The Role of Astrocytes in Synaptic Dysfunction and Memory Deficits in Alzheimer’s Disease
Abstract
1. Introduction
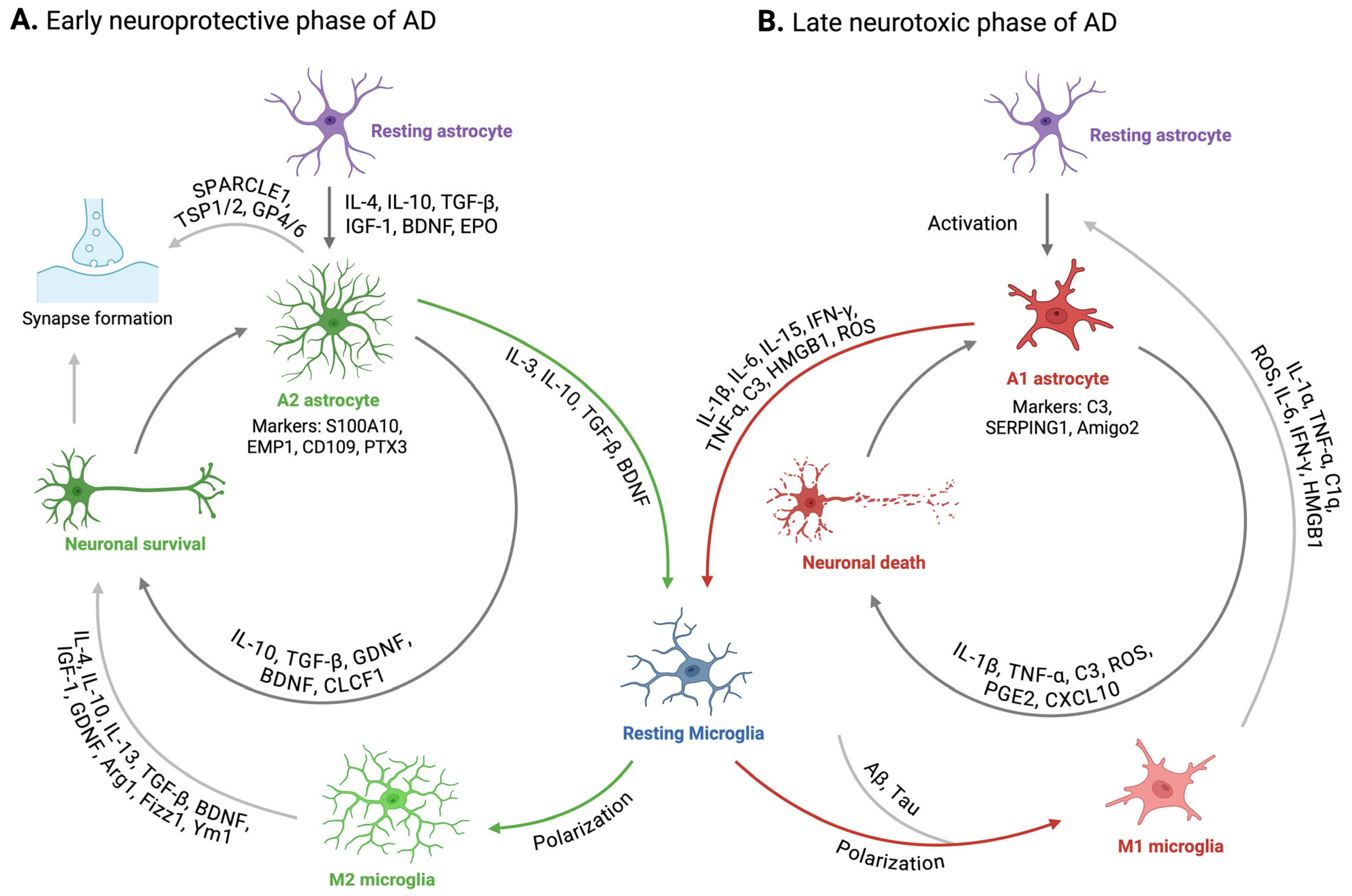
2. Reactive Astrogliosis in Alzheimer’s Disease
3. Dysregulation in Microglia-Astrocyte Interaction in Alzheimer’s Disease
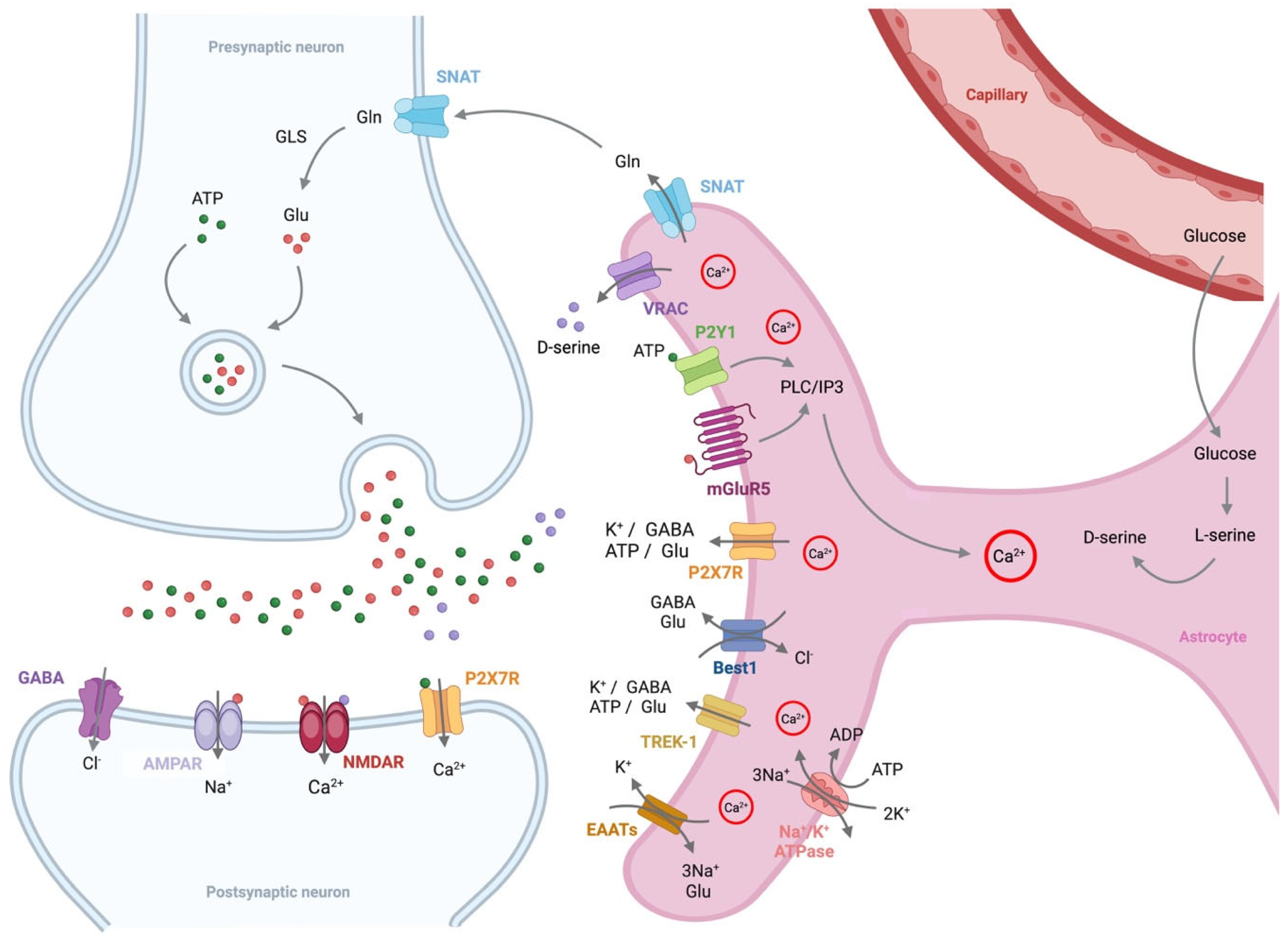
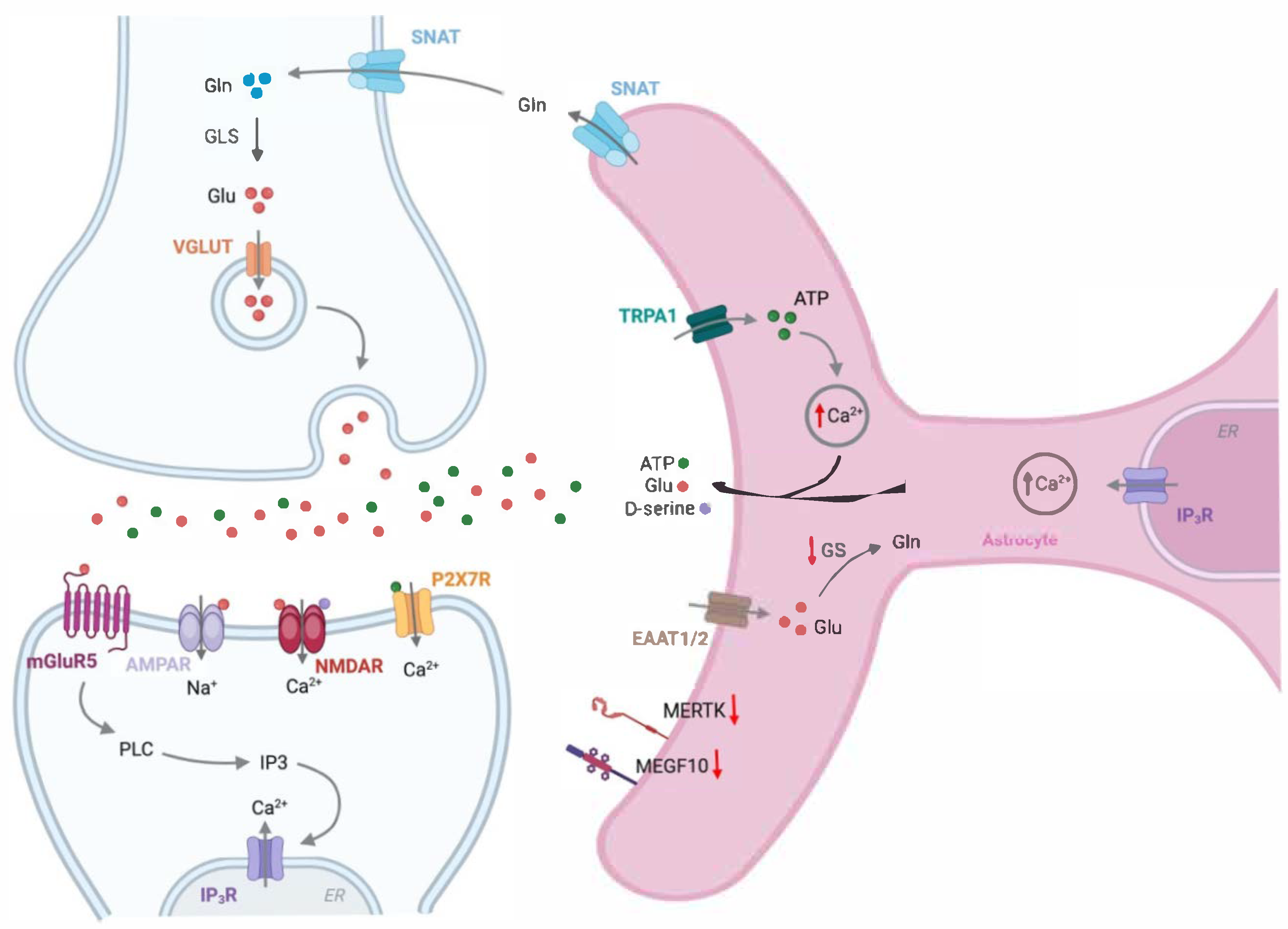
4. Alterations in Astrocyte Function in Alzheimer’s Disease
4.1. Glutamate
4.2. D-Serine
4.3. ATP
4.4. GABA
4.5. Potassium
4.6. Reactive Oxygen
5. Astrocytes in Synaptic Dysfunction in Alzheimer’s Disease
5.1. Synaptic Plasticity
5.1.1. Hebbian Synaptic Plasticity
Long-Term Potentiation
Long-Term Depression
Spike-Timing-Dependent Plasticity
5.1.2. Homeostatic Synaptic Plasticity
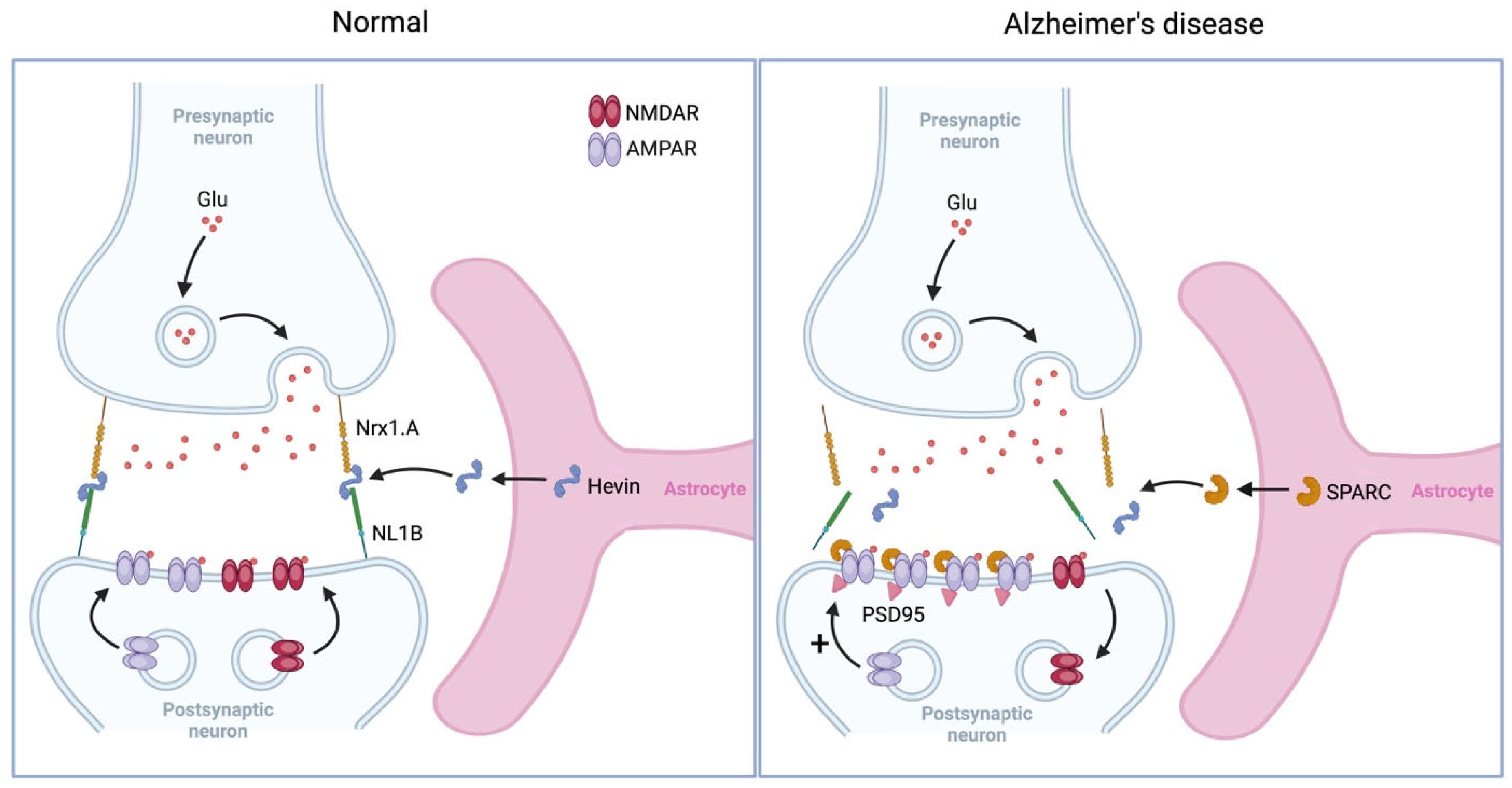
5.2. Synaptic Remodeling
5.2.1. Synaptogenesis
5.2.2. Synaptic Pruning
6. Astrocytes in Memory Deficits in Alzheimer’s Disease
6.1. Recognition Memory
6.2. Spatial Memory
6.3. Fear Memory
6.4. Working Memory
7. Astrocytes in the Treatment of Alzheimer’s Disease
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirrlinger, J.; Nimmerjahn, A. A Perspective on Astrocyte Regulation of Neural Circuit Function and Animal Behavior. Glia 2022, 70, 1554–1580. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; Deneen, B. The Emerging Nature of Astrocyte Diversity. Annu. Rev. Neurosci. 2019, 42, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Ben Haim, L.; Rowitch, D.H. Functional Diversity of Astrocytes in Neural Circuit Regulation. Nat. Rev. Neurosci. 2016, 18, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Lanjakornsiripan, D.; Pior, B.J.; Kawaguchi, D.; Furutachi, S.; Tahara, T.; Katsuyama, Y.; Suzuki, Y.; Fukazawa, Y.; Gotoh, Y. Layer-Specific Morphological and Molecular Differences in Neocortical Astrocytes and Their Dependence on Neuronal Layers. Nat. Commun. 2018, 9, 1623. [Google Scholar] [CrossRef]
- Chai, H.; Diaz-Castro, B.; Shigetomi, E.; Monte, E.; Octeau, J.C.; Yu, X.; Cohn, W.; Rajendran, P.S.; Vondriska, T.M.; Whitelegge, J.P.; et al. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 2017, 95, 531–549.e9. [Google Scholar] [CrossRef]
- Khakh, B.S.; Sofroniew, M.V. Diversity of Astrocyte Functions and Phenotypes in Neural Circuits. Nat. Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef]
- Nagai, J.; Yu, X.; Papouin, T.; Cheong, E.; Freeman, M.R.; Monk, K.R.; Hastings, M.H.; Haydon, P.G.; Rowitch, D.; Shaham, S.; et al. Behaviorally Consequential Astrocytic Regulation of Neural Circuits. Neuron 2021, 109, 576–596. [Google Scholar] [CrossRef]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.R.; Robitaille, R.; Volterra, A. Gliotransmitters Travel in Time and Space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef]
- Rocchi, A.; Valensin, D.; Aldinucci, C.; Giani, G.; Barbucci, R.; Gaggelli, E.; Kozlowski, H.; Valensin, G. NMR Metabolomic Investigation of Astrocytes Interacted with Aβ42 or Its Complexes with Either Copper(II) or Zinc(II). J. Inorg. Biochem. 2012, 117, 326–333. [Google Scholar] [CrossRef]
- Figley, C.R.; Stroman, P.W. The Role(s) of Astrocytes and Astrocyte Activity in Neurometabolism, Neurovascular Coupling, and the Production of Functional Neuroimaging Signals. Eur. J. Neurosci. 2011, 33, 577–588. [Google Scholar] [CrossRef]
- Acosta, C.; Anderson, H.D.; Anderson, C.M. Astrocyte Dysfunction in Alzheimer Disease. J. Neurosci. Res. 2017, 95, 2430–2447. [Google Scholar] [CrossRef] [PubMed]
- González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Phatnani, H.; Maniatis, T. Astrocytes in Neurodegenerative Disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a020628. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; McCabe, C.; Medina, S.; Varshavsky, M.; Kitsberg, D.; Dvir-Szternfeld, R.; Green, G.; Dionne, D.; Nguyen, L.; Marshall, J.L.; et al. Disease-Associated Astrocytes in Alzheimer’s Disease and Aging. Nat. Neurosci. 2020, 23, 701–706. [Google Scholar] [CrossRef]
- Preman, P.; Alfonso-Triguero, M.; Alberdi, E.; Verkhratsky, A.; Arranz, A.M. Astrocytes in Alzheimer’s Disease: Pathological Significance and Molecular Pathways. Cells 2021, 10, 540. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s Disease: Genes, Proteins, and Therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s Disease: The Challenge of the Second Century. Sci. Transl. Med. 2011, 3, 77sr1. [Google Scholar] [CrossRef]
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global Estimates on the Number of Persons across the Alzheimer’s Disease Continuum. Alzheimer’s Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef]
- Buckwalter, M.S.; Wyss-Coray, T. Modelling Neuroinflammatory Phenotypes in Vivo. J. Neuroinflammation 2004, 1, 10. [Google Scholar] [CrossRef][Green Version]
- Zhang, B.; Gaiteri, C.; Bodea, L.G.; Wang, Z.; McElwee, J.; Podtelezhnikov, A.A.; Zhang, C.; Xie, T.; Tran, L.; Dobrin, R.; et al. Integrated Systems Approach Identifies Genetic Nodes and Networks in Late-Onset Alzheimer’s Disease. Cell 2013, 153, 707–720. [Google Scholar] [CrossRef]
- Hinkle, J.T.; Dawson, V.L.; Dawson, T.M. The A1 Astrocyte Paradigm: New Avenues for Pharmacological Intervention in Neurodegeneration. Mov. Disord. 2019, 34, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.; Luo, J.; Harischandra, D.S.; Gordon, R.; Sarkar, S.; Jin, H.; Anantharam, V.; Désaubry, L.; Kanthasamy, A.; Kanthasamy, A. Prokineticin-2 Promotes Chemotaxis and Alternative A2 Reactivity of Astrocytes. Glia 2018, 66, 2137–2157. [Google Scholar] [CrossRef] [PubMed]
- Haim, L.B.; Carrillo-de Sauvage, M.A.; Ceyzériat, K.; Escartin, C. Elusive Roles for Reactive Astrocytes in Neurodegenerative Diseases. Front. Cell. Neurosci. 2015, 9, 278. [Google Scholar] [CrossRef]
- Escartin, C.; Guillemaud, O.; Carrillo-de Sauvage, M.A. Questions and (Some) Answers on Reactive Astrocytes. Glia 2019, 67, 2221–2247. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Muzikansky, A.; Gómez-Isla, T.; Growdon, J.H.; Betensky, R.A.; Frosch, M.P.; Hyman, B.T. Differential Relationships of Reactive Astrocytes and Microglia to Fibrillar Amyloid Deposits in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2013, 72, 462–471. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Ziar, R.; Tesar, P.J.; Clayton, B.L.L. Astrocyte and Oligodendrocyte Pathology in Alzheimer’s Disease. Neurotherapeutics 2025, 22, e00540. [Google Scholar] [CrossRef]
- Alberdi, E.; Wyssenbach, A.; Alberdi, M.; Sánchez-Gómez, M.V.; Cavaliere, F.; Rodríguez, J.J.; Verkhratsky, A.; Matute, C. Ca2+-dependent Endoplasmic Reticulum Stress Correlates with Astrogliosis in Oligomeric Amyloid Β-treated Astrocytes and in a Model of Alzheimer’s Disease. Aging Cell 2013, 12, 292–302. [Google Scholar] [CrossRef]
- Ries, M.; Sastre, M. Mechanisms of Aβ Clearance and Degradation by Glial Cells. Front. Aging Neurosci. 2016, 8, 160. [Google Scholar] [CrossRef]
- Nedergaard, M. Garbage Truck of the Brain. Science (1979) 2013, 340, 1529–1530. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.W.; Hu, X.; Yoon, H.; Yan, P.; Xiao, Q.; Wang, Y.; Gil, S.C.; Brown, J.; Wilhelmsson, U.; Restivo, J.L.; et al. Attenuating Astrocyte Activation Accelerates Plaque Pathogenesis in APP/PS1 Mice. FASEB J. 2013, 27, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Wu, C.; Yang, L.; Feng, S.; Zhu, L.; Yang, L.; Liu, T.C.-Y.; Duan, R. Therapeutic Non-Invasive Brain Treatments in Alzheimer’s Disease: Recent Advances and Challenges. Inflamm. Regen. 2022, 42, 31. [Google Scholar] [CrossRef]
- Deng, Q.; Wu, C.; Parker, E.; Liu, T.C.-Y.; Duan, R.; Yang, L. Microglia and Astrocytes in Alzheimer’s Disease: Significance and Summary of Recent Advances. Aging Dis. 2024, 15, 1537. [Google Scholar] [CrossRef]
- Filipcik, P.; Cente, M.; Zilka, N.; Smolek, T.; Hanes, J.; Kucerak, J.; Opattova, A.; Kovacech, B.; Novak, M. Intraneuronal Accumulation of Misfolded Tau Protein Induces Overexpression of Hsp27 in Activated Astrocytes. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2015, 1852, 1219–1229. [Google Scholar] [CrossRef]
- Piacentini, R.; Li Puma, D.D.; Mainardi, M.; Lazzarino, G.; Tavazzi, B.; Arancio, O.; Grassi, C. Reduced Gliotransmitter Release from Astrocytes Mediates Tau-induced Synaptic Dysfunction in Cultured Hippocampal Neurons. Glia 2017, 65, 1302–1316. [Google Scholar] [CrossRef]
- Richetin, K.; Steullet, P.; Pachoud, M.; Perbet, R.; Parietti, E.; Maheswaran, M.; Eddarkaoui, S.; Bégard, S.; Pythoud, C.; Rey, M.; et al. Tau Accumulation in Astrocytes of the Dentate Gyrus Induces Neuronal Dysfunction and Memory Deficits in Alzheimer’s Disease. Nat. Neurosci. 2020, 23, 1567–1579. [Google Scholar] [CrossRef]
- Bouvier, D.S.; Murai, K.K. Synergistic Actions of Microglia and Astrocytes in the Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 45, 1001–1014. [Google Scholar] [CrossRef]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, S.; Wang, L.; Liu, X.; Liu, G.; Tan, Q.; Li, W.; Zhang, S.; Du, Y. Refining the Interactions between Microglia and Astrocytes in Alzheimer’s Disease Pathology. Neuroscience 2025, 573, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Tsering, W.; de la Rosa, A.; Ruan, I.Y.; Philips, J.L.; Bathe, T.; Villareal, J.A.; Prokop, S. Preferential Clustering of Microglia and Astrocytes around Neuritic Plaques during Progression of Alzheimer’s Disease Neuropathological Changes. J. Neurochem. 2025, 169, e16275. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Kang, S.; Lee, W.; Choi, H.; Chung, S.; Kim, J.-I.; Mook-Jung, I. A Breakdown in Metabolic Reprogramming Causes Microglia Dysfunction in Alzheimer’s Disease. Cell Metab. 2019, 30, 493–507.e6. [Google Scholar] [CrossRef]
- McAlpine, C.S.; Park, J.; Griciuc, A.; Kim, E.; Choi, S.H.; Iwamoto, Y.; Kiss, M.G.; Christie, K.A.; Vinegoni, C.; Poller, W.C.; et al. Astrocytic Interleukin-3 Programs Microglia and Limits Alzheimer’s Disease. Nature 2021, 595, 701–706. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Z.; Zhang, P.; Yu, Q.; Cheng, Q.; Lu, Z.; Zong, S. The Roles of Microglia and Astrocytes in Neuroinflammation of Alzheimer’s Disease. Front. Neurosci. 2025, 19, 1575453. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Shi, S.X.; Li, Y.-J.; Shi, K.; Wood, K.; Ducruet, A.F.; Liu, Q. IL (Interleukin)-15 Bridges Astrocyte-Microglia Crosstalk and Exacerbates Brain Injury Following Intracerebral Hemorrhage. Stroke 2020, 51, 967–974. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kiyota, T.; Horiba, M.; Buescher, J.L.; Walsh, S.M.; Gendelman, H.E.; Ikezu, T. Interferon-γ and Tumor Necrosis Factor-α Regulate Amyloid-β Plaque Deposition and β-Secretase Expression in Swedish Mutant APP Transgenic Mice. Am. J. Pathol. 2007, 170, 680–692. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, S.; Jin, S.M.; Hwang, E.; Kim, Y.S.; Huh, K.; Mook-Jung, I. IFN-γ-induced BACE1 Expression Is Mediated by Activation of JAK2 and ERK1/2 Signaling Pathways and Direct Binding of STAT1 to BACE1 Promoter in Astrocytes. Glia 2007, 55, 253–262. [Google Scholar] [CrossRef]
- Leyns, C.E.G.; Holtzman, D.M. Glial Contributions to Neurodegeneration in Tauopathies. Mol. Neurodegener. 2017, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Reyes, R.C.; Gottipati, M.K.; Lewis, K.; Lesort, M.; Parpura, V.; Gray, M. Enhanced Ca2+-Dependent Glutamate Release from Astrocytes of the BACHD Huntington’s Disease Mouse Model. Neurobiol. Dis. 2013, 58, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Scemes, E.; Giaume, C. Astrocyte Calcium Waves: What They Are and What They Do. Glia 2006, 54, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Dulla, C.G.; Farzampour, Z.; Taylor-Weiner, A.; Huguenard, J.R.; Reimer, R.J. A Local Glutamate-Glutamine Cycle Sustains Synaptic Excitatory Transmitter Release. Neuron 2014, 81, 888–900. [Google Scholar] [CrossRef]
- Robinson, S.R. Changes in the Cellular Distribution of Glutamine Synthetase in Alzheimer’s Disease. J. Neurosci. Res. 2001, 66, 972–980. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Furukawa, H.; Wollmuth, L.P.; Gibb, A.J.; Traynelis, S.F. Structure, Function, and Allosteric Modulation of NMDA Receptors. J. Gen. Physiol. 2018, 150, 1081–1105. [Google Scholar] [CrossRef]
- Panatier, A.; Theodosis, D.T.; Mothet, J.P.; Touquet, B.; Pollegioni, L.; Poulain, D.A.; Oliet, S.H.R. Glia-Derived D-Serine Controls NMDA Receptor Activity and Synaptic Memory. Cell 2006, 125, 775–784. [Google Scholar] [CrossRef]
- Wolosker, H.; Balu, D.T. D-Serine as the Gatekeeper of NMDA Receptor Activity: Implications for the Pharmacologic Management of Anxiety Disorders. Transl. Psychiatry 2020, 10, 184. [Google Scholar] [CrossRef]
- Madeira, C.; Lourenco, M.V.; Vargas-Lopes, C.; Suemoto, C.K.; Brandão, C.O.; Reis, T.; Leite, R.E.P.; Laks, J.; Jacob-Filho, W.; Pasqualucci, C.A.; et al. D-Serine Levels in Alzheimer’s Disease: Implications for Novel Biomarker Development. Transl. Psychiatry 2015, 5, e561. [Google Scholar] [CrossRef]
- Balu, D.T.; Pantazopoulos, H.; Huang, C.C.Y.; Muszynski, K.; Harvey, T.L.; Uno, Y.; Rorabaugh, J.M.; Galloway, C.R.; Botz-Zapp, C.; Berretta, S.; et al. Neurotoxic Astrocytes Express the D-Serine Synthesizing Enzyme, Serine Racemase, in Alzheimer’s Disease. Neurobiol. Dis. 2019, 130, 104511. [Google Scholar] [CrossRef]
- Piubelli, L.; Pollegioni, L.; Rabattoni, V.; Mauri, M.; Princiotta Cariddi, L.; Versino, M.; Sacchi, S. Serum D-Serine Levels Are Altered in Early Phases of Alzheimer’s Disease: Towards a Precocious Biomarker. Transl. Psychiatry 2021, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Historical Review: ATP as a Neurotransmitter. Trends Pharmacol. Sci. 2006, 27, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Kamiya, T.; Tsuboi, T. Gliotransmitter Release from Astrocytes: Functional, Developmental, and Pathological Implications in the Brain. Front. Neurosci. 2016, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Erb, L.; Woods, L.T.; Khalafalla, M.G.; Weisman, G.A. Purinergic Signaling in Alzheimer’s Disease. Brain Res. Bull. 2019, 151, 25–37. [Google Scholar] [CrossRef]
- Haughey, N.J.; Mattson, M.P. Alzheimer’s Amyloid β-Peptide Enhances ATP/Gap Junction-Mediated Calcium-Wave Propagation in Astrocytes. Neuromol. Med. 2003, 3, 173–180. [Google Scholar] [CrossRef]
- Beard, E.; Lengacher, S.; Dias, S.; Magistretti, P.J.; Finsterwald, C. Astrocytes as Key Regulators of Brain Energy Metabolism: New Therapeutic Perspectives. Front. Physiol. 2022, 12, 825816. [Google Scholar] [CrossRef]
- An, Y.; Varma, V.R.; Varma, S.; Casanova, R.; Dammer, E.; Pletnikova, O.; Chia, C.W.; Egan, J.M.; Ferrucci, L.; Troncoso, J.; et al. Evidence for Brain Glucose Dysregulation in Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 318–329. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.-H.; Liu, L.; Gu, Z.; You, Y.; Hao, J.-R.; Sun, N.; Gao, C. Regulation of Glycolysis-Derived L-Lactate Production in Astrocytes Rescues the Memory Deficits and Aβ Burden in Early Alzheimer’s Disease Models. Pharmacol. Res. 2024, 208, 107357. [Google Scholar] [CrossRef]
- Yoon, B.E.; Lee, C.J. GABA as a Rising Gliotransmitter. Front. Neural Circuits 2014, 8, 141. [Google Scholar] [CrossRef]
- Yoon, B.E.; Woo, J.; Chun, Y.E.; Chun, H.; Jo, S.; Bae, J.Y.; An, H.; Min, J.O.; Oh, S.J.; Han, K.S.; et al. Glial GABA, Synthesized by Monoamine Oxidase B, Mediates Tonic Inhibition. J. Physiol. 2014, 592, 4951–4968. [Google Scholar] [CrossRef]
- Mederos, S.; Sánchez-Puelles, C.; Esparza, J.; Valero, M.; Ponomarenko, A.; Perea, G. GABAergic Signaling to Astrocytes in the Prefrontal Cortex Sustains Goal-Directed Behaviors. Nat. Neurosci. 2021, 24, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Garaschuk, O. GABAergic Astrocytes in Alzheimer’s Disease. Aging 2019, 11, 1602–1604. [Google Scholar] [CrossRef]
- Jo, S.; Yarishkin, O.; Hwang, Y.J.; Chun, Y.E.; Park, M.; Woo, D.H.; Bae, J.Y.; Kim, T.; Lee, J.; Chun, H.; et al. GABA from Reactive Astrocytes Impairs Memory in Mouse Models of Alzheimer’s Disease. Nat. Med. 2014, 20, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Laming, P.R. Potassium Signalling in the Brain: Its Role in Behaviour. Neurochem. Int. 2000, 36, 271–290. [Google Scholar] [CrossRef]
- Hertz, L.; Chen, Y. Importance of Astrocytes for Potassium Ion (K+) Homeostasis in Brain and Glial Effects of K+ and Its Transporters on Learning. Neurosci. Biobehav. Rev. 2016, 71, 484–505. [Google Scholar] [CrossRef]
- Dallérac, G.; Rouach, N. Astrocytes as New Targets to Improve Cognitive Functions. Prog. Neurobiol. 2016, 144, 48–67. [Google Scholar] [CrossRef]
- Ding, F.; Sun, Q.; Long, C.; Rasmussen, R.N.; Peng, S.; Xu, Q.; Kang, N.; Song, W.; Weikop, P.; Goldman, S.A.; et al. Dysregulation of Extracellular Potassium Distinguishes Healthy Ageing from Neurodegeneration. Brain 2024, 147, 1726–1739. [Google Scholar] [CrossRef]
- Wilcock, D.M.; Vitek, M.P.; Colton, C.A. Vascular Amyloid Alters Astrocytic Water and Potassium Channels in Mouse Models and Humans with Alzheimer’s Disease. Neuroscience 2009, 159, 1055–1069. [Google Scholar] [CrossRef]
- Graham, S.F.; Nasarauddin, M.B.; Carey, M.; McGuinness, B.; Holscher, C.; Kehoe, P.G.; Love, S.; Passmore, A.P.; Elliott, C.T.; Meharg, A.; et al. Quantitative Measurement of [Na+] and [K+] in Postmortem Human Brain Tissue Indicates Disturbances in Subjects with Alzheimer’s Disease and Dementia with Lewy Bodies. J. Alzheimer’s Dis. 2015, 44, 851–857. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The Role of Astrocytes in Oxidative Stress of Central Nervous System: A Mixed Blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef]
- Stephen, T.-L.; Gupta-Agarwal, S.; Kittler, J.T. Mitochondrial Dynamics in Astrocytes. Biochem. Soc. Trans. 2014, 42, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative Stress Induced-Neurodegenerative Diseases: The Need for Antioxidants That Penetrate the Blood Brain Barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef] [PubMed]
- Volterra, A.; Trotti, D.; Tromba, C.; Floridi, S.; Racagni, G. Glutamate Uptake Inhibition by Oxygen Free Radicals in Rat Cortical Astrocytes. J. Neurosci. 1994, 14, 2924–2932. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, S.; Holsinger, R.M.D. Cyclical Amyloid Beta-Astrocyte Activity Induces Oxidative Stress in Alzheimer’s Disease. Biochimie 2020, 171–172, 38–42. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Duchen, M.R. The Role of an Astrocytic NADPH Oxidase in the Neurotoxicity of Amyloid Beta Peptides. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 2309–2314. [Google Scholar] [CrossRef]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Glutamate-Dependent Astrocyte Modulation of Synaptic Transmission between Cultured Hippocampal Neurons. Eur. J. Neurosci. 1998, 10, 2129–2142. [Google Scholar] [CrossRef]
- Jourdain, P.; Bergersen, L.H.; Bhaukaurally, K.; Bezzi, P.; Santello, M.; Domercq, M.; Matute, C.; Tonello, F.; Gundersen, V.; Volterra, A. Glutamate Exocytosis from Astrocytes Controls Synaptic Strength. Nat. Neurosci. 2007, 10, 331–339. [Google Scholar] [CrossRef]
- Bonansco, C.; Couve, A.; Perea, G.; Ferradas, C.Á.; Roncagliolo, M.; Fuenzalida, M. Glutamate Released Spontaneously from Astrocytes Sets the Threshold for Synaptic Plasticity. Eur. J. Neurosci. 2011, 33, 1483–1492. [Google Scholar] [CrossRef]
- Ota, Y.; Zanetti, A.T.; Hallock, R.M. The Role of Astrocytes in the Regulation of Synaptic Plasticity and Memory Formation. Neural Plast. 2013, 2013, 185463. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, Y.; Xi, W.; Lou, H.F.; Zhu, L.; Guo, Z.; Mei, L.; Duan, S. Glia-Derived ATP Inversely Regulates Excitability of Pyramidal and CCK-Positive Neurons. Nat. Commun. 2017, 8, 13772. [Google Scholar] [CrossRef]
- Sasaki, T.; Matsuki, N.; Ikegaya, Y. Action-Potential Modulation during Axonal Conduction. Science (1979) 2011, 331, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Lezmy, J.; Arancibia-Cárcamo, I.L.; Quintela-López, T.; Sherman, D.L.; Brophy, P.J.; Attwell, D. Astrocyte Ca2+-Evoked ATP Release Regulates Myelinated Axon Excitability and Conduction Speed. Science (1979) 2021, 374, eabh2858. [Google Scholar] [CrossRef]
- Hamilton, N.; Vayro, S.; Kirchhoff, F.; Verkhratsky, A.; Robbins, J.; Gorecki, D.C.; Butt, A.M. Mechanisms of ATP- and Glutamate-Mediated Calcium Signaling in White Matter Astrocytes. Glia 2008, 56, 734–749. [Google Scholar] [CrossRef] [PubMed]
- Pasti, L.; Volterra, A.; Pozzan, T.; Carmignoto, G. Intracellular Calcium Oscillations in Astrocytes: A Highly Plastic, Bidirectional Form of Communication between Neurons and Astrocytes In Situ. J. Neurosci. 1997, 17, 7817–7830. [Google Scholar] [CrossRef]
- Li, C.; Zhao, R.; Gao, K.; Wei, Z.; Yin, M.Y.; Lau, L.T.; Chui, D.; Cheung, A.; Yu, H.; Kang, B.H. Astrocytes: Implications for Neuroinflammatory Pathogenesis of Alzheimer’s Disease. Curr. Alzheimer Res. 2011, 8, 67–80. [Google Scholar] [CrossRef]
- Patel, N.S.; Paris, D.; Mathura, V.; Quadros, A.N.; Crawford, F.C.; Mullan, M.J. Inflammatory Cytokine Levels Correlate with Amyloid Load in Transgenic Mouse Models of Alzheimer’s Disease. J. Neuroinflamm. 2005, 2, 9. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, I.D.; Lim, J.; Moon, J.S. Pathological Phenotypes of Astrocytes in Alzheimer’s Disease. Exp. Mol. Med. 2024, 56, 95–99. [Google Scholar] [CrossRef]
- Talantova, M.; Sanz-Blasco, S.; Zhang, X.; Xia, P.; Akhtar, M.W.; Okamoto, S.; Dziewczapolski, G.; Nakamura, T.; Cao, G.; Pratt, A.E.; et al. Aβ Induces Astrocytic Glutamate Release, Extrasynaptic NMDA Receptor Activation, and Synaptic Loss. Proc. Natl. Acad. Sci. USA 2013, 110, E2518–E2527. [Google Scholar] [CrossRef]
- Magee, J.C.; Grienberger, C. Synaptic Plasticity Forms and Functions. Annu. Rev. Neurosci. 2020, 43, 95–117. [Google Scholar] [CrossRef]
- Morris, R.G.M.D.O. Hebb: The Organization of Behavior. Brain Res. Bull. 1999, 50, 437. [Google Scholar] [CrossRef]
- Pozo, K.; Goda, Y. Unraveling Mechanisms of Homeostatic Synaptic Plasticity. Neuron 2010, 66, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, M.W.; Arizono, M.; Hisatsune, C.; Bannai, H.; Ebisui, E.; Sherwood, J.L.; Panatier, A.; Oliet, S.H.R.; Mikoshiba, K. Astrocytic IP3Rs: Contribution to Ca2+ Signalling and Hippocampal LTP. Glia 2017, 65, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.; Perea, G.; de Sevilla, D.F.; Gómez-Gonzalo, M.; Núñez, A.; Martín, E.D.; Araque, A. Astrocytes Mediate in Vivo Cholinergic-Induced Synaptic Plasticity. PLoS Biol. 2012, 10, e1001259. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Zhang, M.; Wang, Q.; Wu, D.Y.; Jie, W.; Hu, N.Y.; Lan, J.Z.; Zeng, K.; Li, S.J.; Li, X.W.; et al. Distinct Roles of Astroglia and Neurons in Synaptic Plasticity and Memory. Mol. Psychiatry 2022, 27, 873–885. [Google Scholar] [CrossRef]
- Henneberger, C.; Papouin, T.; Oliet, S.H.R.; Rusakov, D.A. Long-Term Potentiation Depends on Release of d-Serine from Astrocytes. Nature 2010, 463, 232–236. [Google Scholar] [CrossRef]
- Papouin, T.; Dunphy, J.M.; Tolman, M.; Dineley, K.T.; Haydon, P.G. Septal Cholinergic Neuromodulation Tunes the Astrocyte-Dependent Gating of Hippocampal NMDA Receptors to Wakefulness. Neuron 2017, 94, 840–854.e7. [Google Scholar] [CrossRef]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef]
- Navarrete, M.; Araque, A. Endocannabinoids Potentiate Synaptic Transmission through Stimulation of Astrocytes. Neuron 2010, 68, 113–126. [Google Scholar] [CrossRef]
- Robin, L.M.; Oliveira da Cruz, J.F.; Langlais, V.C.; Martin-Fernandez, M.; Metna-Laurent, M.; Busquets-Garcia, A.; Bellocchio, L.; Soria-Gomez, E.; Papouin, T.; Varilh, M.; et al. Astroglial CB1 Receptors Determine Synaptic D-Serine Availability to Enable Recognition Memory. Neuron 2018, 98, 935–944.e5. [Google Scholar] [CrossRef]
- Nalbantoglu, J.; Tirado-Santiago, G.; Lahsaïni, A.; Poirier, J.; Goncalves, O.; Verge, G.; Momoli, F.; Welner, S.A.; Massicotte, G.; Julien, J.-P.; et al. Impaired Learning and LTP in Mice Expressing the Carboxy Terminus of the Alzheimer Amyloid Precursor Protein. Nature 1997, 387, 500–505. [Google Scholar] [CrossRef]
- Åbjørsbråten, K.S.; Skaaraas, G.H.S.; Cunen, C.; Bjørnstad, D.M.; Binder, K.M.G.; Bojarskaite, L.; Jensen, V.; Nilsson, L.N.; Rao, S.B.; Tang, W.; et al. Impaired Astrocytic Ca2+ Signaling in Awake-Behaving Alzheimer’s Disease Transgenic Mice. Elife 2022, 11, e75055. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.P.; Koutsilieri, E.; Bartl, J.; Neuen-Jacob, E.; Arzberger, T.; Zander, N.; Ravid, R.; Roggendorf, W.; Riederer, P.; Grünblatt, E. Alterations in Expression of Glutamatergic Transporters and Receptors in Sporadic Alzheimer’s Disease. J. Alzheimer’s Dis. 2007, 11, 97–116. [Google Scholar] [CrossRef] [PubMed]
- de Pins, B.; Cifuentes-Díaz, C.; Thamila Farah, A.; López-Molina, L.; Montalban, E.; Sancho-Balsells, A.; López, A.; Ginés, S.; Delgado-García, J.M.; Alberch, J.; et al. Conditional BDNF Delivery from Astrocytes Rescues Memory Deficits, Spine Density and Synaptic Properties in the 5xFAD Mouse Model of Alzheimer Disease. J. Neurosci. 2019, 39, 2441–2458. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kesner, P.; Metna-Laurent, M.; Duan, T.; Xu, L.; Georges, F.; Koehl, M.; Abrous, D.N.; Mendizabal-Zubiaga, J.; Grandes, P.; et al. Acute Cannabinoids Impair Working Memory through Astroglial CB1 Receptor Modulation of Hippocampal LTD. Cell 2012, 148, 1039–1050. [Google Scholar] [CrossRef]
- Duffy, S.; Labrie, V.; Roder, J.C. D-Serine Augments NMDA-NR2B Receptor-Dependent Hippocampal Long-Term Depression and Spatial Reversal Learning. Neuropsychopharmacology 2008, 33, 1004–1018. [Google Scholar] [CrossRef]
- Zhang, Z.; Gong, N.; Wang, W.; Xu, L.; Xu, T.-L. Bell-Shaped D-Serine Actions on Hippocampal Long-Term Depression and Spatial Memory Retrieval. Cereb. Cortex 2008, 18, 2391–2401. [Google Scholar] [CrossRef]
- Lalo, U.; Pankratov, Y. Role for Astrocytes in MGluR-Dependent LTD in the Neocortex and Hippocampus. Brain Sci. 2022, 12, 1718. [Google Scholar] [CrossRef]
- Cavaccini, A.; Durkee, C.; Kofuji, P.; Tonini, R.; Araque, A. Astrocyte Signaling Gates Long-Term Depression at Corticostriatal Synapses of the Direct Pathway. J. Neurosci. 2020, 40, 5757–5768. [Google Scholar] [CrossRef]
- Navarrete, M.; Cuartero, M.I.; Palenzuela, R.; Draffin, J.E.; Konomi, A.; Serra, I.; Colié, S.; Castaño-Castaño, S.; Hasan, M.T.; Nebreda, Á.R.; et al. Astrocytic P38α MAPK Drives NMDA Receptor-Dependent Long-Term Depression and Modulates Long-Term Memory. Nat. Commun. 2019, 10, 2968. [Google Scholar] [CrossRef]
- Noriega-Prieto, J.A.; Maglio, L.E.; Zegarra-Valdivia, J.A.; Pignatelli, J.; Fernandez, A.M.; Martinez-Rachadell, L.; Fernandes, J.; Núñez, Á.; Araque, A.; Torres-Alemán, I.; et al. Astrocytic IGF-IRs Induce Adenosine-Mediated Inhibitory Downregulation and Improve Sensory Discrimination. J. Neurosci. 2021, 41, 4768–4781. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, X.; Zimmermann, H.R.; Cavener, D.R.; Klann, E.; Ma, T. Repression of the EIF2α Kinase PERK Alleviates MGluR-LTD Impairments in a Mouse Model of Alzheimer’s Disease. Neurobiol. Aging 2016, 41, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, X.; Sava, V.; Weeber, E.J.; Sanchez-Ramos, J. In Vivo Administration of Granulocyte Colony-stimulating Factor Restores Long-term Depression in Hippocampal Slices Prepared from Transgenic APP/PS1 Mice. J. Neurosci. Res. 2014, 92, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Bedse, G.; Romano, A.; Cianci, S.; Lavecchia, A.M.; Lorenzo, P.; Elphick, M.R.; LaFerla, F.M.; Vendemiale, G.; Grillo, C.; Altieri, F.; et al. Altered Expression of the CB1 Cannabinoid Receptor in the Triple Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 40, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Xu, F.; Liu, Z.; Zhao, Y.; Yang, L.Z.; Fang, W. Progress of Astrocyte-Neuron Crosstalk in Central Nervous System Diseases. Neurochem. Res. 2024, 49, 3187–3207. [Google Scholar] [CrossRef]
- Hulshof, L.A.; van Nuijs, D.; Hol, E.M.; Middeldorp, J. The Role of Astrocytes in Synapse Loss in Alzheimer’s Disease: A Systematic Review. Front. Cell. Neurosci. 2022, 16, 899251. [Google Scholar] [CrossRef]
- Brzosko, Z.; Mierau, S.B.; Paulsen, O. Neuromodulation of Spike-Timing-Dependent Plasticity: Past, Present, and Future. Neuron 2019, 103, 563–581. [Google Scholar] [CrossRef]
- Andrade-Talavera, Y.; Pérez-Rodríguez, M.; Prius-Mengual, J.; Rodríguez-Moreno, A. Neuronal and Astrocyte Determinants of Critical Periods of Plasticity. Trends Neurosci. 2023, 46, 566–580. [Google Scholar] [CrossRef]
- Falcón-Moya, R.; Pérez-Rodríguez, M.; Prius-Mengual, J.; Andrade-Talavera, Y.; Arroyo-García, L.E.; Pérez-Artés, R.; Mateos-Aparicio, P.; Guerra-Gomes, S.; Oliveira, J.F.; Flores, G.; et al. Astrocyte-Mediated Switch in Spike Timing-Dependent Plasticity during Hippocampal Development. Nat. Commun. 2020, 11, 4388. [Google Scholar] [CrossRef]
- Martinez-Gallego, I.; Perez-Rodriguez, M.; Coatl-Cuaya, H.; Flores, G.; Rodriguez-Moreno, A. Adenosine and Astrocytes Determine the Developmental Dynamics of Spike Timing-Dependent Plasticity in the Somatosensory Cortex. J. Neurosci. 2022, 42, 6038–6052. [Google Scholar] [CrossRef]
- Martínez-Gallego, I.; Coatl-Cuaya, H.; Rodriguez-Moreno, A. Astrocytes Mediate Two Forms of Spike Timing-Dependent Depression at Entorhinal Cortex-Hippocampal Synapses. Elife 2024, 13, RP98031. [Google Scholar] [CrossRef]
- Min, R.; Nevian, T. Astrocyte Signaling Controls Spike Timing-Dependent Depression at Neocortical Synapses. Nat. Neurosci. 2012, 15, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Ponzo, V.; Motta, C.; Bonnì, S.; Picazio, S.; Caltagirone, C.; Bozzali, M.; Martorana, A.; Koch, G. Impaired Spike Timing Dependent Cortico-Cortical Plasticity in Alzheimer’s Disease Patients. J. Alzheimer’s Dis. 2018, 66, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Garad, M.; Edelmann, E.; Leßmann, V. Impairment of Spike-Timing-Dependent Plasticity at Schaffer Collateral-CA1 Synapses in Adult APP/PS1 Mice Depends on Proximity of Aβ Plaques. Int. J. Mol. Sci. 2021, 22, 1378. [Google Scholar] [CrossRef] [PubMed]
- Shemer, I.; Holmgren, C.; Min, R.; Fülöp, L.; Zilberter, M.; Sousa, K.M.; Farkas, T.; Härtig, W.; Penke, B.; Burnashev, N.; et al. Non-fibrillar Β-amyloid Abates Spike-timing-dependent Synaptic Potentiation at Excitatory Synapses in Layer 2/3 of the Neocortex by Targeting Postsynaptic AMPA Receptors. Eur. J. Neurosci. 2006, 23, 2035–2047. [Google Scholar] [CrossRef]
- Kucukdereli, H.; Allen, N.J.; Lee, A.T.; Feng, A.; Ozlu, M.I.; Conatser, L.M.; Chakraborty, C.; Workman, G.; Weaver, M.; Sage, E.H.; et al. Control of Excitatory CNS Synaptogenesis by Astrocyte-Secreted Proteins Hevin and SPARC. Proc. Natl. Acad. Sci. USA 2011, 108, E440–E449. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, W.Y.; Cheung, K.; Hung, K.W.; Chen, C.; Geng, H.; Yung, W.-H.; Qu, J.Y.; Fu, A.K.Y.; Ip, N.Y. Astrocyte-Secreted IL-33 Mediates Homeostatic Synaptic Plasticity in the Adult Hippocampus. Proc. Natl. Acad. Sci. USA 2021, 118, e2020810118. [Google Scholar] [CrossRef]
- Heir, R.; Abbasi, Z.; Komal, P.; Altimimi, H.F.; Franquin, M.; Moschou, D.; Chambon, J.; Stellwagen, D. Astrocytes Are the Source of TNF Mediating Homeostatic Synaptic Plasticity. J. Neurosci. 2024, 44, e2278222024. [Google Scholar] [CrossRef]
- Stellwagen, D.; Malenka, R.C. Synaptic Scaling Mediated by Glial TNF-α. Nature 2006, 440, 1054–1059. [Google Scholar] [CrossRef]
- Heir, R.; Stellwagen, D. TNF-Mediated Homeostatic Synaptic Plasticity: From in Vitro to in Vivo Models. Front. Cell. Neurosci. 2020, 14, 565841. [Google Scholar] [CrossRef]
- Jones, E.V.; Bernardinelli, Y.; Tse, Y.C.; Chierzi, S.; Wong, T.P.; Murai, K.K. Astrocytes Control Glutamate Receptor Levels at Developing Synapses through SPARC–β-Integrin Interactions. J. Neurosci. 2011, 31, 4154–4165. [Google Scholar] [CrossRef]
- Lalo, U.; Bogdanov, A.; Moss, G.W.; Pankratov, Y. Astroglia-Derived BDNF and MSK-1 Mediate Experience- and Diet-Dependent Synaptic Plasticity. Brain Sci. 2020, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Reimers, J.M.; Loweth, J.A.; Wolf, M.E. BDNF Contributes to Both Rapid and Homeostatic Alterations in AMPA Receptor Surface Expression in Nucleus Accumbens Medium Spiny Neurons. Eur. J. Neurosci. 2014, 39, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- de León-López, C.A.M.; Carretero-Rey, M.; Khan, Z.U. AMPA Receptors in Synaptic Plasticity, Memory Function, and Brain Diseases. Cell. Mol. Neurobiol. 2025, 45, 14. [Google Scholar] [CrossRef] [PubMed]
- Forloni, G.; Mangiarotti, F.; Angeretti, N.; Lucca, E.; De Simoni, M.G. β-Amyloid Fragment Potentiates IL-6 And TNF-α Secretion by LPS in Astrocytes But Not in Microglia. Cytokine 1997, 9, 759–762. [Google Scholar] [CrossRef]
- Brosseron, F.; Krauthausen, M.; Kummer, M.; Heneka, M.T. Body Fluid Cytokine Levels in Mild Cognitive Impairment and Alzheimer’s Disease: A Comparative Overview. Mol. Neurobiol. 2014, 50, 534–544. [Google Scholar] [CrossRef]
- Plantone, D.; Pardini, M.; Righi, D.; Manco, C.; Colombo, B.M.; De Stefano, N. The Role of TNF-α in Alzheimer’s Disease: A Narrative Review. Cells 2023, 13, 54. [Google Scholar] [CrossRef]
- Strunz, M.; Jarrell, J.T.; Cohen, D.S.; Rosin, E.R.; Vanderburg, C.R.; Huang, X. Modulation of SPARC/Hevin Proteins in Alzheimer’s Disease Brain Injury. J. Alzheimer’s Dis. 2019, 68, 695–710. [Google Scholar] [CrossRef]
- Cabral-Miranda, F.; Araujo, A.P.B.; Medinas, D.B.; Gomes, F.C.A. Astrocytic Hevin/SPARCL-1 Regulates Cognitive Decline in Pathological and Normal Brain Aging. Aging Cell 2025, 24, e14493. [Google Scholar] [CrossRef]
- Jiang, T.; Zheng, T.; Li, W.; Liu, N.; Wang, M. IL-33/ST2 Signaling Pathway and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Clin. Neurol. Neurosurg. 2023, 230, 107773. [Google Scholar] [CrossRef]
- Gilbert, J.; Shu, S.; Yang, X.; Lu, Y.; Zhu, L.-Q.; Man, H.-Y. β-Amyloid Triggers Aberrant over-Scaling of Homeostatic Synaptic Plasticity. Acta Neuropathol. Commun. 2016, 4, 131. [Google Scholar] [CrossRef]
- Palop, J.J.; Chin, J.; Roberson, E.D.; Wang, J.; Thwin, M.T.; Bien-Ly, N.; Yoo, J.; Ho, K.O.; Yu, G.-Q.; Kreitzer, A.; et al. Aberrant Excitatory Neuronal Activity and Compensatory Remodeling of Inhibitory Hippocampal Circuits in Mouse Models of Alzheimer’s Disease. Neuron 2007, 55, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Small, D.H. Network Dysfunction in Alzheimer’s Disease: Does Synaptic Scaling Drive Disease Progression? Trends Mol. Med. 2008, 14, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.S.; Chung, W.S. Astrocytes Regulate Neuronal Network Activity by Mediating Synapse Remodeling. Neurosci. Res. 2023, 187, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.T.; Eroglu, C. Molecular Mechanisms of Astrocyte-Induced Synaptogenesis. Curr. Opin. Neurobiol. 2017, 45, 113–120. [Google Scholar] [CrossRef]
- Sadick, J.S.; Liddelow, S.A. Don’t Forget Astrocytes When Targeting Alzheimer’s Disease. Br. J. Pharmacol. 2019, 176, 3585–3598. [Google Scholar] [CrossRef]
- Christopherson, K.S.; Ullian, E.M.; Stokes, C.C.A.; Mullowney, C.E.; Hell, J.W.; Agah, A.; Lawler, J.; Mosher, D.F.; Bornstein, P.; Barres, B.A. Thrombospondins Are Astrocyte-Secreted Proteins That Promote CNS Synaptogenesis. Cell 2005, 120, 421–433. [Google Scholar] [CrossRef]
- Rao, K.V.R.; Curtis, K.M.; Johnstone, J.T.; Norenberg, M.D. Amyloid-β Inhibits Thrombospondin 1 Release From Cultured Astrocytes: Effects on Synaptic Protein Expression. J. Neuropathol. Exp. Neurol. 2013, 72, 735–744. [Google Scholar] [CrossRef]
- Son, S.M.; Nam, D.W.; Cha, M.Y.; Kim, K.H.; Byun, J.; Ryu, H.; Mook-Jung, I. Thrombospondin-1 Prevents Amyloid Beta-Mediated Synaptic Pathology in Alzheimer’s Disease. Neurobiol. Aging 2015, 36, 3214–3227. [Google Scholar] [CrossRef]
- Kang, S.; Byun, J.; Son, S.M.; Mook-Jung, I. Thrombospondin-1 Protects against Aβ-Induced Mitochondrial Fragmentation and Dysfunction in Hippocampal Cells. Cell Death Discov. 2018, 4, 31. [Google Scholar] [CrossRef]
- Chung, W.S.; Clarke, L.E.; Wang, G.X.; Stafford, B.K.; Sher, A.; Chakraborty, C.; Joung, J.; Foo, L.C.; Thompson, A.; Chen, C.; et al. Astrocytes Mediate Synapse Elimination through MEGF10 and MERTK Pathways. Nature 2013, 504, 394–400. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.Y.; Noh, S.; Lee, H.; Lee, S.Y.; Mun, J.Y.; Park, H.; Chung, W.S. Astrocytes Phagocytose Adult Hippocampal Synapses for Circuit Homeostasis. Nature 2021, 590, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arboledas, A.; Davila, J.C.; Sanchez-Mejias, E.; Navarro, V.; Nuñez-Diaz, C.; Sanchez-Varo, R.; Sanchez-Mico, M.V.; Trujillo-Estrada, L.; Fernandez-Valenzuela, J.J.; Vizuete, M.; et al. Phagocytic Clearance of Presynaptic Dystrophies by Reactive Astrocytes in Alzheimer’s Disease. Glia 2018, 66, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Vainchtein, I.D.; Chin, G.; Cho, F.S.; Kelley, K.W.; Miller, J.G.; Chien, E.C.; Liddelow, S.A.; Nguyen, P.T.; Nakao-Inoue, H.; Dorman, L.C.; et al. Astrocyte-Derived Interleukin-33 Promotes Microglial Synapse Engulfment and Neural Circuit Development. Science (1979) 2018, 359, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Ghetti, A.; Pinto-Duarte, A.; Wang, X.; Dziewczapolski, G.; Galimi, F.; Huitron-Resendiz, S.; Piña-Crespo, J.C.; Roberts, A.J.; Verma, I.M.; et al. Astrocytes Contribute to Gamma Oscillations and Recognition Memory. Proc. Natl. Acad. Sci. USA 2014, 111, E3343–E3352. [Google Scholar] [CrossRef]
- Cheung, G.; Bataveljic, D.; Visser, J.; Kumar, N.; Moulard, J.; Dallérac, G.; Mozheiko, D.; Rollenhagen, A.; Ezan, P.; Mongin, C.; et al. Physiological Synaptic Activity and Recognition Memory Require Astroglial Glutamine. Nat. Commun. 2022, 13, 753. [Google Scholar] [CrossRef]
- Delcourte, S.; Bouloufa, A.; Rovera, R.; Bétry, C.; Abrial, E.; Dkhissi-Benyahya, O.; Heinrich, C.; Marcy, G.; Raineteau, O.; Haddjeri, N.; et al. Chemogenetic Activation of Prefrontal Astroglia Enhances Recognition Memory Performance in Rat. Biomed. Pharmacother. 2023, 166, 115384. [Google Scholar] [CrossRef]
- Vicente-Gutierrez, C.; Bonora, N.; Bobo-Jimenez, V.; Jimenez-Blasco, D.; Lopez-Fabuel, I.; Fernandez, E.; Josephine, C.; Bonvento, G.; Enriquez, J.A.; Almeida, A.; et al. Astrocytic Mitochondrial ROS Modulate Brain Metabolism and Mouse Behaviour. Nat. Metab. 2019, 1, 201–211. [Google Scholar] [CrossRef]
- Vezzoli, E.; Calì, C.; De Roo, M.; Ponzoni, L.; Sogne, E.; Gagnon, N.; Francolini, M.; Braida, D.; Sala, M.; Muller, D.; et al. Ultrastructural Evidence for a Role of Astrocytes and Glycogen-Derived Lactate in Learning-Dependent Synaptic Stabilization. Cereb. Cortex 2020, 30, 2114–2127. [Google Scholar] [CrossRef]
- Vignoli, B.; Battistini, G.; Melani, R.; Blum, R.; Santi, S.; Berardi, N.; Canossa, M. Peri-Synaptic Glia Recycles Brain-Derived Neurotrophic Factor for LTP Stabilization and Memory Retention. Neuron 2016, 92, 873–887. [Google Scholar] [CrossRef]
- Ameen-Ali, K.E.; Simpson, J.E.; Wharton, S.B.; Heath, P.R.; Sharp, P.S.; Brezzo, G.; Berwick, J. The Time Course of Recognition Memory Impairment and Glial Pathology in the HAPP-J20 Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 68, 609–624. [Google Scholar] [CrossRef]
- Ardiles, Á.O.; Tapia-Rojas, C.C.; Mandal, M.; Alexandre, F.; Kirkwood, A.; Inestrosa, N.C.; Palacios, A.G. Postsynaptic Dysfunction Is Associated with Spatial and Object Recognition Memory Loss in a Natural Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2012, 109, 13835–13840. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.R.; Stricker, N.H.; Libon, D.J.; Delano-Wood, L.; Salmon, D.P.; Delis, D.C.; Bondi, M.W. Yes/No Versus Forced-Choice Recognition Memory in Mild Cognitive Impairment and Alzheimer’s Disease: Patterns of Impairment and Associations with Dementia Severity. Clin. Neuropsychol. 2012, 26, 1201–1216. [Google Scholar] [CrossRef] [PubMed]
- Antuono, P.G.; Jones, J.L.; Wang, Y.; Li, S.-J. Decreased Glutamate + Glutamine in Alzheimer’s Disease Detected in Vivo with 1 H-MRS at 0.5 T. Neurology 2001, 56, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Angeli, S.; Kousiappa, I.; Stavrou, M.; Sargiannidou, I.; Georgiou, E.; Papacostas, S.S.; Kleopa, K.A. Altered Expression of Glial Gap Junction Proteins Cx43, Cx30, and Cx47 in the 5XFAD Model of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 582934. [Google Scholar] [CrossRef]
- Gupta, S.; Yadav, K.; Mantri, S.S.; Singhal, N.K.; Ganesh, S.; Sandhir, R. Evidence for Compromised Insulin Signaling and Neuronal Vulnerability in Experimental Model of Sporadic Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 8916–8935. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Role of Mitochondrial ROS in the Brain: From Physiology to Neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef]
- Meeker, R.; Williams, K. The P75 Neurotrophin Receptor: At the Crossroad of Neural Repair and Death. Neural Regen. Res. 2015, 10, 721. [Google Scholar] [CrossRef]
- Doron, A.; Rubin, A.; Benmelech-Chovav, A.; Benaim, N.; Carmi, T.; Refaeli, R.; Novick, N.; Kreisel, T.; Ziv, Y.; Goshen, I. Hippocampal Astrocytes Encode Reward Location. Nature 2022, 609, 772–778. [Google Scholar] [CrossRef]
- Curreli, S.; Bonato, J.; Romanzi, S.; Panzeri, S.; Fellin, T. Complementary Encoding of Spatial Information in Hippocampal Astrocytes. PLoS Biol. 2022, 20, e3001530. [Google Scholar] [CrossRef]
- Pannasch, U.; Vargová, L.; Reingruber, J.; Ezan, P.; Holcman, D.; Giaume, C.; Syková, E.; Rouach, N. Astroglial Networks Scale Synaptic Activity and Plasticity. Proc. Natl. Acad. Sci. USA 2011, 108, 8467–8472. [Google Scholar] [CrossRef]
- Hösli, L.; Binini, N.; Ferrari, K.D.; Thieren, L.; Looser, Z.J.; Zuend, M.; Zanker, H.S.; Berry, S.; Holub, M.; Möbius, W.; et al. Decoupling Astrocytes in Adult Mice Impairs Synaptic Plasticity and Spatial Learning. Cell Rep. 2022, 38, 110484. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, F.; de Concini, V.; Larrigaldie, V.; Benmerzoug, S.; Briault, S.; Togbé, D.; Ryffel, B.; Quesniaux, V.F.J.; Menuet, A. Hippocampal Interleukin-33 Mediates Neuroinflammation-Induced Cognitive Impairments. J. Neuroinflamm. 2020, 17, 268. [Google Scholar] [CrossRef] [PubMed]
- Lester, A.W.; Moffat, S.D.; Wiener, J.M.; Barnes, C.A.; Wolbers, T. The Aging Navigational System. Neuron 2017, 95, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Carlesimo, G.A.; Fadda, L.; Lorusso, S.; Caltagirone, C. Verbal and Spatial Memory Spans in Alzheimer’s and Multi-Infarct Dementia. Acta Neurol. Scand. 2009, 89, 132–138. [Google Scholar] [CrossRef]
- Stewart, S.; Cacucci, F.; Lever, C. Which Memory Task for My Mouse? A Systematic Review of Spatial Memory Performance in the Tg2576 Alzheimer’s Mouse Model. J. Alzheimer’s Dis. 2011, 26, 105–126. [Google Scholar] [CrossRef]
- Le Douce, J.; Maugard, M.; Veran, J.; Matos, M.; Jégo, P.; Vigneron, P.A.; Faivre, E.; Toussay, X.; Vandenberghe, M.; Balbastre, Y.; et al. Impairment of Glycolysis-Derived l-Serine Production in Astrocytes Contributes to Cognitive Deficits in Alzheimer’s Disease. Cell Metab. 2020, 31, 503–517.e8. [Google Scholar] [CrossRef]
- Riera, J.; Hatanaka, R.; Uchida, T.; Ozaki, T.; Kawashima, R. Quantifying the Uncertainty of Spontaneous Ca2+ Oscillations in Astrocytes: Particulars of Alzheimer’s Disease. Biophys. J. 2011, 101, 554–564. [Google Scholar] [CrossRef]
- Koulakoff, A.; Mei, X.; Orellana, J.A.; Sáez, J.C.; Giaume, C. Glial Connexin Expression and Function in the Context of Alzheimer’s Disease. Biochim. Biophys. Acta (BBA) Biomembr. 2012, 1818, 2048–2057. [Google Scholar] [CrossRef]
- Chapuis, J.; Hot, D.; Hansmannel, F.; Kerdraon, O.; Ferreira, S.; Hubans, C.; Maurage, C.A.; Huot, L.; Bensemain, F.; Laumet, G.; et al. Transcriptomic and Genetic Studies Identify IL-33 as a Candidate Gene for Alzheimer’s Disease. Mol. Psychiatry 2009, 14, 1004–1016. [Google Scholar] [CrossRef]
- Fu, A.K.Y.; Hung, K.-W.; Yuen, M.Y.F.; Zhou, X.; Mak, D.S.Y.; Chan, I.C.W.; Cheung, T.H.; Zhang, B.; Fu, W.-Y.; Liew, F.Y.; et al. IL-33 Ameliorates Alzheimer’s Disease-like Pathology and Cognitive Decline. Proc. Natl. Acad. Sci. USA 2016, 113, E2705–E2713. [Google Scholar] [CrossRef]
- Zhang, K.; Förster, R.; He, W.; Liao, X.; Li, J.; Yang, C.; Qin, H.; Wang, M.; Ding, R.; Li, R.; et al. Fear Learning Induces A7-Nicotinic Acetylcholine Receptor-Mediated Astrocytic Responsiveness That Is Required for Memory Persistence. Nat. Neurosci. 2021, 24, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Wu, J.; Zhu, Z.; Feng, X.; Qin, L.; Zhu, Y.; Sun, L.; Liu, Y.; Qiu, Z.; et al. Activation of Astrocytes in Hippocampus Decreases Fear Memory through Adenosine A1 Receptors. Elife 2020, 9, e57155. [Google Scholar] [CrossRef] [PubMed]
- Martin-Fernandez, M.; Jamison, S.; Robin, L.M.; Zhao, Z.; Martin, E.D.; Aguilar, J.; Benneyworth, M.A.; Marsicano, G.; Araque, A. Synapse-Specific Astrocyte Gating of Amygdala-Related Behavior. Nat. Neurosci. 2017, 20, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Li, W.P.; Su, X.H.; Hu, N.Y.; Hu, J.; Li, X.W.; Yang, J.M.; Gao, T.M. Astrocytes Mediate Cholinergic Regulation of Adult Hippocampal Neurogenesis and Memory Through M1 Muscarinic Receptor. Biol. Psychiatry 2022, 92, 984–998. [Google Scholar] [CrossRef]
- Pinto-Duarte, A.; Roberts, A.J.; Ouyang, K.; Sejnowski, T.J. Impairments in Remote Memory Caused by the Lack of Type 2 IP3 Receptors. Glia 2019, 67, 1976–1989. [Google Scholar] [CrossRef]
- Steele, J.W.; Brautigam, H.; Short, J.A.; Sowa, A.; Shi, M.; Yadav, A.; Weaver, C.M.; Westaway, D.; Fraser, P.E.; St George-Hyslop, P.H.; et al. Early Fear Memory Defects Are Associated with Altered Synaptic Plasticity and Molecular Architecture in the TgCRND8 Alzheimer’s Disease Mouse Model. J. Comp. Neurol. 2014, 522, 2319–2335. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Fukumoto, K.; Nagai, M.; Mizuguchi, A.; Kobashi, Y. Early Contextual Fear Memory Deficits in a Double-Transgenic Amyloid-β Precursor Protein/Presenilin 2 Mouse Model of Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Hirata, K.; Matsuoka, K.; Tagai, K.; Endo, H.; Tatebe, H.; Ono, M.; Kokubo, N.; Oyama, A.; Shinotoh, H.; Takahata, K.; et al. Altered Brain Energy Metabolism Related to Astrocytes in Alzheimer’s Disease. Ann. Neurol. 2024, 95, 104–115. [Google Scholar] [CrossRef]
- Lu, W.; Huang, J.; Sun, S.; Huang, S.; Gan, S.; Xu, J.; Yang, M.; Xu, S.; Jiang, X. Changes in Lactate Content and Monocarboxylate Transporter 2 Expression in Aβ25-35-Treated Rat Model of Alzheimer’s Disease. Neurol. Sci. 2015, 36, 871–876. [Google Scholar] [CrossRef]
- Bonzanni, M.; Braga, A.; Saito, T.; Saido, T.C.; Tesco, G.; Haydon, P.G. Adenosine Deficiency Facilitates CA1 Synaptic Hyperexcitability in the Presymptomatic Phase of a Knockin Mouse Model of Alzheimer Disease. iScience 2025, 28, 111616. [Google Scholar] [CrossRef]
- Fontana, I.C.; Kumar, A.; Nordberg, A. The Role of Astrocytic A7 Nicotinic Acetylcholine Receptors in Alzheimer Disease. Nat. Rev. Neurol. 2023, 19, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Trinh, P.N.H.; Baltos, J.-A.; Hellyer, S.D.; May, L.T.; Gregory, K.J. Adenosine Receptor Signalling in Alzheimer’s Disease. Purinergic Signal 2022, 18, 359–381. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Kitazawa, M.; Caccamo, A.; Baglietto-Vargas, D.; Estrada-Hernandez, T.; Cribbs, D.H.; Fisher, A.; LaFerla, F.M. Loss of Muscarinic M1 Receptor Exacerbates Alzheimer’s Disease–Like Pathology and Cognitive Decline. Am. J. Pathol. 2011, 179, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, M.W.; Arizono, M.; Panatier, A.; Mikoshiba, K.; Oliet, S.H.R. Astrocytic IP3Rs: Beyond IP3R2. Front. Cell. Neurosci. 2021, 15, 695817. [Google Scholar] [CrossRef]
- Grolla, A.A.; Fakhfouri, G.; Balzaretti, G.; Marcello, E.; Gardoni, F.; Canonico, P.L.; DiLuca, M.; Genazzani, A.A.; Lim, D. Aβ Leads to Ca2+ Signaling Alterations and Transcriptional Changes in Glial Cells. Neurobiol. Aging 2013, 34, 511–522. [Google Scholar] [CrossRef]
- Tyurikova, O.; Zheng, K.; Rings, A.; Drews, A.; Klenerman, D.; Rusakov, D.A. Monitoring Ca 2+ Elevations in Individual Astrocytes upon Local Release of Amyloid Beta in Acute Brain Slices. Brain Res. Bull. 2018, 136, 85–90. [Google Scholar] [CrossRef]
- Kater, M.S.J.; Badia-Soteras, A.; van Weering, J.R.T.; Smit, A.B.; Verheijen, M.H.G. Electron Microscopy Analysis of Astrocyte-Synapse Interactions Shows Altered Dynamics in an Alzheimer’s Disease Mouse Model. Front. Cell. Neurosci. 2023, 17, 1085690. [Google Scholar] [CrossRef]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–71.e14. [Google Scholar] [CrossRef]
- Nagai, J.; Bellafard, A.; Qu, Z.; Yu, X.; Ollivier, M.; Gangwani, M.R.; Diaz-Castro, B.; Coppola, G.; Schumacher, S.M.; Golshani, P.; et al. Specific and Behaviorally Consequential Astrocyte Gq GPCR Signaling Attenuation in Vivo with IβARK. Neuron 2021, 109, 2256–2274.e9. [Google Scholar] [CrossRef]
- Matos, M.; Shen, H.Y.; Augusto, E.; Wang, Y.; Wei, C.J.; Wang, Y.T.; Agostinho, P.; Boison, D.; Cunha, R.A.; Chen, J.F. Deletion of Adenosine A2A Receptors from Astrocytes Disrupts Glutamate Homeostasis Leading to Psychomotor and Cognitive Impairment: Relevance to Schizophrenia. Biol. Psychiatry 2015, 78, 763–774. [Google Scholar] [CrossRef]
- Logan, S.; Pharaoh, G.A.; Marlin, M.C.; Masser, D.R.; Matsuzaki, S.; Wronowski, B.; Yeganeh, A.; Parks, E.E.; Premkumar, P.; Farley, J.A.; et al. Insulin-like Growth Factor Receptor Signaling Regulates Working Memory, Mitochondrial Metabolism, and Amyloid-β Uptake in Astrocytes. Mol. Metab. 2018, 9, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.-D.; Liu, Z.-R.; Zhang, Y.-Q.; Zhang, X.-H. Connexin43 Hemichannels Contribute to Working Memory and Excitatory Synaptic Transmission of Pyramidal Neurons in the Prefrontal Cortex of Rats. Life Sci. 2021, 286, 120049. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.P.; Nagaraja, R.Y.; Yarbrough, H.P.; Owen, D.B.; Masingale, A.M.; Ranjit, R.; Stiles, M.A.; Murphy, A.; Agbaga, M.P.; Ahmad, M.; et al. Selective Ablation of Sod2 in Astrocytes Induces Sex-Specific Effects on Cognitive Function, D-Serine Availability, and Astrogliosis. J. Neurosci. 2022, 42, 5992–6006. [Google Scholar] [CrossRef] [PubMed]
- Wirths, O.; Breyhan, H.; Schäfer, S.; Roth, C.; Bayer, T.A. Deficits in Working Memory and Motor Performance in the APP/PS1ki Mouse Model for Alzheimer’s Disease. Neurobiol. Aging 2008, 29, 891–901. [Google Scholar] [CrossRef]
- Dudkin, K.N.; Chueva, l.V.; Makarov, F.N.; Bich, T.G.; Roer, A.E. Impairments in Working Memory and Decision-Taking Processes in Monkeys in a Model of Alzheimer’s Disease. Neurosci. Behav. Physiol. 2005, 35, 281–289. [Google Scholar] [CrossRef]
- Baddeley, A.D.; Bressi, S.; Della Sala, S.; Logie, R.; Spinnler, H. The Decline of Working Memory in Alzheimer’s Disease. A Longitudinal Study. Brain 1991, 114 Pt 6, 2521–2542. [Google Scholar] [CrossRef]
- Chu, D.C.M.; Penney, J.B.; Young, A.B. Cortical GABAB and GABA A Receptors in Alzheimer’s Disease. Neurology 1987, 37, 1454. [Google Scholar] [CrossRef]
- Martín-Belmonte, A.; Aguado, C.; Alfaro-Ruíz, R.; Moreno-Martínez, A.E.; de la Ossa, L.; Martínez-Hernández, J.; Buisson, A.; Shigemoto, R.; Fukazawa, Y.; Luján, R. Density of GABAB Receptors Is Reduced in Granule Cells of the Hippocampus in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2459. [Google Scholar] [CrossRef]
- Lopes, C.R.; Cunha, R.A.; Agostinho, P. Astrocytes and Adenosine A2A Receptors: Active Players in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 666710. [Google Scholar] [CrossRef]
- Frölich, L.; Blum-Degen, D.; Riederer, P.; Hoyer, S. A Disturbance in the Neuronal Insulin Receptor Signal Transduction in Sporadic Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 1999, 893, 290–293. [Google Scholar] [CrossRef]
- Miao, J.; Zhang, Y.; Su, C.; Zheng, Q.; Guo, J. Insulin-Like Growth Factor Signaling in Alzheimer’s Disease: Pathophysiology and Therapeutic Strategies. Mol. Neurobiol. 2025, 62, 3195–3225. [Google Scholar] [CrossRef] [PubMed]
- Madeira, D.; Domingues, J.; Lopes, C.R.; Canas, P.M.; Cunha, R.A.; Agostinho, P. Modification of Astrocytic Cx43 Hemichannel Activity in Animal Models of AD: Modulation by Adenosine A2A Receptors. Cell. Mol. Life Sci. 2023, 80, 340. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Kato, Y.; Takatsu, H.; Fukui, K. Relationship between Cognitive Dysfunction and Age-Related Variability in Oxidative Markers in Isolated Mitochondria of Alzheimer’s Disease Transgenic Mouse Brains. Biomedicines 2022, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Massaad, C.A.; Washington, T.M.; Pautler, R.G.; Klann, E. Overexpression of SOD-2 Reduces Hippocampal Superoxide and Prevents Memory Deficits in a Mouse Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13576–13581. [Google Scholar] [CrossRef]
- Esposito, L.; Raber, J.; Kekonius, L.; Yan, F.; Yu, G.-Q.; Bien-Ly, N.; Puoliväli, J.; Scearce-Levie, K.; Masliah, E.; Mucke, L. Reduction in Mitochondrial Superoxide Dismutase Modulates Alzheimer’s Disease-Like Pathology and Accelerates the Onset of Behavioral Changes in Human Amyloid Precursor Protein Transgenic Mice. J. Neurosci. 2006, 26, 5167–5179. [Google Scholar] [CrossRef]
- Rodríguez-Arellano, J.J.; Parpura, V.; Zorec, R.; Verkhratsky, A. Astrocytes in Physiological Aging and Alzheimer’s Disease. Neuroscience 2016, 323, 170–182. [Google Scholar] [CrossRef]
- Reichenbach, N.; Delekate, A.; Plescher, M.; Schmitt, F.; Krauss, S.; Blank, N.; Halle, A.; Petzold, G.C. Inhibition of Stat3-mediated Astrogliosis Ameliorates Pathology in an Alzheimer’s Disease Model. EMBO Mol. Med. 2019, 11, e9665. [Google Scholar] [CrossRef]
- Emílio dos Santos Frizzo, M.; Patrícia Dall, L.; Borges Dalcin, K.; Onofre Souza, D. Riluzole Enhances Glutamate Uptake in Rat Astrocyte Cultures. Cell. Mol. Neurobiol. 2004, 24, 123–128. [Google Scholar] [CrossRef]
- Ji, H.F.; Shen, L.; Zhang, H.Y. β-Lactam Antibiotics Are Multipotent Agents to Combat Neurological Diseases. Biochem. Biophys. Res. Commun. 2005, 333, 661–663. [Google Scholar] [CrossRef]
- Kirvell, S.L.; Esiri, M.; Francis, P.T. Down-regulation of Vesicular Glutamate Transporters Precedes Cell Loss and Pathology in Alzheimer’s Disease. J. Neurochem. 2006, 98, 939–950. [Google Scholar] [CrossRef]
- Coomans, E.M.; Schoonhoven, D.N.; Tuncel, H.; Verfaillie, S.C.J.; Wolters, E.E.; Boellaard, R.; Ossenkoppele, R.; den Braber, A.; Scheper, W.; Schober, P.; et al. In Vivo Tau Pathology Is Associated with Synaptic Loss and Altered Synaptic Function. Alzheimer’s Res. Ther. 2021, 13, 35. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz de León-López, C.A.; Navarro-Lobato, I.; Khan, Z.U. The Role of Astrocytes in Synaptic Dysfunction and Memory Deficits in Alzheimer’s Disease. Biomolecules 2025, 15, 910. https://doi.org/10.3390/biom15070910
Muñoz de León-López CA, Navarro-Lobato I, Khan ZU. The Role of Astrocytes in Synaptic Dysfunction and Memory Deficits in Alzheimer’s Disease. Biomolecules. 2025; 15(7):910. https://doi.org/10.3390/biom15070910
Chicago/Turabian StyleMuñoz de León-López, Cristina A., Irene Navarro-Lobato, and Zafar U. Khan. 2025. "The Role of Astrocytes in Synaptic Dysfunction and Memory Deficits in Alzheimer’s Disease" Biomolecules 15, no. 7: 910. https://doi.org/10.3390/biom15070910
APA StyleMuñoz de León-López, C. A., Navarro-Lobato, I., & Khan, Z. U. (2025). The Role of Astrocytes in Synaptic Dysfunction and Memory Deficits in Alzheimer’s Disease. Biomolecules, 15(7), 910. https://doi.org/10.3390/biom15070910






