1. Introduction
Colorectal cancer (CRC) is one of the most commonly diagnosed cancers and represents the leading cause of cancer-related mortality. While its pathogenesis involves complex interactions between genetic predisposition and environmental factors, emerging evidence highlights the major contribution of the gut microbiota in modulating colorectal carcinogenesis [
1,
2]. In the last decade, significant efforts and advancements have been made to comprehend the intricate relationship between the human gut microbiome and CRC, but due to multi-factorial interactions, the precise mechanisms underlying this relationship remain unclear. The intestinal microbiome, comprising trillions of microorganisms, contributes to host homeostasis under normal conditions. The emergence of an imbalance in the microbiota, known as dysbiosis, disrupts intestinal homeostasis and drives chronic inflammation, genotoxicity, and immune dysregulation, thereby promoting tumor development. Dysbiosis is characterized by a decrease in the level of beneficial bacteria, such as
Bifidobacterium spp. and
Lactobacillus spp., accompanied by an excessive growth of pathogenic, harmful bacteria, such as
Clostridium difficile (
CD),
Fusobacterium nucleatum (
Fn), or
Escherichia coli (
E. coli) [
3,
4]. In the context of colorectal cancer (CRC), research has yet to identify a single pathogenic agent. Recent advancements in the fields of microbiology, immunology, and oncology suggest that CRC should be viewed as a condition influenced by the complex interactions between the host and its microbiome rather than being caused by a specific infectious agent [
5]. The diverse bacterial species that constitute the microbiota coexist with the host and engage in continuous interactions with immune and epithelial cells, resulting in a bidirectional communication process. The immune response of the host can shape the composition of the microbiota, while the microbiota simultaneously exerts an influence over the host’s immune system. The dysbiosis observed in CRC is not attributable to a solitary microbe but rather arises from an imbalanced ecosystem that compromises the integrity of the intestinal barrier, promotes chronic inflammation, and affects DNA stability [
6]. A consortium of bacterial species—including
Fn,
Enterotoxigenic bacteroides fragilis (ETBF),
Streptococcus gallolyticus (
S. gallolyticus),
Enterococcus faecalis, toxigenic
E. coli (phylogroup B2),
Helicobacter pylori (
H. pylori), and
Clostridium septicum (
CS)—has been implicated in CRC pathogenesis. These microorganisms contribute to tumorigenesis via diverse but often converging mechanisms such as chronic inflammation, genotoxicity, epigenetic reprogramming, immune modulation, and the disruption of epithelial integrity. Rather than acting independently, many of these species may function synergistically, modifying the intestinal microenvironment in ways that favor malignant transformation and progression [
7].
Although there is still no unanimously accepted sequence of the appearance of pathogenic bacteria in the dysbiosis that precedes colorectal cancer, some longitudinal studies and animal models suggest a probable three-stage pattern: (1) the pre-neoplastic phase associated with the appearance of dysbiosis due to environmental factors (high-fat diet, antibiotics, obesity) that reduce the abundance of beneficial bacteria (such as
Roseburia spp.) allowing the development of pathogens [
8]; (2) early colonization with “inflammophiles”, pathogenic species that settle in an already permeable epithelium generating oxidative stress and DNA damage, accelerating the transformation of adenoma into carcinoma [
9]; (3) the overlap of “tumor promoters” at the time of tumor formation, when the microenvironment becomes hypoxic and rich in necroinflammatory nutrients, proliferating bacteria that can amplify local inflammation and tumor proliferation (for example,
Fn and
ETBF and, in advanced stages,
S. gallolyticus and
CS (opportunistic species that thrive in necrotic and hypoxic tumor tissue)) [
10]. The phases are not strictly sequential and may vary from one individual to another; rather, they represent an ecological dynamic in which niches created by one microbial group are exploited by the next [
11,
12]. A complex network of interactions facilitates the relationship between the immune system and the microbiota, which is essential for maintaining an equilibrium between immune tolerance and immunogenicity [
13]. The immune system develops two distinct types of immune responses, innate and adaptive, which act synergistically against pathogens. Adaptive immune responses can be mediated by antibodies (humoral immune response) or by cells (cellular immune response) [
14], with both of them being modulated by the microbiota in CRC [
15]. Certain microbial species can either promote immune evasion and tumor progression or enhance anti-tumor immunity, thereby affecting the disease outcome and response to therapy [
16,
17,
18].
Dysbiosis leads to changes in microbial metabolites, resulting in a decrease in beneficial substances like short-chain fatty acids (SCFAs), particularly butyrate. At the same time, dysbiosis increases the production of harmful metabolites, such as lipopolysaccharides (LPS) [
19]. Furthermore, dysbiosis contributes to heightened intestinal permeability, which facilitates the entry of microbial products, such as LPS, into the bloodstream. This process can induce chronic inflammation, a factor that plays a significant role in immune dysregulation [
20].
Immunological mechanisms are essential and ubiquitous in CRC development and progression, but they work in synergy with genetic, epigenetic, and metabolic mechanisms. Depending on the bacterium and the host context, one of these mechanisms may become dominant. Understanding these complex and context-dependent interactions is essential for developing personalized therapeutic strategies, such as microbiota manipulation by probiotics, targeted bacteriotherapy, or dietary interventions that restore immune homeostasis and inhibit microbially activated oncogenic pathways [
21].
This review seeks to elucidate the impact of the gut microbiota on tumor development and progression from an immunological point of view. The initial section examines how gut microbiota influences immune responses that may either promote or inhibit the progression of CRC. We present the strategies employed by pathogenic bacteria that induce chronic inflammation and immune suppression, as well as the mechanisms through which beneficial bacteria can enhance anti-tumor immunity. Additionally, we analyze microbiota-derived metabolites and their role in immune modulation within the context of CRC, aiming to identify how these metabolites affect the efficacy of CRC therapies.
2. The Impact of Bacterial Pathogens on Immune Response and Intestinal Barrier Integrity in CRC
Certain gut bacteria serve as more than passive observers in the context of CRC; they function as active participants in the disease’s progression by altering both host immunity and the architecture of epithelial tissue in ways that facilitate tumor development [
22,
23]. This section summarizes the current evidence on two interconnected fronts: (1) key strategies used by bacterial pathogens to impact immune modulation and evasion in CRC; (2) the mechanisms through which pathogenic bacteria compromise the integrity of the intestinal barrier in this disease. Together, these pathogenic maneuvers create a permissive niche in which neoplastic clones can emerge, expand, and metastasize.
2.1. Key Strategies by Which Bacterial Pathogens Modulate and Evade Immunity in CRC
Colorectal carcinogenesis unfolds within a microenvironment in which host immunity is continually shaped by the gut microbiota. Pathogenic bacteria exploit this context through a repertoire of immune-oriented strategies that both activate and evade host defenses, ultimately tipping the balance toward tumor promotion via: (1) the activation of innate immune responses, (2) the suppression and evasion of adaptive immunity, (3) crosstalk among pathogenic bacteria, reactive oxygen species (ROS) production, and the immune response (
Figure 1).
2.1.1. Activation of Innate Immune Responses by Pathogenic Bacteria
The activation of the innate immune response represents the initial line of defense the body employs against pathogenic incursions. This process is critical for the recognition and response to harmful microorganisms [
24]. Pathogenic bacteria can activate the innate immune system by interacting with pattern recognition receptors (PRRs) on immune and epithelial cells, triggering pro-inflammatory signaling pathways [
25,
26]. Some pathogenic bacteria release molecules such as lipopolysaccharides (LPS), flagellin, or peptidoglycans that bind to receptors on epithelial cells and immune cells (e.g., toll-like receptor 4—TLR4, toll-like receptor 5—TLR5) and trigger the activation of the nuclear factor-κB (NF-κB) and mitogen-activated protein kinase signaling pathways (MAPK) that lead to the increased synthesis and release of pro-inflammatory cytokines, such as interleukine-6 (IL-6), interleukine-1beta (IL-1β), and tumor necrosis factor alpha (TNF-α) [
27]. Thus, the generated inflammatory process is sustained and contributes to DNA damage, epithelial barrier disruption, and tumor progression. The activation of nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) in epithelial cells by pathogens such as ETBF or certain strains of
E. coli leads to the formation of the inflammasome (e.g., the NLRP3 inflammasome) and the release of IL-1β and IL-18, which support chronic inflammation and tumorigenesis [
28,
29]. In addition, as a consequence of the increased synthesis of pro-inflammatory cytokines, there is a massive recruitment of myeloid cells, including macrophages and neutrophils, which produce reactive oxygen species (ROS) and reactive nitrogen species (RNS) in large quantities, leading to DNA damage in epithelial cells [
30]. Consequently, chronic inflammation promotes angiogenesis by increasing the production of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), supporting tumor growth (
Figure 1).
2.1.2. Immune Suppression and Evasion
The suppression and evasion of adaptive immunity encompass the mechanisms employed by pathogens to evade or diminish the body’s specific immune response. This phenomenon typically occurs subsequent to the activation of the adaptive immune system. T cells, including CD4
+ helper cells and CD8
+ cytotoxic cells, as well as B cells, play a role in inhibiting the presentation of major histocompatibility complex (MHC) molecules. They also contribute to the suppression of the immune response through the action of regulatory T cells (Tregs). As a result of these interactions, pathogens can evade detection, creating conditions that allow for chronic infections [
31]. Pathogenic bacteria like
Fn can impair the function of anti-tumor immune cells. Fn interacts with immune cells through the fusobacterium adhesin A (FadA) adhesin, which binds to E-cadherin on tumor cells, activating Wnt/β-catenin signaling and promoting cell proliferation and invasion. Also,
Fn expresses the fibroblast activation protein-2 (Fap2), which binds to the immune inhibitory receptor known as T cell immunoreceptor with Ig and ITIM domains (TIGIT) on T cells and natural killers (NK) cells, inhibiting their functions. The result of this process is reduced tumor cell killing and allowing cancer cells to evade immune surveillance. Pathogens may recruit or induce populations of immune cells that facilitate tumor growth and suppress anti-tumor immunity [
32]. For example, Fn recruits myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), both of which suppress cytotoxic T lymphocyte (CTL) functions, which are crucial for attacking tumor cells (
Figure 1). Additionally, Fn secretes various factors that impair antigen-presenting cells (APCs), like dendritic cells, preventing an effective anti-tumor immune response (
Table 1).
Pathogenic bacteria produce metabolites that interfere with immune functions. Bacterial toxins like colibactin from
E. coli not only damage DNA but also lead to chronic inflammation which promotes immunosuppressive cell recruitment, making the immune system less effective at targeting tumor cells. These bacterial toxins contribute to promoting immune evasion in CRC (
Table 1). Metabolites produced by certain bacteria, like secondary bile acids (e.g., deoxycholic acid), promote oxidative stress and DNA damage [
40]. In addition, hydrogen sulfide (H2S) generated by sulfur-reducing bacteria damages epithelial DNA and impairs mitochondrial function. These toxins and metabolites create a pro-carcinogenic environment and enhance tumor evolution [
41]. Pathogenic bacteria can suppress anti-tumor immune responses, allowing tumors to grow unchecked and spread to distant sites.
Pathogenic bacteria alter the balance between pro-inflammatory and anti-inflammatory cytokines in the tumor microenvironment. Many bacteria trigger the release of IL-10 and TGF-β, which suppress effector T cells and promote immune tolerance. At the same time, they inhibit interferon-gamma (IFN-γ) and TNF-α, which are crucial for the activation of macrophages, dendritic cells, and T cells that attack tumors. Reduced levels of IFN-γ and IL-12 impair the activation of CTLs and NK cells. Persistent inflammation can exhaust effector T cells, promote Treg activity, and create a tumor-promoting cytokine milieu [
42,
43]. This shift in cytokine balance makes the tumor microenvironment immunosuppressive, allowing cancer cells to evade immune attack.
2.1.3. Interactions Between the Pathogenic Bacteria, ROS Production, and the Immune Response in Colorectal Cancer
Elucidating how the gut microbiota drives ROS generation reveals that these microbes shape colorectal carcinogenesis by stimulating pro-tumor inflammation, reprogramming immune cells toward a suppressive phenotype, and facilitating tumor immune evasion [
44]. Some of the pathogenic bacteria that significantly influence the production of ROS in CRC initiation and progression, and several interconnected mechanisms are presented in
Table 2. These bacteria contribute either directly through toxins or genotoxins or indirectly modulating host cells to produce them by inducing inflammation that promotes ROS release by immune cells [
45].
Bacteroides fragilis (particularly
enterotoxigenic B. fragilis) releases BFT, which activates nicotine adenine dinucleotide phosphate oxidase (NOX) in colon epithelial cells, leading to ROS production.
E. coli strains with the pks+ island produce colibactin, a genotoxin that induces DNA double-strand breaks, partly through ROS-mediated mechanisms. These ROS can cause mutagenesis (DNA damage), lipid peroxidation, and protein oxidation that contribute to genomic instability, a hallmark of cancer initiation. In early CRC, microbial ROS activation may break the tolerance of the gut immune system leading to the over-activation of innate immunity [
51].
Immune system interactions with microbial imbalance lead to chronic inflammation, and pathogenic bacteria facilitate the infiltration of inflammatory cells like neutrophils and macrophages in the colon mucosa and release high levels of ROS. Chronic exposure to this oxidative environment promotes the oncogenic mutations and activation of pro-cancer signaling pathways (e.g., NF-κB, or Signal Transducer and Activator of Transcription 3-STAT3) [
52]. In late-stage CRC, some pathogen bacteria increase oxidative stress which suppresses anti-tumor immunity, for example,
Fn inhibits NK and T cell activity. However, ROS can suppress APCs and impair dendritic cell (DC) function [
53].
In addition, ROS alters immune cell function and polarization. Low ROS levels activate immune responses, including antigen presentation and pro-inflammatory cytokine release. High levels of ROS induce T cell exhaustion, impair CTLs, promote differentiation of Tregs and Th17 cells (linked to tumor growth) or lead to M2-like macrophage polarization (pro-tumor), rather than M1 (anti-tumor) [
54,
55]. In consequence, ROS reprogram the immune landscape to support immune evasion and tumor tolerance (
Table 2).
Some pathogenic bacteria act to induce antioxidant pathway suppression, downregulating host antioxidant enzymes like glutathione peroxidase (GPx) and superoxide dismutase (SOD). This increases intracellular ROS, pushing epithelial cells toward transformation.
Fusobacterium nucleatum,
E. coli, and
B. fragilis together create a biofilm and facilitate a hypoxic, inflammatory microenvironment that promote sustained ROS production at the mucosal surface. This further encourages epithelial-to-mesenchymal transition (EMT), tumor growth, and invasion. ROS can contribute to more microbial translocation and dysbiosis but when ROS is locally higher concentrated ROS feedback appears to alter the microbiome and form a vicious cycle responsible for CRC progression [
56].
Understanding these relationships between the microbiome, ROS production, and the immune response can help pave the way for innovative therapeutic strategies and enhance our approach to prevention and treatment.
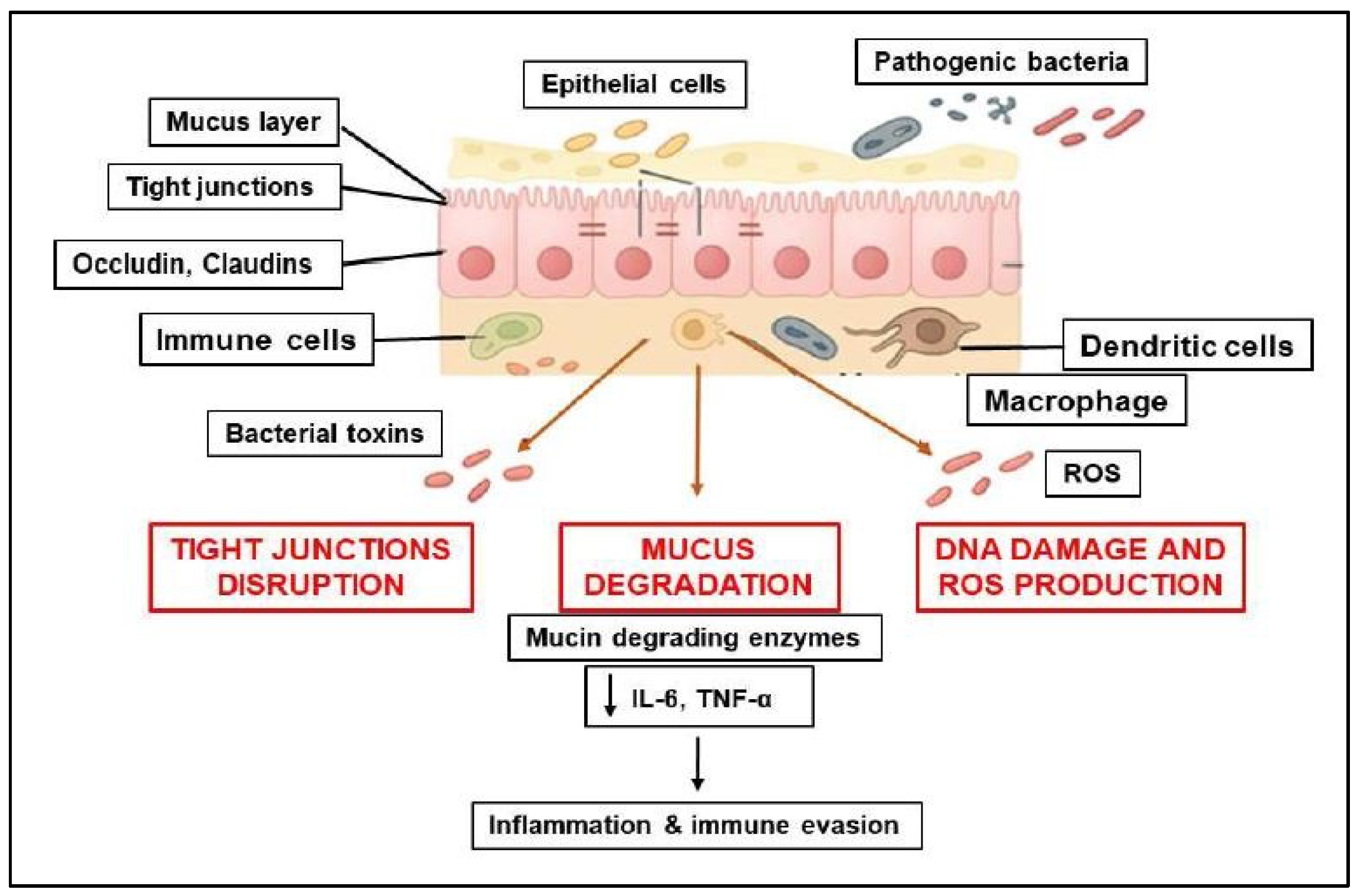
2.2. Mechanisms by Which Pathogenic Bacteria Disrupt the Integrity of the Intestinal Barrier in CRC
The intestinal epithelial barrier is a crucial defense system comprising various types of epithelial cells. This barrier is supported by a thin layer of connective tissue known as the lamina propria, which facilitates communication between the microbiome and immune cells. Additionally, the intestinal epithelial system hosts immune cells, including dendritic cells, T cells, B cells, and macrophages. Together with the epithelial cells, these immune cells contribute to the maintenance of intestinal homeostasis [
57].
Pathogenic bacteria can act as harmful agents that compromise the integrity of the intestinal barrier in CRC through several mechanisms, including: (1) disrupting tight junctions, (2) degrading the mucus layer, (3) inducing inflammation, (4) secreting toxins and causing genotoxic effects. As a result, these actions lead to increased permeability, chronic inflammation, and changes in the intestinal microenvironment that promote tumor development [
58] (
Figure 2).
2.2.1. Disruption of Tight Junctions and Epithelial Integrity
Bacterial pathogens can contribute to tumorigenesis by disrupting tight junctions, increasing intestinal permeability, and triggering inflammation. BFT produced by
Bacteroides fragilis cleaves E-cadherin, disrupts tight junction proteins (such as ZO-1, occludin, and claudins), and increases epithelial permeability, facilitating tumor development [
59,
60].
Fusobacterium nucleatum can activate inflammatory pathways, including NF-κB and β-catenin, which lead to the downregulation of tight junction proteins and a weakening of barrier integrity [
61]. Furthermore,
F. nucleatum enhances bacterial invasion and colorectal cancer progression through the adhesin FadA, which binds to E-cadherin and disrupts tight junctions. Additionally,
Escherichia coli (pks+ strains) secrete colibactin, which can modify epithelial adhesion molecules and impact both DNA integrity and epithelial barriers [
62].
2.2.2. Degradation of the Mucus Layer
The mucus in the intestinal epithelium contains a highly glycosylated polymeric protein called mucin, which reduces the exposure of epithelial cells to bacteria and viruses from the human microbiome. Recent studies have provided compelling evidence for the significant role of the intestinal microbiota at both the metabolic and immunological levels [
63].
Akkermansia muciniphila contributes under dysbiotic conditions to the degradation of mucins, thinning the protective mucus layer and thus allowing pathogens to reach the epithelial surface. Some species of
Bacteroides spp. can act to degrade mucins, exposing epithelial cells to bacterial toxins and inflammatory signals.
S. gallolyticus also produces virulence factors that promote bacterial adhesion and disrupt epithelial integrity [
64].
2.2.3. Effects of the Chronic Inflammation Process Induced by Bacterial Pathogens on Epithelial Integrity
The intestinal barrier, composed of the intestinal mucosa, epithelial cells, and a protective mucus layer, plays a critical role in regulating the passage of substances between the intestine and the bloodstream while preventing the entry of pathogens and endotoxins. The degradation of this barrier by pathogenic bacteria can significantly influence the immune response in CRC. Both systemic and local immune activation, driven by various molecular and cellular mechanisms, are associated with the disruption of the intestinal barrier [
65]. In CRC, it has been demonstrated that specific pathogens, including
Fn,
B. fragilis, and
E. coli, facilitate the translocation of bacteria across the mucosa, thereby initiating a local inflammatory response. This process compromises the integrity of the intestinal barrier and is linked to intestinal dysbiosis, which may contribute to tumor progression [
66].
When pathogenic bacteria, such as
CD,
E. coli, and
B. fragilis, compromise the intestinal barrier, they can create epithelial lesions that enable the penetration of endotoxins and bacteria. This process can initiate a systemic inflammatory response, highlighting the importance of maintaining intestinal health. Key to this response are pattern recognition receptors (PRRs), including TLR4 (which recognizes LPS), NOD1/NOD2 (which detect peptidoglycan), STING, and cGAS pathway (which respond to bacterial DNA). The activation of these receptors triggers significant intracellular pathways, such as NF-κB and IRF3, ultimately leading to the production of inflammatory cytokines. For example, the bacterium
Fn activates the NF-κB pathway through TLR4, resulting in the increased secretion of IL-6 and TNF-α, which can negatively impact epithelial integrity. Moreover, bacterial components like LPS, flagellin, and BFT engage TLR4/NF-κB signaling, further contributing to the release of pro-inflammatory cytokines (including IL-6, TNF-α, and IL-1β) that can disrupt the intestinal barrier [
67,
68]. In response to these challenges, macrophages and dendritic cells act as APCs. The cells capture bacteria or their components, undergo a process of activation, and subsequently migrate to the lymph nodes, where they present antigens to T lymphocytes. This process triggers the activation of CD4
+ (helper) and CD8
+ (cytotoxic) T lymphocytes, facilitating a robust adaptive immune response. By comprehending these mechanisms, we can develop strategies that promote intestinal health and enhance immune function [
69,
70,
71] (
Figure 1).
Also, dysbiosis accompanied by inflammation generates a polarization of T cells, translated by favoring Th17 cells involved in tumor angiogenesis and Th1 cells responsible for the secretion of pro-inflammatory cytokines associated with the reduction in the Treg population. This immune imbalance process favors the establishment of chronic inflammation and the suppression of immunological tolerance [
72,
73]. For example, ETBF and
Fn activate the IL-17/Th17 pathway, which is responsible for the increase in IL-17 secretion. This cytokine is responsible for promoting chronic inflammation and intestinal barrier damage, thus creating a microenvironment favorable to tumor development [
74].
E. coli pks+, capable of secreting colibactin, intervenes in the activation of the Wnt/β-catenin pathway and TGF-β that affects EMT and induces intestinal barrier dysfunction, thus promoting CRC progression [
75,
76]. At the same time, systemic inflammation contributes to the repeated stimulation of the immune system, which can result in T cell exhaustion and reduced effective anti-tumor response, favoring tumor progression [
77].
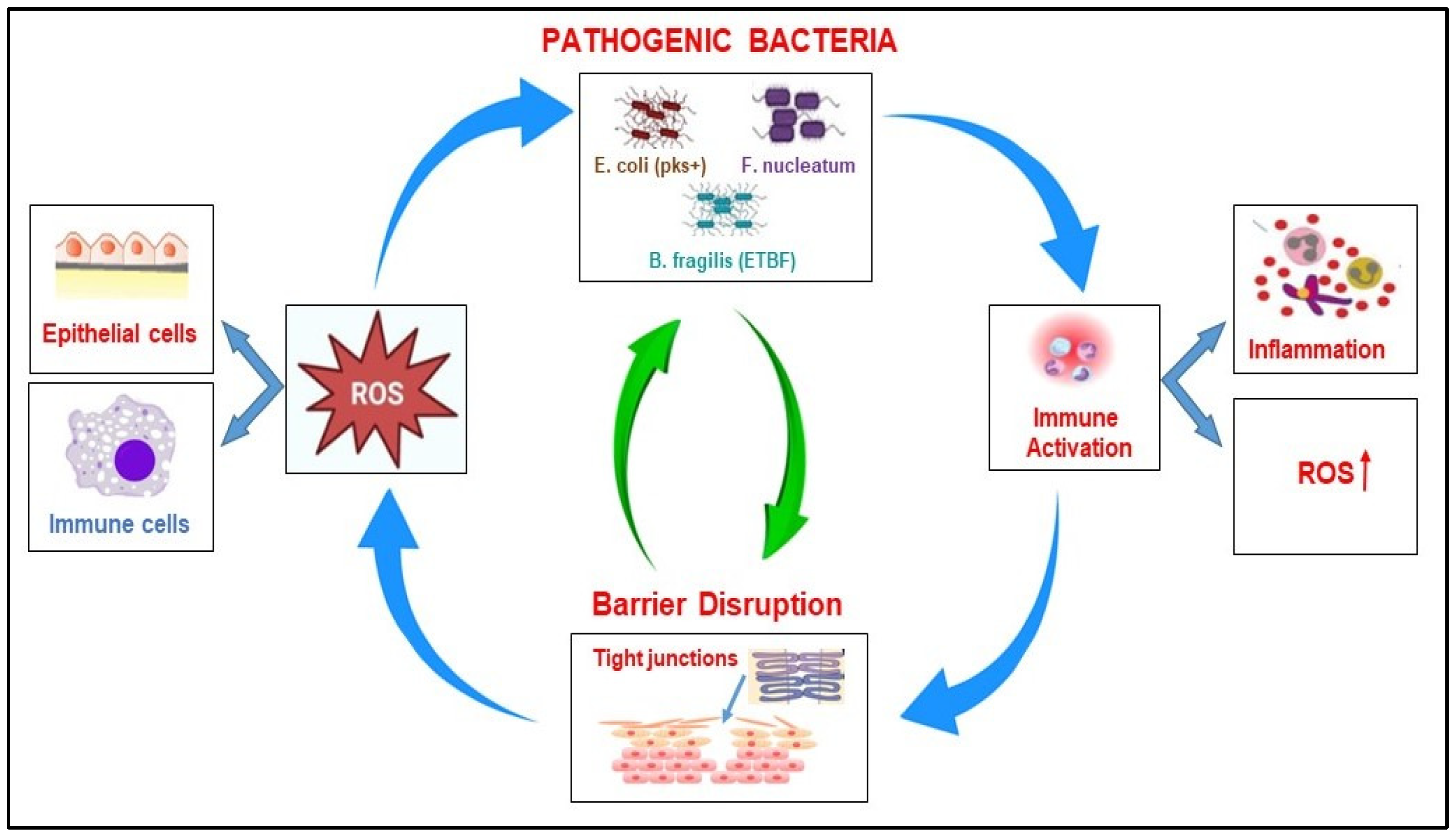
2.2.4. The Correlation Between Microbiome, ROS Production, and Barrier Disruption in CRC
When the intestinal flora’s homeostasis is disrupted, many harmful bacteria can induce the oxidative stress-related pathway in the host cell, leading to complex interactions, such as the abnormal differentiation and apoptosis of intestinal cells, the disruption of the epithelial barrier, and inflammatory response (
Table 2). Pathogenic bacteria such as
Bacteroides fragilis ETBF,
E. coli (pks+), and
Fn invade the intestine and release toxins (e.g., BFT, colibactin), triggering inflammation. This process causes increased ROS production by host epithelial cells (via the activation of NOX enzymes) or by activated immune cells such as macrophages and neutrophils. Increased ROS production causes oxidative damage to DNA, proteins, and lipids and promotes genomic instability. In addition, high levels of ROS induce epithelial stress and apoptosis, contributing to the weakening of the intestinal barrier and the amplification of the process [
78]. The disruption of the intestinal barrier is accompanied by the loss of tight junction proteins (e.g., ZO-1, E-cadherin), a thinning of the mucus layer, and epithelial cell death. As a consequence of these processes, microbial translocation into the submucosa occurs, and immune cells are exposed to bacterial antigens, which results in an amplification of the immune response followed by the increased secretion of TNF-α and IL-6, but also an increase in ROS production, which will generate more tissue damage. Consequently, a weakened barrier allows more pathogenic bacteria to infiltrate and colonize; thus, the cycle repeats and intensifies. These processes facilitate the accumulation of DNA mutations, result in immune suppression, and contribute to the progression of cancer [
79].
The detrimental cycle involving pathogenic bacteria, the production of ROS, and the disruption of the intestinal barrier in CRC represents the central loop to tumor initiation, chronic inflammation, and progression (
Figure 3).
Clearly, these mechanisms collectively weaken the intestinal barrier, promote chronic inflammation, and create a microenvironment favorable for tumor development. Understanding and combating these mechanisms is a crucial task for our audience in the fields of microbiology, oncology, and gastroenterology.
3. Beneficial Bacteria and Their Role in Activating Anti-Tumor Immune Defenses in CRC
While pathogenic microbes can sustain colorectal tumorigenesis, a distinct cohort of commensal and probiotic bacteria counterbalances this threat by strengthening host immunity. These beneficial species, such as
Faecalibacterium prausnitzii,
Bifidobacterium spp.,
Lactobacillus spp., and
Akkermansia muciniphila, can modulate immune responses through various mechanisms that activate anti-inflammatory pathways, increase the efficiency of anti-tumor immunity, and support the immune system’s balance. These actions help maintain the integrity of the intestinal barrier and modulate innate and adaptive immunity, ensuring the counteraction of the effects of pathogenic bacteria on tumor promotion [
80,
81]. Therefore, knowledge of these mechanisms may lead to the development of therapies that directly address the microbiome through new promising approaches to improve CRC treatment outcomes. The most important beneficial bacterial species that play a role in modulating the immune response in CRC are presented in
Table 3.
3.1. Key Strategies Used by Beneficial Bacteria to Enhance the Anti-Tumor Immune Response in CRC
Beneficial gut microbes counteract colorectal carcinogenesis through several converging immunological tactics: (1) the modulation of the immune response by SCFAs; (2) the induction of anti-tumor immunity and the regulation of innate immunity; (3) the modulation of dendritic cell function; (4) strengthening the intestinal barrier (
Figure 4).
3.1.1. Modulation of the Immune Response by SCFAs
Beneficial bacteria, such as
Bifidobacterium and
Lactobacillus, produce SCFAs like butyrate, propionate, and acetate through the fermentation of dietary fiber. These SCFAs and other bacterial metabolites suppress NF-κB activation and reduce inflammation by suppressing pro-inflammatory cytokines, such as IL-6 and IL-1β, while simultaneously promoting the production of anti-inflammatory cytokines such as IL-10 [
96]. Moreover, SCFAs have a significant impact on the differentiation, recruitment, and activation of immune cells, including neutrophils, DCs, macrophages, and T lymphocytes [
97]. Furthermore, SCFAs facilitate the differentiation of regulatory Tregs, which are essential for the modulation of chronic inflammation. They also play a crucial role in the differentiation of regulatory Tregs, which are important for controlling chronic inflammation. Additionally, SCFAs are involved in epigenetic regulation by inhibiting histone deacetylases (HDACs) [
98], which enhances the expression of anticancer genes and helps to slow the progression of CRC [
99,
100]. Beneficial bacteria play a vital role in repairing and regenerating damaged epithelial cells. SCFAs enhance the expression of antimicrobial peptides on the external surface of epithelial cells and modulate their production of immune mediators, including IL-18, which is critical for the maintenance and restoration of epithelial integrity [
101]. Notably, butyrate, a type of SCFA, activates the Wnt signaling pathways that promote the proliferation and differentiation of epithelial cells. This process is essential for the continuous renewal of the epithelial layer, which helps maintain barrier integrity in CRC [
102].
3.1.2. Induction of Anti-Tumor Immunity and Regulation of Innate Immunity
Some bacteria directly activate anti-tumor immune responses. Beneficial bacteria can promote immune tolerance by stimulating regulatory Tregs or encouraging a balanced immune response between Th1 and Th2 cells, which is important for anti-tumor effects [
103]. Furthermore, CTLs can directly activate anti-tumor immune responses. This activation can be enhanced by improving antigen presentation, such as with Bifidobacterium, or by increasing the cytotoxic activity of NK cells, both of which help destroy tumor cells [
104]. Additionally, these beneficial bacteria can influence the regulation of innate immunity, helping to maintain immune homeostasis. They do this by encouraging macrophage polarization towards the anti-inflammatory M2 phenotype and by modulating neutrophil activity to prevent excessive tissue damage and inflammation [
105,
106].
3.1.3. Modulation of Dendritic Cell Function
Immune system cells receive signals from various bacterial substances, which integrate and initiate the activation of appropriate immune response mechanisms. DCs exhibit a broad spectrum of receptors that facilitate the recognition of various bacterial constituents. For instance, specific free fatty acid receptors (FFARs) are capable of detecting bacterial metabolites classified as SCFAs. The activation of these receptors serves as an indication of the bacteria’s viability and demonstrates how their metabolites interact with the immune system to promote anti-inflammatory functions [
107]. Furthermore, the diverse array of receptors expressed by DCs enables them to activate multiple signaling cascades concurrently. This ability to integrate several signals simultaneously ensures the generation of an appropriate response to both antigenic and tissue-related stimuli [
108]. The modulation of dendritic cell function by beneficial bacteria influences DCs to act as antigen-presenting cells for T cells. Consequently, beneficial bacteria promote the development of tolerogenic DCs, which enhance the induction of Treg cells or stimulate DCs to produce anti-inflammatory cytokines and prevent the over-activation of the immune system [
109]. Furthermore, these bacteria can stimulate dendritic cells to produce anti-inflammatory cytokines, such as IL-10, thereby contributing to the balance of immune responses and preventing the excessive activation of the immune system, particularly in the context of CRC [
110].
3.1.4. Strengthening the Intestinal Barrier
The intestinal barrier is a crucial barrier for an organism against invasion by foreign pathogens, including mechanical, chemical, immune, and microbial barriers. Beneficial bacteria play a crucial role in strengthening the intestinal barrier in CRC by maintaining intestinal homeostasis and reducing inflammation (
Figure 4).
Lactobacillus,
Bifidobacterium, and
Akkermansia muciniphila act beneficially and promote mucin secretion by goblet cells, forming a protective barrier that prevents the entry of pathogens and minimizes the activation of the immune system, thus promoting overall gut health [
111].
Several beneficial bacteria (
Lactobacillus rhamnosus GG,
Lactobacillus plantarum, Lactobacillus reuteri, Bifidobacterium breve, Bifidobacterium longum,
Bifidobacterium bifidum,
Akkermansia muciniphila, and
Faecalibacterium prausnitzii) play a significant role in improving the integrity of the intestinal epithelial barrier by upregulating the expression of tight junction proteins (occludin, claudins, and zonula occludens) and preventing CRC progression (
Figure 4).
Beneficial bacteria are instrumental in safeguarding the epithelial barrier by scavenging ROS and mitigating oxidative stress. For example, species within the Lactobacillus genus are known to synthesize antioxidants and detoxify harmful metabolites, thereby preventing oxidative damage to epithelial cells [
112]. Furthermore, specific bacterial metabolites can diminish epithelial inflammation and reinforce the integrity of the barrier [
113]. Indole derivatives resulting from tryptophan metabolism are known to activate the aryl hydrocarbon receptor (AhR) in epithelial cells, facilitating both barrier integrity and immune regulation. Moreover, certain intestinal bacteria possess the capacity to degrade toxic compounds, such as bile acids and polyamines, which are implicated in the progression of CRC [
114]. Additionally, beneficial bacteria occupy competing niches, effectively outcompeting harmful bacteria for the occupation of adhesion niches on the mucosa by secreting bacteriocins and organic acids that directly inhibit pathobionts, maintaining a local pH unfavorable to pathogenic species, and preferentially consuming essential nutrients, thus preventing pathogenic bacterial colonization that could compromise the intestinal barrier (
Figure 4) [
115].
Collectively, these mechanisms underscore the significance of beneficial bacteria in fortifying tight junctions, reducing intestinal permeability, and providing protection against the progression of CRC. These mechanisms emphasize the crucial role of beneficial bacteria in enhancing the integrity of tight junctions, which are vital barriers within the intestinal lining. Furthermore, their protective effects play a significant role in reducing the risk of developing CRC, offering an important defense in the battle against this disease [
116].
4. Role of the Microbiome in Current and Future Therapeutic Strategies for Colorectal Cancer
The composition of the colorectal microbiota plays a significant role in modulating local inflammation, epithelial permeability, and the efficacy of various treatments, including chemotherapy and immunotherapy. This observation supports the notion that true personalization in colorectal cancer (CRC) management necessitates the consideration of a triad comprising: (1) tumor genotype, (2) immune signature, which includes microsatellite instability (MSI), tumor mutational burden (TMB), and CD8+ T cell infiltration, (3) the microbial ecosystem. Each component contributes distinct targets and biomarkers, facilitating the development of genuinely adaptive multimodal therapies. Furthermore, the microbiome is emerging as a critical factor in both the pathogenesis of CRC and the responsiveness to treatment. Consequently, contemporary and future therapeutic strategies increasingly incorporate the microbiome as a target or modulator of treatment efficacy and toxicity.
4.1. Microbiome Impact on Current Therapies in CRC
Currently, CRC treatment has become increasingly personalized, combining curative surgery with molecularly guided systemic regimens and, more recently, with immunotherapy. Numerous studies demonstrate that the microbiome has a significant impact on the efficacy, toxicity, and outcomes of current therapies in CRC [
117], intervening in a complex way in drug metabolism, immune response, and the development of tumor resistance.
4.1.1. Microbiome Modulation of Chemotherapy Efficacy and Toxicity
Chemotherapy remains the standard first-line treatment in CRC, with an essential role in controlling the disease, and preparing the patient for combination treatments or surgery, where possible. For localized stages (local disease), oncological resection remains the foundation, complemented by chemotherapy (FOLFOX/CAPOX) and, for rectal tumors, preoperative radio- or chemoradio-therapy (“short course” (5 × 5 Gy) or long chemoradiotherapy (50 Gy + capecitabine), to reduce tumor volume and preserve sphincter function) [
118]. At the advanced or metastatic stages, chemotherapy is often combined with other types of therapies, depending on the stage of the disease and the molecular profile of the tumor.
Microbiome bacteria can modulate the activation or deactivation of chemotherapy drugs before their delivery to tumors, thereby influencing their efficacy and toxicity. Certain microbial species produce enzymes, such as β-glucuronidases, which can convert inactive drug metabolites back into their active and toxic forms, resulting in adverse side effects. A pertinent example is Irinotecan (CPT-11), which is metabolized in the liver to an inactive form (SN-38G) and subsequently reactivated by bacterial β-glucuronidase, leading to inflammatory responses [
119]. Furthermore, the microbiome exerts a considerable influence on immune cell recruitment and the inflammatory pathways that are critical to chemotherapy-induced tumor cell death. Empirical studies have demonstrated that the composition of the microbiota can impact immune responses to treatments such as 5-Fluorouracil (5-FU), thereby modulating both the effectiveness and side effects of the treatment oxaliplatin, another chemotherapeutic agent, which induces tumor cell death through the generation of ROS and enhances the anti-tumor immune response by activating mechanisms of innate immunity [
120]. Additionally, specific bacteria, including
Alistipes and
Lactobacillus, have been identified as enhancers of the immune-mediated anti-tumor effects associated with oxaliplatin. Conversely, other bacteria may promote chemoresistance through mechanisms such as inflammation, the disruption of the mucosal barrier, or immune system suppression. For instance,
Fn has been implicated in promoting chemoresistance via pathways that inhibit apoptosis and facilitate autophagy [
121].
4.1.2. Microbiome Impact on Immunotherapy
In metastatic disease, treatment is based on the genetic profile of the tumor (RAS, BRAF, HER2, or MSI/dMMR) and the extent of the disease [
122]. Programmed cell death 1 (PD-1) immunotherapy (pembrolizumab, nivolumab) has become the standard in patients with microsatellite instability-high (MSI-H) or mismatch repair deficiency (dMMR) tumors, conferring durable responses, and current research is exploring bimodal synergies (ICI and anti VEGF/EGFR, radiotherapy, or microbiota manipulation) for refractory microsatellite stable (MSS) tumors. This multimodal and biomarker-targeted approach transforms CRC from a uniformly treated disease to one managed by sequential strategies tailored to each patient. Anti-PD-1 immunotherapy has decisively changed the treatment paradigm for the subgroup of CRC with MSI-H or dMMR [
123]. MSI H/dMMR tumors accumulate thousands of “frameshift” mutations that generate abnormal peptides, neoantigens, which are processed by the proteasome, loaded on major histocompatibility complex-1 (MHC I) molecules, and presented on the surface of tumor cells. Due to their uniqueness, they are recognized as non-self by naive T lymphocytes in the mesenteric nodes, inducing intense proliferation and differentiation into effector cells in CTLs. The large number and diversity of these neoantigens leads to the polyclonal clonal expansion of distinct T cells, each with a unique specificity, from which some dominant clones are selected and multiplied that exert a strong immune pressure on the tumor, secreting IFNγ and TNFα and releasing granzymes/perforin that induce the apoptosis of malignant cells [
124]. In the absence of PD-1 blockade, the response is rapidly exhausted by tumor evasion mechanisms, and the therapeutic inhibition of PD-1 re-energizes these expanded clones and prolongs their activity. PD-1 inhibition restructures the tumor microenvironment: re-energized CTLs secrete IFN-γ that polarizes macrophages towards an M1 phenotype and stimulates stromal cells to secrete the chemokines CXCL9/10, which additionally attract NK cells to the tumor [
125]. Concomitantly, the density of suppressor cells, such as Treg and myeloid-derived suppressor cells (MDSCs), decreases [
126], either through apoptosis induced by the pro-inflammatory environment or through unfavorable competitive chemotaxis, and MHC-I/II expression on tumor cells increases, enhancing antigen presentation. The synergy between high neoantigen load, clonal expansion, and PD-1 re-energization supports a robust polyclonal response, explaining the durable remissions observed in MSI-H/dMMR patients. For patients with MSI-H/dMMR tumors, the introduction of PD-1 inhibitors has transformed the prognosis from less-than-one-year survival to five-year survival rates exceeding 55% [
127], validating the concept of “durable benefit” and establishing immunotherapy as the backbone of treatment in this molecular subset of CRC.
The efficacy of immunotherapy, particularly in relation to immune checkpoint inhibitors (ICIs), can be significantly impacted by the composition of the gut microbiota. Immunotherapy, particularly ICIs, shows great promise in treating CRC that is characterized by MSI-H or dMMR [
128]. Conversely, it exhibits limited effectiveness in microsatellite stable (MSS) CRC, unless influenced by the microbiome. Certain beneficial microbes can potentially enhance the therapeutic response to ICIs by improving antigen presentation and promoting T cell activation. For instance,
Akkermansia muciniphila has been shown to stimulate DCs, thereby augmenting the effectiveness of PD-1 blockade. Additionally,
Bacteroides fragilis (non-toxigenic) can induce Th1 immunity, which increases the efficacy of anti-cytotoxic T lymphocyte antigen 4 (anti-CTLA-4) treatment. Furthermore,
Faecalibacterium prausnitzii plays a crucial role in maintaining mucosal integrity and is also noteworthy for highlighting the potential of microbiome modulation in cancer therapies [
81]. Moreover, while the use of antibiotics is often necessary, it is important to be aware that their administration before or during ICI therapy can lead to adverse effects on response rates due to gut dysbiosis [
129]. Addressing this issue and understanding the implications on the gut microbiota, such as the impact of
Fn related to immune evasion and therapy resistance, will be crucial in optimizing treatment strategies and improving patient outcomes [
130]. By focusing on these microbial interactions, we can potentially enhance the effectiveness of immunotherapy in colorectal cancer patients.
4.1.3. Microbiome Impact on Targeted Therapy
Targeted therapies constitute an essential aspect of CRC management, particularly in cases of metastasis. This therapy involves the use of molecules that block angiogenesis and are known as anti-VEGF (Vascular Endothelial Growth Factor) inhibitors, such as Bevacizumab (Avastin), Ziv-aflibercept (Zaltrap), and Ramucirumab (Cyramza). Furthermore, in the context of RAS wild-type and BRAF wild-type tumors, anti-EGFR (Epidermal Growth Factor Receptor) inhibitors are employed to obstruct signaling pathways that promote cell growth. These agents are marketed under various trade names, including Cetuximab (Erbitux) and Panitumumab (Vectibix). The microbiota may interact with the tumor microenvironment, influencing the responses to anti-EGFR or anti-angiogenic therapies; thus, in metastatic disease, chemotherapy is supplemented with targeted therapies against EGFR or VEGF, and newly approved combinations—encorafenib + cetuximab for BRAFV600E, adagrasib + cetuximab for KRASG12C, and trastuzumab deruxtecan for HER2 positive—expanding the options in advanced lines [
131,
132,
133].
Emerging research on the therapeutic interplay between the gut microbiota and targeted treatments reveals a fascinating landscape where specific gut bacteria, like
Akkermansia muciniphila and
Bacteroides fragilis, play pivotal roles. These microorganisms possess the remarkable ability to invigorate immune responses and mitigate inflammation, which may significantly bolster the effectiveness of anti-EGFR therapies. Furthermore,
Bifidobacterium emerges as a powerful ally, capable of enhancing the therapeutic impact of anti-VEGF treatments [
134].
These studies emphasize the increasing interest in the integration of microbiome modulation into treatment strategies for CRC by synergistically blending traditional therapies with innovative interventions designed to influence the gut microbiota. The aspiration is to significantly elevate treatment effectiveness, surmount resistance barriers, and alleviate the burden of adverse effects, ultimately paving the way for more holistic and patient-centered care.
4.2. Therapeutic Modulation of the Microbiome in CRC
Combinatory therapies for CRC that integrate microbiome components are part of a rapidly evolving field aimed at enhancing therapeutic efficacy, overcoming resistance, and reducing toxicity. These strategies involve combining conventional treatments (chemotherapy, immunotherapy, and targeted therapy) with microbiome-modulating interventions, such as probiotics, prebiotics, antibiotics, or fecal microbiota transplantation.
4.2.1. Probiotics, Prebiotics, Synbiotics, and Postbiotics in Therapeutic Approaches in CRC
The introduction of microecological agents such as probiotics, prebiotics, synbiotics, and postbiotics, in CRC management hold the potential to modulate gut microbiota. These agents contribute to both the prevention and treatment of CRC by restoring microbial balance, enhancing immune responses, reducing inflammation, and suppressing tumor-promoting pathways.
Probiotics, living microorganisms which are specific non-pathogenic strains of bacteria or yeast, are beneficial to human health when consumed in adequate amounts, helping to support a balanced and healthy gut microbiota. They belong to the genus
Lactobacillus and
Bifidobacterium but also include
Bacillus,
Pediococcus, or yeasts and are found in fermented food and drinks (yogurt, kefir) or as dietary supplements [
135]. Certain probiotic strains from the genera
Lactobacillus and
Bifidobacterium (especially
Lactobacillus rhamnosus GG,
Lactobacillus casei, and
Bifidobacterium longum) have demonstrated the ability to reduce the activity of pro-carcinogenic bacterial enzymes such as β-glucuronidase, azoreductase, and nitro-reductase, which are involved in the conversion of food or bile pro-carcinogens into active metabolites. Moreover, these probiotics can sequester mutagens (e.g., heterocyclic amines from processed meat), reducing their bioavailability.
Prebiotics, indigestible fibers or substances found in specific foods that nourish the beneficial bacteria in the gut, support the growth and activity of good bacteria, helping to maintain a healthy digestive system. Inulin [
136], fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS) [
137], and pectin [
138], are among the most investigated prebiotics, with the aim of evaluating their potential role in CRC prevention and treatment [
139].
A recent study underlines that, in contrast to the conventional methods of vaccination that stimulate systemic immune response, the administration of probiotics as oral vaccines holds several advantages, such as the ability to target both systemic and mucosal immunity, convenient storage and transport without the need for refrigeration or cold chain systems, and inherent acid and bile tolerance, which aids survival in the gut environment. Unlike attenuated vaccines, probiotics eliminate the risk of reversion to virulence. Advanced strategies that employ CRISPR-Cas editing, the insertion of heterologous genes into bacterial chromosomes, and Phage-Mu transposition improve the genetic stability of probiotics. Moreover, the association of probiotics with prebiotics, encapsulation, and enhancing acid tolerance leads to improved host colonization, thereby optimizing probiotic vaccine performance [
140].
Synbiotics combine probiotics and prebiotics to promote a healthy gut microbiome. The synergy of probiotics with prebiotic fibers enhances the production of SCFA and contributes to the restoration of the intestinal barrier integrity (by increasing the expression of zonulin and occludins), reducing bacterial translocation and systemic inflammation, which are critical factors in the progression of CRC. Thus, their administration can modulate the intestinal metabolic profile in a protective sense [
141]. In colorectal oncology,
postbiotics, structural or metabolic products of viable bacteria that exert host benefits even in the absence of the living microorganism, offer the advantage of controllable bioavailability, eliminating the risk of bacterial translocation in immunocompromised patients. Compared with probiotics, postbiotics have been shown to have a safer and more stable chemical structure. Postbiotics include SCFAs (butyrate, acetate, and propionate), enzymes, cell wall fragments (peptidoglycan), bacterial metabolites, exopolysaccharides, bacteriocins, vitamins, and amino acids [
142]. A recent article explores the potential of bacteriocins—specifically Lactacin B, Lactacin F, and Doderlin—produced by lactic acid bacteria (LAB) as safe and effective therapeutic agents for colorectal cancer (CRC). The study utilized in silico analysis to identify strong binding affinities between these compounds and key CRC-associated genes, such as CTNNB1 and LRP5. This indicates their potential to modulate the Wnt signaling pathway and to provide a less toxic therapeutic option compared with current CRC treatments [
143].
Used both as prevention and adjuvant therapy in CRC treatment, postbiotics exert their anticancer role through multiple mechanisms including the modulation of the intestinal microbiota, the enhancement of the intestinal mucosal barrier, the regulation of the immune response, and their influence on systemic metabolism [
144,
145].
The incorporation of beneficial microbes or compounds into supplementation strategies serves to promote their growth. This approach not only aids in reducing the inflammation associated with colorectal cancer (CRC) but also enhances the mucosal barrier function and improves tolerance to chemotherapy.
4.2.2. Fecal Microbiota Transplantation (FMT)
FMT is an innovative medical procedure in which fecal matter from a healthy donor is transferred into the intestine of a person with dysbiosis to restore the balance of the intestinal microbiota [
146]. FMT contains the bacteria and viruses naturally present in healthy stools. FMT is currently widely accepted and recommended as an effective therapeutic strategy for the management of recurrent
Clostridioides difficile infection [
147], and its use in experimental studies for other intestinal diseases such as inflammatory bowel disease (IBD), constipation, short bowel syndrome (SBS), and irritable bowel syndrome (IBS) showed promising results. In obesity, type 2 diabetes, and metabolic syndrome, FMT is being investigated for its potential to modulate the metabolism and systemic inflammation by intervening in the composition of the intestinal microbiota [
148,
149]. A growing number of experimental studies are currently exploring the potential of FMT to inhibit the progression of CRC. Using a chemically induced murine model of CRC with intestinal microbial dysbiosis, a recent study [
150] investigated the effects of introducing healthy gut bacteria through FMT. This strategy restored the composition and diversity of the gut microbiota in CRC mice, facilitated the recruitment of intestinal immune cells, improved host immune activity targeting CRC, modulated cytokine expression within the CRC microenvironment, and thereby inhibited tumor growth and prolonged survival.
Immunotherapy can enhance the immune system’s ability to recognize and attack cancer cells, while also potentially disrupting tumor growth and metastasis. Combining FMT with pembrolizumab or nivolumab may offer a synergistic effect, improving disease control. A phase II clinical trial, conducted by the MD Anderson Cancer Centre (NCT04729322), is currently exploring the efficacy of FMT in combination with the reintroduction of anti-PD-1 therapy (pembrolizumab or nivolumab) in patients with advanced metastatic CRC who have failed anti-PD-1 therapy [
151].
The interaction between the microbiota and radiotherapy is a complex process. Bidirectional anticancer treatments can alter the microbiome, leading to dysbiosis, and these microbial changes, in turn, can affect both the efficacy of treatments and the severity of treatment-related gastrointestinal toxicities. In this context, FMT may be associated with radiotherapy and could restore the diversity of the intestinal microbiota, consequently improving the response to radiation and reducing side effects [
152]. These data support the beneficial effect of transferring a healthy microbiome to a patient, enhancing the efficacy of immunotherapy and reducing dysbiosis induced by chemotherapy, radiotherapy, or antibiotics.
4.2.3. Therapeutic Bacteriophages
Bacteriophages, commonly referred to as phages, are specific viruses that infect bacteria and play a vital role in regulating bacterial populations. These phages can be modified to deliver therapeutic agents. Preliminary research and preclinical studies have explored the use of lytic phages targeting
Fusobacterium nucleatum, a bacterium linked to enhanced tumor cell proliferation and the promotion of chemoresistance by shielding tumors from immune cell attacks. Furthermore, the increased abundance and prevalence of
Fusobacterium nucleatum correlate with more advanced tumor stages and a poorer prognosis in patients diagnosed with CRC [
153,
154]. These phage therapies have shown potential in reducing tumor growth in CRC xenograft mouse models achieved through a decrease in bacterial load, a reduction in inflammation, and an increase in T cell infiltration [
155].
Innovations derived from the CRISPR-Cas (clustered regularly interspaced short palindromic repeats) immune system of prokaryotes have facilitated the application of CRISPR-Cas tools for human gene editing. These tools may also be employed to target the pathogenic and commensal bacteria that colonize the human body, thereby providing novel avenues for treating infections and modulating the microbiome [
156].
Phages can be engineered to selectively delete virulence genes or to eliminate antibiotic-resistant bacteria associated with CRC by utilizing CRISPR-Cas3 or CRISPR-Cas9 technologies. CRISPR-Cas tools possess the capability to eliminate or modify specific bacteria through DNA and RNA targeting strategies, effectively disarming them by deleting, altering, or silencing particular genes [
157,
158]. Additionally, the delivery of CRISPR-Cas tools via bacteriophages or conjugation highlights promising therapeutic opportunities for treating infectious diseases and modifying the microbiome, underscoring both the advancements achieved and the challenges that remain in translating these methodologies into clinical practice.
One notable advantage of phage therapy is its high specificity, which ensures the targeted elimination of pathogens while preserving the diversity of the gut microbiome, thus offering a potential strategy to address antibiotic resistance. Therapeutic bacteriophage approaches in CRC are an emerging and promising area of research that targets pro-tumor bacteria in the gut, modulates the tumor microenvironment, and potentially enhances the efficacy of existing cancer therapies [
159,
160].
4.3. Perspectives in CRC Therapy
The perspectives in the treatment of CRC encompass the development of novel pharmaceuticals, advancements in immunotherapy, targeting of the microbiome, and the implementation of precision medicine. A primary focus of research is the translation of these innovations into clinical applications to enhance patient outcomes through personalized medicine, early detection, and combination therapies. Research efforts are focused on the comprehensive genomic profiling of CRC, examining mutations in key genes such as KRAS, NRAS, BRAF, MSI, HER2, and PIK3CA to identify new actionable mutations and signaling pathways. A range of targeted therapies is currently being evaluated in clinical trials for specific patient populations, including BRAF mutant CRC therapies utilizing combinations such as encorafenib and cetuximab, HER2-targeted therapies (trastuzumab and pertuzumab) for HER2-positive CRC, and KRAS G12C inhibitors, such as sotorasib [
161,
162]. Additionally, investigations into the roles of immune cell exhaustion, the tumor microenvironment, and the microbiota have facilitated the clinical translation of various combination therapies. These include ICIs combined with chemotherapy, anti-VEGF agents, and oncolytic viruses [
163]. The development of bispecific antibodies, CAR-T cells, and vaccines that target tumor-associated antigens is also underway. Significant research is focused on understanding the impact of microbial influences on drug metabolism, immune modulation, and the development of resistance. This knowledge has led to the clinical application of FMT to improve responses to immunotherapy, as well as the exploration of phage therapy in clinical trials. Furthermore, it is essential to monitor the synergistic effects of combining existing pharmaceuticals with immunotherapy or targeted agents [
164]. Ongoing clinical trials are investigating combinations such as ICIs with MEK inhibitors, chemotherapy, or checkpoint inhibitors in the presence of microbiota modulators [
165]. Finally, the analysis of circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) through liquid biopsies is instrumental in enabling the early detection of minimal residual disease (MRD) and in providing critical guidance for therapeutic interventions.
5. Challenge and Future Directions
Colorectal cancer represents a major public health problem, and the intestinal microbiota influences both tumor initiation and progression, as well as therapeutic response, transforming itself from a possible risk factor into a true therapeutic resource. The links between the microbiota and colorectal cancer form an extremely complex bidirectional network: bacteria, bacteriophages, and bacterial metabolites interact with the tumor genome, the epithelial barrier, and host immunity.
Multi-cohort studies have shown that a group of pathobionts and the decrease in butyrate-producing bacteria can discriminate patients with adenomas/CRC from healthy controls. Thus, colorectal microbiota profiling has diagnostic potential for establishing a microbial signature for early detection, but has not yet become a “routine” test. Inter-individual variability (diet, antibiotics, or comorbidities), lack of standards for collection/storage, and sequencing costs prevent, for now, large-scale clinical validation. Circulating microbial DNA (cmDNA) from liquid biopsy contains fragments from tumor-associated bacteria; their profiling, correlated with ctDNA mutations, may suggest the colorectal tumor origin from the early stages. Oncologists can use fecal or plasma signatures for risk stratification and for the selection of patients eligible for immunotherapy/synbiotics, making it necessary to integrate microbial biomarkers into oncological decision making. Fecal microbiota profiling is a promising biomarker for screening and risk stratification, but for now, remains complementary to established tests.
The adjuvant role of probiotics/synbiotics/postbiotics in CRC, especially perioperatively and during chemotherapy, through detoxification, barrier strengthening, and immune modulation, is proven. The challenge is the standardization of strains and doses, and the integration of response biomarkers as essential steps towards large-scale clinical implementation. Studies have shown that FMT and bacteriophages can selectively remodel the colonic ecosystem in CRC, but critical barriers remain: FMT risks pathogen transmission and provides variable results between donors, and phage therapy faces pharmacokinetic limitations.
The future depends on: (i) obtaining standardized microbial consortia grown in good manufacturing practice standards, (ii) personalized phage cocktails based on tumor/fecal sequencing, (iii) adaptive clinical trials integrated with immunotherapy. If these obstacles are overcome through regulations and bioengineering, FMT and bacteriophages may become safe and precise adjuvants in the multimodal therapy of colorectal cancer.
Exploring innovative approaches has the potential to facilitate the establishment of comprehensive databases concerning microbiome–drug interactions. By employing machine learning and large language model platforms, it is possible to integrate diverse data types—including genomics, transcriptomics, pathology images, and microbiome data—to determine which drugs a tumor or other diseased tissue may be sensitive to or resistant against. Furthermore, this methodology can assist in predicting prognosis, recurrence risk, and the likelihood of side effects.
6. Conclusions
This review provides a comprehensive overview that supports the essential role of the composition and activity of the gut microbiota in colorectal carcinogenesis. Both pathogenic and beneficial microbes influence host immune pathways, which are critical for tumor suppression or promotion. While some pathogens inhibit antigen presentation and cytotoxic T cell function, beneficial bacteria enhance immune surveillance and support immune cell-mediated anti-tumor activity. These processes are intricate and are mediated by microbial metabolites, surface molecules, and signaling pathways that facilitate communication between the host and microbes.
Moreover, we highlighted the significant potential of microbiota as a therapeutic target for CRC. Strategies such as probiotics, prebiotics, dietary modifications, and FMT are currently being investigated to restore microbial balance and improve the efficacy of standard treatments, including immunotherapy and chemotherapy. Integrating microbiome-targeted interventions with conventional and emerging therapies may lead to more effective, durable, and personalized treatment outcomes for CRC. However, it is essential to emphasize that further studies and well-controlled clinical trials are necessary to fully understand the causal relationships between specific microbes and the progression or regression of CRC.
Author Contributions
Conceptualization, A.I.N., M.B. and C.B.; validation, C.B., C.C.D. (Camelia Cristina Diaconu), and C.C.D. (Carmen Cristina Diaconu); S.I.S. and C.M.H. searched the literature for inclusion in the study; formal analysis, M.M. and V.A.I.; writing—original draft preparation, A.I.N., V.R., C.M.H., C.B. and G.G.; writing—review and editing, C.B., M.B. and A.I.N.; visualization and graphics, C.M.H. and M.M.; supervision, C.B., C.C.D. (Camelia Cristina Diaconu), and S.M.R.; project administration, C.B., C.C.D. (Carmen Cristina Diaconu) and S.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by The Ministry of Investments and European Projects, through the Managing Authority for the Health Program, PS/272/PS_P5/OP1/RSO1.1/PS_P5_RSO1.1_A9-ROGEN Project (MySMIS 324809). Publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, W.; An, Y.; Qin, X.; Wu, X.; Wang, X.; Hou, H.; Song, X.; Liu, T.; Wang, B.; Huang, X.; et al. Gut Microbiota-Derived Metabolites in Colorectal Cancer: The Bad and the Challenges. Front. Oncol. 2021, 11, 739648. [Google Scholar] [CrossRef] [PubMed]
- Nobels, A.; van Marcke, C.; Jordan, B.F.; Van Hul, M.; Cani, P.D. The gut microbiome and cancer: From tumorigenesis to therapy. Nat. Metab. 2025, 7, 895–917. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Strati, F.; Pujolassos, M.; Burrello, C.; Giuffrè, M.R.; Lattanzi, G.; Caprioli, F.; Troisi, J.; Facciotti, F. Antibiotic-Associated Dysbiosis Affects the Ability of the Gut Microbiota to Control Intestinal Inflammation upon Fecal Microbiota Transplantation in Experimental Colitis Models. Microbiome 2021, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Pfeilmeier, S.; Werz, A.; Ote, M.; Bortfeld-Miller, M.; Kirner, P.; Keppler, A.; Hemmerle, L.; Gäbelein, C.G.; Petti, G.C.; Wolf, S.; et al. Leaf microbiome dysbiosis triggered by T2SS-dependent enzyme secretion from opportunistic Xanthomonas pathogens. Nat. Microbiol. 2024, 9, 136–149. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Hu, T.; Huang, H.; Chen, G.; Jin, B.; Zeng, G.; Liu, J. Entero-toxigenic Bacteroides fragilis contributes to intestinal barrier injury and colorectal cancer progression by mediating the BFT/STAT3/ZEB2 pathway. Cell Cycle 2024, 23, 70–82. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Diaconu, C.C.; Gheorghe, G.; Mihai, M.-M.; Diaconu, C.C.; Bostan, M.; Bleotu, C. Gut Microbiota and Colorectal Cancer: A Balance Between Risk and Protection. Int. J. Mol. Sci. 2025, 26, 3733. [Google Scholar] [CrossRef]
- Rebersek, M. Gut microbiome and its role in colorectal cancer. BMC Cancer 2021, 21, 1325. [Google Scholar] [CrossRef]
- Lee, M.H. Harness the functions of gut microbiome in tumorigenesis for cancer treatment. Cancer Commun. 2021, 41, 937–967. [Google Scholar] [CrossRef]
- Nakatsu, G.; Li, X.; Zhou, H.; Sheng, J.; Wong, S.H.; Wu, W.K.K.; Ng, S.C.; Tsoi, H.; Dong, Y.; Zhang, N.; et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat. Commun. 2015, 6, 8727. [Google Scholar] [CrossRef]
- Tjalsma, H.; Boleij, A.; Marchesi, J.; Dutilh, B.E. A bacterial driver–passenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Microbiol. 2012, 10, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef] [PubMed]
- Erturk-Hasdemir, D.; Oh, S.F.; Okan, N.A.; Stefanetti, G.; Gazzaniga, F.S.; Seeberger, P.H.; Plevy, S.E.; Kasper, D.L. Symbionts exploit complex signaling to educate the immune system. Proc. Natl. Acad. Sci. USA 2019, 116, 26157–26166. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.; Wu, L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef]
- Fakharian, F.; Asgari, B.; Nabavi-Rad, A.; Sadeghi, A.; Soleimani, N.; Yadegar, A.; Zali, M.R. The interplay between Helicobacter pylori and the gut microbiota: An emerging driver influencing the immune system homeostasis and gastric carcinogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 953718. [Google Scholar] [CrossRef]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Aghamajidi, A.; Maleki Vareki, S. The Effect of the Gut Microbiota on Systemic and Anti-Tumor Immunity and Response to Systemic Therapy against Cancer. Cancers 2022, 14, 3563. [Google Scholar] [CrossRef]
- Senevirathne, A.; Lloren, K.K.S.; Aganja, R.P.; Kwon, J.; Lee, J.H. Transforming bacterial pathogens into wonder tools in cancer immunotherapy. Mol. Ther. 2025, 33, 866–882. [Google Scholar] [CrossRef]
- Pujo, J.; Petitfils, C.; Le Faouder, P.; Eeckhaut, V.; Payros, G.; Maurel, S.; Perez-Berezo, T.; Van Hul, M.; Barreau, F.; Blanpied, C.; et al. Bacteria-derived long-chain fatty acid exhibits anti-inflammatory properties in colitis. Gut 2021, 70, 1088–1097. [Google Scholar] [CrossRef]
- Liu, J.; ∙Kang, ∙R.; Tang, D. Lipopolysaccharide delivery systems in innate immunity. Trends Immunol. 2024, 45, 274–287. [Google Scholar] [CrossRef]
- Roelands, J.; Kuppen, P.J.K.; Vermeulen, L.; Maccalli, C.; Decock, J.; Wang, E.; Marincola, F.M.; Bedognetti, D.; Hendrickx, W. Immunogenomic Classification of Colorectal Cancer and Therapeutic Implications. Int. J. Mol. Sci. 2017, 18, 2229. [Google Scholar] [CrossRef]
- Acosta, I.C.; Alonzo, F. The Intersection Between Bacterial Metabolism and Innate Immunity. J. Innate Immun. 2023, 15, 782–803. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Artis, D.; Becker, C. The intestinal barrier: A pivotal role in health, inflammation, and cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef]
- Stiel, L.; Gaudet, A.; Thietart, S.; Vallet, H.; Bastard, P.; Voiriot, G.; Oualha, M.; Sarton, B.; Kallel, H.; Brechot, N.; et al. Innate immune response in acute critical illness: A narrative review. Ann. Intensive Care 2024, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Dayang, E.Z.; Plantinga, J.; Ter Ellen, B.; van Meurs, M.; Molema, G.; Moser, J. Identification of LPS-activated endothelial subpopulations with distinct inflammatory phenotypes and regulatory signaling mechanisms. Front. Immunol. 2019, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Reddick, L.E.; Alto, N.M. Bacteria fighting back: How pathogens target and subvert the host innate immune system. Mol. Cell 2014, 54, 321–328. [Google Scholar] [CrossRef]
- Wang, M.; Feng, J.; Zhou, D.; Wang, J. Bacterial lipopolysaccharide-induced endothelial activation and dysfunction: A new predictive and therapeutic paradigm for sepsis. Eur. J. Med. Res. 2023, 28, 339. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Ramachandran, R.; Manan, A.; Kim, J.; Choi, S. NLRP3 inflammasome: A key player in the pathogenesis of life-style disorders. Exp. Mol. Med. 2024, 56, 1488–1500. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Z.; Chang, Z. Lipopolysaccharide induces vascular endothelial cell pyroptosis via the SP1/RCN2/ROS signaling pathway. Eur. J. Cell Biol. 2021, 100, 151164. [Google Scholar] [CrossRef]
- Rady, M.; Ashraf, A.; Abdelaziz, H.; El-Azizi, M. Adaptive immune changes in colorectal cancer: A focus on T and B cell activation genes. Discov. Oncol. 2025, 16, 1032. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F.R.; McLaren, J.E. T Cell Immunity to Bacterial Pathogens: Mechanisms of Immune Control and Bacterial Evasion. Int. J. Mol. Sci. 2020, 21, 6144. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Zhang, J.; Cao, P.; Su, W.; Deng, Y.; Zhan, N.; Fu, X.; Huang, Y.; Dong, W. Fusobacterium nucleatum Promotes Metastasis in Colorectal Cancer by Activating Autophagy Signaling via the Upregulation of CARD3 Expression. Theranostics 2020, 10, 323–339. [Google Scholar] [CrossRef]
- Nieto, P.A.; Pardo-Roa, C.; Salazar-Echegarai, F.J.; Tobar, H.E.; Coronado-Arrázola, I.; Riedel, C.A.; Kalergis, A.M.; Bueno, S.M. New insights about excisable pathogenicity islands in Salmonella and their contribution to virulence. Microbes Infect. 2016, 18, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhao, Y.; Ma, Q.; Li, Q.; Wang, S.; Shi, H. Manipulation of host immune defenses by effector proteins delivered from multiple secretion systems of Salmonella and its application in vaccine research. Front. Immunol. 2023, 14, 1152017. [Google Scholar] [CrossRef]
- Pellegrino, A.; Coppola, G.; Santopaolo, F.; Gasbarrini, A.; Ponziani, F.R. Role of Akkermansia in Human Diseases: From Causation to Therapeutic Properties. Nutrients 2023, 15, 1815. [Google Scholar] [CrossRef]
- Jans, M.; Kolata, M.; Blancke, G.; D’Hondt, A.; Gräf, C.; Ciers, M.; Sze, M.; Thiran, A.; Petta, I.; Andries, V.; et al. Colibactin-driven colon cancer requires adhesin-mediated epithelial binding. Nature 2024, 635, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Engelsberger, V.; Gerhard, M.; Mejías-Luque, R. Effects of Helicobacter pylori infection on intestinal microbiota, immunity and colorectal cancer risk. Front. Cell. Infect. Microbiol. 2024, 14, 1339750. [Google Scholar] [CrossRef]
- Öberg, J.; Rasmussen, M.; Buchwald, P.; Nilson, B.; Inghammar, M. Streptococcus bovis-bacteremia: Subspecies distribution and association with colorectal cancer: A retrospective cohort study. Epidemiol. Infect. 2021, 150, e8. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Geisbrecht, B.V.; Rueter, C.; Hardwidge, P.R. Enterotoxigenic Escherichia coli Flagellin Inhibits TNF-Induced NF-κB Activation in Intestinal Epithelial Cells. Pathogens 2017, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Dordević, D.; Kollar, P.; Vítězová, M.; Drago, L. Hydrogen Sulfide as a Toxic Product in the Small–Large Intestine Axis and its Role in IBD Development. J. Clin. Med. 2019, 8, 1054. [Google Scholar] [CrossRef]
- Janney, A.; Powrie, F.; Mann, E.H. Host-microbiota maladaptation in colorectal cancer. Nature 2018, 585, 509–517. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Gerner, R.R.; Moschen, A.R. The intestinal microbiota in colorectal cancer. Cancer Cell 2018, 33, 954–964. [Google Scholar] [CrossRef]
- Catalano, T.; Selvaggi, F.; Cotellese, R.; Aceto, G.M. The Role of Reactive Oxygen Species in Colorectal Cancer Initiation and Progression: Perspectives on Theranostic Approaches. Cancers 2025, 17, 752. [Google Scholar] [CrossRef]
- González, A.; Fullaondo, A.; Odriozola, A. Microbiota-associated mechanisms in colorectal cancer. Adv. Genet. 2024, 112, 123–205. [Google Scholar]
- Kong, X.; Zhang, Y.; Xiang, L.; You, Y.; Duan, Y.; Zhao, Y.; Li, S.; Wu, R.; Zhang, J.; Zhou, L.; et al. Fusobacterium nucleatum-triggered neutrophil extracellular traps facilitate colorectal carcinoma progression. J. Exp. Clin. Cancer Res. CR 2023, 42, 236. [Google Scholar] [CrossRef] [PubMed]
- Zulpa, A.K.; Barathan, M.; Iyadorai, T.; Mariappan, V.; Vadivelu, J.; Teh, C.S.J.; Vellasamy, K.M. Selective pks+ Escherichia coli strains induce cell cycle arrest and apoptosis in colon cancer cell line. World J. Microbiol. Biotechnol. 2023, 39, 333. [Google Scholar] [CrossRef]
- Yekani, M.; Baghi, H.B.; Naghili, B.; Vahed, S.Z.; Sóki, J.; Memar, M.Y. To resist and persist: Important factors in the pathogenesis of Bacteroides fragilis. Microb. Pathog. 2020, 149, 104506. [Google Scholar] [CrossRef]
- Dong, R.; Liang, Y.; He, S.; Cui, Y.; Shi, C.; He, Y.; Shi, X. DsrA Modulates Central Carbon Metabolism and Redox Balance by Directly Repressing pflB Expression in Salmonella typhimurium. Microbiol. Spectr. 2022, 10, e0152221. [Google Scholar] [CrossRef]
- Ralser, A.; Dietl, A.; Jarosch, S.; Engelsberger, V.; Wanisch, A.; Janssen, K.P.; Middelhoff, M.; Vieth, M.; Quante, M.; Haller, D.; et al. Helicobacter pylori promotes colorectal carcinogenesis by deregulating intestinal immunity and inducing a mucus-degrading microbiota signature. Gut 2023, 72, 1258–1270. [Google Scholar] [CrossRef]
- Garvey, M. Intestinal Dysbiosis: Microbial Imbalance Impacts on Colorectal Cancer Initiation, Progression and Disease Mitigation. Biomedicines 2024, 12, 740. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Lasry, A.; Zinger, A.; Ben-Neriah, Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol 2016, 17, 230–240. [Google Scholar]
- Kennel, K.B.; Greten, F.R. Immune cell—produced ROS and their impact on tumor growth and metastasis. Redox Biol. 2021, 42, 101891. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Pal, S.; Das, M.; Dam, S. Microbe-Induced Oxidative Stress in Cancer Development and Efficacy of Probiotics as Therapeutics in Preventing its Onset and Progression. In Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Chakraborti, S., Ed.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zheng, X.; Ren, L.; Yang, Y.; Li, W.; Fu, W.; Wang, J.; Du, G. Tumorigenic bacteria in colorectal cancer: Mechanisms and treatments. Cancer Biol. Med. 2021, 19, 147–162. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotte, R.P.D. The role of the intestinal microbiota in health and chronic gastrointestinal diseases: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Boleij, A.; Hechenbleikner, E.M.; Goodwin, A.C.; Badani, R.; Stein, E.M.; Lazarev, M.G.; Ellis, B.; Carroll, K.C.; Albesiano, E.; Wick, E.C.; et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 60, 208–215. [Google Scholar] [CrossRef]
- Wei, S.C.; Yang-Yen, H.F.; Tsao, P.N.; Weng, M.T.; Tung, C.C.; Yu, L.C.H.; Lai, L.C.; Hsiao, J.H.; Chuang, E.Y.; Shun, C.T.; et al. SHANK3 Regulates Intestinal Barrier Function Through Modulating ZO-1 Expression Through the PKCepsilon-Dependent Pathway. Inflamm. Bowel Dis. 2017, 23, 1730–1740. [Google Scholar] [CrossRef]
- Jin, M.; Ventilator, Q.; Shang, F.; Zhang, T.; Ogino, S.; Liu, H. Fusobacteria alterations are associated with liver metastasis of colorectal cancer and poor prognosis. Oncol. Lett. 2024, 27, 235. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal barrier in human health and disease. Int. J. Environ. Res. Public. Health 2021, 18, 12836. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Chai, Z.; Chen, S.; Zhang, H.; Zhang, X.; Zhou, Y. Intestinal mucus components and secretion mechanisms: What we do and do not know. Exp. Mol. Med. 2023, 55, 681–691. [Google Scholar] [CrossRef]
- Pothuraju, R.; Chaudhary, S.; Rachagani, S.; Kaur, S.; Roy, H.K.; Bouvet, M.; Batra, S.K. Mucins, gut microbiota, and postbiotics role in colorectal cancer. Gut Microbes 2021, 13, 1974795. [Google Scholar] [CrossRef]
- Lewis, K.; Lutgendorff, F.; Phan, V.; Soderholm, J.D.; Sherman, P.M.; McKay, D.M. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm. Bowel Dis. 2010, 16, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Kendong, S.M.; Raja Ali, R.A.; Nawawi, K.N.M.; Ahmad, H.F.; Mokhtar, N.M. Gut dysbiosis and intestinal barrier dysfunction: Potential explanation for earlyonset colorectal cancer. Front. Cell. Infect. Microbiol. 2021, 11, 744606. [Google Scholar] [CrossRef]
- Murota, Y.; Jobin, C. Bacteria break barrier to promote metastasis. Cancer Cell 2021, 39, 598–600. [Google Scholar] [CrossRef]
- Okumura, S.; Konishi, Y.; Narukawa, M.; Sugiura, Y.; Yoshimoto, S.; Arai, Y.; Sato, S.; Yoshida, Y.; Tsuji, S.; Uemura, K.; et al. Gut bacteria identified in colorectal cancer patients promote tumourigenesis via butyrate secretion. Nat. Commun. 2021, 12, 5674. [Google Scholar] [CrossRef]
- Meng, E.X.; Verne, G.N.; Zhou, Q. Macrophages and Gut Barrier Function: Guardians of Gastrointestinal Health in Post-Inflammatory and Post-Infection Responses. Int. J. Mol. Sci. 2024, 25, 9422. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Munari, F.; Sanchez-Rodriguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Dharmasiri, S.; Garrido-Martin, E.M.; Harris, R.J.; Bateman, A.C.; Collins, J.E.; Cummings, J.R.F.; Sanchez-Elsner, T. Human Intestinal Macrophages Are Involved in the Pathology of Both Ulcerative Colitis and Crohn Disease. Inflamm. Bowel Dis. 2021, 27, 1641–1652. [Google Scholar] [CrossRef]
- Tanaka, S.; Jiang, Y.; Martinez, G.J.; Tanaka, K.; Yan, X.; Kurosaki, T.; Kaartinen, V.; Feng, X.H.; Tian, Q.; Wang, X.; et al. Trim33 mediates the proinflammatory function of Th17 cells. J. Exp. Med. 2018, 215, 1853–1868. [Google Scholar] [CrossRef]
- Schnell, A.; Littman, D.R.; Kuchroo, V.K. TH17 cell heterogeneity and its role in tissue inflammation. Nat. Immunol. 2023, 24, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Fesneau, O.; Thevin, V.; Pinet, V.; Goldsmith, C.; Vieille, B.; M’Homa Soudja, S.; Lattanzio, R.; Hahne, M.; Dardalhon, V.; Hernandez-Vargas, H.; et al. An intestinal TH17 cell-derived subset can initiate cancer. Nat. Immunol. 2024, 25, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.-Q.; Luo, Q.; Wang, L.; Song, G.-B.; Sheng, J.-P.; Xu, B. Signaling pathways involved in colorectal cancer: Pathogenesis and targeted therapy. Sig. Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Goel, A.; Chung, D.C. Pathways of colorectal carcinogenesis. Gastroenterology 2020, 158, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, Y.; Chen, C.; Shao, B.; Zhao, L.; Zhou, Q.; Liu, J.; Wang, G.; Yuan, W.; Sun, Z. Interaction between intestinal microbiota and tumour immunity in the tumour microenvironment. Immunology 2021, 164, 476–493. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Shakhpazyan, N.K.; Mikhaleva, L.M.; Bedzhanyan, A.L.; Gioeva, Z.V.; Mikhalev, A.I.; Midiber, K.Y.; Pechnikova, V.V.; Biryukov, A.E. Exploring the Role of the Gut Microbiota in Modulating Colorectal Cancer Immunity. Cells 2024, 13, 1437. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Singh, A. Gut Microbiome and Human Health: Exploring How the Probiotic Genus Lactobacillus Modulate Immune Responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef]
- Guo, S.; Gillingham, T.; Guo, Y.; Meng, D.; Zhu, W.; Walker, W.A.; Gangul, I.K. Secretions of Bifidobacterium infantis and Lactobacillus acidophilus Protect Intestinal Epithelial Barrier Function. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 404–412. [Google Scholar] [CrossRef]
- Mi, H.; Dong, Y.; Zhang, B.; Wang, H.; Peter, C.C.K.; Gao, P.; Fu, H.; Gao, Y. Bifidobacterium Infantis Ameliorates Chemotherapy-Induced Intestinal Mucositis Via Regulating T Cell Immunity in Colorectal CancerRats. Cell Physiol. Biochem. 2017, 42, 2330–2341. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, H.; Chen, H.; Liu, X.; Zhao, J.; Stanton, C.; Ross, R.P.; Chen, W.; Yang, B. Bifidobacterium inhibits the progression of colorectal tumorigenesis in mice through fatty acid isomerization and gut microbiota modulation. Gut Microbes 2025, 17, 2464945. [Google Scholar] [CrossRef] [PubMed]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Chiu, H.T.; Alshawsh, M.A. Faecalibacterium prausnitzii Ameliorates Colorectal Tumorigenesis and Suppresses Proliferation of HCT116 Colorectal Cancer Cells. Biomedicines 2022, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cui, W.; Li, X.; Yang, H. Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease. Front. Immunol. 2021, 12, 761981. [Google Scholar] [CrossRef] [PubMed]
- Molaaghaee-Rouzbahani, S.; Asri, N.; Sapone, A.; Baghaei, K.; Yadegar, A.; Amani, D.; Rostami-Nejad, M. Akkermansia muciniphila Exerts Immunomodulatory and Anti-Inflammatory Effects on Gliadin-Stimulated THP-1 Derived Macrophages. Sci. Rep. 2023, 13, 3237. [Google Scholar] [CrossRef]
- Wade, H.; Pan, K.; Duan, Q.; Kaluzny, S.; Pandey, E.; Fatumoju, L.; Saraswathi, V.; Wu, R.; Harris, E.N.; Su, Q. Akkermansia muciniphila and Its Membrane Protein Ameliorates Intestinal Inflammatory Stress and Promotes Epithelial Wound Healing via CREBH and miR-143/145. J. Biomed. Sci. 2023, 30, 38. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Lazar, V.; Ditu, L.M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 2018, 9, 1830. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, D. The synthesis of the novel Escherichia coli toxin—Colibactin and its mechanisms of tumorigenesis of colorectal cancer. Front. Microbiol. 2024, 15, 1501973. [Google Scholar] [CrossRef]
- Cheng, W.T.; Kantilal, H.K.; Davamani, F. The Mechanism of Bacteroides fragilis Toxin Contributes to Colon Cancer Formation. Malays. J. Med. Sci. MJMS 2020, 27, 9–21. [Google Scholar] [CrossRef]
- Hadad, S.E.; Alsolami, M.; Aldahlawi, A.; Alrahimi, J.; Basingab, F.; Hassoubah, S.; Alothaid, H. In vivo evidence: Repression of mucosal immune responses in mice with colon cancer following sustained administration of Streptococcus thermophiles. Saudi J. Biol. Sci. 2021, 28, 4751–4761. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Tang, J.; Zhou, J.; Dong, M. Short-chain fatty acids play a positive role in colorectal cancer. Discov. Oncol. 2024, 15, 425. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Hu, M.; Alashkar Alhamwe, B.; Santner-Nanan, B.; Miethe, S.; Harb, H.; Renz, H.; Potaczek, D.P.; Nanan, R.K. Short-Chain Fatty Acids Augment Differentiation and Function of Human Induced Regulatory T Cells. Int. J. Mol. Sci. 2022, 23, 5740. [Google Scholar] [CrossRef]
- Liu, X.; Shao, J.; Liao, Y.-T.; Wang, L.-N.; Jia, Y.; Dong, P.; Liu, Z.; He, D.; Li, C.; Zhang, X. Regulation of Short-Chain Fatty Acids in the Immune System. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Nshanian, M.; Gruber, J.J.; Geller, B.S.; Chleilat, F.; Lancaster, S.M.; White, S.M.; Alexandrova, L.; Camarillo, J.M.; Kelleher, N.L.; Zhao, Y.; et al. Short-chain fatty acid metabolites propionate and butyrate are unique epigenetic regulatory elements linking diet, metabolism and gene expression. Nat. Metab. 2025, 7, 196–211. [Google Scholar] [CrossRef]
- Olguín, J.E.; Medina-Andrade, I.; Rodríguez, T.; Rodríguez-Sosa, M.; Terrazas, L.I. Relevance of Regulatory T Cells During Colorectal Cancer Development. Cancers 2020, 12, 1888. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.B.; Uzonna, J.E. The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front. Immunol. 2019, 10, 680. [Google Scholar] [CrossRef]
- Waniczek, D.; Lorenc, Z.; Snietura, M.; Wesecki, M.; Kopec, A.; Muc-Wierzgon, M. Tumor-Associated Macrophages and Regulatory T Cells Infiltration and the Clinical Outcome in Colorectal Cancer. Arch. Immunol. Ther. Exp. 2017, 65, 445–454. [Google Scholar] [CrossRef]
- Krijgsman, D.; de Vries, N.L.; Skovbo, A.; Andersen, M.N.; Swets, M.; Bastiaannet, E.; Vahrmeijer, A.L.; van de Velde, C.J.H.; Heemskerk, M.H.M.; Hokland, M.; et al. Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: The peripheral blood immune cell profile. Cancer Immunol. Immunother. CII 2019, 68, 1011–1024. [Google Scholar] [CrossRef]
- Del Prete, A.; Salvi, V.; Soriani, A.; Laffranchi, M.; Sozio, F.; Bosisio, D.; Sozzani, S. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell. Mol. Immunol. 2023, 20, 432–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, Y.; Xin, V.W.; Wang, X.W.; Peng, X.C.; Liu, X.Q.; Wang, D.; Li, N.; Cheng, J.T.; Lyv, Y.N.; et al. Dendritic cell biology and its role in tumor immunotherapy. J. Hematol. Oncol. 2020, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Garrido, N.; Badia, J.; Baldom`a, L. Modulation of dendritic cells by microbiota extracellular vesicles influences the cytokine profile and exosome cargo. Nutrients 2022, 14, 344. [Google Scholar] [CrossRef]
- Kim, C.H. Immune regulation by microbiome metabolites. Immunology 2018, 154, 220–229. [Google Scholar] [CrossRef]
- Yoshihara, T.; Oikawa, Y.; Kato, T.; Kessoku, T.; Kobayashi, T.; Kato, S.; Misawa, N.; Ashikari, K.; Fuyuki, A.; Ohkubo, H.; et al. The protective effect of Bifidobacterium bifidum G9-1 against mucus degradation by Akkermansia muciniphila following small intestine injury caused by a proton pump inhibitor and aspirin. Gut Microbes 2020, 11, 1385–1404. [Google Scholar] [CrossRef]
- Luchan, J.; Choi, C.; Carrier, R.L. Reactive oxygen species limit intestinal mucosa-bacteria homeostasis in vitro. Sci Rep. 2021, 11, 23727. [Google Scholar] [CrossRef]
- Luissint, A.C.; Parkos, C.A.; Nusrat, A. Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef]
- Mandal, P. Molecular mechanistic pathway of colorectal carcinogenesis associated with intestinal microbiota. Anaerobe 2018, 49, 63–70. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. reviews. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Lee, A.; Santin, E. Microbiome and pathogen interaction with the immune system. Poult. Sci. 2020, 99, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, S.; Liu, J.; Chen, F.; Li, X.; Wu, G. Gut microbiota as a new target for anticancer therapy: From mechanism to means of regulation. npj Biofilms Microbiomes 2025, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gautam, V.; Sandhu, A.; Rawat, K.; Sharma, A.; Saha, L. Current and emerging therapeutic approaches for colorectal cancer: A comprehensive review. World J. Gastrointest. Surg. 2023, 15, 495–519. [Google Scholar] [CrossRef]
- Palko-Łabuz, A.; Maksymowicz, J.; Sobieszczańska, B.; Wikiera, A.; Skonieczna, M.; Wesołowska, O.; Środa-Pomianek, K. Newly Obtained Apple Pectin as an Adjunct to Irinotecan Therapy of Colorectal Cancer Reducing, E. coli Adherence and β-Glucuronidase Activity. Cancers 2021, 13, 2952. [Google Scholar] [CrossRef]
- Deng, Y.; Hou, X.; Wang, H.; Du, H.; Liu, Y. Influence of Gut Microbiota-Mediated Immune Regulation on Response to Chemotherapy. Pharmaceuticals 2024, 17, 604. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millãn, J.; Queipo-Ortuño, J. The role of the gut microbiome in colorectal cancer development and therapy response. Cancers 2020, 12, 1406. [Google Scholar] [CrossRef]
- Cervantes, A.; Martinelli, E. Updated treatment recommendation for third-line treatment in advanced colorectal cancer from the ESMO Metastatic Colorectal Cancer Living Guideline. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2024, 35, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Zhao, J.; Luo, R.; Da, L.; Li, E.; Zhu, H.; Li, Y.; Luo, Y.; Tian, K.; Wang, Z.; et al. Efficacy and challenges of anti-PD1 in MSI-H mCRC: A case report on concurrent infections and ir-AIHA. Front. Oncol. 2024, 14, 1407312. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Li, X.; Chen, T.T.; Wang, J.X.; Zhou, Y.X.; Mu, X.L.; Du, Y.; Wang, J.L.; Tang, J.; Liu, J.Y. Personalised neoantigen-based therapy in colorectal cancer. Clin. Transl. Med. 2023, 13, e1461. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Zielińska, M.K.; Ciążyńska, M.; Sulejczak, D.; Rutkowski, P.; Czarnecka, A.M. Mechanisms of Resistance to Anti-PD-1 Immunotherapy in Melanoma and Strategies to Overcome it. Biomolecules 2025, 15, 269. [Google Scholar] [CrossRef] [PubMed]
- Motta, R.; Cabezas-Camarero, S.; Torres-Mattos, C.; Riquelme, A.; Calle, A.; Figueroa, A.; Sotelo, M.J. Immunotherapy in microsatellite instability metastatic colorectal cancer: Current status and future perspectives. J. Clin. Transl. Res. 2021, 7, 511–522. [Google Scholar]
- Zhuang, Y.-P.; Zhou, H.-L.; Chen, H.-B.; Zheng, M.-Y.; Liang, Y.-W.; Gu, Y.-T.; Li, W.-T.; Qiu, W.-L.; Zhou, H.-G. Gut microbiota interactions with antitumor immunity in colorectal cancer: From understanding to application. Biomed. Pharmacother. 2023, 165, 115040. [Google Scholar] [CrossRef]
- Xu, J.; Tian, Y.; Zhao, B.; Hu, D.; Wu, S.; Ma, J.; Yang, L. Gut microbiome influences efficacy of Endostatin combined with PD-1 blockade against colorectal cancer. Mol. Biomed. 2024, 5, 37. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Salesse, L.; Garcia-Weber, D.; Marinelli, L.; Beguet-Crespel, F.; Brochard, V.; Le Gléau, C.; Jamet, A.; Doré, J.; Blottière, H.M.; et al. Fusobacterium nucleatum promotes inflammatory and anti-apoptotic responses in colorectal cancer cells via ADP-heptose release and ALPK1/TIFA axis activation. Gut Microbes 2024, 16, 2295384. [Google Scholar] [CrossRef]
- Kopetz, S.; Yoshino, T.; Van Cutsem, E.; Eng, C.; Kim, T.W.; Wasan, H.S.; Desai, J.; Ciardiello, F.; Yaeger, R.; Maughan, T.S.; et al. Encorafenib, cetuximab and chemotherapy in BRAF-mutant colorectal cancer: A randomized phase 3 trial. Nat. Med. 2025, 31, 901–908. [Google Scholar] [CrossRef]
- Yaeger, R.; Weiss, J.; Pelster, M.S.; Spira, A.I.; Barve, M.; Ou, S.I.; Leal, T.A.; Bekaii-Saab, T.S.; Paweletz, C.P.; Heavey, G.A.; et al. Adagrasib with or Without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 388, 44–54. [Google Scholar] [CrossRef]
- Roodhart, J.M.L.; Koopman, M. Trastuzumab deruxtecan in HER2-positive metastatic colorectal cancer: Less is more? Lancet Oncol. 2024, 25, 1104–1105. [Google Scholar] [CrossRef] [PubMed]
- Qasem, H.H.; El-Sayed, W.M. The bacterial microbiome and cancer: Development, diagnosis, treatment, and future directions. Clin. Exp. Med. 2025, 25, 12. [Google Scholar] [CrossRef] [PubMed]
- Soemarie, Y.B.; Milanda, T.; Barliana, M.I. Fermented Foods as Probiotics: A Review. J. Adv. Pharm. Technol. Res. 2021, 12, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Oliero, M.; Alaoui, A.A.; McCartney, C.; Santos, M.M. Colorectal cancer and inulin supplementation: The good, the bad, and the unhelpful. Gastroenterol. Rep. 2024, 12, goae058. [Google Scholar] [CrossRef]
- Turati, F.; Concina, F.; Rossi, M.; Fiori, F.; Parpinel, M.; Taborelli, M.; Giacosa, A.; Crispo, A.; Pagan, E.; Rosato, V.; et al. Association of prebiotic fiber intake with colorectal cancer risk: The PrebiotiCa study. Eur. J. Nutr. 2023, 62, 455–464. [Google Scholar] [CrossRef]
- Ornelas, A.C.; Ferguson, S.; DePlaza, M.; Adekunle, T.; Basha, R. Anti-Cancer Pectins and Their Role in Colorectal Cancer Treatment. Oncol. Ther. 2022, 9, 43–55. [Google Scholar] [CrossRef]
- Shrifteylik, A.; Maiolini, M.; Dufault, M.; Austin, D.L.; Subhadra, B.; Lamichhane, P.; Deshmukh, R.R. A Current Review on the Role of Prebiotics in Colorectal Cancer. Biologics 2023, 3, 209–231. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.; Gaur, S. Probiotics as multifaceted oral vaccines against colon cancer: A review. Front. Immunol. 2022, 13, 1002674. [Google Scholar] [CrossRef]
- Polakowski, C.B.; Kato, M.; Preti, V.B.; Schieferdecker, M.E.M.; Ligocki Campos, A.C. Impact of the preoperative use of synbiotics in colorectal cancer patients: A prospective, randomized, double-blind, placebo-controlled study. Nutrition 2019, 58, 40–46. [Google Scholar] [CrossRef]
- Kvakova, M.; Kamlarova, A.; Stofilova, J.; Benetinova, V.; Bertkova, I. Probiotics and postbiotics in colorectal cancer: Prevention and complementary therapy. World J. Gastroenterol. 2022, 28, 3370–3382. [Google Scholar] [CrossRef] [PubMed]
- Arokiaraj, S.R.; Prathiviraj, R.; Chaiyasut, C.; Sivamaruthi, B.S. Bacteriocins from Lactic Acid Bacteria Could Modulate the Wnt Pathway: A Possible Therapeutic Candidate for the Management of Colorectal Cancer—An In silico Study. Anti-Cancer Agents Med. Chem. 2025, 25, 1239–1252. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhong, Y.S.; Li, X.J.; Kang, Y.K.; Peng, Q.Y.; Ying, H.Z. Postbiotics in colorectal cancer: Intervention mechanisms and perspectives. Front. Microbiol. 2024, 15, 1360225. [Google Scholar] [CrossRef] [PubMed]
- Balendra, V.; Rosenfeld, R.; Amoroso, C.; Castagnone, C.; Rossino, M.G.; Garrone, O.; Ghidini, M. Postbiotics as Adjuvant Therapy in Cancer Care. Nutrients 2024, 16, 2400. [Google Scholar] [CrossRef]
- Hou, S.; Yu, J.; Li, Y.; Zhao, D.; Zhang, Z. Advances in Fecal Microbiota Transplantation for Gut Dysbiosis-Related Diseases. Adv. Sci. 2025, 12, e2413197. [Google Scholar] [CrossRef]
- Khoruts, A.; Sadowsky, M.J. Understanding the mechanisms of faecal microbiota transplantation. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 508–516. [Google Scholar] [CrossRef]
- da Ponte Neto, A.M.; Clemente, A.C.O.; Rosa, P.W.; Ribeiro, I.B.; Funari, M.P.; Nunes, G.C.; Moreira, L.; Sparvoli, L.G.; Cortez, R.; Taddei, C.R.; et al. Fecal microbiota transplantation in patients with metabolic syndrome and obesity: A randomized controlled trial. World J. Clin. Cases 2023, 11, 4612–4624. [Google Scholar] [CrossRef]
- Zecheng, L.; Donghai, L.; Runchuan, G.; Yuan, Q.; Qi, J.; Yijia, Z.; Shuaman, R.; Xiaoqi, L.; Yi, W.; Ni, M.; et al. Fecal microbiota transplantation in obesity metabolism: A meta analysis and systematic review. Diabetes Res. Clin. Pract. 2023, 202, 110803. [Google Scholar] [CrossRef]
- Yu, H.; Li, X.X.; Han, X.; Chen, B.X.; Zhang, X.H.; Gao, S.; Xu, D.Q.; Wang, Y.; Gao, Z.K.; Yu, L.; et al. Fecal microbiota transplantation inhibits colorectal cancer progression: Reversing intestinal microbial dysbiosis to enhance anti-cancer immune responses. Front. Microbiol. 2023, 14, 1126808. [Google Scholar] [CrossRef]
- Fecal Microbiota Transplant and Re-introduction of Anti-PD-1 Therapy (Pembrolizumab or Nivolumab) for the Treatment of Metastatic Colorectal Cancer in Anti-PD-1 Non-responders. 2024. Available online: https://clinicaltrials.gov/study/NCT04729322 (accessed on 6 July 2025).
- Xiao, H.W.; Cui, M.; Li, Y.; Dong, J.L.; Zhang, S.Q.; Zhu, C.C.; Jiang, M.; Zhu, T.; Wang, B.; Wang, H.C.; et al. Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome 2020, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.W.; Roh, T.Y. Opportunistic detection of Fusobacterium nucleatum as a marker for the early gut microbial dysbiosis. BMC Microbiol. 2020, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.Y.P.; Lai, M.-J.; Wang, P.-C.; Wu, W.-J.; Chen, L.-K.; Fan, H.-W.; Tseng, C.-C.; Peng, S.-Y.; Chang, K.-C. A Novel Bacteriophage with the Potential to Inhibit Fusobacterium nucleatum-Induced Proliferation of Colorectal Cancer Cells. Antibiotics 2025, 14, 45. [Google Scholar] [CrossRef]
- Kabwe, M.; Brown, T.; Ku, H.; Dashper, S.; Tucci, J. Isolation and Functional Characterization of Fusobacterium nucleatum Bacteriophage. Methods Mol. Biol. 2021, 2327, 51–68. [Google Scholar]
- Benz, F.; Beamud, B.; Laurenceau, R.; Maire, A.; Duportet, X.; Decrulle, A.; Bikard, D. CRISPR–Cas therapies targeting bacteria. Nat. Rev. Bioeng. 2025. [Google Scholar] [CrossRef]
- Gupta, M.K.; Gouda, G.; Moazzam-Jazi, M.; Vadde, R.; Nagaraju, G.P.; El-Rayes, B.F. CRISPR/Cas9-directed epigenetic editing in colorectal cancer. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2025, 1880, 189338. [Google Scholar] [CrossRef]
- Kolanu, N.D. CRISPR–Cas9 Gene Editing: Curing Genetic Diseases by Inherited Epigenetic Modifications. Glob. Med. Genet. 2024, 11, 113–122. [Google Scholar] [CrossRef]
- Dong, X.; Pan, P.; Zheng, D.W.; Bao, P.; Zeng, X.; Zhang, X.Z. Bioinorganic hybrid bacteriophage for modulation of intestinal microbiota to remodel tumor-immune microenvironment against colorectal cancer. Sci. Adv. 2020, 6, eaba1590. [Google Scholar] [CrossRef]
- Kabwe, M.; Meehan-Andrews, T.; Ku, H.; Petrovski, S.; Batinovic, S.; Chan, H.T.; Tucci, J. Lytic Bacteriophage EFA1 Modulates HCT116 Colon Cancer Cell Growth and Upregulates ROS Production in an Enterococcus faecalis Co-culture System. Front. Microbiol. 2021, 12, 650849. [Google Scholar] [CrossRef]
- Elez, E.; Kopetz, S.; Tabernero, J.; Bekaii-Saab, T.; Taieb, J.; Yoshino, T.; Manji, G.; Fernandez, K.; Abbattista, A.; Zhang, X.; et al. SEAMARK: Phase II study of first-line encorafenib and cetuximab plus pembrolizumab for MSI-H/dMMR BRAFV600E-mutant mCRC. Future Oncol. 2024, 20, 653–663. [Google Scholar] [CrossRef]
- Raghav, K.; Siena, S.; Takashima, A.; Takeshi, K.; Van den Eynde, M.; Pietrantonio, F.; Komatsu, Y.; Kawakami, H.; Peeters, M.; Andre, T.; et al. Trastuzumab deruxtecan in patients with HER2-positive advanced colorectal cancer (DESTINY-CRC02): Primary results from a multicentre, randomised, phase 2 trial. Lancet Oncol. 2024, 25, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Varayathu, H.; Sarathy, V.; Thomas, B.E.; Mufti, S.S.; Naik, R. Combination Strategies to Augment Immune Check Point Inhibitors Efficacy—Implications for Translational Research. Front. Oncol. 2021, 11, 559161. [Google Scholar] [CrossRef] [PubMed]
- Borgers, J.S.W.; Burgers, F.H.; Terveer, E.M.; van Leerdam, M.E.; Korse, C.M.; Kessels, R.; Flohil, C.C.; Blank, C.U.; Schumacher, T.N.; van Dijk, M.; et al. Conversion of unresponsiveness to immune checkpoint inhibition by fecal microbiota transplantation in patients with metastatic melanoma: Study protocol for a randomized phase Ib/IIa trial. BMC Cancer 2022, 22, 1366. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Q. Gut microbiota influences the efficiency of immune checkpoint inhibitors by modulating the immune system (Review). Oncol. Lett. 2024, 27, 87. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).