Ankyrin-G and Its Binding Partners in Neurons: Orchestrating the Molecular Structure of the Axon Initial Segment
Abstract
1. Introduction
2. AIS Proteome
| Ran King | Previously Reported AIS Protein Names (Shadow: High Confidence AIS Proteins, White: Lower Confidence AIS Proteins) | Gene Names | AIS Localization | References |
|---|---|---|---|---|
| 1 | Tripartite motif-containing protein 46 | Trim46 | ++ | [22] |
| 2 | Spectrin beta chain, non-erythrocytic 4 isoform sigma6 | Sptbn4 | ++ | [23] |
| 3 | Ankyrin-3 (Ankyrin-G) | Ank3 | ++ | [12] |
| 4 | Sodium channel protein type 2 subunit alpha (voltage-gated sodium channel subunit alpha Nav1.2) | Scn2a | ++ | [12] |
| 5 | Neurofascin | Nfasc | + | [24] |
| 6 | WD repeat-containing protein 7 (TGF-beta resistance-associated protein TRAG) | Wdr7 | + | [17] |
| 7 | Tenascin-R (TN-R) | Tnr | + | [25] |
| 8 | Scribble planar cell polarity protein | Scrib | + | [17] |
| 9 | WD repeat domain 47 | Wdr47 | + | [17] |
| 16 | Band 4.1 (erythrocyte membrane protein band 4.1) | Epb41 Epb4.1 | para-AIS | [26] |
| 18 | Versican core protein (chondroitin sulfate proteoglycan core protein 2) | Vcan Cspg2 | + | [27] |
| 20 | Lissencephaly-1 protein (LIS-1) | Lis-1 | + | [28] |
| 23 | Voltage-dependent P/Q-type calcium channel subunit alpha-1A | Cacna1a | Only physiologically certified | [29] |
| 29 | Brevican core protein | Bcan Behab | + | [24] |
| 34 | Sodium channel subunit beta-2 | Scn2b | Other isoforms expressed | [30,31] |
| 38 | Glutamate decarboxylase 2 (GAD-65) | Gad2 Gad65 | + | [32] |
| 86 | Potassium voltage-gated channel subfamily B member 1 (delayed rectifier potassium channel 1) (DRK1) (voltage-gated potassium channel subunit Kv2.1) | Kcnb1 | + | [33] |
| 165 | PH and SEC7 domain-containing protein 1 (exchange factor for ADP-ribosylation factor guanine nucleotide factor 6) (exchange factor for ARF6) | Psd Efa6 | + | [34] |
| 183 | Sodium/potassium-transporting ATPase subunit alpha-1 (Na(+)/K(+) ATPase alpha-1 subunit) (sodium pump subunit alpha-1) | Atp1a1 | + | [17] |
| 200 | Voltage-dependent N-type calcium channel subunit alpha-1B (brain calcium channel III) (BIII) (calcium channel, L type, alpha-1 polypeptide isoform 5) (voltage-gated calcium channel subunit alpha Cav2.2) | Cacna1b | Only physiologically + | [29] |
| 207 | Potassium voltage-gated channel subfamily KQT member 2 (KQT-like 2) (potassium channel subunit alpha KvLQT2) (voltage-gated potassium channel subunit Kv7.2) | Kcnq2 | + | [35] |
| 228 | Nuclear distribution protein nude-like 1 (Protein Nudel) | Ndel1 | + | [28] |
| 246 | Voltage-gated potassium channel subunit beta-2 (K (+) channel subunit beta-2) (Kv-beta-2) | Kcnab2 | + | [36] |
| 269 | Gamma-aminobutyric acid receptor subunit beta-1 (GABA (A) receptor subunit beta-1) | Gabrb1 | Other isoforms + | [37] |
| 355 | Casein kinase II subunit alpha (CK II alpha) | Csnk2a1 | + | [38] |
| 371 | F-actin monooxygenase | Mical3 | + | [16] |
| 455 | Rho GTPase-activating protein 21 | Arhgap21 | + | [16] |
| 470 | Neuronal cell adhesion molecule (NrCAM) | Nrcam | + | [24] |
| 507 | Glycogen synthase kinase-3 beta (GSK-3 beta) (Factor A) (FA) (serine/threonine-protein kinase GSK3B) | Gsk3b | n/a | [39] |
| 534 | Gamma-aminobutyric acid receptor subunit beta-3 (GABA (A) receptor subunit beta-3) | Gabrb3 Gabrb-3 | Other isoforms + | [37] |
| 543 | Inositol 1,4,5-trisphosphate receptor type 1 (IP3 receptor isoform 1) (IP-3-R) (IP3R 1) (InsP3R1) (type 1 inositol 1,4,5-trisphosphate receptor) (type 1 InsP3 receptor) | Itpr1 Insp3r | + | [40] |
| 585 | Leucine-rich repeat-containing protein 7 (Densin-180) (Densin) (Protein LAP1) | Lrrc7 Lap1 | + | [41] |
| 663 | Microtubule-actin cross-linking factor 1 (actin cross-linking family 7) | Macf1 | + | [16] |
| 676 | Neuroligin-2 | Nlgn2 | + | [42] |
| 699 | Potassium voltage-gated channel subfamily A member 2 (RAK) (RBK2) (RCK5) (voltage-gated potassium channel subunit Kv1.2) | Kcna2 | + | [43] |
| 709 | Gamma-aminobutyric acid receptor subunit gamma-2 (GABA(A) receptor subunit gamma-2) | Gabrg2 | Other isoforms + | [37] |
| 748 | Gamma-aminobutyric acid receptor subunit alpha-3 (GABA(A) receptor subunit alpha-3) | Gabra3 | + | [37] |
| 765 | Tubulin alpha-4A chain (alpha-tubulin 4) (tubulin alpha-4 chain) | Tuba4a | n/a | [44] |
| 768 | Casein kinase 2 alpha 2 (casein kinase 2, alpha prime polypeptide) | Csnk2a2 | n/a | [38] |
| 800 | Spectrin alpha chain, non-erythrocytic 1 (alpha-II spectrin) (fodrin alpha chain) | Sptan1 | + | [45] |
| 827 | Septin-7 (CDC10 protein homolog) | Septin7 | + | [16] |
| 862 | Calcium/calmodulin-dependent protein kinase type II subunit alpha | Camk2a | n/a | [46] |
| 874 | Disks large homolog 2 (channel-associated protein of synapse-110) (Chapsyn-110) (postsynaptic density protein PSD-93) | Dlg2 Dlgh2 | + | [47] |
| 985 | Synaptopodin | Synpo | + | [48,49] |
| 1000 | Myc box-dependent-interacting protein 1 (Amphiphysin II) (Amphiphysin-like protein) (bridging integrator 1) | Amph2 | + | [50] |
| 1050 | ADAM metallopeptidase domain 22 | Adam22 | + | [51] |
| 1058 | Septin-11 | Septin11 | + | [16] |
| 1063 | Calcium/calmodulin-dependent protein kinase type II subunit beta | Camk2b | n/a | [46] |
| 1068 | Microtubule-associated protein 6 (MAP-6) (145-kDa STOP) (STOP145) (stable tubule-only polypeptide) (STOP) | Map6 Mtap6 | + | [52] |
| 1271 | Microtubule-associated protein 1A (MAP-1A) [cleaved into MAP1A heavy chain and MAP1 light chain LC2] | Map1a Mtap1a | + | [53] |
| 1281 | Microtubule-associated protein RP/EB family member 1 (APC-binding protein EB1) (end-binding protein 1) (EB1) | Mapre1 | + | [54] |
| 1307 | Microtubule-associated protein RP/EB family member 3 (EB1 protein family member 3) (EB3) | Mapre3 | + | [54] |
| 1324 | Kinesin-1 heavy chain (conventional kinesin heavy chain) (ubiquitous kinesin heavy chain) (UKHC) | Kif5b Khc | + | [55] |
| 1371 | Contactin-1 (neural cell surface protein F3) | Cntn1 | + | [56] |
| 1396 | Tubulin beta-3 chain (neuron-specific class III beta-tubulin) | Tubb3 | + | [57] |
3. Ankyrin-G
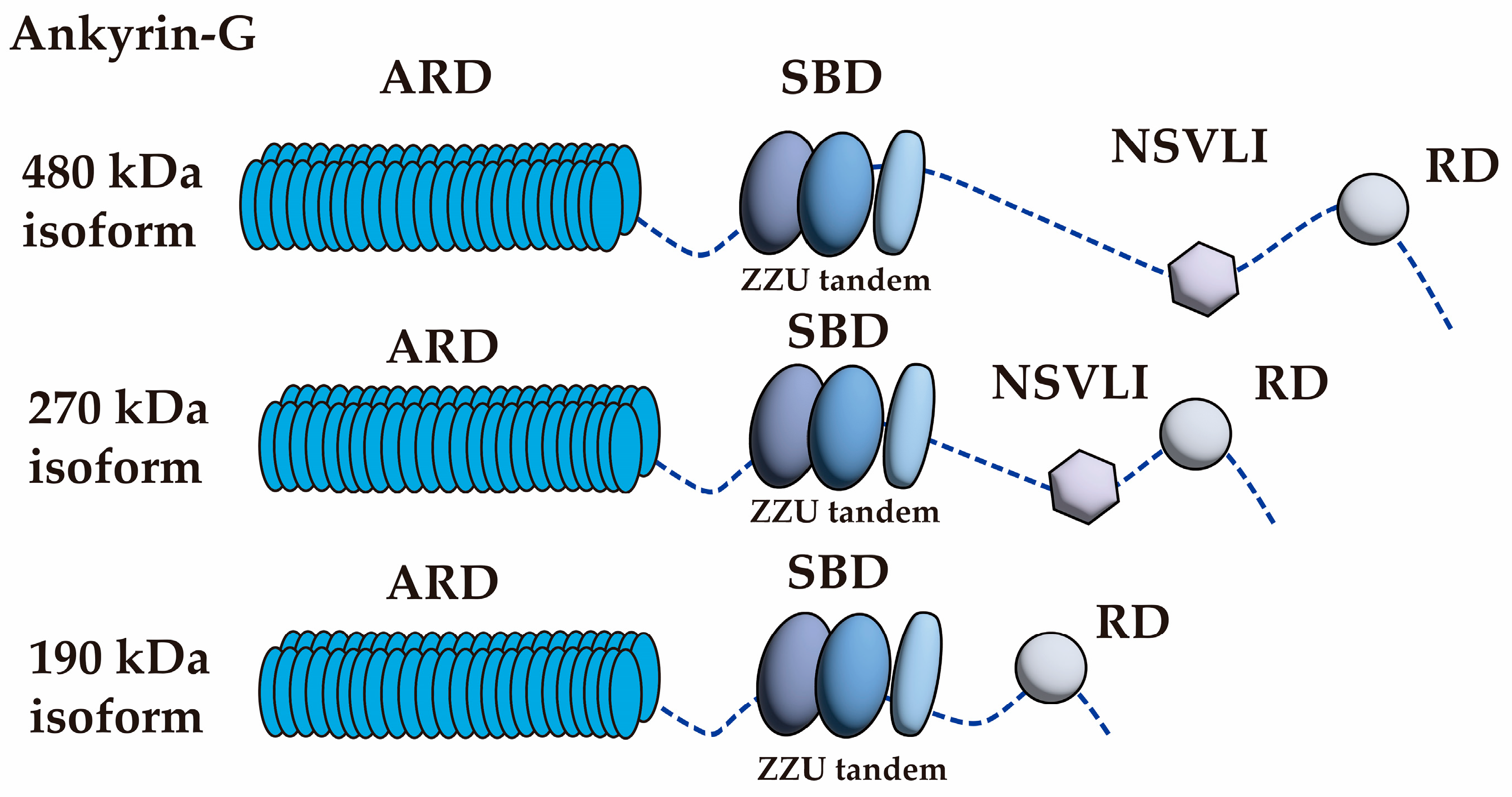
| AIS Localized Protein Names | Binding Domain of Ankyrin-G | Method | References |
|---|---|---|---|
| Nav1.2 and Nav1.6 | N/A | They determined the AIS localization signal at the cytoplasmic II–III region of Nav1.2 | [70] |
| ARD | Pull-down assay | [71] | |
| Kv7.2 and Kv7.3 (KCNQ2/3) | Reduced rate of fluorescence recovery after photobleaching (FRAP) | [72] | |
| Neurofascin | Co-immunoprecipitation and in vitro ankyrin-binding assays | [73] | |
| Neurofascin, NrCAM | Immunoblotting analysis and immunofluorescence staining | [74] | |
| KIF5 (KIF5B) | Pull-down assay | [55] | |
| βIV spectrin | SBD SBD (Zu5) | Co-immunoprecipitation | [23] |
| Co-immunoprecipitation | [75] | ||
| Surface plasmon resonance | [76] | ||
| Ndel1 | NSVLI | Isothermal Titration Calorimetry (ITC) assays | [77] |
| GABARAP | Proximity ligation assay | [18] | |
| IQCJ-SCHIP-1 | ARD | Pull-down assay | [60] |
| TRAAK (K2P4.1) | N/A | Single-molecule pull-down assay Co-immunoprecipitation | [61] [62] |
| EB1/EB3 | RD | Pull-down assay | [54] |
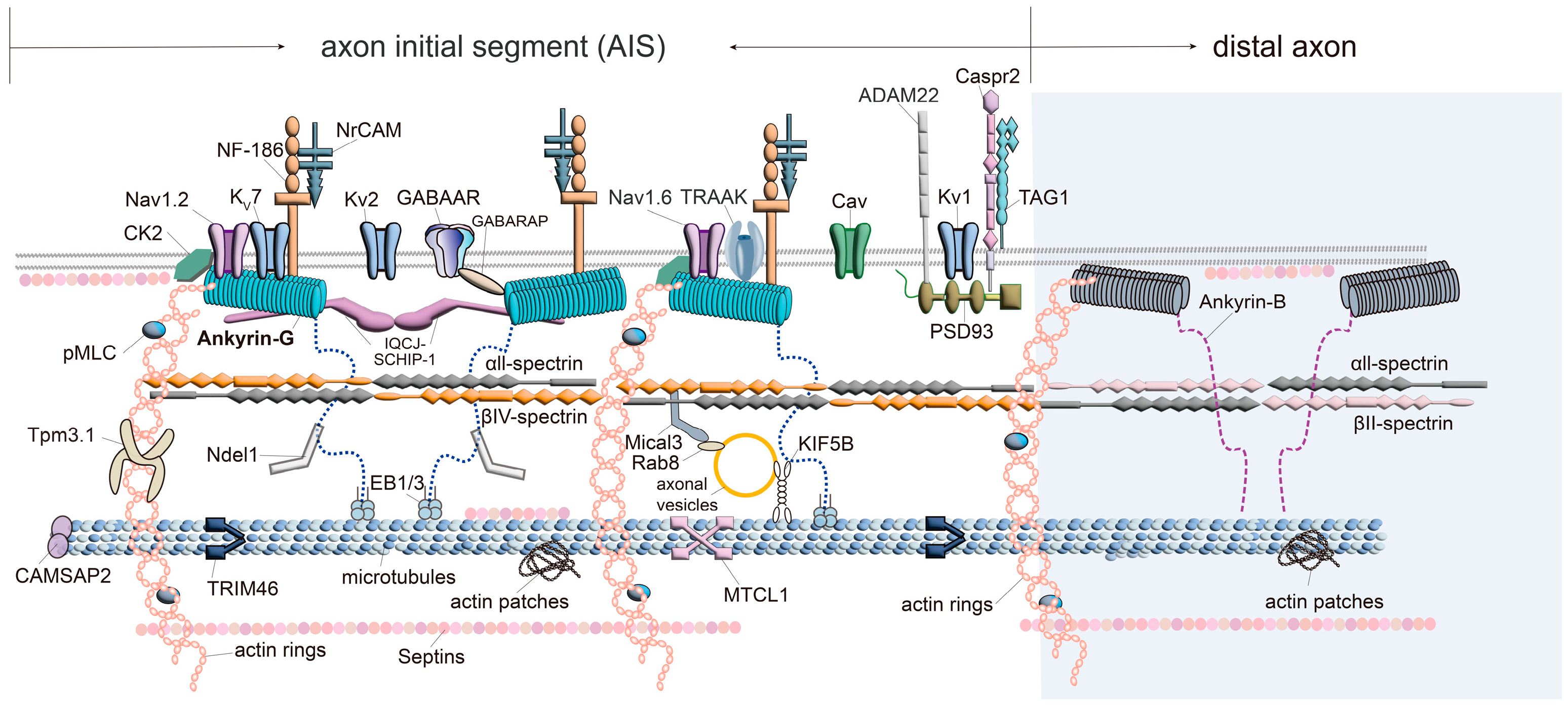
4. Ion Channels/Transporters
5. Spectrins
6. Actin Cytoskeleton and the Related Proteins
7. Microtubule-Associated Proteins
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| 3D-STORM | three-dimensional stochastic optical reconstruction microscopy |
| ADAM22 | a disintegrin and metallopeptidase domain 22 |
| AIS | axon initial segment |
| ARD | ankyrin repeat domain |
| CAMs | cell adhesion molecules |
| CAMSAP2 | calmodulin-regulated spectrin-associated protein 2 |
| Caspr2 | contactin-associated protein-like 2 |
| Cav | voltage-gated calcium channel |
| CK2 | casein kinase 2 |
| EB1/EB3 | end-binding proteins 1 and 3 |
| GABAAR | gamma-aminobutyric acid type A receptor |
| GABARAP | gamma-aminobutyric acid receptor associated protein |
| IQCJ-SCHIP-1 | IQ motif-containing J-Schwannomin-Interacting Protein 1 |
| KIF5B | Kinesin family member 5B |
| Kv | voltage-gated potassium channels |
| MAPs | microtubule-associated proteins |
| Mical3 | molecule interacting with CasL 3 |
| MLC | myosin light chain |
| MTCL1 | microtubule cross-linking factor 1 |
| Nav | voltage-gated sodium channels |
| Ndel1 | nuclear distribution element-like 1 |
| NF-186 | neurofascin-186 |
| NrCAM | neuronal cell adhesion molecule |
| NSVLI | neuron-specific variable long insert |
| PAEZ | proximal axon enrichment zone |
| PSD-93 | postsynaptic density 93 |
| RD | regulatory domain |
| SRD | spectrin-binding domain |
| Tag1 | transient axonal glycoprotein 1 |
References
- Huang, C.Y.; Rasband, M.N. Axon initial segments: Structure, function, and disease. Ann. N. Y. Acad. Sci. 2018, 1420, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, C. The Axon Initial Segment: An Updated Viewpoint. J. Neurosci. 2018, 38, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.M.; Bender, K.J. Axon initial segment structure and function in health and disease. Physiol. Rev. 2025, 105, 765–801. [Google Scholar] [CrossRef]
- Freal, A.; Hoogenraad, C.C. The dynamic axon initial segment: From neuronal polarity to network homeostasis. Neuron 2025, 113, 649–669. [Google Scholar] [CrossRef]
- Jones, S.L.; Svitkina, T.M. Axon Initial Segment Cytoskeleton: Architecture, Development, and Role in Neuron Polarity. Neural Plast. 2016, 2016, 6808293. [Google Scholar] [CrossRef] [PubMed]
- Rasband, M.N. The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 2010, 11, 552–562. [Google Scholar] [CrossRef]
- Nakada, C.; Ritchie, K.; Oba, Y.; Nakamura, M.; Hotta, Y.; Iino, R.; Kasai, R.S.; Yamaguchi, K.; Fujiwara, T.; Kusumi, A. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat. Cell Biol. 2003, 5, 626–632. [Google Scholar] [CrossRef]
- Smith, K.R.; Penzes, P. Ankyrins: Roles in synaptic biology and pathology. Mol. Cell. Neurosci. 2018, 91, 131–139. [Google Scholar] [CrossRef]
- Yoon, S.; Piguel, N.H.; Penzes, P. Roles and mechanisms of ankyrin-G in neuropsychiatric disorders. Exp. Mol. Med. 2022, 54, 867–877. [Google Scholar] [CrossRef]
- Bennett, V.; Lorenzo, D.N. Spectrin- and ankyrin-based membrane domains and the evolution of vertebrates. Curr. Top. Membr. 2013, 72, 1–37. [Google Scholar] [CrossRef]
- Jenkins, S.M.; Bennett, V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J. Cell Biol. 2001, 155, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Lambert, S.; Malen, P.L.; Carpenter, S.; Boland, L.M.; Bennett, V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 1998, 143, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, M.; Otani, Y.; Miyajima, H. Pathophysiological Roles of Abnormal Axon Initial Segments in Neurodevelopmental Disorders. Cells 2021, 10, 2110. [Google Scholar] [CrossRef]
- Garrido, J.J. Contribution of Axon Initial Segment Structure and Channels to Brain Pathology. Cells 2023, 12, 1210. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Kuba, H. Structural and Functional Plasticity at the Axon Initial Segment. Front. Cell. Neurosci. 2016, 10, 250. [Google Scholar] [CrossRef]
- Hamdan, H.; Lim, B.C.; Torii, T.; Joshi, A.; Konning, M.; Smith, C.; Palmer, D.J.; Ng, P.; Leterrier, C.; Oses-Prieto, J.A.; et al. Mapping axon initial segment structure and function by multiplexed proximity biotinylation. Nat. Commun. 2020, 11, 100. [Google Scholar] [CrossRef]
- Zhang, W.; Fu, Y.; Peng, L.; Ogawa, Y.; Ding, X.; Rasband, A.; Zhou, X.; Shelly, M.; Rasband, M.N.; Zou, P. Immunoproximity biotinylation reveals the axon initial segment proteome. Nat. Commun. 2023, 14, 8201. [Google Scholar] [CrossRef]
- Tseng, W.C.; Jenkins, P.M.; Tanaka, M.; Mooney, R.; Bennett, V. Giant ankyrin-G stabilizes somatodendritic GABAergic synapses through opposing endocytosis of GABAA receptors. Proc. Natl. Acad. Sci. USA 2015, 112, 1214–1219. [Google Scholar] [CrossRef]
- Koroll, M.; Rathjen, F.G.; Volkmer, H. The neural cell recognition molecule neurofascin interacts with syntenin-1 but not with syntenin-2, both of which reveal self-associating activity. J. Biol. Chem. 2001, 276, 10646–10654. [Google Scholar] [CrossRef]
- Gunn-Moore, F.J.; Hill, M.; Davey, F.; Herron, L.R.; Tait, S.; Sherman, D.; Brophy, P.J. A functional FERM domain binding motif in neurofascin. Mol. Cell. Neurosci. 2006, 33, 441–446. [Google Scholar] [CrossRef]
- Davey, F.; Hill, M.; Falk, J.; Sans, N.; Gunn-Moore, F.J. Synapse associated protein 102 is a novel binding partner to the cytoplasmic terminus of neurone-glial related cell adhesion molecule. J. Neurochem. 2005, 94, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- van Beuningen, S.F.B.; Will, L.; Harterink, M.; Chazeau, A.; van Battum, E.Y.; Frias, C.P.; Franker, M.A.M.; Katrukha, E.A.; Stucchi, R.; Vocking, K.; et al. TRIM46 Controls Neuronal Polarity and Axon Specification by Driving the Formation of Parallel Microtubule Arrays. Neuron 2015, 88, 1208–1226. [Google Scholar] [CrossRef] [PubMed]
- Komada, M.; Soriano, P. [Beta]IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J. Cell Biol. 2002, 156, 337–348. [Google Scholar] [CrossRef]
- Hedstrom, K.L.; Xu, X.; Ogawa, Y.; Frischknecht, R.; Seidenbecher, C.I.; Shrager, P.; Rasband, M.N. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J. Cell Biol. 2007, 178, 875–886. [Google Scholar] [CrossRef]
- Bruckner, G.; Szeoke, S.; Pavlica, S.; Grosche, J.; Kacza, J. Axon initial segment ensheathed by extracellular matrix in perineuronal nets. Neuroscience 2006, 138, 365–375. [Google Scholar] [CrossRef]
- Duflocq, A.; Chareyre, F.; Giovannini, M.; Couraud, F.; Davenne, M. Characterization of the axon initial segment (AIS) of motor neurons and identification of a para-AIS and a juxtapara-AIS, organized by protein 4.1B. BMC Biol. 2011, 9, 66. [Google Scholar] [CrossRef]
- Susuki, K.; Chang, K.J.; Zollinger, D.R.; Liu, Y.; Ogawa, Y.; Eshed-Eisenbach, Y.; Dours-Zimmermann, M.T.; Oses-Prieto, J.A.; Burlingame, A.L.; Seidenbecher, C.I.; et al. Three mechanisms assemble central nervous system nodes of Ranvier. Neuron 2013, 78, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, M.; van de Willige, D.; Freal, A.; Chazeau, A.; Franker, M.A.; Hofenk, J.; Rodrigues, R.J.; Kapitein, L.C.; Akhmanova, A.; Jaarsma, D.; et al. Dynein Regulator NDEL1 Controls Polarized Cargo Transport at the Axon Initial Segment. Neuron 2016, 89, 461–471. [Google Scholar] [CrossRef]
- Bender, K.J.; Trussell, L.O. The physiology of the axon initial segment. Annu. Rev. Neurosci. 2012, 35, 249–265. [Google Scholar] [CrossRef]
- Buffington, S.A.; Rasband, M.N. Na+Channel-Dependent Recruitment of Navβ4 to Axon Initial Segments and Nodes of Ranvier. J. Neurosci. 2013, 33, 6191–6202. [Google Scholar] [CrossRef]
- Wimmer, V.C.; Harty, R.C.; Richards, K.L.; Phillips, A.M.; Miyazaki, H.; Nukina, N.; Petrou, S. Sodium channel beta1 subunit localizes to axon initial segments of excitatory and inhibitory neurons and shows regional heterogeneity in mouse brain. J. Comp. Neurol. 2015, 523, 814–830. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Eisinger, B.; Gammie, S.C. Characterization of GABAergic neurons in the mouse lateral septum: A double fluorescence in situ hybridization and immunohistochemical study using tyramide signal amplification. PLoS ONE 2013, 8, e73750. [Google Scholar] [CrossRef] [PubMed]
- Sarmiere, P.D.; Weigle, C.M.; Tamkun, M.M. The Kv2.1 K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ. BMC Neurosci. 2008, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Eva, R.; Koseki, H.; Kanamarlapudi, V.; Fawcett, J.W. EFA6 regulates selective polarised transport and axon regeneration from the axon initial segment. J. Cell Sci. 2017, 130, 3663–3675. [Google Scholar] [CrossRef]
- Chung, H.J.; Jan, Y.N.; Jan, L.Y. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc. Natl. Acad. Sci. USA 2006, 103, 8870–8875. [Google Scholar] [CrossRef]
- Zhang, W.; Palfini, V.L.; Wu, Y.; Ding, X.; Melton, A.J.; Gao, Y.; Ogawa, Y.; Rasband, M.N. A hierarchy of PDZ domain scaffolding proteins clusters the Kv1 K(+) channel protein complex at the axon initial segment. Sci. Adv. 2025, 11, eadv1281. [Google Scholar] [CrossRef]
- Gao, Y.; Heldt, S.A. Enrichment of GABAA Receptor alpha-Subunits on the Axonal Initial Segment Shows Regional Differences. Front. Cell. Neurosci. 2016, 10, 39. [Google Scholar] [CrossRef]
- Brechet, A.; Fache, M.P.; Brachet, A.; Ferracci, G.; Baude, A.; Irondelle, M.; Pereira, S.; Leterrier, C.; Dargent, B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J. Cell Biol. 2008, 183, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.; Del Puerto, A.; Puime, A.; Sanchez-Ponce, D.; Fronzaroli-Molinieres, L.; Pallas-Bazarra, N.; Carlier, E.; Giraud, P.; Debanne, D.; Wandosell, F.; et al. GSK3 and beta-catenin determines functional expression of sodium channels at the axon initial segment. Cell. Mol. Life Sci. 2013, 70, 105–120. [Google Scholar] [CrossRef]
- Gomez, L.C.; Kawaguchi, S.-y.; Collin, T.; Jalil, A.; Gomez, M.d.P.; Nasi, E.; Marty, A.; Llano, I. Influence of spatially segregated IP3-producing pathways on spike generation and transmitter release in Purkinje cell axons. Proc. Natl. Acad. Sci. USA 2020, 117, 11097–11108. [Google Scholar] [CrossRef]
- Apperson, M.L.; Moon, I.S.; Kennedy, M.B. Characterization of densin-180, a new brain-specific synaptic protein of the O-sialoglycoprotein family. J. Neurosci. 1996, 16, 6839–6852. [Google Scholar] [CrossRef] [PubMed]
- Panzanelli, P.; Gunn, B.G.; Schlatter, M.C.; Benke, D.; Tyagarajan, S.K.; Scheiffele, P.; Belelli, D.; Lambert, J.J.; Rudolph, U.; Fritschy, J.M. Distinct mechanisms regulate GABAA receptor and gephyrin clustering at perisomatic and axo-axonic synapses on CA1 pyramidal cells. J. Physiol. 2011, 589, 4959–4980. [Google Scholar] [CrossRef] [PubMed]
- Lorincz, A.; Nusser, Z. Cell-type-dependent molecular composition of the axon initial segment. J. Neurosci. 2008, 28, 14329–14340. [Google Scholar] [CrossRef]
- Leterrier, C.; Potier, J.; Caillol, G.; Debarnot, C.; Rueda Boroni, F.; Dargent, B. Nanoscale Architecture of the Axon Initial Segment Reveals an Organized and Robust Scaffold. Cell Rep. 2015, 13, 2781–2793. [Google Scholar] [CrossRef]
- Huang, C.Y.; Zhang, C.; Ho, T.S.; Oses-Prieto, J.; Burlingame, A.L.; Lalonde, J.; Noebels, J.L.; Leterrier, C.; Rasband, M.N. alphaII Spectrin Forms a Periodic Cytoskeleton at the Axon Initial Segment and Is Required for Nervous System Function. J. Neurosci. 2017, 37, 11311–11322. [Google Scholar] [CrossRef]
- Hund, T.J.; Koval, O.M.; Li, J.; Wright, P.J.; Qian, L.; Snyder, J.S.; Gudmundsson, H.; Kline, C.F.; Davidson, N.P.; Cardona, N.; et al. A beta(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J. Clin. Investig. 2010, 120, 3508–3519. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Horresh, I.; Trimmer, J.S.; Bredt, D.S.; Peles, E.; Rasband, M.N. Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2. J. Neurosci. 2008, 28, 5731–5739. [Google Scholar] [CrossRef]
- Schluter, A.; Del Turco, D.; Deller, T.; Gutzmann, A.; Schultz, C.; Engelhardt, M. Structural Plasticity of Synaptopodin in the Axon Initial Segment during Visual Cortex Development. Cereb. Cortex 2017, 27, 4662–4675. [Google Scholar] [CrossRef]
- Sanchez-Ponce, D.; Blazquez-Llorca, L.; DeFelipe, J.; Garrido, J.J.; Munoz, A. Colocalization of -actinin and Synaptopodin in the Pyramidal Cell Axon Initial Segment. Cereb. Cortex 2011, 22, 1648–1661. [Google Scholar] [CrossRef]
- Butler, M.H.; David, C.; Ochoa, G.C.; Freyberg, Z.; Daniell, L.; Grabs, D.; Cremona, O.; De Camilli, P. Amphiphysin II (SH3P9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of Ranvier in brain and around T tubules in skeletal muscle. J. Cell Biol. 1997, 137, 1355–1367. [Google Scholar] [CrossRef]
- Ogawa, Y.; Oses-Prieto, J.; Kim, M.Y.; Horresh, I.; Peles, E.; Burlingame, A.L.; Trimmer, J.S.; Meijer, D.; Rasband, M.N. ADAM22, a Kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons. J. Neurosci. 2010, 30, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Tortosa, E.; Adolfs, Y.; Fukata, M.; Pasterkamp, R.J.; Kapitein, L.C.; Hoogenraad, C.C. Dynamic Palmitoylation Targets MAP6 to the Axon to Promote Microtubule Stabilization during Neuronal Polarization. Neuron 2017, 94, 809–825.e7. [Google Scholar] [CrossRef] [PubMed]
- Galiano, M.R.; Jha, S.; Ho, T.S.; Zhang, C.; Ogawa, Y.; Chang, K.J.; Stankewich, M.C.; Mohler, P.J.; Rasband, M.N. A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell 2012, 149, 1125–1139. [Google Scholar] [CrossRef]
- Leterrier, C.; Vacher, H.; Fache, M.P.; d’Ortoli, S.A.; Castets, F.; Autillo-Touati, A.; Dargent, B. End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proc. Natl. Acad. Sci. USA 2011, 108, 8826–8831. [Google Scholar] [CrossRef]
- Barry, J.; Gu, Y.; Jukkola, P.; O’Neill, B.; Gu, H.; Mohler, P.J.; Rajamani, K.T.; Gu, C. Ankyrin-G directly binds to kinesin-1 to transport voltage-gated Na+ channels into axons. Dev. Cell 2014, 28, 117–131. [Google Scholar] [CrossRef]
- Ogawa, Y.; Lim, B.C.; George, S.; Oses-Prieto, J.A.; Rasband, J.M.; Eshed-Eisenbach, Y.; Hamdan, H.; Nair, S.; Boato, F.; Peles, E.; et al. Antibody-directed extracellular proximity biotinylation reveals that Contactin-1 regulates axo-axonic innervation of axon initial segments. Nat. Commun. 2023, 14, 6797. [Google Scholar] [CrossRef]
- Radwitz, J.; Hausrat, T.J.; Heisler, F.F.; Janiesch, P.C.; Pechmann, Y.; Rubhausen, M.; Kneussel, M. Tubb3 expression levels are sensitive to neuronal activity changes and determine microtubule growth and kinesin-mediated transport. Cell. Mol. Life Sci. 2022, 79, 575. [Google Scholar] [CrossRef] [PubMed]
- Pinatel, D.; Hivert, B.; Saint-Martin, M.; Noraz, N.; Savvaki, M.; Karagogeos, D.; Faivre-Sarrailh, C. The Kv1-associated molecules TAG-1 and Caspr2 are selectively targeted to the axon initial segment in hippocampal neurons. J. Cell Sci. 2017, 130, 2209–2220. [Google Scholar] [CrossRef]
- Hivert, B.; Marien, L.; Agbam, K.N.; Faivre-Sarrailh, C. ADAM22 and ADAM23 modulate the targeting of the Kv1 channel-associated protein LGI1 to the axon initial segment. J. Cell Sci. 2019, 132, jcs219774. [Google Scholar] [CrossRef]
- Martin, P.M.; Carnaud, M.; Garcia del Cano, G.; Irondelle, M.; Irinopoulou, T.; Girault, J.A.; Dargent, B.; Goutebroze, L. Schwannomin-interacting protein-1 isoform IQCJ-SCHIP-1 is a late component of nodes of Ranvier and axon initial segments. J. Neurosci. 2008, 28, 6111–6117. [Google Scholar] [CrossRef]
- Luque-Fernandez, V.; Vanspauwen, S.K.; Landra-Willm, A.; Arvedsen, E.; Besquent, M.; Sandoz, G.; Rasmussen, H.B. An ankyrin G-binding motif mediates TRAAK periodic localization at axon initial segments of hippocampal pyramidal neurons. Proc. Natl. Acad. Sci. USA 2024, 121, e2310120121. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, G., Jr.; Wu, Y.; Ogawa, Y.; Ding, X.; Rasband, M.N. An evolutionarily conserved AnkyrinG-dependent motif clusters axonal K2P K+ channels. J. Cell Biol. 2024, 223, e202401140. [Google Scholar] [CrossRef]
- Michaely, P.; Tomchick, D.R.; Machius, M.; Anderson, R.G. Crystal structure of a 12 ANK repeat stack from human ankyrinR. EMBO J. 2002, 21, 6387–6396. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, C.; Ye, F.; Wei, Z.; Zhang, M. Structure of the ZU5-ZU5-UPA-DD tandem of ankyrin-B reveals interaction surfaces necessary for ankyrin function. Proc. Natl. Acad. Sci. USA 2012, 109, 4822–4827. [Google Scholar] [CrossRef]
- Wang, R.; Wei, Z.; Jin, H.; Wu, H.; Yu, C.; Wen, W.; Chan, L.N.; Wen, Z.; Zhang, M. Autoinhibition of UNC5b revealed by the cytoplasmic domain structure of the receptor. Mol. Cell 2009, 33, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Kopeikina, K.J.; Fawcett-Patel, J.M.; Leaderbrand, K.; Gao, R.; Schurmann, B.; Myczek, K.; Radulovic, J.; Swanson, G.T.; Penzes, P. Psychiatric risk factor ANK3/ankyrin-G nanodomains regulate the structure and function of glutamatergic synapses. Neuron 2014, 84, 399–415. [Google Scholar] [CrossRef]
- Zhang, X.; Bennett, V. Restriction of 480/270-kD ankyrin G to axon proximal segments requires multiple ankyrin G-specific domains. J. Cell Biol. 1998, 142, 1571–1581. [Google Scholar] [CrossRef]
- Rueckert, E.H.; Barker, D.; Ruderfer, D.; Bergen, S.E.; O’Dushlaine, C.; Luce, C.J.; Sheridan, S.D.; Theriault, K.M.; Chambert, K.; Moran, J.; et al. Cis-acting regulation of brain-specific ANK3 gene expression by a genetic variant associated with bipolar disorder. Mol. Psychiatry 2013, 18, 922–929. [Google Scholar] [CrossRef]
- Yoshimura, T.; Stevens, S.R.; Leterrier, C.; Stankewich, M.C.; Rasband, M.N. Developmental Changes in Expression of betaIV Spectrin Splice Variants at Axon Initial Segments and Nodes of Ranvier. Front. Cell. Neurosci. 2016, 10, 304. [Google Scholar] [CrossRef]
- Garrido, J.J.; Giraud, P.; Carlier, E.; Fernandes, F.; Moussif, A.; Fache, M.P.; Debanne, D.; Dargent, B. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 2003, 300, 2091–2094. [Google Scholar] [CrossRef]
- Lemaillet, G.; Walker, B.; Lambert, S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J. Biol. Chem. 2003, 278, 27333–27339. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Kao, T.; Horvath, Z.; Lemos, J.; Sul, J.Y.; Cranstoun, S.D.; Bennett, V.; Scherer, S.S.; Cooper, E.C. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J. Neurosci. 2006, 26, 2599–2613. [Google Scholar] [CrossRef] [PubMed]
- Garver, T.D.; Ren, Q.; Tuvia, S.; Bennett, V. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. J. Cell Biol. 1997, 137, 703–714. [Google Scholar] [CrossRef]
- Jenkins, S.M.; Kizhatil, K.; Kramarcy, N.R.; Sen, A.; Sealock, R.; Bennett, V. FIGQY phosphorylation defines discrete populations of L1 cell adhesion molecules at sites of cell-cell contact and in migrating neurons. J. Cell Sci. 2001, 114, 3823–3835. [Google Scholar] [CrossRef]
- Yang, Y.; Ogawa, Y.; Hedstrom, K.L.; Rasband, M.N. betaIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J. Cell Biol. 2007, 176, 509–519. [Google Scholar] [CrossRef]
- Ipsaro, J.J.; Huang, L.; Gutierrez, L.; MacDonald, R.I. Molecular epitopes of the ankyrin-spectrin interaction. Biochemistry 2008, 47, 7452–7464. [Google Scholar] [CrossRef]
- Ye, J.; Li, J.; Ye, F.; Zhang, Y.; Zhang, M.; Wang, C. Mechanistic insights into the interactions of dynein regulator Ndel1 with neuronal ankyrins and implications in polarity maintenance. Proc. Natl. Acad. Sci. USA 2020, 117, 1207–1215. [Google Scholar] [CrossRef]
- Stevens, S.R.; Rasband, M.N. Pleiotropic Ankyrins: Scaffolds for Ion Channels and Transporters. Channels 2022, 16, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Kole, M.H.; Ilschner, S.U.; Kampa, B.M.; Williams, S.R.; Ruben, P.C.; Stuart, G.J. Action potential generation requires a high sodium channel density in the axon initial segment. Nat. Neurosci. 2008, 11, 178–186. [Google Scholar] [CrossRef]
- Xu, M.; Cooper, E.C. An Ankyrin-G N-terminal Gate and Protein Kinase CK2 Dually Regulate Binding of Voltage-gated Sodium and KCNQ2/3 Potassium Channels. J. Biol. Chem. 2015, 290, 16619–16632. [Google Scholar] [CrossRef]
- Papandreou, M.J.; Vacher, H.; Fache, M.P.; Klingler, E.; Rueda-Boroni, F.; Ferracci, G.; Debarnot, C.; Piperoglou, C.; Garcia Del Cano, G.; Goutebroze, L.; et al. CK2-regulated schwannomin-interacting protein IQCJ-SCHIP-1 association with AnkG contributes to the maintenance of the axon initial segment. J. Neurochem. 2015, 134, 527–537. [Google Scholar] [CrossRef]
- Boiko, T.; Van Wart, A.; Caldwell, J.H.; Levinson, S.R.; Trimmer, J.S.; Matthews, G. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J. Neurosci. 2003, 23, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Duflocq, A.; Le Bras, B.; Bullier, E.; Couraud, F.; Davenne, M. Nav1.1 is predominantly expressed in nodes of Ranvier and axon initial segments. Mol. Cell. Neurosci. 2008, 39, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Tian, C.; Li, T.; Yang, M.; Hou, H.; Shu, Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat. Neurosci. 2009, 12, 996–1002. [Google Scholar] [CrossRef]
- Compans, B.; Burrone, J. Chandelier cells shine a light on the formation of GABAergic synapses. Curr. Opin. Neurobiol. 2023, 80, 102697. [Google Scholar] [CrossRef]
- Inan, M.; Anderson, S.A. The chandelier cell, form and function. Curr. Opin. Neurobiol. 2014, 26, 142–148. [Google Scholar] [CrossRef]
- Somogyi, P. A specific ’axo-axonal’ interneuron in the visual cortex of the rat. Brain Res. 1977, 136, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Ango, F.; di Cristo, G.; Higashiyama, H.; Bennett, V.; Wu, P.; Huang, Z.J. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell 2004, 119, 257–272. [Google Scholar] [CrossRef]
- King, A.N.; Manning, C.F.; Trimmer, J.S. A unique ion channel clustering domain on the axon initial segment of mammalian neurons. J. Comp. Neurol. 2014, 522, 2594–2608. [Google Scholar] [CrossRef]
- Nelson, A.D.; Caballero-Floran, R.N.; Rodriguez Diaz, J.C.; Hull, J.M.; Yuan, Y.; Li, J.; Chen, K.; Walder, K.K.; Lopez-Santiago, L.F.; Bennett, V.; et al. Ankyrin-G regulates forebrain connectivity and network synchronization via interaction with GABARAP. Mol. Psychiatry 2020, 25, 2800–2817. [Google Scholar] [CrossRef]
- Genetet, S.; Ripoche, P.; Le Van Kim, C.; Colin, Y.; Lopez, C. Evidence of a structural and functional ammonium transporter RhBG.anion exchanger 1.ankyrin-G complex in kidney epithelial cells. J. Biol. Chem. 2015, 290, 6925–6936. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P.; Scaramuzzino, D.A.; Morrow, J.S. Ankyrin binds to two distinct cytoplasmic domains of Na,K-ATPase alpha subunit. Proc. Natl. Acad. Sci. USA 1994, 91, 2965–2969. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Rasband, M.N. Axonal Spectrins: Nanoscale Organization, Functional Domains and Spectrinopathies. Front. Cell. Neurosci. 2019, 13, 234. [Google Scholar] [CrossRef]
- Berghs, S.; Aggujaro, D.; Dirkx, R.; Maksimova, E.; Stabach, P.; Hermel, J.M.; Zhang, J.P.; Philbrick, W.; Slepnev, V.; Ort, T.; et al. beta IV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J. Cell Biol. 2000, 151, 985–1001. [Google Scholar] [CrossRef]
- Xu, K.; Zhong, G.; Zhuang, X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 2013, 339, 452–456. [Google Scholar] [CrossRef]
- Watanabe, K.; Al-Bassam, S.; Miyazaki, Y.; Wandless, T.J.; Webster, P.; Arnold, D.B. Networks of polarized actin filaments in the axon initial segment provide a mechanism for sorting axonal and dendritic proteins. Cell Rep. 2012, 2, 1546–1553. [Google Scholar] [CrossRef]
- Micinski, D.; Hotulainen, P. Actin polymerization and longitudinal actin fibers in axon initial segment plasticity. Front. Mol. Neurosci. 2024, 17, 1376997. [Google Scholar] [CrossRef]
- Berger, S.L.; Leo-Macias, A.; Yuen, S.; Khatri, L.; Pfennig, S.; Zhang, Y.; Agullo-Pascual, E.; Caillol, G.; Zhu, M.S.; Rothenberg, E.; et al. Localized Myosin II Activity Regulates Assembly and Plasticity of the Axon Initial Segment. Neuron 2018, 97, 555–570.e6. [Google Scholar] [CrossRef] [PubMed]
- Abouelezz, A.; Stefen, H.; Segerstrale, M.; Micinski, D.; Minkeviciene, R.; Lahti, L.; Hardeman, E.C.; Gunning, P.W.; Hoogenraad, C.C.; Taira, T.; et al. Tropomyosin Tpm3.1 Is Required to Maintain the Structure and Function of the Axon Initial Segment. iScience 2020, 23, 101053. [Google Scholar] [CrossRef]
- Palay, S.L.; Sotelo, C.; Peters, A.; Orkand, P.M. The axon hillock and the initial segment. J. Cell Biol. 1968, 38, 193–201. [Google Scholar] [CrossRef]
- Peters, A.; Proskauer, C.C.; Kaiserman-Abramof, I.R. The small pyramidal neuron of the rat cerebral cortex. The axon hillock and initial segment. J. Cell Biol. 1968, 39, 604–619. [Google Scholar] [CrossRef]
- Sobotzik, J.M.; Sie, J.M.; Politi, C.; Del Turco, D.; Bennett, V.; Deller, T.; Schultz, C. AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 17564–17569. [Google Scholar] [CrossRef] [PubMed]
- Freal, A.; Fassier, C.; Le Bras, B.; Bullier, E.; De Gois, S.; Hazan, J.; Hoogenraad, C.C.; Couraud, F. Cooperative Interactions between 480 kDa Ankyrin-G and EB Proteins Assemble the Axon Initial Segment. J. Neurosci. 2016, 36, 4421–4433. [Google Scholar] [CrossRef] [PubMed]
- Freal, A.; Rai, D.; Tas, R.P.; Pan, X.; Katrukha, E.A.; van de Willige, D.; Stucchi, R.; Aher, A.; Yang, C.; Altelaar, A.F.M.; et al. Feedback-Driven Assembly of the Axon Initial Segment. Neuron 2019, 104, 305–321.e8. [Google Scholar] [CrossRef]
- Sohn, P.D.; Huang, C.T.; Yan, R.; Fan, L.; Tracy, T.E.; Camargo, C.M.; Montgomery, K.M.; Arhar, T.; Mok, S.A.; Freilich, R.; et al. Pathogenic Tau Impairs Axon Initial Segment Plasticity and Excitability Homeostasis. Neuron 2019, 104, 458–470.e5. [Google Scholar] [CrossRef]
- Yau, K.W.; van Beuningen, S.F.; Cunha-Ferreira, I.; Cloin, B.M.; van Battum, E.Y.; Will, L.; Schatzle, P.; Tas, R.P.; van Krugten, J.; Katrukha, E.A.; et al. Microtubule minus-end binding protein CAMSAP2 controls axon specification and dendrite development. Neuron 2014, 82, 1058–1073. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, C.S.; Plowinske, C.R.; Montgomery, A.C.; Quinones, G.B.; Banker, G.; Bentley, M. Kinesin Regulation in the Proximal Axon is Essential for Dendrite-selective Transport. Mol. Biol. Cell 2024, 35, ar81. [Google Scholar] [CrossRef]
- Satake, T.; Yamashita, K.; Hayashi, K.; Miyatake, S.; Tamura-Nakano, M.; Doi, H.; Furuta, Y.; Shioi, G.; Miura, E.; Takeo, Y.H.; et al. MTCL1 plays an essential role in maintaining Purkinje neuron axon initial segment. EMBO J. 2017, 36, 1227–1242. [Google Scholar] [CrossRef]
- Melton, A.J.; Palfini, V.L.; Ogawa, Y.; Oses Prieto, J.A.; Vainshtein, A.; Burlingame, A.L.; Peles, E.; Rasband, M.N. TRIM46 Is Required for Microtubule Fasciculation In Vivo But Not Axon Specification or Axon Initial Segment Formation. J. Neurosci. 2024, 44, e0976242024. [Google Scholar] [CrossRef]
- Harterink, M.; Vocking, K.; Pan, X.; Soriano Jerez, E.M.; Slenders, L.; Freal, A.; Tas, R.P.; van de Wetering, W.J.; Timmer, K.; Motshagen, J.; et al. TRIM46 Organizes Microtubule Fasciculation in the Axon Initial Segment. J. Neurosci. 2019, 39, 4864–4873. [Google Scholar] [CrossRef]
- Ichinose, S.; Ogawa, T.; Jiang, X.; Hirokawa, N. The Spatiotemporal Construction of the Axon Initial Segment via KIF3/KAP3/TRIM46 Transport under MARK2 Signaling. Cell Rep. 2019, 28, 2413–2426.e7. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Yu, Y.; Jiang, Z.; Otani, Y.; Fujitani, M. Ankyrin-G and Its Binding Partners in Neurons: Orchestrating the Molecular Structure of the Axon Initial Segment. Biomolecules 2025, 15, 901. https://doi.org/10.3390/biom15060901
Zhu X, Yu Y, Jiang Z, Otani Y, Fujitani M. Ankyrin-G and Its Binding Partners in Neurons: Orchestrating the Molecular Structure of the Axon Initial Segment. Biomolecules. 2025; 15(6):901. https://doi.org/10.3390/biom15060901
Chicago/Turabian StyleZhu, Xiaowei, Yanyan Yu, Zhuqian Jiang, Yoshinori Otani, and Masashi Fujitani. 2025. "Ankyrin-G and Its Binding Partners in Neurons: Orchestrating the Molecular Structure of the Axon Initial Segment" Biomolecules 15, no. 6: 901. https://doi.org/10.3390/biom15060901
APA StyleZhu, X., Yu, Y., Jiang, Z., Otani, Y., & Fujitani, M. (2025). Ankyrin-G and Its Binding Partners in Neurons: Orchestrating the Molecular Structure of the Axon Initial Segment. Biomolecules, 15(6), 901. https://doi.org/10.3390/biom15060901







