volatiles in interactions with insect herbivores. J. Exp. Bot. 2022, 73, 449–462. [Google Scholar] [CrossRef]
- Dicke, M.; Lucas-Barbosa, D. Herbivore-induced plant volatiles as a source of information in plant–insect networks. In Biology of Plant Volatiles; CRC Press: Boca Raton, FL, USA, 2020; pp. 327–346. [Google Scholar]
- Kessler, A.; Chautá, A. The ecological consequences of herbivore-induced plant responses on plant–pollinator interactions. Emerg. Top. Life Sci. 2020, 4, 33–43. [Google Scholar] [PubMed]
- Moreira, X.; Abdala-Roberts, L.; Gols, R.; Lago-Núñez, B.; Rasmann, S.; Röder, G.; Soengas, P.; Vázquez-González, C.; Cartea, M.E. Insect herbivory but not plant pathogen infection drive floral volatile-mediated indirect effects on pollinators and plant fitness in Brassica rapa. J. Ecol. 2024, 112, 402–415. [Google Scholar] [CrossRef]
- Rattray, J.B.; Thomas, S.A.; Wang, Y.; Molotkova, E.; Gurney, J.; Varga, J.J.; Brown, S.P. Bacterial quorum sensing allows graded and bimodal cellular responses to variations in population density. MBio 2022, 13, e0074522. [Google Scholar] [CrossRef]
- Li, X.; Jin, J.; Zhang, X.; Xu, F.; Zhong, J.; Yin, Z.; Qi, H.; Wang, Z.; Shuai, J. Quantifying the optimal strategy of population control of quorum sensing network in Escherichia coli. Npj Syst. Biol. Appl. 2021, 7, 35. [Google Scholar] [CrossRef]
- Striednig, B.; Hilbi, H. Bacterial quorum sensing and phenotypic heterogeneity: How the collective shapes the individual. Trends Microbiol. 2022, 30, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, J.; Liu, C.; Yang, A.; Qiao, J. Quorum sensing for population-level control of bacteria and potential therapeutic applications. Cell. Mol. Life Sci. 2020, 77, 1319–1343. [Google Scholar] [CrossRef] [PubMed]
- Buchinger, T.J.; Li, W. Chemical communication and its role in sexual selection across Animalia. Commun. Biol. 2023, 6, 1178. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Alborn, H.; von Reuss, S.; Ajredini, R.; Ali, J. Interspecific Nematode Signals Regulate Dispersal Behavior. PLoS ONE 2012, 7, e38735. [Google Scholar] [CrossRef]
- Srinivasan, J.; von Reuss, S.H.; Bose, N.; Zaslaver, A.; Mahanti, P.; Ho, M.C.; O’Doherty, O.G.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol. 2012, 10, e1001237. [Google Scholar] [CrossRef]
- Ludewig, A.H.; Schroeder, F.C. Ascaroside signaling in C. elegans. In WormBook: The Online Review of C. elegans Biology [Internet]; Cold Spring Harbor Laboratory: Pasadena, CA, USA, 2018. [Google Scholar] [CrossRef]
- Fielenbach, N.; Antebi, A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008, 22, 2149–2165. [Google Scholar] [CrossRef]
- Manohar, M.; Tenjo-Castano, F.; Chen, S.; Zhang, Y.K.; Kumari, A.; Williamson, V.M.; Wang, X.; Klessig, D.F.; Schroeder, F.C. Plant metabolism of nematode pheromones mediates plant-nematode interactions. Nat. Commun. 2020, 11, 208. [Google Scholar] [CrossRef]
- Michailidu, J.; Maťátková, O.; Čejková, A.; Masák, J. Chemical Conversations. Molecules 2025, 30, 431. [Google Scholar] [CrossRef]
- Law, J.H.; Regnier, F.E. Pheromones. Annu. Rev. Biochem. 1971, 40, 533–548. [Google Scholar] [CrossRef]
- Combarnous, Y.; Nguyen, T.M.D. Cell communications among microorganisms, plants, and animals: Origin, evolution, and interplays. Int. J. Mol. Sci. 2020, 21, 8052. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.M. Pheromones and chemical communication in insects. Pests Weeds Dis. Agric. Crop Anim. Husb. Prod. 2020, 1–13. [Google Scholar]
- Yang, B.; Wang, J.; Zheng, X.; Wang, X. Nematode pheromones: Structures and functions. Molecules 2023, 28, 2409. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Tay, R.J.; Lin, H.-C.; Juan, S.-C.; Vidal-Diez de Ulzurrun, G.; Chang, Y.-C.; Hoki, J.; Schroeder, F.C.; Hsueh, Y.-P. The nematode-trapping fungus Arthrobotrys oligospora detects prey pheromones via G protein-coupled receptors. Nat. Microbiol. 2024, 9, 1738–1751. [Google Scholar] [CrossRef]
- Kuo, T.-H.; Yang, C.-T.; Chang, H.-Y.; Hsueh, Y.-P.; Hsu, C.-C. Nematode-trapping fungi produce diverse metabolites during predator–prey interaction. Metabolites 2020, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-P.; Mahanti, P.; Schroeder, F.C.; Sternberg, P.W. Nematode-trapping fungi eavesdrop on nematode pheromones. Curr. Biol. 2013, 23, 83–86. [Google Scholar] [CrossRef]
- Zou, J.; Kyndt, T.; Yu, J.; Zhou, J. Plant–nematode battle: Engagement of complex signaling network. Trends Parasitol. 2024, 40, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-P.; Leighton, D.H.; Sternberg, P.W. Nematode Communication. In Biocommunication of Animals; Springer: Berlin/Heidelberg, Germany, 2013; pp. 383–407. [Google Scholar]
- Braendle, C. Pheromones: Evolving language of chemical communication in nematodes. Curr. Biol. 2012, 22, R294–R296. [Google Scholar] [CrossRef]
- Machado, R.A.; Von Reuss, S.H. Chemical ecology of nematodes. Chimia 2022, 76, 945–953. [Google Scholar]
- Srinivasan, J.; Durak, O.; Sternberg, P.W. Evolution of a polymodal sensory response network. BMC Biol. 2008, 6, 1–15. [Google Scholar] [CrossRef]
- Jeong, P.-Y.; Jung, M.; Yim, Y.-H.; Kim, H.; Park, M.; Hong, E.; Lee, W.; Kim, Y.H.; Kim, K.; Paik, Y.-K. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature 2005, 433, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Butcher, R.A.; Fujita, M.; Schroeder, F.C.; Clardy, J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat. Chem. Biol. 2007, 3, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, J.; Kaplan, F.; Ajredini, R.; Zachariah, C.; Alborn, H.T.; Teal, P.E.; Malik, R.U.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature 2008, 454, 1115–1118. [Google Scholar] [CrossRef]
- Butcher, R.A.; Ragains, J.R.; Kim, E.; Clardy, J. A potent dauer pheromone component in Caenorhabditis elegans that acts synergistically with other components. Proc. Natl. Acad. Sci. USA 2008, 105, 14288–14292. [Google Scholar] [CrossRef]
- Pungaliya, C.; Srinivasan, J.; Fox, B.W.; Malik, R.U.; Ludewig, A.H.; Sternberg, P.W.; Schroeder, F.C. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 7708–7713. [Google Scholar] [CrossRef]
- Butcher, R.A.; Ragains, J.R.; Clardy, J. An indole-containing dauer pheromone component with unusual dauer inhibitory activity at higher concentrations. Org. Lett. 2009, 11, 3100–3103. [Google Scholar] [CrossRef]
- Izrayelit, Y.; Srinivasan, J.; Campbell, S.L.; Jo, Y.; von Reuss, S.H.; Genoff, M.C.; Sternberg, P.W.; Schroeder, F.C. Targeted metabolomics reveals a male pheromone and sex-specific ascaroside biosynthesis in Caenorhabditis elegans. ACS Chem. Biol. 2012, 7, 1321–1325. [Google Scholar] [CrossRef]
- Choe, A.; von Reuss, S.H.; Kogan, D.; Gasser, R.B.; Platzer, E.G.; Schroeder, F.C.; Sternberg, P.W. Ascaroside signaling is widely conserved among nematodes. Curr. Biol. 2012, 22, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Laing, S.T.; Ivens, A.; Laing, R.; Ravikumar, S.; Butler, V.; Woods, D.J.; Gilleard, J.S. Characterization of the xenobiotic response of Caenorhabditis elegans to the anthelmintic drug albendazole and the identification of novel drug glucoside metabolites. Biochem. J. 2010, 432, 505–516. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Kim, M.; Kim, E.; Choi, H.; Kim, Y.; Lee, J. Indole-associated predator–prey interactions between the nematode Caenorhabditis elegans and bacteria. Environ. Microbiol. 2017, 19, 1776–1790. [Google Scholar] [CrossRef]
- Stupp, G.S.; von Reuss, S.H.; Izrayelit, Y.; Ajredini, R.; Schroeder, F.C.; Edison, A.S. Chemical detoxification of small molecules by Caenorhabditis elegans. ACS Chem. Biol. 2013, 8, 309–313. [Google Scholar] [CrossRef]
- Motola, D.L.; Cummins, C.L.; Rottiers, V.; Sharma, K.K.; Li, T.; Li, Y.; Suino-Powell, K.; Xu, H.E.; Auchus, R.J.; Antebi, A. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell 2006, 124, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Papsdorf, K.; Miklas, J.W.; Hosseini, A.; Cabruja, M.; Morrow, C.S.; Savini, M.; Yu, Y.; Silva-García, C.G.; Haseley, N.R.; Murphy, L.M. Lipid droplets and peroxisomes are co-regulated to drive lifespan extension in response to mono-unsaturated fatty acids. Nat. Cell Biol. 2023, 25, 672–684. [Google Scholar] [CrossRef]
- Ludewig, A.H.; Artyukhin, A.B.; Aprison, E.Z.; Rodrigues, P.R.; Pulido, D.C.; Burkhardt, R.N.; Panda, O.; Zhang, Y.K.; Gudibanda, P.; Ruvinsky, I. An excreted small molecule promotes C. elegans reproductive development and aging. Nat. Chem. Biol. 2019, 15, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Chantab, K.; Rao, Z.; Zheng, X.; Han, R.; Cao, L. Ascarosides and Symbiotic Bacteria of Entomopathogenic Nematodes Regulate Host Immune Response in Galleria mellonella Larvae. Insects 2024, 15, 514. [Google Scholar] [CrossRef]
- Kaplan, F.; Srinivasan, J.; Mahanti, P.; Ajredini, R.; Durak, O.; Nimalendran, R.; Sternberg, P.W.; Teal, P.E.; Schroeder, F.C.; Edison, A.S. Ascaroside expression in Caenorhabditis elegans is strongly dependent on diet and developmental stage. PLoS ONE 2011, 6, e17804. [Google Scholar] [CrossRef] [PubMed]
- Hartley, C.J.; Lillis, P.E.; Owens, R.A.; Griffin, C.T. Infective juveniles of entomopathogenic nematodes (Steinernema and Heterorhabditis) secrete ascarosides and respond to interspecific dispersal signals. J. Invertebr. Pathol. 2019, 168, 107257. [Google Scholar] [CrossRef]
- Dong, C. Small Molecule Signaling in Nematodes; Friedrich-Schiller-Universität Jena: Jena, Germany, 2017. [Google Scholar]
- Von Reuss, S.H.; Schroeder, F.C. Combinatorial chemistry in nematodes: Modular assembly of primary metabolism-derived building blocks. Nat. Prod. Rep. 2015, 32, 994–1006. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.K.; Manohar, M.; Artyukhin, A.B.; Kumari, A.; Tenjo-Castano, F.J.; Nguyen, H.; Routray, P.; Choe, A.; Klessig, D.F. Nematode signaling molecules are extensively metabolized by animals, plants, and microorganisms. ACS Chem. Biol. 2021, 16, 1050–1058. [Google Scholar] [CrossRef]
- Wrobel, C.J.; Schroeder, F.C. Repurposing degradation pathways for modular metabolite biosynthesis in nematodes. Nat. Chem. Biol. 2023, 19, 676–686. [Google Scholar] [CrossRef]
- Salzer, L.; Witting, M. Quo vadis Caenorhabditis elegans metabolomics—A review of current methods and applications to explore metabolism in the nematode. Metabolites 2021, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Bento, G.; Ogawa, A.; Sommer, R.J. Co-option of the hormone-signalling module dafachronic acid–DAF-12 in nematode evolution. Nature 2010, 466, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Kotowska, A.M.; Hiramatsu, F.; Alexander, M.R.; Scurr, D.J.; Lightfoot, J.W.; Chauhan, V.M. Surface Lipids in Nematodes are Influenced by Development and Species-specific Adaptations. J. Am. Chem. Soc. 2025, 147, 6439–6449. [Google Scholar] [CrossRef]
- C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 1998, 282, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Abad, P.; Gouzy, J.; Aury, J.-M.; Castagnone-Sereno, P.; Danchin, E.G.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef]
- Dong, C.; Reilly, D.K.; Bergame, C.; Dolke, F.; Srinivasan, J.; von Reuss, S.H. Comparative ascaroside profiling of Caenorhabditis exometabolomes reveals species-specific (ω) and (ω–2)-hydroxylation downstream of peroxisomal β-oxidation. J. Org. Chem. 2018, 83, 7109–7120. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Perez, D.H.; Jones Lipinski, R.A.; Butcher, R.A.; Lipinski, R. Acyl-CoA Oxidases Fine-Tune the Production of Ascaroside Pheromones with Specific Side Chain Lengths. ACS Chem. Biol. 2018, 13, 1048–1056. [Google Scholar] [CrossRef]
- Park, J.Y.; Joo, H.-J.; Park, S.; Paik, Y.-K. Ascaroside pheromones: Chemical biology and pleiotropic neuronal functions. Int. J. Mol. Sci. 2019, 20, 3898. [Google Scholar] [CrossRef]
- Bono, M.d.; Villu Maricq, A. Neuronal substrates of complex behaviors in C. elegans. Annu. Rev. Neurosci. 2005, 28, 451–501. [Google Scholar] [CrossRef]
- Wang, J.; Cao, L.; Huang, Z.; Gu, X.; Cui, Y.; Li, J.; Li, Y.; Xu, C.; Han, R. Influence of the ascarosides on the recovery, yield and dispersal of entomopathogenic nematodes. J. Invertebr. Pathol. 2022, 188, 107717. [Google Scholar] [CrossRef]
- Bargmann, C. Chemosensation in C. elegans. In WormBook: The Online Review of C. elegans Biology; Cold Spring Harbor Laboratory: Pasadena, CA, USA, 2006. [Google Scholar] [CrossRef]
- Ferkey, D.M.; Sengupta, P.; Noelle, D.L. Chemosensory signal transduction in Caenorhabditis elegans. Genetics 2021, 217, iyab004. [Google Scholar] [CrossRef]
- Hart, A.C.; Chao, M.Y. From odors to behaviors in Caenorhabditis elegans. In The Neurobiology of Olfaction; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; p. 21882435. [Google Scholar]
- De Bono, M.; Bargmann, C.I. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell 1998, 94, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Macosko, E.Z.; Pokala, N.; Feinberg, E.H.; Chalasani, S.H.; Butcher, R.A.; Clardy, J.; Bargmann, C.I. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 2009, 458, 1171–1175. [Google Scholar] [CrossRef]
- White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. B Biol. Sci. 1986, 314, 1–340. [Google Scholar] [PubMed]
- Poinar Jr, G.O. Nematoda and nematomorpha. In Ecology and Classification of North American Freshwater Invertebrates; Elsevier: Amsterdam, The Netherlands, 2010; pp. 237–276. [Google Scholar]
- Godoy, L.F.; Hochbaum, D. Transcriptional and spatiotemporal regulation of the dauer program. Transcription 2023, 14, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.J. Dauer. In WormBook: The Online Review of C. elegans Biology [Internet]; Cold Spring Harbor Laboratory: Pasadena, CA, USA, 2018; doi: 10.1895/wormbook.1.144.1. [Google Scholar]
- Vlaar, L.E.; Bertran, A.; Rahimi, M.; Dong, L.; Kammenga, J.E.; Helder, J.; Goverse, A.; Bouwmeester, H.J. On the role of dauer in the adaptation of nematodes to a parasitic lifestyle. Parasites Vectors 2021, 14, 1–20. [Google Scholar] [CrossRef]
- Golden, J.W.; Riddle, D.L. The Caenorhabditis elegans dauer larva: Developmental effects of pheromone, food, and temperature. Dev. Biol. 1984, 102, 368–378. [Google Scholar] [CrossRef]
- Crook, M. The dauer hypothesis and the evolution of parasitism: 20 years on and still going strong. Int. J. Parasitol. 2014, 44, 1–8. [Google Scholar] [CrossRef]
- Golden, J.W.; Riddle, D.L. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science 1982, 218, 578–580. [Google Scholar] [CrossRef]
- Diaz, S.A.; Brunet, V.; Lloyd-Jones, G.C.; Spinner, W.; Wharam, B.; Viney, M. Diverse and potentially manipulative signalling with ascarosides in the model nematode C. elegans. BMC Evol. Biol. 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Cohen, S.M.; Wrobel, C.J.; Prakash, S.J.; Schroeder, F.C.; Sternberg, P.W. Formation and function of dauer ascarosides in the nematodes Caenorhabditis briggsae and Caenorhabditis elegans. G3 2022, 12, jkac014. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Sato, K.; Shibuya, M.; Zeiger, D.M.; Butcher, R.A.; Ragains, J.R.; Clardy, J.; Touhara, K.; Sengupta, P. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science 2009, 326, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Lim, C.-S.; Johnsen, R.; Albert, P.S.; Pilgrim, D.; Riddle, D.L. Control of C. elegans larval development by neuronal expression of a TGF-β homolog. Science 1996, 274, 1389–1391. [Google Scholar] [CrossRef]
- Schackwitz, W.S.; Inoue, T.; Thomas, J.H. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron 1996, 17, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.G.; Sternberg, P.W. Entry to and exit from diapause arrest in Caenorhabditis elegans are both regulated by a steroid hormone pathway. BioRxiv 2021, 149, dev200173. [Google Scholar] [CrossRef]
- Simon, J.M.; Sternberg, P.W. Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 1598–1603. [Google Scholar] [CrossRef]
- Wu, T.; Ge, M.; Wu, M.; Duan, F.; Liang, J.; Chen, M.; Gracida, X.; Liu, H.; Yang, W.; Dar, A.R. Pathogenic bacteria modulate pheromone response to promote mating. Nature 2023, 613, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, A.H.; Izrayelit, Y.; Park, D.; Malik, R.U.; Zimmermann, A.; Mahanti, P.; Fox, B.W.; Bethke, A.; Doering, F.; Riddle, D.L. Pheromone sensing regulates Caenorhabditis elegans lifespan and stress resistance via the deacetylase SIR-2.1. Proc. Natl. Acad. Sci. USA 2013, 110, 5522–5527. [Google Scholar] [CrossRef]
- Vidal-Diez de Ulzurrun, G.; Hsueh, Y.-P. Predator-prey interactions of nematode-trapping fungi and nematodes: Both sides of the coin. Appl. Microbiol. Biotechnol. 2018, 102, 3939–3949. [Google Scholar] [CrossRef]
- Clarke, D.J.; Eberl, L. Interactions between bacteria and nematodes. Intest. Microorg. Termit. Other Invertebr. 2006, 55–64. [Google Scholar]
- Horiuchi, J.-i.; Prithiviraj, B.; Bais, H.P.; Kimball, B.A.; Vivanco, J.M. Soil nematodes mediate positive interactions between legume plants and rhizobium bacteria. Planta 2005, 222, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Williamson, V.M.; Gleason, C.A. Plant–nematode interactions. Curr. On. Plant Biol. 2003, 6, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Eves-van den Akker, S. Plant–nematode interactions. Curr. Opin. Plant Biol. 2021, 62, 102035. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M. Understanding molecular plant–nematode interactions to develop alternative approaches for nematode control. Plants 2022, 11, 2141. [Google Scholar] [CrossRef]
- Akhtar, M.S. Opportunistic Fungi, Nematode and Plant Interactions: Interplay and Mechanisms; Springer Nature: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Zhang, Y.; Li, G.; Zhang, K. A review on the research of nematophagous fungal species. Mycosystema 2011, 30, 836–845. [Google Scholar]
- Karakaş, M. Nematode-destroying fungi: Infection structures, interaction mechanisms and biocontrol. Commun. Fac. Sci. Univ. Ank. Ser. C Biol. 2020, 29, 176–201. [Google Scholar]
- Nordbring-Hertz, B.; Jansson, H.-B.; Tunlid, A. Nematophagous fungi. Encycl. Life Sci. 2006, 10. [Google Scholar]
- Yadav, B.; Singh, U.B.; Malviya, D.; Vishwakarma, S.K.; Ilyas, T.; Shafi, Z.; Shahid, M.; Singh, H.V. Nematophagous fungi: Biology, ecology and potential application. In Detection, Diagnosis and Management of Soil-Borne Phytopathogens; Springer: Berlin/Heidelberg, Germany, 2023; pp. 309–328. [Google Scholar]
- Hu, X.; Hoffmann, D.S.; Wang, M.; Schuhmacher, L.; Stroe, M.C.; Schreckenberger, B.; Elstner, M.; Fischer, R. GprC of the nematode-trapping fungus Arthrobotrys flagrans activates mitochondria and reprograms fungal cells for nematode hunting. Nat. Microbiol. 2024, 9, 1752–1763. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Shen, Q. Dual function of a fungal GPCR in activating mitochondrial respiration and initiating prey hunting in fungus-nematode interactions. Innov. Life 2024, 2, 100087. [Google Scholar] [CrossRef]
- Hsueh, Y.-P.; Gronquist, M.R.; Schwarz, E.M.; Nath, R.D.; Lee, C.-H.; Gharib, S.; Schroeder, F.C.; Sternberg, P.W. Nematophagous fungus Arthrobotrys oligospora mimics olfactory cues of sex and food to lure its nematode prey. Elife 2017, 6, e20023. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, N.; Chen, Y.; Zhang, D.; Yan, J.; Zou, W.; Zhang, K.; Huang, X. The signaling pathway of Caenorhabditis elegans mediates chemotaxis response to the attractant 2-heptanone in a trojan horse-like pathogenesis. J. Biol. Chem. 2016, 291, 23618–23627. [Google Scholar] [CrossRef] [PubMed]
- Pender, C.L.; Dishart, J.G.; Gildea, H.K.; Nauta, K.M.; Page, E.M.; Siddiqi, T.F.; Cheung, S.S.; Joe, L.; Burton, N.O.; Dillin, A. Perception of a pathogenic signature initiates intergenerational protection. Cell 2025, 188, 594–605.e510. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yuan, Y.; Lewis, C.; Kud, J.; Kuhl, J.C.; Caplan, A.; Dandurand, L.-M.; Zasada, I.; Xiao, F. NILR1 perceives a nematode ascaroside triggering immune signaling and resistance. Curr. Biol. 2023, 33, 3992–3997.e3993. [Google Scholar] [CrossRef] [PubMed]
- Klessig, D.F.; Manohar, M.; Baby, S.; Koch, A.; Danquah, W.B.; Luna, E.; Park, H.J.; Kolkman, J.M.; Turgeon, B.G.; Nelson, R. Nematode ascaroside enhances resistance in a broad spectrum of plant–pathogen systems. J. Phytopathol. 2019, 167, 265–272. [Google Scholar] [CrossRef]
- Kamboj, A.; Thielmann, J.; Delfan, S.; Kloppe, T.; Schulz, P.; Manohar, M.; Schroeder, F.C.; Klessig, D.F.; Kogel, K.-H. The Nematode Signaling Molecule ascr# 18 Induces Prehaustorial Defenses in Wheat Against a Leaf Rust Fungus. J. Plant Dis. Prot. 2024, 131, 2053–2062. [Google Scholar]
- Mendy, B.; Wang’ombe, M.W.; Radakovic, Z.S.; Holbein, J.; Ilyas, M.; Chopra, D.; Holton, N.; Zipfel, C.; Grundler, F.M.; Siddique, S. Arabidopsis leucine-rich repeat receptor–like kinase NILR1 is required for induction of innate immunity to parasitic nematodes. PLoS Pathog. 2017, 13, e1006284. [Google Scholar] [CrossRef]
- Zhao, M.; Wickham, J.D.; Zhao, L.; Sun, J. Major ascaroside pheromone component asc-C5 influences reproductive plasticity among isolates of the invasive species pinewood nematode. Integr. Zool. 2021, 16, 893–907. [Google Scholar] [CrossRef]
- Zhao, L.; Ahmad, F.; Lu, M.; Zhang, W.; Wickham, J.D.; Sun, J. Ascarosides promote the prevalence of ophiostomatoid fungi and an invasive pathogenic nematode, Bursaphelenchus xylophilus. J. Chem. Ecol. 2018, 44, 701–710. [Google Scholar] [CrossRef]
- von Reuss, S.H. Exploring modular glycolipids involved in nematode chemical communication. Chimia 2018, 72, 297–303. [Google Scholar] [CrossRef]
- Peng, J.-Y.; Liu, X.; Zeng, X.-T.; Hao, Y.; Zhang, J.-H.; Li, Q.; Tong, X.-J. Early pheromone perception remodels neurodevelopment and accelerates neurodegeneration in adult C. elegans. Cell Rep. 2023, 42, 112598. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, F.C. Modular assembly of primary metabolic building blocks: A chemical language in C. elegans. Chem. Biol. 2015, 22, 7–16. [Google Scholar] [CrossRef]
- von Reuss, S.H.; Dolke, F.; Dong, C. Ascaroside profiling of Caenorhabditis elegans using gas chromatography–electron ionization mass spectrometry. Anal. Chem. 2017, 89, 10570–10577. [Google Scholar] [CrossRef] [PubMed]
- Von Reuss, S.H.; Bose, N.; Srinivasan, J.; Yim, J.J.; Judkins, J.C.; Sternberg, P.W.; Schroeder, F.C. Comparative metabolomics reveals biogenesis of ascarosides, a modular library of small-molecule signals in C. elegans. J. Am. Chem. Soc. 2012, 134, 1817–1824. [Google Scholar] [CrossRef]
- Zagoriy, V.; Matyash, V.; Kurzchalia, T. Long-Chain O-Ascarosyl-alkanediols Are Constitutive Components of Caenorhabditis elegans but Do Not Induce Dauer Larva Formation. Chem. Biodivers. 2010, 7, 2016–2022. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, L.; Chinta, S.; Singh, P.; Wang, Y.; Nunnery, J.K.; Butcher, R.A. Acyl-CoA oxidase complexes control the chemical message produced by Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2015, 112, 3955–3960. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vogt, M.C.; Fox, B.W.; Wrobel, C.J.; Fajardo Palomino, D.; Curtis, B.J.; Zhang, B.; Le, H.H.; Tauffenberger, A.; Hobert, O. Parallel pathways for serotonin biosynthesis and metabolism in C. elegans. Nat. Chem. Biol. 2023, 19, 141–150. [Google Scholar] [CrossRef]
- Le, H.H.; Wrobel, C.J.; Cohen, S.M.; Yu, J.; Park, H.; Helf, M.J.; Curtis, B.J.; Kruempel, J.C.; Rodrigues, P.R.; Hu, P.J. Modular metabolite assembly in Caenorhabditis elegans depends on carboxylesterases and formation of lysosome-related organelles. Elife 2020, 9, e61886. [Google Scholar] [CrossRef]
- Espada, M.; Silva, A.C.; Eves van den Akker, S.; Cock, P.J.; Mota, M.; Jones, J.T. Identification and characterization of parasitism genes from the pinewood nematode Bursaphelenchus xylophilus reveals a multilayered detoxification strategy. Mol. Plant Pathol. 2016, 17, 286–295. [Google Scholar] [CrossRef]
- Wrobel, C.J.J. Combinatorial Assembly of Modular Metabolites via Carboxylesterases in Nematodes; Cornell University: Ithaca, NY, USA, 2023. [Google Scholar]
- Szeto, S.S.; Reinke, S.N.; Lemire, B.D. 1 H NMR-based metabolic profiling reveals inherent biological variation in yeast and nematode model systems. J. Biomol. NMR 2011, 49, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kowsalya, K.; Vidya, N.; Halka, J.; Preetha, J.S.Y.; Saradhadevi, M.; Sahayarayan, J.J.; Gurusaravanan, P.; Arun, M. Plant glycosides and glycosidases: Classification, sources, and therapeutic insights in current medicine. Glycoconj. J. 2025, 42, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Stasiuk, S.; MacNevin, G.; Workentine, M.; Gray, D.; Redman, E.; Bartley, D.; Morrison, A.; Sharma, N.; Colwell, D.; Ro, D. Similarities and differences in the biotransformation and transcriptomic responses of Caenorhabditis elegans and Haemonchus contortus to five different benzimidazole drugs. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Kellerová, P.; Raisová Stuchlíková, L.; Matoušková, P.; Štěrbová, K.; Lamka, J.; Navrátilová, M.; Vokřál, I.; Szotáková, B.; Skálová, L. Sub-lethal doses of albendazole induce drug metabolizing enzymes and increase albendazole deactivation in Haemonchus contortus adults. Vet. Res. 2020, 51, 1–17. [Google Scholar] [CrossRef]
- Fontaine, P.; Choe, K. The transcription factor SKN-1 and detoxification gene ugt-22 alter albendazole efficacy in Caenorhabditis elegans. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 312–319. [Google Scholar] [CrossRef]
- Wrobel, C.J.; Yu, J.; Rodrigues, P.R.; Ludewig, A.H.; Curtis, B.J.; Cohen, S.M.; Fox, B.W.; O’Donnell, M.P.; Sternberg, P.W.; Schroeder, F.C. Combinatorial assembly of modular glucosides via carboxylesterases regulates C. elegans starvation survival. J. Am. Chem. Soc. 2021, 143, 14676–14683. [Google Scholar] [CrossRef]
- Mahanti, P.; Bose, N.; Bethke, A.; Judkins, J.C.; Wollam, J.; Dumas, K.J.; Zimmerman, A.M.; Campbell, S.L.; Hu, P.J.; Antebi, A. Comparative metabolomics reveals endogenous ligands of DAF-12, a nuclear hormone receptor, regulating C. elegans development and lifespan. Cell Metab. 2014, 19, 73–83. [Google Scholar] [CrossRef]
- Wollam, J.; Magner, D.B.; Magomedova, L.; Rass, E.; Shen, Y.; Rottiers, V.; Habermann, B.; Cummins, C.L.; Antebi, A. A novel 3-hydroxysteroid dehydrogenase that regulates reproductive development and longevity. PLoS Biol. 2012, 10, e1001305. [Google Scholar] [CrossRef]
- Cui, G.Z.; Zhu, J.J. Pheromone-based pest management in China: Past, present, and future prospects. J. Chem. Ecol. 2016, 42, 557–570. [Google Scholar] [CrossRef]
- Mota, M.M.; Vieira, P. Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Degenhardt, J.; Hiltpold, I.; Köllner, T.G.; Frey, M.; Gierl, A.; Gershenzon, J.; Hibbard, B.E.; Ellersieck, M.R.; Turlings, T.C. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc. Natl. Acad. Sci. USA 2009, 106, 13213–13218. [Google Scholar] [CrossRef]
- Bruce, T.J.; Aradottir, G.I.; Smart, L.E.; Martin, J.L.; Caulfield, J.C.; Doherty, A.; Sparks, C.A.; Woodcock, C.M.; Birkett, M.A.; Napier, J.A. The first crop plant genetically engineered to release an insect pheromone for defence. Sci. Rep. 2015, 5, 11183. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Barros-Parada, W.; Bosch, D.; Escudero-Colomar, L.A.; Fuentes-Contreras, E.; Hernández-Sánchez, J.; Yung, C.; Kim, Y.; Kovanci, O.; Levi, A. Similar worldwide patterns in the sex pheromone signal and response in the oriental fruit moth, Grapholita molesta (Lepidoptera: Tortricidae). Bull. Entomol. Res. 2015, 105, 23–31. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, L.; Lv, C.; Ge, S.; Zhang, H.; Jiang, S.; Chu, B.; Yang, X.; Wyckhuys, K.A.; Wu, K. Use of food attractants to monitor and forecast Spodoptera frugiperda (JE Smith) seasonal abundance in southern China. J. Pest Sci. 2023, 96, 1509–1521. [Google Scholar] [CrossRef]
- Sullivan, N.; Butler, R.; Salehi, L.; Twidle, A.; Baker, G.; Suckling, D. Deployment of the sex pheromone of Pseudococcus calceolariae (Hemiptera: Pseudococcidae) as a potential new tool for mass trapping in citrus in South Australia. N. Zealand Entomol. 2019, 42, 1–12. [Google Scholar] [CrossRef]
- Cherif, A.; Harbaoui, K.; Zappala, L.; Grissa-Lebdi, K. Efficacy of mass trapping and insecticides to control Tuta absoluta in Tunisia. J. Plant Dis. Prot. 2018, 125, 51–61. [Google Scholar] [CrossRef]
- Madhu, T.; Shah, V.; Prabhulinga, T.; Chakravarthy, A.; Ashok Kumar, C. Optimization of pheromone trap densities and impact of insecticides on pheromone catches for mass trapping Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) in chickpea. J. Entomol. Zool. Stud. 2019, 7, 78–84. [Google Scholar]
- Piñero, J.C.; Dudenhoeffer, A.P. Mass trapping designs for organic control of the Japanese beetle, Popillia japonica (Coleoptera: Scarabaeidae). Pest Manag. Sci. 2018, 74, 1687–1693. [Google Scholar] [CrossRef]
- Harari, A.R.; Zahavi, T.; Steinitz, H. Female detection of the synthetic sex pheromone contributes to the efficacy of mating disruption of the European grapevine moth, Lobesia botrana. Pest Manag. Sci. 2015, 71, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Walgenbach, J.F.; Schoof, S.C.; Bosch, D.; Escudero-Colomar, L.-A.; Lingren, B.; Krawczyk, G. Comparison of sex pheromone and kairomone-enhanced pheromone lures for monitoring oriental fruit moth (Lepidoptera: Tortricidae) in mating disruption and non-disruption tree fruit orchards. Environ. Entomol. 2021, 50, 1063–1074. [Google Scholar] [CrossRef]
- Liang, Y.-y.; Luo, M.; Fu, X.-g.; Zheng, L.-x.; Wei, H.-y. Mating disruption of Chilo suppressalis from sex pheromone of another pyralid rice pest Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). J. Insect Sci. 2020, 20, 19. [Google Scholar] [CrossRef]
- Ricciardi, R.; Di Giovanni, F.; Cosci, F.; Ladurner, E.; Savino, F.; Iodice, A.; Benelli, G.; Lucchi, A. Mating Disruption for Managing the Honeydew Moth, Cryptoblabes gnidiella (Millière), in Mediterranean Vineyards. Insects 2021, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Hofman, C.; Kaplan, F.; Stevens, G.; Lewis, E.; Wu, S.; Alborn, H.T.; Perret-Gentil, A.; Shapiro-Ilan, D.I. Pheromone extracts act as boosters for entomopathogenic nematodes efficacy. J. Invertebr. Pathol. 2019, 164, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, G.U.; Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F. Development and commercialization of pheromone-based biopesticides: A global perspective. In Development and Commercialization of Biopesticides; Elsevier: Amsterdam, The Netherlands, 2023; pp. 37–56. [Google Scholar]
- Koul, O. Development and Commercialization of Biopesticides: Costs and Benefits; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Fox, B.W.; Ponomarova, O.; Lee, Y.-U.; Zhang, G.; Giese, G.E.; Walker, M.; Roberto, N.M.; Na, H.; Rodrigues, P.R.; Curtis, B.J. C. elegans as a model for inter-individual variation in metabolism. Nature 2022, 607, 571–577. [Google Scholar] [CrossRef]
- Robinette, S.L.; Brüschweiler, R.; Schroeder, F.C.; Edison, A.S. NMR in metabolomics and natural products research: Two sides of the same coin. Acc. Chem. Res. 2012, 45, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Salzer, L.; Schmitt-Kopplin, P.; Witting, M. Capillary electrophoresis-mass spectrometry as a tool for Caenorhabditis elegans metabolomics research. Metabolomics 2023, 19, 61. [Google Scholar] [CrossRef]
- Dong, C.; Weadick, C.J.; Truffault, V.; Sommer, R.J. Convergent evolution of small molecule pheromones in Pristionchus nematodes. Elife 2020, 9, e55687. [Google Scholar] [CrossRef]
- Butcher, R.A. Small-molecule pheromones and hormones controlling nematode development. Nat. Chem. Biol. 2017, 13, 577–586. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Sanchez-Ayala, M.A.; Sternberg, P.W.; Srinivasan, J.; Schroeder, F.C. Improved synthesis for modular ascarosides uncovers biological activity. Org. Lett. 2017, 19, 2837–2840. [Google Scholar] [CrossRef]
- Pathak, V.M. Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 1–31. [Google Scholar] [CrossRef]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale drug delivery systems: From medicine to agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial seed coating: An attractive tool for sustainable agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef] [PubMed]
- Manosalva, P.; Manohar, M.; Von Reuss, S.H.; Chen, S.; Koch, A.; Kaplan, F.; Choe, A.; Micikas, R.J.; Wang, X.; Kogel, K.-H. Conserved nematode signalling molecules elicit plant defenses and pathogen resistance. Nat. Commun. 2015, 6, 7795. [Google Scholar] [CrossRef] [PubMed]
- González-Guzmán, A.; Sánchez-Rodríguez, A.R.; Quesada-Moraga, E.; del Campillo, M.C.; Yousef-Yousef, M. Optimizing wheat seed treatment with entomopathogenic fungi for improving plant growth at early development stages. Span. J. Agric. Res. 2021, 19, e1004. [Google Scholar] [CrossRef]
- Muirhead, C.S.; Srinivasan, J. Small molecule signals mediate social behaviors in C. elegans. J. Neurogenet. 2020, 34, 395–403. [Google Scholar] [CrossRef]
| Dauer Pheromone ascr#1 [33] (very low activity) ascr#2 [34] (high activity) ascr#3 [34] (high activity) ascr#4 [35] (low activity) ascr#5 [36] (high activity) ascr#8 [37] (moderate activity) icas#9 [38] (moderate activity) | 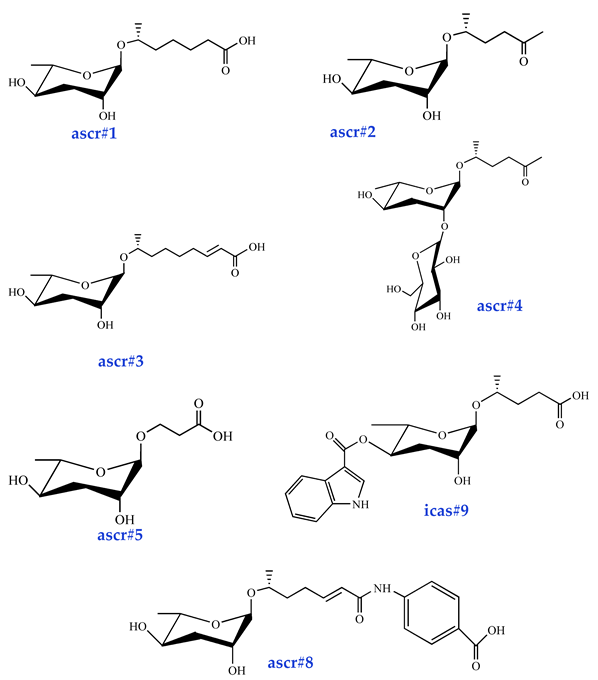 |
| Sex pheromones ascr#2 [34] (moderate activity) ascr#3 [34] (high activity) ascr#4 [35] (low activity) ascr#6.1 [37] (moderate activity) ascr#6.2 [37] (activity?) ascr#8 [37] (high activity) ascr#10 [39] (high activity) | 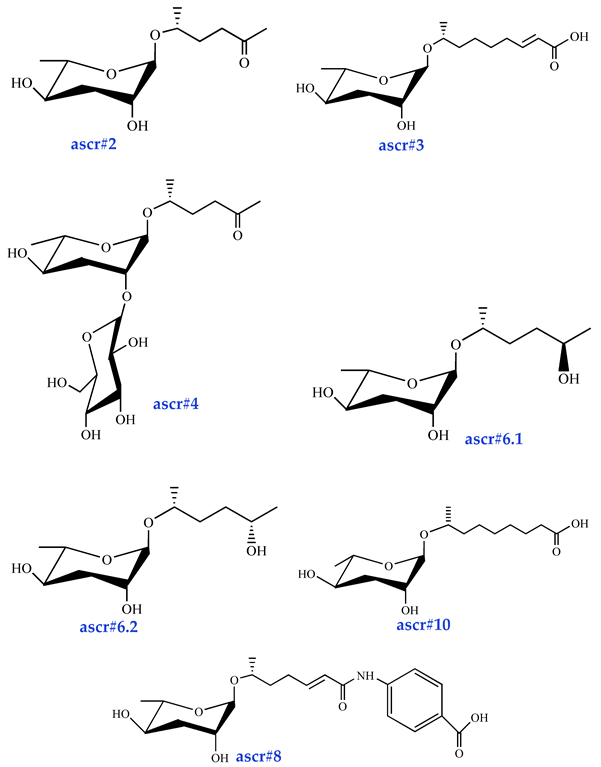 |
| Aggregation pheromones icas#3 [16] (high activity) icas#9 [38] (high activity) | 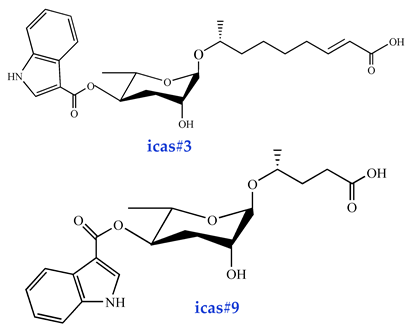 |
| Behavioral Pheromone (retention, avoidance, and attraction) ascr#7 [37] ascr#9 [40] ascr#12 [40] ascr#14 [40] ascr#10 [40] ascr#18 [40] ascr#20 [40] ascr#22 [40] ascr#24 [40] ascr#26 [40] oscr#9 [40] oscr#10 [40] oscr#18 [40] | 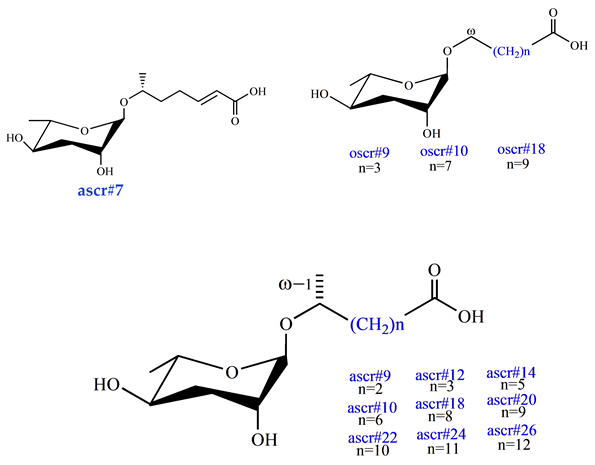 |
| Glucosides (detoxification capability) ABZ–glucoside [41] N-(β-D-glucopyranosyl)indole [42] N-(3′-O-phospho-β-D-glucopyranosyl)-indol [42] 1-O-(3′-O-phospho-β-Dglucopyranosyl)-phenazinee [43] | 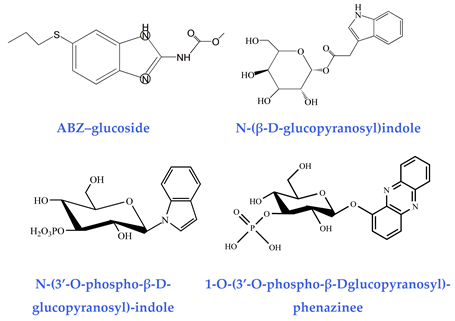 |
| Non-Glycosidic Pheromones (regulation of development and behavior) dafachronic acids [44] oleic acids [45] elaidic acids [45] nacq#1 [46] | 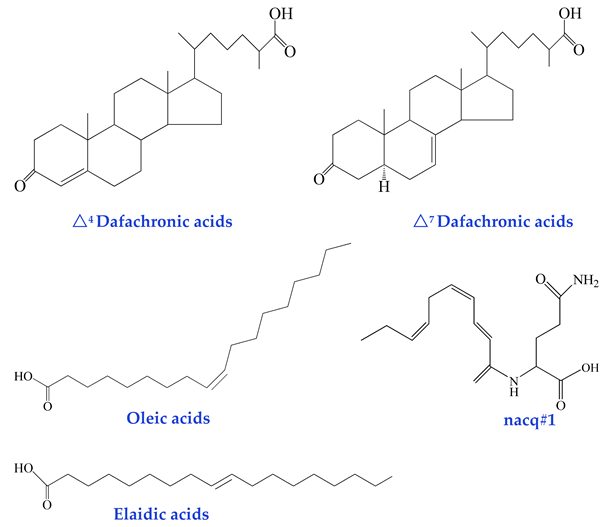 |
| Neuron | Receptors | G proteins | Signal Transduction | Regulators | Function | Chemical Stimulus |
|---|---|---|---|---|---|---|
| ASE | Receptor guanylate cyclases? | gpa-3 | tax-4, tax-2, daf-11, cGMP | osm-9, gpc-1, tax-6, ttx-4, adp-1 | Water-soluble chemotaxis | cAMP, cGMP, Lysine, Histidine, Cysteine, Methionine, Biotin, Na+, K+, Cl− |
| AWA | GPCRs (odr-10) | odr-3, gpa-3, gpa-5, gpa-13; gpa-6 | osm-9, ocr-2, fat-3, PUFA, odr-4, odr-8 | egl-4, grk-2, tax-6, ttx-4 | Volatile chemotaxis, lifespan (minor) | Pyrazine, Diacetyl (low), 2, 4, 5-Trimethylthiazole (low), Butyric acid, Isobutyric acid, Benzyl proprionate, soamyl alcohol (low) |
| AWC | GPCRs (str-2) | odr-3(major), gpa-3, gpa-2, gpa-5, gpa-13 | tax-4, tax-2, daf-11, odr-1, odr-4, odr-8, cGMP | osm-9, eg/-4, grk-2, tax-6, ttx-4, sdf-13, adp-1, let-60, goa-1 | Volatile chemotaxis, lifespan, navigation | Diacetyl, 2, 4, 5-Trimethylthiazole (low), Benzyl proprionate, Benzaldehyde, Isoamyl alcohol, Acetone, Dimethylthiazole, 1--Methylpyrrole, 1-Pentanol, 2-Cyclohexylethanol, 2-Ethoxythiazole, Benzyl mercaptan, 2-Heptanone, 3-Pentanedione |
| AWB | GPCRs | odr-3 | tax-4, tax-2, daf-11, odr-1, cGMP | kin-29 | Volatile avoidance | Ketones, 2-Nonanone, Serrawettin W2 |
| ASH | GPCRs | odr-3(major), gpa-3(major), gpa-11, gpa-1, gpa-13, gpa-14, gpa-15 | osm-9, ocr-2, fat-3, PUFA, qui-1(chemical only), osm-10(osmo only), odr-4 | grk-2(chemical, partial osmo)tax-6, ttx-4, gpc-1, kin-29 | Nociception: osmotic avoidance, nose touch avoidance, chemical avoidance, social feeding | Acidic pH, Basic pH (>10.5), Copper, Cadmium, SDS, Bitters quinine, Diacetyl (high), Benzaldehyde (high), Isoamyl alcohol (high), 1-Octanol, 2-Nonanone |
| ASI | GPCRs; | gpa-1, gpa-3, gpa-4, gpa-5, gpa-6, gpa-10, gpa-14 | tax-4, tax-2, daf-11, cGMPodr-1, odr-4 | Dauer formation, chemotaxis (minor), navigation | cAMP, cGMP, Lysine, Histidine, Na+, K+, Cl−, Biotin, SDS | |
| ADF | GPCRs; | odr-3, gpa-3, gpa-10, gpa-13 | osm-9, ocr-2, odr-4 | Dauer formation, chemotaxis (minor) | cAMP, cGMP, Na+, K+, Cl−, Biotin, Acidic pH | |
| ASG | GPCRs; | gpa-3 | tax-4, tax-2, cGMP, odr-4 | Dauer formation (minor), lifespan, chemotaxis (minor) | cAMP, cGMP, Lysine, Histidine, Cysteine, Biotin, Na+, K+, Cl− | |
| ASJ | GPCRs; | gpa-1, gpa-3, gpa-9, gpa-10, gpa-14 | tax-4, tax-2, daf-11, daf-21, cGMP, odr-1, odr-4 | Dauer formation and recovery, chemotaxis (minor), lifespan | Phenazine-1-carboxamide, Pyochelin, SDS | |
| ASK | GPCRs; | gpa-2, gpa-3, gpa-14, gpa-15 | tax-4, tax-2, cGMP, odr-1, daf-11, odr-4 | kin-29 | avoidance (minor), chemotaxis (minor), lifespan, navigation | Lysine, Histidine, Cysteine, Methionine, Acidic pH, Bitters quinine |
| ADL | GPCRs; | gpa-1, gpa-3, gpa-11, gpa-15 | osm-9, ocr-2, odr-4 | avoidance (minor), social feeding | Copper, Cadmium, Isoamyl alcohol (high) | |
| URX, AQR, PQR | gcy-35, gcy-36 | gpa-8 | tax-4, tax-2, cGMP | npr-1 | oxygen/aerotaxis, social feeding | ? |
| PHA, PHB | GPCRs | gpa-1, gpa-2, gpa-3, gpa-6, gpa-9, gpa-13, gpa-14, gpa-15 | osm-9, ocr-2, odr-4 | Avoidance (antagonistic) | SDS, Dodecanoic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Liu, J.; Wang, X. Nematode Pheromones as Key Mediators of Behavior, Development, and Ecological Interactions. Biomolecules 2025, 15, 981. https://doi.org/10.3390/biom15070981
Zheng X, Liu J, Wang X. Nematode Pheromones as Key Mediators of Behavior, Development, and Ecological Interactions. Biomolecules. 2025; 15(7):981. https://doi.org/10.3390/biom15070981
Chicago/Turabian StyleZheng, Xi, Junjie Liu, and Xin Wang. 2025. "Nematode Pheromones as Key Mediators of Behavior, Development, and Ecological Interactions" Biomolecules 15, no. 7: 981. https://doi.org/10.3390/biom15070981
APA StyleZheng, X., Liu, J., & Wang, X. (2025). Nematode Pheromones as Key Mediators of Behavior, Development, and Ecological Interactions. Biomolecules, 15(7), 981. https://doi.org/10.3390/biom15070981







