Abstract
Large-animal models are playing a pivotal role in bridging the translational research gap. Positron emission tomography (PET) imaging is preferred in disease research involving large-animal models. Its ability to non-invasively monitor metabolic activity, receptor–ligand interactions, and pharmacokinetics in real time makes PET imaging an essential tool for evaluating therapeutic efficacy and advancing the development of targeted treatments. This review focuses on recent advancements in dedicated large-animal PET scanners, the utilization of large-animal models for simulating human diseases, and their applications in PET studies. It specifically highlights the critical role of PET imaging in facilitating the development of more effective and safer treatments for infections, chronic heart disease, diabetes, cancer, central nervous system disorders, and addiction, emphasizing its importance in the translational research landscape.
1. Introduction
The physiological and anatomical similarities between large animals and humans—particularly in organ size and complexity—make large animals invaluable for modeling human diseases, including complex conditions such as cardiovascular disorders, neurological pathologies, and oncological malignancies. For clinically oriented research, the validation of preliminary findings from rodent studies in large-animal models with high genetic homology to humans is essential [1].Common large-animal models, including pigs (Sus scrifa), dogs (Canis lupus), and non-human primates (NHPs, such as rhesus monkey [Macaca mulatta] and cynomolgus macaque [Macaca fascicularis]), are widely employed in translational research. Biomedical imaging in large animals provides non-invasive tools to longitudinally monitor disease progression and therapeutic efficacy. These imaging techniques enable researchers to accelerate the development of safer, more effective diagnostics and therapies for human applications.
Among imaging modalities, positron emission tomography (PET) is particularly prominent in large-animal disease research. Unlike optical imaging, which is commonly used in basic and translational studies in small animals [2], PET imaging distinguishes itself by providing precise quantitative insights into molecular, pharmacological, and metabolic processes at the tissue level. Following the introduction of the first PET scanner [3], researchers conducted experiments on canine subjects to visualize myocardial activity and intracardiac blood distribution. PET’s capacity to non-invasively monitor metabolic activity, receptor–ligand interactions, and pharmacokinetic behavior in real time has since established it as a critical tool for evaluating therapeutic efficacy and advancing targeted treatments. In 2001, a clinical PET scanner integrated with a 16-row computed tomography (CT) system was commercialized as a hybrid PET/CT platform. Subsequently, a small-animal PET scanner with simultaneous magnetic resonance imaging (MRI) capability emerged in 2007, designated as PET/MRI [4].
While conventional human PET scanners may partially meet the demand for dedicated systems in large-animal research, substantial opportunities remain for further improving this equipment. In recent years, a range of dedicated large-animal PET prototypes and commercial scanners with various configurations and architectural designs have been developed, manufactured, and evaluated. These systems bridge the gap between clinical scanners, which are inadequate for imaging specific anatomical structures of large animals, and conventional preclinical scanners with bore sizes too small to accommodate these species.
In this review, we examined the technical aspects of PET scanner design, introduced several state-of-the-art systems for large animals, and explored their applications in large-animal models, such as pigs and NHPs. Additionally, we elucidated tracer developments and methodological innovations that have solidified PET as a cornerstone of molecular imaging.
2. The PET Scanner for Large Animals
2.1. The Design of the PET Scanner
A traditional PET scanner consists of block detectors arranged in various configurations, typically arranged in circular or polygonal arrays [5]. Each block detector contains a scintillator and a photodetector. The scintillator is composed of crystals, such as bismuth germanate (BGO), lutetium oxyorthosilicate (LSO), lutetium yttrium orthosilicate (LYSO), and lutetium gadolinium oxyorthosilicate (LGSO), among others. These crystals are segmented into arrays of small elements and secured to photodetectors beneath them.
A block detector typically comprises a scintillator segmented into an array of elements with dimensions of approximately 4 mm × 4 mm × 20 mm for clinical systems and 1 mm × 1 mm × 10 mm for preclinical systems, which are read out using single-channel photodetectors [6]. An alternative design is the quadrant-sharing detector, where each scintillator block spans the corners of four photodetectors. This configuration reduces the number of photodetectors required to read out the scintillator elements by nearly 75%.
Photomultiplier tubes (PMTs) and silicon photomultipliers (SiPMs) are commonly used as photodetectors. PMTs were predominant in early PET scanners, whereas SiPMs are now the preferred choice for modern PET systems due to their compact size, magnetic insusceptibility (enabling use in hybrid PET/MRI systems), high photon detection efficiency, and low timing jitter [6].
The depth of interaction (DOI) measurement, which identifies the positron annihilation location within matter, further enables state-of-the-art PET systems, especially for those with a small axial field of view (FOV), like animal PET scanners. Thick detector elements in multi-coincidence-mode systems can degrade the spatial resolution, a phenomenon known as the DOI effect [7]. Scintillation crystals like LSO, LYSO, or gadolinium oxyorthosilicate (GSO) convert gamma-ray energy into optical signals. These signals are captured by PMTs or SiPMs, enabling DOI estimation via the analysis of optical signal arrival time differences across detector layers. DOI measurement is integral to modern PET detector design, as it enhances positron localization accuracy and overall image resolution. However, PET manufacturers vary in their design priorities; some emphasize DOI integration, while others focus on optimizing alternative performance metrics.
2.2. Dedicated Large-Animal PET
The transaxial FOV of clinical PET scanners is typically larger than 60 cm, providing high sensitivity and relatively low resolution, whereas small-animal PET scanners usually have transaxial FOVs smaller than 15 cm, offering low sensitivity and relatively high resolution [8,9,10,11,12,13,14]. Consequently, imaging large-animal models with these devices often fails to achieve a satisfactory balance between sensitivity and resolution, necessitating specialized designs tailored to specific species [15,16]. After comprehensive evaluation, a lateral FOV of 20–30 cm is widely accepted for large animals, particularly NHPs, as it balances high resolution and sensitivity for imaging needs [15]. Naidoo-Variawa et al. [17] employed the microPET Focus 220 small-animal scanner (Siemens Preclinical Solutions, Knoxville, TN, USA) to image baboon brains, observing resolution degradation and mismatches in scatter correction algorithms. Notably, its transaxial FOV range aligns with clinical dedicated brain PET systems. However, most major manufacturers have discontinued dedicated high-performance brain PET scanners, and clinical neuroPET studies now predominantly rely on general-purpose devices [18].
UC Davis, Siemens, and other institutions have developed PET/CT systems for large-animal imaging. To date, few dedicated large-animal PET systems exist commercially, with most designed for NHPs [15,16,18,19]. In 2018, the mini-EXPLORER I for NHPs featured a large FOV and PMT photodetectors, but systems for other large animals (e.g., pigs and sheep) remain scarce [20]. In 2019, China’s Institute of High Energy Physics introduced the Eplus-260 primate PET system, utilizing a cerium-doped crystal array and Hamamatsu Photonics (Shizuoka, Japan) photomultiplier tubes [16]. Mediso Ltd. (Budapest, Hungary) and UC Davis later launched high-resolution systems, such as the LFER 150 PET/CT and mini-EXPLORER II, employing LYSO crystals and SiPM photodetectors for veterinary and human brain imaging [15,18]. The LFER 150 is specifically designed for NHP brain imaging, as 3 [15]. In 2022, Inviscan SAS (Strasbourg, France) released the IRIS XL-220 PET/CT, a system tailored for NHP imaging with multi-anode PMTs [19]. For larger animals like pigs, researchers often adapt clinical PET scanners due to their size.
A detailed comparison of these systems alongside other small-animal and clinical PET scanners is provided in Figure 1, indicating their volumetric resolutions and sensitivity against the axial FOV (AFOV). Additional specifications are also provided in Table 1. Representative imaging results from the mini-EXPLORER I are shown in Figure 2.
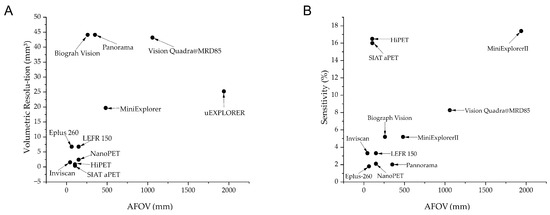
Figure 1.
Comparison between different systems. (A) Differences in volumetric resolution of different systems; (B) differences in sensitivity of different systems. Volumetric resolution refers to the ability of an imaging system to distinguish between two or more objects in three-dimensional space. It is a measure of how well the system can resolve details in all three dimensions: radial (r), tangential (t), and axial (z). Volumetric Resolution (mm3) = (Radial × Tangential × Axial).

Table 1.
The performance of representative small-animal and large-animal and clinical PET scanners.
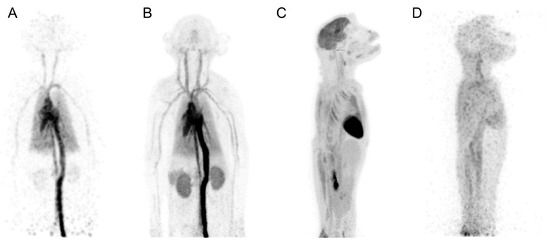
Figure 2.
Maximum-intensity-projection images from [18F]FDG rhesus monkey study: 1 s frame at 5 s after injection (A), 0–30 s after injection (B), 55–60 min after injection (C), and 18 h after injection (40 min scan) (D). This research was originally published in JNM. Eric Berg et al. Development and Evaluation of mini-EXPLORER: A Long Axial Field-of-View PET Scanner for Non-human Primate Imaging. J Nucl Med. June 2018, 59(6), 993–998. © SNMMI.
3. Large-Animal Model
3.1. Pig Model
Pigs have emerged as a highly relevant animal model across biomedical research, owing to their organ size and weight similarity to humans. Porcine tissues and fluids can be sampled repeatedly with minimal discomfort [21]. The integration of efficient cloning and transgenic technologies, coupled with stable cell lines, position the pigs as an excellent model for scientific research. After nearly half a century of specialized reproduction, large-scale breeding has become feasible, enabling rapid growth cycles and accessibility at a relatively low cost. The versatility of pigs in research is underscored by their widespread applications in studying human developmental processes, congenital diseases, pathogen response mechanisms, xenotransplantation, and vaccine/drug development [22].
In PET imaging, two prominent swine models, the Yucatan pig and the Göttingen pig, are widely adopted, emphasizing the critical role of porcine models in studying complex biological processes. While large-size pig breeds such as Landrace or Yorkshire pose handling challenges during medical procedures, smaller-size models reduce feeding and maintenance costs [21].
The Yucatan pig, or Yucatan miniature pig (YMP), was domesticated from the Yucatan Peninsula of Mexico. YMPs reach an adult body weight of 70–90 kg, aligning with human radiation dose requirements for PET imaging. YMPs are characterized by their gentle temperament, intelligence, disease resistance, and minimal odor [23]. They also exhibit metabolic and pharmacokinetic properties closely resembling humans [24,25], critical for evaluating radiolabeled tracer distribution, metabolism, and excretion in PET.
The Göttingen pig, also known as the Göttingen Minipig (GMP), developed in the 1960s at the University of Göttingen, reaches 12–16 kg at 6 months and 20–27 kg at 12 months. Despite their smaller size, GMPs retain body proportions comparable to standard pigs [26]. Genetic isolation through inbreeding ensures homogeneity and reduced experimental variability [27]. Similar to YMPs, GMPs share human-like physiological, metabolic, and pharmacokinetic traits [28,29], enhancing their utility in PET studies. Both breeds are easily bred, sensitive to environmental conditions, and fed 1–2 times daily. Sows re-enter estrus within 7–10 days post-weaning, enabling rapid reproductive cycles.
Obesity induction in pigs via high-energy diets provides a valuable method for metabolic studies. To model type 1 diabetes, β-cell dysfunction is induced using streptozotocin (STZ), generating hyperglycemic phenotypes [30]. STZ-induced diabetic pigs exhibit metabolic changes in carbohydrate, fat, and amino acid metabolism, mirroring those in humans with untreated diabetes [31]. The porcine pancreas and islets also share functional similarities with humans [32]. In contrast, rodent models fail to fully replicate phenotypes such as insulin resistance, obesity, and hypercholesterolemia, which contribute to glucose intolerance and type 2 diabetes [33]. Thus, STZ-induced and diet-induced diabetic pigs serve as robust large-animal models for diabetes research.
As animal models approach human body or heart weight, differences diminish, establishing pigs as a preferred model for chronic heart disease (CHD) studies [1,34]. Their coronary arterial anatomy and ischemic tolerance similarities to humans further suit CHD pathophysiological research [35]. However, inducing large myocardial infarction with global left ventricular dysfunction remains challenging, as pigs often experience fatal arrhythmias and low ischemia tolerance [36]. Genetically engineered pigs have demonstrated their potential in elucidating human disease mechanisms (Table 2), solidifying PET imaging as a key tool for dissecting disease pathways.

Table 2.
Genetically engineered pig models.
3.2. Non-Human Primate Model
The NHP model is indispensable in human disease research due to its genetic similarity to humans and comparable drug metabolism and pharmacokinetics [15,42]. NHPs also exhibit physiological congruence with humans in organ structure, immune responses, metabolism, and neurobiology. Their advanced cognitive abilities and complex behaviors further distinguish them as ideal models for studying neurological, cognitive, and behavioral disorders. Consequently, NHPs are widely used in infectious diseases, central nervous system (CNS) diseases, and substance abuse research [15,42]. For instance, in tuberculosis studies, NHPs replicate the full spectrum of human Mycobacterium tuberculosis (Mtb) infection, including latent tuberculosis—a feature that is absent in mouse models, which fail to form human-like granulomas [43,44,45].
While small-animal models dominate CNS research, their limitations are obvious. For example, rodents exhibit higher absolute expression of the drug efflux transporter P-glycoprotein in the brain compared to humans, pigs, and NHPs, limiting their relevance for certain CNS studies [46]. PET studies confirm divergent P-glycoprotein activity across species [47,48]. Additionally, primate Betz/extratelencephalic neurons display unique gene expression and electrophysiological features—such as pauses, bursting, and spike-frequency acceleration—observed in NHPs but absent in rodents [49]. In a Parkinson’s disease (PD) study, 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced animal models are common, yet they fail to replicate human PD hallmarks, like Lewy bodies [50]. MPTP-induced mouse models exhibit strain- and age-dependent responses to MPTP, with acute injury and rapid recovery complicating long-term therapeutic evaluation [51]. In contrast, MPTP-induced NHP models recapitulate both motor and non-motor PD symptoms, solidifying their superiority for PD studies [52].
The genetic overlap between humans and NHPs has spurred widespread use of genetically engineered NHP models and gene therapies (Table 3), particularly in CNS research. Alongside genetic tools, toxin-induced models are employed to study CNS disorders [53]. NHPs also remain irreplaceable in infectious disease research (Table 4), enabling the investigation of single- and multi-pathogen interactions [54].

Table 3.
Genetically engineered NHP models.
Beyond infectious diseases, the cognitive capacities and complex behaviors of NHPs provide a critical platform for studying drug effects on the brain and substance abuse mechanisms. Although rodent models are widely used in addiction research, key physiological and behavioral differences persist between rodents and primates. For example, opioid receptor expression and localization in the rodent CNS differ significantly from primates, with δ-opioid binding sites in the rodent spinal cord being far more extensive than in primates. This makes it likely that the pharmacological effects of such drugs are more numerous than in the primate spinal cord, especially in humans [59]. Furthermore, NHPs’ capacity for higher-order cognitive tasks offers unique insights into psychiatric and psychological impairments in human drug users [60].
To support these investigations, PET imaging serves as a pivotal tool. By capturing in vivo metabolic processes and integrating with MRI or CT, PET enables researchers to dissect the complex interactions of genetics, toxins, and drug effects on the NHP CNS (Table 4).

Table 4.
Infectious disease NHP models.
Table 4.
Infectious disease NHP models.
| Pathogen | Phenotype | Potential Applications and Significance |
|---|---|---|
| Simian immunodeficiency virus (SIV) [61] | High levels of virus replication associated with a high magnitude of cytokine/chemokine response | Difference between progressive and non-progressive disease courses |
| Simian–human immunodeficiency virus (SHIV) [62] | Lower viral burden and viral control during cART; exhibited less peripheral CD4 depletion and lower gut immune dysfunction and immune activation | SHIV macaque model may be better for identifying initial vaccine candidates |
| Sudan virus [63] | Early stage: viremia, granulocytosis, lymphopenia, albuminemia, thrombocytopenia, and decreased expression of HLA-class transcripts; mid-to-late stage: fever and petechial rashes, high levels of pro-inflammatory mediators and pro-thrombotic factors; end stage: shock and multi-organ failure | The development of vaccines and therapeutics |
| Mycobacterium tuberculosis (Mtb) [64] | Complement C1q increased after Mtb infection; C1q increased after Bacillus Calmette Guérin (BCG) vaccination | C1q can serve as a marker of progressive TB disease |
| Severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2) [65,66] | Increased levels of monocytes and chemokines; interstitial macrophages accumulate in the lungs | The immune events of the host response and viral replication and disease progression; therapeutic strategies |
| Both intranasal and intragastric inoculation with SARS-CoV-2 caused pneumonia and GI dysfunction | Inflammatory cytokines are possible connections for the pathogenesis of SARS-CoV-2 between the respiratory and digestive systems |
3.3. Sheep Model
Sheep are a widely used model in reproductive research due to their anatomical similarity to humans, including comparable fetus-to-pelvis ratios and spontaneous pelvic organ prolapse [67]. Their accessibility, affordability, and docility further enhance their practicality [68]. In CNS studies, sheep brains demonstrate high structural homology with humans [69]. Critically, their neurodegenerative processes recapitulate human pathology, making them invaluable for studying disorders like Huntington’s disease (HD) [70]. Similarities in spinal anatomy and cerebrospinal fluid (CSF) volume further reinforce their translational relevance [71]. Snell et al. [72,73] have extensively developed sheep CNS disease models, most notably the OVT73 HD model. This transgenic line, generated via DNA injection into zygotes, carries CAG repeats within the human disease-causing range—unlike rodent models with artificially elongated repeats [70,72,74]. OVT73 sheep live up to 10 years, enabling longitudinal studies that track HD progression and accelerate therapeutic development. When integrated with PET imaging, OVT73 provides a powerful platform for HD research.
4. Application
4.1. Infection
Respiratory infections have long been a major research focus. The coronavirus disease 2019 (COVID-19) pandemic has profoundly influenced PET research. During the initial stages of the pandemic, the limited understanding of SARS-CoV-2 necessitated animal models to study infection pathology and develop therapeutics and vaccines. In NHP models, infected NHPs exhibit varied COVID-19 progression, making them valuable for investigating infection pathology, therapeutics, and vaccines [75,76,77,78]. However, these findings were predominantly obtained through invasive methods, underscoring the critical need for non-invasive tools to visualize infection and treatment responses. PET imaging, with its high resolution and non-invasive nature, emerged as a complementary tool for monitoring NHPs. The advent of PET technology has revolutionized the ability to study respiratory infections, offering a powerful method to explore infection outcomes without invasive procedures (Figure 3). Using [18F]FDG PET imaging, researchers gain insights into the molecular-level onset, progression, and spread of COVID-19-induced inflammation in the lungs and nervous system [79,80].
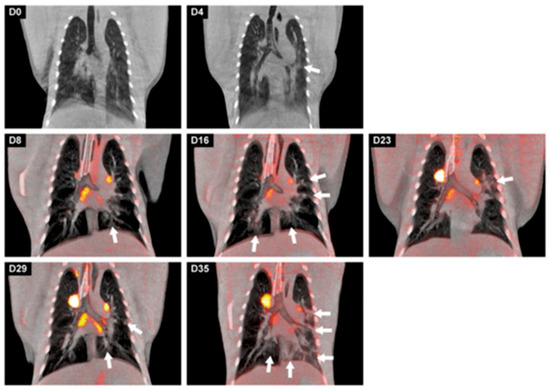
Figure 3.
Longitudinal development of both lung lesions and metabolic activity in tracheobronchial lymph nodes of a cynomolgus macaque over time after a SARS-CoV-2 infection. Representative coronal slices with a thickness of 3 mm of the cynomolgus macaque were used for visualization. On days 0 and 4, only CT images were obtained; afterwards, until day 35, CT was combined with PET (LFER 150). The location of the lesions, marked with arrows, differed almost per time point but are most prominently localized in the left lung on day 35. This research was originally published in Viruses. Böszörményi, K.P. et al., The Post-Acute Phase of SARS-CoV-2 Infection in Two Macaque Species Is Associated with Signs of Ongoing Virus Replication and Pathology in Pulmonary and Extrapulmonary Tissues. Viruses, 2021. 13(8).
Prior to COVID-19, the scientific community focused on Mtb, a respiratory disease that has afflicted humanity for millennia. Given its inflammatory pathogenesis, [18F]FDG is well suited for imaging tuberculosis. Decades of research have revealed intricate mechanisms underlying lymph node and granuloma dynamics [81,82,83], findings with significant implications for immunization strategies in vulnerable populations. By elucidating complex tuberculosis mechanisms, PET technology advances interventions against this persistent disease.
Human immunodeficiency virus (HIV) is a key focus in large-animal studies. While humanized mouse models allow for direct HIV infection and provide insights into antiretroviral therapy efficacy, they cannot fully recapitulate HIV-1 pathogenesis [82]. However, HIV is unable to infect NHPs in the wild, leading to the discovery of simian immunodeficiency virus (SIV) in Africa. Although SIV shares similarity with HIV-1, it lacks key structural features and is non-pathogenic in most natural hosts despite high viral replication levels [84]. Nevertheless, SIV offers insights into HIV mechanisms, such as significant enteric virome expansion linked to SIV-induced immunopathogenesis [85,86]. Additionally, simian–human immunodeficiency virus (SHIV), a chimeric virus combining SIV and HIV genetic elements, was developed to further study HIV.
In HIV vaccine research, both the virion and the subunit vaccine entities exhibit an extended biodistribution profile, and their in vivo distribution is more compatible with 64Cu [87,88]. Research on SIV and SHIV, combined with PET imaging, provides critical tools for studying HIV pathology, vaccines, therapies, and immune responses, accelerating progress toward prevention and treatment strategies. PET imaging in large animals facilitates vaccine development, evaluation of novel drug treatments, and testing of new tracers.
4.2. Chronic Heart Disease
PET imaging stands as an invaluable asset in the diagnosis and monitoring of CHD, providing a comprehensive evaluation of cardiac function. Central to this application is myocardial perfusion imaging (MPI) [89,90], which quantifies relative differences in myocardial blood flow distribution under rest and stress conditions (induced via exercise or pharmacological agents). Myocardial arterioles distal to significant coronary stenosis dilate via autoregulation to maintain resting blood flow. During stress, normal vascular beds undergo significant vasodilation, whereas stenotic regions exhibit limited dilation, resulting in perfusion disparities that manifest as "defects" in MPI. Large-animal studies commonly model CHD through artificially induced coronary artery occlusion [90].
MPI primarily assesses coronary artery disease by evaluating myocardial blood flow. Key radiotracers include [15O]H2O, a metabolically inert, freely diffusible tracer for myocardial viability assessment [91,92,93], and [13N]NH3, whose uptake directly reflects blood flow [90,94]. In large-animal studies, MPI is often integrated with [18F]FDG to simultaneously assess tissue viability [36]. Researchers have also tested novel tracers in a permanent occlusion model and occlusion–reperfusion model, demonstrating efficacy in these systems [90,94,95]. Collectively, PET imaging has significantly advanced CHD research, providing critical insights to guide therapeutic innovation and refine clinical management.
4.3. Diabetes
The loss of functional β-cell mass (BCM) is a hallmark feature of both type 1 and type 2 diabetes (T1D and T2D). The current understanding of progressive BCM changes during diabetes pathogenesis relies on post-mortem biopsies [96]. Notably, BCM declines before T2D onset, and emerging evidence suggests that impaired glucose tolerance—a precursor to T2D—is linked to reduced BCM [97,98,99]. Hypotheses proposed that T2D progression involves reduced β-cell regeneration or accelerated β-cell loss [100]. PET imaging offers a non-invasive method to visualize BCM, allowing for high-resolution quantification of pancreatic β-cells in diabetes research [96].
Beyond BCM assessment, glucagon-like peptide-1 (GLP-1) has emerged as a critical focus in diabetes studies. GLP-1 is produced in the gut, brainstem, and endocrine pancreas, exerting energy balance regulation via the GLP-1 receptor (GLP-1R). Its primary physiological role is insulinotropic action, accompanied by the suppression of glucagon release, mediated through somatostatin from pancreatic δ cells. GLP-1 also exhibits glucose-lowering properties, including CNS-mediated satiety, blood pressure reduction, and modulation of postprandial lipid metabolism [99]. Advances in GLP-1R-targeted tracers have enabled PET imaging to elucidate the signaling dynamics of GLP-1, offering insights into BCM and therapeutic development [32,101]. Collectively, PET imaging holds revolutionary potential for diabetes research, linking mechanistic understanding with innovative diagnostic and therapeutic strategies.
4.4. Cancer
PET imaging is a tool in cancer research involving large animals, offering detailed insights into biochemical processes within tumors. This capability significantly enhances cancer diagnosis, staging, monitoring, and treatment. The radiotracer [18F]FDG, widely used in species (including humans, large animals, and small animals), capitalizes on elevated glucose uptake driven by rapid tumor cell proliferation. Similarly, [68Ga]Ga-DOTATATE, targeting somatostatin receptor type 2 overexpression, is a cornerstone in neuroendocrine tumor diagnosis and management [102]. Currently, researchers focus on developing new radiotracers and testing them in large-animal models [103,104,105,106]. Although the use of large-animal models in oncology has declined in recent years due to advances in humanized mice, which offer a cost-effective platform for studying tumor biology, including the human immune microenvironment [107], these models retain relevance in translational medicine, particularly in drug development, owing to their anatomical and physiological similarity to humans [108,109]. Moreover, large-animal models play a pivotal role in evaluating cancer therapeutics, as PET imaging enables real-time monitoring of treatment progression and efficacy, accelerating the development of innovative diagnostic and therapeutic strategies [110].
4.5. Central Nervous System Disease
4.5.1. α-Synucleinopathies
PET imaging has proven instrumental in investigating α-synuclein (α-syn) pathologies, including PD and Lewy body disease (LBD) [111]. These disorders are characterized by the misfolding and abnormal aggregation of pathological α-syn in neurons, eventually forming insoluble α-syn inclusion bodies (the primary component of Lewy bodies) [112]. These aggregates propagate across the central and peripheral nervous systems, causing diverse motor and non-motor symptoms. Diagnosing α-synucleinopathies remains challenging, relying on clinical history, signs, and symptoms; accurate diagnosis necessitates multidisciplinary collaboration [111]. The radiotracer [18F]F-dihydroxyphenylalanine ([18F]F-DOPA), a marker of monoaminergic nerve terminal function, has been extensively used to evaluate the severity and progression of presynaptic nigrostriatal dysfunction in PD, as striatal [18F]F-DOPA uptake reflects aromatic amino acid decarboxylase activity [113]. Similarly, [18F]FDG has revealed distinct metabolic declines in glucose utilization in NHP brains affected by α-synucleinopathies [114,115]. MPTP-induced PD models in large animals have further delineated presymptomatic and symptomatic phases of nigrostriatal neurodegeneration [53,116,117]. Together, PET imaging provides an indispensable foundation for elucidating α-synucleinopathy pathophysiology and advancing therapeutic strategies.
4.5.2. Alzheimer’s Disease
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline, memory impairment, and the loss of functional independence. Despite extensive research, its pathogenesis remains incompletely understood. The predominant hypothesis posits that dysregulated amyloid-β (Aβ) production and clearance triggers neuronal degeneration and cognitive decline [118]. Aβ plaques disrupt interneuronal communication and contribute to neurodegeneration. Another key pathological feature involves neurofibrillary tangles—tau (τ) protein aggregates within neurons—which directly impair neuronal microtubular transport [119]. To unravel AD pathology, researchers have advanced PET imaging techniques. The 11C-labeled Pittsburgh compound B ([11C] PIB), a radiotracer specifically designed to bind to β-amyloid plaques, enables detection of early AD pathological processes and subsequent neurodegeneration [120,121]. In the field of AD research in large animals, a current prominent research direction involves exploring novel radiotracers in large-animal models to detect Aβ deposition and abnormal τ protein aggregation and facilitate new drug development, particularly in NHP models [122,123,124,125,126]. These advancements underscore PET’s pivotal role in deciphering AD mechanisms and accelerating therapeutic and diagnostic innovations. By leveraging these novel radiotracers, studies in NHPs have refined our understanding of AD pathology, paving the way to targeted interventions.
4.5.3. Huntington’s Disease
HD is a rare inherited neurological disorder characterized by progressive motor dysfunction, cognitive decline, and emotional dysregulation. The disorder stems from a mutation in the huntingtin (HTT) gene, involving an abnormal expansion of cytosine–adenine–guanine (CAG) repeats in exon 1, which leads to the production of toxic mutant HTT protein [127]. This aberrant protein accumulates as neuronal aggregates, disrupting cellular homeostasis and inducing neurodegeneration, particularly within the cerebral cortex and striatum, driving progressive motor and cognitive deterioration.
Current diagnostic frameworks lack standardized criteria for diagnosing HD prior to clinical onset, relying primarily on motor symptom assessment [128]. Williams et al. [129] demonstrated the feasibility of PET imaging in HD sheep using [18F]FDG and [18F]FDOPA to map cerebral glucose metabolism and dopaminergic activity, establishing a translational tool for preclinical research (Figure 4). NHPs are similarly important models, with novel radiotracers developed to probe HD pathophysiology [130,131,132,133]. In summary, preclinical large-animal studies are rapidly advancing, offering transformative insights for clinical diagnostics and therapeutics.
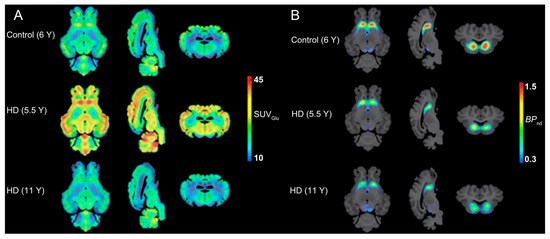
Figure 4.
PET examination on HD sheep. (A) Average glucose uptake images showing the coronal, sagittal, and axial views in each group (n = 3/group). The averaged PET images are overlaid onto the MRI template for anatomical reference. (B) Imaging dopamine metabolism using [18F]FDOPA. Tracer uptake assessment and averaged [18F]FDOPA parametric maps for control, 5.5 Y, and 11 Y HD sheep (n = 3/group) are overlaid onto the T1w MRI template for anatomical reference. Reprinted (adapted) with permission from Williams, G. K., Akkermans, J., Lawson, M., Syta, P., Staelens, S., Adhikari, M. H., Morton, A. J., Nitzsche, B., Boltze, J., Christou, C., Bertoglio, D., & Ahamed, M. (2024). Imaging Glucose Metabolism and Dopaminergic Dysfunction in Sheep (Ovis aries) Brain Using Positron Emission Tomography Imaging Reveals Abnormalities in OVT73 Huntington’s Disease Sheep. ACS chemical neuroscience, 15(21), 4082–4091. https://doi.org/10.1021/acschemneuro.4c00561. Copyright 2025 American Chemical Society.
4.5.4. Psychiatric Disorders
In neuroscience, PET has emerged as an indispensable tool, not only in investigating α-synucleinopathies, AD, and HD but also in exploring psychiatric disorders, such as schizophrenia, stress-related disorders, anxiety, and depression. These conditions, each with distinct pathogenic mechanisms, pose significant challenges to developing effective diagnostics and therapies. Smucny et al. [134] investigated the link between maternal immune activation during pregnancy and schizophrenia risk in offspring, employing PET with the radiotracer [18F]fluoro-l-m-tyrosine (an L-DOPA analog) in rhesus monkeys. Their findings revealed that maternal immune activation increases susceptibility to schizophrenia-related outcomes. Wokeford et al. [135] explored the influence of gender and social status on the efficacy of serotonergic drugs using the radiotracer [18F]MPPF (4-(2′-methoxyphenyl)-1-[2′-(N-2″-pyridinyl)-p-fluorobenzamido]ethylpiperazine). They demonstrated that these factors significantly modulate treatment responses. Furthermore, PET studies in NHPs have uncovered associations between childhood anxious temperament and increased risks for anxiety, depression, and substance abuse, providing mechanistic insights for refining interventions [136,137,138]. Together, PET imaging in NHPs provides a versatile platform to elucidate the neurobiological underpinnings of psychiatric disorders. These advances highlight PET’s ability to illuminate pathogenic pathways and accelerate translational breakthroughs in neuroscience.
4.6. Addiction
Addiction refers to a chronic, recurring disorder characterized by an inability to control the pursuit, continued use, or compulsive behaviors despite harmful consequences. It is typically associated with physical or psychological dependence on a substance or behavior. Ethical constraints governing human addiction studies necessitate reliance on observational methodologies that limit systematic control of variables, particularly for invasive experimental protocols and genetic heterogeneity among subjects. Animal models, despite their inability to replicate the psychosocial complexities of substance abuse, provide critical experimental control over pharmacokinetic parameters and enable longitudinal monitoring of neurocognitive adaptations through controlled dosing regimens [139]. These models have proven invaluable for elucidating neurobiological mechanisms, such as reward-related learning. Central to this pathology is dysregulation of the mesolimbic dopamine system, which includes dopaminergic projections from the ventral tegmental area to the ventral striatum (nucleus accumbens) and cortical regions [140].
PET studies have consistently shown that long-term cocaine use on NHPs is associated with reduced dopamine D2/D3 receptor (D2/D3R) availability [141,142]. A study conducted by Allen et al. [143] on female cynomolgus monkeys extended the previous research findings based on male monkeys to females, indicating gender differences in the availability of D2/D3R related to vulnerability and long-term cocaine use. The study utilized [11C]raclopride (a D2R selective antagonist) to quantify dopamine D2R availability during addictive drug exposure and [18F]FECNT (a novel tracer targeting the dopamine transporter) to assess dopamine transporter availability [143]. Furthermore, studies using [11C]raclopride and its analog [18F]fluoroclebopride on NHPs have demonstrated nicotine-induced dopamine release in reward-related circuits [144,145]. Together, these findings underscore PET imaging’s unique capacity to unravel neurochemical dynamics in addiction, from substance-induced receptor adaptation to withdrawal pathophysiology. Such insights are pivotal for advancing targeted therapeutic strategies.
In summary, a summary of radiotracers used in large-animal models is listed in Table 5.

Table 5.
Summary of radiotracers used in large-animal models.
Table 5.
Summary of radiotracers used in large-animal models.
| Tracer | Application | Mechanism | Reference |
|---|---|---|---|
| [11C]acetate | Myocardial perfusion imaging (MPI) | [11C]acetate is rapidly metabolized in cardiomyocytes to acetyl-coenzyme A; acetyl-CoA is a key intermediate in fatty acid oxidation and glucose metabolism in cardiomyocytes | [93] |
| [11C]dihydrotetrabenazine ([11C]DTBZ) | Parkinson’s disease (PD) | [11C]DTBZ binds to the VMAT2 of synaptic vesicles in monoaminergic neurons, which reflects the density and functional state of VMAT2 | [116,117] |
| 11C-labeled Pittsburgh compound B ([11C]PIB) | Alzheimer’s disease (AD) | [11C]PIB is specifically designed to bind to β-amyloid plaques in the brain | [120,121] |
| [11C]raclopride | Addiction | D2R sensitivity to reward decreases in addiction; [11C]raclopride can Quantify dopamine D2R availability in the brain | [141,144] |
| [11C]AZ12204657 and [11C]MK-7246 | Diabetes | Target to GPR44 to β-cell imaging | [96] |
| [N-methyl-11C]-cholylsarcosine ([11C]CSar) | Cancer | [11C]CSar can assess bile acid excretion in the liver | [110] |
| [11C]MPC-6827 | AD | MPC-6827 is a microtubule-targeting agent that binds to tubulin sites with high affinity | [122] |
| [11C]GSK215083 | AD | [11C]GSK215083 is a selective 5-HT6 tracer | [124] |
| [11C]BIO-1819578 | AD | BIO-1819578 is an O-GlcNAcase PET ligand that is an enzyme associated with the development of τ | [126] |
| [11C]PHNO | Addiction (nicotine) | [11C]PHNO has a higher affinity for D3 vs. D2 DA receptors, which allows for regional interpretation of D3 and D2 receptors | [144] |
| [11C]CHDI-180R, [11C]CHDI-626 and [11C]CHDI-650 | Huntington disease (HD) | [11C]CHDI-180R, [11C]CHDI-626, and [11C]CHDI-650 are mHTT aggregate-specific PET ligands | [131,132,133] |
| [13N]NH3 | MPI | [13N]NH3 can be taken up by cardiomyocytes via an amino acid transport system, and its uptake is proportional to myocardial blood flow | [90,94] |
| [15O]H2O | MPI | [15O]H2O is a freely diffusible and metabolically inert tracer; can be used to establish myocardial blood flow | [91,92,93] |
| [18F]FDG | Cancer, tuberculosis, COVID-19 infection, and psychiatric disorders | Increased glucose metabolism in tumor tissue or inflammation | [79,81,82,83,136,138] |
| Myocardial viability imaging, AD, HD, and α-syn disease | Decreased glucose metabolism in damaged cardiomyocytes, AD brain, or α-syn disease brain | [36,114,115,116,129] | |
| [18F]F-DOPA | PD | Striatal uptake of [18F]F-DOPA reflects aromatic amino acid decarboxylase activity | [113] |
| [18F]Flurpiridaz | MPI | [18F]Flurpiridaz works by binding to mitochondrial complex 1 in the heart | [94,95] |
| [18F]KS1 | Cancer | [18F]KS1 is a fluoroethoxy furanose ring-containing ascorbate derivative, to track ROS in prostate tumor | [103] |
| [18F]TTDP | Cancer | Target to T cell immunoglobulin and ITIM domain | [105] |
| [18F]BMS-986229 | Cancer | A 18F-labeled macrocyclic peptide-based PET ligand for imaging PD-L1 | [106] |
| [18F]fluoro-2-deoxy-D-galactose ([18F]FDGal) | Cancer | [18F]FDGal can assess galactose metabolism | [110] |
| [18F]fluoro-l-m-tyrosine | Schizophrenia | L-m-tyrosine is an analogue of L-DOPA | [134] |
| [18F]1 | HD | An mHTT aggregate-specific PET ligand | [130] |
| [18F]3 | AD | A BACE1 PET ligand | [125] |
| [18F]92 | AD | A new tracer that is designed to combined Aβ | [123] |
| [18F]MPPF (4-(2′-methoxyphenyl)-1-[2′-(N-2′′-pyridinyl)-p-fluorobenzamido]ethylpiperazine) | Psychiatric disorders | A radioligand employed for imaging 5-HT1A receptors | [135] |
| [18F]FECNT | Addiction (cocaine) | Target to dopamine transporter | [141] |
| [18F]fluoroclebopride | Addiction (cocaine) | An 18F-labeled raclopride | [142] |
| [18F]Fallypride | Psychiatric disorders and addiction (nicotine) | A high-affinity dopamine D2/D3 receptor antagonist | [137,145] |
| 64Cu labeled to Photoactivatable-Green Fluorescent Protein-HIV-BaL | HIV infection | PET/CT and fluorescent microscopy dual-modal imaging | [87] |
| 64Cu labeled to vaccine-loaded nanoparticle | HIV infection | A combination of PET and fluorescence imaging | [88] |
| [68Ga]Ga-DOTATATE | Cancer | Based on somatostatin receptor (SSTR) type 2 | [102] |
| [68Ga]Ga-DO3A-VS-Cys(40)-Exendin-4 | Diabetes | Target to GLP1-R | [32,101] |
| [89Zr]hu5A10 | Cancer | A humanized uncomplexed and catalytically active prostate-specific antigen-targeting IgG1-mAb | [104] |
5. Conclusions
PET has evolved into an indispensable tool for investigating physiological and pathological processes in large animals. The integration of advanced PET technology, tailored disease models, and diverse applications establishes large-animal PET studies as pivotal bridges between preclinical research and clinical outcomes. Due to the closer physiological and anatomical resemblance of large-animal models to humans, molecular probes and drug mechanisms tested in these models exhibit higher predictive value, enhancing their utility in translational medicine. By labeling radioisotopes onto drug candidates, PET dynamically tracks their distribution in organs and lesions. PET further enables the evaluation of pharmacodynamic efficacy through metabolic monitoring in large animals. Future advancements in large-animal PET imaging will likely focus on developing dedicated systems with higher resolution, sensitivity, and specificity, or designing tri-modal/multimodal imaging platforms to acquire multidimensional data. As methodologies and insights expand, PET’s integration into large-animal studies promises to drive transformative breakthroughs in basic research and clinical medicine.
Author Contributions
Conceptualization, Z.D.; writing—original draft preparation, Z.D., P.X., D.Z. and Z.X.; writing—review and editing, X.M. and Q.R.; visualization, Z.D.; supervision, X.M. and Q.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the 2024 China Industrial Technology Infrastructure Public Service Platform Project (GN2024-31-4700).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Camacho, P.; Fan, H.; Liu, Z.; He, J.Q. Large Mammalian Animal Models of Heart Disease. J. Cardiovasc Dev. Dis. 2016, 3, 30. [Google Scholar] [CrossRef]
- Pirovano, G.; Roberts, S.; Kossatz, S.; Reiner, T. Optical Imaging Modalities: Principles and Applications in Preclinical Research and Clinical Settings. J. Nucl. Med. 2020, 61, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Ter-Pogossian, M.M.; Phelps, M.E.; Hoffman, E.J.; Mullani, N.A. A positron-emission transaxial tomograph for nuclear imaging (PETT). Radiology 1975, 114, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Catana, C.; Procissi, D.; Wu, Y.; Judenhofer, M.S.; Qi, J.; Pichler, B.J.; Jacobs, R.E.; Cherry, S.R. Simultaneous in vivo positron emission tomography and magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 3705–3710. [Google Scholar] [CrossRef] [PubMed]
- Zanzonico, P. Positron emission tomography: A review of basic principles, scanner design and performance, and current systems. Semin. Nucl. Med. 2004, 34, 87–111. [Google Scholar] [CrossRef]
- Berg, E.; Cherry, S.R. Innovations in Instrumentation for Positron Emission Tomography. Semin. Nucl. Med. 2018, 48, 311–331. [Google Scholar] [CrossRef]
- Cherry, S.R.; Sorenson, J.A.; Phelps, M.E. chapter 18-Positron Emission Tomography. In Physics in Nuclear Medicine, 4th ed.; Cherry, S.R., Sorenson, J.A., Phelps, M.E., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2012; pp. 307–343. [Google Scholar] [CrossRef]
- Li, G.; Ma, W.; Li, X.; Yang, W.; Quan, Z.; Ma, T.; Wang, J.; Wang, Y.; Kang, F.; Wang, J. Performance Evaluation of the uMI Panorama PET/CT System in Accordance with the National Electrical Manufacturers Association NU 2-2018 Standard. J. Nucl. Med. 2024, 65, 652–658. [Google Scholar] [CrossRef]
- van Sluis, J.; de Jong, J.; Schaar, J.; Noordzij, W.; van Snick, P.; Dierckx, R.; Borra, R.; Willemsen, A.; Boellaard, R. Performance Characteristics of the Digital Biograph Vision PET/CT System. J. Nucl. Med. 2019, 60, 1031–1036. [Google Scholar] [CrossRef]
- Prenosil, G.A.; Sari, H.; Fürstner, M.; Afshar-Oromieh, A.; Shi, K.; Rominger, A.; Hentschel, M. Performance Characteristics of the Biograph Vision Quadra PET/CT System with a Long Axial Field of View Using the NEMA NU 2-2018 Standard. J. Nucl. Med. 2022, 63, 476–484. [Google Scholar] [CrossRef]
- Spencer, B.A.; Berg, E.; Schmall, J.P.; Omidvari, N.; Leung, E.K.; Abdelhafez, Y.G.; Tang, S.; Deng, Z.; Dong, Y.; Lv, Y.; et al. Performance Evaluation of the uEXPLORER Total-Body PET/CT Scanner Based on NEMA NU 2-2018 with Additional Tests to Characterize PET Scanners with a Long Axial Field of View. J. Nucl. Med. 2021, 62, 861–870. [Google Scholar] [CrossRef]
- Nagy, K.; Tóth, M.; Major, P.; Patay, G.; Egri, G.; Häggkvist, J.; Varrone, A.; Farde, L.; Halldin, C.; Gulyás, B. Performance Evaluation of the Small-Animal nanoScan PET/MRI System. J. Nucl. Med. 2013, 54, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Taschereau, R.; Vu, N.T.; Prout, D.L.; Lee, J.; Chatziioannou, A.F. Performance evaluation of HiPET, a high sensitivity and high resolution preclinical PET tomograph. Phys. Med. Biol. 2020, 65, 045009. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.; Wang, X.; Ren, N.; Wu, S.; Gao, J.; Zeng, T.; Gao, D.; Zhang, C.; Sang, Z.; Hu, Z.; et al. Design and performance of SIAT aPET: A uniform high-resolution small animal PET scanner using dual-ended readout detectors. Phys. Med. Biol. 2020, 65, 235013. [Google Scholar] [CrossRef]
- Sarnyai, Z.; Nagy, K.; Patay, G.; Molnar, M.; Rosenqvist, G.; Toth, M.; Takano, A.; Gulyas, B.; Major, P.; Halldin, C.; et al. Performance Evaluation of a High-Resolution Nonhuman Primate PET/CT System. J. Nucl. Med. 2019, 60, 1818–1824. [Google Scholar] [CrossRef]
- Chai, P.; Feng, B.T.; Zhang, Z.M.; Tang, H.H.; Liu, S.Q.; Sun, X.L.; Wang, P.L.; Wang, X.M.; Zhao, X.D.; Wei, L. NEMA NU-4 performance evaluation of a non-human primate animal PET. Phys. Med. Biol. 2019, 64, 105018. [Google Scholar] [CrossRef]
- Naidoo-Variawa, S.; Hey-Cunningham, A.J.; Lehnert, W.; Kench, P.L.; Kassiou, M.; Banati, R.; Meikle, S.R. High-resolution imaging of the large non-human primate brain using microPET: A feasibility study. Phys. Med. Biol. 2007, 52, 6627–6638. [Google Scholar] [CrossRef]
- Lv, Y.; Lv, X.Y.; Liu, W.P.; Judenhofer, M.S.; Zwingenberger, A.; Wisner, E.; Berg, E.; McKenney, S.; Leung, E.; Spencer, B.A.; et al. Mini EXPLORER II: A prototype high-sensitivity PET/CT scanner for companion animal whole body and human brain scanning. Phys. Med. Biol. 2019, 64, 075004. [Google Scholar] [CrossRef] [PubMed]
- Boisson, F.; Serriere, S.; Cao, L.J.; Bodard, S.; Pilleri, A.; Thomas, L.; Sportelli, G.; Vercouillie, J.; Emond, P.; Tauber, C.; et al. Performance evaluation of the IRIS XL-220 PET/CT system, a new camera dedicated to non-human primates. Nucl. Med. Biol. 2022, 108, S173. [Google Scholar]
- Berg, E.; Zhang, X.Z.; Bec, J.; Judenhofer, M.S.; Patel, B.; Peng, Q.Y.; Kapusta, M.; Schmand, M.; Casey, M.E.; Tarantal, A.F.; et al. Development and Evaluation of mini-EXPLORER: A Long Axial Field-of-View PET Scanner for Nonhuman Primate Imaging. J. Nucl. Med. 2018, 59, 993–998. [Google Scholar] [CrossRef]
- Curtasu, M.V.; Knudsen, K.E.B.; Callesen, H.; Purup, S.; Stagsted, J.; Hedemann, M.S. Obesity Development in a Miniature Yucatan Pig Model: A Multi-compartmental Metabolomics Study on Cloned and Normal Pigs Fed Restricted or Ad Libitum High-Energy Diets. J. Proteome Res. 2019, 18, 30–47. [Google Scholar] [CrossRef]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Song, K.D.; Kim, H.J.; Park, W.; Kim, J.; Lee, T.; Shin, D.H.; Kwak, W.; Kwon, Y.J.; Sung, S.; et al. Exploring the genetic signature of body size in Yucatan miniature pig. PLoS ONE 2015, 10, e0121732. [Google Scholar] [CrossRef]
- Oberle, R.L.; Das, H.; Wong, S.L.; Chan, K.K.; Sawchuk, R.J. Pharmacokinetics and metabolism of diclofenac sodium in Yucatan miniature pigs. Pharm. Res. 1994, 11, 698–703. [Google Scholar] [CrossRef]
- Tang, H.; Mayersohn, M. Porcine Prediction of Pharmacokinetic Parameters in People: A Pig in a Poke? Drug Metab. Dispos. 2018, 46, 1712–1724. [Google Scholar] [CrossRef]
- Simianer, H.; Kohn, F. Genetic management of the Gottingen Minipig population. J. Pharmacol. Toxicol. Methods 2010, 62, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Reimer, C.; Ha, N.T.; Sharifi, A.R.; Geibel, J.; Mikkelsen, L.F.; Schlather, M.; Weigend, S.; Simianer, H. Assessing breed integrity of Gottingen Minipigs. BMC Genom. 2020, 21, 308. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 738. [Google Scholar] [CrossRef]
- Lignet, F.; Sherbetjian, E.; Kratochwil, N.; Jones, R.; Suenderhauf, C.; Otteneder, M.B.; Singer, T.; Parrott, N. Characterization of Pharmacokinetics in the Gottingen Minipig with Reference Human Drugs: An In Vitro and In Vivo Approach. Pharm. Res. 2016, 33, 2565–2579. [Google Scholar] [CrossRef]
- von Wilmowsky, C.; Stockmann, P.; Metzler, P.; Harsch, I.A.; Amann, K.; Schlegel, K.A. Establishment of a streptozotocin-induced diabetic domestic pig model and a systematic evaluation of pathological changes in the hard and soft tissue over a 12-month period. Clin. Oral. Implant. Res. 2010, 21, 709–717. [Google Scholar] [CrossRef]
- Jensen-Waern, M.; Andersson, M.; Kruse, R.; Nilsson, B.; Larsson, R.; Korsgren, O.; Essén-Gustavsson, B. Effects of streptozotocin-induced diabetes in domestic pigs with focus on the amino acid metabolism. Lab. Anim. 2009, 43, 249–254. [Google Scholar] [CrossRef]
- Nalin, L.; Selvaraju, R.K.; Velikyan, I.; Berglund, M.; Andreasson, S.; Wikstrand, A.; Ryden, A.; Lubberink, M.; Kandeel, F.; Nyman, G.; et al. Positron emission tomography imaging of the glucagon-like peptide-1 receptor in healthy and streptozotocin-induced diabetic pigs. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1800–1810. [Google Scholar] [CrossRef]
- Bellinger, D.A.; Merricks, E.P.; Nichols, T.C. Swine models of type 2 diabetes mellitus: Insulin resistance, glucose tolerance, and cardiovascular complications. Ilar J. 2006, 47, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Milani-Nejad, N.; Janssen, P.M. Small and large animal models in cardiac contraction research: Advantages and disadvantages. Pharmacol. Ther. 2014, 141, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Millard, R.W. Induction of functional coronary collaterals in the swine heart. Basic. Res. Cardiol. 1981, 76, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, N.; Koshino, K.; Yokoyama, I.; Miyagawa, S.; Zeniya, T.; Hirano, Y.; Fukuda, H.; Enmi, J.; Sawa, Y.; Knuuti, J.; et al. Experimental pig model of old myocardial infarction with long survival leading to chronic left ventricular dysfunction and remodeling as evaluated by PET. J. Nucl. Med. 2011, 52, 761–768. [Google Scholar] [CrossRef]
- Renner, S.; Fehlings, C.; Herbach, N.; Hofmann, A.; von Waldthausen, D.C.; Kessler, B.; Ulrichs, K.; Chodnevskaja, I.; Moskalenko, V.; Amselgruber, W.; et al. Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function. Diabetes 2010, 59, 1228–1238. [Google Scholar] [CrossRef]
- Renner, S.; Braun-Reichhart, C.; Blutke, A.; Herbach, N.; Emrich, D.; Streckel, E.; Wunsch, A.; Kessler, B.; Kurome, M.; Bahr, A.; et al. Permanent neonatal diabetes in INS(C94Y) transgenic pigs. Diabetes 2013, 62, 1505–1511. [Google Scholar] [CrossRef]
- Goldfracht, I.; Efraim, Y.; Shinnawi, R.; Kovalev, E.; Huber, I.; Gepstein, A.; Arbel, G.; Shaheen, N.; Tiburcy, M.; Zimmermann, W.H.; et al. Engineered heart tissue models from hiPSC-derived cardiomyocytes and cardiac ECM for disease modeling and drug testing applications. Acta Biomater. 2019, 92, 145–159. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; Wagner Florencio, L.; Tang, L.; Heallen, T.R.; Leach, J.P.; Wang, Y.; Grisanti, F.; Willerson, J.T.; Perin, E.C.; et al. Gene therapy knockdown of Hippo signaling induces cardiomyocyte renewal in pigs after myocardial infarction. Sci. Transl. Med. 2021, 13, eabd6892. [Google Scholar] [CrossRef]
- Tilemann, L.; Lee, A.; Ishikawa, K.; Aguero, J.; Rapti, K.; Santos-Gallego, C.; Kohlbrenner, E.; Fish, K.M.; Kho, C.; Hajjar, R.J. SUMO-1 gene transfer improves cardiac function in a large-animal model of heart failure. Sci. Transl. Med. 2013, 5, 211ra159. [Google Scholar] [CrossRef]
- Takano, A.; Varrone, A.; Gulyás, B.; Salvadori, P.; Gee, A.; Windhorst, A.; Vercouillie, J.; Bormans, G.; Lammertsma, A.A.; Halldin, C. Guidelines to PET measurements of the target occupancy in the brain for drug development. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Capuano, S.V., 3rd; Croix, D.A.; Pawar, S.; Zinovik, A.; Myers, A.; Lin, P.L.; Bissel, S.; Fuhrman, C.; Klein, E.; Flynn, J.L. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect. Immun. 2003, 71, 5831–5844. [Google Scholar] [CrossRef]
- Flynn, J.L.; Capuano, S.V.; Croix, D.; Pawar, S.; Myers, A.; Zinovik, A.; Klein, E. Non-human primates: A model for tuberculosis research. Tuberculosis 2003, 83, 116–118. [Google Scholar] [CrossRef]
- Russell, D.G.; Barry, C.E., 3rd; Flynn, J.L. Tuberculosis: What we don’t know can, and does, hurt us. Science 2010, 328, 852–856. [Google Scholar] [CrossRef]
- Chu, X.; Bleasby, K.; Evers, R. Species differences in drug transporters and implications for translating preclinical findings to humans. Expert. Opin. Drug Metab. Toxicol. 2013, 9, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Shalgunov, V.; Xiong, M.; L’Estrade, E.T.; Raval, N.R.; Andersen, I.V.; Edgar, F.G.; Speth, N.R.; Baerentzen, S.L.; Hansen, H.D.; Donovan, L.L.; et al. Blocking of efflux transporters in rats improves translational validation of brain radioligands. EJNMMI Res. 2020, 10, 124. [Google Scholar] [CrossRef]
- Syvänen, S.; Lindhe, O.; Palner, M.; Kornum, B.R.; Rahman, O.; Långström, B.; Knudsen, G.M.; Hammarlund-Udenaes, M. Species differences in blood-brain barrier transport of three positron emission tomography radioligands with emphasis on P-glycoprotein transport. Drug Metab. Dispos. 2009, 37, 635–643. [Google Scholar] [CrossRef]
- Bakken, T.E.; Jorstad, N.L.; Hu, Q.; Lake, B.B.; Tian, W.; Kalmbach, B.E.; Crow, M.; Hodge, R.D.; Krienen, F.M.; Sorensen, S.A.; et al. Comparative cellular analysis of motor cortex in human, marmoset and mouse. Nature 2021, 598, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Lal, R.; Singh, A.; Watts, S.; Chopra, K. Experimental models of Parkinson’s disease: Challenges and Opportunities. Eur. J. Pharmacol. 2024, 980, 176819. [Google Scholar] [CrossRef]
- Le, W.; Sayana, P.; Jankovic, J. Animal Models of Parkinson’s Disease: A Gateway to Therapeutics? Neurotherapeutics 2014, 11, 92–110. [Google Scholar] [CrossRef]
- Deffains, M.; Canron, M.H.; Teil, M.; Li, Q.; Dehay, B.; Bezard, E.; Fernagut, P.O. L-DOPA regulates α-synuclein accumulation in experimental parkinsonism. Neuropathol. Appl. Neurobiol. 2021, 47, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Lillethorup, T.P.; Noer, O.; Alstrup, A.K.O.; Real, C.C.; Stokholm, K.; Thomsen, M.B.; Zaer, H.; Orlowski, D.; Mikkelsen, T.W.; Glud, A.N.; et al. Spontaneous partial recovery of striatal dopaminergic uptake despite nigral cell loss in asymptomatic MPTP-lesioned female minipigs. Neurotoxicology 2022, 91, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Gough, M.; Singh, D.K.; Singh, B.; Kaushal, D.; Mehra, S. System-wide identification of myeloid markers of TB disease and HIV-induced reactivation in the macaque model of Mtb infection and Mtb/SIV co-infection. Front. Immunol. 2022, 13, 777733. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Cheng, P.H.; Banta, H.; Piotrowska-Nitsche, K.; Yang, J.J.; Cheng, E.C.; Snyder, B.; Larkin, K.; Liu, J.; Orkin, J.; et al. Towards a transgenic model of Huntington’s disease in a non-human primate. Nature 2008, 453, 921–924. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Tu, Z.; Xiao, C.; Yan, S.; Ma, X.; Guo, X.; Chen, X.; Yin, P.; Yang, Z.; et al. CRISPR/Cas9-mediated PINK1 deletion leads to neurodegeneration in rhesus monkeys. Cell Res. 2019, 29, 334–336. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Kang, Y.; Yang, W.; Niu, Y.; Guo, X.; Tu, Z.; Si, C.; Wang, H.; Xing, R.; et al. Functional disruption of the dystrophin gene in rhesus monkey using CRISPR/Cas9. Hum. Mol. Genet. 2015, 24, 3764–3774. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Zhang, J.T.; Cai, Y.J.; Cheng, T.L.; Cheng, C.; Wang, Y.; Zhang, C.C.; Nie, Y.H.; Chen, Z.F.; et al. Autism-like behaviours and germline transmission in transgenic monkeys overexpressing MeCP2. Nature 2016, 530, 98–102. [Google Scholar] [CrossRef]
- Mennicken, F.; Zhang, J.; Hoffert, C.; Ahmad, S.; Beaudet, A.; O’Donnell, D. Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J. Comp. Neurol. 2003, 465, 349–360. [Google Scholar] [CrossRef]
- Porrino, L.J.; Smith, H.R.; Nader, M.A.; Beveridge, T.J. The effects of cocaine: A shifting target over the course of addiction. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1593–1600. [Google Scholar] [CrossRef]
- Keating, S.M.; Heitman, J.W.; Wu, S.; Deng, X.; Stacey, A.R.; Zahn, R.C.; de la Rosa, M.; Finstad, S.L.; Lifson, J.D.; Piatak, M., Jr.; et al. Magnitude and Quality of Cytokine and Chemokine Storm during Acute Infection Distinguish Nonprogressive and Progressive Simian Immunodeficiency Virus Infections of Nonhuman Primates. J. Virol. 2016, 90, 10339–10350. [Google Scholar] [CrossRef]
- O’Connor, M.A.; Munson, P.V.; Dross, S.E.; Tunggal, H.C.; Lewis, T.B.; Osborn, J.; Peterson, C.W.; Huang, M.L.W.; Moats, C.; Smedley, J.; et al. A Gut Reaction to SIV and SHIV Infection: Lower Dysregulation of Mucosal T Cells during Acute Infection Is Associated with Greater Viral Suppression during cART. Viruses 2021, 13, 1609. [Google Scholar] [CrossRef]
- Woolsey, C.; Fears, A.C.; Borisevich, V.; Agans, K.N.; Dobias, N.S.; Prasad, A.N.; Deer, D.J.; Geisbert, J.B.; Fenton, K.A.; Geisbert, T.W.; et al. Natural history of Sudan ebolavirus infection in rhesus and cynomolgus macaques. Emerg. Microbes Infect. 2022, 11, 1635–1646. [Google Scholar] [CrossRef]
- Dijkman, K.; Lubbers, R.; Borggreven, N.V.; Ottenhoff, T.H.M.; Joosten, S.A.; Trouw, L.A.; Verreck, F.A.W. Systemic and pulmonary C1q as biomarker of progressive disease in experimental non-human primate tuberculosis. Sci. Rep. 2020, 10, 6290. [Google Scholar] [CrossRef]
- Fahlberg, M.D.; Blair, R.V.; Doyle-Meyers, L.A.; Midkiff, C.C.; Zenere, G.; Russell-Lodrigue, K.E.; Monjure, C.J.; Haupt, E.H.; Penney, T.P.; Lehmicke, G.; et al. Cellular events of acute, resolving or progressive COVID-19 in SARS-CoV-2 infected non-human primates. Nat. Commun. 2020, 11, 6078. [Google Scholar] [CrossRef]
- Jiao, L.; Li, H.Y.; Xu, J.W.; Yang, M.L.; Ma, C.X.; Li, J.M.; Zhao, S.W.; Wang, H.X.; Yang, Y.; Yu, W.H.; et al. The Gastrointestinal Tract Is an Alternative Route for SARS-CoV-2 Infection in a Nonhuman Primate Model. Gastroenterology 2021, 160, 1647–1661. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.M.; Calderon, G.A.; Robinson, A.J.; Sullivan, C.N.; Cosgriff-Hernandez, E.; Hakim, J.C.E. Animal Models and Alternatives in Vaginal Research: A Comparative Review. Reprod. Sci. 2021, 28, 1759–1773. [Google Scholar] [CrossRef] [PubMed]
- Hympanova, L.; Rynkevic, R.; Urbankova, I.; Blacher, S.; de Landsheere, L.; Mackova, K.; Krofta, L.; Deprest, J. Morphological and Functional Changes in the Vagina following Critical Lifespan Events in the Ewe. Gynecol. Obstet. Investig. 2019, 84, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.J.; Mitchell, N.L. The Translational Benefits of Sheep as Large Animal Models of Human Neurological Disorders. Front. Vet. Sci. 2022, 9, 831838. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.J. Large-Brained Animal Models of Huntington’s Disease: Sheep. Methods Mol. Biol. 2018, 1780, 221–239. [Google Scholar] [CrossRef]
- Mageed, M.; Berner, D.; Jülke, H.; Hohaus, C.; Brehm, W.; Gerlach, K. Morphometrical dimensions of the sheep thoracolumbar vertebrae as seen on digitised CT images. Lab. Anim. Res. 2013, 29, 138–147. [Google Scholar] [CrossRef]
- Jacobsen, J.C.; Bawden, C.S.; Rudiger, S.R.; McLaughlan, C.J.; Reid, S.J.; Waldvogel, H.J.; MacDonald, M.E.; Gusella, J.F.; Walker, S.K.; Kelly, J.M.; et al. An ovine transgenic Huntington’s disease model. Human. Mol. Genet. 2010, 19, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- McKean, N.; McMurray, C.; Handley, R.; Rudiger, S.; Verma, P.; Kelly, J.; Reid, S.; Pearson, J.; Hardy, J.; Gusella, J.; et al. A Sheep Model of Alzheimer’s Disease; Centre for Brain Research: Bengaluru, India, 2023. [Google Scholar] [CrossRef]
- Pouladi, M.A.; Morton, A.J.; Hayden, M.R. Choosing an animal model for the study of Huntington’s disease. Nat. Rev. Neurosci. 2013, 14, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Rockx, B.; Kuiken, T.; Herfst, S.; Bestebroer, T.; Lamers, M.M.; Oude Munnink, B.B.; de Meulder, D.; van Amerongen, G.; van den Brand, J.; Okba, N.M.A.; et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 2020, 368, 1012–1015. [Google Scholar] [CrossRef]
- Singh, D.K.; Singh, B.; Ganatra, S.R.; Gazi, M.; Cole, J.; Thippeshappa, R.; Alfson, K.J.; Clemmons, E.; Gonzalez, O.; Escobedo, R.; et al. Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets. Nat. Microbiol. 2021, 6, 73–86. [Google Scholar] [CrossRef]
- Baum, A.; Ajithdoss, D.; Copin, R.; Zhou, A.; Lanza, K.; Negron, N.; Ni, M.; Wei, Y.; Mohammadi, K.; Musser, B.; et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science 2020, 370, 1110–1115. [Google Scholar] [CrossRef]
- Vogel, A.B.; Kanevsky, I.; Che, Y.; Swanson, K.A.; Muik, A.; Vormehr, M.; Kranz, L.M.; Walzer, K.C.; Hein, S.; Guler, A.; et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 2021, 592, 283–289. [Google Scholar] [CrossRef]
- Böszörményi, K.P.; Stammes, M.A.; Fagrouch, Z.C.; Kiemenyi-Kayere, G.; Niphuis, H.; Mortier, D.; van Driel, N.; Nieuwenhuis, I.; Vervenne, R.A.W.; Haaksma, T.; et al. The Post-Acute Phase of SARS-CoV-2 Infection in Two Macaque Species Is Associated with Signs of Ongoing Virus Replication and Pathology in Pulmonary and Extrapulmonary Tissues. Viruses 2021, 13, 1673. [Google Scholar] [CrossRef] [PubMed]
- Naninck, T.; Kahlaoui, N.; Lemaitre, J.; Maisonnasse, P.; De Mori, A.; Pascal, Q.; Contreras, V.; Marlin, R.; Relouzat, F.; Delache, B.; et al. Computed tomography and [18F]-FDG PET imaging provide additional readouts for COVID-19 pathogenesis and therapies evaluation in non-human primates. iScience 2022, 25, 104101. [Google Scholar] [CrossRef]
- Darrah, P.A.; Zeppa, J.J.; Maiello, P.; Hackney, J.A.; Wadsworth, M.H., 2nd; Hughes, T.K.; Pokkali, S.; Swanson, P.A., 2nd; Grant, N.L.; Rodgers, M.A.; et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 2020, 577, 95–102. [Google Scholar] [CrossRef]
- White, A.G.; Maiello, P.; Coleman, M.T.; Tomko, J.A.; Frye, L.J.; Scanga, C.A.; Lin, P.L.; Flynn, J.L. Analysis of 18FDG PET/CT Imaging as a Tool for Studying Mycobacterium tuberculosis Infection and Treatment in Non-human Primates. JoVE-J. Vis. Exp. 2017, 127, e56375. [Google Scholar] [CrossRef]
- Ganchua, S.K.C.; Cadena, A.M.; Maiello, P.; Gideon, H.P.; Myers, A.J.; Junecko, B.F.; Klein, E.C.; Lin, P.L.; Mattila, J.T.; Flynn, J.L. Lymph nodes are sites of prolonged bacterial persistence during Mycobacterium tuberculosis infection in macaques. PLoS Pathog. 2018, 14, e1007337. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, C.; Robertson, D.L.; Marx, P.A. The history of SIVS and AIDS: Epidemiology, phylogeny and biology of isolates from naturally SIV infected non-human primates (NHP) in Africa. Front. Biosci. 2004, 9, 225–254. [Google Scholar] [CrossRef]
- Schmitz, J.E.; Korioth-Schmitz, B. Immunopathogenesis of simian immunodeficiency virus infection in nonhuman primates. Curr. Opin. Hiv. Aids 2013, 8, 273–279. [Google Scholar] [CrossRef]
- Kim, I.; Srinivasula, S.; DeGrange, P.; Long, B.; Jang, H.; Carrasquillo, J.A.; Lane, H.C.; Di Mascio, M. Quantitative PET imaging of the CD4 pool in nonhuman primates. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; McRaven, M.D.; Carias, A.M.; Anderson, M.R.; Matias, E.; Arainga, M.; Allen, E.J.; Rogers, K.A.; Gupta, S.; Kulkarni, V.; et al. Localization of infection in neonatal rhesus macaques after oral viral challenge. PLoS Pathog. 2021, 17, e1009855. [Google Scholar] [CrossRef]
- Martin, J.T.; Hartwell, B.L.; Kumarapperuma, S.C.; Melo, M.B.; Carnathan, D.G.; Cossette, B.J.; Adams, J.; Gong, S.; Zhang, W.; Tokatlian, T.; et al. Combined PET and whole-tissue imaging of lymphatic-targeting vaccines in non-human primates. Biomaterials 2021, 275, 120868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiang, Z.; Wang, F.; Pan, X.; Zhang, Q.; Wang, P.; Jiang, L.; Yuan, H. 13N-NH3 myocardial perfusion imaging with reduced scan duration: A feasibility study in the era of total-body PET/CT. EJNMMI Phys. 2025, 12, 18. [Google Scholar] [CrossRef]
- Wang, J.; Mpharm, S.L.; Liu, T.W.; Zhang, J.M.; Chen, Y.; Li, J.M.; Xu, W.G. Preliminary and Comparative Experiment Study Between 18F-Flurpiridaz and 13N-NH3·H2O Myocardial Perfusion Imaging with PET/CT in Miniature Pigs. Mol. Imaging 2020, 19, 1536012120947506. [Google Scholar] [CrossRef]
- Maaniitty, T.; Knuuti, J.; Saraste, A. 15O-Water PET MPI: Current Status and Future Perspectives. Semin. Nucl. Med. 2020, 50, 238–247. [Google Scholar] [CrossRef]
- Gronman, M.; Tarkia, M.; Stark, C.; Vahasilta, T.; Kiviniemi, T.; Lubberink, M.; Halonen, P.; Kuivanen, A.; Saunavaara, V.; Tolvanen, T.; et al. Assessment of myocardial viability with [O-15]water PET: A validation study in experimental myocardial infarction. J. Nucl. Cardiol. 2021, 28, 1271–1280. [Google Scholar] [CrossRef]
- Ballo, H.; Tarkia, M.; Haavisto, M.; Stark, C.; Strandberg, M.; Vahasilta, T.; Saunavaara, V.; Tolvanen, T.; Teras, M.; Hynninen, V.V.; et al. Determinants of Myocardial Strain in Experimental Chronic Myocardial Infarction. Ultrasound Med. Biol. 2019, 45, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Mizoguchi, H.; Fukushima, S.; Imanishi, Y.; Watabe, T.; Harada, A.; Sakai, Y.; Sawa, Y. New regional drug delivery system by direct epicardial placement of slow-release prostacyclin agonist promise therapeutic angiogenesis in a porcine chronic myocardial infarction. J. Artif. Organs 2021, 24, 465–472. [Google Scholar] [CrossRef]
- Werner, R.A.; Koshino, K.; Arimitsu, K.; Lapa, C.; Javadi, M.S.; Rowe, S.P.; Nose, N.; Kimura, H.; Fukushima, K.; Higuchi, T. Stability of Distribution of F18 Flurpiridaz After Transient Coronary Occlusion in Pigs. JACC Cardiovasc. Imaging 2019, 12, 2269–2271. [Google Scholar] [CrossRef]
- Eriksson, O. GPR44 as a Target for Imaging Pancreatic Beta-Cell Mass. Curr. Diabetes Rep. 2019, 19, 49. [Google Scholar] [CrossRef]
- Meier, J.J.; Menge, B.A.; Breuer, T.G.; Muller, C.A.; Tannapfel, A.; Uhl, W.; Schmidt, W.E.; Schrader, H. Functional assessment of pancreatic beta-cell area in humans. Diabetes 2009, 58, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, H.; Takahashi, K.; Inaba, W.; Tsuboi, K.; Osonoi, S.; Yoshida, T.; Yagihashi, S. Involvement of oxidative stress-induced DNA damage, endoplasmic reticulum stress, and autophagy deficits in the decline of beta-cell mass in Japanese type 2 diabetic patients. Diabetes Care 2014, 37, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Breuer, T.G.; Bonadonna, R.C.; Tannapfel, A.; Uhl, W.; Schmidt, W.E.; Schrader, H.; Menge, B.A. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia 2012, 55, 1346–1354. [Google Scholar] [CrossRef]
- Inaishi, J.; Saisho, Y. Beta-Cell Mass in Obesity and Type 2 Diabetes, and Its Relation to Pancreas Fat: A Mini-Review. Nutrients 2020, 12, 3846. [Google Scholar] [CrossRef]
- Malbert, C.H.; Chauvin, A.; Horowitz, M.; Jones, K.L. Glucose Sensing Mediated by Portal Glucagon-Like Peptide 1 Receptor Is Markedly Impaired in Insulin-Resistant Obese Animals. Diabetes 2021, 70, 99–110. [Google Scholar] [CrossRef]
- Deppen, S.A.; Blume, J.; Bobbey, A.J.; Shah, C.; Graham, M.M.; Lee, P.; Delbeke, D.; Walker, R.C. 68Ga-DOTATATE Compared with 111In-DTPA-Octreotide and Conventional Imaging for Pulmonary and Gastroenteropancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. J. Nucl. Med. 2016, 57, 872–878. [Google Scholar] [CrossRef]
- Damuka, N.; Bashetti, N.; Mintz, A.; Bansode, A.H.; Miller, M.; Krizan, I.; Furdui, C.; Bhoopal, B.; Gollapelli, K.K.; Shanmukha Kumar, J.V.; et al. [18F]KS1, a novel ascorbate-based ligand images ROS in tumor models of rodents and nonhuman primates. Biomed. Pharmacother. 2022, 156, 113937. [Google Scholar] [CrossRef] [PubMed]
- Veach, D.R.; Storey, C.M.; Lückerath, K.; Braun, K.; von Bodman, C.; Lamminmäki, U.; Kalidindi, T.; Strand, S.E.; Strand, J.; Altai, M.; et al. PSA-Targeted Alpha-, Beta-, and Positron-Emitting Immunotheranostics in Murine Prostate Cancer Models and Nonhuman Primates. Clin. Cancer Res. 2021, 27, 2050–2060. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, X.; Wang, Y.; Li, X.; Sun, Y.; Guan, Z.; Li, X.; Wu, Y.; Wang, J.; Zhao, F.; et al. Development and characterisation of [18F]TTDP, a novel T cell immunoglobulin and ITIM domain tracer, in humanised mice and non-human primates. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.J.; Kim, J.; Tran, T.; Scola, P.M.; Tenney, D.; Pena, A.; Petrone, T.; Zhang, Y.; Boy, K.M.; Poss, M.A.; et al. The discovery and evaluation of [18F]BMS-986229, a novel macrocyclic peptide PET radioligand for the measurement of PD-L1 expression and in-vivo PD-L1 target engagement. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 978–990. [Google Scholar] [CrossRef]
- Brauer, J.; Tumani, M.; Frey, N.; Lehmann, L.H. The cardio-oncologic burden of breast cancer: Molecular mechanisms and importance of preclinical models. Basic. Res. Cardiol. 2025, 120, 91–112. [Google Scholar] [CrossRef]
- Wang, L.; Piao, Y.; Guo, F.; Wei, J.; Chen, Y.; Dai, X.; Zhang, X. Current progress of pig models for liver cancer research. Biomed. Pharmacother. 2023, 165, 115256. [Google Scholar] [CrossRef]
- Choen, S.; Kent, M.S.; Loucks, F.A.; Winger, J.A.; Zwingenberger, A.L. Assessment of tumor hypoxia in spontaneous canine tumors after treatment with OMX, a novel H-NOX oxygen carrier, with [18F]FMISO PET/CT. BMC Vet. Res. 2024, 20, 196. [Google Scholar] [CrossRef]
- Kjaergaard, K.; Weber, B.; Alstrup, A.K.O.; Petersen, J.B.B.; Hansen, R.; Hamilton-Dutoit, S.J.; Mortensen, F.V.; Sorensen, M. Hepatic regeneration following radiation-induced liver injury is associated with increased hepatobiliary secretion measured by PET in Gottingen minipigs. Sci. Rep. 2020, 10, 10858. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, Z.; Wu, S.; Ye, K. Positron emission tomography tracers for synucleinopathies. Mol. Neurodegener. 2025, 20, 1. [Google Scholar] [CrossRef]
- Yuan, X.; Nie, S.; Yang, Y.; Liu, C.; Xia, D.; Meng, L.; Xia, Y.; Su, H.; Zhang, C.; Bu, L.; et al. Propagation of pathologic α-synuclein from kidney to brain may contribute to Parkinson’s disease. Nat. Neurosci. 2025, 28, 577–588. [Google Scholar] [CrossRef]
- Pavese, N.; Rivero-Bosch, M.; Lewis, S.J.; Whone, A.L.; Brooks, D.J. Progression of monoaminergic dysfunction in Parkinson’s disease: A longitudinal 18F-dopa PET study. Neuroimage 2011, 56, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Fayard, A.; Fenyi, A.; Lavisse, S.; Dovero, S.; Bousset, L.; Bellande, T.; Lecourtois, S.; Jouy, C.; Guillermier, M.; Jan, C.; et al. Functional and neuropathological changes induced by injection of distinct alpha-synuclein strains: A pilot study in non-human primates. Neurobiol. Dis. 2023, 180, 106086. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, M.; Onoe, H.; Tsukada, H.; Isa, K.; Yamakado, H.; Okuda, S.; Ikuno, M.; Hatanaka, Y.; Murayama, S.; Uemura, N.; et al. Lewy Body Disease Primate Model with alpha-Synuclein Propagation from the Olfactory Bulb. Mov. Disord. 2022, 37, 2033–2044. [Google Scholar] [CrossRef]
- Molinet-Dronda, F.; Blesa, J.; Del Rey, N.L.; Juri, C.; Collantes, M.; Pineda-Pardo, J.A.; Trigo-Damas, I.; Iglesias, E.; Hernandez, L.F.; Rodriguez-Rojas, R.; et al. Cerebral metabolic pattern associated with progressive parkinsonism in non-human primates reveals early cortical hypometabolism. Neurobiol. Dis. 2022, 167, 105669. [Google Scholar] [CrossRef]
- López-Ornelas, A.; Escobedo-Avila, I.; Ramírez-García, G.; Lara-Rodarte, R.; Meléndez-Ramírez, C.; Urrieta-Chavez, B.; Barrios-García, T.; Cáceres-Chávez, V.A.; Flores-Ponce, X.; Carmona, F.; et al. Human Embryonic Stem Cell-Derived Immature Midbrain Dopaminergic Neurons Transplanted in Parkinsonian Monkeys. Cells 2023, 12, 2738. [Google Scholar] [CrossRef]
- Pokrzyk, J.; Kulczyńska-Przybik, A.; Guzik-Makaruk, E.; Winkel, I.; Mroczko, B. Clinical Importance of Amyloid Beta Implication in the Detection and Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 1935. [Google Scholar] [CrossRef]
- Nabizadeh, F. Connectomics and neurotransmitter receptor profile explain regional tau pathology in Alzheimer’s disease. Cereb. Cortex 2025, 35, bhaf053. [Google Scholar] [CrossRef] [PubMed]
- Maetzler, W.; Reimold, M.; Liepelt, I.; Solbach, C.; Leyhe, T.; Schweitzer, K.; Eschweiler, G.W.; Mittelbronn, M.; Gaenslen, A.; Uebele, M.; et al. [11C]PIB binding in Parkinson’s disease dementia. Neuroimage 2008, 39, 1027–1033. [Google Scholar] [CrossRef]
- Cohen, A.D.; Klunk, W.E. Early detection of Alzheimer’s disease using PiB and FDG PET. Neurobiol. Dis. 2014, 72 Pt A, 117–122. [Google Scholar] [CrossRef]
- Bhoopal, B.; Gollapelli, K.K.; Damuka, N.; Miller, M.; Krizan, I.; Bansode, A.; Register, T.; Frye, B.M.; Kim, J.; Mintz, A.; et al. Preliminary PET Imaging of Microtubule-Based PET Radioligand [11C]MPC-6827 in a Nonhuman Primate Model of Alzheimer’s Disease. Acs Chem. Neurosci. 2023, 14, 3745–3751. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, K.; Zhang, X.; Zhao, H.; Wang, X.; Dong, R.; Wang, Y.; Chen, B.; Yan, X.X.; Dai, J.; et al. Fluorine-18-Labeled Diaryl-azines as Improved beta-Amyloid Imaging Tracers: From Bench to First-in-Human Studies. J. Med. Chem. 2023, 66, 4603–4616. [Google Scholar] [CrossRef] [PubMed]
- Sawant-Basak, A.; Chen, L.; Lockwood, P.; Boyden, T.; Doran, A.C.; Mancuso, J.; Zasadny, K.; McCarthy, T.; Morris, E.D.; Carson, R.E.; et al. Investigating CNS distribution of PF-05212377, a P-glycoprotein substrate, by translation of 5-HT(6) receptor occupancy from non-human primates to humans. Biopharm. Drug Dispos. 2023, 44, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, L.; Dutra, J.K.; Beck, E.M.; Nag, S.; Takano, A.; Amini, N.; Arakawa, R.; Brodney, M.A.; Buzon, L.M.; et al. Identification of a Novel Positron Emission Tomography (PET) Ligand for Imaging beta-Site Amyloid Precursor Protein Cleaving Enzyme 1 (BACE-1) in Brain. J. Med. Chem. 2018, 61, 3296–3308. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.E.; Nag, S.; Arakawa, R.; Lin, E.Y.; Stratman, N.; Guckian, K.; Hering, H.; Lulla, M.; Choi, J.; Salinas, C.; et al. Development of a PET Tracer for OGA with Improved Kinetics in the Living Brain. J. Nucl. Med. 2023, 64, 1588–1593. [Google Scholar] [CrossRef]
- Stoker, T.B.; Mason, S.L.; Greenland, J.C.; Holden, S.T.; Santini, H.; Barker, R.A. Huntington’s disease: Diagnosis and management. Pract. Neurol. 2022, 22, 32–41. [Google Scholar] [CrossRef]
- Aqel, S.; Ahmad, J.; Saleh, I.; Fathima, A.; Al Thani, A.A.; Mohamed, W.M.Y.; Shaito, A.A. Advances in Huntington’s Disease Biomarkers: A 10-Year Bibliometric Analysis and a Comprehensive Review. Biology 2025, 14, 129. [Google Scholar] [CrossRef]
- Williams, G.K.; Akkermans, J.; Lawson, M.; Syta, P.; Staelens, S.; Adhikari, M.H.; Morton, A.J.; Nitzsche, B.; Boltze, J.; Christou, C.; et al. Imaging Glucose Metabolism and Dopaminergic Dysfunction in Sheep (Ovis aries) Brain Using Positron Emission Tomography Imaging Reveals Abnormalities in OVT73 Huntington’s Disease Sheep. Acs Chem. Neurosci. 2024, 15, 4082–4091. [Google Scholar] [CrossRef]
- Kaur, T.; Brooks, A.F.; Lapsys, A.; Desmond, T.J.; Stauff, J.; Arteaga, J.; Winton, W.P.; Scott, P.J.H. Synthesis and Evaluation of a Fluorine-18 Radioligand for Imaging Huntingtin Aggregates by Positron Emission Tomographic Imaging. Front. Neurosci. 2021, 15, 766176. [Google Scholar] [CrossRef]
- Liu, L.; Prime, M.E.; Lee, M.R.; Khetarpal, V.; Brown, C.J.; Johnson, P.D.; Miranda-Azpiazu, P.; Chen, X.; Clark-Frew, D.; Coe, S.; et al. Imaging Mutant Huntingtin Aggregates: Development of a Potential PET Ligand. J. Med. Chem. 2020, 63, 8608–8633. [Google Scholar] [CrossRef]
- Liu, L.; Johnson, P.D.; Prime, M.E.; Khetarpal, V.; Lee, M.R.; Brown, C.J.; Chen, X.; Clark-Frew, D.; Coe, S.; Conlon, M.; et al. [(11)C]CHDI-626, a PET Tracer Candidate for Imaging Mutant Huntingtin Aggregates with Reduced Binding to AD Pathological Proteins. J. Med. Chem. 2021, 64, 12003–12021. [Google Scholar] [CrossRef]
- Liu, L.B.; Johnson, P.D.; Prime, M.E.; Khetarpal, V.; Brown, C.J.; Anzillotti, L.; Bertoglio, D.; Chen, X.M.; Coe, S.; Davis, R.; et al. Design and Evaluation of [18F]CHDI-650 as a Positron Emission Tomography Ligand to Image Mutant Huntingtin Aggregates. J. Med. Chem. 2023, 66, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Smucny, J.; Vlasova, R.M.; Lesh, T.A.; Rowland, D.J.; Wang, G.; Chaudhari, A.J.; Chen, S.; Iosif, A.M.; Hogrefe, C.E.; Bennett, J.L.; et al. Increased Striatal Presynaptic Dopamine in a Nonhuman Primate Model of Maternal Immune Activation: A Longitudinal Neurodevelopmental Positron Emission Tomography Study With Implications for Schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 505–513. [Google Scholar] [CrossRef]
- Wakeford, A.; Nye, J.A.; Grieb, Z.A.; Voisin, D.A.; Mun, J.; Huhman, K.L.; Albers, E.; Michopoulos, V. Sex influences the effects of social status on socioemotional behavior and serotonin neurochemistry in rhesus monkeys. Biol. Sex. Differ. 2023, 14, 75. [Google Scholar] [CrossRef]
- Birn, R.M.; Shackman, A.J.; Oler, J.A.; Williams, L.E.; McFarlin, D.R.; Rogers, G.M.; Shelton, S.E.; Alexander, A.L.; Pine, D.S.; Slattery, M.J.; et al. Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Mol. Psychiatry 2014, 19, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.S.; Oler, J.A.; Shackman, A.J.; Shelton, S.E.; Raveendran, M.; McKay, D.R.; Converse, A.K.; Alexander, A.; Davidson, R.J.; Blangero, J.; et al. Intergenerational neural mediators of early-life anxious temperament. Proc. Natl. Acad. Sci. USA 2015, 112, 9118–9122. [Google Scholar] [CrossRef] [PubMed]
- Shackman, A.J.; Fox, A.S.; Oler, J.A.; Shelton, S.E.; Oakes, T.R.; Davidson, R.J.; Kalin, N.H. Heightened extended amygdala metabolism following threat characterizes the early phenotypic risk to develop anxiety-related psychopathology. Mol. Psychiatry 2017, 22, 724–732. [Google Scholar] [CrossRef]
- Belin, D.; Dalley, J.W. Animal Models in Addiction Research. In Drug Abuse and Addiction in Medical Illness: Causes, Consequences and Treatment; Verster, J.C., Brady, K., Galanter, M., Conrod, P., Eds.; Springer: New York, NY, USA, 2012; pp. 73–93. [Google Scholar] [CrossRef]
- Trifilieff, P.; Ducrocq, F.; van der Veldt, S.; Martinez, D. Blunted Dopamine Transmission in Addiction: Potential Mechanisms and Implications for Behavior. Semin. Nucl. Med. 2017, 47, 64–74. [Google Scholar] [CrossRef]
- Allen, M.I.; Duke, A.N.; Nader, S.H.; Adler-Neal, A.; Solingapuram Sai, K.K.; Reboussin, B.A.; Gage, H.D.; Voll, R.J.; Mintz, A.; Goodman, M.M.; et al. PET imaging of dopamine transporters and D2/D3 receptors in female monkeys: Effects of chronic cocaine self-administration. Neuropsychopharmacology 2023, 48, 1436–1445. [Google Scholar] [CrossRef]
- Nader, M.A.; Morgan, D.; Gage, H.D.; Nader, S.H.; Calhoun, T.L.; Buchheimer, N.; Ehrenkaufer, R.; Mach, R.H. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat. Neurosci. 2006, 9, 1050–1056. [Google Scholar] [CrossRef]
- Gould, R.W.; Porrino, L.J.; Nader, M.A. Nonhuman primate models of addiction and PET imaging: Dopamine system dysregulation. Curr. Top. Behav. Neurosci. 2012, 11, 25–44. [Google Scholar] [CrossRef]
- Gallezot, J.D.; Kloczynski, T.; Weinzimmer, D.; Labaree, D.; Zheng, M.Q.; Lim, K.; Rabiner, E.A.; Ridler, K.; Pittman, B.; Huang, Y.; et al. Imaging nicotine- and amphetamine-induced dopamine release in rhesus monkeys with [(11)C]PHNO vs [(11)C]raclopride PET. Neuropsychopharmacology 2014, 39, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Naylor, J.E.; Hiranita, T.; Matazel, K.S.; Zhang, X.; Paule, M.G.; Goodwin, A.K. Positron emission tomography (PET) imaging of nicotine-induced dopamine release in squirrel monkeys using [18F]Fallypride. Drug Alcohol. Depend. 2017, 179, 254–259. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).