Circadian Biomarkers in Humans: Methodological Insights into the Detection of Melatonin and Cortisol
Abstract
1. Introduction
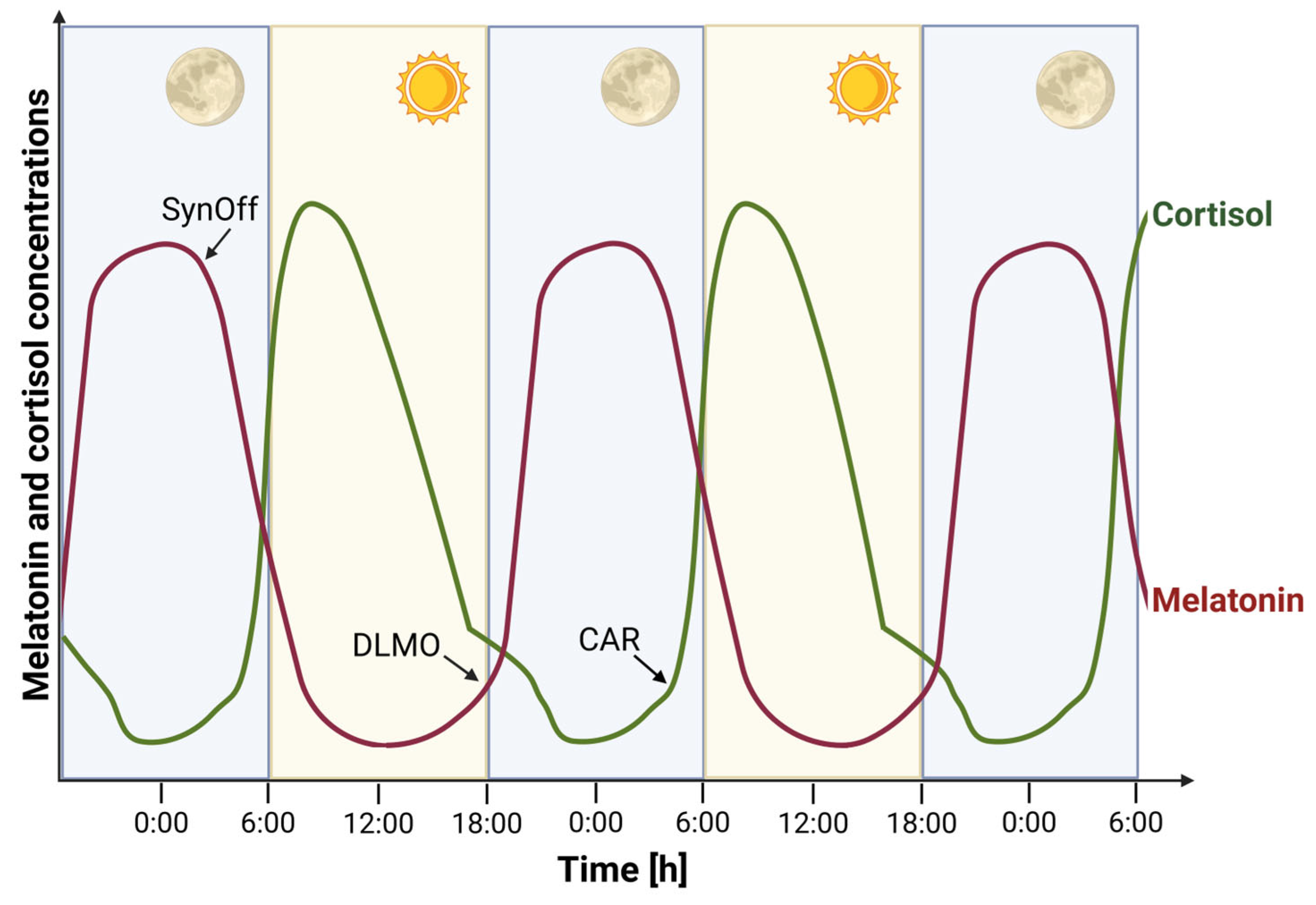
2. Melatonin and Cortisol as Endocrine Markers of Circadian Rhythms
2.1. Melatonin
2.2. Cortisol
2.3. Cofounding Factors
2.4. Alternative Circadian Biomarkers
3. Sampling Strategies in Different Matrices
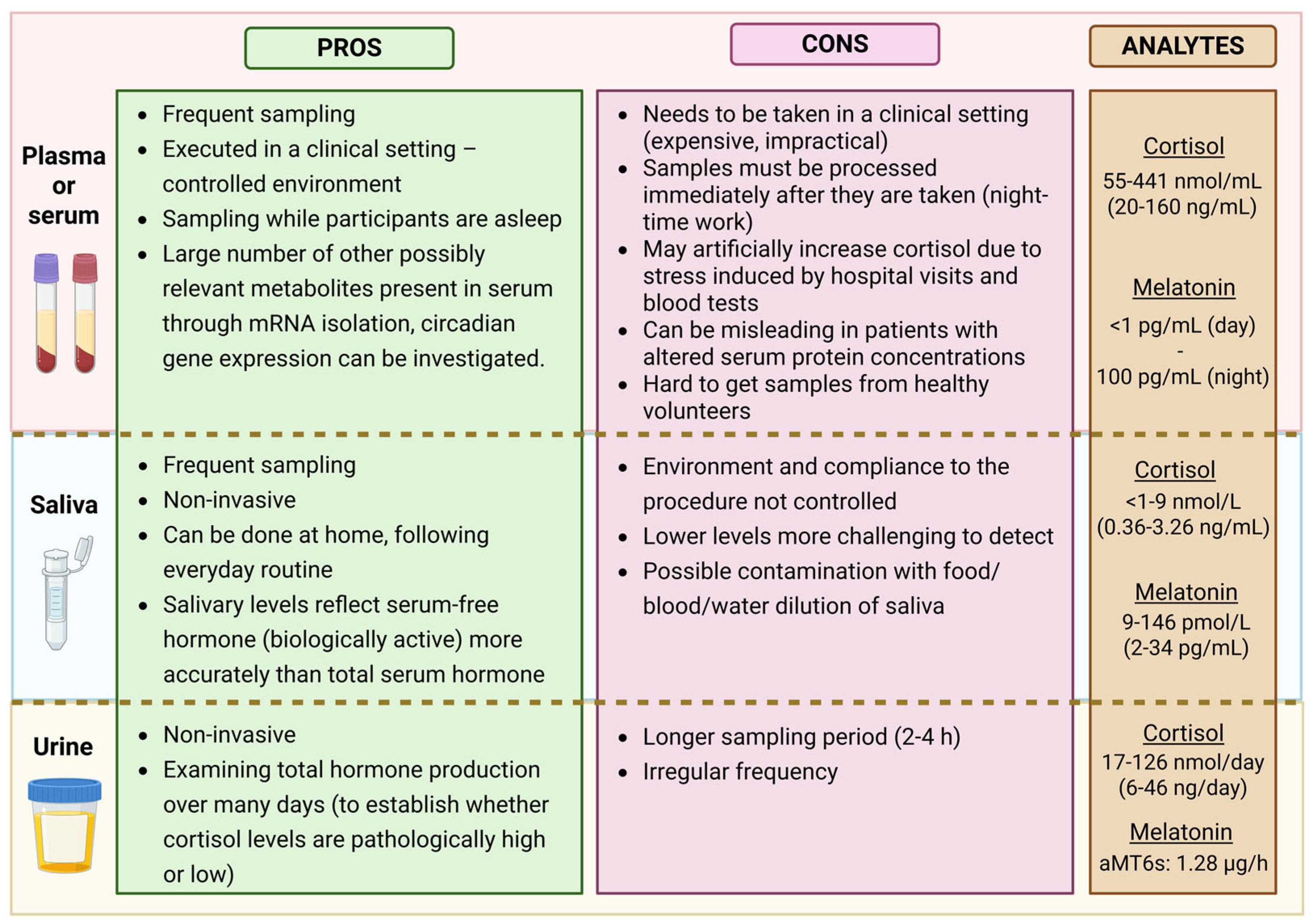
3.1. Serum or Plasma
3.2. Saliva
3.3. Urine
3.4. Alternative Sampling Options
4. Analytical Techniques for Melatonin and Cortisol Quantification
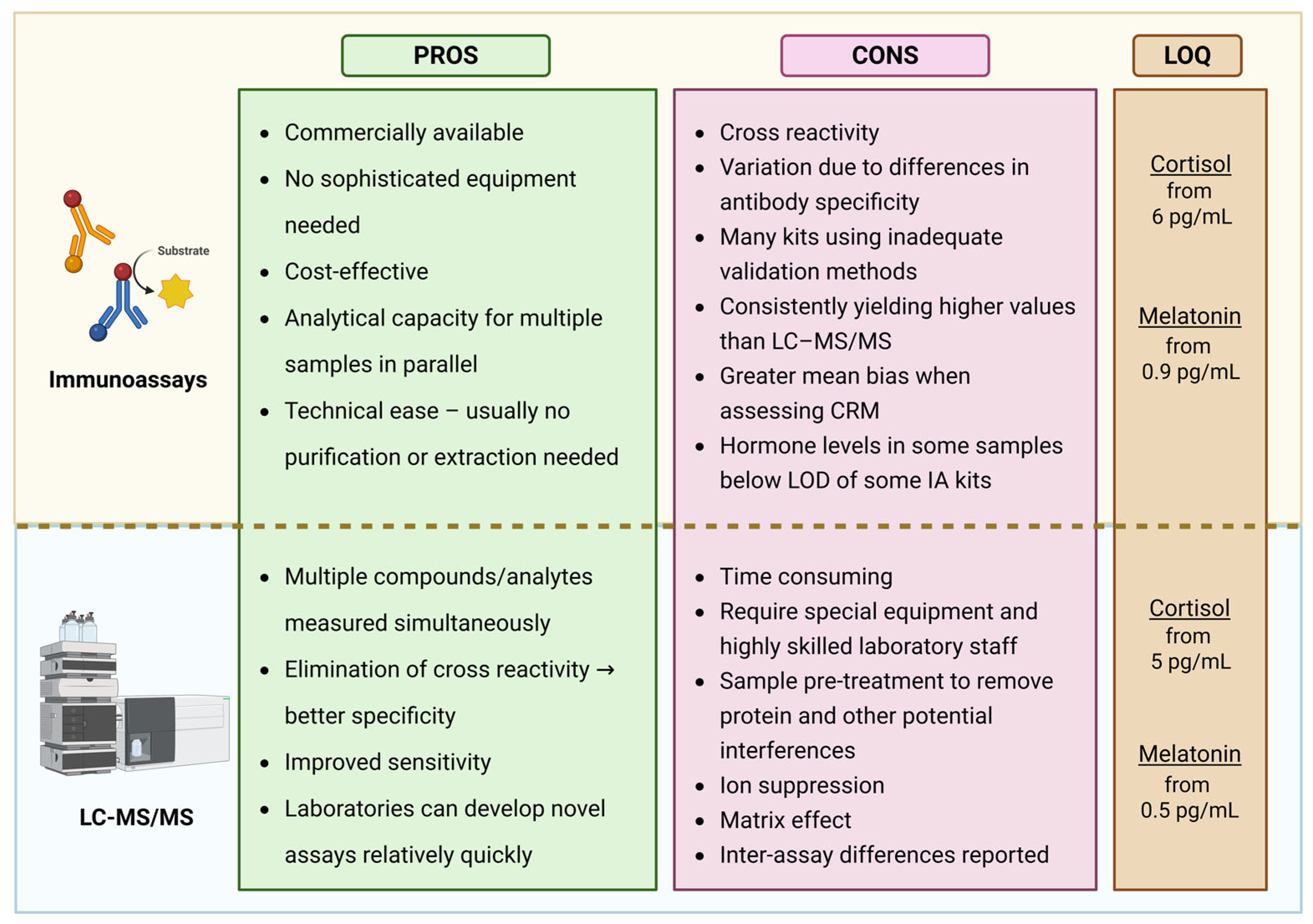
4.1. Immunoassays (IA)
4.2. Liquid Chromatography–Tandem Mass Spectrometry
| Reference | What Did They Compare? | Conclusion |
|---|---|---|
| [20] | LC–MS/MS vs. ELISA (melatonin) and ECLIA (cortisol) in 121 salivary samples from healthy subjects. | Strong correlation (r = 0.910 for melatonin, r = 0.955 for cortisol), but IA showed significant positive bias; 30% of cortisol samples fell below ECLIA LLOQ; LC–MS/MS required less sample volume. |
| [107] | Two LC–MS/MS methods and RIA (Bühlmann) on salivary melatonin from 39 patients. | LC–MS/MS methods showed strong agreement (r = 0.99); RIA had greater variance (r = 0.74, mean bias −11.7%). LC–MS/MS was superior in precision and trueness. |
| [106] | ELISA vs. LC–MS/MS on 35 salivary melatonin samples. | Good agreement in low range; above 30 pmol/L, ELISA underestimates. Mean bias 7.9 pmol/L. Calibration difference excluded as source. |
| [108] | Two CLIA (ADVIA, LIAISON) vs. LC–MS/MS on 24 h urinary cortisol in 174 patients. | Strong correlation overall; discrepancies at high cortisol (>500 µg/mL), with IA reading 2–9× higher than LC–MS/MS. |
| [101] | IA vs. LC–MS/MS on 2703 salivary cortisol samples from children | IA measured values ~2.39× higher. Cross-reactivity with cortisone affected results <5 nmol/L. Over 50% of samples were in this range. |
| [97] | ECLIA vs. LC–MS/MS on 324 late-night salivary cortisol samples. | High correlation (r² = 0.892), but high bias at low concentrations. In total, 68.8% of reference samples were under ECLIA detection limit. |
| [102] | Routine immunoassays and LC–MS/MS vs. cRMP in serum cortisol across multiple cohorts. | LC–MS/MS closely matched cRMP. IA results varied by cohort: underestimation in pregnancy, overestimation in metyrapone/prednisolone groups. |
| [105] | ECLIA (Cortisol I and II) vs. LC–MS/MS on stimulated serum cortisol. | Cortisol II showed small bias (~4% lower); Cortisol I overestimated by ~36%. LC–MS/MS supports lower diagnostic cutoffs. |
| [103] | ELISA, RIA, and LC–MS/MS in inter-laboratory salivary melatonin and cortisol comparison. | High inter-lab variability. ELISA overestimated melatonin (6.90 vs. 0.278 pmol/L). LC–MS/MS results varied among labs. |
| [104] | EIA vs. LC–MS/MS on salivary cortisol (bedtime and morning) in 53 subjects. | Excellent correlation (r² = 0.97); LC–MS/MS consistently yielded lower values, likely due to reduced cross-reactivity. |
4.3. Comparison of Immunoassay and LC–MS/MS Methods
5. Determination of DLMO and CAR in Pediatric Population with Psychiatric Disorders
Recommendations for Standardized Protocols
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DLMO | Dim light melatonin onset |
| CAR | Cortisol awakening response |
| LC–MS/MS | Liquid chromatography–tandem mass spectrometry |
| MRM | Multiple reaction monitoring |
| LOQ | Limit of quantification |
| SCN | The suprachiasmatic nucleus |
| RIA | Radioimmunoassay |
| PPT | Protein precipitation |
| LLE | Liquid–liquid extraction |
| SPE | Solid-phase extraction |
| GC–MS | Gas chromatography–mass spectrometry |
| ASD | Autism spectrum disorder |
| ADHD | Attention-deficit/hyperactivity disorder |
| WASO | Wake after sleep onset |
References
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, S.J.; Craig, L.M.; Duffy, J.F. Introduction to Chronobiology. Cold Spring Harb Perspect. Biol. 2018, 10, a033613. [Google Scholar] [CrossRef] [PubMed]
- Korenčič, A.; Košir, R.; Bordyugov, G.; Lehmann, R.; Rozman, D.; Herzel, H. Timing of circadian genes in mammalian tissues. Sci. Rep. 2014, 4, 5782. [Google Scholar] [CrossRef]
- Fagiani, F.; Di Marino, D.; Romagnoli, A.; Travelli, C.; Voltan, D.; Di Cesare Mannelli, L.; Racchi, M.; Govoni, S.; Lanni, C. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct. Target. Ther. 2022, 7, 41. [Google Scholar] [CrossRef]
- Videnovic, A.; Lazar, A.S.; Barker, R.A.; Overeem, S. ‘The clocks that time us’—Circadian rhythms in neurodegenerative disorders. Nat. Rev. Neurol. 2014, 10, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Sassone-Corsi, P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018, 24, 1795–1803. [Google Scholar] [CrossRef]
- Kovač, U.; Skubic, C.; Bohinc, L.; Rozman, D.; Režen, T. Oxysterols and Gastrointestinal Cancers Around the Clock. Front. Endocrinol. 2019, 10, 483. [Google Scholar] [CrossRef]
- Morris, C.J.; Purvis, T.E.; Mistretta, J.; Scheer, F.A.J.L. Effects of the Internal Circadian System and Circadian Misalignment on Glucose Tolerance in Chronic Shift Workers. J. Clin. Endocrinol. Metab. 2016, 101, 1066–1074. [Google Scholar] [CrossRef]
- Maemura, K.; Takeda, N.; Nagai, R. Circadian Rhythms in the CNS and Peripheral Clock Disorders: Role of the Biological Clock in Cardiovascular Diseases. J. Pharmacol. Sci. 2007, 103, 134–138. [Google Scholar] [CrossRef]
- Hombali, A.; Seow, E.; Yuan, Q.; Chang, S.H.S.; Satghare, P.; Kumar, S.; Verma, S.K.; Mok, Y.M.; Chong, S.A.; Subramaniam, M. Prevalence and correlates of sleep disorder symptoms in psychiatric disorders. Psychiatry Res. 2019, 279, 116–122. [Google Scholar] [CrossRef]
- Plavc, L.; Skubic, C.; Dolenc Grošelj, L.; Rozman, D. Variants in the circadian clock genes PER2 and PER3 associate with familial sleep phase disorders. Chronobiol. Int. 2024, 41, 757–766. [Google Scholar] [CrossRef]
- Flynn-Evans, E.E.; Shekleton, J.A.; Miller, B.; Epstein, L.J.; Kirsch, D.; Brogna, L.A.; Burke, L.M.; Bremer, E.; Murray, J.M.; Gehrman, P.; et al. Circadian Phase and Phase Angle Disorders in Primary Insomnia. Sleep 2017, 40, zsx163. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, B.; Scholtze, J.; Schmitt, J. Circadian rhythms in blood pressure, heart rate, hormones, and on polysomnographic parameters in severe obstructive sleep apnea syndrome patients: Effect of continuous positive airway pressure. Blood Press. Monit. 2016, 21, 136–143. [Google Scholar] [CrossRef]
- Šmon, J.; Kočar, E.; Pintar, T.; Dolenc-Grošelj, L.; Rozman, D. Is obstructive sleep apnea a circadian rhythm disorder? J. Sleep Res. 2023, 32, e13875. [Google Scholar] [CrossRef]
- Zmrzljak, U.P.; Rozman, D. Circadian regulation of the hepatic endobiotic and xenobitoic detoxification pathways: The time matters. Chem. Res. Toxicol. 2012, 25, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Okyar, A.; Civelek, D.O.; Akyel, Y.K.; Surme, S.; Kara, Z.P.; Kavakli, İ.H. The role of the circadian timing system on drug metabolism and detoxification: An update. Expert Opin. Drug Metab. Toxicol. 2024, 20, 503–517. [Google Scholar] [CrossRef]
- Walton, J.C.; Walker, W.H.; Bumgarner, J.R.; Meléndez-Fernández, O.H.; Liu, J.A.; Hughes, H.L.; Kaper, A.L.; Nelson, R.J. Circadian Variation in Efficacy of Medications. Clin. Pharma Ther. 2021, 109, 1457–1488. [Google Scholar] [CrossRef]
- Reid, K.J. Assessment of Circadian Rhythms. Neurol. Clin. 2019, 37, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Clow, A.; Hucklebridge, F.; Stalder, T.; Evans, P.; Thorn, L. The cortisol awakening response: More than a measure of HPA axis function. Neurosci. Biobehav. Rev. 2010, 35, 97–103. [Google Scholar] [CrossRef]
- Shin, S.; Oh, H.; Park, H.R.; Joo, E.Y.; Lee, S.-Y. A Sensitive and Specific Liquid Chromatography-Tandem Mass Spectrometry Assay for Simultaneous Quantification of Salivary Melatonin and Cortisol: Development and Comparison With Immunoassays. Ann. Lab. Med. 2021, 41, 108–113. [Google Scholar] [CrossRef]
- Kennaway, D.J. The appropriate and inappropriate uses of saliva melatonin measurements. Chronobiol. Int. 2024, 41, 1351–1364. [Google Scholar] [CrossRef]
- Lewy, A.J.; Cutler, N.L.; Sack, R.L. The Endogenous Melatonin Profile as a Marker for Circadian Phase Position. J. Biol. Rhythm. 1999, 14, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Choi, S.J.; Song, Y.M.; Park, H.R.; Joo, E.Y.; Kim, J.K. Enhanced Circadian Phase Tracking: A 5 h DLMO Sampling Protocol Using Wearable Data. J. Biol. Rhythm. 2025, 40, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Srinivasan, V.; Spence, D.W.; Cardinali, D.P. Role of the melatonin system in the control of sleep: Therapeutic implications. CNS Drugs 2007, 21, 995–1018. [Google Scholar] [CrossRef]
- Burgess, H.J.; Emens, J.S. Circadian-Based Therapies for Circadian Rhythm Sleep-Wake Disorders. Curr. Sleep Med. Rep. 2016, 2, 158–165. [Google Scholar] [CrossRef][Green Version]
- Sack, R.L.; Brandes, R.W.; Kendall, A.R.; Lewy, A.J. Entrainment of free-running circadian rhythms by melatonin in blind people. N. Engl. J. Med. 2000, 343, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Meyrel, M.; Rolland, B.; Geoffroy, P.A. Alterations in circadian rhythms following alcohol use: A systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 99, 109831. [Google Scholar] [CrossRef]
- Voultsios, A.; Kennaway, D.J.; Dawson, D. Salivary melatonin as a circadian phase marker: Validation and comparison to plasma melatonin. J. Biol. Rhythm. 1997, 12, 457–466. [Google Scholar] [CrossRef]
- Danilenko, K.V.; Verevkin, E.G.; Antyufeev, V.S.; Wirz-Justice, A.; Cajochen, C. The hockey-stick method to estimate evening dim light melatonin onset (DLMO) in humans. Chronobiol. Int. 2014, 31, 349–355. [Google Scholar] [CrossRef]
- Crowley, S.J.; Suh, C.; Molina, T.A.; Fogg, L.F.; Sharkey, K.M.; Carskadon, M.A. Estimating the dim light melatonin onset of adolescents within a 6-h sampling window: The impact of sampling rate and threshold method. Sleep Med. 2016, 20, 59–66. [Google Scholar] [CrossRef]
- Molina, T.A.; Burgess, H.J. Calculating the dim light melatonin onset: The impact of threshold and sampling rate. Chronobiol. Int. 2011, 28, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Grima, N.A.; Ponsford, J.L.; St. Hilaire, M.A.; Mansfield, D.; Rajaratnam, S.M. Circadian Melatonin Rhythm Following Traumatic Brain Injury. Neurorehabil. Neural Repair 2016, 30, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Smolensky, M.H.; Sackett-Lundeen, L.L.; Portaluppi, F. Nocturnal light pollution and underexposure to daytime sunlight: Complementary mechanisms of circadian disruption and related diseases. Chronobiol. Int. 2015, 32, 1029–1048. [Google Scholar] [CrossRef]
- Srinivasan, V.; Spence, D.W.; Pandi-Perumal, S.R.; Trakht, I.; Cardinali, D.P. Jet lag: Therapeutic use of melatonin and possible application of melatonin analogs. Travel Med. Infect. Dis. 2008, 6, 17–28. [Google Scholar] [CrossRef]
- Nous, A.; Engelborghs, S.; Smolders, I. Melatonin levels in the Alzheimer’s disease continuum: A systematic review. Alzheimers. Res. Ther. 2021, 13, 52. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, P.; Bendlin, B.B.; Zetterberg, H.; De Felice, F.; Tan, X.; Benedict, C. Melatonin: A potential nighttime guardian against Alzheimer’s. Mol. Psychiatry 2025, 30, 237–250. [Google Scholar] [CrossRef]
- da Silveira Cruz-Machado, S.; Guissoni Campos, L.M.; Fadini, C.C.; Anderson, G.; Markus, R.P.; Pinato, L. Disrupted nocturnal melatonin in autism: Association with tumor necrosis factor and sleep disturbances. J. Pineal Res. 2021, 70, e12715. [Google Scholar] [CrossRef]
- Luo, Z.; Tang, Y.Y.; Zhou, L. Melatonin as an adjunctive therapy in cardiovascular disease management. Sci. Prog. 2024, 107, 368504241299993. [Google Scholar] [CrossRef]
- Weibel, L.; Brandenberger, G. The start of the quiescent period of cortisol remains phase locked to the melatonin onset despite circadian phase alterations in humans working the night schedule. Neurosci. Lett. 2002, 318, 89–92. [Google Scholar] [CrossRef]
- Klerman, E.B.; Gershengorn, H.B.; Duffy, J.F.; Kronauer, R.E. Comparisons of the variability of three markers of the human circadian pacemaker. J. Biol. Rhythm. 2002, 17, 181–193. [Google Scholar] [CrossRef]
- Honma, A.; Revell, V.L.; Gunn, P.J.; Davies, S.K.; Middleton, B.; Raynaud, F.I.; Skene, D.J. Effect of acute total sleep deprivation on plasma melatonin, cortisol and metabolite rhythms in females. Eur. J. Neurosci. 2020, 51, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.; Gorenstein, C.; Moreno, R.; Pariante, C.; Markus, R. Effect of antidepressants on melatonin metabolite in depressed patients. J. Psychopharmacol. 2009, 23, 315–321. [Google Scholar] [CrossRef]
- Burgess, H.J.; Fogg, L.F. Individual differences in the amount and timing of salivary melatonin secretion. PLoS ONE 2008, 3, e3055. [Google Scholar] [CrossRef] [PubMed]
- Stoschitzky, K.; Sakotnik, A.; Lercher, P.; Zweiker, R.; Maier, R.; Liebmann, P.; Lindner, W. Influence of beta-blockers on melatonin release. E. J. Clin. Pharmacol. 1999, 55, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Fries, E.; Dettenborn, L.; Kirschbaum, C. The cortisol awakening response (CAR): Facts and future directions. Int. J. Psychophysiol. 2009, 72, 67–73. [Google Scholar] [CrossRef]
- Serwinski, B.; Salavecz, G.; Kirschbaum, C.; Steptoe, A. Associations between hair cortisol concentration, income, income dynamics and status incongruity in healthy middle-aged women. Psychoneuroendocrinology 2016, 67, 182–188. [Google Scholar] [CrossRef]
- Chida, Y.; Steptoe, A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biol. Psychol. 2009, 80, 265–278. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.K.; Findling, J.W.; Murad, M.H.; Newell-Price, J.; Savage, M.O.; Tabarin, A. Treatment of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2015, 100, 2807–2831. [Google Scholar] [CrossRef]
- Ouanes, S.; Popp, J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2019, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Mohd Azmi, N.A.; Juliana, N.; Azmani, S.; Mohd Effendy, N.; Abu, I.F.; Mohd Fahmi Teng, N.I.; Das, S. Cortisol on Circadian Rhythm and Its Effect on Cardiovascular System. Int. J. Environ. Res. Public Health 2021, 18, 676. [Google Scholar] [CrossRef]
- Onton, J.; Le, L.D. Amount of <1Hz deep sleep correlates with melatonin dose in military veterans with PTSD. Neurobiol. Sleep Circadian Rhythm. 2021, 11, 100072. [Google Scholar] [CrossRef]
- Lee, K.F.A.; Tong, H.; Jin, R.R.; Lee, T.M.C. Effects of Exposure to Life Stressors, Perceived Stress, and Psychopathological Symptoms on Cortisol Awakening Response: Individual Differences in Resilience. Stress Health 2025, 41, e70048. [Google Scholar] [CrossRef]
- Bogudzińska, B.; Maciaszek, J.; Stańczykiewicz, B.; Bielawski, T.; Dybek, A.; Alejnikowa, J.; Pawłowski, T.; Misiak, B. Blunted Cortisol Awakening Response Is Associated with External Attribution Bias Among Individuals with Personality Disorders. Brain Sci. 2024, 14, 1040. [Google Scholar] [CrossRef]
- Reinberg, A.E.; Touitou, Y.; Soudant, É.; Bernard, D.; Bazin, R.; Mechkouri, M. Oral Contraceptives Alter Circadian Rhythm Parameters of Cortisol, Melatonin, Blood Pressure, Heart Rate, Skin Blood Flow, Transepidermal Water Loss, and Skin Amino Acids of Healthy Young Women. Chronobiol. Int. 1996, 13, 199–211. [Google Scholar] [CrossRef]
- Zeitzer, J.M.; Duffy, J.F.; Lockley, S.W.; Dijk, D.-J.; Czeisler, C.A. Plasma Melatonin Rhythms In Young and Older Humans During Sleep, Sleep Deprivation, and Wake. Sleep 2007, 30, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Deacon, S.; Arendt, J. Posture influences melatonin concentrations in plasma and saliva in humans. Neurosci. Lett. 1994, 167, 191–194. [Google Scholar] [CrossRef]
- Kozaki, T.; Arata, T.; Kubokawa, A. Salivary Melatonin Concentrations in a Sitting and a Standing Position. J. Horm. 2013, 2013, 1–4. [Google Scholar] [CrossRef]
- Hofstra, W.A.; de Weerd, A.W. How to assess circadian rhythm in humans: A review of literature. Epilepsy Behav. 2008, 13, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; Colecchia, E.F.; L’Hermite-Balériaux, M.; Van Cauter, E. Transition from Dim to Bright Light in the Morning Induces an Immediate Elevation of Cortisol Levels1. J. Clin. Endocrinol. Metab. 2001, 86, 151–157. [Google Scholar] [CrossRef][Green Version]
- Sellami, M.; Dhahbi, W.; Hayes, L.D.; Kuvacic, G.; Milic, M.; Padulo, J. The effect of acute and chronic exercise on steroid hormone fluctuations in young and middle-aged men. Steroids 2018, 132, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Baid, S.K.; Sinaii, N.; Wade, M.; Rubino, D.; Nieman, L.K. Radioimmunoassay and Tandem Mass Spectrometry Measurement of Bedtime Salivary Cortisol Levels: A Comparison of Assays to Establish Hypercortisolism. J. Clin. Endocrinol. Metab. 2007, 92, 3102–3107. [Google Scholar] [CrossRef] [PubMed]
- El-Farhan, N.; Pickett, A.; Ducroq, D.; Bailey, C.; Mitchem, K.; Morgan, N.; Armston, A.; Jones, L.; Evans, C.; Rees, D.A. Method-specific serum cortisol responses to the adrenocorticotrophin test: Comparison of gas chromatography-mass spectrometry and five automated immunoassays. Clin. Endocrinol. 2013, 78, 673–680. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Steiner, R.; Blattner, P.; Oelhafen, P.; Götz, T.; Cajochen, C. Non-Visual Effects of Light on Melatonin, Alertness and Cognitive Performance: Can Blue-Enriched Light Keep Us Alert? PLoS ONE 2011, 6, e16429. [Google Scholar] [CrossRef]
- Rahman, S.A.; Wright, K.P.; Lockley, S.W.; Czeisler, C.A.; Gronfier, C. Characterizing the temporal Dynamics of Melatonin and Cortisol Changes in Response to Nocturnal Light Exposure. Sci. Rep. 2019, 9, 19720. [Google Scholar] [CrossRef]
- Wright, H.R.; Lack, L.C. Effect of light wavelength on suppression and phase delay of the melatonin rhythm. Chronobiol. Int. 2001, 18, 801–808. [Google Scholar] [CrossRef]
- Monteleone, P.; Maj, M.; Fusco, M.; Orazzo, C.; Kemali, D. Physical exercise at night blunts the nocturnal increase of plasma melatonin levels in healthy humans. Life Sci. 1990, 47, 1989–1995. [Google Scholar] [CrossRef]
- Shilo, L.; Sabbah, H.; Hadari, R.; Kovatz, S.; Weinberg, U.; Dolev, S.; Dagan, Y.; Shenkman, L. The effects of coffee consumption on sleep and melatonin secretion. Sleep Med. 2002, 3, 271–273. [Google Scholar] [CrossRef]
- Kozaki, T.; Lee, S.; Nishimura, T.; Katsuura, T.; Yasukouchi, A. Effects of saliva collection using cotton swabs on melatonin enzyme immunoassay. J. Circadian Rhythm. 2011, 9, 1. [Google Scholar] [CrossRef]
- Baker, F.C.; Driver, H.S. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007, 8, 613–622. [Google Scholar] [CrossRef]
- Bhatti, P.; Mirick, D.K.; Davis, S. Racial Differences in the Association Between Night Shift Work and Melatonin Levels Among Women. Am. J. Epidemiol. 2013, 177, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, H.; Harris, R.; Dong, Y.; Su, S.; Tingen, M.; Kapuku, G.; Pollock, J.; Pollock, D.; Harshfield, G. Ethnic difference in nighttime melatonin can partially explain the ethnic difference in nighttime blood pressure: A study in European Americans and African Americans. FASEB J. 2019, 33, 533.15. [Google Scholar] [CrossRef]
- DeSantis, A.S.; Adam, E.K.; Hawkley, L.C.; Kudielka, B.M.; Cacioppo, J.T. Racial and Ethnic Differences in Diurnal Cortisol Rhythms: Are They Consistent Over Time? Psychosom. Med. 2015, 77, 6–15. [Google Scholar] [CrossRef]
- Bellastella, G.; Maiorino, M.I.; De Bellis, A.; Vietri, M.T.; Mosca, C.; Scappaticcio, L.; Pasquali, D.; Esposito, K.; Giugliano, D. Serum but not salivary cortisol levels are influenced by daily glycemic oscillations in type 2 diabetes. Endocrine 2016, 53, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J.K.; Vidafar, P.; Burns, A.C.; McGlashan, E.M.; Anderson, C.; Rajaratnam, S.M.W.; Lockley, S.W.; Cain, S.W. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc. Natl. Acad. Sci. USA 2019, 116, 12019–12024. [Google Scholar] [CrossRef]
- Borisenkov, M.F.; Popov, S.V.; Smirnov, V.V.; Martinson, E.A.; Solovieva, S.V.; Danilova, L.A.; Gubin, D.G. The Association between Melatonin-Containing Foods Consumption and Students’ Sleep-Wake Rhythm, Psychoemotional, and Anthropometric Characteristics: A Semi-Quantitative Analysis and Hypothetical Application. Nutrients 2023, 15, 3302. [Google Scholar] [CrossRef]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Dietary factors and fluctuating levels of melatonin. Food Nutr. Res. 2012, 56, 17252. [Google Scholar] [CrossRef]
- Windred, D.P.; Anderson, C.; Jeppe, K.J.; Ftouni, S.; Grant, L.K.; Nijagal, B.; Rajaratnam, S.M.W.; McConville, M.; Tull, D.; Lockley, S.W.; et al. Higher central circadian temperature amplitude is associated with greater metabolite rhythmicity in humans. Sci. Rep. 2024, 14, 16796. [Google Scholar] [CrossRef]
- Shin, M.; Carpenter, J.S.; Park, S.H.; Janiszewski, C.; Tonini, E.; McKenna, S.; Hindmarsh, G.; Iorfino, F.; Nichles, A.; Zmicerevska, N.; et al. Twenty-four-hour Skin Temperature Rhythms in Young People With Emerging Mood Disorders: Relationships With Illness Subtypes and Clinical Stage. J. Biol. Rhythm. 2025, 40, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.P.; Kim, D.W.; Fang, Y.; Kim, R.; Bohnert, A.S.B.; Sen, S.; Forger, D.B. The real-world association between digital markers of circadian disruption and mental health risks. npj Digit. Med. 2024, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.A.; Baba, K.; DeBruyne, J.P.; Davidson, A.J.; Ehlen, C.; Powell, M.; Tosini, G. Environmental circadian disruption re-writes liver circadian proteomes. Nat. Commun. 2024, 15, 5537. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J. Brain circadian clocks timing the 24h rhythms of behavior. npj Biol. Timing Sleep 2025, 2, 13. [Google Scholar] [CrossRef]
- Titman, A.; Price, V.; Hawcutt, D.; Chesters, C.; Ali, M.; Cacace, G.; Lancaster, G.A.; Peak, M.; Blair, J.C. Salivary cortisol, cortisone and serum cortisol concentrations are related to age and body mass index in healthy children and young people. Clin. Endocrinol 2020, 93, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Ashman, S.B.; Dawson, G.; Panagiotides, H.; Yamada, E.; Wilkinson, C.W. Stress hormone levels of children of depressed mothers. Dev. Psychopathol. 2002, 14, 333–349. [Google Scholar] [CrossRef]
- Pullman, R.E.; Roepke, S.E.; Duffy, J.F. Laboratory validation of an in-home method for assessing circadian phase using dim light melatonin onset (DLMO). Sleep Med. 2012, 13, 703–706. [Google Scholar] [CrossRef]
- Cutando, A.; Gómez-Moreno, G.; Arana, C.; Acuña-Castroviejo, D.; Reiter, R.J. Melatonin: Potential Functions in the Oral Cavity. J. Periodontol. 2007, 78, 1094–1102. [Google Scholar] [CrossRef]
- Meulenberg, P.M.; Hofman, J.A. Differences between concentrations of salivary cortisol and cortisone and of free cortisol and cortisone in plasma during pregnancy and postpartum. Clin. Chem. 1990, 36, 70–75. [Google Scholar] [CrossRef]
- El-Farhan, N.; Rees, D.A.; Evans, C. Measuring cortisol in serum, urine and saliva—Are our assays good enough? Ann. Clin. Biochem. 2017, 54, 308–322. [Google Scholar] [CrossRef]
- Roach, G.D.; Reid, K.J.; Ferguson, S.; Dawson, D. The relationship between the rate of melatonin excretion and sleep consolidation for locomotive engineers in natural sleep settings. J. Circadian Rhythm. 2006, 4, 8. [Google Scholar] [CrossRef][Green Version]
- Torrente-Rodríguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W.; et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2020, 2, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yin, L.; Sempionatto, J.R.; Moon, J.-M.; Teymourian, H.; Wang, J. Touch-Based Stressless Cortisol Sensing. Adv. Mater 2021, 33, e2008465. [Google Scholar] [CrossRef] [PubMed]
- Ku, M.; Kim, J.; Won, J.-E.; Kang, W.; Park, Y.-G.; Park, J.; Lee, J.-H.; Cheon, J.; Lee, H.H.; Park, J.-U. Smart, soft contact lens for wireless immunosensing of cortisol. Sci. Adv. 2020, 6, eabb2891. [Google Scholar] [CrossRef] [PubMed]
- Isherwood, C.M.; van der Veen, D.R.; Chowdhury, N.R.; Lightman, S.T.; Upton, T.J.; Skene, D.J. Simultaneous plasma and interstitial profiles of hormones and metabolites using URHYTHM: A novel ambulatory collection device. Proc. Nutr. Soc. 2024, 83, E242. [Google Scholar] [CrossRef]
- Greff, M.J.E.; Levine, J.M.; Abuzgaia, A.M.; Elzagallaai, A.A.; Rieder, M.J.; van Uum, S.H.M. Hair cortisol analysis: An update on methodological considerations and clinical applications. Clin. Biochem. 2019, 63, 1–9. [Google Scholar] [CrossRef]
- Gow, R.; Koren, G.; Rieder, M.; Van Uum, S. Hair cortisol content in patients with adrenal insufficiency on hydrocortisone replacement therapy. Clin. Endocrinol. 2011, 74, 687–693. [Google Scholar] [CrossRef]
- Mészáros, K.; Karvaly, G.; Márta, Z.; Magda, B.; Tőke, J.; Szücs, N.; Tóth, M.; Rácz, K.; Patócs, A. Diagnostic performance of a newly developed salivary cortisol and cortisone measurement using an LC–MS/MS method with simple and rapid sample preparation. J. Endocrinol. Investig. 2018, 41, 315–323. [Google Scholar] [CrossRef]
- Miller, R.; Plessow, F.; Kirschbaum, C.; Stalder, T. Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: Evaluation of salivary cortisol pulse detection in panel designs. Psychosom. Med. 2013, 75, 832–840. [Google Scholar] [CrossRef]
- Kennaway, D.J. Measuring melatonin by immunoassay. J. Pineal Res. 2020, 69, e12657. [Google Scholar] [CrossRef]
- Burgess, H.J.; Kagan, D.; Rizvydeen, M.; Swanson, L.M.; Kim, H.M. An independent comparison of the Novolytix salivary melatonin radioimmunoassay with the new Novolytix salivary melatonin enzyme-linked immunosorbent assay. J. Pineal Res. 2024, 76, e12933. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.J.; Gaudl, A.; Jaeger, S.; Stadelmann, S.; Hiemisch, A.; Kiess, W.; Willenberg, A.; Schaab, M.; von Klitzing, K.; Thiery, J.; et al. Immunoassay or LC-MS/MS for the measurement of salivary cortisol in children? Clin. Chem. Lab Med. 2016, 54, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.M.; Keevil, B.G. Endogenous glucocorticoid analysis by liquid chromatography-tandem mass spectrometry in routine clinical laboratories. J. Steroid Biochem. Mol. Biol. 2016, 162, 27–40. [Google Scholar] [CrossRef]
- Jensen, M.A.; Mortier, L.; Koh, E.; Keevil, B.; Hyttinen, S.; Hansen, Å.M. An interlaboratory comparison between similar methods for determination of melatonin, cortisol and testosterone in saliva. Scand. J. Clin. Lab Investig. 2014, 74, 454–461. [Google Scholar] [CrossRef]
- Raff, H.; Phillips, J.M. Bedtime Salivary Cortisol and Cortisone by LC-MS/MS in Healthy Adult Subjects: Evaluation of Sampling Time. J. Endocr. Soc. 2019, 3, 1631–1640. [Google Scholar] [CrossRef]
- Grassi, G.; Morelli, V.; Ceriotti, F.; Polledri, E.; Fustinoni, S.; D’Agostino, S.; Mantovani, G.; Chiodini, I.; Arosio, M. Minding the gap between cortisol levels measured with second-generation assays and current diagnostic thresholds for the diagnosis of adrenal insufficiency: A single-center experience. Hormones 2020, 19, 425–431. [Google Scholar] [CrossRef]
- van Faassen, M.; Bischoff, R.; Kema, I.P. Relationship between plasma and salivary melatonin and cortisol investigated by LC-MS/MS. Clin. Chem. Lab Med. 2017, 55, 1340–1348. [Google Scholar] [CrossRef]
- Karel, P.; Schutten, E.; van Faassen, M.; Wanschers, H.; Brouwer, R.; Mulder, A.L.; Kema, I.P.; Reichman, L.J.; Krabbe, J.G. A comparison of two LC-MS/MS methods and one radioimmunoassay for the analysis of salivary melatonin. Ann. Clin. Biochem. 2021, 58, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Oßwald, A.; Wang, R.; Beuschlein, F.; Hartmann, M.F.; Wudy, S.A.; Bidlingmaier, M.; Zopp, S.; Reincke, M.; Ritzel, K. Performance of LC-MS/MS and immunoassay based 24-h urine free cortisol in the diagnosis of Cushing’s syndrome. J. Steroid Biochem. Mol. Biol. 2019, 190, 193–197. [Google Scholar] [CrossRef]
- Yu, S.; Zou, Y.; Ma, X.; Wang, D.; Luo, W.; Tang, Y.; Mu, D.; Zhang, R.; Cheng, X.; Qiu, L. Evolution of LC-MS/MS in clinical laboratories. Clin. Chim. Acta 2024, 555, 117797. [Google Scholar] [CrossRef]
- Kennaway, D.J. The dim light melatonin onset across ages, methodologies, and sex and its relationship with morningness/eveningness. Sleep 2023, 46, zsad033. [Google Scholar] [CrossRef]
- Bormes, G.; Love, J.; Akeju, O.; Cherry, J.; Kunorozva, L.; Qadri, S.; Rahman, S.A.; Westover, B.; Winkelman, J.; Lane, J.M. Self-Directed Home-Based Dim-Light Melatonin Onset Collection: The Circadia Pilot Study. medRxiv Prepr. Serv. Health Sci. 2023. preprint. [Google Scholar] [CrossRef]
- Mandrell, B.N.; Avent, Y.; Walker, B.; Loew, M.; Tynes, B.L.; Crabtree, V.M. In-home salivary melatonin collection: Methodology for children and adolescents. Dev. Psychobiol. 2018, 60, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Atlas, M.; Genc, B.O.; Kilinç, İ.; Gurses, A.A. Relationship between Juvenile Myoclonic Epilepsy and Melatonin. Selçuk Tıp Derg. 2023, 39, 35–40. [Google Scholar]
- Esposito, S.; Laino, D.; D’Alonzo, R.; Mencarelli, A.; Di Genova, L.; Fattorusso, A.; Argentiero, A.; Mencaroni, E. Pediatric sleep disturbances and treatment with melatonin. J. Transl. Med. 2019, 17, 77. [Google Scholar] [CrossRef]
- Leu, R.M.; Beyderman, L.; Botzolakis, E.J.; Surdyka, K.; Wang, L.; Malow, B.A. Relation of Melatonin to Sleep Architecture in Children with Autism. J. Autism Dev. Disord. 2011, 41, 427–433. [Google Scholar] [CrossRef]
- Carlucci, M.; Kriščiūnas, A.; Li, H.; Gibas, P.; Koncevičius, K.; Petronis, A.; Oh, G. DiscoRhythm: An easy-to-use web application and R package for discovering rhythmicity. Bioinformatics 2020, 36, 1952–1954. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Moreira, J.P.; de Oliveira Guarnieri, L.; Passos, M.C.; Emrich, F.; Bargi-Souza, P.; Peliciari-Garcia, R.A.; Moraes, M.F.D. CircadiPy: An open-source toolkit for analyzing chronobiology time series. J. Neurosci. Methods 2024, 411, 110245. [Google Scholar] [CrossRef]
- Moškon, M. CosinorPy: A python package for cosinor-based rhythmometry. BMC Bioinform. 2020, 21, 485. [Google Scholar] [CrossRef]
- Parsons, R.; Parsons, R.; Garner, N.; Oster, H.; Rawashdeh, O. CircaCompare: A method to estimate and statistically support differences in mesor, amplitude and phase, between circadian rhythms. Bioinformatics 2020, 36, 1208–1212. [Google Scholar] [CrossRef]
| Change | Melatonin | Cortisol |
|---|---|---|
| Increase ↑ | Major: Contraceptives [45,56], Minor: Sleep deprivation [43,57], certain antidepressants [44], standing position versus sitting [58,59]. | Major: Stress [60], morning light (induces an immediate, greater than 50% elevation of cortisol levels—even after a sleepless night) [61], awakening [60], exercise [62], aging (also shifts cycle), contamination of saliva samples with blood [63], oral contraceptives (women treated with the OCP displayed a 1.7–2.2-fold increase in total plasma cortisol levels) [64]. Minor: high protein meals [60] and smoking before saliva collection [63]. |
| Decrease ↓ | Major: Light (with light at the blue end of the spectrum having the biggest impact) [65,66,67] and certain beta blockers [46] Minor: Nonsteroidal anti-inflammatory drugs [18], nocturnal physical activity [68], caffeine (consumed a few hours before measurement) [69], saliva collection with cotton swabs compared with that from passive saliva collection [70], possibly reduced melatonin secretion in the luteal phase in women [71]. | Minor: Possibly reduced amplitude of cortisol in the luteal phase in women [71]. |
| Masking ↑ and ↓ | Major: Ethnicity, ancestry and genetics: Caucasian participants were found to have higher daily melatonin levels than Asians [72], African Americans excreted less 6-sulphatoxymelatonin compared to European Americans [73]. Nevertheless, DLMO was not found to vary between races [45]. | Major: Ethnicity, ancestry: Africans and Hispanics/Latinos have flatter diurnal cortisol slopes [74]. Minor: In patients with type 2 diabetes, morning serum cortisol was shown to depend on morning fasting glycemia, while salivary cortisol did not [75]. |
| LC–MS/MS | Immunoassay |
|---|---|
| Sample collection | Assay Selection |
| Clearly define time of sample collection relative to expected DLMO (e.g., every 30–60 min, from 18:00 to 00:00). Clearly define time of sample collection relative to expected CAR (immediately upon waking and every additional 15–45 min). Store samples at −80 °C immediately after collection. Use appropriate sample collection tools (e.g., salivettes). | Validated commercial kits should be used. Kit sensitivity: <0.5 pg/mL for melatonin, <1 ng/mL for cortisol. Kit should report cross-reactivity profile and should be validated for studied population. Kit should have <10% intra-and inter-assay coefficient of variation. |
| Analytical procedure | Sample collection |
| Use solid-phase extraction or protein precipitation method for interfering compounds removal. Use stable isotope-labeled internal standards (e.g., D4-cortisol, D4-melatonin) for quantification. Reverse-phase C18 columns are commonly used for chromatography. Use multiple reaction monitoring (MRM) with high specificity transitions (e.g., melatonin m/z 233 → 174) in MS parameters. Limit of quantification (LOQ) should be ≤1 pg/mL for melatonin and ≤0.5 ng/mL for cortisol in saliva. | Avoid food and/or drinks 30 min before saliva sampling. Collect samples under dim light for DLMO assessment. Store samples at −20 °C or lower, and analyze within a defined time window (e.g., <3 months if not frozen at −80 °C). |
| Quality control | Normalization and interpretation |
| Prepare calibration curves using matrix-matched calibrators covering the full physiological range. Include low, mid and high concentration controls in every batch of samples. | CAR needs to be normalized to the baseline. DLMO threshold must be defined and consistent (e.g., 3 pg/mL of melatonin in saliva). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skubic, C.; Zevnik, U.; Nahtigal, K.; Dolenc Grošelj, L.; Rozman, D. Circadian Biomarkers in Humans: Methodological Insights into the Detection of Melatonin and Cortisol. Biomolecules 2025, 15, 1006. https://doi.org/10.3390/biom15071006
Skubic C, Zevnik U, Nahtigal K, Dolenc Grošelj L, Rozman D. Circadian Biomarkers in Humans: Methodological Insights into the Detection of Melatonin and Cortisol. Biomolecules. 2025; 15(7):1006. https://doi.org/10.3390/biom15071006
Chicago/Turabian StyleSkubic, Cene, Urša Zevnik, Katarina Nahtigal, Leja Dolenc Grošelj, and Damjana Rozman. 2025. "Circadian Biomarkers in Humans: Methodological Insights into the Detection of Melatonin and Cortisol" Biomolecules 15, no. 7: 1006. https://doi.org/10.3390/biom15071006
APA StyleSkubic, C., Zevnik, U., Nahtigal, K., Dolenc Grošelj, L., & Rozman, D. (2025). Circadian Biomarkers in Humans: Methodological Insights into the Detection of Melatonin and Cortisol. Biomolecules, 15(7), 1006. https://doi.org/10.3390/biom15071006






