Macropinocytosis: Both a Target and a Tool for Cancer Therapy
Abstract
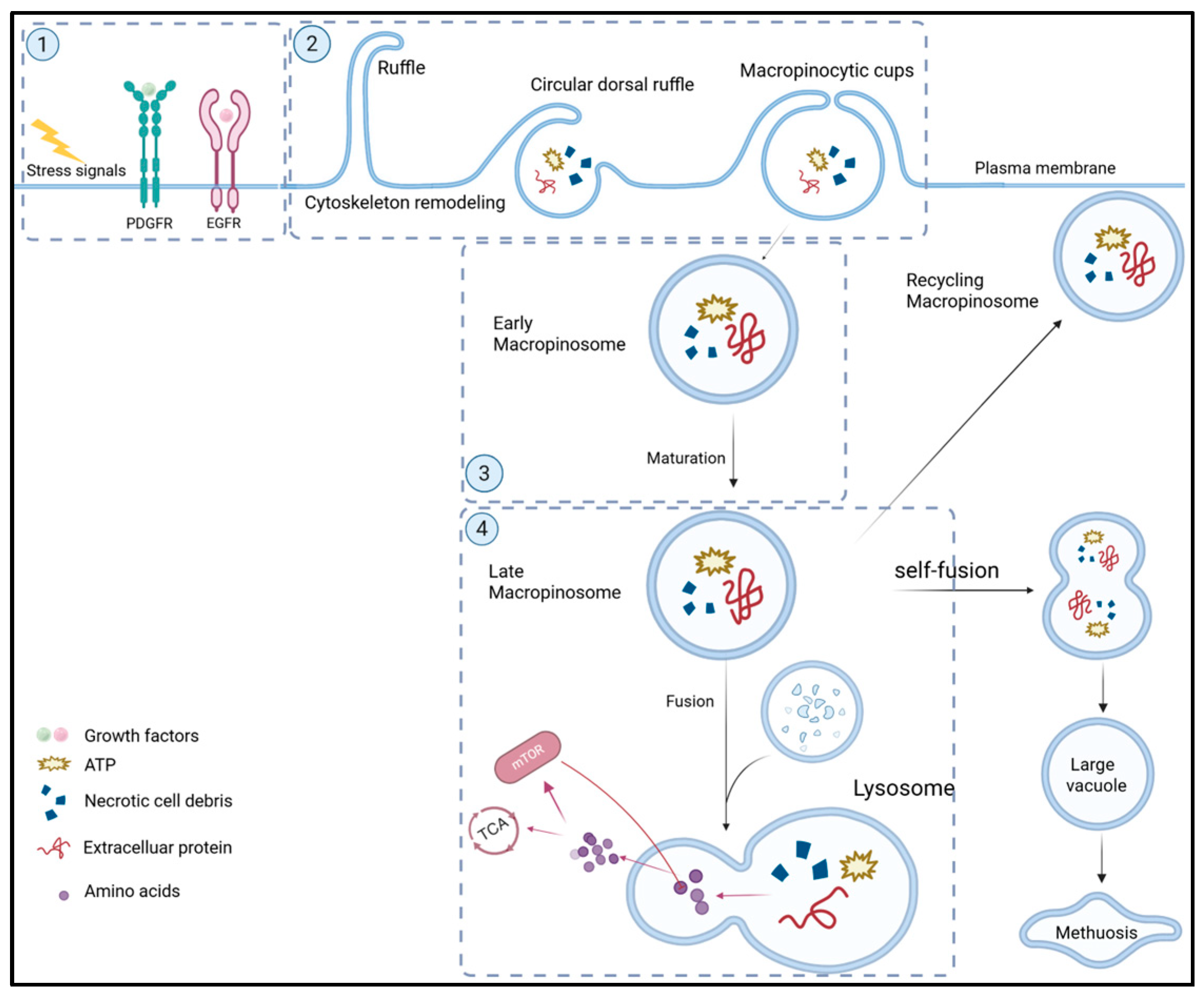
1. Overview of Macropinocytosis
2. Roles of Macropinocytosis in Cancers
2.1. Providing Nutrients for Cancer Cell Growth
- (a)
- Uptake of proteins and ATP: It has been observed that the majority of biomolecules in macropinosomes are proteins [11]. These proteins are digested into free amino acids in lysosomes that are used to synthesize new proteins or catabolized to generate ATP for energy [6]. The main protein internalized by macropinocytosis is extracellular albumin, the most abundant protein in human plasma (~50%) [12]. In addition, given that albumin serves as a carrier protein, albumin-bound molecules such as fatty acids (FAs) and cholesterol, which are essential components for maintaining the structural integrity of the cell membrane, are also internalized by macropinocytosis [12]. Apparently, macropinocytosis enables tumor cells to sustain their metabolic and biosynthetic needs and promotes tumor cell growth under nutritional stress conditions [11,13].
- (b)
- Uptake of necrotic cell debris: In addition to extracellular proteins and ATP, cancer cells assimilate other extracellular bioactive macromolecules through macropinocytosis. Tumor cell necrosis occurs under hypoxic or nutrient-deprived conditions and generates abundant cell debris containing proteins, fatty acids (FAs), nucleotides, and triacylglycerols (TAGs) [16,17]. These necrotic fragments are captured by neighbor tumor cells via macropinocytosis and processed by lysosomes to produce nutrients for fueling tumor cell growth. In addition, the uptake of necrotic cells by macropinocytosis may also contribute to resistance against antimetabolite chemotherapeutics [18].
2.2. Counteracting Oxidative Stress
2.3. Promoting Immune Escape of Cancers
2.4. Induction of Cancer Cell Death (Methuosis) upon Excessive Macropinocytosis
3. The Role of Macropinocytosis in Cancer Therapy
3.1. Targeting Macropinocytosis for Cancer Therapy
3.1.1. Application of Macropinocytosis Inhibitors for Cancer Therapy
| Inhibitor Type | Drug | Mechanism | Cancer Type | References |
|---|---|---|---|---|
| Macropinocytosis inhibitors | NSC23766 | Inhibition of Rac1 activity | Ras-mutant tumors | [44] |
| EHT1864 | Inhibition of Rac1 activity | Ras-mutant tumors | [45] | |
| 1D-242 | Inhibition of Rac1-mediated nuclear translocation | Ras-mutant tumors | [46] | |
| MG53 | Ubiquitination modification at Lys5 | Ras-mutant tumors | [47] | |
| Wortmannin | Inhibition of PI3K/AKT pathway | Ras-mutant tumors | [48] | |
| EIPA | Lowering the pH value below the plasma membrane | Ras-mutant tumors | [49] | |
| LY294002 | Blocking PI3K/AKT signaling | Ras-mutant tumors | [50] | |
| Cytochalasin D | Inhibition of actin polymerization | Ras-mutant tumors | [9] | |
| NaN3 | Blocking ATP dependence | Ras-mutant tumors | [51] | |
| Poziotinib | Targeting EGFR | Kras-mutant tumors | [52] | |
| Ivermectin | Inhibiting PAK1 | Kras-mutant tumors | [52] | |
| Tyrphostin A9 | Targeting PDGFR | Kras-mutant tumors | [53] | |
| LY2090314 | Inhibiting GSK-3 | Kras-mutant tumors | [54] | |
| Pyrvinium pamoate | Inhibiting Wnt/β-catenin | Kras-mutant tumors | [55] | |
| Metabolic regulators | CQ, HCQ | Inhibition of cytoprotective autophagy | Pancreatic cancer, etc. | [56] |
| Lys05 | Lysosomal deacidification | Glioblastoma | [57] | |
| Bafilomycin A1 | V-ATPase inhibitors | Various cancers | [58] | |
| Telaglenastat | Inhibition of glutamine conversion | Melanoma | [59] | |
| Methuosis inducers | MIPP, MOMIPP | Inducing plasma membrane shrinkage | Glioblastoma, Breast cancer | [60,61] |
| Bacoside A | Induction of macropinocytosis | Glioblastoma | [62] | |
| Nerve growth factor | Induction of macropinocytosis | Medulloblastoma | [63] | |
| Trehalose | Forced induction of macropinocytosis | Glioblastoma | [64] | |
| 12A | Induction of macropinocytosis | Breast cancer, etc. | [65] |
3.1.2. Macropinocytosis-Associated Metabolic Regulators
3.1.3. Methuosis Inducers
3.1.4. Targeting Macropinocytosis for Drug Resistance
3.2. Utilizing Macropinocytosis for Anti-Cancer Drug Delivery
3.2.1. The Macropinocytosis-Mediated Carrier Delivery System
- (a)
- Nanoparticles
- (b)
- Virus vectors
- (c)
- Exosomes
3.2.2. Macropinocytosis-Mediated Conjugated Targeted Delivery Systems
- (a)
- Antibody–Drug Conjugates (ADCs)
- (b)
- Peptide–Drug Conjugates (PDCs)
3.2.3. Current Clinical Application of the Macropinocytosis-Mediated Anti-Cancer Drug Delivery for Cancer Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| Ad | Adenovirus |
| ADCs | Antibody–drug conjugates |
| ATRA | All-trans retinoic acid |
| BCRP | Breast cancer resistance protein |
| CK2 | Casein kinase 2 |
| CPPs | Cell-penetrating peptides |
| CQ | Chloroquine |
| CSCs | Cancer stem cells |
| DCs | Dendritic cells |
| eATP | Extracellular ATP |
| EMT | Epithelial–mesenchymal transition |
| FAP | Fibroblast activation protein |
| FAs | Fatty acids |
| FDA | Food and Drug Administration |
| GEF | Guanine nucleotide exchange factor |
| Gln | L-glutamine |
| GLSi | Glutaminase inhibitor |
| HCQ | Hydroxychloroquine |
| HDL | High-density lipoprotein |
| HEP-2 | Human epithelioma-2 |
| HCC | Hepatocellular carcinoma |
| LDL | Low-density lipoprotein |
| LNPs | Lipid nanoparticles |
| MK | Megakaryocyte |
| MP | Macropinocytosis |
| MRP2 | Multidrug resistance protein 2 |
| mSCLC | Metastatic small-cell lung cancer |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| NSCLC | Non-small-cell lung cancer |
| PDAC | Pancreatic ductal adenocarcinoma |
| PDCs | Peptide–drug conjugates |
| P-gp | P-Glycoprotein |
| PPCBA | pH-sensitive and bio-reducible polymer |
| PSCs | Pancreatic stellate cells |
| PTX | Paclitaxel |
| EMA | European Medicines Agency |
| ROS | Reactive oxygen species |
| TAGs | Triacylglycerols |
| TAMs | Tumor-associated macrophages |
| TME | Tumor microenvironment |
References
- Wu, Y.; Hu, X.; Wei, Z.; Lin, Q. Cellular Regulation of Macropinocytosis. Int. J. Mol. Sci. 2024, 25, 6963. [Google Scholar] [CrossRef] [PubMed]
- Lambies, G.; Commisso, C. Macropinocytosis and Cancer: From Tumor Stress to Signaling Pathways. In Macropinocytosis; Commisso, C., Ed.; Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2022; Volume 98, pp. 15–40. ISBN 978-3-030-94003-4. [Google Scholar]
- Yang, C.; Zhang, F.; Chen, F.; Chang, Z.; Zhao, Y.; Shao, D.; Sun, W.; Dong, W.; Wang, Z. Biomimetic Nanovaccines Potentiating Dendritic Cell Internalization via CXCR4-Mediated Macropinocytosis. Adv. Healthc. Mater. 2023, 12, 2202064. [Google Scholar] [CrossRef] [PubMed]
- Hall, A. Ras-Related GTPases and the Cytoskeleton. Mol. Biol. Cell 1992, 3, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Salloum, G.; Jakubik, C.T.; Erami, Z.; Heitz, S.D.; Bresnick, A.R.; Backer, J.M. PI3Kβ Is Selectively Required for Growth Factor-Stimulated Macropinocytosis. Cell Sci. 2019, 132, jcs231639. [Google Scholar] [CrossRef]
- Recouvreux, M.V.; Commisso, C. Macropinocytosis: A Metabolic Adaptation to Nutrient Stress in Cancer. Front. Endocrinol. 2017, 8, 261. [Google Scholar] [CrossRef]
- Swanson, J.A. Shaping Cups into Phagosomes and Macropinosomes. Nat. Rev. Mol. Cell Biol. 2008, 9, 639–649. [Google Scholar] [CrossRef]
- Murugan, A.K.; Grieco, M.; Tsuchida, N. RAS Mutations in Human Cancers: Roles in Precision Medicine. Semin. Cancer Biol. 2019, 59, 23–35. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Q.; Cheng, R.; Qu, J.; Li, W. Survival Strategies of Cancer Cells: The Role of Macropinocytosis in Nutrient Acquisition, Metabolic Reprogramming, and Therapeutic Targeting. Autophagy 2025, 21, 693–718. [Google Scholar] [CrossRef]
- Zhang, Y.; Commisso, C. Macropinocytosis in Cancer: A Complex Signaling Network. Trends Cancer 2019, 5, 332–334. [Google Scholar] [CrossRef]
- Commisso, C.; Davidson, S.M.; Soydaner-Azeloglu, R.G.; Parker, S.J.; Kamphorst, J.J.; Hackett, S.; Grabocka, E.; Nofal, M.; Drebin, J.A.; Thompson, C.B.; et al. Macropinocytosis of Protein Is an Amino Acid Supply Route in Ras-Transformed Cells. Nature 2013, 497, 633–637. [Google Scholar] [CrossRef]
- Commisso, C.; Debnath, J. Macropinocytosis Fuels Prostate Cancer. Cancer Discov. 2018, 8, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheng, D.; Chu, J.; Zhang, T.; Dong, Z.; Lou, H.; Zhu, L.; Liu, Y. A Novel Method to Image Macropinocytosis in Vivo. Front. Neurosci. 2018, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, F.U.; Sufiyan Chhipa, A.; Mishra, V.; Gupta, V.K.; Rawat, S.G.; Kumar, A.; Pathak, C. Molecular and Cellular Paradigms of Multidrug Resistance in Cancer. Cancer Rep. 2022, 5, e1291. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, X.; Liu, Y.; Li, Y.; Colvin, R.A.; Tong, L.; Wu, S.; Chen, X. Extracellular ATP Is Internalized by Macropinocytosis and Induces Intracellular ATP Increase and Drug Resistance in Cancer Cells. Cancer Lett. 2014, 351, 242–251. [Google Scholar] [CrossRef]
- Tsuchihara, K.; Fujii, S.; Esumi, H. Autophagy and Cancer: Dynamism of the Metabolism of Tumor Cells and Tissues. Cancer Lett. 2009, 278, 130–138. [Google Scholar] [CrossRef]
- Petan, T.; Jarc, E.; Jusović, M. Lipid Droplets in Cancer: Guardians of Fat in a Stressful World. Molecules 2018, 23, 1941. [Google Scholar] [CrossRef]
- Jayashankar, V.; Edinger, A.L. Macropinocytosis Confers Resistance to Therapies Targeting Cancer Anabolism. Nat. Commun. 2020, 11, 1121. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of Oxidative Stress as an Anticancer Strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-Induced Nrf2 Transcription Promotes ROS Detoxification and Tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef]
- Su, H.; Yang, F.; Fu, R.; Li, X.; French, R.; Mose, E.; Pu, X.; Trinh, B.; Kumar, A.; Liu, J.; et al. Cancer Cells Escape Autophagy Inhibition via NRF2-Induced Macropinocytosis. Cancer Cell 2021, 39, 678–693.e11. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Li, W.; Li, H.; Bao, J.; Yang, C.; Wang, A.; Wei, J.; Chen, S.; Jin, H. Role of Nrf2 in the Antioxidation and Oxidative Stress Induced Developmental Toxicity of Honokiol in Zebrafish. Toxicol. Appl. Pharmacol. 2019, 373, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yao, T.; Liu, X.; Fan, Z.; Liu, Y. A Macropinocytosis-Related Gene Signature Predicts the Prognosis and Immune Microenvironment in Hepatocellular Carcinoma. Front. Oncol. 2023, 13, 1143013. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, Q.; Shi, X.; Jiang, M. Targeting Endocytosis and Cell Communications in the Tumor Immune Microenvironment. Cell Commun. Signal. 2022, 20, 161. [Google Scholar] [CrossRef]
- Galassi, C.; Chan, T.A.; Vitale, I.; Galluzzi, L. The Hallmarks of Cancer Immune Evasion. Cancer Cell 2024, 42, 1825–1863. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, H.; Liu, G.; Wu, J.; Yuan, Y.; Shang, A. Tumor Microenvironment: Lactic Acid Promotes Tumor Development. Immunol. Res. 2022, 2022, 3119375. [Google Scholar] [CrossRef]
- Meng, D.; Yang, Q.; Jeong, M.-H.; Curukovic, A.; Tiwary, S.; Melick, C.H.; Lama-Sherpa, T.D.; Wang, H.; Huerta-Rosario, M.; Urquhart, G.; et al. SNAT7 Regulates mTORC1 via Macropinocytosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2123261119. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Yashiro, R. Molecular Docking and Intracellular Translocation of Extracellular Vesicles for Efficient Drug Delivery. Int. J. Mol. Sci. 2022, 23, 12971. [Google Scholar] [CrossRef]
- Koh, V.; Chakrabarti, J.; Torvund, M.; Steele, N.; Hawkins, J.A.; Ito, Y.; Wang, J.; Helmrath, M.A.; Merchant, J.L.; Ahmed, S.A.; et al. Hedgehog Transcriptional Effector GLI Mediates mTOR-Induced PD-L1 Expression in Gastric Cancer Organoids. Cancer Lett. 2021, 518, 59–71. [Google Scholar] [CrossRef]
- Palm, W. Metabolic Functions of Macropinocytosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180285. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen Presentation in Cancer: Insights into Tumour Immunogenicity and Immune Evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef]
- Gide, T.N.; Quek, C.; Menzies, A.M.; Tasker, A.T.; Shang, P.; Holst, J.; Madore, J.; Lim, S.Y.; Velickovic, R.; Wongchenko, M.; et al. Distinct Immune Cell Populations Define Response to Anti-PD-1 Monotherapy and Anti-PD-1/Anti-CTLA-4 Combined Therapy. Cancer Cell 2019, 35, 238–255.e6. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, L.H.; Najjar, Y.G. Immunotherapy Combination Approaches: Mechanisms, Biomarkers and Clinical Observations. Nat. Rev. Immunol. 2024, 24, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Maltese, W.A.; Overmeyer, J.H. Methuosis: Nonapoptotic Cell Death Associated with Vacuolization of Macropinosome and Endosome Compartments. Am. J. Pathol. 2014, 184, 1630–1642. [Google Scholar] [CrossRef]
- Park, W.; Wei, S.; Kim, B.-S.; Kim, B.; Bae, S.-J.; Chae, Y.C.; Ryu, D.; Ha, K.-T. Diversity and Complexity of Cell Death: A Historical Review. Exp. Mol. Med. 2023, 55, 1573–1594. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Li, Y.; Shao, Y.; Li, L.; Song, F. Osmotic Pressure and Its Biological Implications. Int. J. Mol. Sci. 2024, 25, 3310. [Google Scholar] [CrossRef]
- Roffay, C.; Molinard, G.; Kim, K.; Urbanska, M.; Andrade, V.; Barbarasa, V.; Nowak, P.; Mercier, V.; García-Calvo, J.; Matile, S.; et al. Passive Coupling of Membrane Tension and Cell Volume during Active Response of Cells to Osmosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2103228118. [Google Scholar] [CrossRef]
- Yan, Q.; Gomis Perez, C.; Karatekin, E. Cell Membrane Tension Gradients, Membrane Flows, and Cellular Processes. Physiology 2024, 39, 231–245. [Google Scholar] [CrossRef]
- Overmeyer, J.H.; Kaul, A.; Johnson, E.E.; Maltese, W.A. Active Ras Triggers Death in Glioblastoma Cells through Hyperstimulation of Macropinocytosis. Mol. Cancer Res. 2008, 6, 965–977. [Google Scholar] [CrossRef]
- Puccini, J.; Badgley, M.A.; Bar-Sagi, D. Exploiting Cancer’s Drinking Problem: Regulation and Therapeutic Potential of Macropinocytosis. Trends Cancer 2022, 8, 54–64. [Google Scholar] [CrossRef]
- Bielsa, N.; Casasampere, M.; Abad, J.L.; Enrich, C.; Delgado, A.; Fabriàs, G.; Lizcano, J.M.; Casas, J. Methuosis Contributes to Jaspine-B-Induced Cell Death. Int. J. Mol. Sci. 2022, 23, 7257. [Google Scholar] [CrossRef]
- Bartoszewska, E.; Florek, K.; Zagórski, K.; Gachowska, M.; Wietrzyk, A.; Hutny, A.; Nowakowska-Toporowska, A.; Kulbacka, J. Methuosis, Alkaliptosis, and Oxeiptosis and Their Significance in Anticancer Therapy. Cells 2024, 13, 2095. [Google Scholar] [CrossRef] [PubMed]
- Onesto, C.; Shutes, A.; Picard, V.; Schweighoffer, F.; Der, C.J. Characterization of EHT 1864, a Novel Small Molecule Inhibitor of Rac Family Small GTPases. Methods Enzymol. 2008, 439, 111–129. [Google Scholar] [CrossRef]
- Gao, Y.; Dickerson, J.B.; Guo, F.; Zheng, J.; Zheng, Y. Rational Design and Characterization of a Rac GTPase-Specific Small Molecule Inhibitor. Proc. Natl. Acad. Sci. USA 2004, 101, 7618–7623. [Google Scholar] [CrossRef]
- Shutes, A.; Onesto, C.; Picard, V.; Leblond, B.; Schweighoffer, F.; Der, C.J. Specificity and Mechanism of Action of EHT 1864, a Novel Small Molecule Inhibitor of Rac Family Small GTPases. Biol. Chem. 2007, 282, 35666–35678. [Google Scholar] [CrossRef]
- Ciarlantini, M.S.; Barquero, A.; Bayo, J.; Wetzler, D.; Dodes Traian, M.M.; Bucci, H.A.; Fiore, E.J.; Gandolfi Donadío, L.; Defelipe, L.; Turjanski, A.; et al. Development of an Improved Guanidine-Based Rac1 Inhibitor with in Vivo Activity against Non-Small Cell Lung Cancer. ChemMedChem 2021, 16, 1011–1021. [Google Scholar] [CrossRef]
- Ma, X.; Ma, X.; Zhu, L.; Zhao, Y.; Chen, M.; Li, T.; Lin, Y.; Ma, D.; Sun, C.; Han, L. The E3 Ubiquitin Ligase MG53 Inhibits Hepatocellular Carcinoma by Targeting RAC1 Signaling. Oncogenesis 2022, 11, 40. [Google Scholar] [CrossRef]
- Bani, N.; Rahmani, F.; Shakour, N.; Amerizadeh, F.; Khalili-Tanha, G.; Khazaei, M.; Hassanian, S.M.; Kerachian, M.A.; Abbaszadegan, M.R.; Mojarad, M.; et al. Wortmannin Inhibits Cell Growth and Induces Apoptosis in Colorectal Cancer Cells by Suppressing the PI3K/AKT Pathway. Anticancer. Agents Med. Chem. 2024, 24, 916–927. [Google Scholar] [CrossRef]
- Huang, A.; Zeng, P.; Li, Y.; Lu, W.; Lai, Y. LY294002 Is a Promising Inhibitor to Overcome Sorafenib Resistance in FLT3-ITD Mutant AML Cells by Interfering with PI3K/Akt Signaling Pathway. Front. Oncol. 2021, 11, 782065. [Google Scholar] [CrossRef]
- Koivusalo, M.; Welch, C.; Hayashi, H.; Scott, C.C.; Kim, M.; Alexander, T.; Touret, N.; Hahn, K.M.; Grinstein, S. Amiloride Inhibits Macropinocytosis by Lowering Submembranous pH and Preventing Rac1 and Cdc42 Signaling. Cell Biol. 2010, 188, 547–563. [Google Scholar] [CrossRef]
- Ida, H.; Taira, N.; Azuma, K.; Kumatani, A.; Akishiba, M.; Futaki, S.; Takahashi, Y.; Shiku, H. Surface Morphology Live-Cell Imaging Reveals How Macropinocytosis Inhibitors Affect Membrane Dynamics. Electrochim. Acta 2023, 441, 141783. [Google Scholar] [CrossRef]
- Brambillasca, S.; Cera, M.R.; Andronache, A.; Dey, S.K.; Fagá, G.; Fancelli, D.; Frittoli, E.; Pasi, M.; Robusto, M.; Varasi, M.; et al. Novel Selective Inhibitors of Macropinocytosis-Dependent Growth in Pancreatic Ductal Carcinoma. Biomed. Pharmacother. 2024, 177, 116991. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Park, Y.J.; Shin, J.H.; Kim, E.S.; Hwang, J.J.; Jin, D.-H.; Kim, J.C.; Cho, D.-H. A Receptor Tyrosine Kinase Inhibitor, Tyrphostin A9 Induces Cancer Cell Death through Drp1 Dependent Mitochondria Fragmentation. Biochem. Biophys. Res. Commun. 2011, 408, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Zamek-Gliszczynski, M.J.; Abraham, T.L.; Alberts, J.J.; Kulanthaivel, P.; Jackson, K.A.; Chow, K.H.; McCann, D.J.; Hu, H.; Anderson, S.; Furr, N.A.; et al. Pharmacokinetics, Metabolism, and Excretion of the Glycogen Synthase Kinase-3 Inhibitor LY2090314 in Rats, Dogs, and Humans: A Case Study in Rapid Clearance by Extensive Metabolism with Low Circulating Metabolite Exposure. Drug Metab. Dispos. 2013, 41, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, L.; Hu, C.; Liang, S.; Fei, X.; Yan, N.; Zhang, Y.; Zhang, F. WNT Pathway Inhibitor Pyrvinium Pamoate Inhibits the Self-Renewal and Metastasis of Breast Cancer Stem Cells. Int. J. Oncol. 2016, 48, 1175–1186. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; de Sousa, R.W.R.; Ferreira, J.R.d.O.; Militão, G.C.G.; Bezerra, D.P. Chloroquine and Hydroxychloroquine in Antitumor Therapies Based on Autophagy-Related Mechanisms. Pharmacol. Res. 2021, 168, 105582. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Winkler, J.D. Lys05: A New Lysosomal Autophagy Inhibitor. Autophagy 2012, 8, 1383–1384. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Hassan, A.; Lee, C.-H.; Xie, X.-S.; Li, X. Molecular Basis of V-ATPase Inhibition by Bafilomycin A1. Nat. Commun. 2021, 12, 1782. [Google Scholar] [CrossRef]
- Varghese, S.; Pramanik, S.; Williams, L.J.; Hodges, H.R.; Hudgens, C.W.; Fischer, G.M.; Luo, C.K.; Knighton, B.; Tan, L.; Lorenzi, P.L.; et al. The Glutaminase Inhibitor CB-839 (Telaglenastat) Enhances the Antimelanoma Activity of T-Cell-Mediated Immunotherapies. Mol. Cancer Ther. 2021, 20, 500–511. [Google Scholar] [CrossRef]
- Robinson, M.W.; Overmeyer, J.H.; Young, A.M.; Erhardt, P.W.; Maltese, W.A. Synthesis and Evaluation of Indole-Based Chalcones as Inducers of Methuosis, a Novel Type of Nonapoptotic Cell Death. Med. Chem. 2012, 55, 1940–1956. [Google Scholar] [CrossRef]
- Huang, W.; Sun, X.; Li, Y.; He, Z.; Li, L.; Deng, Z.; Huang, X.; Han, S.; Zhang, T.; Zhong, J.; et al. Discovery and Identification of Small Molecules as Methuosis Inducers with in Vivo Antitumor Activities. Med. Chem. 2018, 61, 5424–5434. [Google Scholar] [CrossRef]
- John, S.; Sivakumar, K.C.; Mishra, R. Bacoside A Induces Tumor Cell Death in Human Glioblastoma Cell Lines through Catastrophic Macropinocytosis. Front. Mol. Neurosci. 2017, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; MacDonald, J.I.S.; Talebian, A.; Leuenberger, J.; Seah, C.; Pasternak, S.H.; Michnick, S.W.; Meakin, S.O. Unravelling the Mechanism of TrkA-Induced Cell Death by Macropinocytosis in Medulloblastoma Daoy Cells. Mol. Cell. Biol. 2016, 36, 2596–2611. [Google Scholar] [CrossRef] [PubMed]
- Del Bello, B.; Gamberucci, A.; Marcolongo, P.; Maellaro, E. The Autophagy Inducer Trehalose Stimulates Macropinocytosis in NF1-Deficient Glioblastoma Cells. Cancer Cell Int. 2022, 22, 232. [Google Scholar] [CrossRef]
- Wu, J.; Hu, H.; Ao, M.; Cui, Z.; Zhou, X.; Qin, J.; Guo, Y.; Chen, J.; Xue, Y.; Fang, M. Design, Synthesis, and Biological Evaluation of 5-((4-(Pyridin-3-Yl)Pyrimidin-2-Yl)Amino)-1H-Indole-2-Carbohydrazide Derivatives: The Methuosis Inducer 12A as a Novel and Selective Anticancer Agent. Enzym. Inhib. Med. Chem. 2021, 36, 1436–1453. [Google Scholar] [CrossRef] [PubMed]
- Silva-Pavez, E.; Villar, P.; Trigo, C.; Caamaño, E.; Niechi, I.; Pérez, P.; Muñoz, J.P.; Aguayo, F.; Burzio, V.A.; Varas-Godoy, M.; et al. CK2 Inhibition with Silmitasertib Promotes Methuosis-like Cell Death Associated to Catastrophic Massive Vacuolization of Colorectal Cancer Cells. Cell Death Dis. 2019, 10, 73. [Google Scholar] [CrossRef]
- Schinkel, A.H.; Jonker, J.W. Mammalian Drug Efflux Transporters of the ATP Binding Cassette (ABC) Family: An Overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Yang, Y.; Cai, C.-Y.; Teng, Q.-X.; Cui, Q.; Lin, J.; Assaraf, Y.G.; Chen, Z.-S. Multidrug Resistance Proteins (MRPs): Structure, Function and the Overcoming of Cancer Multidrug Resistance. Drug Resist. Updat. 2021, 54, 100743. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Ashby, C.R.; Assaraf, Y.G.; Chen, Z.-S.; Liu, H.-M. Chemical Molecular-Based Approach to Overcome Multidrug Resistance in Cancer by Targeting P-Glycoprotein (P-Gp). Med. Res. Rev. 2021, 41, 525–555. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X. Abstract 405: A New Mechanism of Drug Resistance in Cancer: Extracellular ATP-Induced Resistance by Macropinocytosis-Mediated Internalization and Redox Changes. Cancer Res. 2022, 82, 405. [Google Scholar] [CrossRef]
- Toschi, L.; Finocchiaro, G.; Bartolini, S.; Gioia, V.; Cappuzzo, F. Role of Gemcitabine in Cancer Therapy. Future Oncol. Lond. Engl. 2005, 1, 7–17. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Blum, R.H.; Carter, S.K. Adriamycin. A New Anticancer Drug with Significant Clinical Activity. Ann. Intern. Med. 1974, 80, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-Based Drugs for Cancer Therapy and Anti-Tumor Strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-L.; Gong, Y.; Ji, P.; Xie, Y.-F.; Jiang, Y.-Z.; Liu, G.-Y. Targeting Nucleotide Metabolism: A Promising Approach to Enhance Cancer Immunotherapy. Hematol. Oncol. 2022, 15, 45. [Google Scholar] [CrossRef]
- Khine, H.E.E.; Suriya, U.; Rungrotmongkol, T.; Chamni, S.; Lu, Y.; Bénard, A.; Lan, B.; Mukhopadhyay, D.; Chang, D.; Biankin, A.; et al. Jorunnamycin A Induces Apoptosis in Pancreatic Ductal Adenocarcinoma Cells, Spheroids, and Patient-Derived Organoids by Modulating KRAS-Mediated Survival Pathways. Sci. Rep. 2025, 15, 11376. [Google Scholar] [CrossRef]
- Hubert, A.; Lyass, O.; Pode, D.; Gabizon, A. Doxil (Caelyx): An Exploratory Study with Pharmacokinetics in Patients with Hormone-Refractory Prostate Cancer. Anticancer Drugs 2000, 11, 123–127. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, Y.; Wang, M.; Chen, D.; Lv, J.; Yan, J.; Zhou, D.; Pang, Y.; Liang, H.; Zhang, D.; et al. FAP-Activated Liposomes Achieved Specific Macropinocytosis Uptake by Pancreatic Stellate Cells for Efficient Desmoplasia Reversal. Chem. Eng. 2024, 495, 153369. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, M.; Yang, J.; Zhou, X.; Chen, Y.; Silli, E.K.; Tang, J.; Gong, S.; Yuan, Y.; Zong, Y.; et al. Growth Inhibition of Pancreatic Cancer by Targeted Delivery of Gemcitabine via Fucoidan-Coated pH-Sensitive Liposomes. Int. J. Biol. Macromol. 2024, 277, 134517. [Google Scholar] [CrossRef]
- Yan, J.; Wang, M.; Lv, S.; Chen, D.; Wu, Z.; Zhou, D.; Zhang, S.; Lv, J.; Xu, K.; Xu, C.; et al. SiATG5-Loaded Cancer Cell Membrane-Fused Liposomes Induced Increased Uptake of Albumin-Bound Chemotherapeutics by Pancreatic Cancer Cells. Control. Release 2024, 367, 620–636. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, K.; Wang, D.; Yao, S.; Lu, L.; Wang, H.; Song, J.; Zhou, J.; Fan, X.; Wang, Y.; et al. Core-Shell Lipoplexes Inducing Active Macropinocytosis Promote Intranasal Delivery of c-Myc siRNA for Treatment of Glioblastoma. Acta Biomater. 2022, 138, 478–490. [Google Scholar] [CrossRef]
- Li, R.; Ng, T.S.C.; Wang, S.J.; Prytyskach, M.; Rodell, C.B.; Mikula, H.; Kohler, R.H.; Garlin, M.A.; Lauffenburger, D.A.; Parangi, S.; et al. Therapeutically Reprogrammed Nutrient Signalling Enhances Nanoparticulate Albumin Bound Drug Uptake and Efficacy in KRAS-Mutant Cancer. Nat. Nanotechnol. 2021, 16, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sheng, W.; Wang, Y.; Li, L.; Li, Y.; Zhang, S.; Liu, X.; Chen, S.; Zhen, Y. A Macropinocytosis-Intensifying Albumin Domain-Based scFv Antibody and Its Conjugate Directed against K-Ras Mutant Pancreatic Cancer. Mol. Pharm. 2018, 15, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Shang, B.-Y.; Sheng, W.-J.; Zhang, S.-H.; Li, Y.; Miao, Q.-F.; Zhen, Y.-S. A Recombinantly Tailored β-Defensin That Displays Intensive Macropinocytosis-Mediated Uptake Exerting Potent Efficacy against K-Ras Mutant Pancreatic Cancer. Oncotarget 2016, 7, 58418–58434. [Google Scholar] [CrossRef] [PubMed]
- Maranhão, R.C.; Vital, C.G.; Tavoni, T.M.; Graziani, S.R. Clinical Experience with Drug Delivery Systems as Tools to Decrease the Toxicity of Anticancer Chemotherapeutic Agents. Expert Opin. Drug Deliv. 2017, 14, 1217–1226. [Google Scholar] [CrossRef]
- Yin, X.; Lu, Y.; Zou, M.; Wang, L.; Zhou, X.; Zhang, Y.; Su, M. Synthesis and Characterization of Salinomycin-Loaded High-Density Lipoprotein and Its Effects on Cervical Cancer Cells and Cervical Cancer Stem Cells. Int. J. Nanomed. 2021, 16, 6367–6382. [Google Scholar] [CrossRef]
- Huang, J.-L.; Jiang, G.; Song, Q.-X.; Gu, X.; Hu, M.; Wang, X.-L.; Song, H.-H.; Chen, L.-P.; Lin, Y.-Y.; Jiang, D.; et al. Lipoprotein-Biomimetic Nanostructure Enables Efficient Targeting Delivery of siRNA to Ras-Activated Glioblastoma Cells via Macropinocytosis. Nat. Commun. 2017, 8, 15144. [Google Scholar] [CrossRef]
- Persano, S.; Guevara, M.L.; Li, Z.; Mai, J.; Ferrari, M.; Pompa, P.P.; Shen, H. Lipopolyplex Potentiates Anti-Tumor Immunity of mRNA-Based Vaccination. Biomaterials 2017, 125, 81–89. [Google Scholar] [CrossRef]
- Al-Humaidi, R.B.; Fayed, B.; Shakartalla, S.B.; Jagal, J.; Jayakumar, M.N.; Al Shareef, Z.M.; Sharif, S.I.; Noreddin, A.; Semreen, M.H.; Omar, H.A.; et al. Optimum Inhibition of MCF-7 Breast Cancer Cells by Efficient Targeting of the Macropinocytosis Using Optimized Paclitaxel-Loaded Nanoparticles. Life Sci. 2022, 305, 120778. [Google Scholar] [CrossRef]
- Xiang, H.-L.; Chen, Y.; Wang, J.-W.; Wang, H.-J.; Gao, X.-F.; Li, H.; Mao, S.-J. Enhancing Cytotoxicity of Daunorubicin on Drug-resistant Leukaemia Cells with Microparticle-Mediated Drug Delivery System. J. Microencapsul. 2019, 36, 291–304. [Google Scholar] [CrossRef]
- Moon, C.Y.; Choi, J.-W.; Kasala, D.; Jung, S.-J.; Kim, S.W.; Yun, C.-O. Dual Tumor Targeting with pH-Sensitive and Bioreducible Polymer-Complexed Oncolytic Adenovirus. Biomaterials 2015, 41, 53–68. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, H.; Zhang, Y.; Luo, S.; Du, Z.; Lin, Q.; Zhang, Z.; Zhang, L. An Exosome-Mimicking Membrane Hybrid Nanoplatform for Targeted Treatment toward Kras-Mutant Pancreatic Carcinoma. Biomater. Sci. 2021, 9, 5599–5611. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Kobayashi, N.B.; Takatani-Nakase, T.; Yoshida, T. Active Macropinocytosis Induction by Stimulation of Epidermal Growth Factor Receptor and Oncogenic Ras Expression Potentiates Cellular Uptake Efficacy of Exosomes. Sci. Rep. 2015, 5, 10300. [Google Scholar] [CrossRef] [PubMed]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-Based Analysis of Lipid Nanoparticle-Mediated siRNA Delivery, Intracellular Trafficking and Endosomal Escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef]
- Liu, H.; Sun, M.; Liu, Z.; Kong, C.; Kong, W.; Ye, J.; Gong, J.; Huang, D.C.S.; Qian, F. KRAS-Enhanced Macropinocytosis and Reduced FcRn-Mediated Recycling Sensitize Pancreatic Cancer to Albumin-Conjugated Drugs. Control. Release 2019, 296, 40–53. [Google Scholar] [CrossRef]
- Liu, H.; Qian, F. Exploiting Macropinocytosis for Drug Delivery into KRAS Mutant Cancer. Theranostics 2022, 12, 1321–1332. [Google Scholar] [CrossRef]
- Xia, F.; Hu, X.; Zhang, B.; Wang, X.; Guan, Y.; Lin, P.; Ma, Z.; Sheng, J.; Ling, D.; Li, F. Ultrasmall Ruthenium Nanoparticles with Boosted Antioxidant Activity Upregulate Regulatory T Cells for Highly Efficient Liver Injury Therapy. Small 2022, 18, e2201558. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, Q.; Qin, X.; Bhavsar, D.; Yang, L.; Chen, Q.; Zheng, W.; Chen, L.; Liu, J. Improving the Anticancer Efficacy of Laminin Receptor-Specific Therapeutic Ruthenium Nanoparticles (RuBB-Loaded EGCG-RuNPs) via ROS-Dependent Apoptosis in SMMC-7721 Cells. ACS Appl. Mater. Interfaces 2016, 8, 15000–15012. [Google Scholar] [CrossRef]
- Su, L.; Sun, Z.; Qi, F.; Su, H.; Qian, L.; Li, J.; Zuo, L.; Huang, J.; Yu, Z.; Li, J.; et al. GRP75-Driven, Cell-Cycle-Dependent Macropinocytosis of Tat/pDNA-Ca2+ Nanoparticles Underlies Distinct Gene Therapy Effect in Ovarian Cancer. Nanobiotechnology 2022, 20, 340. [Google Scholar] [CrossRef]
- Gobeil, L.-A.; Lodge, R.; Tremblay, M.J. Macropinocytosis-like HIV-1 Internalization in Macrophages Is CCR5 Dependent and Leads to Efficient but Delayed Degradation in Endosomal Compartments. J. Virol. 2013, 87, 735–745. [Google Scholar] [CrossRef]
- Meier, O.; Greber, U.F. Adenovirus Endocytosis. J. Gene Med. 2004, 6, S152–S163. [Google Scholar] [CrossRef]
- Shan, L.; Cui, S.; Du, C.; Wan, S.; Qian, Z.; Achilefu, S.; Gu, Y. A Paclitaxel-Conjugated Adenovirus Vector for Targeted Drug Delivery for Tumor Therapy. Biomaterials 2012, 33, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes Facilitate Therapeutic Targeting of Oncogenic KRAS in Pancreatic Cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Atkinson, J.; Gulesserian, S.; Zeng, Z.; Nater, J.; Ou, J.; Yang, P.; Morrison, K.; Coleman, J.; Malik, F.; et al. Modulation of Macropinocytosis-Mediated Internalization Decreases Ocular Toxicity of Antibody-Drug Conjugates. Cancer Res. 2018, 78, 2115–2126. [Google Scholar] [CrossRef]
- Zhao, H.; Gulesserian, S.; Ganesan, S.K.; Ou, J.; Morrison, K.; Zeng, Z.; Robles, V.; Snyder, J.; Do, L.; Aviña, H.; et al. Inhibition of Megakaryocyte Differentiation by Antibody-Drug Conjugates (ADCs) Is Mediated by Macropinocytosis: Implications for ADC-Induced Thrombocytopenia. Mol. Cancer Ther. 2017, 16, 1877–1886. [Google Scholar] [CrossRef]
- Jiang, G.; Chen, H.; Huang, J.; Song, Q.; Chen, Y.; Gu, X.; Jiang, Z.; Huang, Y.; Lin, Y.; Feng, J.; et al. Tailored Lipoprotein-Like miRNA Delivery Nanostructure Suppresses Glioma Stemness and Drug Resistance through Receptor-Stimulated Macropinocytosis. Adv. Sci. 2020, 7, 1903290. [Google Scholar] [CrossRef]
- Nakamura, T.; Moriguchi, R.; Kogure, K.; Shastri, N.; Harashima, H. Efficient MHC Class I Presentation by Controlled Intracellular Trafficking of Antigens in Octaarginine-Modified Liposomes. Mol. Ther. Am. Soc. Gene Ther. 2008, 16, 1507–1514. [Google Scholar] [CrossRef]
- Nakase, I.; Akita, H.; Kogure, K.; Gräslund, A.; Langel, Ü.; Harashima, H.; Futaki, S. Efficient Intracellular Delivery of Nucleic Acid Pharmaceuticals Using Cell-Penetrating Peptides. Acc. Chem. Res. 2012, 45, 1132–1139. [Google Scholar] [CrossRef]
- Wei, Y.; Tang, T.; Pang, H.-B. Cellular Internalization of Bystander Nanomaterial Induced by TAT-Nanoparticles and Regulated by Extracellular Cysteine. Nat. Commun. 2019, 10, 3646. [Google Scholar] [CrossRef]
- Tanaka, G.; Nakase, I.; Fukuda, Y.; Masuda, R.; Oishi, S.; Shimura, K.; Kawaguchi, Y.; Takatani-Nakase, T.; Langel, U.; Gräslund, A.; et al. CXCR4 Stimulates Macropinocytosis: Implications for Cellular Uptake of Arginine-Rich Cell-Penetrating Peptides and HIV. Chem. Biol. 2012, 19, 1437–1446. [Google Scholar] [CrossRef]
- Cullis, J.; Siolas, D.; Avanzi, A.; Barui, S.; Maitra, A.; Bar-Sagi, D. Macropinocytosis of Nab-Paclitaxel Drives Macrophage Activation in Pancreatic Cancer. Cancer Immunol. Res. 2017, 5, 182–190. [Google Scholar] [CrossRef]
- Holder, J.E.; Ferguson, C.; Oliveira, E.; Lodeiro, C.; Trim, C.M.; Byrne, L.J.; Bertolo, E.; Wilson, C.M. The Use of Nanoparticles for Targeted Drug Delivery in Non-Small Cell Lung Cancer. Front. Oncol. 2023, 13, 1154318. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Huang, J.; Su, L.; Li, J.; Qi, F.; Su, H.; Chen, Y.; Zhang, Q.; Zhang, Q.; Li, Z.; et al. Arf6-Mediated Macropinocytosis-Enhanced Suicide Gene Therapy of C16TAB-Condensed Tat/pDNA Nanoparticles in Ovarian Cancer. Nanoscale 2021, 13, 14538–14551. [Google Scholar] [CrossRef] [PubMed]
| Subtype | Carrier | Payloads | Cancer Type | Reference |
|---|---|---|---|---|
| Nanoparticles | Liposomes | Adriamycin | Kaposi sarcoma | [77] |
| All-trans retinoic acid | Pancreatic ductal adenocarcinoma | [78] | ||
| Gemcitabine | Pancreatic cancer | [79] | ||
| siRNA(ATG5) | Pancreatic cancer | [80] | ||
| siRNA | Glioma | [81] | ||
| Albumin | Paclitaxel | Multiple types of cancer cells | [82] | |
| Adriamycin | Pancreatic cancer | [83] | ||
| Anti-EGFR antibodies | Pancreatic cancer | [83] | ||
| β-Defensin-2 | Multiple types of cancer cells | [84] | ||
| Lipoprotein | Paclitaxel | Multiple types of cancer cells | [85] | |
| Salinomycin | Cervical cancer | [86] | ||
| siRNA | Glioblastoma | [87] | ||
| Lipid shell | mRNA | Melanoma | [88] | |
| Adriamycin | Breast cancer, etc. | [89] | ||
| Virus vectors | Lentivirus | siRNA | Leukemia | [90] |
| Oncolytic viruses | PPCBA | Primary tumor and metastatic tumors | [91] | |
| Exosomes | Exosomes | Celastrol | Kras-mutant tumors | [92] |
| Exosomes | Saporin | Ras-mutant tumors | [93] |
| Subtype | Carrier | Payloads | Cancer Type | References |
|---|---|---|---|---|
| Antibody–Drug Conjugates (ADCs) | Antibody | Chemotherapy drugs | Multiple types of cancer cells | [104,105] |
| Peptide–Drug Conjugates (PDCs) | Elastin-like peptide | miRNA-34a | Glioma | [106] |
| R8 | NAP | Multiple types of cancer cells | [107,108] | |
| R12 | NAP | Multiple types of cancer cells | [107,108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Zhou, L.; Zhai, Y.; Sun, A.; Shao, G.; Lin, Q. Macropinocytosis: Both a Target and a Tool for Cancer Therapy. Biomolecules 2025, 15, 936. https://doi.org/10.3390/biom15070936
Zhao M, Zhou L, Zhai Y, Sun A, Shao G, Lin Q. Macropinocytosis: Both a Target and a Tool for Cancer Therapy. Biomolecules. 2025; 15(7):936. https://doi.org/10.3390/biom15070936
Chicago/Turabian StyleZhao, Manhan, Liming Zhou, Yifei Zhai, Aiqin Sun, Genbao Shao, and Qiong Lin. 2025. "Macropinocytosis: Both a Target and a Tool for Cancer Therapy" Biomolecules 15, no. 7: 936. https://doi.org/10.3390/biom15070936
APA StyleZhao, M., Zhou, L., Zhai, Y., Sun, A., Shao, G., & Lin, Q. (2025). Macropinocytosis: Both a Target and a Tool for Cancer Therapy. Biomolecules, 15(7), 936. https://doi.org/10.3390/biom15070936






