A Novel Copper Ionophore Nanoshuttle (Winged Cu) for Inducing Cuproptosis in B16 Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Soluplus Concentration Confirmation
2.3. Synthesis of Cu(HEDTC)2@Soluplus Nanomicelles
2.4. Characterization of Cu(HEDTC)2@Soluplus Nanomicelles
2.5. Intracellular Cu Content Assay
2.6. Cellular Uptake
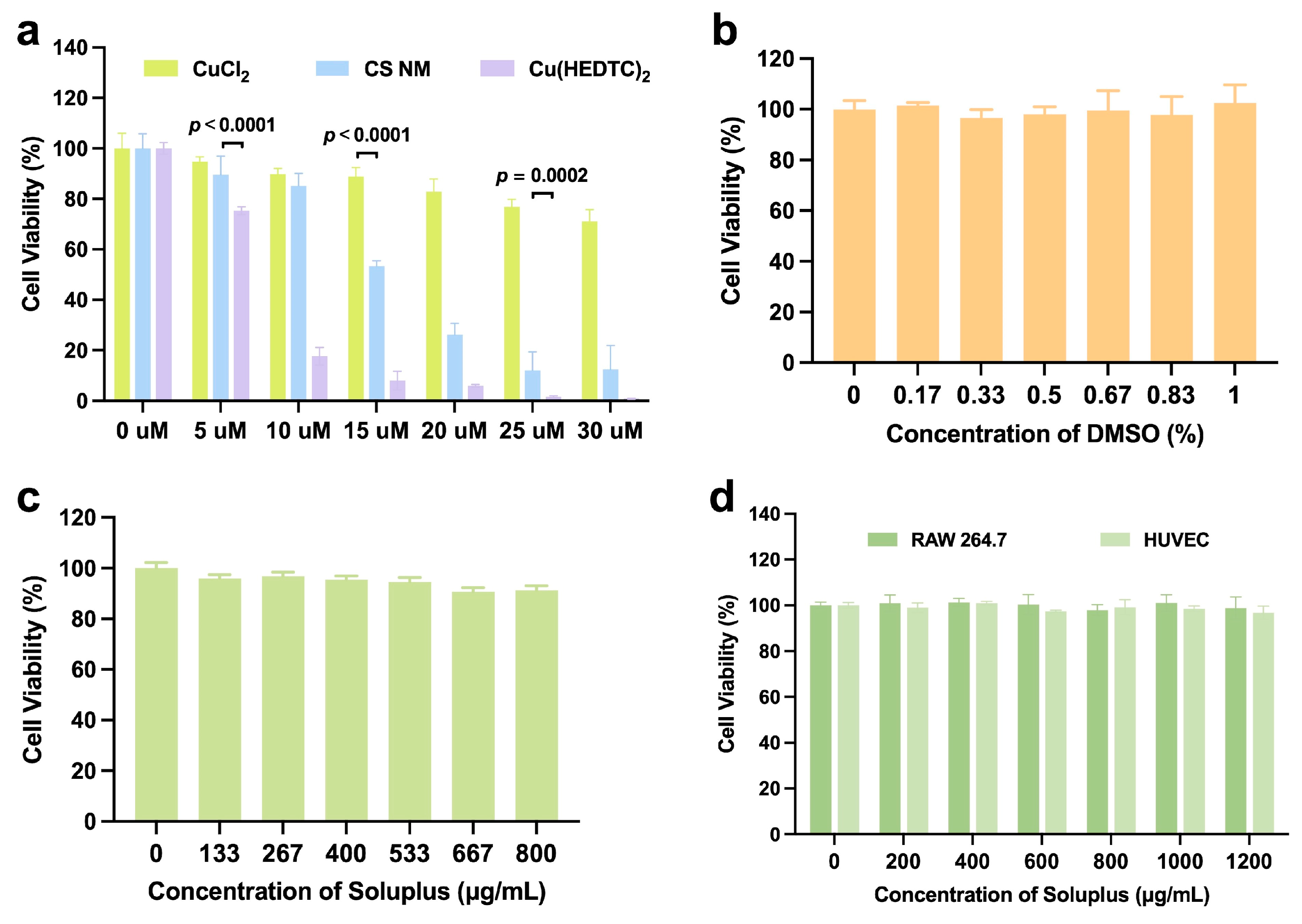
2.7. Cytotoxicity Assay
2.8. Biocompatibility Test
2.9. Antioxidant Rescue Assay
2.10. Intracellular GSH Level Detection
2.11. Intracellular ROS Generation Assay
2.12. Cell Death Form Confirmation
2.13. Cell Mitochondrial Morphology
2.14. Immunofluorescence of DLAT Oligomerization and FDX1, LIAS and ATP7A Expression
2.15. Western Blot
2.16. Statistical Analysis
3. Results and Discussion
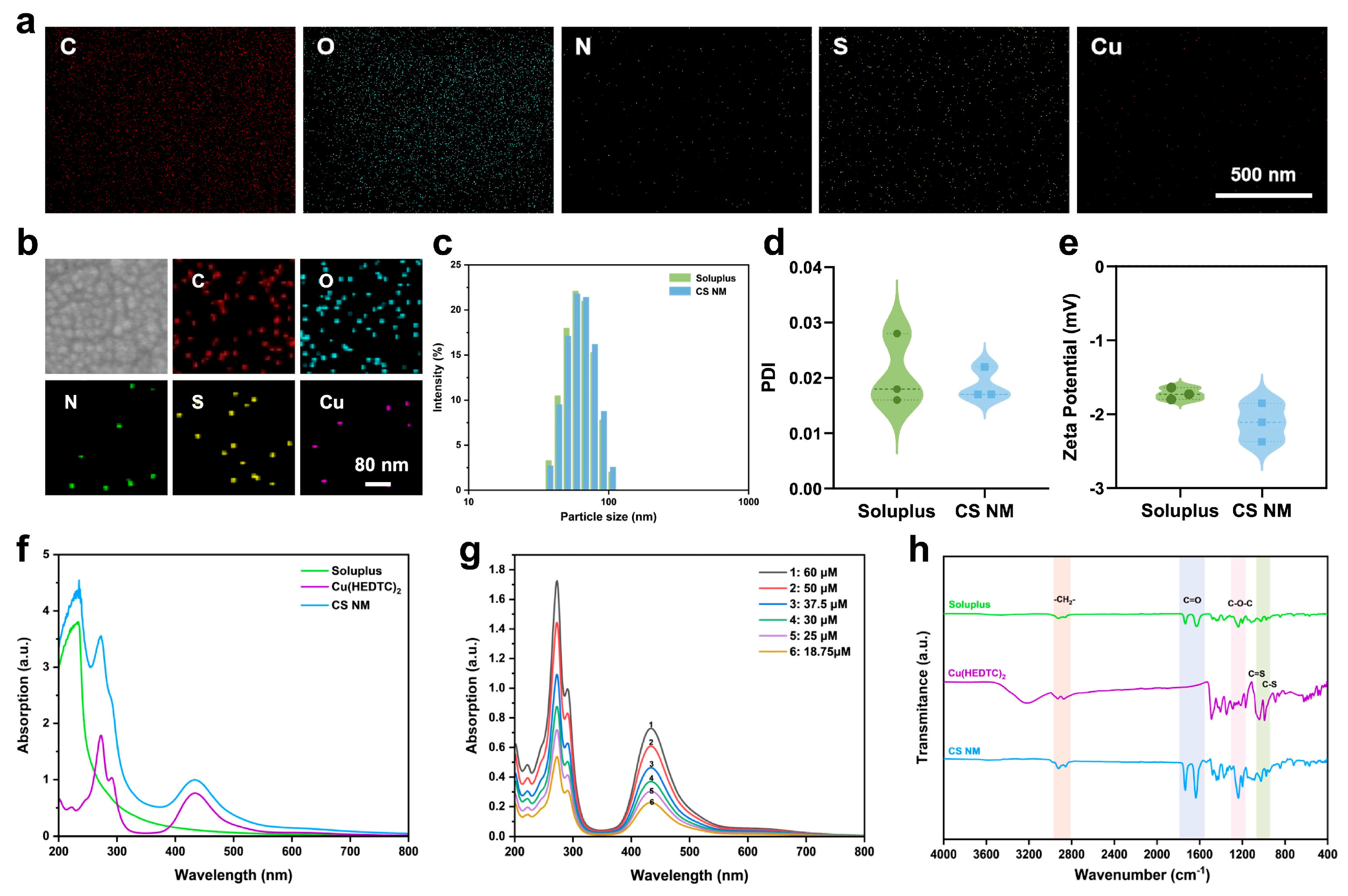
3.1. Preparation and Characterization of the CS NM
3.2. Efficient Delivery of Copper and Anti-Cancer Effect Using CS NM In Vitro
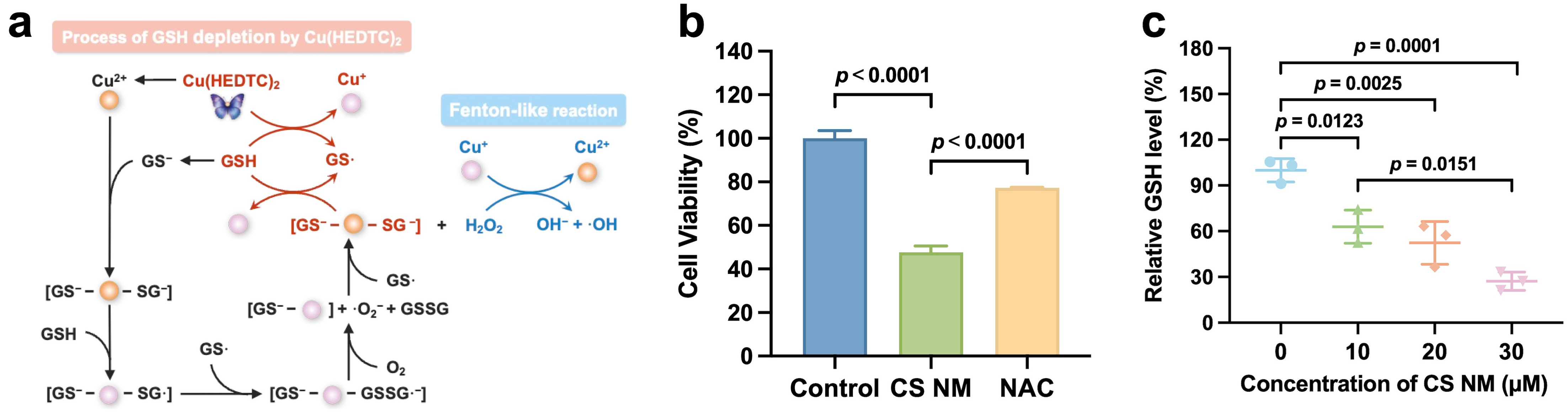
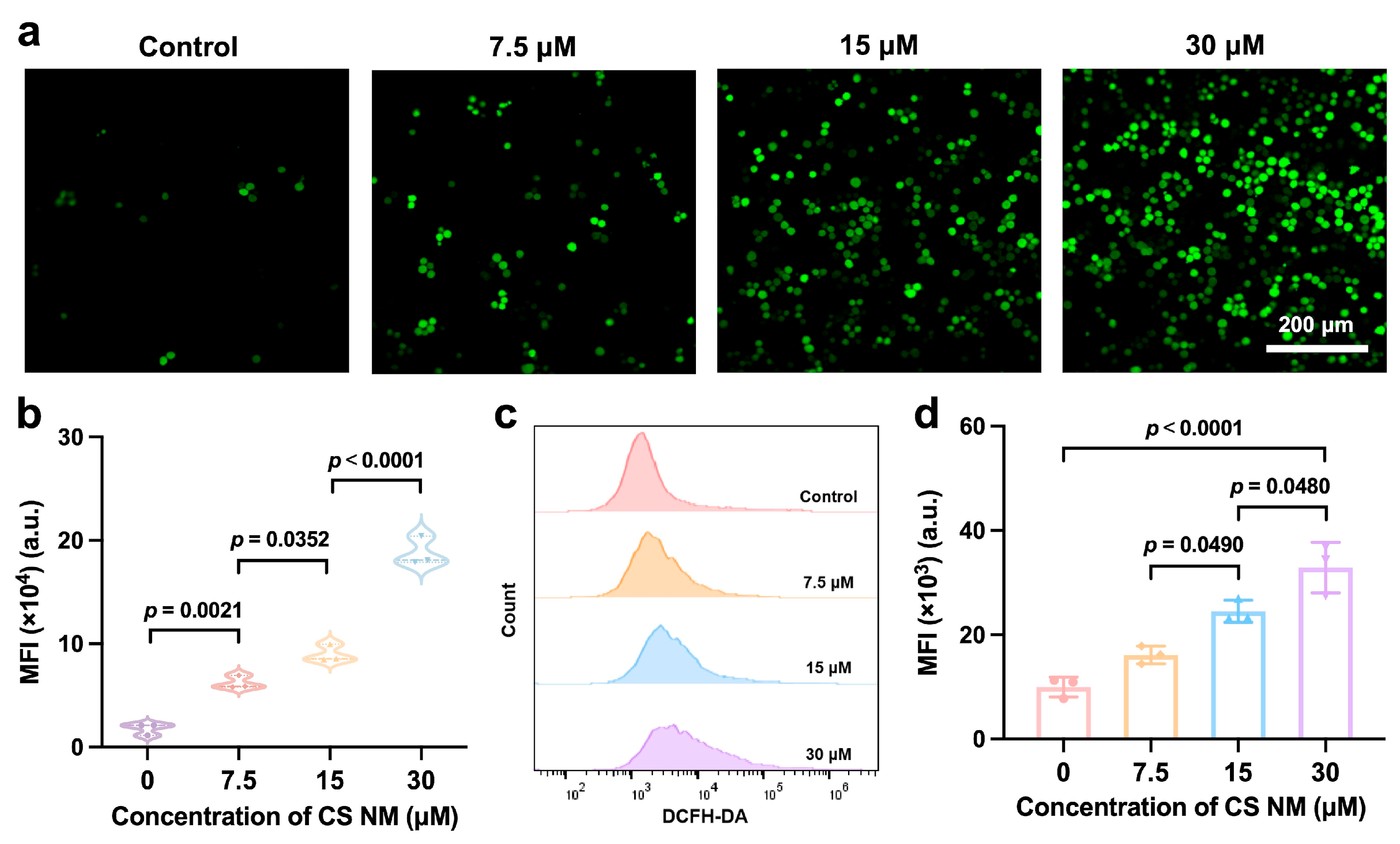
3.3. Evaluation of Oxidative Stress Induced by CS NM In Vitro
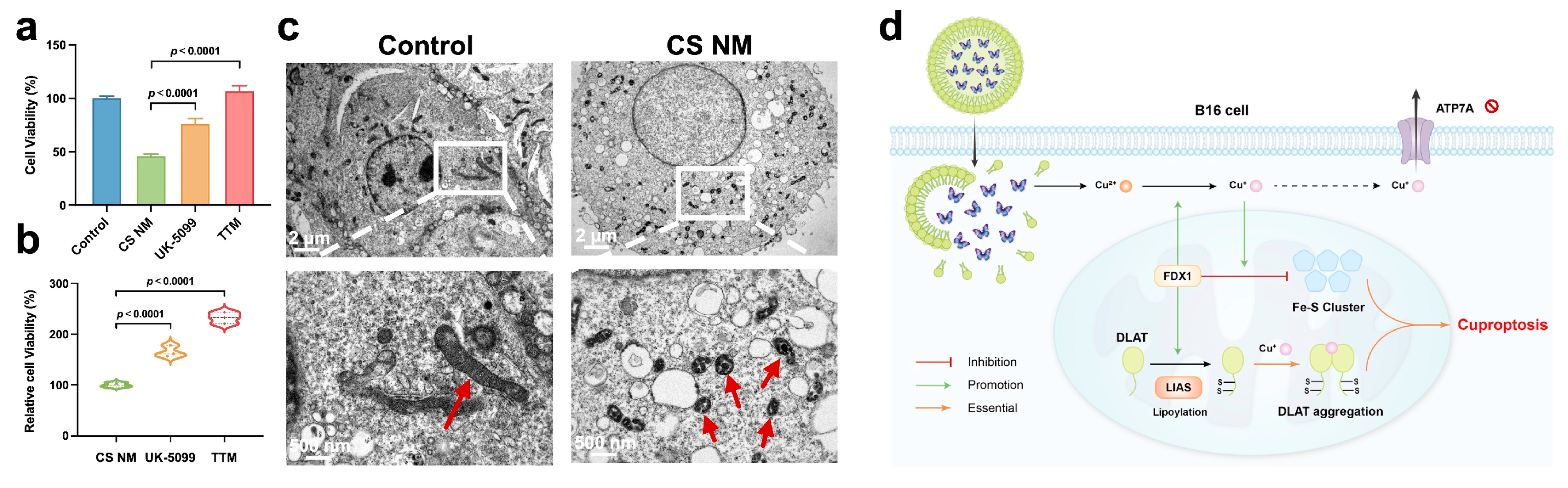
3.4. CS NM Induced Cuproptosis in B16 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CS NM | Cu(HEDTC)2@Soluplus nanomicelle |
| TCA | Tricarboxylic acid |
| DLAT | Dihydrolipoamide S-acetyltransferase |
| Fe-S | Iron–sulfur |
| DSF | Disulfiram |
| Cu(HEDTC)2 | Bis(2-hydroxyethyl)dithiocarbamic acid copper(II) |
| PEG | Polyethylene glycol |
| PVCL | Polyvinyl caprolactam |
| PVAc | Polyvinyl acetate |
| EPR | Enhanced permeability and retention |
| GSH | Glutathione |
| ROS | Reactive oxygen species |
References
- Wang, Y.; Chen, Y.; Zhang, J.; Yang, Y.; Fleishman, J.; Wang, Y.; Wang, J.; Chen, J.; Li, Y.; Wang, H. Cuproptosis: A novel therapeutic target for overcoming cancer drug resistance. Drug Resist. Updat. 2024, 72, 101018. [Google Scholar] [CrossRef] [PubMed]
- Bedoui, S.; Herold, M.J.; Strasser, A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 2020, 21, 678–695. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Chrzan, N.; Hartman, M.L. Copper in melanoma: At the crossroad of protumorigenic and anticancer roles. Redox Biol. 2025, 81, 103552. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shen, J.; Huang, K.; Wang, L.; Sethi, G.; Ma, Z. Cuproptosis in cancers: Function and implications from bench to bedside. Biomed. Pharmacother. 2024, 176, 116874. [Google Scholar] [CrossRef]
- Toh, E.; Xing, Y.; Gao, X.; Jordan, S.; Batteiger, T.; Batteiger, B.; Pol, B.V.; Muzny, C.A.; Gebregziabher, N.; Williams, J.A.; et al. Sexual behavior shapes male genitourinary microbiome composition. Cell Rep. Med. 2023, 4, 100981. [Google Scholar] [CrossRef]
- Tang, D.; Kroemer, G.; Kang, R. Targeting cuproplasia and cuproptosis in cancer. Nat. Rev. Clin. Oncol. 2024, 21, 370–388. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, M.; Liu, Y.; Si, Z. Cope with copper: From copper linked mechanisms to copper-based clinical cancer therapies. Cancer Lett. 2023, 561, 216157. [Google Scholar] [CrossRef]
- Nagai, M.; Vo, N.H.; Ogawa, L.S.; Chimmanamada, D.; Inoue, T.; Chu, J.; Beaudette-Zlatanova, B.C.; Lu, R.Z.; Blackman, R.K.; Barsoum, J.; et al. The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free Radical Biol. Med. 2012, 52, 2142–2150. [Google Scholar] [CrossRef]
- Zheng, P.; Zhou, C.; Lu, L.; Liu, B.; Ding, Y. Elesclomol: A copper ionophore targeting mitochondrial metabolism for cancer therapy. J. Exp. Clin. Cancer Res. 2022, 41, 271. [Google Scholar] [CrossRef]
- Oliveri, V. Selective Targeting of Cancer Cells by Copper Ionophores: An Overview. Front. Mol. Biosci. 2022, 9, 841814. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.W.; Du, S.Y.; Liu, Z.Y.; He, B.S.; Pan, Y.C.; Yang, J.; Li, Y.Y.; Kong, D.S.; Han, X.; Song, Y.J. Microneedle-Loaded Gene-Editable Nanohybrids Engineer Cancer Cells by Telomere Stress Induction and Metabolic Interference for Enhanced Cuproptosis and Immunotherapy. Adv. Funct. Mater. 2025, 35, 2416285. [Google Scholar] [CrossRef]
- Lu, X.; Chen, X.; Lin, C.; Yi, Y.; Zhao, S.; Zhu, B.; Deng, W.; Wang, X.; Xie, Z.; Rao, S.; et al. Elesclomol Loaded Copper Oxide Nanoplatform Triggers Cuproptosis to Enhance Antitumor Immunotherapy. Adv. Sci. 2024, 11, 2309984. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, P.; Yu, J.; Deng, H.; Ge, S.; Dou, H.; Ruan, J.; Fan, X. Thermal-triggered mitochondrial oxidative phosphorylation-enhanced nanodrug sensitizes cuproptosis with amplified anti-tumor immunity. Mater. Today 2025, 82, 12–31. [Google Scholar] [CrossRef]
- Shi, H.; Suo, Y.; Zhang, Z.; Liu, R.; Liu, H.; Cheng, Z. Copper(II)-disulfiram loaded melanin-dots for cancer theranostics. Nanomedicine 2021, 32, 102340. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, G.; Guo, Z.; Chen, Q.; Zhang, Y.; Chu, Y.; Guo, Q.; Li, C.; Zhou, W.; Zhang, Y.; et al. Co-delivery of Cu(I) chelator and chemotherapeutics as a new strategy for tumor theranostic. J. Control. Release 2020, 321, 483–496. [Google Scholar] [CrossRef]
- Feng, L.; Wu, T.; Guo, X.; Wang, Y.; Wang, X.; Liu, S.; Zhang, R.; Ma, Y.; Tan, N.; Bian, J.; et al. Discovery of Natural Resorcylic Acid Lactones as Novel Potent Copper Ionophores Covalently Targeting PRDX1 to Induce Cuproptosis for Triple-Negative Breast Cancer Therapy. ACS Cent. Sci. 2025, 11, 357–370. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Zhu, C.; Gong, S.; Ding, J.; Yu, M.; Ahmad, E.; Feng, Y.; Gan, Y. Supersaturated polymeric micelles for oral silybin delivery: The role of the Soluplus-PVPVA complex. Acta Pharm. Sin. B. 2019, 9, 107–117. [Google Scholar] [CrossRef]
- Xia, D.; Yu, H.; Tao, J.; Zeng, J.; Zhu, Q.; Zhu, C.; Gan, Y. Supersaturated polymeric micelles for oral cyclosporine A delivery: The role of Soluplus-sodium dodecyl sulfate complex. Colloids Surf. B Biointerfaces 2016, 141, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Talelli, M.; Barz, M.; Rijcken, C.J.F.; Kiessling, F.; Hennink, W.E.; Lammers, T. Core-crosslinked polymeric micelles: Principles, preparation, biomedical applications and clinical translation. Nano Today 2015, 10, 93–117. [Google Scholar] [CrossRef]
- Cheng, R.; Li, Z.; Luo, W.; Chen, H.; Deng, T.; Gong, Z.; Zheng, Q.; Li, B.; Zeng, Y.; Wang, H.; et al. A Copper-Based Photothermal-Responsive Nanoplatform Reprograms Tumor Immunogenicity via Self-Amplified Cuproptosis for Synergistic Cancer Therapy. Adv. Sci. 2025, 12, e2500652. [Google Scholar] [CrossRef]
- Rostas, A.M.; Badea, M.; Ruta, L.L.; Farcasanu, I.C.; Maxim, C.; Chifiriuc, M.C.; Popa, M.; Luca, M.; Korosin, N.C.; Korosec, R.C.; et al. Copper(II) Complexes with Mixed Heterocycle Ligands as Promising Antibacterial and Antitumor Species. Molecules 2020, 25, 3777. [Google Scholar] [CrossRef]
- Mariani, D.; Ghasemishahrestani, Z.; Freitas, W.; Pezzuto, P.; Costa-da-Silva, A.C.; Tanuri, A.; Kanashiro, M.M.; Fernandes, C.; Horn, A.; Pereira, M.D. Antitumoral synergism between a copper(II) complex and cisplatin improves in vitro and in vivo anticancer activity against melanoma, lung and breast cancer cells. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129963. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Corsaro, R.; Bonaccorso, A.; Zingale, E.; Carbone, C.; Musumeci, T. Soluplus® polymeric nanomicelles improve solubility of BCS-class II drugs. Drug Deliv. Transl. Res. 2022, 12, 1991–2006. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Chen, M.; Li, X.; Fu, Y.; Yang, D.; Wen, T.; Zhao, W.; Sun, Y.; Wang, W.; Lu, C.; et al. ATP-adenosine axis regulation combined with microneedle assisted photoimmunotherapy to boost the immunotherapy efficiency. J. Control. Release 2024, 367, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhong, Z.; Peng, S.; Fu, J.; Chen, M.; Lang, T.; Yue, X.; Fu, Y.; He, J.; Jin, Y.; et al. “All-in-one” metal polyphenol network nanocapsules integrated microneedle patches for lipophagy fueled ferroptosis-mediated multimodal therapy. J. Control. Release 2024, 373, 599–616. [Google Scholar] [CrossRef]
- Lu, S.; Li, Y.; Yu, Y. Glutathione-Scavenging Celastrol-Cu Nanoparticles Induce Self-Amplified Cuproptosis for Augmented Cancer Immunotherapy. Adv. Mater. 2024, 36, 2404971. [Google Scholar] [CrossRef]
- Hayat, M.J.; Powell, A.; Johnson, T.; Cadwell, B.L. Statistical methods used in the public health literature and implications for training of public health professionals. PLoS ONE 2017, 12, e0179032. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef]
- Chang, Z.; Wu, Y.; Chen, Y.; Bai, X.; Peng, T.; Wu, C.; Pan, X.; Huang, Z. Biological Fate Tracking of Nitric Oxide-Propelled Microneedle Delivery System Using an Aggregation-Caused Quenching Probe. Mol. Pharm. 2024, 21, 4541–4552. [Google Scholar] [CrossRef] [PubMed]
- Shamma, R.N.; Basha, M. Solupluse®: A novel polymeric solubilizer for optimization of Carvedilol solid dispersions: Formulation design and effect of method of preparation. Powder Technol. 2013, 237, 406–414. [Google Scholar] [CrossRef]
- Koleva, I.Z.; Tzachev, C.T. Efficient Improvement of Eugenol Water Solubility by Spray Drying Encapsulation in Soluplus® and Lutrol F 127. Pharmaceuticals 2024, 17, 1156. [Google Scholar] [CrossRef] [PubMed]
- Mucha, I.; Karolewicz, B.; Górniak, A. Stability Studies of Amorphous Ibrutinib Prepared Using the Quench-Cooling Method and Its Dispersions with Soluplus®. Polymers 2024, 16, 1961. [Google Scholar] [CrossRef]
- Zi, Y.; Yang, K.; He, J.; Wu, Z.; Liu, J.; Zhang, W. Strategies to enhance drug delivery to solid tumors by harnessing the EPR effects and alternative targeting mechanisms. Adv. Drug Deliv. Rev. 2022, 188, 114449. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Attia, M.S.; Elshahat, A.; Hamdy, A.; Fathi, A.M.; Emad-Eldin, M.; Ghazy, F.E.S.; Chopra, H.; Ibrahim, T.M. Soluplus® as a solubilizing excipient for poorly water-soluble drugs: Recent advances in formulation strategies and pharmaceutical product features. J. Drug Deliv. Sci. Technol. 2023, 84, 104519. [Google Scholar] [CrossRef]
- Liu, S.; Yan, W.; Zhang, W.; Zhang, J.; Li, Z.; Guo, Y.; Chen, H.; Xu, J. Nanoenhanced-Cuproptosis Results From the Synergy of Calcium Overload and GSH Depletion with the Increasing of Intracellular Ca/Mn/Cu Ions. Adv. Sci. 2025, 12, 2412067. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Y.; Zhu, G.; Wang, M.; Qian, Y.; Sun, Q.; Li, C. Mitochondria-targeted H2O2 homeostatic disruptors: Harnessing glutathione autoxidation and reversing GSDME “silencing” for ferroptotic and dual-pathway pyroptotic oncotherapy. Nano Today 2025, 61, 102640. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.; Liu, C.; Si, W.; Wang, W.; Zhang, Y.; Zhong, L.; Dong, X.; Zhao, Y. Copper-doped MOF-based nanocomposite for GSH depleted chemo/photothermal/chemodynamic combination therapy. Chem. Eng. J. 2022, 438, 135567. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, S.; Zhang, L.; Deng, Q.; Ren, J.; Qu, X. A Metabolic Multistage Glutathione Depletion Used for Tumor-Specific Chemodynamic Therapy. ACS Nano 2022, 16, 4228–4238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, J.; Xie, L.; Hao, M.; Liang, W.; Wang, S.; Dong, X.; Li, Y.; Mu, Q. Mitochondria- and anaerobic glycolysis-targeted self-assembled copper complex nanoparticles for boosting cuproptosis-immunotherapy. Adv. Compos. Hybrid. Mater. 2025, 8, 45. [Google Scholar] [CrossRef]




| Category | Limitations of Previous Studies | Distinctiveness of CS NM | Reference |
|---|---|---|---|
| Material | Limited investigational materials (copper ionophores such as elesclomol and disulfiram are commonly used in studies) | Cu(HEDTC)2 was proposed for the first time as a novel copper ionophore for cancer therapy | [8,10,11] |
| Copper delivery efficiency | Low copper delivery efficiency (upon elesclomol treatment, intracellular copper levels increased only approximately 2-fold compared to controls) | Significantly enhanced copper delivery efficiency (CS NM elevated intracellular copper levels approximately 5-fold compared to controls) | [23] |
| Cytotoxicity | Low cytotoxicity (high IC50 values, IC50 > 70 μM) | CS NM enhanced cytotoxicity (IC50: 14.12 μM) | [24,25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Chang, Z.; Wang, W.; Wu, C.; Pan, X.; Huang, Z. A Novel Copper Ionophore Nanoshuttle (Winged Cu) for Inducing Cuproptosis in B16 Melanoma Cells. Biomolecules 2025, 15, 895. https://doi.org/10.3390/biom15060895
Wu Y, Chang Z, Wang W, Wu C, Pan X, Huang Z. A Novel Copper Ionophore Nanoshuttle (Winged Cu) for Inducing Cuproptosis in B16 Melanoma Cells. Biomolecules. 2025; 15(6):895. https://doi.org/10.3390/biom15060895
Chicago/Turabian StyleWu, Yuhuan, Ziyao Chang, Wenhao Wang, Chuanbin Wu, Xin Pan, and Zhengwei Huang. 2025. "A Novel Copper Ionophore Nanoshuttle (Winged Cu) for Inducing Cuproptosis in B16 Melanoma Cells" Biomolecules 15, no. 6: 895. https://doi.org/10.3390/biom15060895
APA StyleWu, Y., Chang, Z., Wang, W., Wu, C., Pan, X., & Huang, Z. (2025). A Novel Copper Ionophore Nanoshuttle (Winged Cu) for Inducing Cuproptosis in B16 Melanoma Cells. Biomolecules, 15(6), 895. https://doi.org/10.3390/biom15060895







