Transcription-Coupled Nucleotide Excision Repair: A Faster Solution or the Only Option?
Abstract
1. Introduction
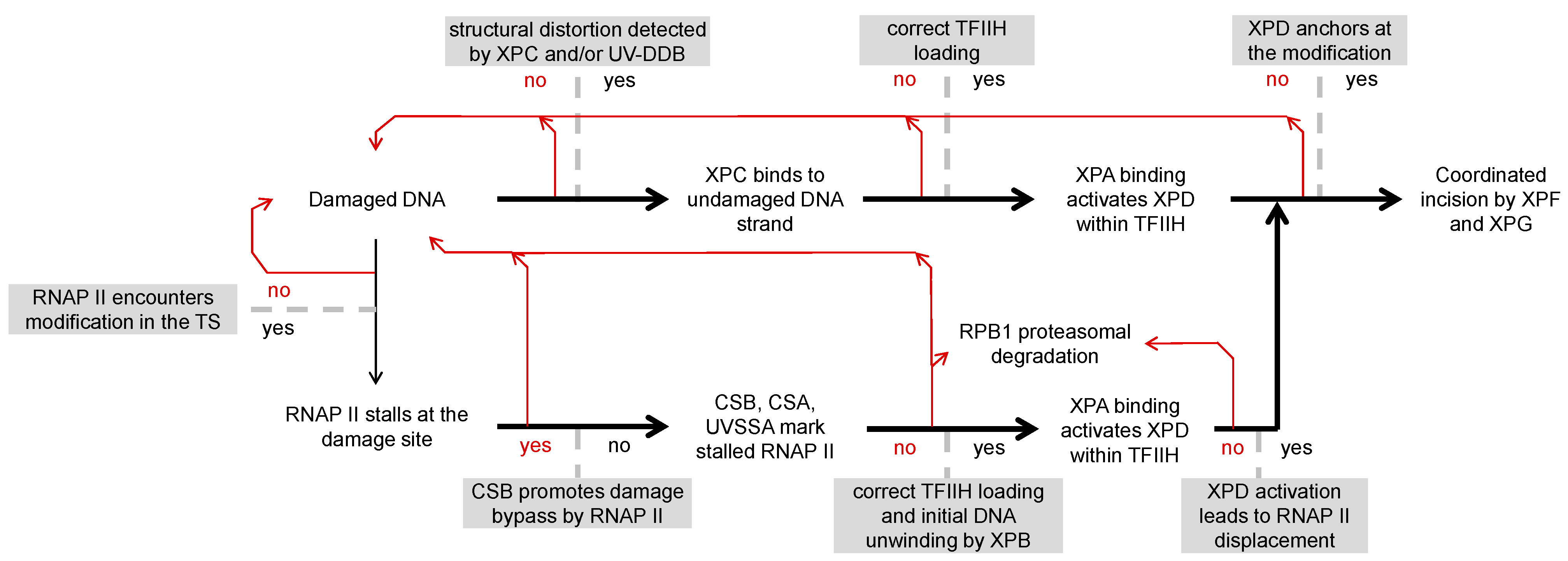
2. Multistep Damage Recognition in NER
3. Molecular Mechanisms of Damage Sensing in TC-NER
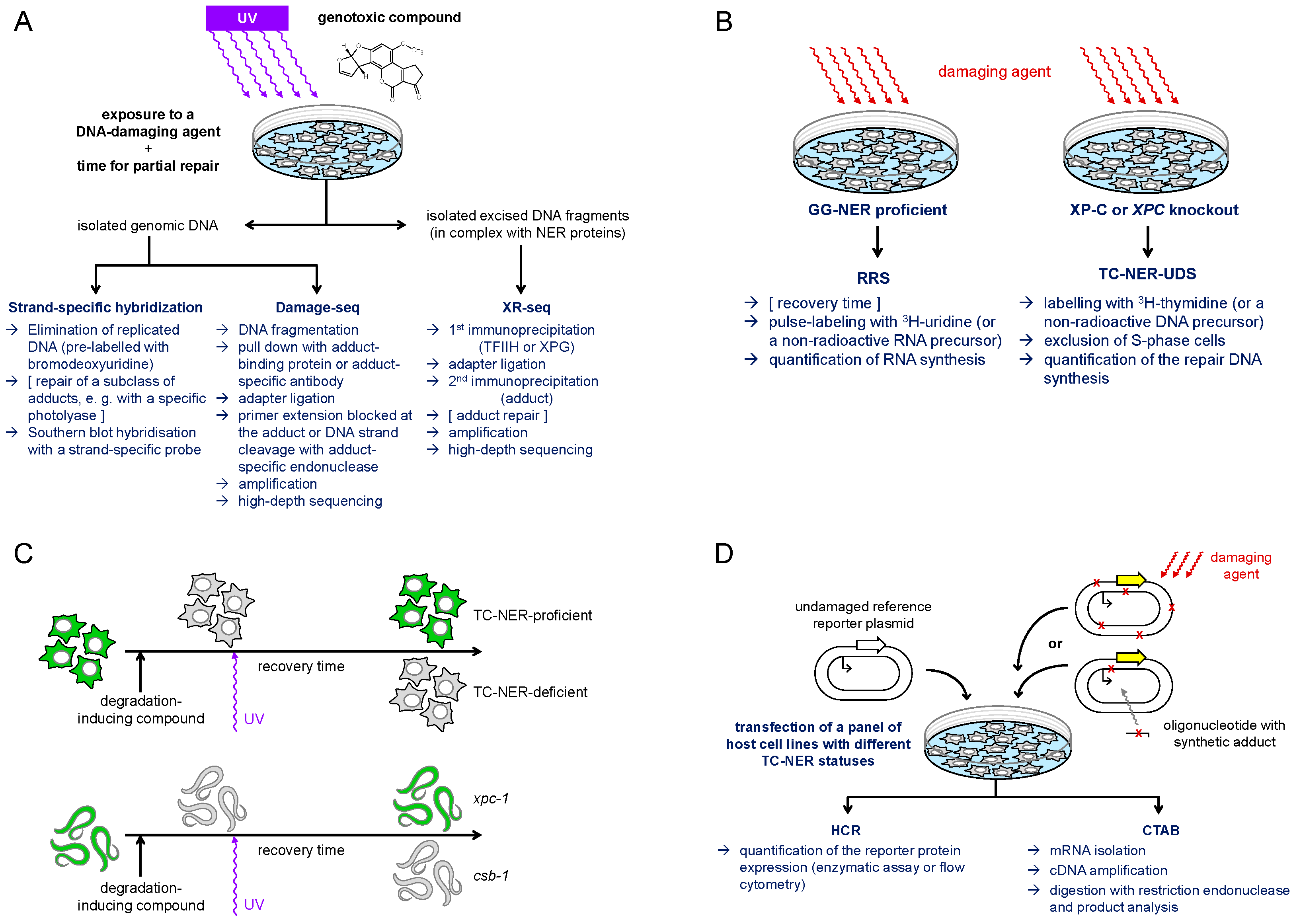
4. Available Methodology for Detection of TC-NER
4.1. Peculiarities of TC-NER Detection Compared to Pathways Active Genome-Wide
4.2. TC-NER Detection in Damaged Cells
4.2.1. Strand-Specific Detection of DNA Damage Removal
4.2.2. Transcription Recovery After Damage
4.2.3. Genome-Wide Mapping of Damage by Advanced Sequencing Techniques
4.3. Biochemical Reconstitution of TC-NER or of RNAP II Stalling
4.4. Repair of Damage in Transfected Vector DNA
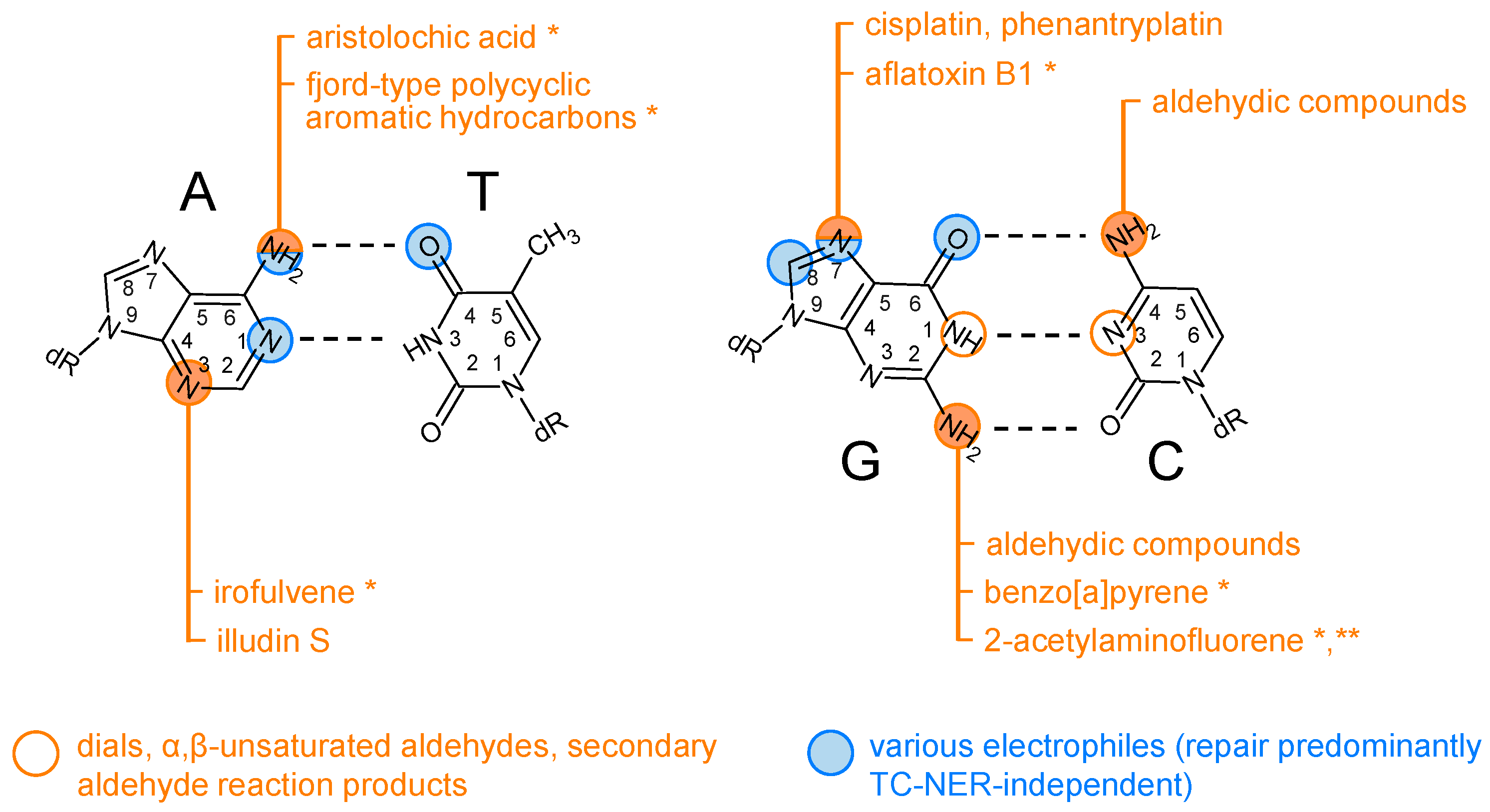
5. Types of DNA Damage Repaired Preferentially or Exclusively by TC-NER
5.1. UV-Induced Dipyrimidine Adducts
5.2. Adducts Formed by Reactive Metabolites of Carcinogenic Chemicals and Toxins
5.3. Adducts Formed by Alkylating(-like) Agents
5.4. Adducts Induced by Aldehydes
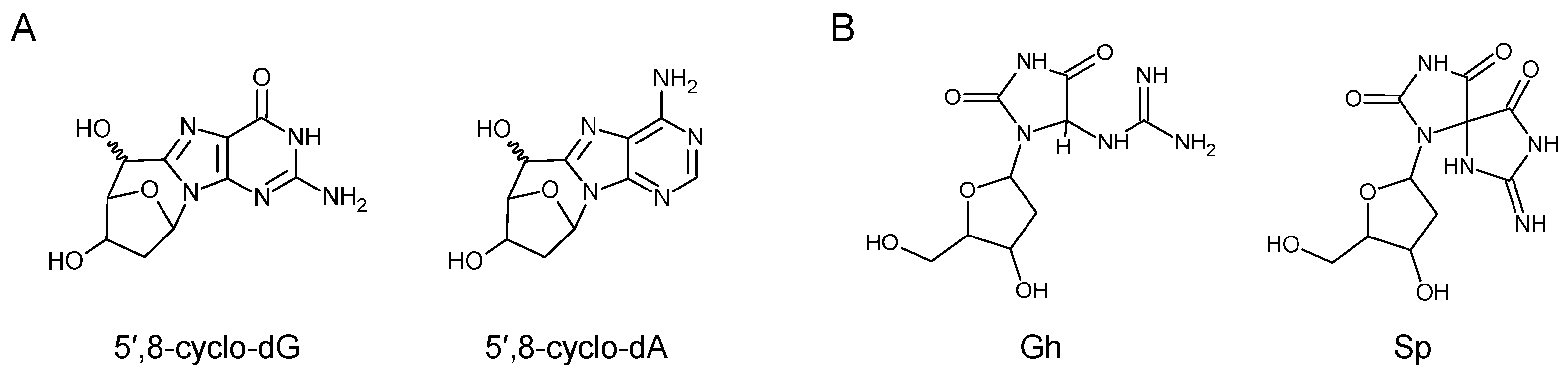
5.5. Damage to Nucleobases Induced by Endogenously Arising Reactive Oxygen Species
5.6. Can Apurinic/Apyrimidinic (AP) Lesions Be Processed by TC-NER?
6. Current Advances and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Jiricny, J. Postreplicative mismatch repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012633. [Google Scholar] [CrossRef]
- Krokan, H.E.; Bjoras, M. Base excision repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012583. [Google Scholar] [CrossRef] [PubMed]
- Scharer, O.D. Nucleotide excision repair in eukaryotes. Cold Spring Harb. Perspect. Biol. 2013, 5, a012609. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, W.; Fousteri, M. Mammalian transcription-coupled excision repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012625. [Google Scholar] [CrossRef]
- D’Souza, A.; Kim, M.; Chazin, W.J.; Scharer, O.D. Protein-protein interactions in the core nucleotide excision repair pathway. DNA Repair 2024, 141, 103728. [Google Scholar] [CrossRef]
- Nieto Moreno, N.; Olthof, A.M.; Svejstrup, J.Q. Transcription-Coupled Nucleotide Excision Repair and the Transcriptional Response to UV-Induced DNA Damage. Annu. Rev. Biochem. 2023, 92, 81–113. [Google Scholar] [CrossRef]
- Kuper, J.; Kisker, C. At the core of nucleotide excision repair. Curr. Opin. Struct. Biol. 2023, 80, 102605. [Google Scholar] [CrossRef]
- Kim, J.; Li, C.L.; Chen, X.; Cui, Y.; Golebiowski, F.M.; Wang, H.; Hanaoka, F.; Sugasawa, K.; Yang, W. Lesion recognition by XPC, TFIIH and XPA in DNA excision repair. Nature 2023, 617, 170–175. [Google Scholar] [CrossRef]
- Geacintov, N.E.; Broyde, S. Repair-Resistant DNA Lesions. Chem. Res. Toxicol. 2017, 30, 1517–1548. [Google Scholar] [CrossRef]
- Llerena Schiffmacher, D.A.; Pai, Y.J.; Pines, A.; Vermeulen, W. Transcription-coupled repair: Tangled up in convoluted repair. FEBS J. 2025. (online ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Lans, H.; Hoeijmakers, J.H.J.; Vermeulen, W.; Marteijn, J.A. The DNA damage response to transcription stress. Nat. Rev. Mol. Cell Biol. 2019, 20, 766–784. [Google Scholar] [CrossRef]
- Bukowska, B.; Karwowski, B.T. Actual state of knowledge in the field of diseases related with defective nucleotide excision repair. Life Sci. 2018, 195, 6–18. [Google Scholar] [CrossRef]
- Karikkineth, A.C.; Scheibye-Knudsen, M.; Fivenson, E.; Croteau, D.L.; Bohr, V.A. Cockayne syndrome: Clinical features, model systems and pathways. Ageing Res. Rev. 2017, 33, 3–17. [Google Scholar] [CrossRef]
- Marteijn, J.A.; Lans, H.; Vermeulen, W.; Hoeijmakers, J.H. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 2014, 15, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Troelstra, C.; van Gool, A.; de Wit, J.; Vermeulen, W.; Bootsma, D.; Hoeijmakers, J.H. ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne’s syndrome and preferential repair of active genes. Cell 1992, 71, 939–953. [Google Scholar] [CrossRef]
- Henning, K.A.; Li, L.; Iyer, N.; McDaniel, L.D.; Reagan, M.S.; Legerski, R.; Schultz, R.A.; Stefanini, M.; Lehmann, A.R.; Mayne, L.V.; et al. The Cockayne syndrome group A gene encodes a WD repeat protein that interacts with CSB protein and a subunit of RNA polymerase II TFIIH. Cell 1995, 82, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Mellon, I.; Spivak, G.; Hanawalt, P.C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell 1987, 51, 241–249. [Google Scholar] [CrossRef]
- Sugasawa, K.; Ng, J.M.; Masutani, C.; Maekawa, T.; Uchida, A.; van der Spek, P.J.; Eker, A.P.; Rademakers, S.; Visser, C.; Aboussekhra, A.; et al. Two human homologs of Rad23 are functionally interchangeable in complex formation and stimulation of XPC repair activity. Mol. Cell Biol. 1997, 17, 6924–6931. [Google Scholar] [CrossRef]
- Sugasawa, K.; Ng, J.M.; Masutani, C.; Iwai, S.; van der Spek, P.J.; Eker, A.P.; Hanaoka, F.; Bootsma, D.; Hoeijmakers, J.H. Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol. Cell 1998, 2, 223–232. [Google Scholar] [CrossRef]
- Sugasawa, K.; Okamoto, T.; Shimizu, Y.; Masutani, C.; Iwai, S.; Hanaoka, F. A multistep damage recognition mechanism for global genomic nucleotide excision repair. Genes. Dev. 2001, 15, 507–521. [Google Scholar] [CrossRef]
- Zhang, E.T.; He, Y.; Grob, P.; Fong, Y.W.; Nogales, E.; Tjian, R. Architecture of the human XPC DNA repair and stem cell coactivator complex. Proc. Natl. Acad. Sci. USA 2015, 112, 14817–14822. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Masutani, C.; Mizukoshi, T.; Kondo, J.; Hanaoka, F.; Iwai, S. Characterization of DNA recognition by the human UV-damaged DNA-binding protein. J. Biol. Chem. 1999, 274, 20027–20033. [Google Scholar] [CrossRef]
- Groisman, R.; Polanowska, J.; Kuraoka, I.; Sawada, J.; Saijo, M.; Drapkin, R.; Kisselev, A.F.; Tanaka, K.; Nakatani, Y. The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell 2003, 113, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.S.; Scrima, A.; Bohm, K.; Matsumoto, S.; Lingaraju, G.M.; Faty, M.; Yasuda, T.; Cavadini, S.; Wakasugi, M.; Hanaoka, F.; et al. The molecular basis of CRL4DDB2/CSA ubiquitin ligase architecture, targeting, and activation. Cell 2011, 147, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Hwang, B.J.; Ford, J.M.; Hanawalt, P.C.; Chu, G. Xeroderma pigmentosum gene enhances global genomic repair and suppresses UV-induced mutagenesis. Mol. Cell 2000, 5, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Slyskova, J.; Muniesa-Vargas, A.; da Silva, I.T.; Drummond, R.; Park, J.; Hackes, D.; Poetsch, I.; Ribeiro-Silva, C.; Moretton, A.; Heffeter, P.; et al. Detection of oxaliplatin- and cisplatin-DNA lesions requires different global genome repair mechanisms that affect their clinical efficacy. NAR Cancer 2023, 5, zcad057. [Google Scholar] [CrossRef]
- Li, C.L.; Golebiowski, F.M.; Onishi, Y.; Samara, N.L.; Sugasawa, K.; Yang, W. Tripartite DNA Lesion Recognition and Verification by XPC, TFIIH, and XPA in Nucleotide Excision Repair. Mol. Cell 2015, 59, 1025–1034. [Google Scholar] [CrossRef]
- Coin, F.; Oksenych, V.; Mocquet, V.; Groh, S.; Blattner, C.; Egly, J.M. Nucleotide excision repair driven by the dissociation of CAK from TFIIH. Mol. Cell 2008, 31, 9–20. [Google Scholar] [CrossRef]
- Kokic, G.; Chernev, A.; Tegunov, D.; Dienemann, C.; Urlaub, H.; Cramer, P. Structural basis of TFIIH activation for nucleotide excision repair. Nat. Commun. 2019, 10, 2885. [Google Scholar] [CrossRef]
- Bralic, A.; Tehseen, M.; Sobhy, M.A.; Tsai, C.L.; Alhudhali, L.; Yi, G.; Yu, J.; Yan, C.; Ivanov, I.; Tsutakawa, S.E.; et al. A scanning-to-incision switch in TFIIH-XPG induced by DNA damage licenses nucleotide excision repair. Nucleic Acids Res. 2023, 51, 1019–1033. [Google Scholar] [CrossRef]
- Naegeli, H.; Modrich, P.; Friedberg, E.C. The DNA helicase activities of Rad3 protein of Saccharomyces cerevisiae and helicase II of Escherichia coli are differentially inhibited by covalent and noncovalent DNA modifications. J. Biol. Chem. 1993, 268, 10386–10392. [Google Scholar] [CrossRef] [PubMed]
- Coin, F.; Oksenych, V.; Egly, J.M. Distinct roles for the XPB/p52 and XPD/p44 subcomplexes of TFIIH in damaged DNA opening during nucleotide excision repair. Mol. Cell 2007, 26, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, N.; Kaczmarek, N.; Naegeli, H. Strand- and site-specific DNA lesion demarcation by the xeroderma pigmentosum group D helicase. Proc. Natl. Acad. Sci. USA 2010, 107, 17545–17550. [Google Scholar] [CrossRef] [PubMed]
- Buechner, C.N.; Heil, K.; Michels, G.; Carell, T.; Kisker, C.; Tessmer, I. Strand-specific recognition of DNA damages by XPD provides insights into nucleotide excision repair substrate versatility. J. Biol. Chem. 2014, 289, 3613–3624. [Google Scholar] [CrossRef]
- Sugasawa, K.; Akagi, J.; Nishi, R.; Iwai, S.; Hanaoka, F. Two-step recognition of DNA damage for mammalian nucleotide excision repair: Directional binding of the XPC complex and DNA strand scanning. Mol. Cell 2009, 36, 642–653. [Google Scholar] [CrossRef]
- Yokoi, M.; Masutani, C.; Maekawa, T.; Sugasawa, K.; Ohkuma, Y.; Hanaoka, F. The xeroderma pigmentosum group C protein complex XPC-HR23B plays an important role in the recruitment of transcription factor IIH to damaged DNA. J. Biol. Chem. 2000, 275, 9870–9875. [Google Scholar] [CrossRef]
- Oksenych, V.; de Jesus, B.B.; Zhovmer, A.; Egly, J.M.; Coin, F. Molecular insights into the recruitment of TFIIH to sites of DNA damage. EMBO J. 2009, 28, 2971–2980. [Google Scholar] [CrossRef]
- van Eeuwen, T.; Shim, Y.; Kim, H.J.; Zhao, T.; Basu, S.; Garcia, B.A.; Kaplan, C.D.; Min, J.H.; Murakami, K. Cryo-EM structure of TFIIH/Rad4-Rad23-Rad33 in damaged DNA opening in nucleotide excision repair. Nat. Commun. 2021, 12, 3338. [Google Scholar] [CrossRef]
- Coin, F.; Proietti De Santis, L.; Nardo, T.; Zlobinskaya, O.; Stefanini, M.; Egly, J.M. p8/TTD-A as a repair-specific TFIIH subunit. Mol. Cell 2006, 21, 215–226. [Google Scholar] [CrossRef]
- Fu, I.; Mu, H.; Geacintov, N.E.; Broyde, S. Mechanism of lesion verification by the human XPD helicase in nucleotide excision repair. Nucleic Acids Res. 2022, 50, 6837–6853. [Google Scholar] [CrossRef]
- Fu, I.; Geacintov, N.E.; Broyde, S. Differing structures and dynamics of two photolesions portray verification differences by the human XPD helicase. Nucleic Acids Res. 2023, 51, 12261–12274. [Google Scholar] [CrossRef]
- Kuper, J.; Hove, T.; Maidl, S.; Neitz, H.; Sauer, F.; Kempf, M.; Schroeder, T.; Greiter, E.; Hobartner, C.; Kisker, C. XPD stalled on cross-linked DNA provides insight into damage verification. Nat. Struct. Mol. Biol. 2024, 31, 1580–1588. [Google Scholar] [CrossRef]
- Wirth, N.; Gross, J.; Roth, H.M.; Buechner, C.N.; Kisker, C.; Tessmer, I. Conservation and Divergence in Nucleotide Excision Repair Lesion Recognition. J. Biol. Chem. 2016, 291, 18932–18946. [Google Scholar] [CrossRef]
- Fagbemi, A.F.; Orelli, B.; Scharer, O.D. Regulation of endonuclease activity in human nucleotide excision repair. DNA Repair 2011, 10, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yan, C.; Paul, T.; Brewer, L.; Tsutakawa, S.E.; Tsai, C.L.; Hamdan, S.M.; Tainer, J.A.; Ivanov, I. Molecular architecture and functional dynamics of the pre-incision complex in nucleotide excision repair. Nat. Commun. 2024, 15, 8511. [Google Scholar] [CrossRef] [PubMed]
- Sugasawa, K. Molecular mechanisms of DNA damage recognition for mammalian nucleotide excision repair. DNA Repair 2016, 44, 110–117. [Google Scholar] [CrossRef]
- Gsell, C.; Richly, H.; Coin, F.; Naegeli, H. A chromatin scaffold for DNA damage recognition: How histone methyltransferases prime nucleosomes for repair of ultraviolet light-induced lesions. Nucleic Acids Res. 2020, 48, 1652–1668. [Google Scholar] [CrossRef] [PubMed]
- Tsutakawa, S.E.; Tsai, C.L.; Yan, C.; Bralic, A.; Chazin, W.J.; Hamdan, S.M.; Scharer, O.D.; Ivanov, I.; Tainer, J.A. Envisioning how the prototypic molecular machine TFIIH functions in transcription initiation and DNA repair. DNA Repair 2020, 96, 102972. [Google Scholar] [CrossRef]
- Theil, A.F.; Hackes, D.; Lans, H. TFIIH central activity in nucleotide excision repair to prevent disease. DNA Repair 2023, 132, 103568. [Google Scholar] [CrossRef]
- Wittschieben, B.O.; Iwai, S.; Wood, R.D. DDB1-DDB2 (xeroderma pigmentosum group E) protein complex recognizes a cyclobutane pyrimidine dimer, mismatches, apurinic/apyrimidinic sites, and compound lesions in DNA. J. Biol. Chem. 2005, 280, 39982–39989. [Google Scholar] [CrossRef] [PubMed]
- Hanawalt, P.C.; Spivak, G. Transcription-coupled DNA repair: Two decades of progress and surprises. Nat. Rev. Mol. Cell Biol. 2008, 9, 958–970. [Google Scholar] [CrossRef]
- Jia, N.; Guo, C.; Nakazawa, Y.; van den Heuvel, D.; Luijsterburg, M.S.; Ogi, T. Dealing with transcription-blocking DNA damage: Repair mechanisms, RNA polymerase II processing and human disorders. DNA Repair 2021, 106, 103192. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, D.; van der Weegen, Y.; Boer, D.E.C.; Ogi, T.; Luijsterburg, M.S. Transcription-Coupled DNA Repair: From Mechanism to Human Disorder. Trends Cell Biol. 2021, 31, 359–371. [Google Scholar] [CrossRef]
- Selby, C.P.; Lindsey-Boltz, L.A.; Li, W.; Sancar, A. Molecular Mechanisms of Transcription-Coupled Repair. Annu. Rev. Biochem. 2023, 92, 115–144. [Google Scholar] [CrossRef]
- Brueckner, F.; Hennecke, U.; Carell, T.; Cramer, P. CPD damage recognition by transcribing RNA polymerase II. Science 2007, 315, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Damsma, G.E.; Alt, A.; Brueckner, F.; Carell, T.; Cramer, P. Mechanism of transcriptional stalling at cisplatin-damaged DNA. Nat. Struct. Mol. Biol. 2007, 14, 1127–1133. [Google Scholar] [CrossRef]
- Selby, C.P.; Sancar, A. Cockayne syndrome group B protein enhances elongation by RNA polymerase II. Proc. Natl. Acad. Sci. USA 1997, 94, 11205–11209. [Google Scholar] [CrossRef]
- Cho, I.; Tsai, P.F.; Lake, R.J.; Basheer, A.; Fan, H.Y. ATP-dependent chromatin remodeling by Cockayne syndrome protein B and NAP1-like histone chaperones is required for efficient transcription-coupled DNA repair. PLoS Genet. 2013, 9, e1003407. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Xu, L.; Chen, J.Y.; Chong, J.; Oh, J.; Leschziner, A.E.; Fu, X.D.; Wang, D. Cockayne syndrome B protein acts as an ATP-dependent processivity factor that helps RNA polymerase II overcome nucleosome barriers. Proc. Natl. Acad. Sci. USA 2020, 117, 25486–25493. [Google Scholar] [CrossRef]
- van Gool, A.J.; Citterio, E.; Rademakers, S.; van Os, R.; Vermeulen, W.; Constantinou, A.; Egly, J.M.; Bootsma, D.; Hoeijmakers, J.H. The Cockayne syndrome B protein, involved in transcription-coupled DNA repair, resides in an RNA polymerase II-containing complex. EMBO J. 1997, 16, 5955–5965. [Google Scholar] [CrossRef] [PubMed]
- van den Boom, V.; Citterio, E.; Hoogstraten, D.; Zotter, A.; Egly, J.M.; van Cappellen, W.A.; Hoeijmakers, J.H.; Houtsmuller, A.B.; Vermeulen, W. DNA damage stabilizes interaction of CSB with the transcription elongation machinery. J. Cell Biol. 2004, 166, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Charlet-Berguerand, N.; Feuerhahn, S.; Kong, S.E.; Ziserman, H.; Conaway, J.W.; Conaway, R.; Egly, J.M. RNA polymerase II bypass of oxidative DNA damage is regulated by transcription elongation factors. EMBO J. 2006, 25, 5481–5491. [Google Scholar] [CrossRef] [PubMed]
- Kokic, G.; Wagner, F.R.; Chernev, A.; Urlaub, H.; Cramer, P. Structural basis of human transcription-DNA repair coupling. Nature 2021, 598, 368–372. [Google Scholar] [CrossRef]
- Xu, J.; Lahiri, I.; Wang, W.; Wier, A.; Cianfrocco, M.A.; Chong, J.; Hare, A.A.; Dervan, P.B.; DiMaio, F.; Leschziner, A.E.; et al. Structural basis for the initiation of eukaryotic transcription-coupled DNA repair. Nature 2017, 551, 653–657. [Google Scholar] [CrossRef]
- van der Weegen, Y.; Golan-Berman, H.; Mevissen, T.E.T.; Apelt, K.; Gonzalez-Prieto, R.; Goedhart, J.; Heilbrun, E.E.; Vertegaal, A.C.O.; van den Heuvel, D.; Walter, J.C.; et al. The cooperative action of CSB, CSA, and UVSSA target TFIIH to DNA damage-stalled RNA polymerase II. Nat. Commun. 2020, 11, 2104. [Google Scholar] [CrossRef]
- Venema, J.; Mullenders, L.H.; Natarajan, A.T.; van Zeeland, A.A.; Mayne, L.V. The genetic defect in Cockayne syndrome is associated with a defect in repair of UV-induced DNA damage in transcriptionally active DNA. Proc. Natl. Acad. Sci. USA 1990, 87, 4707–4711. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Hara, Y.; Oka, Y.; Komine, O.; van den Heuvel, D.; Guo, C.; Daigaku, Y.; Isono, M.; He, Y.; Shimada, M.; et al. Ubiquitination of DNA Damage-Stalled RNAPII Promotes Transcription-Coupled Repair. Cell 2020, 180, 1228–1244.e24. [Google Scholar] [CrossRef]
- Tufegdzic Vidakovic, A.; Mitter, R.; Kelly, G.P.; Neumann, M.; Harreman, M.; Rodriguez-Martinez, M.; Herlihy, A.; Weems, J.C.; Boeing, S.; Encheva, V.; et al. Regulation of the RNAPII Pool Is Integral to the DNA Damage Response. Cell 2020, 180, 1245–1261.e21. [Google Scholar] [CrossRef]
- Fei, J.; Chen, J. KIAA1530 protein is recruited by Cockayne syndrome complementation group protein A (CSA) to participate in transcription-coupled repair (TCR). J. Biol. Chem. 2012, 287, 35118–35126. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Sasaki, K.; Mitsutake, N.; Matsuse, M.; Shimada, M.; Nardo, T.; Takahashi, Y.; Ohyama, K.; Ito, K.; Mishima, H.; et al. Mutations in UVSSA cause UV-sensitive syndrome and impair RNA polymerase IIo processing in transcription-coupled nucleotide-excision repair. Nat. Genet. 2012, 44, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Schwertman, P.; Lagarou, A.; Dekkers, D.H.; Raams, A.; van der Hoek, A.C.; Laffeber, C.; Hoeijmakers, J.H.; Demmers, J.A.; Fousteri, M.; Vermeulen, W.; et al. UV-sensitive syndrome protein UVSSA recruits USP7 to regulate transcription-coupled repair. Nat. Genet. 2012, 44, 598–602. [Google Scholar] [CrossRef]
- Zhang, X.; Horibata, K.; Saijo, M.; Ishigami, C.; Ukai, A.; Kanno, S.; Tahara, H.; Neilan, E.G.; Honma, M.; Nohmi, T.; et al. Mutations in UVSSA cause UV-sensitive syndrome and destabilize ERCC6 in transcription-coupled DNA repair. Nat. Genet. 2012, 44, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Nakazawa, Y.; Guo, C.; Ogi, T.; Nishimura, Y. Common TFIIH recruitment mechanism in global genome and transcription-coupled repair subpathways. Nucleic Acids Res. 2017, 45, 13043–13055. [Google Scholar] [CrossRef]
- Olivieri, M.; Cho, T.; Alvarez-Quilon, A.; Li, K.; Schellenberg, M.J.; Zimmermann, M.; Hustedt, N.; Rossi, S.E.; Adam, S.; Melo, H.; et al. A Genetic Map of the Response to DNA Damage in Human Cells. Cell 2020, 182, 481–496.e421. [Google Scholar] [CrossRef]
- van der Weegen, Y.; de Lint, K.; van den Heuvel, D.; Nakazawa, Y.; Mevissen, T.E.T.; van Schie, J.J.M.; San Martin Alonso, M.; Boer, D.E.C.; Gonzalez-Prieto, R.; Narayanan, I.V.; et al. ELOF1 is a transcription-coupled DNA repair factor that directs RNA polymerase II ubiquitylation. Nat. Cell Biol. 2021, 23, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Geijer, M.E.; Zhou, D.; Selvam, K.; Steurer, B.; Mukherjee, C.; Evers, B.; Cugusi, S.; van Toorn, M.; van der Woude, M.; Janssens, R.C.; et al. Elongation factor ELOF1 drives transcription-coupled repair and prevents genome instability. Nat. Cell Biol. 2021, 23, 608–619. [Google Scholar] [CrossRef]
- Mevissen, T.E.T.; Kummecke, M.; Schmid, E.W.; Farnung, L.; Walter, J.C. STK19 positions TFIIH for cell-free transcription-coupled DNA repair. Cell 2024, 187, 7091–7106.e24. [Google Scholar] [CrossRef]
- Ramadhin, A.R.; Lee, S.H.; Zhou, D.; Salmazo, A.; Gonzalo-Hansen, C.; van Sluis, M.; Blom, C.M.A.; Janssens, R.C.; Raams, A.; Dekkers, D.; et al. STK19 drives transcription-coupled repair by stimulating repair complex stability, RNA Pol II ubiquitylation, and TFIIH recruitment. Mol. Cell 2024, 84, 4740–4757.e12. [Google Scholar] [CrossRef]
- Tan, Y.; Gao, M.; Huang, Y.; Zhan, D.; Wu, S.; An, J.; Zhang, X.; Hu, J. STK19 is a transcription-coupled repair factor that participates in UVSSA ubiquitination and TFIIH loading. Nucleic Acids Res. 2024, 52, 12767–12783. [Google Scholar] [CrossRef]
- van den Heuvel, D.; Rodriguez-Martinez, M.; van der Meer, P.J.; Nieto Moreno, N.; Park, J.; Kim, H.S.; van Schie, J.J.M.; Wondergem, A.P.; D’Souza, A.; Yakoub, G.; et al. STK19 facilitates the clearance of lesion-stalled RNAPII during transcription-coupled DNA repair. Cell 2024, 187, 7107–7125.e25. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, Y.; Asahina, H.; Citterio, E.; Rademakers, S.; Vermeulen, W.; Kamiuchi, S.; Yeo, J.P.; Khaw, M.C.; Saijo, M.; Kodo, N.; et al. XAB2, a novel tetratricopeptide repeat protein involved in transcription-coupled DNA repair and transcription. J. Biol. Chem. 2000, 275, 34931–34937. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, X.; Gao, M.; Huang, Y.; Tan, Y.; Parnas, A.; Wu, S.; Zhan, D.; Adar, S.; Hu, J. Coordination of transcription-coupled repair and repair-independent release of lesion-stalled RNA polymerase II. Nat. Commun. 2024, 15, 7089. [Google Scholar] [CrossRef]
- Paul, T.; Yan, C.; Yu, J.; Tsutakawa, S.E.; Tainer, J.A.; Wang, D.; Ivanov, I. Molecular model of TFIIH recruitment to the transcription-coupled repair machinery. Nat. Commun. 2025, 16, 2341. [Google Scholar] [CrossRef]
- Cleaver, J.E. Defective Repair Replication of DNA in Xeroderma Pigmentosum. Nature 1968, 218, 652–656. [Google Scholar] [CrossRef]
- Limsirichaikul, S.; Niimi, A.; Fawcett, H.; Lehmann, A.; Yamashita, S.; Ogi, T. A rapid non-radioactive technique for measurement of repair synthesis in primary human fibroblasts by incorporation of ethynyl deoxyuridine (EdU). Nucleic Acids Res. 2009, 37, e31. [Google Scholar] [CrossRef] [PubMed]
- Pimpley, M.R.; Foley, M.L.; Latimer, J.J. New Perspectives on Unscheduled DNA Synthesis: Functional Assay for Global Genomic DNA Nucleotide Excision Repair. Methods Mol. Biol. 2020, 2102, 483–507. [Google Scholar] [CrossRef]
- van der Meer, P.J.; Van Den Heuvel, D.; Luijsterburg, M.S. Unscheduled DNA Synthesis at Sites of Local UV-induced DNA Damage to Quantify Global Genome Nucleotide Excision Repair Activity in Human Cells. Bio Protoc. 2023, 13, e4609. [Google Scholar] [CrossRef] [PubMed]
- Wienholz, F.; Vermeulen, W.; Marteijn, J.A. Amplification of unscheduled DNA synthesis signal enables fluorescence-based single cell quantification of transcription-coupled nucleotide excision repair. Nucleic Acids Res. 2017, 45, e68. [Google Scholar] [CrossRef]
- van der Woude, M.; Davo-Martinez, C.; Thijssen, K.L.; Vermeulen, W.; Lans, H. Recovery of protein synthesis to assay DNA repair activity in transcribed genes in living cells and tissues. Nucleic Acids Res. 2023, 51, e93. [Google Scholar] [CrossRef]
- van Hoffen, A.; Venema, J.; Meschini, R.; van Zeeland, A.A.; Mullenders, L.H. Transcription-coupled repair removes both cyclobutane pyrimidine dimers and 6-4 photoproducts with equal efficiency and in a sequential way from transcribed DNA in xeroderma pigmentosum group C fibroblasts. EMBO J. 1995, 14, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Mayne, L.V.; Lehmann, A.R. Failure of RNA synthesis to recover after UV irradiation: An early defect in cells from individuals with Cockayne’s syndrome and xeroderma pigmentosum. Cancer Res. 1982, 42, 1473–1478. [Google Scholar] [CrossRef]
- Lehmann, A.R.; Francis, A.J.; Giannelli, F. Prenatal-Diagnosis of Cockaynes Syndrome. Lancet 1985, 1, 486–488. [Google Scholar] [CrossRef]
- Lehmann, A.R.; Thompson, A.F.; Harcourt, S.A.; Stefanini, M.; Norris, P.G. Cockayne’s syndrome: Correlation of clinical features with cellular sensitivity of RNA synthesis to UV irradiation. J. Med. Genet. 1993, 30, 679–682. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Yamashita, S.; Lehmann, A.R.; Ogi, T. A semi-automated non-radioactive system for measuring recovery of RNA synthesis and unscheduled DNA synthesis using ethynyluracil derivatives. DNA Repair 2010, 9, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Geijer, M.E.; Marteijn, J.A. What happens at the lesion does not stay at the lesion: Transcription-coupled nucleotide excision repair and the effects of DNA damage on transcription in cis and trans. DNA Repair 2018, 71, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Khobta, A.; Epe, B. Interactions between DNA damage, repair, and transcription. Mutat. Res. 2012, 736, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Adar, S.; Selby, C.P.; Lieb, J.D.; Sancar, A. Genome-wide analysis of human global and transcription-coupled excision repair of UV damage at single-nucleotide resolution. Genes. Dev. 2015, 29, 948–960. [Google Scholar] [CrossRef]
- Adar, S.; Hu, J.; Lieb, J.D.; Sancar, A. Genome-wide kinetics of DNA excision repair in relation to chromatin state and mutagenesis. Proc. Natl. Acad. Sci. USA 2016, 113, E2124–E2133. [Google Scholar] [CrossRef]
- Kemp, M.G.; Reardon, J.T.; Lindsey-Boltz, L.A.; Sancar, A. Mechanism of release and fate of excised oligonucleotides during nucleotide excision repair. J. Biol. Chem. 2012, 287, 22889–22899. [Google Scholar] [CrossRef]
- Hu, J.; Li, W.; Adebali, O.; Yang, Y.; Oztas, O.; Selby, C.P.; Sancar, A. Genome-wide mapping of nucleotide excision repair with XR-seq. Nat. Protoc. 2019, 14, 248–282. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, J.; Adebali, O.; Adar, S.; Yang, Y.; Chiou, Y.Y.; Sancar, A. Human genome-wide repair map of DNA damage caused by the cigarette smoke carcinogen benzo[a]pyrene. Proc. Natl. Acad. Sci. USA 2017, 114, 6752–6757. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lieb, J.D.; Sancar, A.; Adar, S. Cisplatin DNA damage and repair maps of the human genome at single-nucleotide resolution. Proc. Natl. Acad. Sci. USA 2016, 113, 11507–11512. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tan, Y.; Li, L.; Xiang, Y.; Huang, Y.; Zhang, X.; Yin, J.; Li, J.; Lan, F.; Qian, M.; et al. Genome-wide mapping of protein-DNA damage interaction by PADD-seq. Nucleic Acids Res. 2023, 51, e32. [Google Scholar] [CrossRef]
- Mao, P.; Smerdon, M.J.; Roberts, S.A.; Wyrick, J.J. Chromosomal landscape of UV damage formation and repair at single-nucleotide resolution. Proc. Natl. Acad. Sci. USA 2016, 113, 9057–9062. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, W.; Lindsey-Boltz, L.A.; Yang, Y.; Li, Y.; Sancar, A. Super hotspots and super coldspots in the repair of UV-induced DNA damage in the human genome. J. Biol. Chem. 2021, 296, 100581. [Google Scholar] [CrossRef]
- Wu, Y.; Adeel, M.M.; Xia, D.; Sancar, A.; Li, W. Nucleotide excision repair of aflatoxin-induced DNA damage within the 3D human genome organization. Nucleic Acids Res. 2024, 52, 11704–11719. [Google Scholar] [CrossRef]
- Haradhvala, N.J.; Polak, P.; Stojanov, P.; Covington, K.R.; Shinbrot, E.; Hess, J.M.; Rheinbay, E.; Kim, J.; Maruvka, Y.E.; Braunstein, L.Z.; et al. Mutational Strand Asymmetries in Cancer Genomes Reveal Mechanisms of DNA Damage and Repair. Cell 2016, 164, 538–549. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Kim, J.; Haradhvala, N.J.; Huang, M.N.; Tian Ng, A.W.; Wu, Y.; Boot, A.; Covington, K.R.; Gordenin, D.A.; Bergstrom, E.N.; et al. The repertoire of mutational signatures in human cancer. Nature 2020, 578, 94–101. [Google Scholar] [CrossRef]
- Pleasance, E.D.; Cheetham, R.K.; Stephens, P.J.; McBride, D.J.; Humphray, S.J.; Greenman, C.D.; Varela, I.; Lin, M.L.; Ordonez, G.R.; Bignell, G.R.; et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 2010, 463, 191–196. [Google Scholar] [CrossRef]
- Laine, J.P.; Egly, J.M. Initiation of DNA repair mediated by a stalled RNA polymerase IIO. EMBO J. 2006, 25, 387–397. [Google Scholar] [CrossRef]
- Araujo, S.J.; Tirode, F.; Coin, F.; Pospiech, H.; Syvaoja, J.E.; Stucki, M.; Hubscher, U.; Egly, J.M.; Wood, R.D. Nucleotide excision repair of DNA with recombinant human proteins: Definition of the minimal set of factors, active forms of TFIIH, and modulation by CAK. Genes. Dev. 2000, 14, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Donahue, B.A.; Yin, S.; Taylor, J.S.; Reines, D.; Hanawalt, P.C. Transcript cleavage by RNA polymerase II arrested by a cyclobutane pyrimidine dimer in the DNA template. Proc. Natl. Acad. Sci. USA 1994, 91, 8502–8506. [Google Scholar] [CrossRef]
- Tornaletti, S.; Reines, D.; Hanawalt, P.C. Structural characterization of RNA polymerase II complexes arrested by a cyclobutane pyrimidine dimer in the transcribed strand of template DNA. J. Biol. Chem. 1999, 274, 24124–24130. [Google Scholar] [CrossRef] [PubMed]
- Tornaletti, S.; Patrick, S.M.; Turchi, J.J.; Hanawalt, P.C. Behavior of T7 RNA polymerase and mammalian RNA polymerase II at site-specific cisplatin adducts in the template DNA. J. Biol. Chem. 2003, 278, 35791–35797. [Google Scholar] [CrossRef] [PubMed]
- Tornaletti, S.; Hanawalt, P.C. Effect of DNA lesions on transcription elongation. Biochimie 1999, 81, 139–146. [Google Scholar] [CrossRef]
- Wang, W.; Xu, J.; Chong, J.; Wang, D. Structural basis of DNA lesion recognition for eukaryotic transcription-coupled nucleotide excision repair. DNA Repair 2018, 71, 43–55. [Google Scholar] [CrossRef]
- Agapov, A.; Olina, A.; Kulbachinskiy, A. RNA polymerase pausing, stalling and bypass during transcription of damaged DNA: From molecular basis to functional consequences. Nucleic Acids Res. 2022, 50, 3018–3041. [Google Scholar] [CrossRef]
- Cheng, T.F.; Hu, X.; Gnatt, A.; Brooks, P.J. Differential blocking effects of the acetaldehyde-derived DNA lesion N2-ethyl-2’-deoxyguanosine on transcription by multisubunit and single subunit RNA polymerases. J. Biol. Chem. 2008, 283, 27820–27828. [Google Scholar] [CrossRef]
- Dimitri, A.; Goodenough, A.K.; Guengerich, F.P.; Broyde, S.; Scicchitano, D.A. Transcription processing at 1,N2-ethenoguanine by human RNA polymerase II and bacteriophage T7 RNA polymerase. J. Mol. Biol. 2008, 375, 353–366. [Google Scholar] [CrossRef]
- Walmacq, C.; Wang, L.; Chong, J.; Scibelli, K.; Lubkowska, L.; Gnatt, A.; Brooks, P.J.; Wang, D.; Kashlev, M. Mechanism of RNA polymerase II bypass of oxidative cyclopurine DNA lesions. Proc. Natl. Acad. Sci. USA 2015, 112, E410–E419. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Fleming, A.M.; Xu, J.; Chong, J.; Burrows, C.J.; Wang, D. RNA polymerase II stalls on oxidative DNA damage via a torsion-latch mechanism involving lone pair-pi and CH-pi interactions. Proc. Natl. Acad. Sci. USA 2020, 117, 9338–9348. [Google Scholar] [CrossRef]
- Walmacq, C.; Cheung, A.C.; Kireeva, M.L.; Lubkowska, L.; Ye, C.; Gotte, D.; Strathern, J.N.; Carell, T.; Cramer, P.; Kashlev, M. Mechanism of translesion transcription by RNA polymerase II and its role in cellular resistance to DNA damage. Mol. Cell 2012, 46, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Selvam, K.; Ko, T.; Li, S. Transcription bypass of DNA lesions enhances cell survival but attenuates transcription coupled DNA repair. Nucleic Acids Res. 2014, 42, 13242–13253. [Google Scholar] [CrossRef]
- Alanazi, J.S.; Latimer, J.J. Host Cell Reactivation: Assay for Actively Transcribed DNA (Nucleotide Excision) Repair Using Luciferase Family Expression Vectors. Methods Mol. Biol. 2020, 2102, 509–528. [Google Scholar] [CrossRef]
- Protic-Sabljic, M.; Kraemer, K.H. One pyrimidine dimer inactivates expression of a transfected gene in xeroderma pigmentosum cells. Proc. Natl. Acad. Sci. USA 1985, 82, 6622–6626. [Google Scholar] [CrossRef]
- Lehmann, A.R.; Oomen, A. Effect of DNA damage on the expression of the chloramphenicol acetyltransferase gene after transfection into diploid human fibroblasts. Nucleic Acids Res. 1985, 13, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, P.J.; Van der Eb, A.J. Host-cell reactivation of ultraviolet-irradiated SV40 DNA in five complementation groups of xeroderma pigmentosum. Mutat. Res. 1976, 35, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Selsky, C.A.; Greer, S. Host-cell reactivation of UV-irradiated and chemically-treated herpes simplex virus-1 by xeroderma pigmentosum, XP heterozygotes and normal skin fibroblasts. Mutat. Res. 1978, 50, 395–405. [Google Scholar] [CrossRef]
- Barrett, S.F.; Robbins, J.H.; Tarone, R.E.; Kraemer, K.H. Evidence for defective repair of cyclobutane pyrimidine dimers with normal repair of other DNA photoproducts in a transcriptionally active gene transfected into Cockayne syndrome cells. Mutat. Res. 1991, 255, 281–291. [Google Scholar] [CrossRef]
- Cordeiro-Stone, M.; Zaritskaya, L.S.; Price, L.K.; Kaufmann, W.K. Replication fork bypass of a pyrimidine dimer blocking leading strand DNA synthesis. J. Biol. Chem. 1997, 272, 13945–13954. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hays, J.B. Simple and rapid preparation of gapped plasmid DNA for incorporation of oligomers containing specific DNA lesions. Mol. Biotechnol. 2001, 19, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Wuenschell, G.; Xia, L.; Termini, J.; Bates, S.E.; Riggs, A.D.; O’Connor, T.R. Nucleotide excision repair eliminates unique DNA-protein cross-links from mammalian cells. J. Biol. Chem. 2007, 282, 22592–22604. [Google Scholar] [CrossRef]
- Burns, J.A.; Dreij, K.; Cartularo, L.; Scicchitano, D.A. O6-methylguanine induces altered proteins at the level of transcription in human cells. Nucleic Acids Res. 2010, 38, 8178–8187. [Google Scholar] [CrossRef]
- Enoiu, M.; Jiricny, J.; Scharer, O.D. Repair of cisplatin-induced DNA interstrand crosslinks by a replication-independent pathway involving transcription-coupled repair and translesion synthesis. Nucleic Acids Res. 2012, 40, 8953–8964. [Google Scholar] [CrossRef] [PubMed]
- Luhnsdorf, B.; Kitsera, N.; Warken, D.; Lingg, T.; Epe, B.; Khobta, A. Generation of reporter plasmids containing defined base modifications in the DNA strand of choice. Anal. Biochem. 2012, 425, 47–53. [Google Scholar] [CrossRef]
- You, C.; Wang, Y. Quantitative measurement of transcriptional inhibition and mutagenesis induced by site-specifically incorporated DNA lesions in vitro and in vivo. Nat. Protoc. 2015, 10, 1389–1406. [Google Scholar] [CrossRef]
- Piett, C.G.; Pecen, T.J.; Laverty, D.J.; Nagel, Z.D. Large-scale preparation of fluorescence multiplex host cell reactivation (FM-HCR) reporters. Nat. Protoc. 2021, 16, 4265–4298. [Google Scholar] [CrossRef]
- Kitsera, N.; Rodriguez-Alvarez, M.; Emmert, S.; Carell, T.; Khobta, A. Nucleotide excision repair of abasic DNA lesions. Nucleic Acids Res. 2019, 47, 8537–8547. [Google Scholar] [CrossRef]
- Sarmini, L.; Meabed, M.; Emmanouil, E.; Atsaves, G.; Robeska, E.; Karwowski, B.T.; Campalans, A.; Gimisis, T.; Khobta, A. Requirement of transcription-coupled nucleotide excision repair for the removal of a specific type of oxidatively induced DNA damage. Nucleic Acids Res. 2023, 51, 4982–4994. [Google Scholar] [CrossRef]
- You, C.; Dai, X.; Yuan, B.; Wang, J.; Wang, J.; Brooks, P.J.; Niedernhofer, L.J.; Wang, Y. A quantitative assay for assessing the effects of DNA lesions on transcription. Nat. Chem. Biol. 2012, 8, 817–822. [Google Scholar] [CrossRef]
- Kitsera, N.; Gasteiger, K.; Luhnsdorf, B.; Allgayer, J.; Epe, B.; Carell, T.; Khobta, A. Cockayne syndrome: Varied requirement of transcription-coupled nucleotide excision repair for the removal of three structurally different adducts from transcribed DNA. PLoS ONE 2014, 9, e94405. [Google Scholar] [CrossRef] [PubMed]
- Nagel, Z.D.; Margulies, C.M.; Chaim, I.A.; McRee, S.K.; Mazzucato, P.; Ahmad, A.; Abo, R.P.; Butty, V.L.; Forget, A.L.; Samson, L.D. Multiplexed DNA repair assays for multiple lesions and multiple doses via transcription inhibition and transcriptional mutagenesis. Proc. Natl. Acad. Sci. USA 2014, 111, E1823–E1832. [Google Scholar] [CrossRef]
- Chaim, I.A.; Gardner, A.; Wu, J.; Iyama, T.; Wilson, D.M., 3rd; Samson, L.D. A novel role for transcription-coupled nucleotide excision repair for the in vivo repair of 3,N4-ethenocytosine. Nucleic Acids Res. 2017, 45, 3242–3252. [Google Scholar] [CrossRef]
- Sarmini, L.; Kitsera, N.; Meabed, M.; Khobta, A. Transcription blocking properties and transcription-coupled repair of N(2)-alkylguanine adducts as a model for aldehyde-induced DNA damage. J. Biol. Chem. 2025, 301, 108459. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Wang, J.; Dai, X.; Wang, Y. Transcriptional inhibition and mutagenesis induced by N-nitroso compound-derived carboxymethylated thymidine adducts in DNA. Nucleic Acids Res. 2015, 43, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Mouret, S.; Baudouin, C.; Charveron, M.; Favier, A.; Cadet, J.; Douki, T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc. Natl. Acad. Sci. USA 2006, 103, 13765–13770. [Google Scholar] [CrossRef]
- Mei Kwei, J.S.; Kuraoka, I.; Horibata, K.; Ubukata, M.; Kobatake, E.; Iwai, S.; Handa, H.; Tanaka, K. Blockage of RNA polymerase II at a cyclobutane pyrimidine dimer and 6-4 photoproduct. Biochem. Biophys. Res. Commun. 2004, 320, 1133–1138. [Google Scholar] [CrossRef]
- Reardon, J.T.; Sancar, A. Recognition and repair of the cyclobutane thymine dimer, a major cause of skin cancers, by the human excision nuclease. Genes. Dev. 2003, 17, 2539–2551. [Google Scholar] [CrossRef]
- Laughery, M.F.; Brown, A.J.; Bohm, K.A.; Sivapragasam, S.; Morris, H.S.; Tchmola, M.; Washington, A.D.; Mitchell, D.; Mather, S.; Malc, E.P.; et al. Atypical UV Photoproducts Induce Non-canonical Mutation Classes Associated with Driver Mutations in Melanoma. Cell Rep. 2020, 33, 108401. [Google Scholar] [CrossRef]
- Hess, M.T.; Gunz, D.; Luneva, N.; Geacintov, N.E.; Naegeli, H. Base pair conformation-dependent excision of benzo[a]pyrene diol epoxide-guanine adducts by human nucleotide excision repair enzymes. Mol. Cell Biol. 1997, 17, 7069–7076. [Google Scholar] [CrossRef] [PubMed]
- Buterin, T.; Hess, M.T.; Luneva, N.; Geacintov, N.E.; Amin, S.; Kroth, H.; Seidel, A.; Naegeli, H. Unrepaired fjord region polycyclic aromatic hydrocarbon-DNA adducts in ras codon 61 mutational hot spots. Cancer Res. 2000, 60, 1849–1856. [Google Scholar] [PubMed]
- Kusumoto, R.; Masutani, C.; Sugasawa, K.; Iwai, S.; Araki, M.; Uchida, A.; Mizukoshi, T.; Hanaoka, F. Diversity of the damage recognition step in the global genomic nucleotide excision repair in vitro. Mutat. Res. 2001, 485, 219–227. [Google Scholar] [CrossRef]
- Mocquet, V.; Kropachev, K.; Kolbanovskiy, M.; Kolbanovskiy, A.; Tapias, A.; Cai, Y.; Broyde, S.; Geacintov, N.E.; Egly, J.M. The human DNA repair factor XPC-HR23B distinguishes stereoisomeric benzo[a]pyrenyl-DNA lesions. EMBO J. 2007, 26, 2923–2932. [Google Scholar] [CrossRef]
- Mu, H.; Kropachev, K.; Wang, L.; Zhang, L.; Kolbanovskiy, A.; Kolbanovskiy, M.; Geacintov, N.E.; Broyde, S. Nucleotide excision repair of 2-acetylaminofluorene- and 2-aminofluorene-(C8)-guanine adducts: Molecular dynamics simulations elucidate how lesion structure and base sequence context impact repair efficiencies. Nucleic Acids Res. 2012, 40, 9675–9690. [Google Scholar] [CrossRef]
- Kropachev, K.; Kolbanovskiy, M.; Liu, Z.; Cai, Y.; Zhang, L.; Schwaid, A.G.; Kolbanovskiy, A.; Ding, S.; Amin, S.; Broyde, S.; et al. Adenine-DNA adducts derived from the highly tumorigenic Dibenzo[a,l]pyrene are resistant to nucleotide excision repair while guanine adducts are not. Chem. Res. Toxicol. 2013, 26, 783–793. [Google Scholar] [CrossRef]
- Nadkarni, A.; Burns, J.A.; Gandolfi, A.; Chowdhury, M.A.; Cartularo, L.; Berens, C.; Geacintov, N.E.; Scicchitano, D.A. Nucleotide Excision Repair and Transcription-coupled DNA Repair Abrogate the Impact of DNA Damage on Transcription. J. Biol. Chem. 2016, 291, 848–861. [Google Scholar] [CrossRef]
- Mu, H.; Geacintov, N.E.; Min, J.H.; Zhang, Y.; Broyde, S. Nucleotide Excision Repair Lesion-Recognition Protein Rad4 Captures a Pre-Flipped Partner Base in a Benzo[a]pyrene-Derived DNA Lesion: How Structure Impacts the Binding Pathway. Chem. Res. Toxicol. 2017, 30, 1344–1354. [Google Scholar] [CrossRef]
- Olivier, M.; Weninger, A.; Ardin, M.; Huskova, H.; Castells, X.; Vallee, M.P.; McKay, J.; Nedelko, T.; Muehlbauer, K.R.; Marusawa, H.; et al. Modelling mutational landscapes of human cancers in vitro. Sci. Rep. 2014, 4, 4482. [Google Scholar] [CrossRef]
- Severson, P.L.; Vrba, L.; Stampfer, M.R.; Futscher, B.W. Exome-wide mutation profile in benzo[a]pyrene-derived post-stasis and immortal human mammary epithelial cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 775–776, 48–54. [Google Scholar] [CrossRef]
- Nik-Zainal, S.; Kucab, J.E.; Morganella, S.; Glodzik, D.; Alexandrov, L.B.; Arlt, V.M.; Weninger, A.; Hollstein, M.; Stratton, M.R.; Phillips, D.H. The genome as a record of environmental exposure. Mutagenesis 2015, 30, 763–770. [Google Scholar] [CrossRef]
- Kucab, J.E.; Zou, X.; Morganella, S.; Joel, M.; Nanda, A.S.; Nagy, E.; Gomez, C.; Degasperi, A.; Harris, R.; Jackson, S.P.; et al. A Compendium of Mutational Signatures of Environmental Agents. Cell 2019, 177, 821–836.e16. [Google Scholar] [CrossRef]
- Mingard, C.; Battey, J.N.D.; Takhaveev, V.; Blatter, K.; Hurlimann, V.; Sierro, N.; Ivanov, N.V.; Sturla, S.J. Dissection of Cancer Mutational Signatures with Individual Components of Cigarette Smoking. Chem. Res. Toxicol. 2023, 36, 714–723. [Google Scholar] [CrossRef]
- Heflich, R.H.; Neft, R.E. Genetic toxicity of 2-acetylaminofluorene, 2-aminofluorene and some of their metabolites and model metabolites. Mutat. Res. 1994, 318, 73–114. [Google Scholar] [CrossRef]
- O’Handley, S.F.; Sanford, D.G.; Xu, R.; Lester, C.C.; Hingerty, B.E.; Broyde, S.; Krugh, T.R. Structural characterization of an N-acetyl-2-aminofluorene (AAF) modified DNA oligomer by NMR, energy minimization, and molecular dynamics. Biochemistry 1993, 32, 2481–2497. [Google Scholar] [CrossRef]
- Zaliznyak, T.; Bonala, R.; Johnson, F.; de Los Santos, C. Structure and stability of duplex DNA containing the 3-(deoxyguanosin-N2-yl)-2-acetylaminofluorene (dG(N2)-AAF) lesion: A bulky adduct that persists in cellular DNA. Chem. Res. Toxicol. 2006, 19, 745–752. [Google Scholar] [CrossRef]
- Cui, X.S.; Eriksson, L.C.; Moller, L. Formation and persistence of DNA adducts during and after a long-term administration of 2-nitrofluorene. Mutat. Res. 1999, 442, 9–18. [Google Scholar] [CrossRef]
- Turesky, R.J.; Markovic, J.; Aeschlimann, J.M. Formation and differential removal of C-8 and N2-guanine adducts of the food carcinogen 2-amino-3-methylimidazo[4,5-f]quinoline in the liver, kidney, and colorectum of the rat. Chem. Res. Toxicol. 1996, 9, 397–402. [Google Scholar] [CrossRef]
- Pfau, W.; Schmeiser, H.H.; Wiessler, M. Aristolochic acid binds covalently to the exocyclic amino group of purine nucleotides in DNA. Carcinogenesis 1990, 11, 313–319. [Google Scholar] [CrossRef]
- Stiborova, M.; Frei, E.; Sopko, B.; Sopkova, K.; Markova, V.; Lankova, M.; Kumstyrova, T.; Wiessler, M.; Schmeiser, H.H. Human cytosolic enzymes involved in the metabolic activation of carcinogenic aristolochic acid: Evidence for reductive activation by human NAD(P)H:quinone oxidoreductase. Carcinogenesis 2003, 24, 1695–1703. [Google Scholar] [CrossRef]
- Sidorenko, V.S.; Yeo, J.E.; Bonala, R.R.; Johnson, F.; Scharer, O.D.; Grollman, A.P. Lack of recognition by global-genome nucleotide excision repair accounts for the high mutagenicity and persistence of aristolactam-DNA adducts. Nucleic Acids Res. 2012, 40, 2494–2505. [Google Scholar] [CrossRef]
- Chen, C.H.; Dickman, K.G.; Moriya, M.; Zavadil, J.; Sidorenko, V.S.; Edwards, K.L.; Gnatenko, D.V.; Wu, L.; Turesky, R.J.; Wu, X.R.; et al. Aristolochic acid-associated urothelial cancer in Taiwan. Proc. Natl. Acad. Sci. USA 2012, 109, 8241–8246. [Google Scholar] [CrossRef]
- Hoang, M.L.; Chen, C.H.; Sidorenko, V.S.; He, J.; Dickman, K.G.; Yun, B.H.; Moriya, M.; Niknafs, N.; Douville, C.; Karchin, R.; et al. Mutational signature of aristolochic acid exposure as revealed by whole-exome sequencing. Sci. Transl. Med. 2013, 5, 197ra102. [Google Scholar] [CrossRef]
- Poon, S.L.; Huang, M.N.; Choo, Y.; McPherson, J.R.; Yu, W.; Heng, H.L.; Gan, A.; Myint, S.S.; Siew, E.Y.; Ler, L.D.; et al. Mutation signatures implicate aristolochic acid in bladder cancer development. Genome Med. 2015, 7, 38. [Google Scholar] [CrossRef]
- Groopman, J.D.; Croy, R.G.; Wogan, G.N. In vitro reactions of aflatoxin B1-adducted DNA. Proc. Natl. Acad. Sci. USA 1981, 78, 5445–5449. [Google Scholar] [CrossRef]
- Chawanthayatham, S.; Valentine, C.C., 3rd; Fedeles, B.I.; Fox, E.J.; Loeb, L.A.; Levine, S.S.; Slocum, S.L.; Wogan, G.N.; Croy, R.G.; Essigmann, J.M. Mutational spectra of aflatoxin B(1) in vivo establish biomarkers of exposure for human hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2017, 114, E3101–E3109. [Google Scholar] [CrossRef]
- Jamieson, E.R.; Lippard, S.J. Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef]
- Huang, J.C.; Zamble, D.B.; Reardon, J.T.; Lippard, S.J.; Sancar, A. HMG-domain proteins specifically inhibit the repair of the major DNA adduct of the anticancer drug cisplatin by human excision nuclease. Proc. Natl. Acad. Sci. USA 1994, 91, 10394–10398. [Google Scholar] [CrossRef]
- Boot, A.; Huang, M.N.; Ng, A.W.T.; Ho, S.C.; Lim, J.Q.; Kawakami, Y.; Chayama, K.; Teh, B.T.; Nakagawa, H.; Rozen, S.G. In-depth characterization of the cisplatin mutational signature in human cell lines and in esophageal and liver tumors. Genome Res. 2018, 28, 654–665. [Google Scholar] [CrossRef]
- Neels, J.F.; Gong, J.; Yu, X.; Sturla, S.J. Quantitative correlation of drug bioactivation and deoxyadenosine alkylation by acylfulvene. Chem. Res. Toxicol. 2007, 20, 1513–1519. [Google Scholar] [CrossRef]
- Pietsch, K.E.; van Midwoud, P.M.; Villalta, P.W.; Sturla, S.J. Quantification of acylfulvene- and illudin S-DNA adducts in cells with variable bioactivation capacities. Chem. Res. Toxicol. 2013, 26, 146–155. [Google Scholar] [CrossRef]
- Gong, J.; Vaidyanathan, V.G.; Yu, X.; Kensler, T.W.; Peterson, L.A.; Sturla, S.J. Depurinating acylfulvene-DNA adducts: Characterizing cellular chemical reactions of a selective antitumor agent. J. Am. Chem. Soc. 2007, 129, 2101–2111. [Google Scholar] [CrossRef]
- Gates, K.S. An overview of chemical processes that damage cellular DNA: Spontaneous hydrolysis, alkylation, and reactions with radicals. Chem. Res. Toxicol. 2009, 22, 1747–1760. [Google Scholar] [CrossRef]
- Jaspers, N.G.; Raams, A.; Kelner, M.J.; Ng, J.M.; Yamashita, Y.M.; Takeda, S.; McMorris, T.C.; Hoeijmakers, J.H. Anti-tumour compounds illudin S and Irofulven induce DNA lesions ignored by global repair and exclusively processed by transcription- and replication-coupled repair pathways. DNA Repair 2002, 1, 1027–1038. [Google Scholar] [CrossRef]
- Koeppel, F.; Poindessous, V.; Lazar, V.; Raymond, E.; Sarasin, A.; Larsen, A.K. Irofulven cytotoxicity depends on transcription-coupled nucleotide excision repair and is correlated with XPG expression in solid tumor cells. Clin. Cancer Res. 2004, 10, 5604–5613. [Google Scholar] [CrossRef]
- Otto, C.; Spivak, G.; Aloisi, C.M.; Menigatti, M.; Naegeli, H.; Hanawalt, P.C.; Tanasova, M.; Sturla, S.J. Modulation of Cytotoxicity by Transcription-Coupled Nucleotide Excision Repair Is Independent of the Requirement for Bioactivation of Acylfulvene. Chem. Res. Toxicol. 2017, 30, 769–776. [Google Scholar] [CrossRef]
- Casimir, L.; Zimmer, S.; Racine-Brassard, F.; Jacques, P.E.; Marechal, A. The mutational impact of Illudin S on human cells. DNA Repair 2023, 122, 103433. [Google Scholar] [CrossRef]
- Malvezzi, S.; Farnung, L.; Aloisi, C.M.N.; Angelov, T.; Cramer, P.; Sturla, S.J. Mechanism of RNA polymerase II stalling by DNA alkylation. Proc. Natl. Acad. Sci. USA 2017, 114, 12172–12177. [Google Scholar] [CrossRef]
- Pommier, Y.; Kohlhagen, G.; Bailly, C.; Waring, M.; Mazumder, A.; Kohn, K.W. DNA sequence- and structure-selective alkylation of guanine N2 in the DNA minor groove by ecteinascidin 743, a potent antitumor compound from the Caribbean tunicate Ecteinascidia turbinata. Biochemistry 1996, 35, 13303–13309. [Google Scholar] [CrossRef]
- Takebayashi, Y.; Pourquier, P.; Zimonjic, D.B.; Nakayama, K.; Emmert, S.; Ueda, T.; Urasaki, Y.; Kanzaki, A.; Akiyama, S.I.; Popescu, N.; et al. Antiproliferative activity of ecteinascidin 743 is dependent upon transcription-coupled nucleotide-excision repair. Nat. Med. 2001, 7, 961–966. [Google Scholar] [CrossRef]
- Son, K.; Takhaveev, V.; Mor, V.; Yu, H.; Dillier, E.; Zilio, N.; Pullen, N.J.L.; Ivanov, D.; Ulrich, H.D.; Sturla, S.J.; et al. Trabectedin derails transcription-coupled nucleotide excision repair to induce DNA breaks in highly transcribed genes. Nat. Commun. 2024, 15, 1388. [Google Scholar] [CrossRef]
- Zewail-Foote, M.; Hurley, L.H. Ecteinascidin 743: A minor groove alkylator that bends DNA toward the major groove. J. Med. Chem. 1999, 42, 2493–2497. [Google Scholar] [CrossRef]
- Bueren-Calabuig, J.A.; Giraudon, C.; Galmarini, C.M.; Egly, J.M.; Gago, F. Temperature-induced melting of double-stranded DNA in the absence and presence of covalently bonded antitumour drugs: Insight from molecular dynamics simulations. Nucleic Acids Res. 2011, 39, 8248–8257. [Google Scholar] [CrossRef]
- Feuerhahn, S.; Giraudon, C.; Martinez-Diez, M.; Bueren-Calabuig, J.A.; Galmarini, C.M.; Gago, F.; Egly, J.M. XPF-dependent DNA breaks and RNA polymerase II arrest induced by antitumor DNA interstrand crosslinking-mimetic alkaloids. Chem. Biol. 2011, 18, 988–999. [Google Scholar] [CrossRef]
- Brooks, P.J.; Zakhari, S. Acetaldehyde and the genome: Beyond nuclear DNA adducts and carcinogenesis. Environ. Mol. Mutagen. 2014, 55, 77–91. [Google Scholar] [CrossRef]
- Xu, L.; Wang, W.; Wu, J.; Shin, J.H.; Wang, P.; Unarta, I.C.; Chong, J.; Wang, Y.; Wang, D. Mechanism of DNA alkylation-induced transcriptional stalling, lesion bypass, and mutagenesis. Proc. Natl. Acad. Sci. USA 2017, 114, E7082–E7091. [Google Scholar] [CrossRef]
- Voulgaridou, G.P.; Anestopoulos, I.; Franco, R.; Panayiotidis, M.I.; Pappa, A. DNA damage induced by endogenous aldehydes: Current state of knowledge. Mutat. Res. 2011, 711, 13–27. [Google Scholar] [CrossRef]
- Brooks, P.J. The case for 8,5’-cyclopurine-2’-deoxynucleosides as endogenous DNA lesions that cause neurodegeneration in xeroderma pigmentosum. Neuroscience 2007, 145, 1407–1417. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Krokidis, M.G.; Masi, A.; Terzidis, M.A. On the relevance of hydroxyl radical to purine DNA damage. Free Radic. Res. 2021, 55, 384–404. [Google Scholar] [CrossRef]
- Brooks, P.J. The cyclopurine deoxynucleosides: DNA repair, biological effects, mechanistic insights, and unanswered questions. Free Radic. Biol. Med. 2017, 107, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Kuraoka, I.; Bender, C.; Romieu, A.; Cadet, J.; Wood, R.D.; Lindahl, T. Removal of oxygen free-radical-induced 5’,8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proc. Natl. Acad. Sci. USA 2000, 97, 3832–3837. [Google Scholar] [CrossRef]
- Kropachev, K.; Ding, S.; Terzidis, M.A.; Masi, A.; Liu, Z.; Cai, Y.; Kolbanovskiy, M.; Chatgilialoglu, C.; Broyde, S.; Geacintov, N.E.; et al. Structural basis for the recognition of diastereomeric 5’,8-cyclo-2’-deoxypurine lesions by the human nucleotide excision repair system. Nucleic Acids Res. 2014, 42, 5020–5032. [Google Scholar] [CrossRef]
- Brooks, P.J.; Wise, D.S.; Berry, D.A.; Kosmoski, J.V.; Smerdon, M.J.; Somers, R.L.; Mackie, H.; Spoonde, A.Y.; Ackerman, E.J.; Coleman, K.; et al. The oxidative DNA lesion 8,5’-(S)-cyclo-2’-deoxyadenosine is repaired by the nucleotide excision repair pathway and blocks gene expression in mammalian cells. J. Biol. Chem. 2000, 275, 22355–22362. [Google Scholar] [CrossRef] [PubMed]
- Kirkali, G.; de Souza-Pinto, N.C.; Jaruga, P.; Bohr, V.A.; Dizdaroglu, M. Accumulation of (5’S)-8,5’-cyclo-2’-deoxyadenosine in organs of Cockayne syndrome complementation group B gene knockout mice. DNA Repair 2009, 8, 274–278. [Google Scholar] [CrossRef]
- Wang, J.; Clauson, C.L.; Robbins, P.D.; Niedernhofer, L.J.; Wang, Y. The oxidative DNA lesions 8,5’-cyclopurines accumulate with aging in a tissue-specific manner. Aging Cell 2012, 11, 714–716. [Google Scholar] [CrossRef]
- Krokidis, M.G.; D’Errico, M.; Pascucci, B.; Parlanti, E.; Masi, A.; Ferreri, C.; Chatgilialoglu, C. Oxygen-Dependent Accumulation of Purine DNA Lesions in Cockayne Syndrome Cells. Cells 2020, 9, 1671. [Google Scholar] [CrossRef]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Reardon, J.T.; Bessho, T.; Kung, H.C.; Bolton, P.H.; Sancar, A. In vitro repair of oxidative DNA damage by human nucleotide excision repair system: Possible explanation for neurodegeneration in xeroderma pigmentosum patients. Proc. Natl. Acad. Sci. USA 1997, 94, 9463–9468. [Google Scholar] [CrossRef]
- Tornaletti, S.; Maeda, L.S.; Lloyd, D.R.; Reines, D.; Hanawalt, P.C. Effect of thymine glycol on transcription elongation by T7 RNA polymerase and mammalian RNA polymerase II. J. Biol. Chem. 2001, 276, 45367–45371. [Google Scholar] [CrossRef] [PubMed]
- Kitsera, N.; Stathis, D.; Luhnsdorf, B.; Muller, H.; Carell, T.; Epe, B.; Khobta, A. 8-Oxo-7,8-dihydroguanine in DNA does not constitute a barrier to transcription, but is converted into transcription-blocking damage by OGG1. Nucleic Acids Res. 2011, 39, 5926–5934. [Google Scholar] [CrossRef]
- Luhnsdorf, B.; Epe, B.; Khobta, A. Excision of uracil from transcribed DNA negatively affects gene expression. J. Biol. Chem. 2014, 289, 22008–22018. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kitsera, N.; Allgayer, J.; Parsa, E.; Geier, N.; Rossa, M.; Carell, T.; Khobta, A. Functional impacts of 5-hydroxymethylcytosine, 5-formylcytosine, and 5-carboxycytosine at a single hemi-modified CpG dinucleotide in a gene promoter. Nucleic Acids Res. 2017, 45, 11033–11042. [Google Scholar] [CrossRef] [PubMed]
- Tornaletti, S.; Maeda, L.S.; Kolodner, R.D.; Hanawalt, P.C. Effect of 8-oxoguanine on transcription elongation by T7 RNA polymerase and mammalian RNA polymerase II. DNA Repair 2004, 3, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Kuraoka, I.; Suzuki, K.; Ito, S.; Hayashida, M.; Kwei, J.S.; Ikegami, T.; Handa, H.; Nakabeppu, Y.; Tanaka, K. RNA polymerase II bypasses 8-oxoguanine in the presence of transcription elongation factor TFIIS. DNA Repair 2007, 6, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Allgayer, J.; Kitsera, N.; von der Lippen, C.; Epe, B.; Khobta, A. Modulation of base excision repair of 8-oxoguanine by the nucleotide sequence. Nucleic Acids Res. 2013, 41, 8559–8571. [Google Scholar] [CrossRef]
- Allgayer, J.; Kitsera, N.; Bartelt, S.; Epe, B.; Khobta, A. Widespread transcriptional gene inactivation initiated by a repair intermediate of 8-oxoguanine. Nucleic Acids Res. 2016, 44, 7267–7280. [Google Scholar] [CrossRef]
- Fleming, A.M.; Burrows, C.J. Formation and processing of DNA damage substrates for the hNEIL enzymes. Free Radic. Biol. Med. 2017, 107, 35–52. [Google Scholar] [CrossRef]
- Shafirovich, V.; Kropachev, K.; Anderson, T.; Liu, Z.; Kolbanovskiy, M.; Martin, B.D.; Sugden, K.; Shim, Y.; Chen, X.; Min, J.H.; et al. Base and Nucleotide Excision Repair of Oxidatively Generated Guanine Lesions in DNA. J. Biol. Chem. 2016, 291, 5309–5319. [Google Scholar] [CrossRef]
- Shafirovich, V.; Kropachev, K.; Kolbanovskiy, M.; Geacintov, N.E. Excision of Oxidatively Generated Guanine Lesions by Competing Base and Nucleotide Excision Repair Mechanisms in Human Cells. Chem. Res. Toxicol. 2019, 32, 753–761. [Google Scholar] [CrossRef]
- Fung, H.; Demple, B. A vital role for Ape1/Ref1 protein in repairing spontaneous DNA damage in human cells. Mol. Cell 2005, 17, 463–470. [Google Scholar] [CrossRef]
- Huang, J.C.; Hsu, D.S.; Kazantsev, A.; Sancar, A. Substrate spectrum of human excinuclease: Repair of abasic sites, methylated bases, mismatches, and bulky adducts. Proc. Natl. Acad. Sci. USA 1994, 91, 12213–12217. [Google Scholar] [CrossRef] [PubMed]
- Tornaletti, S.; Maeda, L.S.; Hanawalt, P.C. Transcription arrest at an abasic site in the transcribed strand of template DNA. Chem. Res. Toxicol. 2006, 19, 1215–1220. [Google Scholar] [CrossRef]
- Wang, W.; Walmacq, C.; Chong, J.; Kashlev, M.; Wang, D. Structural basis of transcriptional stalling and bypass of abasic DNA lesion by RNA polymerase II. Proc. Natl. Acad. Sci. USA 2018, 115, E2538–E2545. [Google Scholar] [CrossRef]
- Okuda, M.; Suwa, T.; Suzuki, H.; Yamaguchi, Y.; Nishimura, Y. Three human RNA polymerases interact with TFIIH via a common RPB6 subunit. Nucleic Acids Res. 2022, 50, 1–16. [Google Scholar] [CrossRef]
- Carnie, C.J.; Acampora, A.C.; Bader, A.S.; Erdenebat, C.; Zhao, S.; Bitensky, E.; van den Heuvel, D.; Parnas, A.; Gupta, V.; D’Alessandro, G.; et al. Transcription-coupled repair of DNA-protein cross-links depends on CSA and CSB. Nat. Cell Biol. 2024, 26, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Nakazawa, Y.; Shimada, M.; Ogi, T. Endogenous aldehyde-induced DNA-protein crosslinks are resolved by transcription-coupled repair. Nat. Cell Biol. 2024, 26, 784–796. [Google Scholar] [CrossRef]
- van Sluis, M.; Yu, Q.; van der Woude, M.; Gonzalo-Hansen, C.; Dealy, S.C.; Janssens, R.C.; Somsen, H.B.; Ramadhin, A.R.; Dekkers, D.H.W.; Wienecke, H.L.; et al. Transcription-coupled DNA-protein crosslink repair by CSB and CRL4(CSA)-mediated degradation. Nat. Cell Biol. 2024, 26, 770–783. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khobta, A.; Sarmini, L. Transcription-Coupled Nucleotide Excision Repair: A Faster Solution or the Only Option? Biomolecules 2025, 15, 1026. https://doi.org/10.3390/biom15071026
Khobta A, Sarmini L. Transcription-Coupled Nucleotide Excision Repair: A Faster Solution or the Only Option? Biomolecules. 2025; 15(7):1026. https://doi.org/10.3390/biom15071026
Chicago/Turabian StyleKhobta, Andriy, and Leen Sarmini. 2025. "Transcription-Coupled Nucleotide Excision Repair: A Faster Solution or the Only Option?" Biomolecules 15, no. 7: 1026. https://doi.org/10.3390/biom15071026
APA StyleKhobta, A., & Sarmini, L. (2025). Transcription-Coupled Nucleotide Excision Repair: A Faster Solution or the Only Option? Biomolecules, 15(7), 1026. https://doi.org/10.3390/biom15071026







