Quantitative Alterations in Short-Chain Fatty Acids in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Literature Search Strategy and Study Selection
2.2. Selection of Studies Involving Measurement of SCFA Concentrations in Stool
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
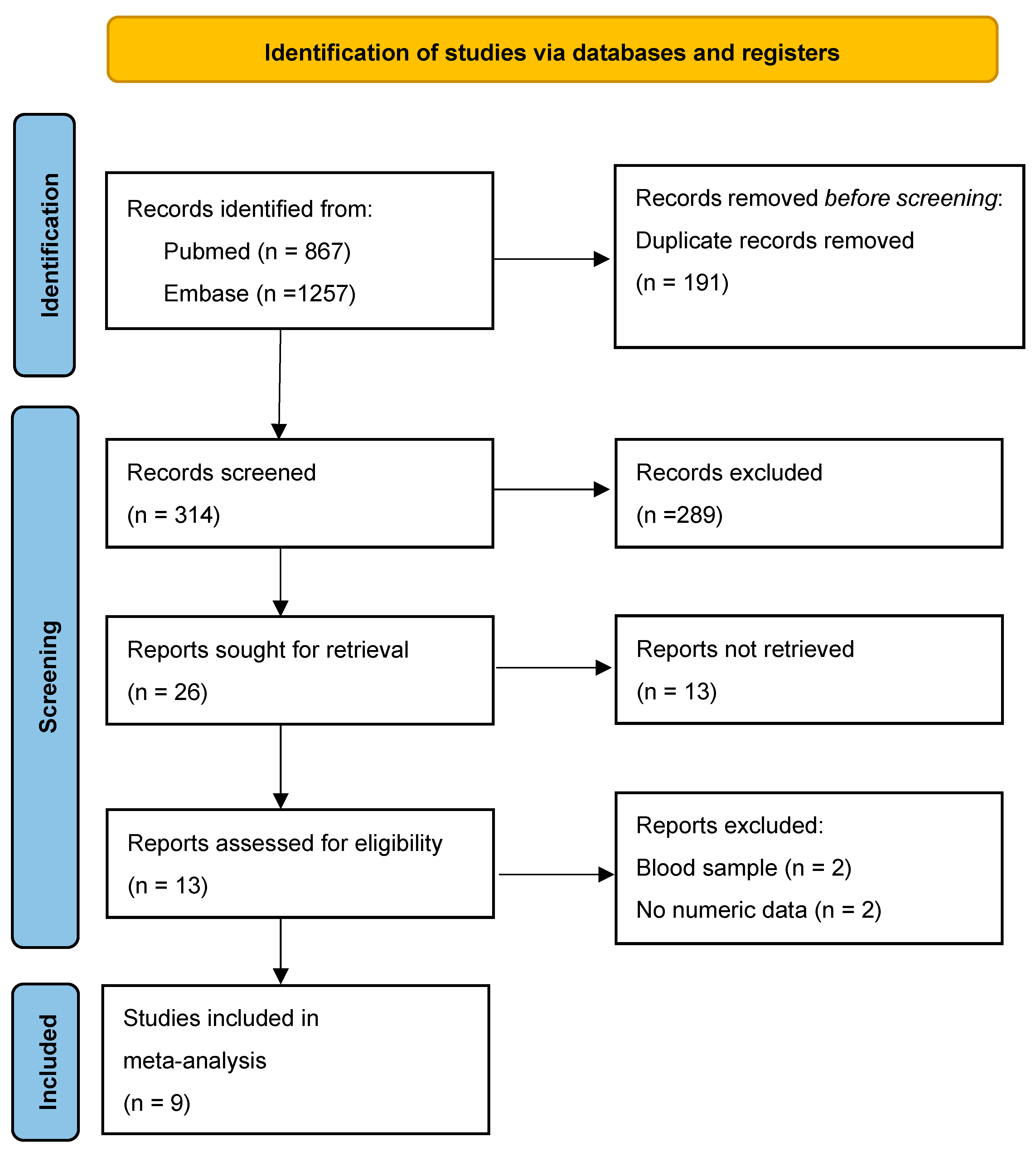
3.1. Literature Search and Study Selection
3.2. Study Characteristics
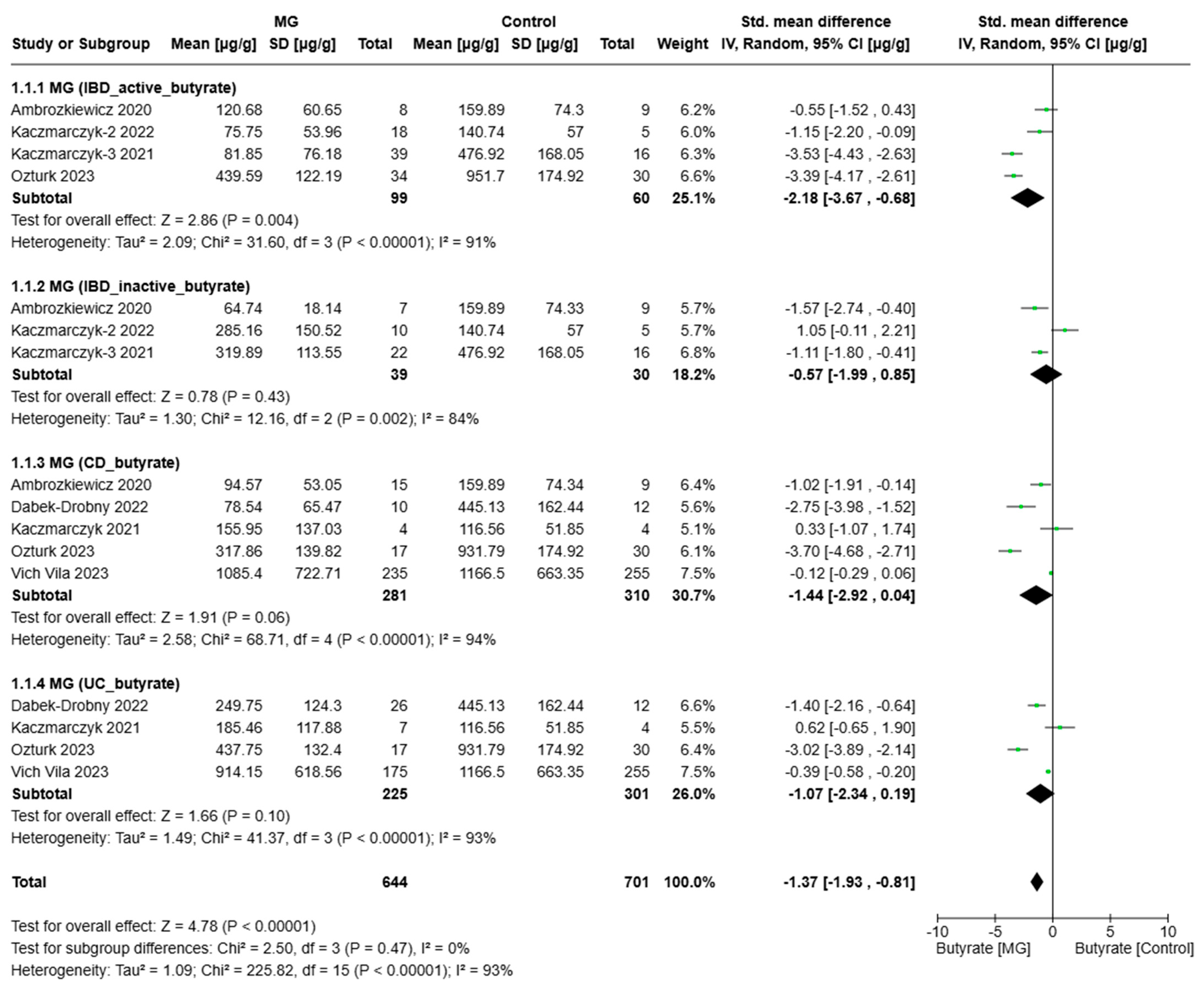
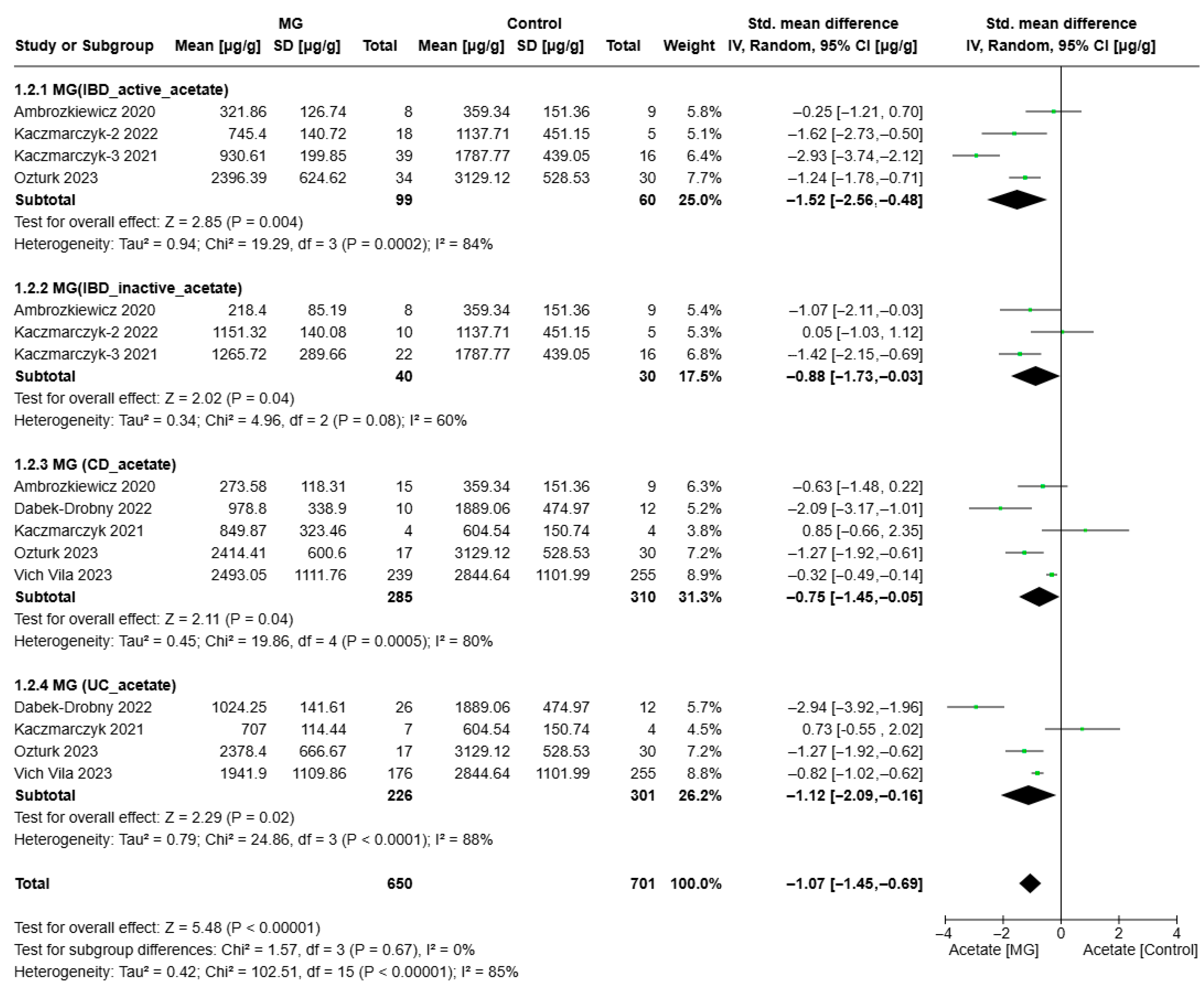
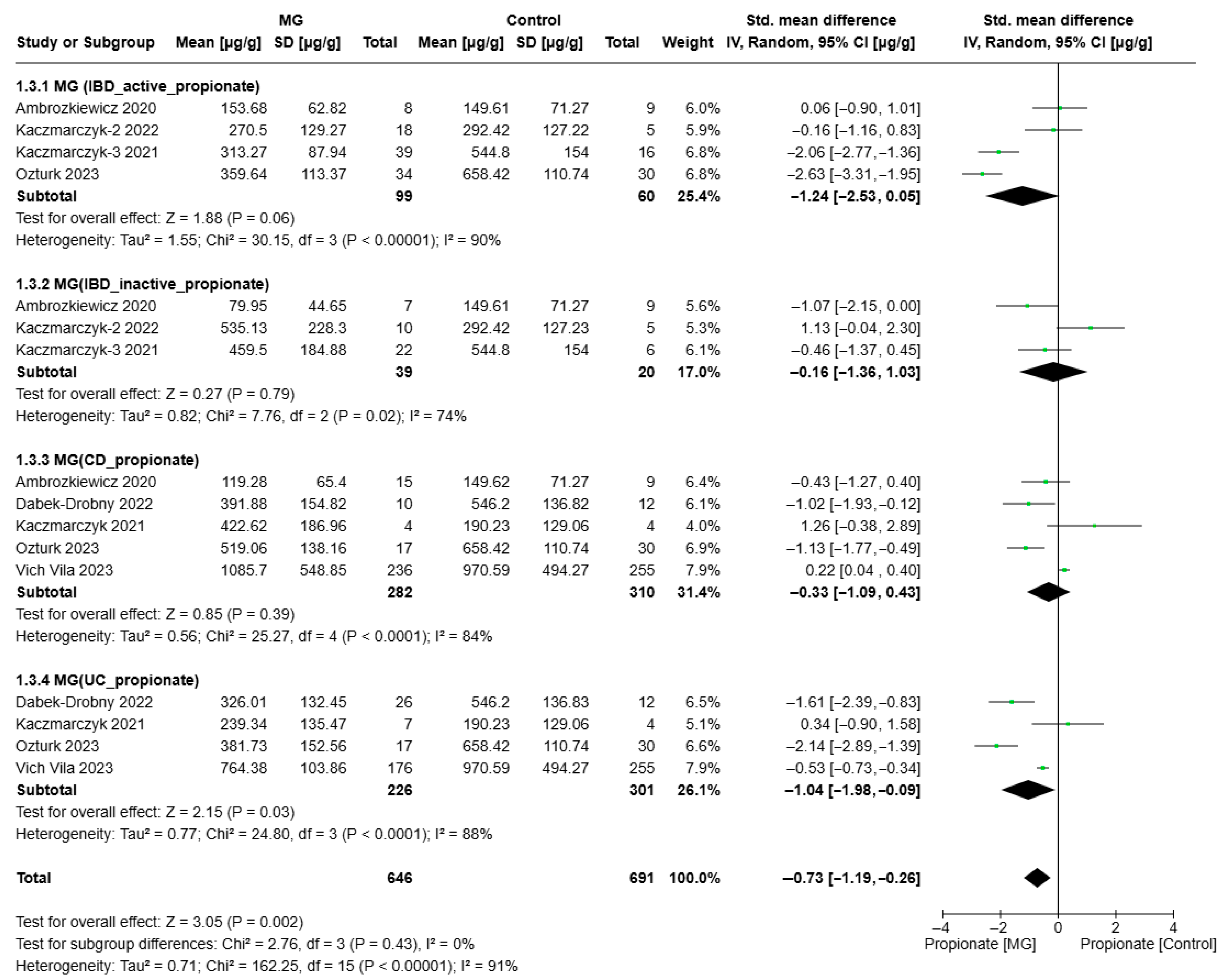
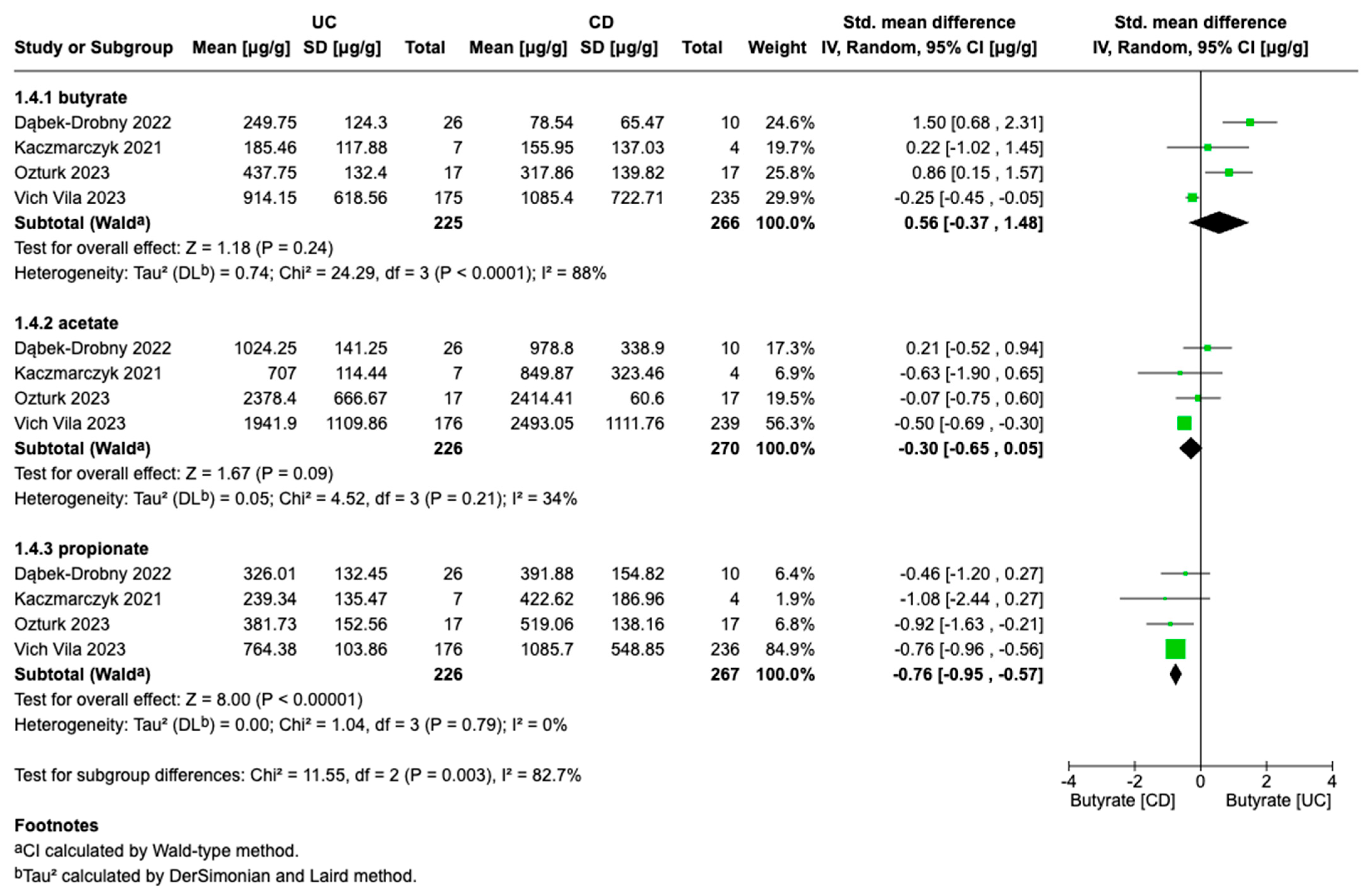
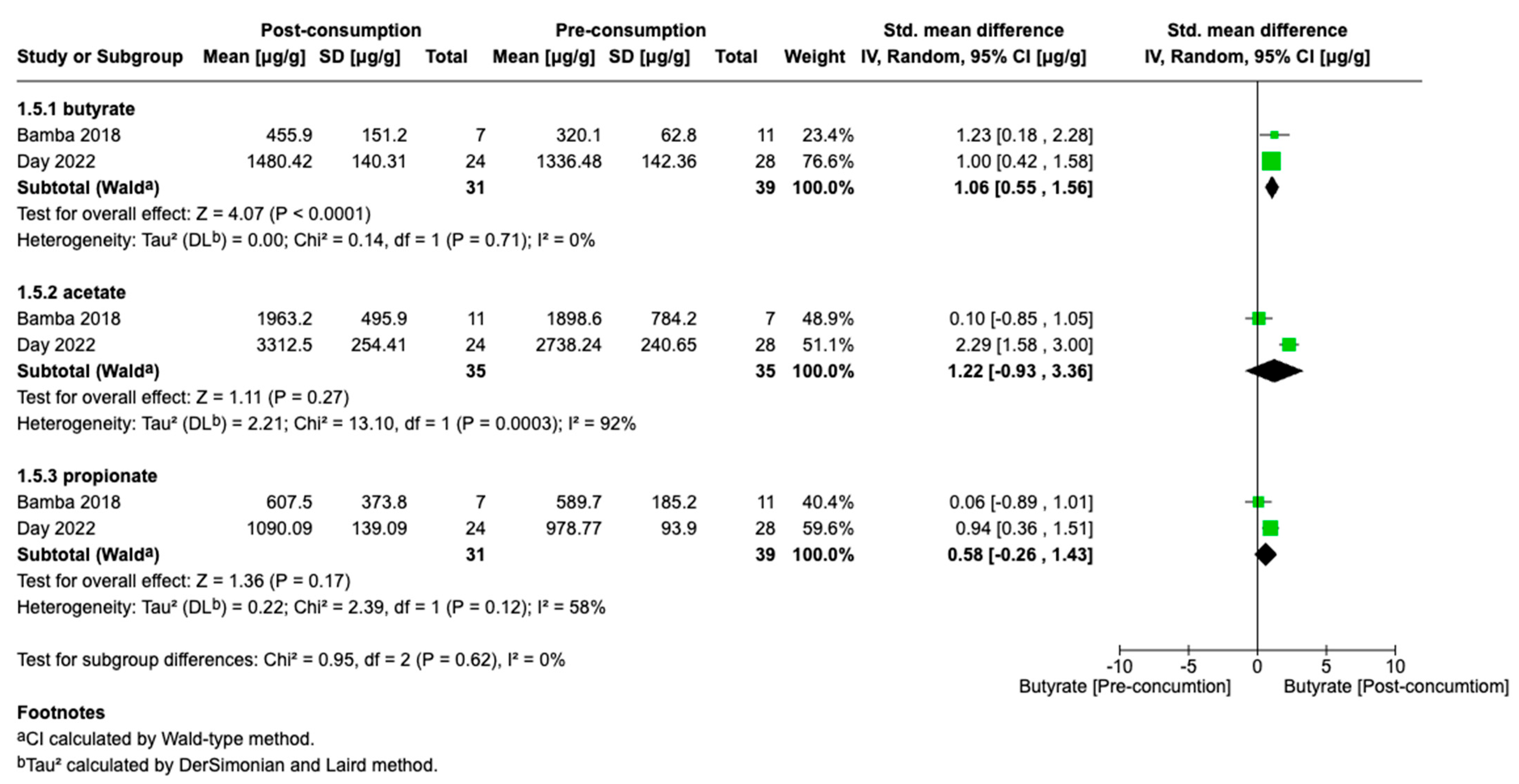
3.3. Meta-Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care-Clin. Off. Pract. 2017, 44, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Segal, J.P.; Jean-Frédéric LeBlanc, A.; Hart, A.L. Ulcerative colitis: An update. Clin. Med. J. R. Coll. Physicians Lond. 2021, 21, 135–139. [Google Scholar] [CrossRef]
- Cockburn, E.; Kamal, S.; Chan, A.; Rao, V.; Liu, T.; Huang, J.Y.; Segal, J.P. Crohn’s disease: An update. Clin. Medicine. J. R. Coll. Physicians Lond. 2023, 23, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Parlett, L.E.; Jonsson Funk, M.L.; Brensinger, C.; Pate, V.; Wu, Q.; Dawwas, G.K.; Weiss, A.; Constant, B.D.; McCauley, M.; et al. Incidence, Prevalence, and Racial and Ethnic Distribution of Inflammatory Bowel Disease in the United States. Gastroenterology 2023, 165, 1197–1205.e2. [Google Scholar] [CrossRef]
- Coward, S.; Benchimol, E.I.; Kuenzig, M.E.; Windsor, J.W.; Bernstein, C.N.; Bitton, A.; Jones, J.L.; Lee, K.; Murthy, S.K.; E Targownik, L.; et al. The 2023 Impact of Inflammatory Bowel Disease in Canada: Epidemiology of IBD. J. Can. Assoc. Gastroenterol. 2023, 6 (Suppl. S2), S9–S15. [Google Scholar] [CrossRef]
- Sarter, H.; Crétin, T.; Savoye, G.; Fumery, M.; Leroyer, A.; Dauchet, L.; Paupard, T.; Coevoet, H.; Wils, P.; Richard, N.; et al. Incidence, Prevalence and Clinical Presentation of Inflammatory Bowel Diseases in Northern France: A 30-Year Population-Based Study [Internet]. 2024. Available online: www.thelancet.com (accessed on 14 April 2025).
- Borowitz, S.M. The epidemiology of inflammatory bowel disease: Clues to pathogenesis? Front. Pediatr. 2023, 10, 1103713. [Google Scholar] [CrossRef]
- Graham, D.B.; Xavier, R.J. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 2020, 578, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, R.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 39–49. [Google Scholar] [CrossRef]
- De Souza, H.S.P.; Fiocchi, C.; Iliopoulos, D. The IBD interactome: An integrated view of aetiology, pathogenesis and therapy. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 739–749. [Google Scholar] [CrossRef]
- Majumder, A.; Bano, S. How the Western Diet Thwarts the Epigenetic Efforts of Gut Microbes in Ulcerative Colitis and Its Association with Colorectal Cancer. Biomolecules 2024, 14, 633. [Google Scholar] [CrossRef]
- Fitzpatrick, J.A.; Halmos, E.P.; Gibson, P.R.; Machado, P.P. Ultra-processed Foods and Risk of Crohn’s Disease: How Much is Too Much? Clin. Gastroenterol. Hepatol. 2023, 21, 2478–2480. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Chang, N.H.; Mohammad, D.; Wong, E.C.L.; Ananthakrishnan, A.N.; Chan, S.S.M.; Carbonnel, F.; Meyer, A. Food Processing and Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 2483–2495.e1. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.; Van Assche, G.; Lindsay, J.O.; Lémann, M.; Söderholm, J.; Colombel, J.F.; Danese, S.; D’HOore, A.; Gassull, M.; Gomollón, F.; et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: Current management. J. Crohn’s Colitis 2010, 4, 28–62. [Google Scholar] [CrossRef]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: Current management. J. Crohns Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Yu, J. Gut microbiome and metabolome: The crucial players in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2023, 38, 5–6. [Google Scholar] [CrossRef]
- Ning, L.; Zhou, Y.L.; Sun, H.; Zhang, Y.; Shen, C.; Wang, Z.; Xuan, B.; Zhao, Y.; Ma, Y.; Yan, Y.; et al. Microbiome and metabolome features in inflammatory bowel disease via multi-omics integration analyses across cohorts. Nat. Commun. 2023, 14, 7135. [Google Scholar] [CrossRef]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef]
- Jauregui-Amezaga, A.; Smet, A. The Microbiome in Inflammatory Bowel Disease. J. Clin. Med. 2024, 13, 4622. [Google Scholar] [CrossRef]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Zhu, S.; Han, M.; Liu, S.; Fan, L.; Shi, H.; Li, P. Composition and diverse differences of intestinal microbiota in ulcerative colitis patients. Front. Cell. Infect. Microbiol. 2022, 12, 953962. [Google Scholar] [CrossRef]
- Liang, B.; Wu, C.; Wang, C.; Sun, W.; Chen, W.; Hu, X.; Liu, N.; Xing, D. New insights into bacterial mechanisms and potential intestinal epithelial cell therapeutic targets of inflammatory bowel disease. Front. Microbiol. 2022, 13, 1065608. [Google Scholar] [CrossRef]
- Lal, S.; Kandiyal, B.; Ahuja, V.; Takeda, K.; Das, B. Gut microbiome dysbiosis in inflammatory bowel disease. In Progress in Molecular Biology and Translational Science; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, J.; Płoska, M.; Płoski, K.; Fichna, J. The role of short-chain fatty acids in inflammatory bowel diseases and colorectal cancer. Postepy Biochem. 2021, 67, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Zhou, P.; Zhang, R. Intestinal Microbiota-Derived Short Chain Fatty Acids in Host Health and Disease. Nutrients 2022, 14, 1977. [Google Scholar] [CrossRef]
- Vinelli, V.; Biscotti, P.; Martini, D.; Del Bo’, C.; Marino, M.; Meroño, T.; Nikoloudaki, O.; Calabrese, F.M.; Turroni, S.; Taverniti, V.; et al. Effects of Dietary Fibers on Short-Chain Fatty Acids and Gut Microbiota Composition in Healthy Adults: A Systematic Review. Nutrients 2022, 14, 2559. [Google Scholar] [CrossRef]
- Kumar, J.; Rani, K.; Datt, C. Molecular link between dietary fibre, gut microbiota and health. Mol. Biol. Rep. 2020, 47, 6229–6237. [Google Scholar] [CrossRef]
- Vich Vila, A.; Hu, S.; Andreu-Sánchez, S.; Collij, V.; Jansen, B.H.; Augustijn, H.E.; Bolte, L.A.; Ruigrok, R.A.A.A.; Abu-Ali, G.; Giallourakis, C.; et al. Faecal metabolome and its determinants in inflammatory bowel disease. Gut 2023, 72, 1472–1485. [Google Scholar] [CrossRef]
- Kaczmarczyk, O.; Dąbek-Drobny, A.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Targosz, A.; Ptak-Belowska, A.; Piątek-Guziewicz, A.; Wcisło, K.; et al. Association between fecal levels of Short-Chain Fatty Acids and serum Pro-and Anti-Inflammatory Cytokines in patients with Inflammatory Bowel Disease. Folia. Med. Cracov. 2022, 62, 43–55. [Google Scholar]
- Bamba, S.; Takahashi, K.; Imaeda, H.; Nishida, A.; Kawahara, M.; Inatomi, O.; Sugimoto, M.; Sasaki, M.; Andoh, A. Effect of fermented vegetable beverage containing pediococcus pentosaceus in patients with mild to moderate ulcerative colitis. Biomed. Rep. 2018, 9, 74–80. [Google Scholar] [CrossRef]
- Ozturk, O.; Celebi, G.; Duman, U.G.; Kupcuk, E.; Uyanik, M.; Sertoglu, E. Short-chain fatty acid levels in stools of patients with inflammatory bowel disease are lower than those in healthy subjects. Eur. J. Gastroenterol. Hepatol. 2024, 36, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Day, A.S.; Yao, C.K.; Costello, S.P.; Ruszkiewicz, A.; Andrews, J.M.; Gibson, P.R.; Bryant, R.V. Therapeutic Potential of the 4 Strategies to SUlfide-REduction (4-SURE) Diet in Adults with Mild to Moderately Active Ulcerative Colitis: An Open-Label Feasibility Study. J. Nutr. 2022, 152, 1690–1701. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, O.; Dąbek-Drobny, A.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Piątek-Guziewicz, A.; Zagrodzki, P.; Mach, T.; Zwolińska-Wcisło, M. Fecal levels of lactic, succinic and short-chain fatty acids in patients with ulcerative colitis and crohn disease: A pilot study. J. Clin. Med. 2021, 10, 4701. [Google Scholar] [CrossRef]
- Dąbek-Drobny, A.; Kaczmarczyk, O.; Piątek-Guziewicz, A.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Targosz, A.; Ptak-Belowska, A.; Zagrodzki, P.; et al. Application of the Clustering Technique to Multiple Nutritional Factors Related to Inflammation and Disease Progression in Patients with Inflammatory Bowel Disease. Nutrients 2022, 14, 3960. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, O.; Dąbek-Drobny, A.; Piątek-Guziewicz, A.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Targosz, A.; Ptak-Belowska, A.; Szczyrk, U.; et al. The Importance of Nutritional Aspects in the Assessment of Inflammation and Intestinal Barrier in Patients with Inflammatory Bowel Disease. Nutrients 2022, 14, 4622. [Google Scholar] [CrossRef]
- Ambrozkiewicz, F.; Karczmarski, J.; Kulecka, M.; Paziewska, A.; Niemira, M.; Zeber-Lubecka, N.; Zagorowicz, E.; Kretowski, A.; Ostrowski, J. In search for interplay between stool microRNAs, microbiota and short chain fatty acids in Crohn’s disease-a preliminary study. BMC Gastroenterol. 2010, 20, 307. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Kaplan, G.G.; Ng, S.C. Changing Global Epidemiology of Inflammatory Bowel Diseases: Sustaining Health Care Delivery Into the 21st Century. Clin. Gastroenterol. Hepatol. 2020, 18, 1252–1260. [Google Scholar] [CrossRef]
- Alam, M.T.; Amos, G.C.A.; Murphy, A.R.J.; Murch, S.; Wellington, E.M.H.; Arasaradnam, R.P. Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog. 2020, 12, 1. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Leontiadis, G.I.; Tse, F.; Yuan, Y.; Surette, M.; Moayyedi, P. Differences in Gut Microbiota in Patients with vs Without Inflammatory Bowel Diseases: A Systematic Review. Gastroenterology 2020, 158, 930–946.e1. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.D.; Son, H.W.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, J.; McEwan, A.G.; Kappler, U. Bacterial acetate metabolism and its influence on human epithelia. Emerg. Top. Life Sci. 2024, 8, 1–13. [Google Scholar]
- Pinhal, S.; Ropers, D.; Geiselmann, J.; De Jong, H. Acetate metabolism and the inhibition of bacterial growth by acetate. J. Bacteriol. 2019, 201, e00147-19. [Google Scholar] [CrossRef]
- Pérez-Reytor, D.; Puebla, C.; Karahanian, E.; García, K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases [Internet]. Front. Immunol. 2019, 10, 277. Available online: https://pubmed.ncbi.nlm.nih.gov/30915065/ (accessed on 23 April 2021).
- Zhang, Z.; Zhang, H.; Chen, T.; Shi, L.; Wang, D.; Tang, D. Regulatory role of short-chain fatty acids in inflammatory bowel disease. Cell Commun. Signal. 2022, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Xia, S.; Xiao, S.; Yu, Q. Short-chain fatty acids affect the development of inflammatory bowel disease through intestinal barrier, immunology, and microbiota: A promising therapy? J. Gastroenterol. Hepatol. 2022, 37, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Martínková, K.; Vítĕzová, M.; Rittmann, S.K.M.R. Intestinal microbiota and perspectives of the use of meta-analysis for comparison of ulcerative colitis studies. J. Clin. Med. 2021, 10, 462. [Google Scholar] [CrossRef]
- Xu, H.M.; Zhao, H.L.; Guo, G.J.; Xu, J.; Zhou, Y.L.; Huang, H.L.; Nie, Y.-Q. Characterization of short-chain fatty acids in patients with ulcerative colitis: A meta-analysis. BMC Gastroenterol. 2022, 22, 117. [Google Scholar] [CrossRef]
- Zhuang, X.; Li, T.; Li, M.; Huang, S.; Qiu, Y.; Feng, R.; Zhang, S.; Chen, M.; Xiong, L.; Zeng, Z. Systematic Review and Meta-analysis: Short-Chain Fatty Acid Characterization in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1751–1763. [Google Scholar] [CrossRef]
- Ferrer-Picón, E.; Dotti, I.; Corraliza, A.M.; Mayorgas, A.; Esteller, M.; Perales, J.C.; Ricart, E.; Masamunt, M.C.; Carrasco, A.; Tristán, E.; et al. Intestinal Inflammation Modulates the Epithelial Response to Butyrate in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 43–55. [Google Scholar] [CrossRef]
- Yang, T.; Magee, K.L.; Colon-Perez, L.M.; Larkin, R.; Liao, Y.S.; Balazic, E.; Cowart, J.R.; Arocha, R.; Redler, T.; Febo, M.; et al. Impaired butyrate absorption in the proximal colon, low serum butyrate and diminished central effects of butyrate on blood pressure in spontaneously hypertensive rats. Acta Physiologica. 2019, 226, e13256. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediat. Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Andoh, A.; Sugimoto, M. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Hajjar, R.; Richard, C.S.; Santos, M.M. The role of butyrate in surgical and oncological outcomes in colorectal cancer. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 320, G601–G608. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, K.N.; Vitetta, L. Effects of intestinal microbial-elaborated butyrate on oncogenic signaling pathways. Nutrients 2019, 11, 1026. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Sakuraba, H. Uptake and Advanced Therapy of Butyrate in Inflammatory Bowel Disease. Immuno 2022, 2, 692–702. [Google Scholar] [CrossRef]
- Salvi, P.S.; Cowles, R.A. Butyrate and the intestinal epithelium: Modulation of proliferation and inflammation in homeostasis and disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Bhati, C.; Minocha, N.; Purohit, D.; Kumar, S.; Makhija, M.; Saini, S.; Kaushik, D.; Pandey, P. High Performance Liquid Chromatography: Recent Patents and Advancement. Biomed. Pharmacol. J. 2022, 15, 729–746. [Google Scholar] [CrossRef]
- Gruber, B.; David, F.; Sandra, P. Capillary gas chromatography-mass spectrometry: Current trends and perspectives. TrAC-Trends Anal. Chem. 2020, 124, 115475. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2014, 74, 13–22. [Google Scholar] [CrossRef]
- Duncan, S.H.; Barcenilla, A.; Stewart, C.S.; Pryde, S.E.; Flint, H.J. Acetate utilization and butyryl coenzyme A (CoA): Acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 2002, 68, 5186–5190. [Google Scholar] [CrossRef]
- Zhan, Z.; Tang, H.; Zhang, Y.; Huang, X.; Xu, M. Potential of gut-derived short-chain fatty acids to control enteric pathogens. Front. Microbiol. 2022, 13, 976406. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Deleu, S.; Arnauts, K.; Deprez, L.; Machiels, K.; Ferrante, M.; Huys, G.R.B.; Thevelein, J.M.; Raes, J.; Vermeire, S. High Acetate Concentration Protects Intestinal Barrier and Exerts Anti-Inflammatory Effects in Organoid-Derived Epithelial Monolayer Cultures from Patients with Ulcerative Colitis. Int. J. Mol. Sci. 2023, 24, 768. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Sun, M.; Chen, F.; Cao, A.T.; Liu, H.; Zhao, Y.; Huang, X.; Xiao, Y.; Yao, S.; Zhao, Q.; et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017, 10, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Miyauchi, E.; Kanaya, T.; Kato, T.; Nakanishi, Y.; Watanabe, T.; Kitami, T.; Taida, T.; Sasaki, T.; Negishi, H.; et al. Acetate differentially regulates IgA reactivity to commensal bacteria. Nature 2021, 595, 560–564. [Google Scholar] [CrossRef]
- Agus, A.; Richard, D.; Faïs, T.; Vazeille, E.; Chervy, M.; Bonnin, V.; Dalmasso, G.; Denizot, J.; Billard, E.; Bonnet, R.; et al. Propionate catabolism by CD-associated adherent-invasive E. coli counteracts its anti-inflammatory effect. Gut Microbes 2021, 13, 1839318. [Google Scholar] [CrossRef]
- Tong, L.C.; Wang, Y.; Wang, Z.B.; Liu, W.Y.; Sun, S.; Li, L.; Su, D.-F.; Zhang, L.-C. Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front. Pharmacol. 2016, 7, 253. [Google Scholar] [CrossRef]
- Hoyles, L.; Snelling, T.; Umlai, U.K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome–host systems interactions: Protective effects of propionate upon the blood–brain barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef]
- Laserna-Mendieta, E.J.; Clooney, A.G.; Carretero-Gomez, J.F.; Moran, C.; Sheehan, D.; Nolan, J.A.; Hill, C.; Gahan, C.G.M.; A Joyce, S.; Shanahan, F.; et al. Determinants of reduced genetic capacity for butyrate synthesis by the gut microbiome in Crohn’s disease and ulcerative colitis. J. Crohns Colitis 2018, 12, 204–216. [Google Scholar] [CrossRef]
- Akkoç, T. Epithelial barrier dysfunction and microbial dysbiosis: Exploring the pathogenesis and therapeutic strategies for Crohn’s disease. Tissue Barriers 2024, 2390705. [Google Scholar] [CrossRef] [PubMed]
- Gasaly, N.; Hermoso, M.A.; Gotteland, M. Butyrate and the fine-tuning of colonic homeostasis: Implication for inflammatory bowel diseases. Int. J. Mol. Sci. 2021, 22, 3061. [Google Scholar] [CrossRef]
- Mohamed Elfadil, O.; Mundi, M.S.; Abdelmagid, M.G.; Patel, A.; Patel, N.; Martindale, R. Butyrate: More Than a Short Chain Fatty Acid. Curr. Nutr. Rep. 2023, 12, 255–262. [Google Scholar] [CrossRef]
- Medina, J.M.; Fernández-López, R.; Crespo, J.; Cruz, F.d.l. Propionate fermentative genes of the gut microbiome decrease in inflammatory bowel disease. J. Clin. Med. 2021, 10, 2176. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Tan, Y.; Yu, D.; Qiu, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The Therapeutic Effect of SCFA-Mediated Regulation of the Intestinal Environment on Obesity. Front. Nutr. 2022, 9, 886902. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, Y.; Wang, X.; Kong, L.; Johnston, L.J.; Lu, L.; Ma, X. Dietary nutrients shape gut microbes and intestinal mucosa via epigenetic modifications. Crit. Rev. Food Sci. Nutr. 2022, 62, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, probiotics, synbiotics, paraprobiotics and postbiotic compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef]
| Study | Country | Study Type | Type of IBD | Case/ Control | Control | IBD | Unit | Data Format Provided for SCFAs | Methods for Measure SCFAs | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UC/CD | |||||||||||||
| N | Median Age, Years | Sex (F/M) | N | Median Age, Years | Sex (F/M) | ||||||||
| Kaczmarczyk, [35] | Poland | Case–control | UC/CD | IBD/control | 4 | 48 | 2/2 | 23/8 | 32/33.5 | (10/13)/(3/5) | µg/g | median (Q1–Q3) | CE-UV |
| Dabek-Drobny, [36] | Poland | Case–control | UC/CD | IBD/control | 12 | 23.5 | 3/9 | 26/10 | 35/28 | (9/17)/(5/5) | µg/g | median (Q1–Q3) | CE-UV |
| Ozturk, [33] | Turkey | Case–control | UC/CD | IBD/control | 30 | 44 | 13/17 | 17/17 | 40/49 | (7/10)/(7/10) | mmol/kg | Mean, SD; median (Q1–Q3) | GC-MS |
| Vich Vila, [30] | The Netherlands | Case–control | UC/CD | IBD/control | 255 | 46.83 | 141/114 | 176/239 | 42.61 | 254/170 | μg/g | Mean, SD | LC-MS-MS |
| Ambrozkiewicz, [38] | Poland | Case–control | CD | IBD/control | 9 | 32 | 6/3 | 15 | 32 | 5/10 | [ppm] | Mean, SD | GC-MS |
| Study | Country | Study Type | Type of IBD | IBD Diagnosis/Activity | Case/Control | Control | IBD | Unit | Data Format Provided for SCFAs | Methods for Measure SCFAs | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inactive/Active | ||||||||||||||
| N | Median Age, Years | Sex (F/M) | N | Median Age, Years | Sex (F/M) | |||||||||
| Kaczmarczyk-2, [31] | Poland | Case–control | UC/CD | Mayo score | IBD/control | 6 | 43.8 | 4/2 | 11/18 | 39.8 | (2/9)/(2/12) | µg/g | median (Q1–Q3) | CE-UV |
| Kaczmarczyk-3, [37] | Poland | Case–control | IBD | Mayo Score/CDAI, colonoscopy, and/or imaging | IBD/control | 16 | 31.7 | 13/3 | 22/39 | 36.8/32.5 | 22/39 | μg/g | median (Q1–Q3) | CE-UV |
| Study | Country | Study Type | Type of IBD | Diet | Case/Control | IBD | Unit | Data Format Provided for SCFAs | Methods for Measure SCFAs | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Consumption/Post-Consumption | |||||||||||
| N | Median Age, Years | Sex (F/M) | |||||||||
| Day, [34] | Australia | prospective, 8 wk, open-label feasibility study | UC | SUlfide-REduction (4-SURE) Diet | W8/W0 | 28/28 | 42 | 15/13 | μmol/g | median (Q1–Q3) | GC |
| Bamba, [32] | Japan | an open-label randomized controlled trial | UC | fermented vegetable beverage | UC before and after diet | 11/11 | 43/58 | 6/5 | µmol/g | median (Q1–Q3) | HPLC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chulenbayeva, L.; Jarmukhanov, Z.; Kaliyekova, K.; Kozhakhmetov, S.; Kushugulova, A. Quantitative Alterations in Short-Chain Fatty Acids in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Biomolecules 2025, 15, 1017. https://doi.org/10.3390/biom15071017
Chulenbayeva L, Jarmukhanov Z, Kaliyekova K, Kozhakhmetov S, Kushugulova A. Quantitative Alterations in Short-Chain Fatty Acids in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Biomolecules. 2025; 15(7):1017. https://doi.org/10.3390/biom15071017
Chicago/Turabian StyleChulenbayeva, Laura, Zharkyn Jarmukhanov, Karlygash Kaliyekova, Samat Kozhakhmetov, and Almagul Kushugulova. 2025. "Quantitative Alterations in Short-Chain Fatty Acids in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis" Biomolecules 15, no. 7: 1017. https://doi.org/10.3390/biom15071017
APA StyleChulenbayeva, L., Jarmukhanov, Z., Kaliyekova, K., Kozhakhmetov, S., & Kushugulova, A. (2025). Quantitative Alterations in Short-Chain Fatty Acids in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Biomolecules, 15(7), 1017. https://doi.org/10.3390/biom15071017






