Synergizing Liquid Biopsy and Hybrid PET Imaging for Prognostic Assessment in Prostate Cancer: A Focus Review
Abstract
1. Introduction
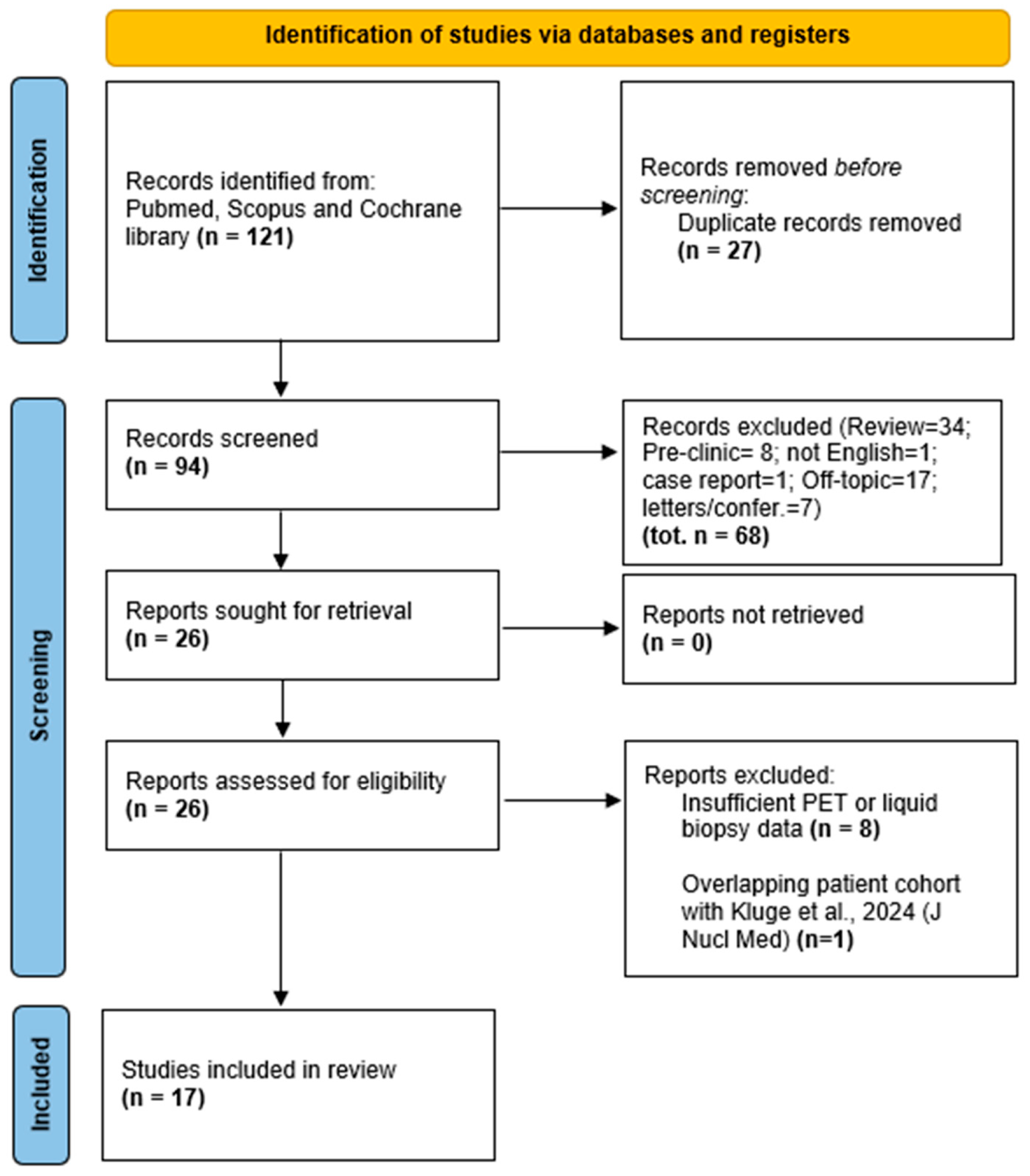
2. Materials and Methods
3. Results
3.1. Study Characteristics
3.2. PET Imaging Findings
3.3. Liquid Biopsy Biomarkers
3.4. Correlations Between PET and Liquid Biopsy
3.5. Complementary Diagnostic Value
3.6. Prognostic Implications
4. Discussion
4.1. Clinical Trials Ongoing
4.2. Challenges
4.3. Comparative Added Value of PET and Liquid Biopsy
4.4. Bridging Clinical Value with Real-World Applications
4.5. Future Directions
- •
- Glycan Score: Offers higher specificity than PSA; correlates with PSMA-PET parameters.
- •
- Met Score: Enhances detection sensitivity; correlates with PSMA-PET in both localized and advanced disease.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCa | prostate cancer |
| cfDNA | cell-free-DNA |
| PSA | prostate-specific antigen |
| SABR | Stereotactic ablative body radiotherapy |
| PSMA | prostate-specific membrane antigen |
| CTC | circulating tumor cell |
| PET | positron emission tomography |
| MR | magnetic resonance |
| TC | computed tomography |
| FCH | fluoroCholine |
| DCFPyL | Piflufolastat |
| SUV | standard uptake volume |
| PVV | positive predictive value |
| NPV | negative predictive value |
| mCRPC | metastatic castration-resistant prostate cancer |
| MTV | molecular tumor volume |
| TLA | total lesion activity |
| NaF | sodium fluoride |
| ptDNA | plasma tumor-DNA |
| EV | extracellular vesicle |
| DSA | digital scoring assay |
| mRNA | messenger-RNA |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 7–33. [Google Scholar] [CrossRef]
- Sekhoacha, M.; Smith, A.; Johnson, P. Advances in molecular imaging and biomarkers for prostate cancer: A review. J. Mol. Diagn. 2023, 25, 234–247. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk pros-tate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomized, multicenter study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven on-cology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, A.W.; Azad, A.A.; Volik, S.V.; Annala, M.; Beja, K.; McConeghy, B.; Haegert, A.; Warner, E.W.; Mo, F.; Brahmbhatt, S.; et al. Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncol. 2016, 2, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.N.; Higano, C.S.; Hahn, N.M.; Taylor, M.H.; Zhang, B.; Zhou, X.; Venkatakrishnan, K.; Leonard, E.J.; Sarantopoulos, J. Open‐label, multicenter, phase 1 study of alisertib (MLN8237), an aurora A kinase inhibitor, with docetaxel in patients with solid tumors. Cancer 2016, 122, 2524–2533. [Google Scholar] [CrossRef]
- Sartor, O. Circulating Tumor DNA Biomarkers for Response Assessment in Prostate Cancer. Clin. Cancer Res. 2023, 2745–2747. [Google Scholar] [CrossRef] [PubMed]
- Fragkiadaki, V. Correlation of PSA blood levels with standard uptake value maximum (SUV max ) and total metabolic tumor volume (TMTV) in 18F-PSMA-1007 and 18F-choline PET/CT in patients with biochemically recurrent prostate cancer. Nucl Med Commun. 2024, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Kluge, K.; Einspieler, H.; Haberl, D.; Spielvogel, C.; Stoiber, S.; Vraka, C.; Papp, L.; Wunsch, S.; Egger, G.; Kramer, G.; et al. Examining the relationship and prognostic significance of cell-free DNA levels and PSMA-positive tumor volume in men with prostate cancer: A retrospective-prospective [68Ga]Ga-PSMA-11 PET/CT study. J. Nucl. Med. 2024, in press. [CrossRef]
- Kluge, K.; Einspieler, H.; Haberl, D.; Spielvogel, C.; Amereller, D.; Egger, G.; Kramer, G.; Grubmüller, B.; Shariat, S.; Hacker, M.; et al. Comparison of discovery rates and prog-nostic utility of [68Ga]Ga-PSMA-11 PET/CT and circulating tumor DNA in prostate cancer—A cross-sectional study. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2833–2842. [Google Scholar] [CrossRef]
- Kwee, S.; Song, M.A.; Cheng, I.; Loo, L.; Tiirikainen, M. Measurement of circulating cell-free DNA in relation to 18F-fluorocholine PET/CT imaging in chemotherapy-treated advanced prostate cancer. Clin. Transl. Sci. 2012, 5, 65–70. [Google Scholar] [CrossRef]
- Aggarwal, R.; Behr, S.C.; Paris, P.; Truillet, C.; Parker, M.F.; Huynh, L.T.; Wei, J.; Hann, B.; Youngren, J.; Huang, J.; et al. Real-time transferrin-based PET detects MYC-positive prostate cancer. Mol. Cancer Res. 2017, 15, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Shi, W.Y.; Deek, M.P.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. A phase II randomized trial of observation versus stereotactic ablative radiation for oli-gometastatic prostate cancer (ORIOLE). JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Eng, P.; Sali, A. Screening for circulating tumour cells allows early detection of cancer and monitoring of treatment effec-tiveness: An observational study. Asian Pac. J. Cancer Prev. 2017, 18, 2133–2139. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Heath, E.I.; Ferrari, A.; Sperger, J.M.; Singh, A.; Perlman, S.B.; Roth, A.R.; Perk, T.G.; Modelska, K.; Porcari, A.; et al. Exploring spatial-temporal changes in 18F-sodium fluoride PET/CT and cir-culating tumor cells in metastatic castration-resistant prostate cancer treated with enzalutamide. J. Clin. Oncol. 2020, 38 (Suppl. S15), 5571. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Tamanna, T.; Matthews, S.; Eng, P.; Sali, A. New screening test improves detection of prostate cancer using circulating tumor cells and prostate-specific markers. Front. Oncol. 2020, 10, 1241. [Google Scholar] [CrossRef]
- Kessel, K.; Seifert, R.; Weckesser, M.; Roll, W.; Humberg, V.; Schlack, K.; Bögemann, M.; Bernemann, C.; Rahbar, K. Molecular analysis of circulating tumor cells of metastatic castration-resistant prostate cancer patients receiving 177Lu-PSMA-617 radioligand therapy. Theranostics 2020, 10, 652–663. [Google Scholar] [CrossRef]
- Emmett, L.; Subramaniam, S.; Joshua, A.M.; Crumbaker, M.; Martin, A.; Zhang, A.Y.; Rana, N.; Langford, A.; Mitchell, J.; Yip, S.; et al. ENZA-p trial protocol: A randomized phase II trial using prostate-specific membrane antigen as a therapeutic target and prognostic indicator in men with metastatic castration-resistant prostate cancer treated with enzalutamide (ANZUP 1901). BJU Int. 2021, in press. [Google Scholar] [CrossRef]
- Conteduca, V.; Scarpi, E.; Caroli, P.; Lolli, C.; Gurioli, G.; Brighi, N.; Poti, G.; Farolfi, A.; Altavilla, A.; Schepisi, G.; et al. Combining liquid biopsy and functional imaging analysis in metastatic castra-tion-resistant prostate cancer helps predict treatment outcome. Mol. Oncol. 2022, 16, 1325–1339. [Google Scholar] [CrossRef]
- Derlin, T.; Riethdorf, S.; Schumacher, U.; Lafos, M.; Peine, S.; Coith, C.; Ross, T.L.; Pantel, K.; Bengel, F.M. PSMA-heterogeneity in metastatic castration-resistant prostate cancer: Circulat-ing tumor cells, metastatic tumor burden, and response to targeted radioligand therapy. Prostate 2023, 83, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Sun, N.; Lee, Y.T.; Kim, M.; Vagner, T.; Rohena-Rivera, K.; Wang, Z.; Chen, Z.; Zhang, R.Y.; Lee, J.; et al. Prostate cancer extracellular vesicle digital scoring assay: A rapid noninvasive approach for quantification of disease-relevant mRNAs. Nano Today 2023, 48, 101733. [Google Scholar] [CrossRef]
- Ghous, M.H.; Afzal, S.; Arooj, M. Alternative and complementary therapies for early diagnosis of prostate cancer. Med. Forum 2023, 34, 48–52. [Google Scholar]
- Thomsen, L.C.V.; Honoré, A.; Reisæter, L.A.R.; Almås, B.; Børretzen, A.; Helle, S.I.; Førde, K.; Kristoffersen, E.K.; Kaada, S.H.; Melve, G.K.; et al. A phase I prospective, non-randomized trial of autologous dendritic cell-based cryoimmunotherapy in patients with metastatic castration-resistant prostate cancer. Cancer Immunol. Immunother. 2023, 72, 2215–2226. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Drozdov, I.A.; Boegemann, M.; Bodei, L.; Kunikowska, J.; Malczewska, A.; Bernemann, C.; Koduru, S.V.; Rahbar, K. Development of a multigenomic liquid biopsy (PROSTest) for prostate cancer in whole blood. Prostate 2024, in press. [Google Scholar] [CrossRef]
- Gupta, S.; Fernande, L.; Bourdon, D.; A Hamid, A.; Pasam, A.; Lam, E.; Wenstrup, R.; Sandhu, S. Detection of PSMA expression on circulating tumor cells by blood-based liquid biopsy in prostate cancer. J. Circ. Biomark. 2024, 13, 5–15. [Google Scholar] [CrossRef]
- Diaz-Fernandez, A.; Jochumsen, M.R.; Christensen, N.L.; Dalsgaard Sørensen, K.; Bouchelouche, K.; Borre, M.; Vendelbo, M.H.; Ferapontova, E.E. Liquid-biopsy glycan score biomarker accurately indicates and stratifies primary and metastatic prostate cancers. Anal. Chem. 2024, 96, 2382–2391. [Google Scholar] [CrossRef] [PubMed]
- Waibel, P.M.A.; Glavynskyi, I.; Fechter, T.; Mix, M.; Kind, F.; Sigle, A.; Jilg, C.A.; Gratzke, C.; Werner, M.; Schilling, O.; et al. Can PSMA PET Detect Intratumour Heterogeneity in His-tological PSMA Expression of Primary Prostate Cancer? Analysis of [68Ga]Ga-PSMA-11 and [18F]PSMA-1007. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Alshamrani, A.F.A. Diagnostic Accuracy of Molecular Imaging Techniques for Detecting Prostate Cancer: A Systematic Review. Diagnostics 2024, 14, 1315. [Google Scholar] [CrossRef]
- Casanova-Salas, I.; Athie, A.; Boutros, P.C.; Del Re, M.; Miyamoto, D.T.; Pienta, K.J.; Posadas, E.M.; Sowalsky, A.G.; Stenzl, A.; Wyatt, A.W.; et al. Quantitative and Qualitative Analysis of Blood-Based Liquid Biopsies to Inform Clinical Decision-Making in Prostate Cancer. Eur. Urol. 2021, 79, 762–771. [Google Scholar] [CrossRef]
- Haffner, M.C.; Zwart, W.; Roudier, M.P.; True, L.D.; Nelson, W.G.; Epstein, J.I.; Yegnasubramanian, S.; Isaacs, W.B. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 2019, 16, 259–270. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Halabi, S.; Luo, J.; Nanus, D.M.; Berry, W.R.; Hars, V.; Stadler, W.M.; Monk, J.P.; Pili, R.; Szmulewitz, R.Z.; et al. Prospective Multicenter Study of Circulating Tumor Cell AR-V7 and Taxane Versus Hormonal Treatment Outcomes in Metastatic Castration-Resistant Prostate Cancer. JCO Precis. Oncol. 2020, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Annala, M.; Vandekerkhove, G.; Khalaf, D.; Taavitsainen, S.; Beja, K.; Warner, E.W.; Leblanc, A.; Thomas, G.; Prager, B.C.; Saini, S.; et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2019, 8, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Matulewicz, R.S.; Narayan, V.; Hahn, N.M.; Appleman, L.J.; Aparicio, A.M.; Alva, A.S.; Szmulewitz, R.Z.; Vaishampayan, U.N.; Harshman, L.C.; et al. PSMA-targeted radionuclide therapy for metastatic castration-resistant prostate cancer: A review. JAMA Oncol. 2022, 8, 80–87. [Google Scholar] [CrossRef]
- Wu, J.; Pan, J.; Wang, B.; Wei, Y.; Ye, D.W. Tumor Heterogeneity and Treatment Response Assessment Using Next Generation Imaging and Liquid Biopsy in Patients with Metastatic Castration Resistant Prostate Cancer Receiving Abiraterone (ANGELA): A Sin-gle-Center Prospective Observational Trial. J. Clin. Oncol. 2023, 41, 5057. [Google Scholar]
- ENZA-p Study Investigators. ENZA-p: Enzalutamide and [^177Lu]Lu-PSMA-617 Versus Enzalutamide Alone in Metastatic Cas-tration-Resistant Prostate Cancer (NCT04419402). ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/study/NCT04419402 (accessed on 1 January 2020).
- Seifert, R.; Gafita, A.; Telli, T.; Voter, A.; Herrmann, K.; Pomper, M.; Hadaschik, B.; Rowe, S.P.; Fendler, W.P. Standardized PSMA-PET Imaging of Advanced Prostate Cancer. In Seminars in Nuclear Medicine; WB Saunders: Philadelphia, PA, USA, 2024; Volume 54, pp. 60–68. [Google Scholar]
- Aide, N.; Lasnon, C.; Veit-Haibach, P.; Sera, T.; Sattler, B.; Boellaard, R. EANM/EARL Harmonization Strategies in PET Quantification: From Daily Practice to Multicentre Oncological Studies. Eur. J. Nucl. Med. Mol. Imaging 2017, 44 (Suppl. S1), 17–31. [Google Scholar] [CrossRef]
- Kaalep, A.; Burggraaff, C.N.; Pieplenbosch, S.; Verwer, E.E.; Sera, T.; Zijlstra, J.; Hoekstra, O.S.; Oprea-Lager, D.E.; Boellaard, R. Quantitative Implications of the Updated EARL 2019 PET-CT Performance Standards. EJNMMI Phys. 2019, 6, 28. [Google Scholar] [CrossRef]
- Hernandez, K.M.; Bramlett, K.S.; Agius, P.; Baden, J.; Cao, R.; Clement, O.; Corner, A.S.; Craft, J.; Dean, D.A., 2nd; Dry, J.R.; et al. Contrived Materials and a Data Set for the Evaluation of Liquid Biopsy Tests: A Blood Profiling Atlas in Cancer (BLOODPAC) Community Study. J. Mol. Diagn. 2023, 25, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Tapper, W.; Carneiro, G.; Mikropoulos, C.; Thomas, S.A.; Evans, P.M.; Boussios, S. The Application of Radiomics and AI to Molecular Imaging for Prostate Cancer. J. Pers. Med. 2024, 14, 287. [Google Scholar] [CrossRef]
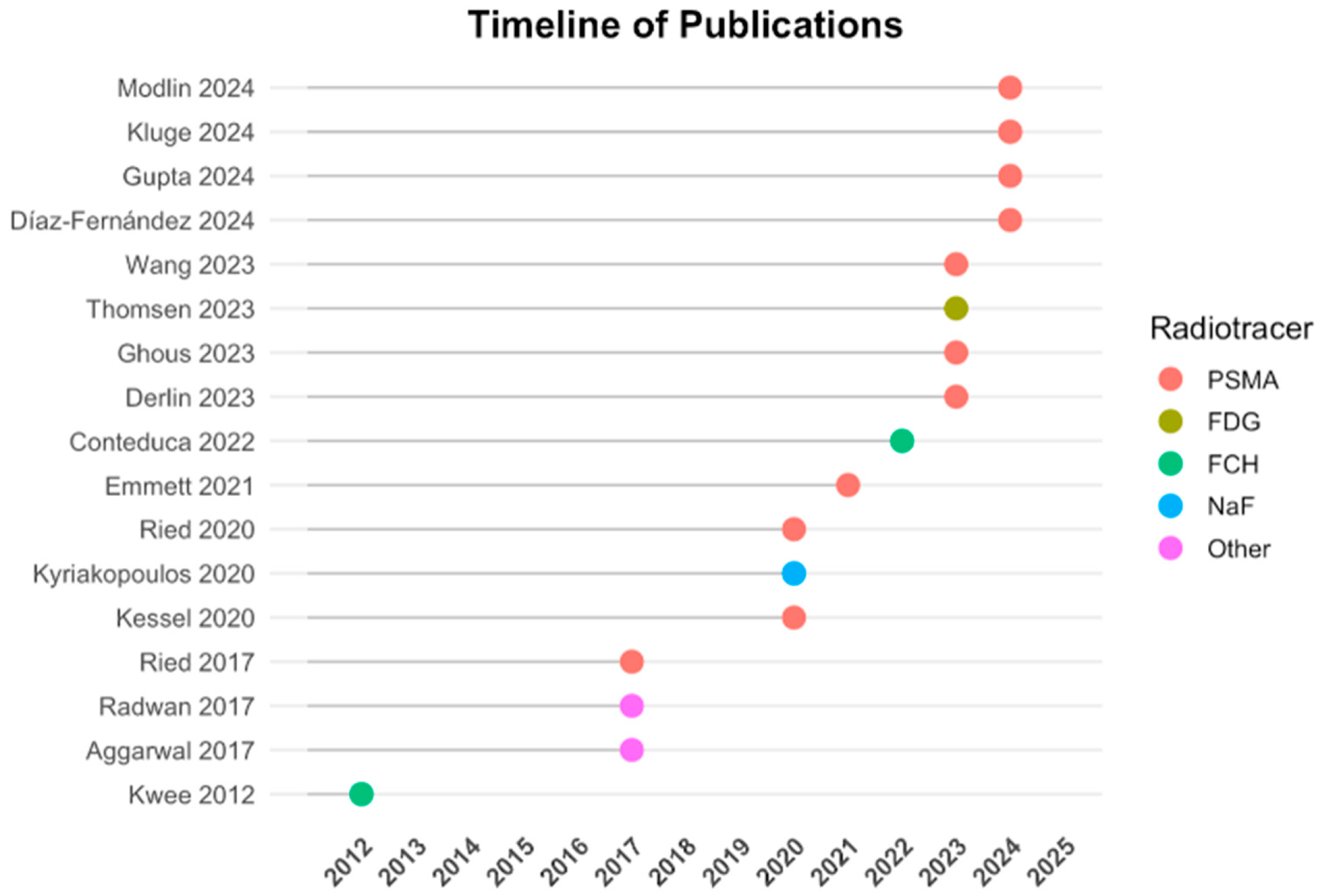
| Author, Year | Study Design | Patients (n) | Clinical Context | Imaging Modality | Liquid Biopsy Markers | Aim | Key Findings |
|---|---|---|---|---|---|---|---|
| Kwee S. et al., 2012 [11] | P | 8 | Metastatic castration-resistant PCa | [18F]FCH PET/TC | cfDNA | Evaluate cfDNA in response to chemotherapy | Significant correlation (r = −0.50, p = 0.01) between cfDNA levels and tumor activity on PET/CT |
| Aggarwal R. et al., 2017 [12] | P | 18 | Metastatic castration-resistant PCa | [68Ga]citrate-PET/TC and PET/MR | cfDNA | Detect MYC-positive prostate tumors | MYC gain detected in cfDNA for all PET-positive patients |
| Philips R. et al., 2017 [13] | CT | 54 | Oligo-metastatic PCa | [18F]DCFPyL PET | ctDNA | Evaluate PSA and imaging-based progression at 6 months post-SABR | SABR improved clinical outcomes; PSMA-PET enabled superior total disease consolidation compared to conventional imaging alone. |
| Ried K. et al., 2017 [14] | R | 69 | Localized disease/biochemical recurrence | [68Ga]Ga-PSMA-PET/CT | CTCs | Assess the relationship between CTC counts and cancer risk/status in patients undergoing treatment or asymptomatic screening. | Higher CTC counts strongly correlated with increased cancer risk; CTC screening demonstrated high sensitivity for early cancer detection. |
| Kyriakopoulos C.E. et al., 2020 [15] | CT | 23 | Metastatic castration-resistant PCa | [18F]NaF PET/TC | CTCs | Inter-lesional response heterogeneity in bone metastases via quantitative PET | SUVhetero changes were the strongest predictors of PSA progression; SUV-total increased at progression despite initial improvement during enzalutamide treatment. |
| Ried K. et al., 2020 [16] | R | 20 | Localized disease | PSMA-PET/TC | CTCs | Evaluate the ISET®-CTC test combined with prostate-specific markers. | The combination of ISET®-CTC and ICC-PSA-marker testing has a positive predictive value (PPV) of 99% and a negative predictive value (NPV) of 97%. |
| Kessel K. et al., 2020 [17] | P | 19 | Metastatic castration-resistant PCa | [18F]PSMA-1007 PET/TC | CTCs, AR-FL, AR-V7 mRNA | Correlate several clinical and molecular parameters with response to PSMA | AR-FL and AR-V7 might serve as prognostic biomarkers displaying high tumor burden in mCRPC patients prior to PSMA-RLT. |
| Emmett L. et al., 2021 [18] | CT | 160 | Metastatic castration-resistant PCa | [68Ga]Ga-PSMA and FDG PET/CT | cfDNA, CTCs | Identification of prognostic and predictive biomarkers from PSMA and FDG PET/CT and ctDNA. | Developed predictive and prognostic biomarkers to better guide treatment decisions. |
| Conteduca V. et al., 2022 [19] | P | 102 | Metastatic castration-resistant PCa | FCH-PET/CT | cfDNA, ptDNA | Investigate whether pretreatment ptDNA reflects metabolic tumor burden in combination with functional imaging. | A significant association was seen between ptDNA and SUVmax, MTV and TLA. |
| Derlin T. et al., 2023 [20] | P | 20 | Metastatic castration-resistant PCa | [68Ga]Ga-PSMA-11 PET/CT | CTCs | Explore the interrelation between CTCs and solid metastatic lesions | Liquid biopsy is complementary to PET for individual PSMA phenotyping of mCRPC. |
| Wang J.J. et al., 2023 [21] | R | 40 | Localized disease/metastatic PCa | [68Ga]Ga-PSMA-11 PET/CT, bone scan | mRNA, AR-V7, antiPSMA | Developing a PCa extracellular vesicle (EV) digital scoring assay (DSA) for detecting metastasis of PCa. | Met score distinguishes metastatic from localized PCa and reflects clinical behavior even when the disease was undetectable by imaging. |
| Ghous M.H. et al., 2023 [22] | R | 55 | Suspected or confirmed prostate cancer | [68Ga]Ga-PSMA-11 PET/CT | CTCs, ctDNA, exosomes | Find the role of liquid biopsy and molecular imaging for early diagnosis of PCa. | Liquid biopsy and molecular imaging have the potential to complement conventional screening methods for early PCa diagnosis. |
| Thomsen L.C.V. et al., 2023 [23] | C | 18 | Metastatic castration-resistant PCa | [18F]FDG PET/CT, 99Tc-bone scan | CTCs | Determined the safety and tolerability of cryoablation without and with checkpoint inhibitors. | Post-treatment symptoms were associated with CTCs presence while CTCs responses correlated with clinical outcomes; cryoimmunotherapy in mCRPC is safe and well tolerated. |
| Modlin I.M. et al., 2024 [24] | P | 178 | Localized disease/metastatic PCa | [68Ga]Ga-PSMA-11 PET/CT | CTCs | Development of a molecular assay from mRNA databases using machine learning in PCa. | Measuring blood expression provides a minimally invasive genomic tool that may facilitate PCa management. |
| Kluge K. et al., 2024 [9] | P/R | 148 | Metastatic hormone sensitive/metastatic castration resistant | [68Ga]Ga-PSMA-11 PET/CT | cfDNA | Evaluate the relationship and prognostic value of cfDNA and PSMA-TV in men with PCa | cfDNA does not reliably reflect total tumor burden or prognosis; although PSMA PET/CT provides a highly prognostic assessment of tumor burden across the spectrum of PCa disease progression. |
| Gupta S. et al., 2024 [25] | P | 24 | Metastatic castration-resistant PCa | [68Ga]Ga-PSMA-11 PET/CT | CTCs | CTCs may identify the patients most likely to benefit from PSMA-targeted RLT. | It demonstrates the potential to detect PSMA protein expression in CTCs from patients with mCRPC. |
| Diaz-Fernández A. et al., 2024 [26] | R | 30 | Localized and metastatic PCa | [18F]PSMA-1007 PET/TC | Glycan score | Using Glycan Score in cancer patients’ serum and proved its facility for stratification of PCa. | The Glycan Score test has a huge potential for accurate diagnosis and staging of PCa. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stracuzzi, F.; Dall’ Armellina, S.; Aghakhanyan, G.; Fanni, S.C.; Aringhieri, G.; Faggioni, L.; Neri, E.; Volterrani, D.; Cioni, D. Synergizing Liquid Biopsy and Hybrid PET Imaging for Prognostic Assessment in Prostate Cancer: A Focus Review. Biomolecules 2025, 15, 1041. https://doi.org/10.3390/biom15071041
Stracuzzi F, Dall’ Armellina S, Aghakhanyan G, Fanni SC, Aringhieri G, Faggioni L, Neri E, Volterrani D, Cioni D. Synergizing Liquid Biopsy and Hybrid PET Imaging for Prognostic Assessment in Prostate Cancer: A Focus Review. Biomolecules. 2025; 15(7):1041. https://doi.org/10.3390/biom15071041
Chicago/Turabian StyleStracuzzi, Federica, Sara Dall’ Armellina, Gayane Aghakhanyan, Salvatore C. Fanni, Giacomo Aringhieri, Lorenzo Faggioni, Emanuele Neri, Duccio Volterrani, and Dania Cioni. 2025. "Synergizing Liquid Biopsy and Hybrid PET Imaging for Prognostic Assessment in Prostate Cancer: A Focus Review" Biomolecules 15, no. 7: 1041. https://doi.org/10.3390/biom15071041
APA StyleStracuzzi, F., Dall’ Armellina, S., Aghakhanyan, G., Fanni, S. C., Aringhieri, G., Faggioni, L., Neri, E., Volterrani, D., & Cioni, D. (2025). Synergizing Liquid Biopsy and Hybrid PET Imaging for Prognostic Assessment in Prostate Cancer: A Focus Review. Biomolecules, 15(7), 1041. https://doi.org/10.3390/biom15071041









