Regeneration of Insulin-Producing β Cells, Reduction in Inflammation and Oxidation Stress, and Improvement in Lipid Profile in a Type 1 Diabetes Rat Model by Intraperitoneal Injection of the Growth Factors-Rich Catfish Skin-Derived Fraction-B: An Introductory Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fraction B from Catfish Skin
2.2. Experimental Animals
2.3. Establishment of Diabetes in Male Rats and Treatment with FB
2.4. Evaluation of General Health Factors After FB Injection
2.5. Animal Sacrifice and Collection of Blood and Tissue Samples
2.6. Investigation of the Activities of the Recovered Pancreas (Production of Insulin and C-Peptide)
2.6.1. Analysis of Insulin in the Serum
2.6.2. Analysis of Fructosamine in the Serum
2.6.3. Analysis of Insulin in the Pancreatic Tissue
2.6.4. Determination of C-Peptide in the Serum
2.7. Histopathology of the Pancreas and Immunohistochemical Staining for Insulin in the Islets
2.7.1. Histopathology of the Pancreas
2.7.2. Immunohistochemical Staining for Insulin
2.8. Improvement of Health Issues Related to Diabetes
2.8.1. Effect of FB Treatment on Oxidation/Antioxidation in the Kidneys and Liver
Concentration of MDA
Concentration of Antioxidants
Concentration of Catalase
2.8.2. Effect of FB Treatment on Inflammatory Cytokine
2.8.3. Effect of FB Treatment on Lipid Profile
2.9. Evaluation of Safety of FB Treatment
2.10. Investigation of the Durability of the Regenerated β Cells After FB Treatment
2.11. Analysis of FB for Growth Factors and Biologically Active Proteins That Can Play Roles in the Recovery of the Diabetic Pancreas and Regeneration of β-Cells
2.12. FB Treatment of Diabetic Female SD Rats Is Given in Supplementary Material
2.13. Unified Graph Legend and Significance Symbols
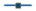 NC = normal control;
NC = normal control;  DC = diabetic control; and
DC = diabetic control; and 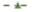 D + FB = fraction B-treated diabetic rats. Graph legend symbols for bar graphs are as follows:
D + FB = fraction B-treated diabetic rats. Graph legend symbols for bar graphs are as follows: 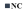 = normal control;
= normal control; 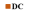 = diabetic control; and
= diabetic control; and  = fraction B-treated diabetic rats.
= fraction B-treated diabetic rats.2.14. Statistical Analysis
3. Results
3.1. Benefits of FB Treatment to Diabetic Animals
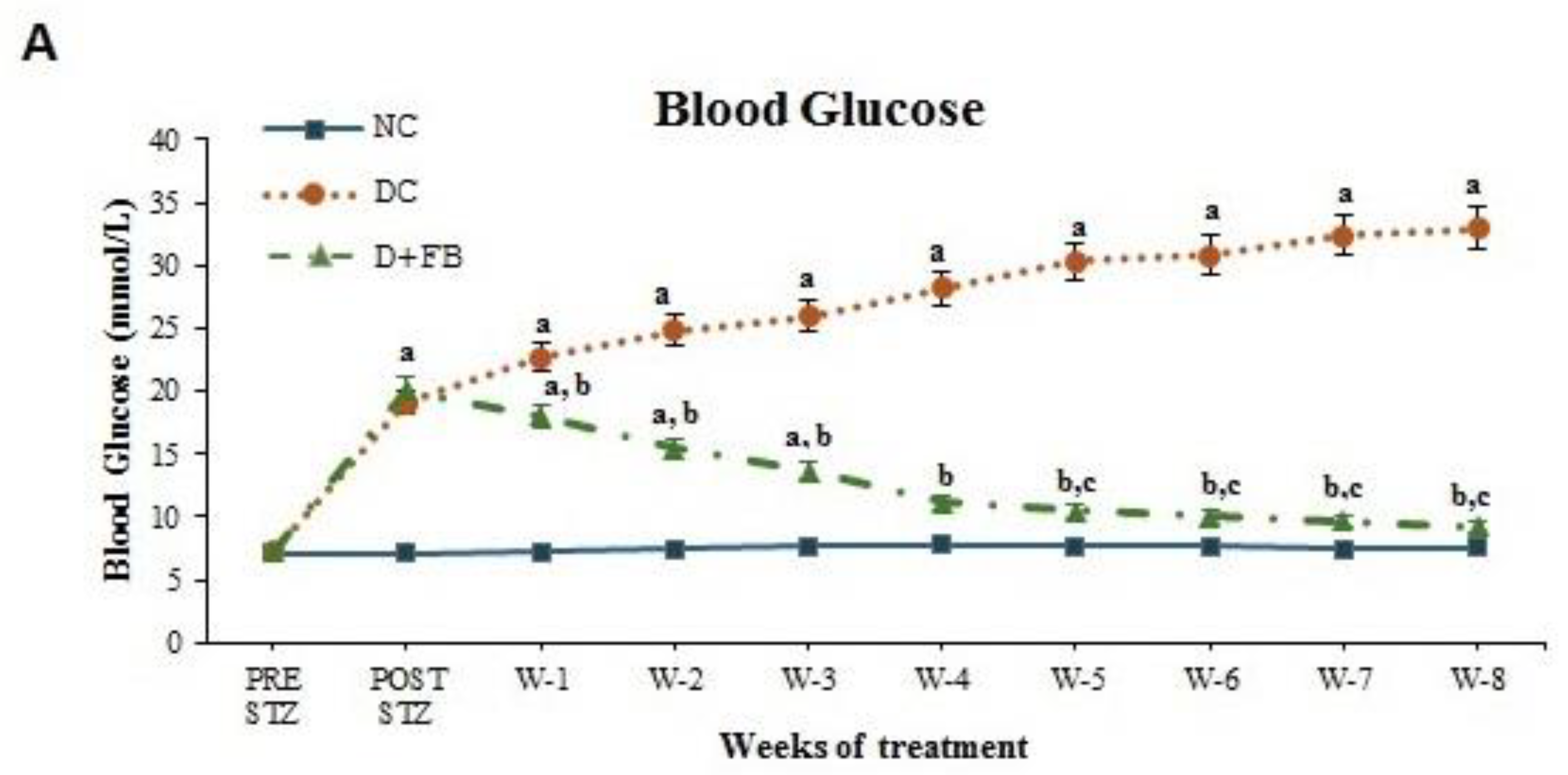
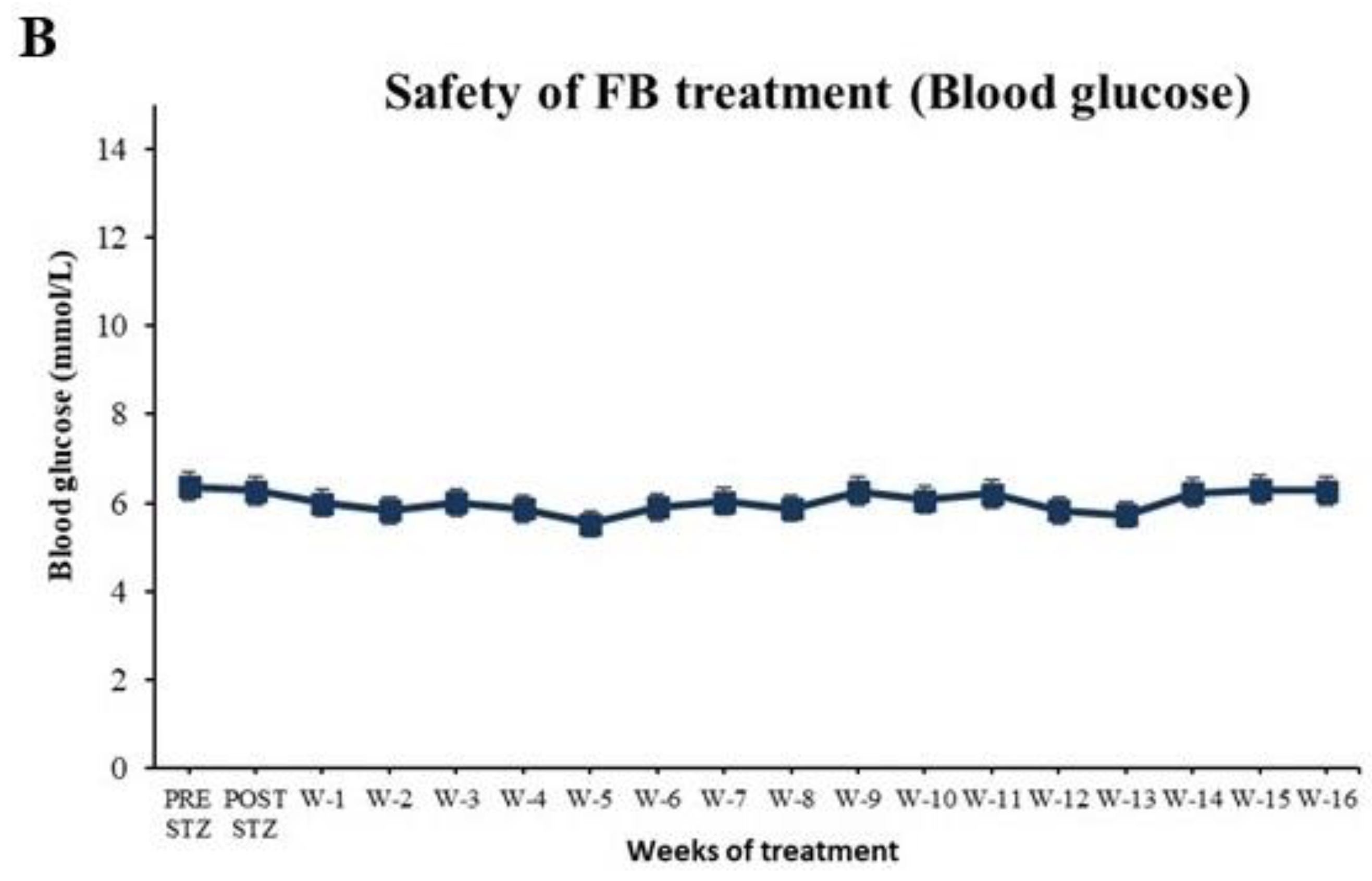
3.1.1. FB Treatment Caused a Reduction in BG Levels in the Diabetic Animals
3.1.2. FB Treatment Caused a Reduction in Serum Fructosamine in the Diabetic Animals
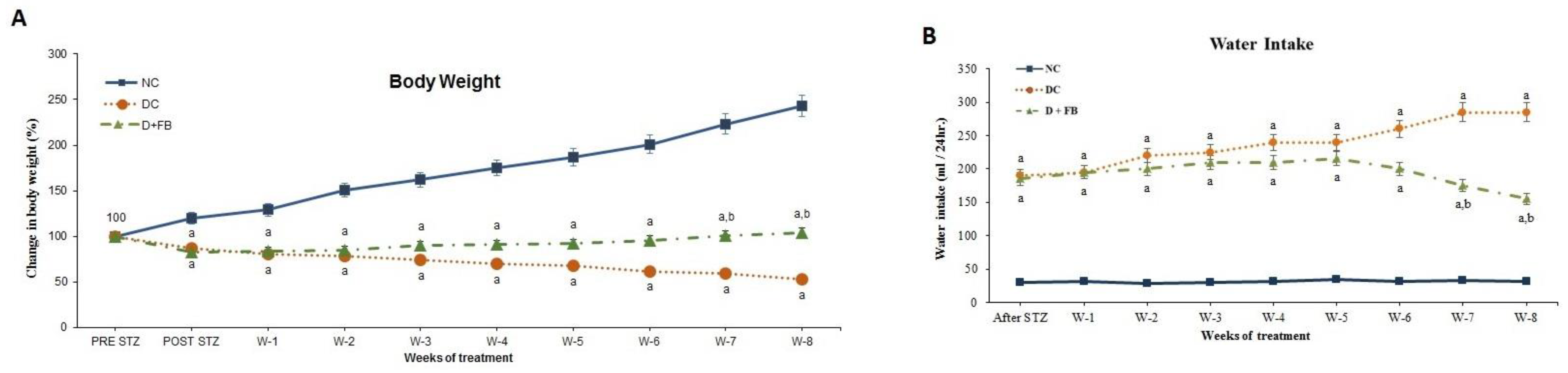
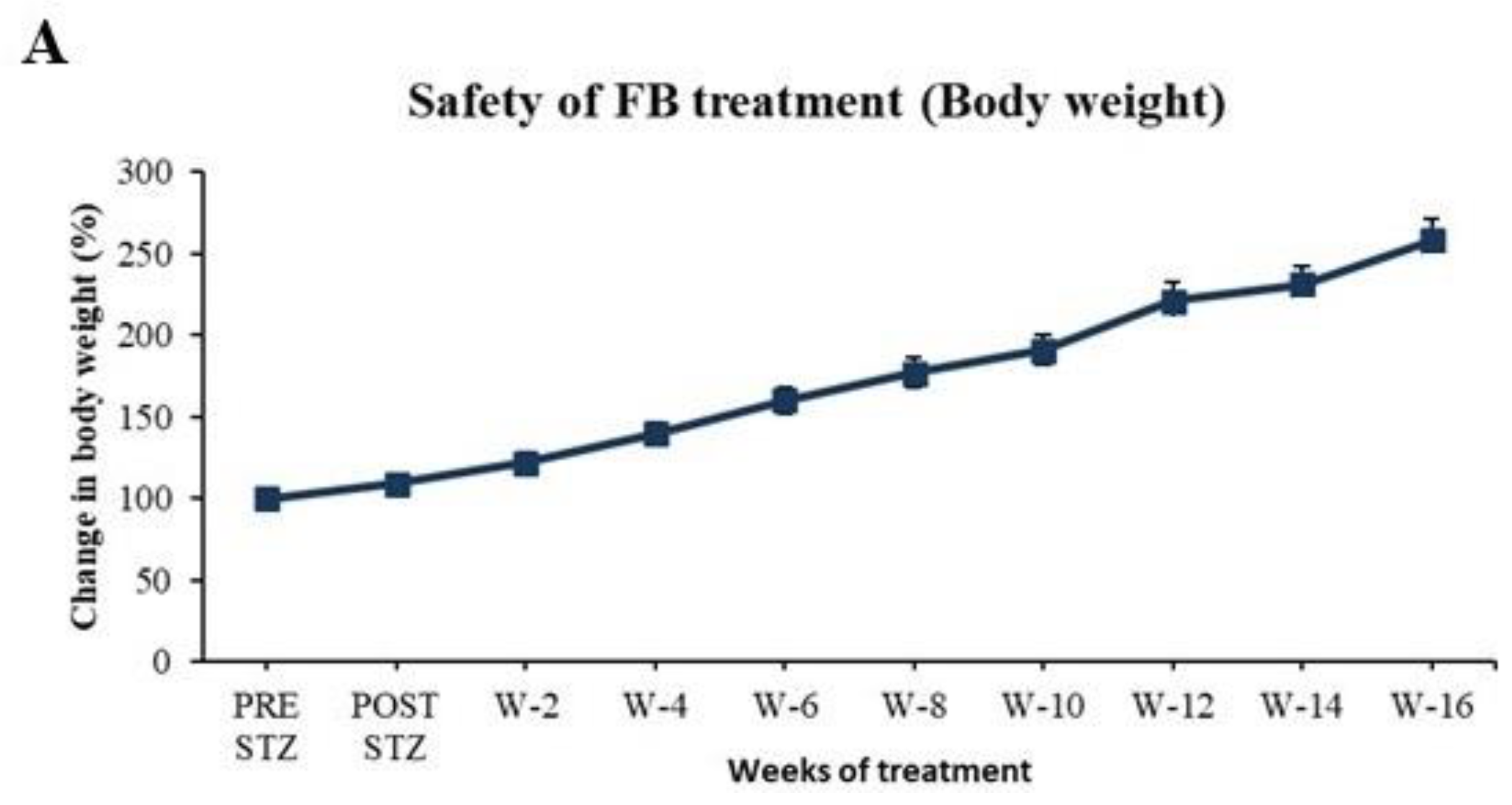
3.1.3. FB Treatment Improved the Body Weight of the Diabetic Animals
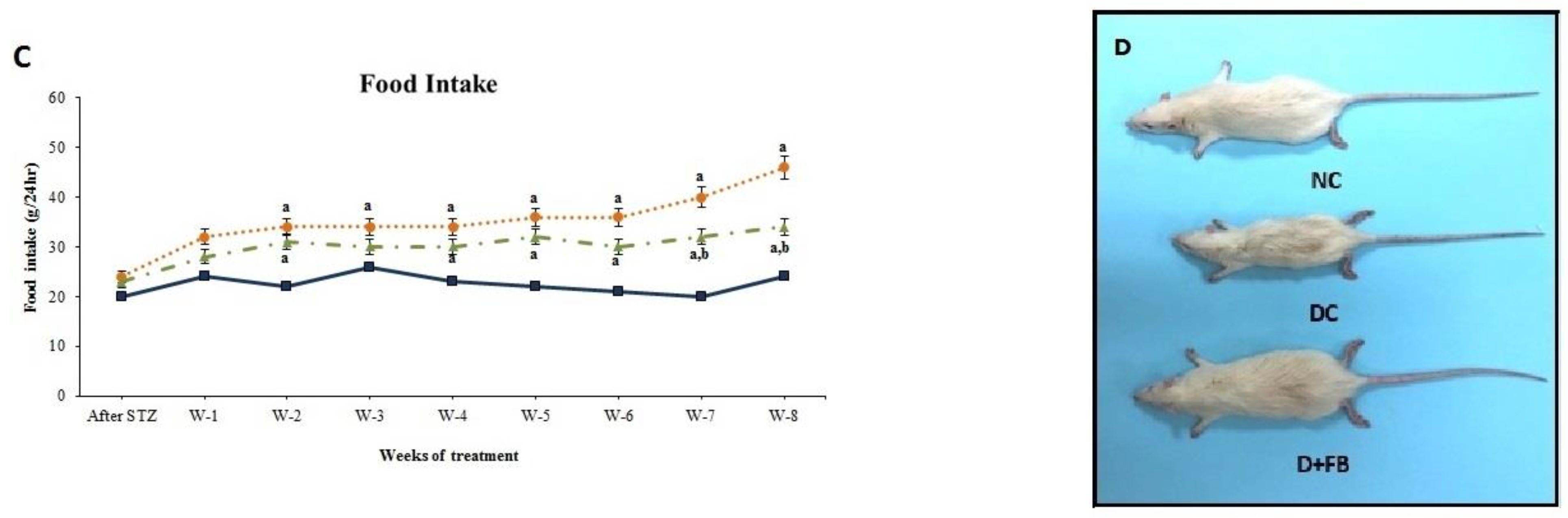
3.1.4. FB Treatment Caused a Reduction in Water and Food Intake in the Diabetic Animals
3.1.5. FB Treatment Improved the Physical Appearance of the Diabetic Animals
3.2. FB Treatment Caused Pancreatic Recovery and the Regeneration of Insulin-Producing β-Cells
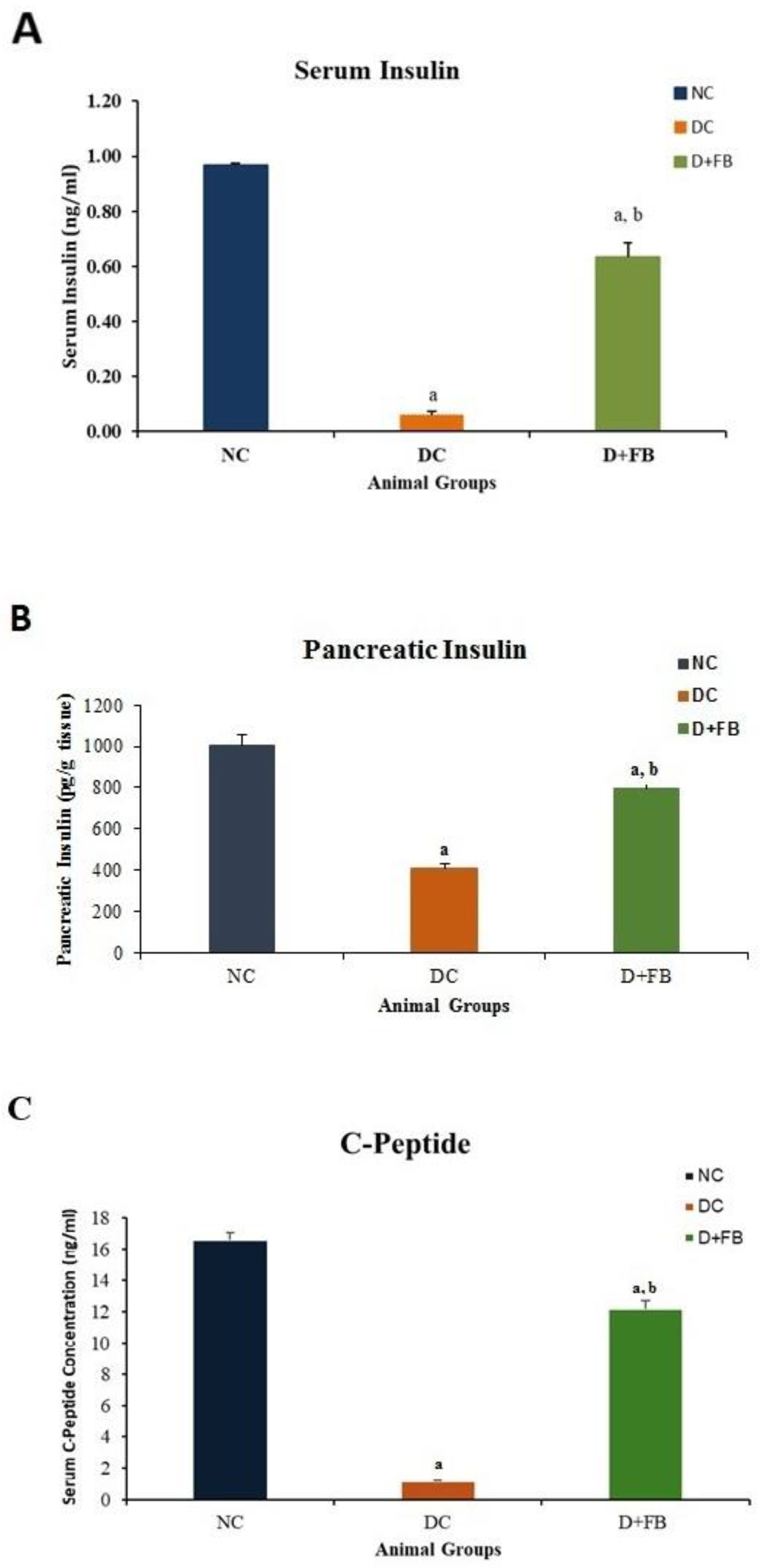
3.2.1. Pancreas of FB-Treated Diabetic Animals Produced Insulin and C-Peptide
Insulin in Serum
Insulin in Pancreatic Tissues
C-Peptide in Serum
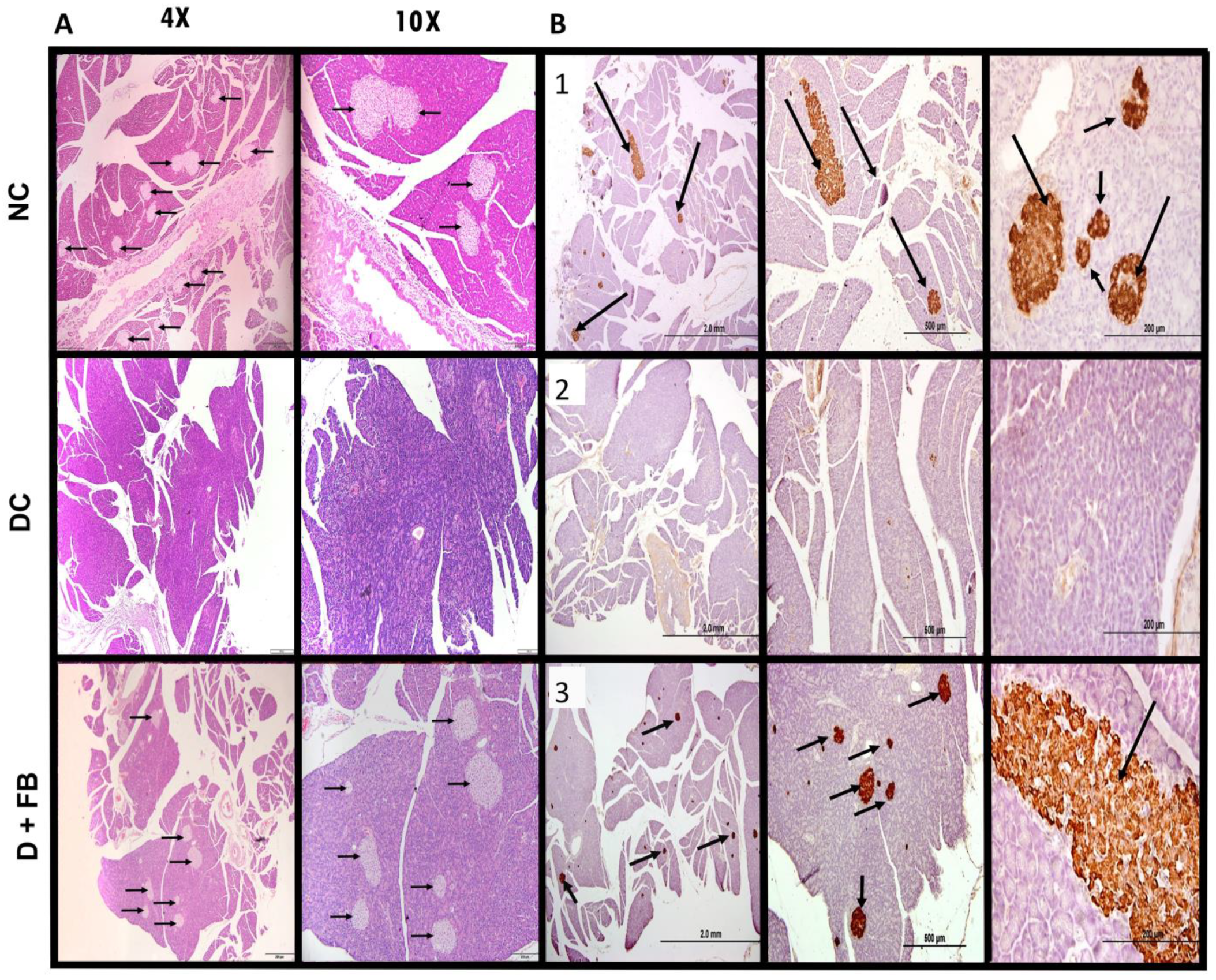
3.3. Comparison of Histopathological Analysis and Immunohistochemical Staining of the Pancreatic Tissues of the Three Groups of Animals
3.3.1. Histopathological Analysis of the Pancreatic Tissues of FB-Treated Rats Confirmed Islet Recovery
3.3.2. Immunohistochemical Staining for Insulin in the Recovered Islets of the Pancreases of the FB-Treated Diabetic Animals Showed Insulin in β-Cells
3.4. FB Treatment Improved Health Issues Related to Diabetes
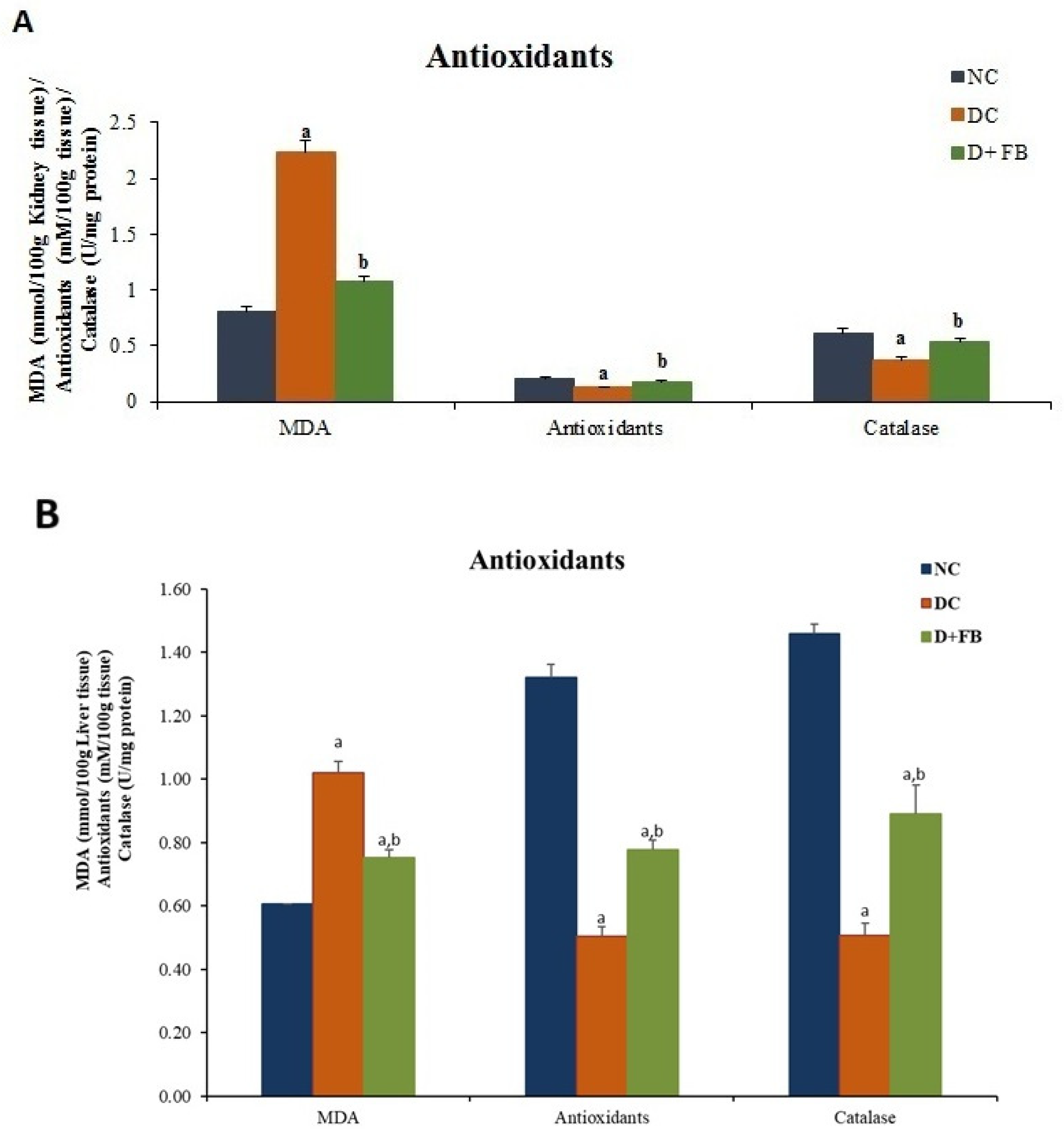
3.4.1. FB Treatment Optimized Oxidation/Antioxidation in the Kidneys and Liver of Diabetic Male Rats
FB Treatment Reduced MDA Concentration in Both the Kidneys and Liver
FB Treatment Increased the Concentration of Antioxidants in Both the Kidneys and Liver
FB Treatment Increased Catalase Concentration in Both the Kidneys and Liver
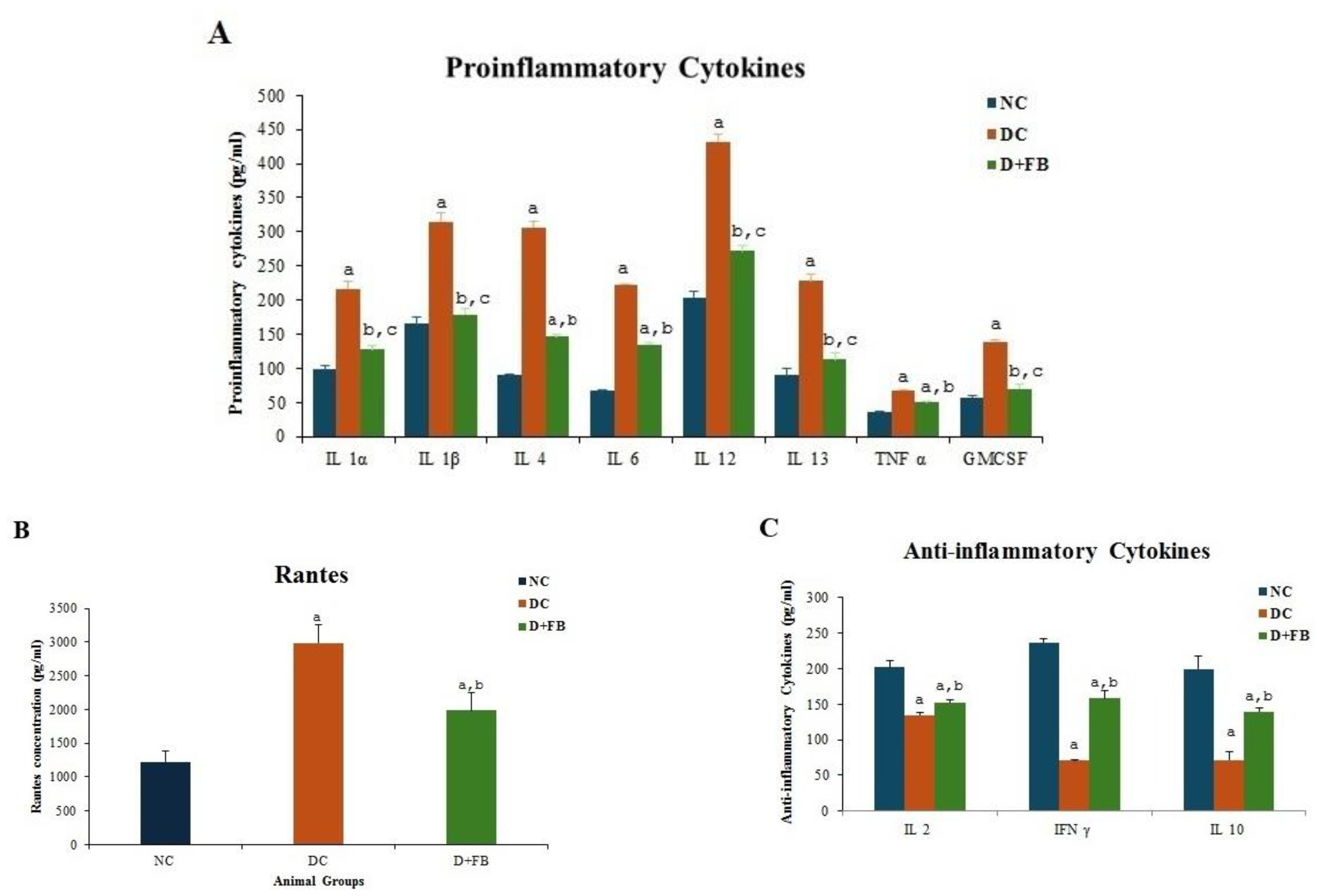
3.5. FB Treatment Reduced the Proinflammatory Cytokine and Elevated the Anti-Inflammatory Cytokine Levels
3.5.1. FB Treatment Reduced the Levels of Proinflammatory Cytokines
3.5.2. FB Treatment Reduced the Levels of Proinflammatory Cytokine RANTES
3.5.3. FB Treatment Elevated the Anti-Inflammatory Cytokine Levels
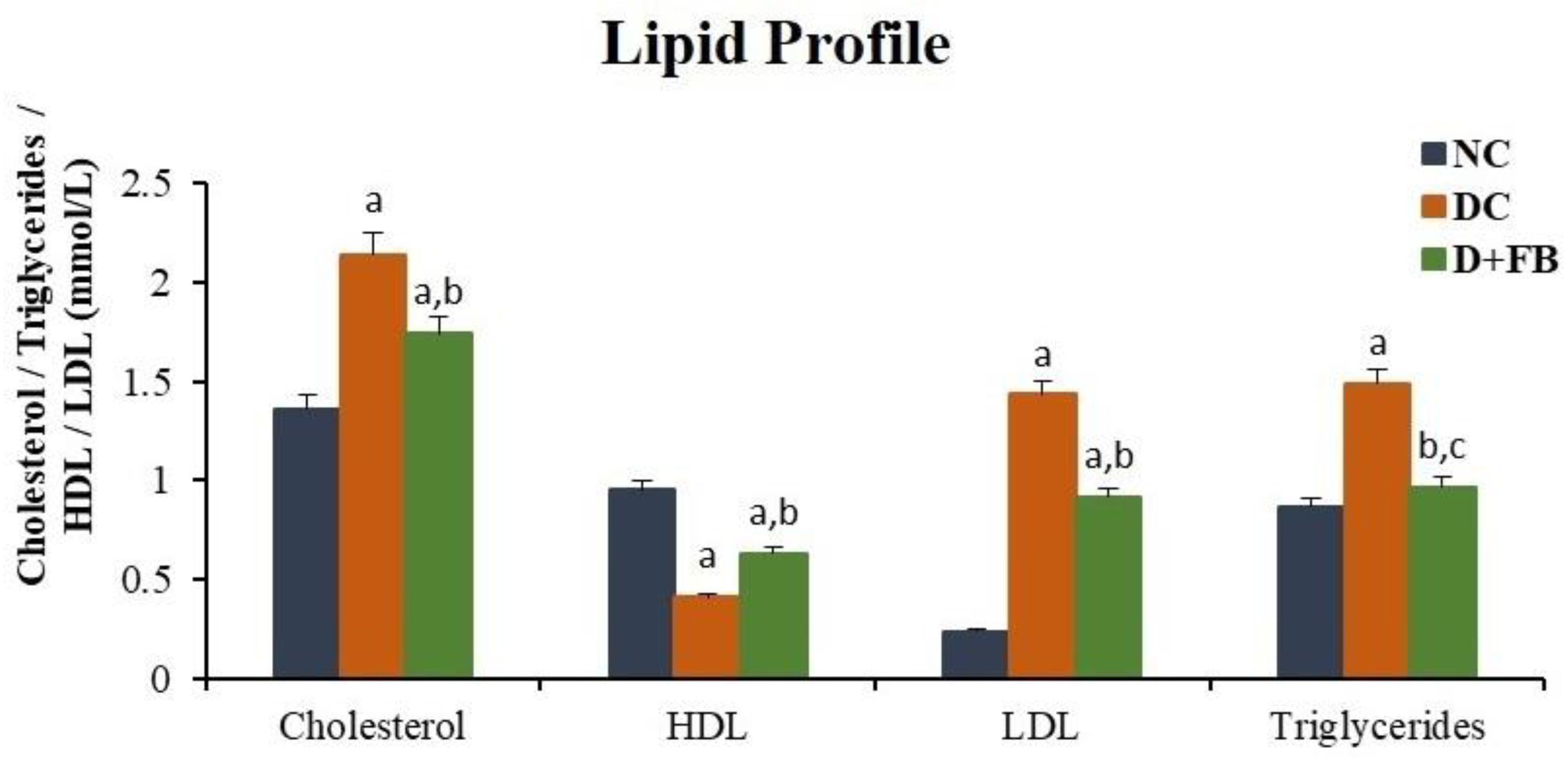
3.6. FB Treatment Improved Lipid Profiles
3.6.1. FB Treatment Decreased Serum Cholesterol
3.6.2. FB Treatment Increased HDL in Serum
3.6.3. FB Treatment Decreased LDL in Serum
3.6.4. FB Treatment Decreased Triglycerides in Serum
3.7. FB Treatment Did Not Cause Side Effects in Normal Animals After 16 Weeks of Treatment
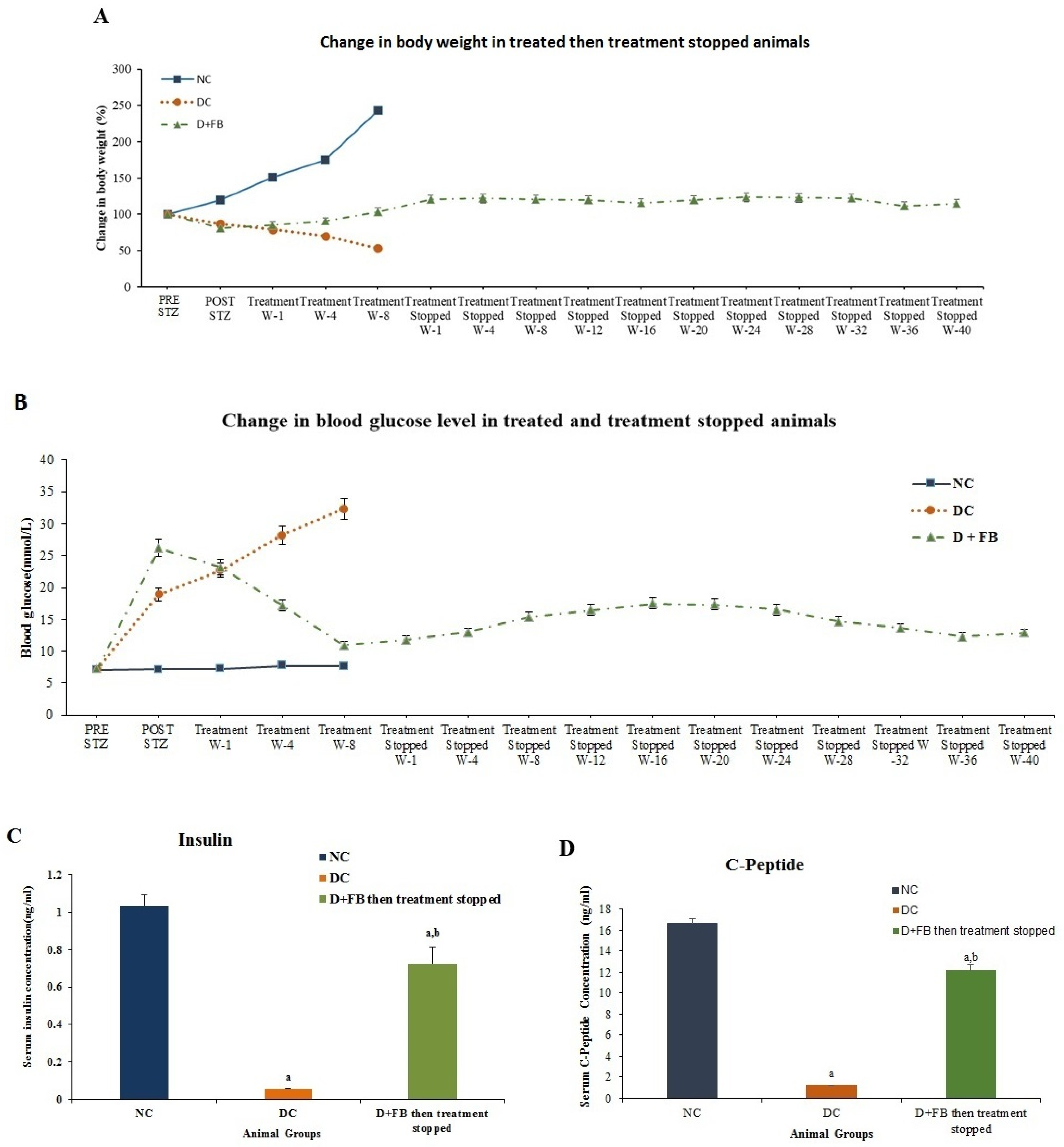
3.8. Regenerated β Cells Remained Active for 48 Weeks Until the Animals Were Sacrificed
3.8.1. Body Weight
3.8.2. Reduction in BG Levels in the FB-Treated Diabetic Animals for 8 Weeks. Their Treatment Was Then Discontinued and the Animals were Monitored for 40 Weeks
3.8.3. Increased Serum Insulin Concentration in the 8 Week Treated Animals Then the Treatment Was Discontinued for 40 Weeks
3.8.4. Increased C-Peptide in the 8-Week Treated Animals Then Their Treatment Was Discontinued for 40 Weeks
3.9. FB Contains Growth Factors and Biologically Active Proteins That Can Play Roles in the Regeneration of β Cells
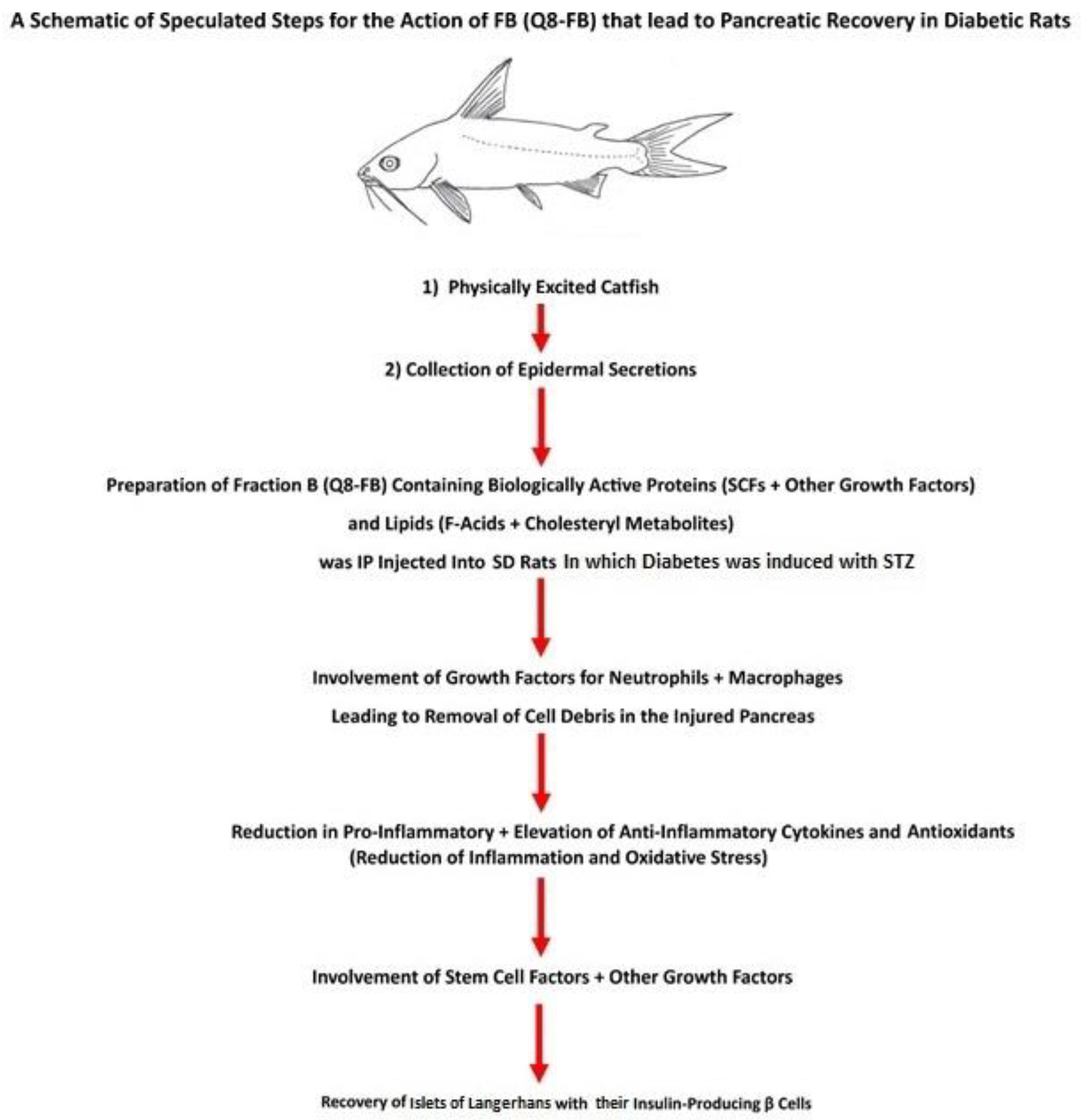
4. Speculated FB Action in Pancreatic Recovery
- 1.
- Among the active proteins found in FB is insulin, which was detected at a concentration of 139.597 ± 3.504 ng/g FB protein, which is equal to an injection of 0.08378 ng insulin/200 g body weight in this study. The literature shows that the daily insulin requirement of a diabetic rat is in the range of 0.1–0.4 units (equivalent to a range of 3470–13,880 ng) per 200 g of animal body weight. The amount of insulin injected via FB into diabetic rats is minute compared to the daily insulin requirement; hence, its effect on BG level is negligible.
- 2.
- FB also contains glucagon at a concentration of 13.581 ± 0.3726 ng/g FB protein. The amount of FB injected into the diabetic rat contained 0.008149 ng glucagon/200 g rat body weight each day. This concentration is very small compared to the insulin concentration required daily by the rat. Accordingly, neither of these two exogenous proteins, insulin and glucagon, were present at high enough concentrations to influence the BG levels in FB-treated rats. This conclusion was supported by the fact that when the diabetic animals were treated with FB for 8 weeks then their treatment was suspended for 40 weeks no elevation in BG levels in the FB-treated experimental animals was observed during the 40 weeks of suspended FB treatment until the animals were sacrificed. Suspending FB injection for 40 weeks resulted in the animals being deprived of these two exogenous proteins (insulin and glucagon). This finding also confirms the lack of effect of both exogenous proteins on the BG levels in FB-treated animals.
- 3.
- It was speculated that the HI, RI, HIGF-1, RIGF-1, HGLP-1, and RGCG found in FB in minute concentrations were components of the total aggregated active proteins and growth factors, whose combined action might have contributed to healing the injured pancreas and islet recovery, as was shown by the regeneration of insulin-producing β-cells. It has been well-established that insulin supports wound healing and that GLP-1 promotes β-cell proliferation through mitogenesis, while decreasing β-cell apoptosis [1,4,49,50,51]. The quantitative data presented in this study supports our hypothesis, which presumed that FB would be able to heal the STZ-injured [90] diabetic pancreas.
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, F.; Jiang, Y. Endogenous pancreatic β cell regeneration: A potential strategy for the recovery of β cell deficiency in diabetes. Front. Endocrinol. 2019, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Poudel, A.; Savari, O.; Striegel, D.A.; Periwal, V.; Taxy, J.; Millis, J.M.; Witkowski, P.; Atkinson, M.A.; Hara, M. Beta-cell destruction and preservation in childhood and adult-onset type 1 diabetes. Endocrine 2015, 49, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Oram, R.A.; Sims, E.K.; Molina, C.E. Beta cells in type 1 diabetes: Mass and function; sleeping or dead? Diabetologia 2019, 62, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Arutyunyan, I.V.; Fatkhudinov, T.K.; Makarov, A.V.; Elchaninov, A.V.; Sukhikh, G.T. Regenerative medicine of pancreatic islets. World J. Gastroenterol. 2020, 26, 2948–2966. [Google Scholar] [CrossRef]
- Mishra, P.K.; Singh, S.R.; Joshua, G.; Tyagi, S.C. Stem cells as a therapeutic target for diabetes. Front. Biosci. 2010, 15, 461–477. [Google Scholar] [CrossRef]
- Vardanyan, M.; Parkin, E.; Gruessner, C.; Rodriguez Rilo, H.L. Pancreas vs. islet transplantation: A call on the future. Curr. Opin. Organ Transplant. 2010, 15, 124–130. [Google Scholar] [CrossRef]
- Ji, Z.; Lu, M.; Xie, H.; Yuan, H.; Chen, Q. β cell regeneration and novel strategies for treatment of diabetes (review). Biomed. Rep. 2022, 17, 72. [Google Scholar] [CrossRef]
- Al-Hassan, J.M.; Thomson, M.; Criddle, R.S. Accelerated wound healing by a preparation from skin of the Arabian Gulf catfish. Lancet 1983, 1, 1043–1044. [Google Scholar] [CrossRef]
- Wakimoto, T.; Kondo, H.; Nii, H.; Kimura, K.; Egami, Y.; Oka, Y.; Akahoshi, S. Furan fatty acid as an anti-inflammatory component from the green-lipped mussel Perna canaliculus. Proc. Natl. Acad. Sci. USA 2011, 108, 17533–17537. [Google Scholar] [CrossRef]
- Khan, M.A.; Pace-Asciak, C.; Al-Hassan, J.M.; Afzal, M.; Liu, Y.F.; Oommen, S.; Paul, B.M.; Nair, D.; Palaniyar, N. Furanoid F-acid F6 uniquely induces NETosis compared to C16 and C18 fatty acids in human neutrophils. Biomolecules 2018, 8, 144–166. [Google Scholar] [CrossRef]
- Al-Hassan, J.M.; Afzal, M.; Oommen, S.; Liu, Y.F.; Pace-Asciak, C. Oxysterols in catfish skin secretions (Arius bilineatus, Val.) exhibit anti-cancer properties. Front. Pharmacol. 2022, 13, 1001067. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassan, J.M.; Liu, Y.F.; Khan, M.A.; Yang, P.; Guan, R.; Wen, X.Y.; Afzal, M.; Oommen, S.; Paul, B.M.; Nair, D.; et al. Furanoic lipid F-6, a novel anti-cancer compound that kills cancer cells by suppressing proliferation and inducing apoptosis. Cancers 2019, 11, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassan, J.M. Diabetic ulcer healing preparations from the skin of the Arabian Gulf catfish (Arius bilineatus Val.): A novel and effective treatment. Int. J. Tissue React. 1990, 12, 121–135. [Google Scholar] [PubMed]
- Al-Hassan, J.M.; Hinek, A.; Renno, W.M.; Wang, Y.; Liu, Y.F.; Guan, R.; Wen, X.Y.; Litvack, M.L.; Lindenmaier, A.; Afzal, M.; et al. Potential mechanism of dermal wound treatment with preparations from the skin gel of Arabian Gulf catfish: A unique furan fatty acid (F6) and cholesta-3,5-diene (S5) recruit neutrophils and fibroblasts to promote wound healing. Front. Pharmacol. 2020, 11, 899. [Google Scholar] [CrossRef]
- Al-Arbeed, T.A.; Renno, W.M.; Al-Hassan, J.M. Neuroregeneration of injured peripheral nerve by fraction B of catfish epidermal secretions through the reversal of the apoptotic pathway and DNA damage. Front. Pharmacol. 2023, 14, 1085314. [Google Scholar] [CrossRef]
- Al-Hassan, J.M.; Wei, D.; Chakraborty, S.; Conway, T.; Rhea, P.; Wei, B.; Tran, M.; Gagea, M.; Afzal, M.; Oommen, S.; et al. Fraction B from catfish epidermal secretions kills pancreatic cancer cells, inhibits CD44 expression and stemness, and alters cancer cell metabolism. Front. Pharmacol. 2021, 12, 659590. [Google Scholar] [CrossRef]
- Al-Hassan, J.M.; Thomson, M.; Ali, M.; Summers, B.; Criddle, R.S. Protein composition of the threat induced epidermal secretion from the Arabian Gulf catfish, Arius thalassinus (Ruppell). Comp. Bioch. Physiol. B Comp. Bioch. 1987, 88, 813–822. [Google Scholar] [CrossRef]
- Al-Hassan, J.M. Method of Making an Anti-Inflammatory Composition. U.S. Patent 8,551,532 B2, 8 October 2013. [Google Scholar]
- Ali, J.M.H. Method of Preparing a Therapeutic Composition Using a Preparation from Epidermal Gel Secretions of Catfish. U.S. Patent 10,568,915 B1, 25 February 2020. [Google Scholar]
- Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Al-Amin, Z.; Thomson, M.; Al-Qattan, K.K.; Peltonen-Shalaby, R.; Ali, M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Brit. J. Nutr. 2006, 96, 660–666. [Google Scholar] [CrossRef]
- Lee, J.H.; Yang, S.H.; Oh, J.M.; Lee, M.G. Pharmacokinetics of drugs in rats with diabetes mellitus induced by alloxan or streptozotocin: Comparison with those in patients with type I diabetes mellitus. J. Pharm. Pharmacol. 2010, 62, 1–23. [Google Scholar] [CrossRef]
- Renno, W.M.; Afzal, M.; Paul, B.; Nair, D.; Kumar, J.; Al-Hassan, J.M. Catfish epidermal preparation accelerates healing of damaged nerve in a sciatic nerve crush injury rat model. Front. Pharmacol. 2021, 12, 632028. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.F.; Lees, H.; Gutman, S.I. Effect of nitroblue tetrazolium concentration on the fructosamine assay for quantifying glycated protein. Clin. Chem. 1988, 34, 2106–2111. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhao, Y.; Gu, D.; Du, L.; Cai, G.; Zhang, Y. Scorpion in Combination with Gypsum: Novel Antidiabetic Activities in Streptozotocin-Induced Diabetic Mice by Up-Regulating Pancreatic PPARγ and PDX-1 Expressions. Evid. Based Complement. Alternat. Med. 2011, 14, 683561. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.M.; Renno, W.M.; Sadek, H.L.; Kayali, N.M.; Al-Salem, A.; Rao, M.S.; Khan, K.M. Monoamine oxidase-B inhibitor protects degenerating spinal neurons, enhances nerve regeneration and functional recovery in sciatic nerve crush injury model. Neuropharmacology 2018, 128, 231–243. [Google Scholar] [CrossRef]
- Al-Adwani, D.G.; Renno, W.M.; Orabi, K.Y. Neurotherapeutic effects of Ginko Biloba extract and its terpene trilactone, ginkgolide B on sciatic crush injury model: A new evidence. PLoS ONE 2019, 14, e0226626. [Google Scholar] [CrossRef]
- Emordi, J.M.; Agbaje, E.O.; Oreagba, I.A.; Iribhogbe, O.I. Antidiabetic and hypolipidemic activities of hydroethanolic root extract of Uvaria chamae in streptozotocin induced diabetic albino rats. BMC Complement. Altern. Med. 2016, 16, 468. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yake, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Drobiova, H.; Thomson, M.; Al-Qattan, K.K.; Shalaby, R.; Al-Amin, Z.; Ali, M. Garlic increases antioxidant in diabetic and hypertensive rats determined by a peroxidase modified method. Evid. Based Complement. Alternat. Med. 2011, 2011, 703049. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods in Enzyme Analysis, 2nd ed.; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; Volume 2, pp. 673–684. [Google Scholar] [CrossRef]
- Georg, P.; Ludvik, B. Lipids and diabetes. J. Clin. Basic Cardiol. 2000, 3, 159–162. [Google Scholar]
- Jun, H.S.; Park, E.Y. Adult stem cells as a renewable source of insulin-producing cells. Int. J. Stem Cells 2009, 2, 115–121. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Chen, X.; Shi, Y.; Xie, J. Epidermal Stem Cells in Wound Healing and Regeneration. Stem Cells Int. 2020, 2020, 9148310. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, Y.L.; Galiano, R.D.; Roth, S.I.; Mustoe, T.A. Macrophage colony-stimulating factor accelerates wound healing and upregulates TGF-beta1 mRNA levels through tissue macrophages. J. Surg. Res. 1997, 72, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Kreimendahl, F.; Marquardt, Y.; Apel, C.; Bartneck, M.; Zwadlo-Klarwasser, G.; Hepp, J.; Jockenhoevel, S.; Baron, J.M. Macrophages significantly enhance wound healing in a vascularized skin model. J. Biomed. Mater. Res. Part A 2019, 107, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Bouchery, T.; Harris, N. Neutrophil-macrophage cooperation and its impact on tissue repair. Immunol. Cell Biol. 2019, 97, 289–2298. [Google Scholar] [CrossRef]
- Aiello, L.P.; Wong, J.S. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int. 2000, 58, 113–119. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Li, W.; Nie, X. Fibroblast growth factor in diabetic foot ulcer: Progress and therapeutic prospects. Front. Endocrinol. 2021, 12, 744868. [Google Scholar] [CrossRef]
- Perry, R.J.; Lee, S.; Ma, L.; Zhang, D.; Schlessinger, J.; Shulman, G.I. FGF1 and FGF19 reverse diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat. Commun. 2015, 6, 6980. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, L.; Lin, X.; Cheng, P.; He, L.; Li, X.; Lu, X.; Tan, Y.; Yang, H.; Cai, L.; et al. Minireview: Roles of Fibroblast Growth Factors 19 and 21 in Metabolic Regulation and Chronic Diseases. Mol. Endocrinol. 2015, 29, 1400–1413. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, J.; Li, R.; Peng, Y.; Huang, J.L.; Huang, L. Basic fibroblast growth factor accelerates myelin debris clearance through activating autophagy to facilitate early peripheral nerve regeneration. J. Cell Mol. Med. 2021, 25, 2596–2608. [Google Scholar] [CrossRef]
- Chen, W.; Hoo, R.L.; Konishi, M.; Itoh, N.; Lee, P.C.; Ye, H.Y.; Lam, K.S.; Xu, A. Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J. Biol. Chem. 2011, 286, 34559–34566. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A cell with many roles in inflammation or several cell types. Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Leal, E.C.; Carvalho, E.; Tellechea, A.; Kafanas, A.; Tecilazich, F.; Kearney, C. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am. J. Pathol. 2015, 185, 1638–1648. [Google Scholar] [CrossRef]
- Um, J.; Yu, J.; Park, K.S. Substance-P accelerates wound healing in type 2 diabetic mice through endothelial progenitor cell mobilization and Yes-associated protein activation. Mol. Med. Rep. 2017, 15, 3035–3040. [Google Scholar] [CrossRef]
- Zhu, F.B.; Fang, X.J.; Liu, D.W.; Shao, Y.; Zhang, H.Y.; Peng, Y.; Zhong, Q.L.; Li, Y.T.; Liu, D.M. Substance-P combined with epidermal stem cells promotes wound healing and nerve regeneration in diabetes mellitus. Neural Reg. Res. 2016, 11, 493–501. [Google Scholar]
- Wang, J.; Xu, J. Effects of topical insulin on wound healing: A review of animal and human evidences. Diabetes Metab. Syndr. Obes. 2020, 13, 719–727. [Google Scholar] [CrossRef]
- Amankwah, N.; Jahangiri, Y.; Landry, G.J.; Moneta, G.L.; Azarbal, A.F. Effect of systemic insulin treatment on diabetic wound healing. Wound Repair Regen. 2017, 25, 288–291. [Google Scholar]
- Lee, Y.S.; Lee, C.; Choung, J.S.; Jung, H.S.; Jun, H.S. Glucagon-like peptide 1 increases β-cell regeneration by promoting α- to β-cell transdifferentiation. Diabetes 2018, 67, 2601–2614. [Google Scholar] [CrossRef]
- Ali, A.; Khan, D.; Dubey, V.; Tarasov, A.I.; Flatt, R.P.; Irwin, N. Comparative Effects of GLP-1 and GLP-2 on Beta-Cell Function, Glucose Homeostasis and Appetite Regulation. Biomolecules 2024, 14, 1520. [Google Scholar] [CrossRef]
- Mysona, B.A.; Shanab, A.Y.; Elshaer, S.L.; El-Remessy, A.B. Nerve growth factor in diabetic retinopathy: Beyond neurons. Expert Rev. Ophthalmol. 2014, 9, 99–107. [Google Scholar] [CrossRef]
- Li, R.; Li, D.; Wu, C.; Ye, L.; Wu, Y.; Yuan, Y.; Yang, S.; Xie, L.; Mao, Y.; Jiang, T.; et al. Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics 2020, 10, 1649–1677. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Tan, W.; Li, B.; Wang, Z.; Li, Y.; Zeng, J.; Jiang, B.; Yoshida, S.; Zhou, Y. Interleukin-19 promotes retinal neovascularization in a mouse model of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2022, 63, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Y.; Zhou, Y.; Fei, B. Interleukin 37 (IL-37) reduces high glucose-induced inflammation, oxidative stress, and apoptosis of podocytes by inhibiting the STAT3–Cyclophilin A (CypA) signaling pathway. Med. Sci. Monit. 2020, 26, e922979. [Google Scholar] [CrossRef] [PubMed]
- Gradwohl, G.; Dierich, A.; LeMeur, M.; Guillemot, F. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 2000, 97, 1607–1611. [Google Scholar] [CrossRef]
- Desgraz, R.; Herrera, P.L. Pancreatic neurogenin3-expressing cells are unipotent islet precursors. Development 2009, 136, 3567–3574. [Google Scholar] [CrossRef]
- Rukstalis, M.J.; Habener, J.F. Neurogenin3: A master regulator of pancreatic islet differentiation and regeneration. Islets 2009, 1, 177–184. [Google Scholar] [CrossRef]
- Albert, S.C.; Hathaway, C.K.; Smithies, O.; Kakoki, M. Transforming growth factor-β1 and diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2016, 310, F689–F696. [Google Scholar]
- Li, J.F.; Duan, H.F.; Wu, C.T.; Zhang, D.J.; Deng, Y.; Yin, H.L.; Han, B.; Gong, H.C.; Wang, H.W.; Wang, Y.L. HGF accelerates wound healing by promoting the dedifferentiation of epidermal cells through β1-integrin/ILK pathway. Biomed. Res. Int. 2013, 2013, 470418. [Google Scholar] [CrossRef]
- Sinha, D.; Valapala, M.; Bhutto, I.; Patek, B.; Zhang, C.; Hose, S.; Yang, F.; Cano, M.; Stark, W.J.; Lutty, G.A.; et al. βA3/A1-crystallin is required for proper astrocyte template formation and vascular remodeling in the retina. Transgenic Res. 2012, 21, 1033–1042. [Google Scholar] [CrossRef]
- Ghosh, S.; Liu, H.; Yazdankhah, M.; Stepicheva, N.; Shang, P.; Vaidya, T.; Hose, S.; Gupta, U.; Calderon, M.J.; Hu, M.W. βA1-crystallin regulates glucose metabolism and mitochondrial function in mouse retinal astrocytes by modulating PTP1B activity. Commun. Biol. 2021, 4, 248. [Google Scholar] [CrossRef]
- Katagiri, T.; Watabe, T. Bone morphogenetic proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef] [PubMed]
- Guercio, M.J.; Natale, B.D.; Gargantini, L.; Garlaschi, C.; Chiumello, G. Effect of somatostatin on blood sugar, plasma growth hormone and glucagon levels in diabetic children. Diabetes 1976, 25, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.Y.; Lichy, J.H.; Waldmann, T.A.; Perera, P. The role of interleukin-15 in inflammation and immune responses to infection: Implications for its therapeutic use. Microbes Infect. 2012, 14, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Li, D.; Li, C.; Muehleisen, B.; Radek, K.A.; Park, H.J.; Jiang, Z.; Li, Z.; Lei, H.; Quan, Y.; et al. The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity 2012, 37, 74–84. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, Q.; Prud’homme, G.J. GABAergic system in the endocrine pancreas: A new target for diabetes treatment. Diabetes Metab. Syndr. Obes. 2015, 8, 79–87. [Google Scholar]
- Jerala, N.K. The role of interferons in inflammation and inflammasome activation. Front. Immunol. 2017, 8, 873. [Google Scholar] [CrossRef]
- Henig, N.; Avidan, N.; Mandel, I.; Ram, E.S.; Ginzburg, E.; Paperna, T.; Pinter, R.Y.; Miller, A. Interferon-beta induces distinct gene expression response patterns in human monocytes versus T cells. PLoS ONE 2013, 8, e62366. [Google Scholar] [CrossRef]
- Dong, J.; Chen, L.; Zhang, Y.; Jayaswal, N.; Mezghani, I.; Zhang, W.; Veves, A. Mast cells in diabetes and diabetic wound healing. Adv. Ther. 2020, 37, 4519–4537. [Google Scholar] [CrossRef]
- Ullrich, S. IGF-1 and insulin receptor signaling in insulin-secreting cells: From function to survival. In Islets of Langerhans, 2nd ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 659–685. [Google Scholar]
- Manshaei, N.; Shakibaei, F.; Fazilati, M.; Salavati, H.; Negahdary, M.; Palizban, A. An investigation of the association between the level of prolactin in serum and type II diabetes. Diabetes Metab. Syndr. 2019, 13, 3035–3041. [Google Scholar] [CrossRef]
- Kanwal, A.; Dsouza, L.A. Sirtuins and diabetes: Optimizing the sweetness in the blood. Trans. Med. Commun. 2019, 4, 3. [Google Scholar] [CrossRef]
- Watroba, M.; Szewczyk, G.; Szukiewicz, D. The Role of Sirtuin-1 (SIRT1) in the Physiology and Pathophysiology of the Human Placenta. Int. J. Mol. Sci. 2023, 24, 16210. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Bin, L. Will Sirtuin 2 be a promising target for neuroinflammatory disorders? Front. Cell. Neurosci. 2022, 16, 915587. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Liu, J.; Xue, Z.; Wang, W.; Li, C.; Xu, F.; Du, Y.; Ly, X. SIRT3 attenuates coronary atherosclerosis in diabetic patients by regulating endothelial cell function. J. Clin. Lab. Anal. 2022, 36, e24586. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.X.; Wang, Q.J.; Li, H.; Huang, Q. SIRT4 overexpression protects against diabetic nephropathy by inhibiting podocyte apoptosis. Exp. Ther. Med. 2017, 13, 342–348. [Google Scholar] [CrossRef]
- Ma, Y.; Fei, X. SIRT5 regulates pancreatic β-cell proliferation and insulin secretion in type 2 diabetes. Exp. Ther. Med. 2018, 16, 1417–1425. [Google Scholar] [CrossRef]
- Harvey, N.L.; Mayrovitz, N. Role of sirtuins in diabetes and age-related processes. Cureus 2022, 14, e28774. [Google Scholar]
- Wu, D.; Li, Y.; Zhu, K.S.; Wang, H.; Zhu, W.G. Advances in cellular characterization of the sirtuin isoform, SIRT7. Front. Endocrinol. 2018, 9, 652. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Yakar, S.; LeRoith, D. The intricate role of growth hormone in metabolism. Front. Endocrinol. 2011, 2, 32. [Google Scholar] [CrossRef]
- Yang, S.; Xue, S.; Zhijun, G.; Yibo, X.; Linyu, X.; Yue, C.; Bohan, L.; Jinran, S.; Lian, W.; Yueyue, W.; et al. IL-11 ameliorates oxidative stress damage in neurons after spinal cord injury by activating the JAK/STAT signaling pathway. Int. Immunopharmacol. 2024, 25, 127. [Google Scholar]
- Daniella, B.R.I.; Carolina, T.A.; Diego, S.C.; Nathalia, S.M.; Maximiliano, R.F.; Tatiana, P.T.F.; Cynthia, M.C.; Andrea, H.-P.; Priscilla, C.O.; Bruno, L.D.; et al. Glucagon reduces airway hyperreactivity, inflammation, and remodeling induced by ovalbumin. Sci. Rep. 2019, 9, 6478. [Google Scholar]
- Chen, J.; Huang, Y.; Liu, C.; Chi, J.; Wang, Y.; Xu, L. The role of C-peptide in diabetes and its complications: An updated review. Front. Endocrinol. 2023, 14, 1256093. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.B.; Ojalvo, A.G.; Montequin, J.F.; Cama, V.F.; Rivero, N.A.; Niet, G.G.; Ferrer, M.P.; Fernandez, M.L.; Avila, M.A.; Eksson, E. Epidermal Growth Factor Intralesional Delivery in Chronic Wounds: The Pioneer and Standalone Technique for Reversing Wound Chronicity and Promoting Sustainable Healing. Int. J. Mol. Sci. 2024, 20, 10883. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.N.; Sun, Q.; Liu, S.Q.; Hu, H.; Lv, J.; Ji, W.J.; Wang, M.; Chen, M.X.; Zhou, J. Erythropoietin improves glucose metabolism and pancreatic β-cell damage in experimental diabetic rats. Mol. Med. Rep. 2015, 12, 5391–5398. [Google Scholar] [CrossRef]
- Shen, S.; Wang, F.; Fernandez, A.; Hu, W. Role of platelet-derived growth factor in type II diabetes mellitus and its complications. Diab. Vasc. Dis. Res. 2020, 17, 1479164120942119. [Google Scholar] [CrossRef]
- Henshaw, F.R.; Boughton, P.; Lo, L.; McLennan, S.V.; Twigg, S.M. Topically Applied Connective Tissue Growth Factor/CCN2 Improves Diabetic Preclinical Cutaneous Wound Healing: Potential Role for CTGF in Human Diabetic Foot Ulcer Healing. J. Diabet. Res. 2015, 2015, 236238. [Google Scholar] [CrossRef]
- Lukić, M.L.; Stosić-Grujicić, S.; Shahin, A. Effector mechanisms in low-dose streptozotocin-induced diabetes. J. Immunol. Res. 1998, 6, 092198. [Google Scholar] [CrossRef]
- Al-Hassan, J.M.; Afzal, M.; Oommen, S.; Liu, Y.F.; Khan, M.; Pace-Asciak, C. Oxidized cholesterol derivatives in fraction B prepared from Gulf catfish (Arius bilineatus, Val.) skin regulate calcium response and neutrophil extracellular traps (NETs) formation. Biomedicines 2024, 2, 1380. [Google Scholar] [CrossRef]

| Sl. No. | Growth Factors and Biologically Active Proteins in FB of A. bilineatus | Details of the ELISA Kit Used for the Assay |
|---|---|---|
| 1 | Human Stem Cell Factor (HSCF) [5,33,34] | HSCF ELISA kit, cat. # MBS4502370, MyBioSource, San Diego, CA, USA. |
| 2 | Rat Stem Cell Factor (RSCF) [5,33,34] | RSCF ELISA kit, cat. # MBS730168, MyBioSource, San Diego, CA, USA. |
| 3 | Human Macrophage Colony Stimulating Factor (HMCSF) [35,36,37] | HMCSF ELISA kit, cat. # MBS165036, MyBioSource, San Diego, CA, USA. |
| 4 | Rat Macrophage Colony Stimulating Factor 1 (RMCSF1) [35,36,37] | RMCSF ELISA kit, cat. # MBS008298, MyBioSource Inc., San Diego, CA, USA. |
| 5 | Human Vascular Endothelial Growth Factor (HVEGF) [38] | HVEGF ELISA kit, Fine Test, cat. # EH0327, Wuhan Fine Biotech, Wuhan China. |
| 6 | Rat Vascular Endothelial Growth Factor (RVEGF) [38] | RVEGF ELISA kit, cat. # EK0540, Boster Biological Technology, Pleasanton, CA 94566, USA. |
| 7 | Human Fibroblast Growth Factor 1 (HFGF-1) [39,40] | HFGF1 ELISA kit, catalog # MBS167170, MyBiosource, Inc., San Diego, CA, USA. |
| 8 | Rat Fibroblast Growth Factor 1 (RFGF-1) [39,40] | RFGF1 ELISA kit, catalog # MBS705077, MyBiosource, Inc., San Diego, CA, USA |
| 9 | Human Fibroblast Growth Factor 19 (HFGF-19) [40,41] | HFGF19 ELISA kit, catalog # MBS938699, MyBiosource, Inc., San Diego, CA, USA. |
| 10 | Rat Fibroblast Growth Factor 19 (RFGF-19) [40,41] | RFGF19 ELISA kit, catalog # MBS102206, MyBiosource, Inc., San Diego, CA, USA. |
| 11 | Human Fibroblast Growth Factor 21 (HFGF-21) [41,42,43] | HFGF21 ELISA kit, catalog # MBS2023437, MyBiosource, Inc., San Diego, CA, USA |
| 12 | Rat Fibroblast Growth Factor 21 (RFGF-21) [41,42,43] | RFGF21 ELISA kit, catalog # MBS2024083, MyBiosource, Inc., San Diego, CA, USA. |
| 13 | Human Neutrophil Activating Protein 2 (HNAP-2) [44,45] | HNAP-2 ELISA kit cat. # CSB-E04562h, Wuhan Huamei Biotech Co., Ltd., Wuhan, Hubei Province, China. |
| 14 | Rat Neutrophil Activating Protein 2 (RNAP-2) [44,45] | RNAP-2 ELISA kit, cat. # MBS760356, MyBioSource, Inc., San Diego, CA, USA. |
| 15 | Human Substance P (HSP) [46,47,48] | HSP ELISA kit, MyBiosource, Cat. # MBS 705162, San Diego, CA, USA. |
| 16 | Rat Substance P (RSP) [46,47,48] | RSP ELISA kit, MyBioSource cat. # MBS 703659, San Diego, CA, USA. |
| 17 | Human Insulin (HI) [49,50] | HI ELISA kit, My BioSource, cat. # MBS 761338, San Diego, CA, USA. |
| 18 | Rat Insulin (RI) [49,50] | RI ELISA kit, My BioSource, cat. # MBS 724709, San Diego, CA, USA. |
| 19 | Human Glucagon-like peptide 1 (HGLP-1) [51,52] | HGLP-1 ELISA kit, cat. # EH1053, Wuhan Fine Biotech Co., Ltd., East Lake High Tech Devt. Zone, Wuhan, China. |
| 20 | Rat Glucogon-like peptide 1 (RGLP-1) [51,52] | RGLP-1 ELISA kit, cat. # E08117r, Cusabio, Houston, USA. |
| 21 | Human Nerve Growth factor (HNGF) [53,54] | HNGF ELISA kit, cat. # MBS2021644, MyBioSource, Inc. San Diego, CA, USA. |
| 22 | Rat Nerve Growth Factor (RNGF) [53,54] | RNGF ELISA kit, cat. # MBS775043, MyBioSource, Inc. San Diego, CA, USA. |
| 23 | Human Interleukin 19 (HIL-19) [55] | HIL-19 ELISA kit, cat. # MBS 2019896, MyBioSource, San Diego, CA, USA. |
| 24 | Rat Interleukin 19 (RIL-19) [55] | RIL-19 ELISA kit, Fine test code: ER1589, Wuhan Fine Biotech Co. Ltd., Wuhan, Hubei, China. |
| 25 | Human Interleukin 37 (HIL-37) [56] | HIL-37 ELISA kit, cat. # MBS761932, MyBioSource, San Diego, CA, USA. |
| 26 | Rat Interleukin 37 (RIL-37) [56] | Rat IL-37 ELISA kit, cat. # EK720300, AFG Bioscience, 1818 Skokie Boulevard, Northbrook, IL 60062, USA. |
| 27 | Human Neurogenin 3 (HNGN-3) [57,58,59] | HNGN-3 ELISA kit, cat. # MBS 2140241, MyBioSource, San Diego, CA, USA. |
| 28 | Rat Neurogenin 3 (RNGN-3) [57,58,59] | RNGN-3 ELISA kit, cat. # MBS 2612446, MyBioSource, San Diego, CA, USA. |
| 29 | Human Transforming Growth Factor Beta 1 (HTGFβ1) [60] | HTGFβ1 ELISA kit, cat. # ORB 437486, Biorbyt LLC, 68 TW Alexander Drive, North Carolina, Durham, 27709, USA. |
| 30 | Rat Transforming Growth Factor Beta 1 (RTGFβ1) [60] | RTGFβ1, ELISA Kit, cat. # MBS 824788, MyBioSource, San Diego, CA, USA. |
| 31 | Human Hepatocyte Growth Factor (HHGF) [61] | HHGF ELISA kit, cat. # CSB-E04573h, Cusabio Wuhan Huamei Biotech Co., Ltd., Wuhan, Hubei Province 430206, P. R. China. |
| 32 | Rat Hepatocyte Growth Factor (RHGF) [61] | RHGF ELISA kit, cat. # CSB-E07346r, Cusabio, Wuhan Huamei Biotech Co., Ltd., Wuhan, Hubei Province 430206, P. R. China. |
| 33 | Human Crystallin Beta A1 (HCRYβA1) [62,63] | HCRYβA1 ELISA kit, cat. # MBS 7252187, MyBioSource, San Diego, CA, USA. |
| 34 | Mouse Crystallin Beta A1 (MCRYβA1) [62,63] | MCRYβA1 ELISA kit, cat. # MBS 2705771, MyBioSource, San Diego, CA, USA. |
| 35 | Human Bone Morphogenetic Protein 3 (HBMP-3) [64] | HBMP-3 ELISA Kit, cat. # MBS 943749, MyBioSource, San Diego, CA, USA. |
| 36 | Rat Bone Morphogenetic Protein 3 (RBMP-3) [64] | RBMP-3 ELISA kit, Fine Test, cat. # ER0769 Wuhan Fine Biotech Co., Ltd., Wuhan, 430074, Hubei, China. |
| 37 | Human Somatostatin (HSS) [65] | HSS ELISA kit, cat. # BSKH61286, Bioss Antibodies, Bioss Inc., Woburn, MA 01801 USA. |
| 38 | Rat Somatostatin (RSS) [65] | RSS ELISA kit, cat. # CSB-E08204r, Cusabio Innovation Centre, Houston, TX 77054, USA. |
| 39 | Human Interleukin 15 (HIL-15) [66] | HIL-15 ELISA kit, cat. # MBS 705189, MyBioSource, San Diego, CA, USA. |
| 40 | Rat Interleukin 15 (RIL-15) [66] | RIL-15 ELISA kit, cat. # MBS 701942, MyBioSource, San Diego, CA, USA. |
| 41 | Human Regenerating Islet Derived Protein 3 Alpha (HREG-3α) [67] | HREG-3α ELISA Kit, cat. # MBS 937542, MyBioSource, San Diego, CA, USA. |
| 42 | Rat Regenerating Islet Derived Protein 3 Alpha (RREG-3α) [67] | RREG-3α ELISA kit, cat. # RTEB0672, AssayGenie, 25 Windsor Place, Dublin 2, Ireland, D02 VY42. |
| 43 | Human Gamma Amino Butyric Acid (HGABA) [68] | HGABA ELISA kit, cat. # EH3098, Fine Test, Wuhan Fine Biotech Co., Ltd., Wuhan, 430074, Hubei, China. |
| 44 | Rat Gamma Amino Butyric Acid (RGABA) [68] | RGABA ELISA kit, cat. # ER1707, Fine Test, Wuhan Fine Biotech Co., Ltd., Wuhan, 430074, Hubei, China. |
| 45 | Human Interferon Alpha (HIFN-α) [69] | HIFN-α ELISA Kit, cat. # MBS 702357, MyBioSource, San Diego, CA, USA. |
| 46 | Rat Interferon Alpha (RIFN-α) [69] | RIFN-α ELISA Kit, cat. # MBS 2022005, MyBioSource, San Diego, CA, USA. |
| 47 | Human Interferon Beta (HIFN-β) [70] | HIFN-β ELISA Kit, cat. # MBS-455331, MyBioSource, San Diego, CA, USA. |
| 48 | Rat Interferon Beta (RIFN-β) [70] | RIFN-β ELISA Kit, cat. # MBS 4500062, MyBioSource, San Diego, CA, USA. |
| 49 | Human Stem Cell Factor/Mast Cell Growth Factor (HSCF/MGF) [71] | HSCF/MGF ELISA Kit, cat. # MBS 161108, MyBioSource, San Diego, CA, USA. |
| 50 | Rat Stem Cell Factor/Mast Cell Growth Factor (RSCF/MGF) [71] | RSCF/MGF ELISA Kit, cat. # MBS 9315966, MyBioSource, San Diego, CA, USA. |
| 51 | Human Insulin-like Growth Factor 1 (HIGF-1) [72] | HIGF-1 ELISA Kit, cat. # MBS 014429, MyBioSource, San Diego, CA, USA. |
| 52 | Rat Insulin-like Growth Factor 1 Receptor (RIGF-1R) [72] | RIGF-1R ELISA kit, cat. # MBS 062253, MyBioSource, San Diego, CA, USA. |
| 53 | Human Prolactin (HPRL) [73] | HPRL ELISA kit, cat. # EH0259, Fine Test, Wuhan Fine Biotech Co., Ltd., Wuhan, 430074, Hubei, China. |
| 54 | Rat Prolactin (RPRL) [73] | RPRL ELISA kit, cat. # ER0076, Fine Test, Wuhan Fine Biotech Co., Ltd., Wuhan, 430074, Hubei, China. |
| 55 | Human Sirtuin 1 (HSIRT1) [74,75] | HSIRT 1 ELISA kit, cat. # MBS 2601311, MyBioSource, San Diego, CA, USA. |
| 56 | Rat Sirtuin 1 (RSIRT1) [74,75] | RSIRT 1 ELISA kit, cat. # MBS 4502504, MyBioSource, San Diego, CA. |
| 57 | Human Sirtuin 2 (HSIRT2) [76] | HSIRT2 ELISA kit, cat. # MBS161729, MyBioSource, San Diego, CA, USA. |
| 58 | Rat Sirtuin 2 (RSIRT2) [76] | RSIRT2 ELISA kit, cat. # MBS079814, MyBioSource, San Diego, CA, USA. |
| 59 | Human Sirtuin 3 (HSIRT3) [77] | HSIRT3 ELISA kit, cat. # MBS 163385, MyBioSource, San Diego, CA, USA. |
| 60 | Rat Sirtuin 3 (RSIRT3) [77] | RSIRT 3 ELISA kit, cat. # MBS 047755, MyBioSource, San Diego, CA, USA. |
| 61 | Human Sirtuin 4 (HSIRT4) [78] | HSIRT4 ELISA kit, cat. # MBS 452110, MyBioSource, San Diego, CA, USA. |
| 62 | Rat Sirtuin 4 (RSIRT4) [78] | RSIRT4 ELISA kit, cat. # MBS 8807375, MyBioSource, San Diego, CA. |
| 63 | Human Sirtuin 5 (HSIRT5) [79] | HSIRT5 ELISA kit, cat. # MBS 2705671, MyBioSource, San Diego, CA, USA. |
| 64 | Rat Sirtuin 5 (RSIRT5) [79] | RSIRT5 ELISA Kit, cat. # MBS 8807376, MyBioSource, San Diego, CA, USA. |
| 65 | Human Sirtuin 6 (HSIRT6) [80] | HSIRT6 ELISA kit, cat. # MBS 162109, MyBioSource, San Diego, CA, USA. |
| 66 | Rat Sirtuin 6 (RSIRT6) [80] | RSIRT6 ELISA kit, cat. # MBS 063651, MyBioSource San Diego, CA, USA. |
| 67 | Human Sirtuin 7 (HSIRT7) [81] | HSIRT7 ELISA kit, cat. # MBS 2021722, MyBioSource, San Diego, CA, USA. |
| 68 | Rat Sirtuin 7 (RSIRT7) [81] | RSIRT7 ELISA kit, cat. # MBS 2705675, MyBioSource, San Diego, CA, USA. |
| 69 | Human Growth Hormone (HGH) [82] | HGH ELISA kit, cat. # EH0152, Fine Test, Wuhan Fine Biotech Co., Ltd., Wuhan, 430074, Hubei, China. |
| 70 | Rat Growth Hormone (RGH) [82] | RGH ELISA kit cat. # SEA044Ra, Cloud-Clone, 23603 W. Fernhurst Dr., Unit 2201, Katy, TX 77494, USA. |
| 71 | Human Interleukin 11 [83] | HIL-11 ELISA kit cat. # MBS2021858, MyBioSource, San Diego, CA, USA. |
| 72 | Rat Interleukin 11 [83] | RIL-11 ELISA kit cat. # MBS2887148, MyBioSource, San Diego, CA, USA. |
| 73 | Rat Glucagon (RGCG) [84] | RGCG ELISA kit, cat. # MBS2887148, MyBioSource, San Diego, CA, USA. |
| 74 | Rat C-Peptide (RC-P) [85] | RC-P ELISA kit, cat. # MBS731074, MyBioSource, San Diego, CA, USA. |
| 75 | Human Epidermal Growth Factor (HEGF) [86] | HEGF ELISA kit, cat. # CSB-E08027h, Cusabio Technology LLC, Houston, Texas, USA. |
| 76 | Rat Epidermal Growth Factor (REGF) [86] | REGF ELISA kit, cat. # CSB-E08029r, Cusabio Technology LLC, Houston, TX, USA. |
| 77 | Human Erythropoietin (HEPO) [87] | HEPO ELISA kit, cat. # MBS2019738, MyBioSource, San Diego, CA, USA. |
| 78 | Rat Erythropoietin (REPO) [87] | REPO ELISA kit cat. # CSB-E07323r, CUSABIO, (CUBIO Innovation Center), Houston, TX 77054, USA. |
| 79 | Human Platelet-Derived Growth Factor (HPDGF) [88] | HPDGF ELISA kit cat. # MBS 021279, MyBioSource, San Diego, CA, USA. |
| 80 | Rat Platelet-Derived Growth Factor (RPDGF) [88] | RPDGF ELISA kit cat. # MBS008389, MyBioSource, San Diego, CA, USA. |
| 81 | Rat Connective Tissue Growth Factor (RCTGF) [89] | RCTGF ELISA kit cat. # CSB-E07876r, CUSABIO, CUBIO Innovation Center, Houston, TX 77054, USA. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hassan, J.M.; Renno, W.M.; Oommen, S.; Nair, D.; Paul, B.M.; Mathew, B.; Kumar, J.; Ummerkutty, A.; Pace-Asciak, C. Regeneration of Insulin-Producing β Cells, Reduction in Inflammation and Oxidation Stress, and Improvement in Lipid Profile in a Type 1 Diabetes Rat Model by Intraperitoneal Injection of the Growth Factors-Rich Catfish Skin-Derived Fraction-B: An Introductory Report. Biomolecules 2025, 15, 929. https://doi.org/10.3390/biom15070929
Al-Hassan JM, Renno WM, Oommen S, Nair D, Paul BM, Mathew B, Kumar J, Ummerkutty A, Pace-Asciak C. Regeneration of Insulin-Producing β Cells, Reduction in Inflammation and Oxidation Stress, and Improvement in Lipid Profile in a Type 1 Diabetes Rat Model by Intraperitoneal Injection of the Growth Factors-Rich Catfish Skin-Derived Fraction-B: An Introductory Report. Biomolecules. 2025; 15(7):929. https://doi.org/10.3390/biom15070929
Chicago/Turabian StyleAl-Hassan, Jassim M., Waleed M. Renno, Sosamma Oommen, Divya Nair, Bincy Maniyalil Paul, Bincy Mathew, Jijin Kumar, Afna Ummerkutty, and Cecil Pace-Asciak. 2025. "Regeneration of Insulin-Producing β Cells, Reduction in Inflammation and Oxidation Stress, and Improvement in Lipid Profile in a Type 1 Diabetes Rat Model by Intraperitoneal Injection of the Growth Factors-Rich Catfish Skin-Derived Fraction-B: An Introductory Report" Biomolecules 15, no. 7: 929. https://doi.org/10.3390/biom15070929
APA StyleAl-Hassan, J. M., Renno, W. M., Oommen, S., Nair, D., Paul, B. M., Mathew, B., Kumar, J., Ummerkutty, A., & Pace-Asciak, C. (2025). Regeneration of Insulin-Producing β Cells, Reduction in Inflammation and Oxidation Stress, and Improvement in Lipid Profile in a Type 1 Diabetes Rat Model by Intraperitoneal Injection of the Growth Factors-Rich Catfish Skin-Derived Fraction-B: An Introductory Report. Biomolecules, 15(7), 929. https://doi.org/10.3390/biom15070929








