Probiotics and Cancer: Mechanistic Insights and Organ-Specific Impact
Abstract
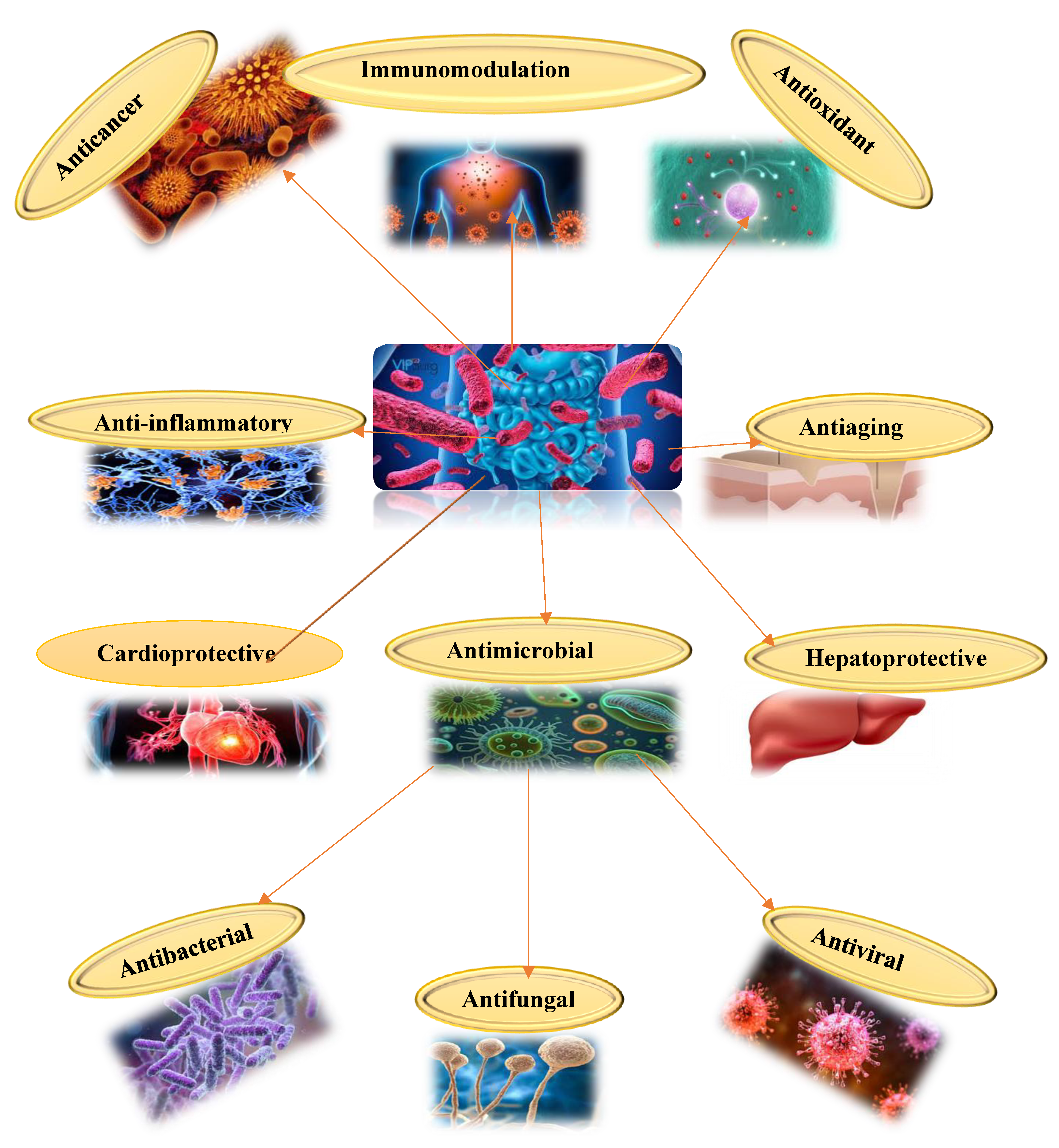
1. Introduction
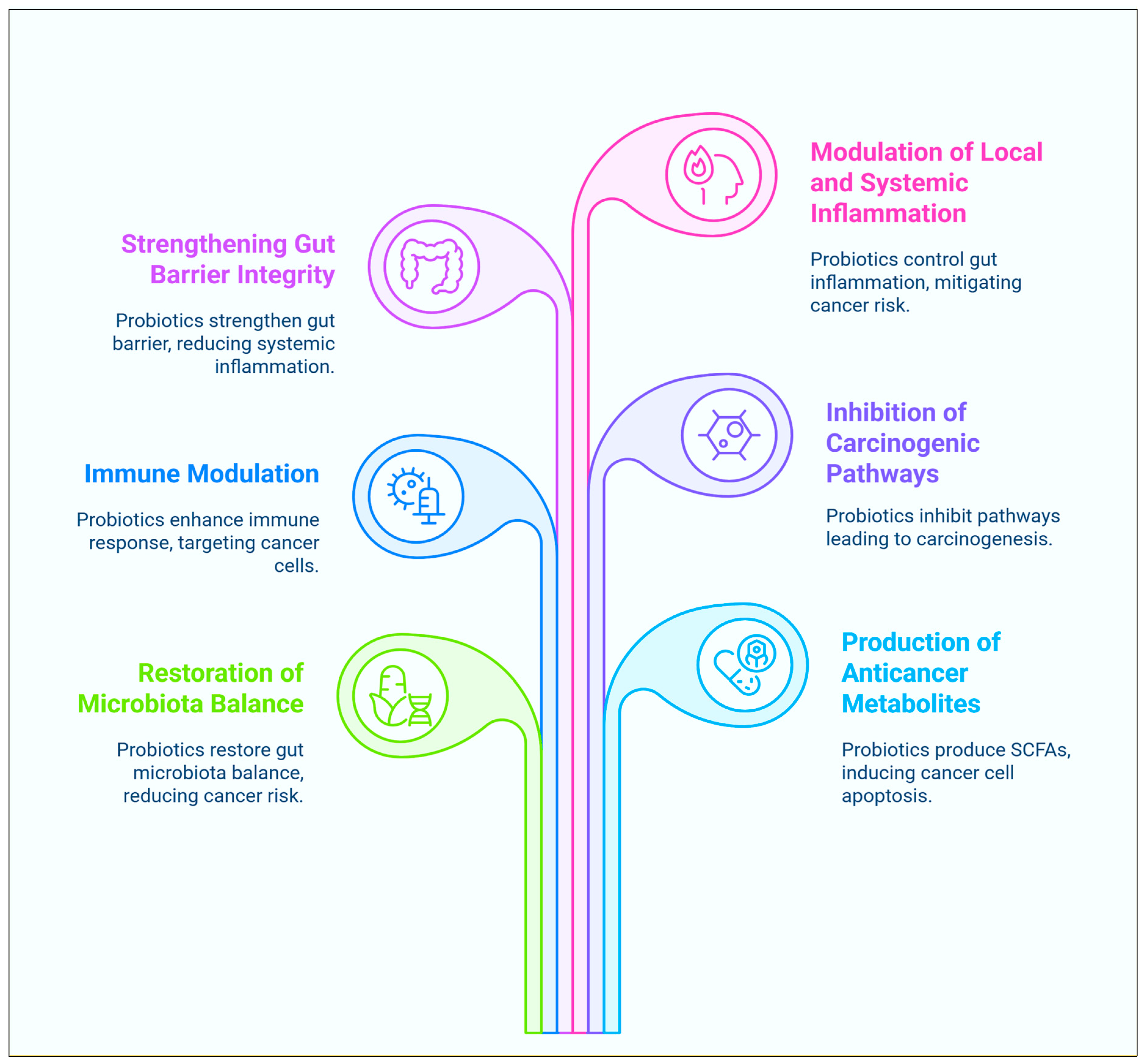
2. Anticancer Mechanisms of Probiotics
2.1. Modulation of the Gastrointestinal Microbiota
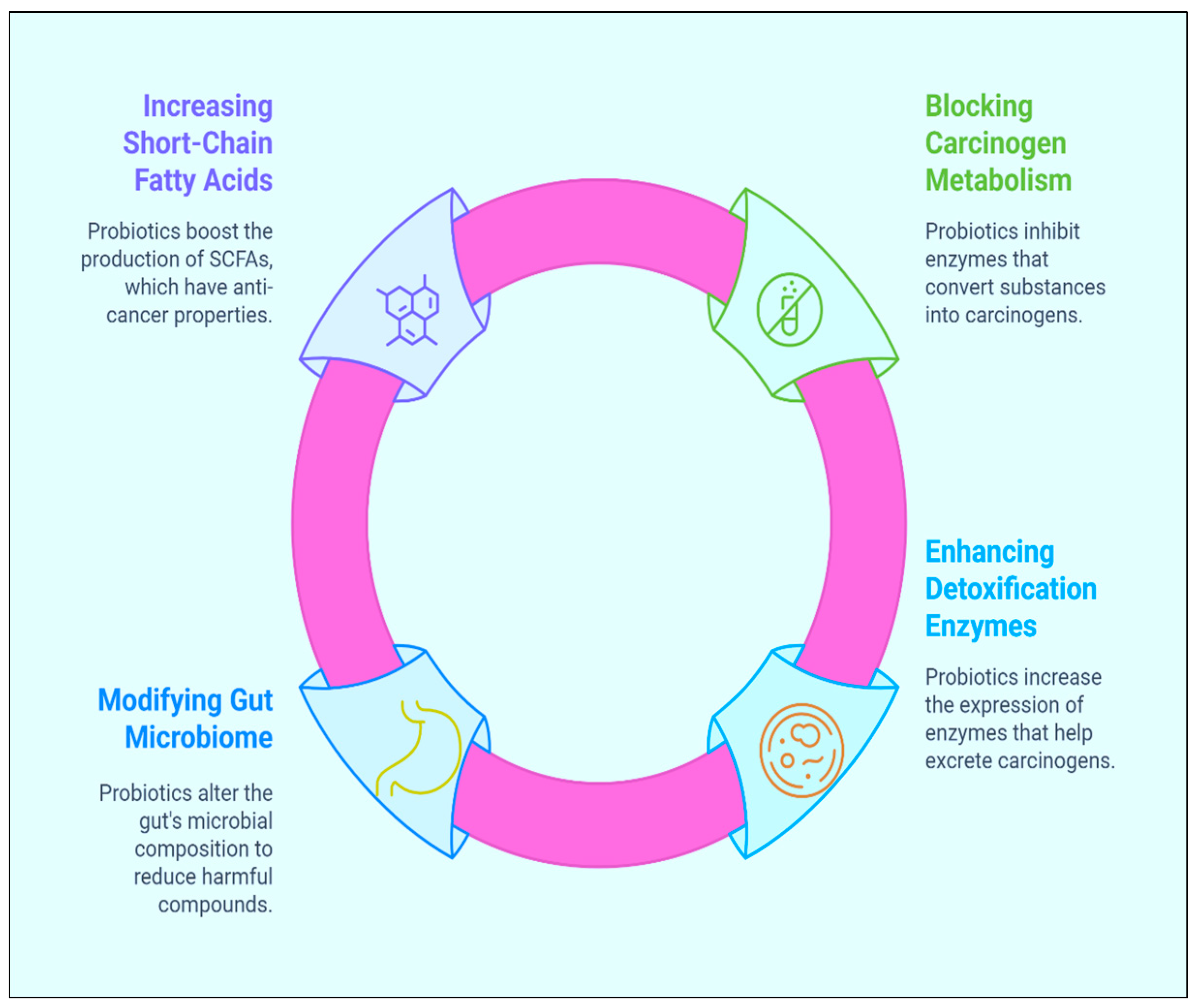
2.2. Inhibition of the Metabolism of Carcinogenic Substances
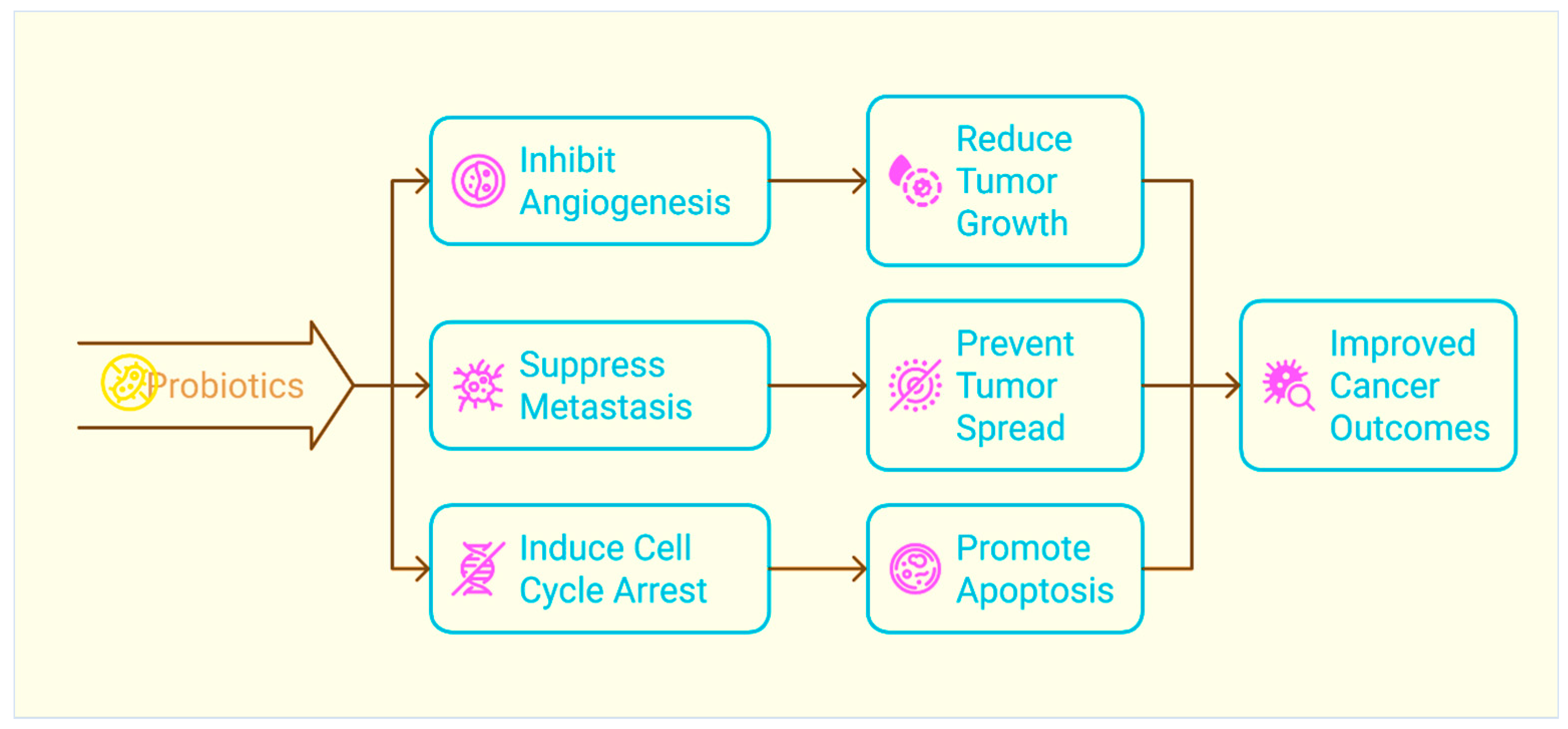
2.3. Limiting the Growth and Proliferation of Tumours
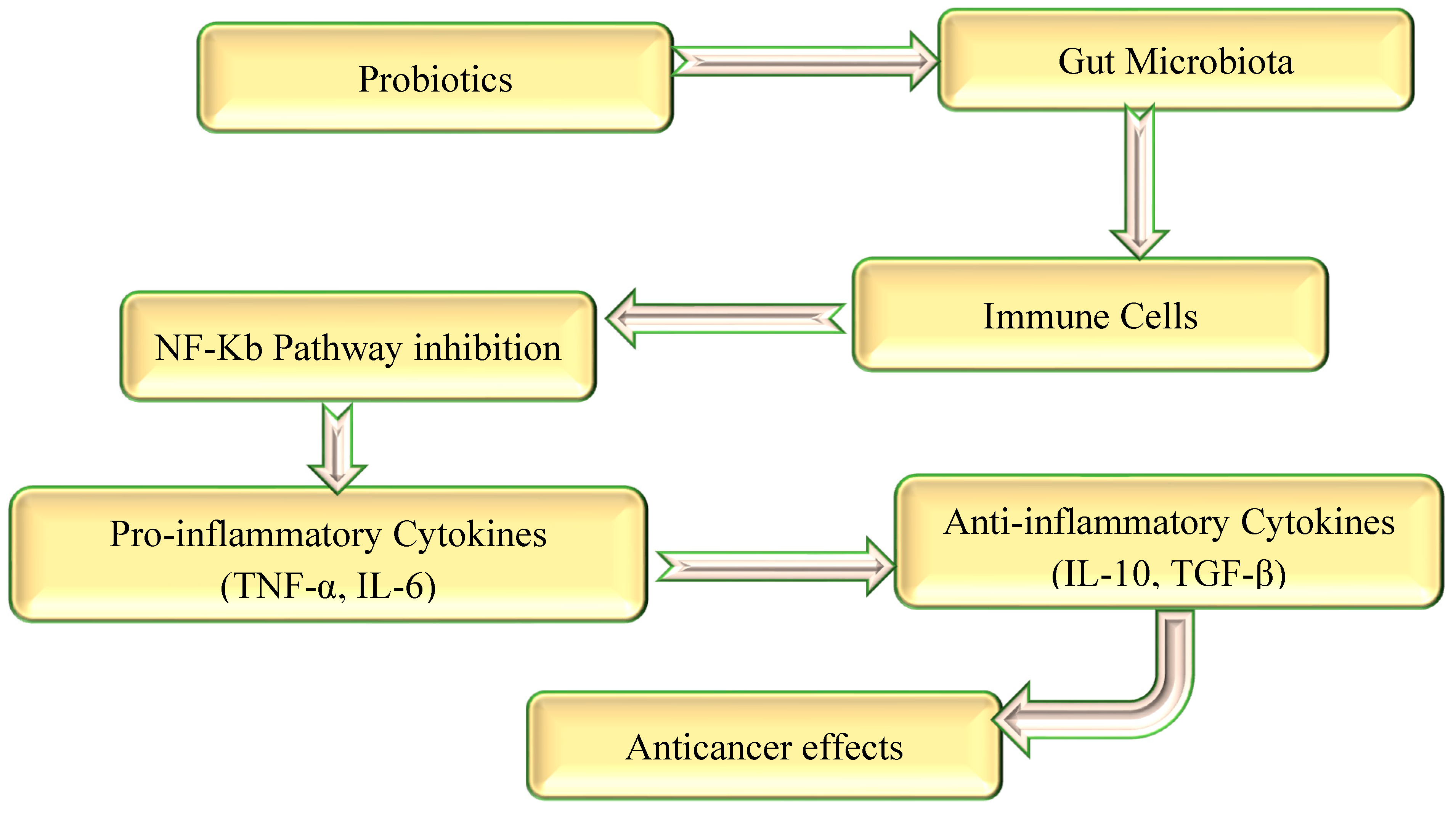
2.4. Anti-Inflammatory and Immunopotentiators Efficacy
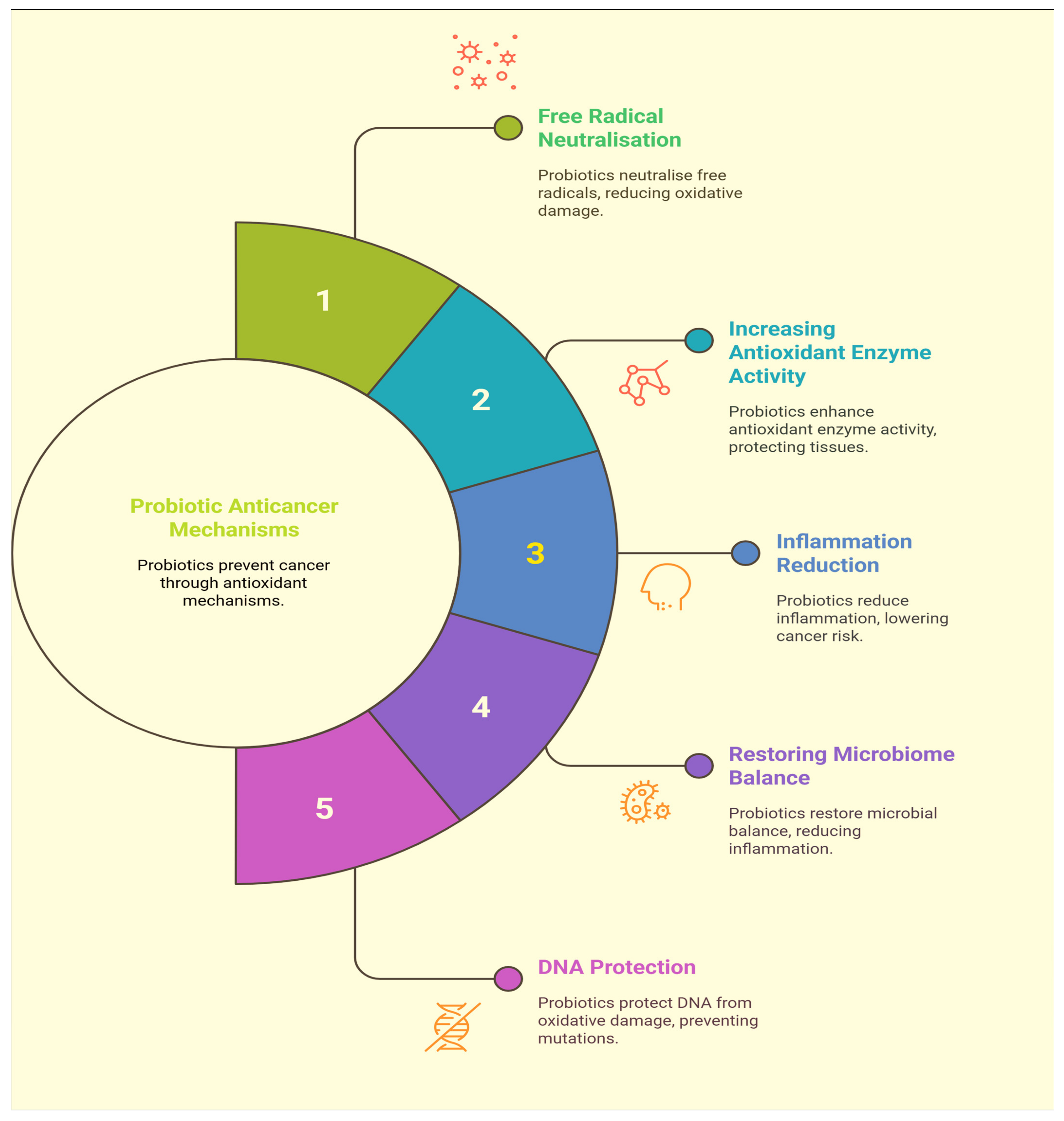
2.5. Antioxidant Properties
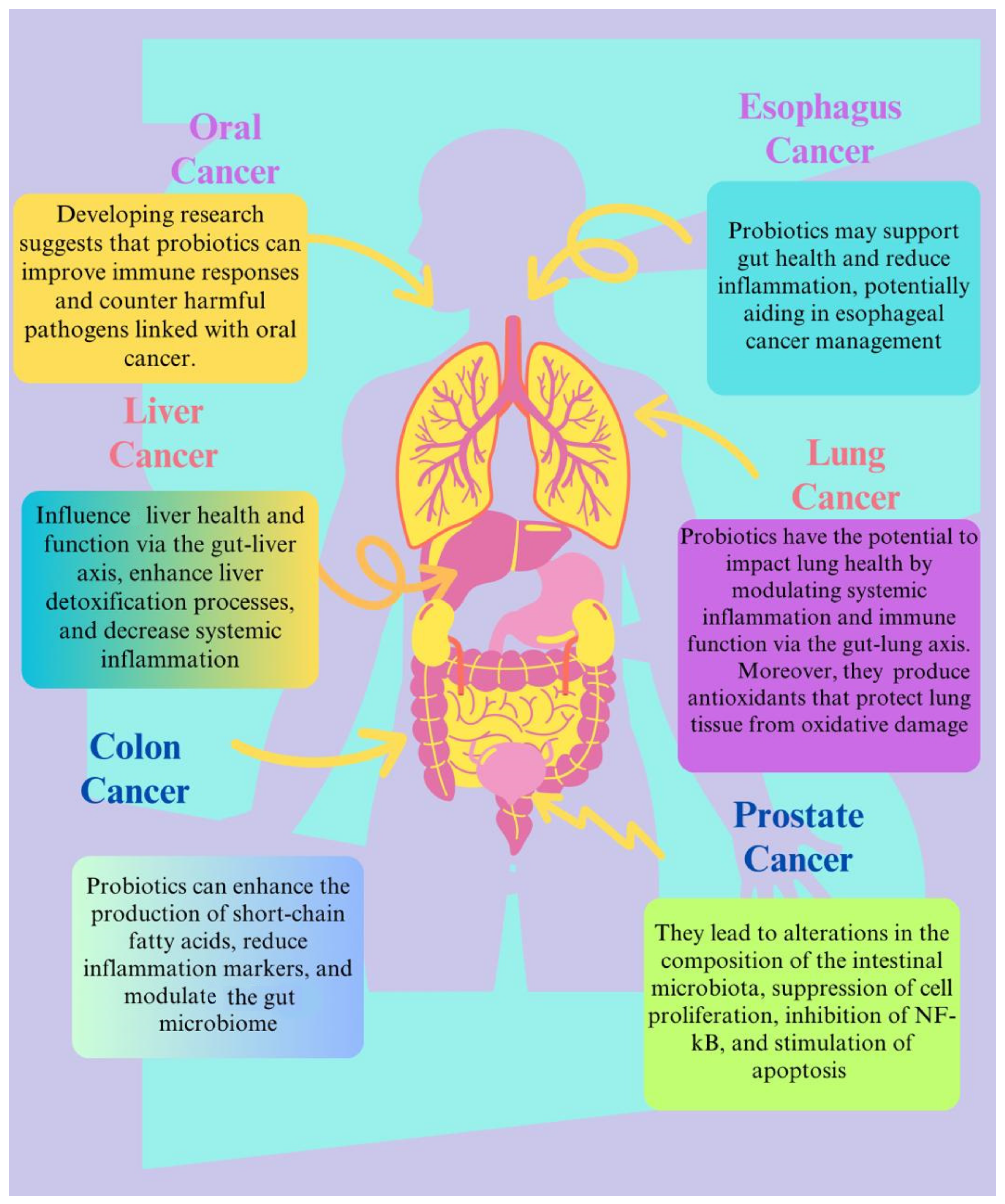
3. The Role of Probiotics in Cancer Management
3.1. Oral and Esophageal Cancer
| Types of Cancer | Probiotic Strains | Mechanisms | Remarks | References |
|---|---|---|---|---|
| Colon cancer | Lactobacillus plantarum YYC-3 | The mechanism involves the modification of the immune system, resulting in the downregulation of inflammatory cytokines interleukin (IL)-6, IL-17F, and IL-22 as well as a decrease in the infiltration of inflammatory cells. | Suppressed colon cancer cell lines and averted tumour development by altering immune response and gut microbiota. | [129] |
| Colorectal cancer | L. rhamnosus | Improves the functioning of the gut barrier, controls the immune system, and stops the growth of tumours. | Studies on animals revealed a lower incidence of tumours. May boost digestive system in general. | [130,131,132] |
| B. longum and L. acidophilus | Generate butyrate and other SCFAs, which have anti-inflammatory properties; strengthen the body’s defences against cancer. | SCFAs have the ability to trigger apoptosis and stop the growth of cancer cells. | ||
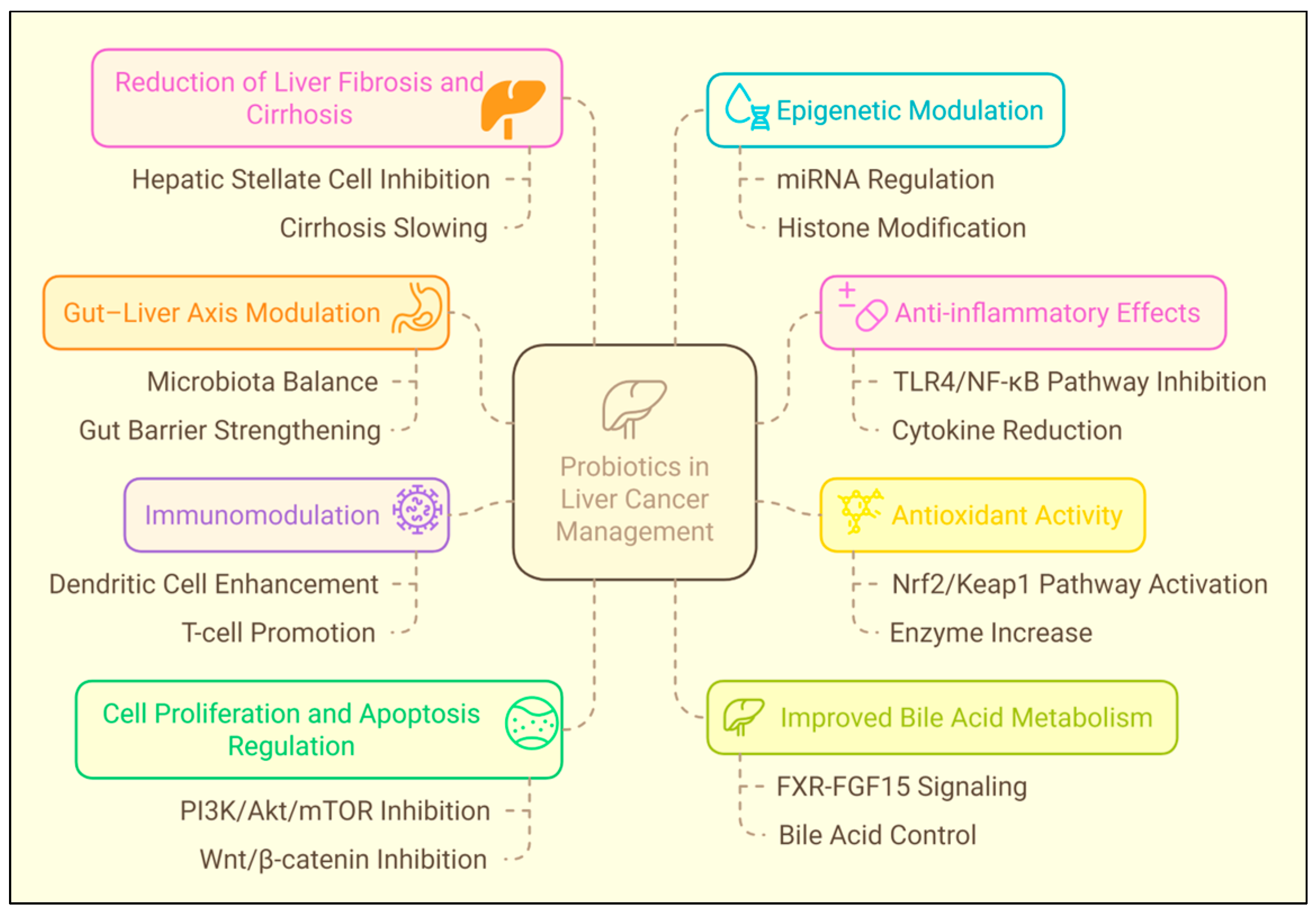
| Liver cancer | L. rhamnosus | Reduces liver inflammation and fibrosis by altering the gut microbiota. | There is little proof; more research is needed to confirm the effects. | [133,134] |
| Gastric cancer | L. acidophilus | Generates lactic acid, which inhibits harmful microorganisms and lowers the pH of the stomach. | May contribute to gut health improvement and a reduction in stomach inflammation. | [135] |
| L. casei | Boosts the immune system and may lessen stomach mucosal irritation. | Few studies have been conducted; additional study is required to confirm effects on stomach cancer. | [136]. | |
| L. plantarum | Improves the immunological response and changes the composition of the gut microbiome. | Potential advantages in lowering inflammation and enhancing gut health were suggested by research. | [137] | |
| Breast cancer | L. acidophilus | Alters the metabolism of oestrogen, which may lower oestrogen levels and strengthens the body’s defences against cancerous cells. | There may be a connection between breast cancer risk and gut bacteria, according to some research. | [138] |
| L. casei | Improves the immune system and, by modifying cytokines, may slow the growth of tumours. | Few studies have been conducted, research is still ongoing, while primary outcomes are promising, additional extensive clinical trials are required to establish certain effects and mechanisms in breast cancer. | [139] | |
| Bladder cancer | L. acidophilus | Creates lactic acid, which can support the preservation of a healthy environment in the urinary system. | Assisted in reducing the incidence of urinary tract infections, which may minimize the risk of bladder cancer. | [140] |
| Bifidobacterium longum | Reveals anti-angiogenesis and antiproliferation of bladder and stomach cancer. | Few studies have been conducted; additional study is required to confirm effects on stomach cancer. | [141] | |
| Oesophageal cancer | L. rhamnosus | Strengthens the immune system and may aid in lessening oesophageal irritation. | Enhancing gut health may have an indirect impact on oesophageal health, according to some research. | [128] |
| Oral cancer | Lactobacillus salivarius (L. salivarius) | Downregulates cyclooxygenase 2 (COX-2) expression, prohibits DNA from oxidative injury, and suppresses tumorigenesis. | Antiproliferative and apoptotic action of L. salivarius reported in oral cancer cell. | [142,143] |
| Cancer therapy-induced oral mucositis | Lactobacillus CD2, Bifidobacterium, and Lactobacillus species | Improve oral tissue repair, decrease inflammation, and strengthen mucosal immunity. | The application of probiotics in cancer therapy patients resulted in a reduction in both the incidence and severity of oral mucositis. | [144,145] |
| Prostate cancer | Lactobacillus acidophilus | Probiotics, including Lactobacillus acidophilus, contribute to regulating rectal volume fluctuations during radiation therapy for prostate cancer by altering gut microbiota, diminishing inflammation, and improving mucosal barrier integrity. | This resulted in reduced rectal volume fluctuations and enhanced tolerance to radiation therapy, ultimately stabilising prostate placement during treatment. | [146] |
| Breast cancer | Lactobacillus casei strains | Prevent the growth of breast tumours, reduce metastases, and prolong survival. | Minimized immunosuppression in metastatic regions while preserving a balanced inflammatory response. | [147,148] |
| Pancreatic cancer | Lactobacillus casei and Aspergillus oryzae | Promote apoptosis and dysregulation of the cell cycle and p38 MAPK pathway activation. | The probiotic exhibited its ability to prevent the proliferation of pancreatic cancer cells. | [149,150] |
| Various different cancers | Lactobacillus rhamnosus, Bifidobacterium longum, and Lactobacillus casei | These probiotics reduce inflammation and encourage cancer cell death by enhancing immune responses, altering the gut microbiota, and inactivating carcinogenic substances. | These probiotics modulated the microbiota in the gut and the immune response, making them effective adjuvants in the treatment and prevention of cancer. | [151,152] |
3.2. Colon Cancer
3.3. Hepatocellular Carcinoma
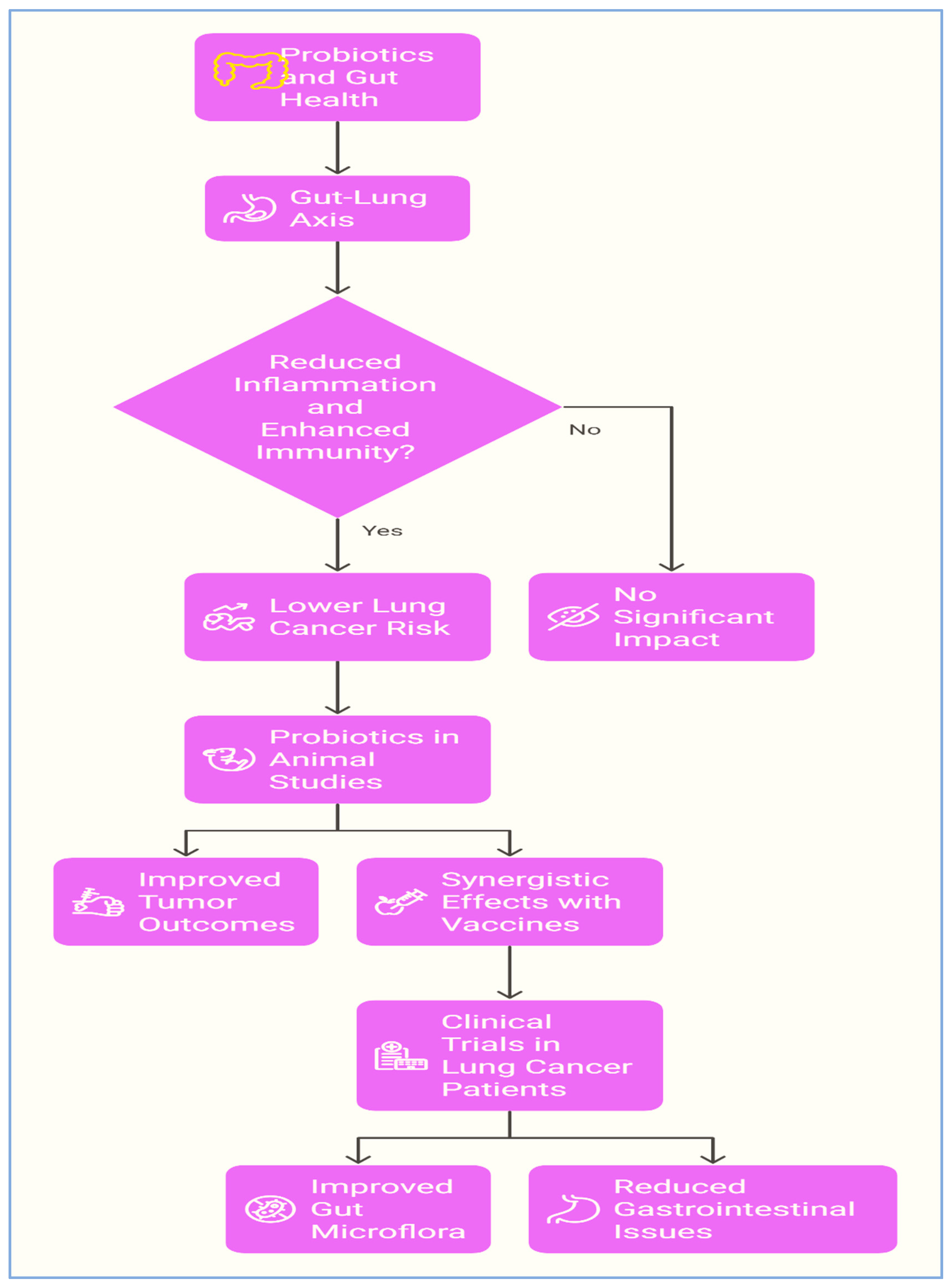
3.4. Lung Cancer
3.5. Breast Cancer
3.6. Prostate Cancer
3.7. Pancreatic Cancer
4. Probiotics Synergistic Relation with Chemotherapy
5. Probiotic-Derived Metabolites as Predictive Biomarkers
6. Clinical Studies
7. Protection from Chemotherapy-Induced Toxicity
8. Safety Considerations of Probiotics in Cancer Therapy
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Labi, V.; Erlacher, M. How cell death shapes cancer. Cell Death Disease 2015, 6, e1675. [Google Scholar] [CrossRef]
- Wahab, S.; Alshahrani, M.Y.; Ahmad, M.F.; Abbas, H. Current trends and future perspectives of nanomedicine for the management of colon cancer. Eur. J. Pharmacol. 2021, 910, 174464. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dunn, J. It is time to close the gap in cancer care. JCO Glob. Oncol. 2023, 9, e2200429. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: A rational pharmacological approach to surmount cancer. J. Ethnopharmacol. 2020, 260, 113047. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Laversanne, M.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F.; et al. Global Cancer Observatory: Cancer Tomorrow; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Conceição, P. Human Development Report 2019: Beyond Income, Beyond Averages, Beyond Today: Inequalities in Human Development in the 21st Century; UNDP: New York, NY, USA, 2019. [Google Scholar]
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Ashraf, S.A.; Elkhalifa, A.E.O.; Ahmad, M.F.; Patel, M.; Adnan, M.; Sulieman, A.M.E. Probiotic fermented foods and health promotion. In African Fermented Food Products—New Trends; Springer: Berlin/Heidelberg, Germany, 2022; pp. 59–88. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Act. Cecropin P1 FA-LL-37 Against Urogenit. Microflora. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. Probiotics: Definition, scope and mechanisms of action. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L. Probiotics: Definition and taxonomy 10 years after the FAO/WHO guidelines. Probiotic Bact. Their Eff. Hum. Health Well-Being 2013, 107, 1–8. [Google Scholar]
- Li, W.; Deng, X.; Chen, T. Exploring the modulatory effects of gut microbiota in anti-cancer therapy. Front. Oncol. 2021, 11, 644454. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Kumar, S.; Ahmad, M.F.; Nath, P.; Roy, R.; Bhattacharjee, R.; Shama, E.; Gahatraj, I.; Sehrawat, M.; Dasriya, V.; Dhillon, H.S.; et al. Controlling intestinal infections and digestive disorders using probiotics. J. Med. Food 2023, 26, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Dasriya, V.L.; Samtiya, M.; Ranveer, S.; Dhillon, H.S.; Devi, N.; Sharma, V.; Nikam, P.; Puniya, M.; Chaudhary, P.; Chaudhary, V.; et al. Modulation of gut-microbiota through probiotics and dietary interventions to improve host health. J. Sci. Food Agric. 2024, 104, 6359–6375. [Google Scholar] [CrossRef]
- Naeem, H.; Hassan, H.U.; Shahbaz, M.; Imran, M.; Memon, A.G.; Hasnain, A.; Murtaza, S.; Alsagaby, S.A.; Al Abdulmonem, W.; Hussain, M.; et al. Role of Probiotics against Human Cancers, Inflammatory Diseases, and Other Complex Malignancies. J. Food Biochem. 2024, 2024, 6632209. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Baretto Penna, A.L.; Venema, K.; Holzapfel, W.H.; Chikindas, M.L. Recommendations for the use of standardised abbreviations for the former Lactobacillus genera, reclassified in the year 2020. Benef. Microbes 2023, 15, 1–4. [Google Scholar] [CrossRef]
- Dos Reis, S.A.; da Conceição, L.L.; Siqueira, N.P.; Rosa, D.D.; da Silva, L.L.; Peluzio, M.d.C.G. Review of the mechanisms of probiotic actions in the prevention of colorectal cancer. Nutr. Res. 2017, 37, 1–19. [Google Scholar] [CrossRef]
- Sankarapandian, V.; Venmathi Maran, B.A.; Rajendran, R.L.; Jogalekar, M.P.; Gurunagarajan, S.; Krishnamoorthy, R.; Gangadaran, P.; Ahn, B.-C. An update on the effectiveness of probiotics in the prevention and treatment of cancer. Life 2022, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Badgeley, A.; Anwar, H.; Modi, K.; Murphy, P.; Lakshmikuttyamma, A. Effect of probiotics and gut microbiota on anti-cancer drugs: Mechanistic perspectives. Biochim. Biophys. Acta—Rev. Cancer 2021, 1875, 188494. [Google Scholar] [CrossRef]
- Molska, M.; Reguła, J. Potential mechanisms of probiotics action in the prevention and treatment of colorectal cancer. Nutrients 2019, 11, 2453. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Gunaratnam, S.; Millette, M.; McFarland, L.V.; DuPont, H.L.; Lacroix, M. Potential role of probiotics in reducing Clostridioides difficile virulence: Interference with quorum sensing systems. Microb. Pathog. 2021, 153, 104798. [Google Scholar] [CrossRef]
- Liu, H.; Yu, Y.; Dong, A.; Elsabahy, M.; Yang, Y.W.; Gao, H. Emerging strategies for combating Fusobacterium nucleatum in colorectal cancer treatment: Systematic review, improvements and future challenges. In Exploration; Wiley Online Library: Hoboken, NJ, USA; p. 20230092.
- Fijan, S.; Šulc, D.; Steyer, A. Study of the in vitro antagonistic activity of various single-strain and multi-strain probiotics against Escherichia coli. Int. J. Environ. Res. Public health 2018, 15, 1539. [Google Scholar] [CrossRef]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Denkova, R.; Goranov, B.; Teneva, D.; Denkova, Z.; Kostov, G. Antimicrobial activity of probiotic microorganisms: Mechanisms of interaction and methods of examination. Antimicrob. Res. Nov. Bioknowl. Educ. Programs 2017, 1, 201–212. [Google Scholar]
- Kobayashi, J. Effect of diet and gut environment on the gastrointestinal formation of N-nitroso compounds: A review. Nitric Oxide 2018, 73, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, M.R.; Shohag, S.; Ahasan, M.T.; Sarkar, N.; Khan, H.; Hasan, A.M.; Cavalu, S.; Rauf, A. Microbiome in cancer: Role in carcinogenesis and impact in therapeutic strategies. Biomed. Pharmacother. 2022, 149, 112898. [Google Scholar] [CrossRef] [PubMed]
- Górska, A.; Przystupski, D.; Niemczura, M.J.; Kulbacka, J. Probiotic bacteria: A promising tool in cancer prevention and therapy. Curr. Microbiol. 2019, 76, 939–949. [Google Scholar] [CrossRef]
- Ocvirk, S.; O’Keefe, S.J. Influence of bile acids on colorectal cancer risk: Potential mechanisms mediated by diet-gut microbiota interactions. Curr. Nutr. Rep. 2017, 6, 315–322. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Sun, B.; Ma, L.; Guo, L.; Huang, L.; Gao, W. Gut liver brain axis in diseases: The implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 443. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Y.; Wang, S.; Zhang, Q.; Zhao, J.; Zhang, H.; Narbad, A.; Tian, F.; Zhai, Q.; Chen, W. Cholestasis: Exploring the triangular relationship of gut microbiota-bile acid-cholestasis and the potential probiotic strategies. Gut Microbes 2023, 15, 2181930. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, prebiotics and epithelial tight junctions: A promising approach to modulate intestinal barrier function. Int. J. Mol. Sci. 2021, 22, 6729. [Google Scholar] [CrossRef] [PubMed]
- Genua, F.; Raghunathan, V.; Jenab, M.; Gallagher, W.M.; Hughes, D.J. The role of gut barrier dysfunction and microbiome dysbiosis in colorectal cancer development. Front. Oncol. 2021, 11, 626349. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Mao, Q.; Xia, W.; Dong, G.; Yu, C.; Jiang, F. Gut microbiota shapes the efficiency of cancer therapy. Front. Microbiol. 2019, 10, 1050. [Google Scholar] [CrossRef]
- Senan, S.; Prajapati, J.B.; Joshi, C.G.; Sreeja, V.; Gohel, M.K.; Trivedi, S.; Patel, R.M.; Pandya, H.; Singh, U.S.; Phatak, A.; et al. Geriatric respondents and non-respondents to probiotic intervention can be differentiated by inherent gut microbiome composition. Front. Microbiol. 2015, 6, 944. [Google Scholar] [CrossRef]
- Zhao, F.; Tie, N.; Kwok, L.-Y.; Ma, T.; Wang, J.; Man, D.; Yuan, X.; Li, H.; Pang, L.; Shi, H.; et al. Baseline gut microbiome as a predictive biomarker of response to probiotic adjuvant treatment in gout management. Pharmacol. Res. 2024, 209, 107445. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.; Szulińska, M.; Łoniewski, I.; Kręgielska-Narożna, M.; Skonieczna-Żydecka, K.; Kosciolek, T.; Bezshapkin, V.; Bogdański, P.; Microbiology, I. Treatment with multi-species probiotics changes the functions, not the composition of gut microbiota in postmenopausal women with obesity: A randomized, double-blind, placebo-controlled study. Front. Cell. Infect. Microbiol. 2022, 12, 815798. [Google Scholar] [CrossRef]
- Vizioli, C.; Jaime-Lara, R.; Daniel, S.G.; Franks, A.; Diallo, A.F.; Bittinger, K.; Tan, T.P.; Merenstein, D.J.; Brooks, B.; Joseph, P.V.; et al. Administration of Bifidobacterium animalis subsp. lactis strain BB-12® in healthy children: Characterization, functional composition, and metabolism of the gut microbiome. Front. Microbiol 2023, 14, 1165771. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Med. 2016, 8, 1–11. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H.J.P. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Sniffen, J.C.; McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Choosing an appropriate probiotic product for your patient: An evidence-based practical guide. PLoS ONE 2018, 13, e0209205. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-specificity and disease-specificity of probiotic efficacy: A systematic review and meta-analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Eid, E.E.M.; Hussin, S.; Alshawsh, M.A. Probiotics supplementation in patients with colorectal cancer: A systematic review of randomized controlled trials. Nutr. Rev. 2022, 80, 22–49. [Google Scholar] [CrossRef]
- Sehrawat, N.; Yadav, M.; Singh, M.; Kumar, V.; Sharma, V.R.; Sharma, A.K. Probiotics in microbiome ecological balance providing a therapeutic window against cancer. Semin. Cancer Biol. 2021, 70, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kang, J.H.; Kim, J.H.; Jeong, J.W.; Kim, H.W.; Oh, D.H.; Yoon, S.H.; Hur, S.J. Relationship between gut microbiota and colorectal cancer: Probiotics as a potential strategy for prevention. Food Res. Int. 2022, 156, 111327. [Google Scholar] [CrossRef]
- Gayathri, D.; Rashmi, B. Anti-cancer properties of probiotics: A natural strategy for cancer prevention. EC Nutr. 2016, 5, 1191–1202. [Google Scholar]
- Knasmüller, S.; Steinkellner, H.; Hirschl, A.M.; Rabot, S.; Nobis, E.C.; Kassie, F. Impact of bacteria in dairy products and of the intestinal microflora on the genotoxic and carcinogenic effects of heterocyclic aromatic amines. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 480, 129–138. [Google Scholar] [CrossRef]
- Schwab, C.E.; Huber, W.W.; Parzefall, W.; Hietsch, G.; Kassie, F.; Schulte-Hermann, R.; Knasmüller, S. Search for compounds that inhibit the genotoxic and carcinogenic effects of heterocyclic aromatic amines. Crit. Rev. Toxicol. 2000, 30, 1–69. [Google Scholar] [CrossRef]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The role of probiotics in cancer prevention. Cancers 2020, 13, 20. [Google Scholar] [CrossRef]
- Nowak, A.; Paliwoda, A.; Błasiak, J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 3456–3467. [Google Scholar] [CrossRef]
- Gao, H.; Li, X.; Chen, X.; Hai, D.; Wei, C.; Zhang, L.; Li, P. The functional roles of Lactobacillus acidophilus in different physiological and pathological processes. J. Microbiol. Biotechnol. 2022, 32, 1226. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.d.M.d.; Perdigón, G. Reduction of b-glucuronidase and nitroreductase activity by yoghurt in a murine colon cancer model. Biocell 2005, 29, 15–24. [Google Scholar]
- Rafter, J.J. The role of lactic acid bacteria in colon cancer prevention. Scand. J. Gastroenterol. 1995, 30, 497–502. [Google Scholar] [CrossRef]
- Seshadri, S.; Gajjar, D.; Joshi, A.; Bhatia, Z.; Kumar, S. Understanding and role of Gut Microbiota on drug response and toxicity. J. Toxicol. Stud. 2024, 2, 1252. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Cui, J.Y. Mechanisms of how the intestinal microbiota alters the effects of drugs and bile acids. Drug Metab. Dispos. 2015, 43, 1505–1521. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.-J.; Hylemon, P.B.; Bajaj, J.S. Gut microbiota, cirrhosis, and alcohol regulate bile acid metabolism in the gut. Dig. Dis. 2015, 33, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Tonacci, A. Tumor Microbial Communities and Thyroid Cancer Development—The Protective Role of Antioxidant Nutrients: Application Strategies and Future Directions. Antioxidants 2023, 12, 1898. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef]
- Sharma, M.; Arora, I.; Stoll, M.L.; Li, Y.; Morrow, C.D.; Barnes, S.; Berryhill, T.F.; Li, S.; Tollefsbol, T.O. Nutritional combinatorial impact on the gut microbiota and plasma short-chain fatty acids levels in the prevention of mammary cancer in Her2/neu estrogen receptor-negative transgenic mice. PLoS ONE 2020, 15, e0234893. [Google Scholar] [CrossRef]
- Mirzaei, R.; Afaghi, A.; Babakhani, S.; Sohrabi, M.R.; Hosseini-Fard, S.R.; Babolhavaeji, K.; Akbari, S.K.A.; Yousefimashouf, R.; Karampoor, S. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed. Pharmacother. 2021, 139, 111619. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Faller, D.V.; Spanjaard, R.A. Short-chain fatty acid inhibitors of histone deacetylases: Promising anticancer therapeutics? Curr. Cancer Drug Targets 2003, 3, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nagpal, R.; Verma, V.; Kumar, A.; Kaur, N.; Hemalatha, R.; Gautam, S.K.; Singh, B. Probiotic metabolites as epigenetic targets in the prevention of colon cancer. Nutr. Rev. 2013, 71, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, A.; Nagpal, R.; Mohania, D.; Behare, P.; Verma, V.; Kumar, P.; Poddar, D.; Aggarwal, P.K.; Henry, C. Cancer-preventing attributes of probiotics: An update. Int. J. Food Sci. Nutr. 2010, 61, 473–496. [Google Scholar] [CrossRef]
- Liotti, F.; Marotta, M.; Sorriento, D.; Pagliuca, C.; Caturano, V.; Mantova, G.; Scaglione, E.; Salvatore, P.; Melillo, R.M.; Prevete, N. Probiotic Lactobacillus rhamnosus GG (LGG) restrains the angiogenic potential of colorectal carcinoma cells by activating a proresolving program via formyl peptide receptor 1. Mol. Oncol. 2022, 16, 2959–2980. [Google Scholar] [CrossRef]
- Amedei, A.; Morbidelli, L. Circulating metabolites originating from gut microbiota control endothelial cell function. Molecules 2019, 24, 3992. [Google Scholar] [CrossRef]
- Chen, X.; Yang, G.; Song, J.-H.; Xu, H.; Li, D.; Goldsmith, J.; Zeng, H.; Parsons-Wingerter, P.A.; Reinecker, H.-C.; Kelly, C.P. Probiotic yeast inhibits VEGFR signaling and angiogenesis in intestinal inflammation. PLoS ONE 2013, 8, e64227. [Google Scholar] [CrossRef]
- Yue, Y.-C.; Yang, B.-Y.; Lu, J.; Zhang, S.-W.; Liu, L.; Nassar, K.; Xu, X.-X.; Pang, X.-Y.; Lv, J.-P. Metabolite secretions of Lactobacillus plantarum YYC-3 may inhibit colon cancer cell metastasis by suppressing the VEGF-MMP2/9 signaling pathway. Microb. Cell Factories 2020, 19, 213. [Google Scholar] [CrossRef]
- Escamilla, J.; Lane, M.A.; Maitin, V. Cell-free supernatants from probiotic Lactobacillus casei and Lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutr. Cancer 2012, 64, 871–878. [Google Scholar] [CrossRef]
- Mangrolia, U.; Osborne, J.W. Probiotics in Counteracting the Role of Neutrophils in Cancer Metastasis. Vaccines 2021, 9, 1306. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial mesenchymal transition in tumor metastasis. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Dong, Y.; Li, M.; Zhang, W.; Ding, Y.; Wang, X.; Chen, D.; Liu, T.; Wang, B.; Cao, H.; et al. Clostridium butyricum inhibits epithelial–mesenchymal transition of intestinal carcinogenesis through downregulating METTL3. Cancer Sci. 2023, 114, 3114–3127. [Google Scholar] [CrossRef]
- Motevaseli, E.; Dianatpour, A.; Ghafouri-Fard, S. The role of probiotics in cancer treatment: Emphasis on their in vivo and in vitro anti-metastatic effects. Int. J. Mol. Cell. Med. 2017, 6, 66. [Google Scholar] [PubMed]
- Liu, J.; Chen, X.; Zhou, X.; Yi, R.; Yang, Z.; Zhao, X. Lactobacillus fermentum ZS09 mediates epithelial–mesenchymal transition (EMT) by regulating the transcriptional activity of the Wnt/β-Catenin signalling pathway to Inhibit colon cancer activity. J. Inflamm. Res. 2021, 14, 7281–7293. [Google Scholar] [CrossRef]
- Vergara, D.; Simeone, P.; Damato, M.; Maffia, M.; Lanuti, P.; Trerotola, M. The cancer microbiota: EMT and inflammation as shared molecular mechanisms associated with plasticity and progression. J. Oncol. 2019, 2019, 1253727. [Google Scholar] [CrossRef]
- Khalil, M.A.; Sonbol, F.I.; Al-Madboly, L.A.; Aboshady, T.A.; Alqurashi, A.S.; Ali, S.S. Exploring the therapeutic potentials of exopolysaccharides derived from lactic acid bacteria and bifidobacteria: Antioxidant, antitumor, and periodontal regeneration. Front. Microbiol. 2022, 13, 803688. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, D.; Yang, Y.; Jiang, X.; Zhang, J.; Zeng, X.; Wu, Z.; Sun, Y.; Guo, Y. Effect of Lactobacillus acidophilus CICC 6074 S-layer protein on colon cancer HT-29 cell proliferation and apoptosis. J. Agric. Food Chem. 2020, 68, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Blottière, H.M.; Buecher, B.; Galmiche, J.-P.; Cherbut, C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc. Natl. Acad. Sci. USA 2003, 62, 101–106. [Google Scholar] [CrossRef]
- Fattahi, Y.; Heidari, H.R.; Khosroushahi, A.Y. Review of short-chain fatty acids effects on the immune system and cancer. Food Biosci. 2020, 38, 100793. [Google Scholar] [CrossRef]
- Bedada, T.L.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar]
- Masheghati, F.; Asgharzadeh, M.R.; Jafari, A.; Masoudi, N.; Maleki-Kakelar, H. The role of gut microbiota and probiotics in preventing, treating, and boosting the immune system in colorectal cancer. Life Sci. 2024, 344, 122529. [Google Scholar] [CrossRef]
- Agrawal, B. New therapeutic targets for cancer: The interplay between immune and metabolic checkpoints and gut microbiota. Clin. Transl. Med. 2019, 8, 23. [Google Scholar] [CrossRef]
- Liu, C.; Fu, L.; Wang, Y.; Yang, W. Influence of the gut microbiota on immune cell interactions and cancer treatment. J. Transl. Med. 2024, 22, 939. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, C.; Yue, J. Radiotherapy and the gut microbiome: Facts and fiction. Radiat. Oncol. 2021, 16, 9. [Google Scholar] [CrossRef]
- Ashraf, R.; Vasiljevic, T.; Day, S.L.; Smith, S.C.; Donkor, O. Lactic acid bacteria and probiotic organisms induce different cytokine profile and regulatory T cells mechanisms. J. Funct. Foods 2014, 6, 395–409. [Google Scholar] [CrossRef]
- Saito, S.; Okuno, A.; Peng, Z.; Cao, D.-Y.; Tsuji, N.M. Probiotic lactic acid bacteria promote anti-tumor immunity through enhanced major histocompatibility complex class I-restricted antigen presentation machinery in dendritic cells. Front. Immunol. 2024, 15, 1335975. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Türker, N.; Toh, Z.Q.; Karagiannis, T.C.; Licciardi, P.V. Anti-inflammatory effects of probiotics and their metabolites: Possible role for epigenetic effects. In Molecular Mechanisms and Physiology of Disease: Implications for Epigenetics and Health; Springer: New York, NY, USA, 2014; pp. 127–150. [Google Scholar]
- Vahed, S.Z.; Barzegari, A.; Saadat, Y.R.; Goreyshi, A.; Omidi, Y. Leuconostoc mesenteroides-derived anticancer pharmaceuticals hinder inflammation and cell survival in colon cancer cells by modulating NF-κB/AKT/PTEN/MAPK pathways. Biomed. Pharmacother. 2017, 94, 1094–1100. [Google Scholar] [CrossRef]
- Dubey, V.; Ghosh, A. Probiotics cross talk with multi cell signaling in colon carcinogenesis. J. Prob. Health 2013, 1, 2–5. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Gomez-Llorente, C.; Fontana, L.; Gil, A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J. Gastroenterol. 2014, 20, 15632. [Google Scholar] [CrossRef]
- Lescheid, D.W. Probiotics as regulators of inflammation: A review. Funct. Foods Health Dis. 2014, 4, 299–311. [Google Scholar] [CrossRef]
- Fernández, J.; Redondo-Blanco, S.; Gutiérrez-del-Río, I.; Miguélez, E.M.; Villar, C.J.; Lombo, F. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: A review. J. Funct. Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Nakkarach, A.; Foo, H.L.; Song, A.A.-L.; Mutalib, N.E.A.; Nitisinprasert, S.; Withayagiat, U. Anti-cancer and anti-inflammatory effects elicited by short chain fatty acids produced by Escherichia coli isolated from healthy human gut microbiota. Microb. Cell Factories 2021, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Hevia, D.; Patchva, S.; Park, B.; Koh, W.; Aggarwal, B.B. Upsides and downsides of reactive oxygen species for cancer: The roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid. Redox Signal. 2012, 16, 1295–1322. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Spyropoulos, B.G.; Misiakos, E.P.; Fotiadis, C.; Stoidis, C.N. Antioxidant properties of probiotics and their protective effects in the pathogenesis of radiation-induced enteritis and colitis. Dig. Dis. Sci. 2011, 56, 285–294. [Google Scholar] [CrossRef]
- Averina, O.V.; Poluektova, E.U.; Marsova, M.V.; Danilenko, V.N. Biomarkers and utility of the antioxidant potential of probiotic Lactobacilli and Bifidobacteria as representatives of the human gut microbiota. Biomedicines 2021, 9, 1340. [Google Scholar] [CrossRef]
- Gao, D.; Gao, Z.; Zhu, G. Antioxidant effects of Lactobacillus plantarum via activation of transcription factor Nrf2. Food Funct. 2013, 4, 982–989. [Google Scholar] [CrossRef]
- Aboulgheit, A.; Karbasiafshar, C.; Zhang, Z.; Sabra, M.; Shi, G.; Tucker, A.; Sodha, N.; Abid, M.R.; Sellke, F.W. Lactobacillus plantarum probiotic induces Nrf2-mediated antioxidant signaling and eNOS expression resulting in improvement of myocardial diastolic function. Am. J. Physiol.-Heart Circ. Physiol. 2021, 321, H839–H849. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Gaisawat, M.B.; Iskandar, M.M.; MacPherson, C.W.; Tompkins, T.A.; Kubow, S. Probiotic supplementation is associated with increased antioxidant capacity and copper chelation in C. difficile-infected fecal water. Nutrients 2019, 11, 2007. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.S.; Saeed, A.; Baig, M.; Asif, N.; Masood, N.; Yasmin, A. Anticarcinogenecity of microbiota and probiotics in breast cancer. Int. J. Food Prop. 2018, 21, 655–666. [Google Scholar] [CrossRef]
- Celebioglu, H.U. Effects of potential synbiotic interaction between Lactobacillus rhamnosus GG and salicylic acid on human colon and prostate cancer cells. Arch. Microbiol. 2021, 203, 1221–1229. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Rodriguez-Arrastia, M.; Martinez-Ortigosa, A.; Rueda-Ruzafa, L.; Folch Ayora, A.; Ropero-Padilla, C. Probiotic supplements on oncology patients’ treatment-related side effects: A systematic review of randomized controlled trials. Int. J. Environ. Res. Public Health 2021, 18, 4265. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Kleniewska, P.; Pawliczak, R. Antioxidative activity of probiotics. Arch. Med. Sci. AMS 2021, 17, 792. [Google Scholar] [CrossRef]
- Geier, M.S.; Butler, R.N.; Howarth, G.S. Probiotics, prebiotics and synbiotics: A role in chemoprevention for colorectal cancer? Cancer Biol. Ther. 2006, 5, 1265–1269. [Google Scholar] [CrossRef]
- Shu, Z.; Li, P.; Yu, B.; Huang, S.; Chen, Y. The effectiveness of probiotics in prevention and treatment of cancer therapy-induced oral mucositis: A systematic review and meta-analysis. Oral Oncol. 2020, 102, 104559. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, C.; Li, W.; Wei, J.; Hong, H.; Li, J.; Feng, L.; Wei, H.; Xin, H.; Chen, T. A phase II randomized clinical trial and mechanistic studies using improved probiotics to prevent oral mucositis induced by concurrent radiotherapy and chemotherapy in nasopharyngeal carcinoma. Front. Immunol. 2021, 12, 618150. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Wu, C.-R.; Huang, T.-W. Preventive effect of probiotics on oral mucositis induced by cancer treatment: A systematic review and meta-analysis. Int. J. Mol. Sci. 2022, 23, 13268. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Zhuang, J.; Lai, J.; Cui, J.; Jiang, D.; Huang, J. Efficacy of probiotics in the treatment of oral mucositis in head and neck cancer patients: A systematic review and meta-analysis. Microb. Pathog. 2024, 193, 106785. [Google Scholar] [CrossRef]
- Vasanthi, V.; Sanjeev, K.; Rajkumar, K.; Divya, B.; Rameshkumar, A.; Swarup, S.; Ramadoss, R. Immune modulation by probiotics in deterring carcinogenesis with an emphasis on oral cancer: A narrative review. Cancer Res. Stat. Treat. 2023, 6, 425–431. [Google Scholar] [CrossRef]
- Kumar, S.S. Can probiotics stop oral cancer progression? Evid.-Based Dent. 2022, 23, 22–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, M.; Liu, Y.; Guo, C.; Zhou, Y.; Li, F.; Xu, R.; Liu, Z.; Deng, Q.; Li, X.; et al. Oral microbiome and risk of malignant esophageal lesions in a high-risk area of China: A nested case-control study. Chin. J. Cancer Res. 2020, 32, 742. [Google Scholar] [CrossRef] [PubMed]
- Reitano, E.; De’Angelis, N.; Gavriilidis, P.; Gaiani, F.; Memeo, R.; Inchingolo, R.; Bianchi, G.; De’Angelis, G.L.; Carra, M.C. Oral bacterial microbiota in digestive cancer patients: A systematic review. Microorganisms 2021, 9, 2585. [Google Scholar] [CrossRef]
- Peters, B.A.; Wu, J.; Pei, Z.; Yang, L.; Purdue, M.P.; Freedman, N.D.; Jacobs, E.J.; Gapstur, S.M.; Hayes, R.B.; Ahn, J. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res. 2017, 77, 6777–6787. [Google Scholar] [CrossRef]
- Stasiewicz, M.; Karpiński, T.M. The oral microbiota and its role in carcinogenesis. Semin. Cancer Biol. 2022, 86, 633–642. [Google Scholar] [CrossRef]
- Kawasaki, M.; Ikeda, Y.; Ikeda, E.; Takahashi, M.; Tanaka, D.; Nakajima, Y.; Arakawa, S.; Izumi, Y.; Miyake, S. Oral infectious bacteria in dental plaque and saliva as risk factors in patients with esophageal cancer. Cancer 2021, 127, 512–519. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, T.; Yan, Y.; Zhang, Y.; Li, Z.; Wang, Y.; Yang, J.; Xia, Y.; Xiao, H.; Han, H.; et al. Alterations of oral microbiota in Chinese patients with esophageal cancer. Front. Cell. Infect. Microbiol. 2020, 10, 541144. [Google Scholar] [CrossRef]
- Jordan, T.; Mastnak, D.M.; Palamar, N.; Kozjek, N.R. Nutritional therapy for patients with esophageal cancer. Nutr. Cancer 2018, 70, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Roviello, G.; Catalano, M.; Polom, K. Gut microbiota modulation in the context of immune-related aspects of Lactobacillus spp. and Bifidobacterium spp. in gastrointestinal cancers. Nutrients 2021, 13, 2674. [Google Scholar] [CrossRef]
- Hashemi-Khah, M.-s.; Arbab-Soleimani, N.; Forghanifard, M.-M.; Gholami, O.; Taheri, S.; Amoueian, S. An In vivo study of Lactobacillus rhamnosus (PTCC 1637) as a new therapeutic candidate in esophageal cancer. BioMed Res. Int. 2022, 2022, 7607470. [Google Scholar] [CrossRef]
- Yue, Y.; Ye, K.; Lu, J.; Wang, X.; Zhang, S.; Liu, L.; Yang, B.; Nassar, K.; Xu, X.; Pang, X.; et al. Probiotic strain Lactobacillus plantarum YYC-3 prevents colon cancer in mice by regulating the tumour microenvironment. Biomed. Pharmacother. 2020, 127, 110159. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Navidifar, T.; Saeb, S.; Barzegari, E.; Jamalan, M. Tumor-targeted induction of intrinsic apoptosis in colon cancer cells by Lactobacillus plantarum and Lactobacillus rhamnosus strains. Mol. Biol. Rep. 2023, 50, 5345–5354. [Google Scholar] [CrossRef] [PubMed]
- Rafter, J.; Bennett, M.; Caderni, G.; Clune, Y.; Hughes, R.; Karlsson, P.C.; Klinder, A.; O’Riordan, M.; O’Sullivan, G.C.; Pool-Zobel, B.; et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007, 85, 488–496. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, X.; Covasa, M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J. Gastroenterol 2014, 20, 7878. [Google Scholar] [CrossRef]
- Won, S.M.; Lee, N.Y.; Oh, K.-K.; Gupta, H.; Sharma, S.P.; Kim, K.H.; Kim, B.K.; Joung, H.C.; Jeong, J.J.; Ganesan, R.; et al. Gut Lactobacillus and probiotics Lactobacillus lactis/rhamnosis ameliorate liver fibrosis in prevention and treatment. J. Microbiol. 2023, 61, 245–257. [Google Scholar] [CrossRef]
- Gu, Z.; Wu, Y.; Wang, Y.; Sun, H.; You, Y.; Piao, C.; Liu, J.; Wang, Y. Lactobacillus rhamnosus granules dose-dependently balance intestinal microbiome disorders and ameliorate chronic alcohol-induced liver injury. J. Med. Food 2020, 23, 114–124. [Google Scholar] [CrossRef]
- Li, Z.-P.; Liu, J.-X.; Lu, L.-L.; Wang, L.-L.; Xu, L.; Guo, Z.-H.; Dong, Q.-J. Overgrowth of Lactobacillus in gastric cancer. World J. Gastrointest. Oncol. 2021, 13, 1099. [Google Scholar] [CrossRef]
- Hwang, J.W.; Baek, Y.-M.; Yang, K.E.; Yoo, H.-S.; Cho, C.-K.; Lee, Y.-W.; Park, J.; Eom, C.-Y.; Lee, Z.-W.; Choi, J.-S.; et al. Lactobacillus casei extract induces apoptosis in gastric cancer by inhibiting NF-κB and mTOR-mediated signaling. Integr. Cancer Ther. 2013, 12, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Maleki-Kakelar, H.; Dehghani, J.; Barzegari, A.; Barar, J.; Shirmohamadi, M.; Sadeghi, J.; Omidi, Y. Lactobacillus plantarum induces apoptosis in gastric cancer cells via modulation of signaling pathways in Helicobacter pylori. BioImpacts 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Maroof, H.; Hassan, Z.M.; Mobarez, A.M.; Mohamadabadi, M.A. Lactobacillus acidophilus could modulate the immune response against breast cancer in murine model. J. Clin. Immunol. 2012, 32, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Aragón, F.; Carino, S.; Perdigón, G.; LeBlanc, A.d.M.d. Inhibition of growth and metastasis of breast cancer in mice by milk fermented with Lactobacillus casei CRL 431. J. Immunother. 2015, 38, 185–196. [Google Scholar] [CrossRef]
- Kitagawa, K.; Tatsumi, M.; Kato, M.; Komai, S.; Doi, H.; Hashii, Y.; Katayama, T.; Fujisawa, M.; Shirakawa, T. An oral cancer vaccine using a Bifidobacterium vector suppresses tumor growth in a syngeneic mouse bladder cancer model. Mol. Ther.—Oncolytics 2021, 22, 592–603. [Google Scholar] [CrossRef]
- Nada, H.G.; Sudha, T.; Darwish, N.H.; Mousa, S.A. Lactobacillus acidophilus and Bifidobacterium longum exhibit antiproliferation, anti-angiogenesis of gastric and bladder cancer: Impact of COX2 inhibition. PharmaNutrition 2020, 14, 100219. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Fang, B.; Chen, L. Effects of Lactobacillus salivarius on oral cancer cell proliferation and apoptosis in vitro. Carpathian J. Food Sci. Technol. 2016, 8, 152–158. [Google Scholar]
- Aghazadeh, Z.; Pouralibaba, F.; Khosroushahi, A.Y. The prophylactic effect of Acetobacter syzygii probiotic species against squamous cell carcinoma. J. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 208. [Google Scholar]
- Feng, J.; Gao, M.; Zhao, C.; Yang, J.; Gao, H.; Lu, X.; Ju, R.; Zhang, X.; Zhang, Y. Oral administration of probiotics reduces chemotherapy-induced diarrhea and oral mucositis: A systematic review and meta-analysis. Front. Nutr. 2022, 9, 823288. [Google Scholar] [CrossRef]
- Lu, K.; Dong, S.; Wu, X.; Jin, R.; Chen, H. Probiotics in cancer. Front. Oncol. 2021, 11, 638148. [Google Scholar] [CrossRef]
- Ki, Y.; Kim, W.; Nam, J.; Kim, D.; Lee, J.; Park, D.; Jeon, H.; Ha, H.; Kim, T.; Kim, D. Probiotics for rectal volume variation during radiation therapy for prostate cancer. Int. J. Radiat. Oncol. 2013, 87, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Aragón, F.; Carino, S.; Perdigón, G.; De Moreno De LeBlanc, A. The administration of milk fermented by the probiotic Lactobacillus casei CRL 431 exerts an immunomodulatory effect against a breast tumour in a mouse model. Immunobiology 2014, 219, 457–464. [Google Scholar] [CrossRef]
- Utz, V.E.M.; Perdigón, G.; LeBlanc, A.d.M.d. Oral administration of milk fermented by Lactobacillus casei CRL431 was able to decrease metastasis from breast cancer in a murine model by modulating immune response locally in the lungs. J. Funct. Foods 2019, 54, 263–270. [Google Scholar]
- Konishi, H.; Isozaki, S.; Kashima, S.; Moriichi, K.; Ichikawa, S.; Yamamoto, K.; Yamamura, C.; Ando, K.; Ueno, N.; Akutsu, H.; et al. Probiotic Aspergillus oryzae produces anti-tumor mediator and exerts anti-tumor effects in pancreatic cancer through the p38 MAPK signaling pathway. Sci. Rep. 2021, 11, 11070. [Google Scholar] [CrossRef] [PubMed]
- Kita, A.; Fujiya, M.; Konishi, H.; Tanaka, H.; Kashima, S.; Iwama, T.; Ijiri, M.; Murakami, Y.; Takauji, S.; Goto, T.; et al. Probiotic-derived ferrichrome inhibits the growth of refractory pancreatic cancer cells. Int. J. Oncol. 2020, 57, 721–732. [Google Scholar] [CrossRef]
- Vivarelli, S.; Falzone, L.; Basile, M.S.; Nicolosi, D.; Genovese, C.; Libra, M.; Salmeri, M. Benefits of using probiotics as adjuvants in anticancer therapy. World Acad. Sci. J. 2019, 1, 125–135. [Google Scholar] [CrossRef]
- Nama, A.A.; Sandeepa, G.M.; Buddolla, V.; Mastan, A. Advances in understanding therapeutic mechanisms of probiotics in cancer management, with special emphasis on breast cancer: A comprehensive review. Eur. J. Pharmacol. 2025, 995, 177410. [Google Scholar] [CrossRef]
- Drago, L. Probiotics and colon cancer. Microorganisms 2019, 7, 66. [Google Scholar] [CrossRef]
- Hatakka, K.; Holma, R.; El-Nezami, H.; Suomalainen, T.; Kuisma, M.; Saxelin, M.; Poussa, T.; Mykkänen, H.; Korpela, R. The influence of Lactobacillus rhamnosus LC705 together with Propionibacterium freudenreichii ssp. shermanii JS on potentially carcinogenic bacterial activity in human colon. Int. J. Food Microbiol. 2008, 128, 406–410. [Google Scholar] [CrossRef]
- Ohara, T.; Yoshino, K.; Kitajima, M. Possibility of preventing colorectal carcinogenesis with probiotics. Hepato-Gastroenterology 2010, 57, 1411–1415. [Google Scholar]
- Liu, Z.; Qin, H.; Yang, Z.; Xia, Y.; Liu, W.; Yang, J.; Jiang, Y.; Zhang, H.; Yang, Z.; Wang, Y. Randomised clinical trial: The effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery—A double-blind study. Aliment. Pharmacol. Ther. 2011, 33, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Gao, R.; Wu, W.; Qin, H. The role of gut microbiota in the pathogenesis of colorectal cancer. Tumor Biol. 2013, 34, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Perdigón, G.; Duarte, J.; Farnworth, E.; Matar, C. Effects of the oral administration of the exopolysaccharide produced by Lactobacillus kefiranofaciens on the gut mucosal immunity. Cytokine 2006, 36, 254–260. [Google Scholar] [CrossRef]
- Delcenserie, V.; Martel, D.; Lamoureux, M.; Amiot, J.; Boutin, Y.; Roy, D. Immunomodulatory effects of probiotics in the intestinal tract. Curr. Issues Mol. Biol. 2008, 10, 37–54. [Google Scholar]
- Madsen, K.L. Enhancement of epithelial barrier function by probiotics. J. Epithel. Biol. Pharmacol. 2012, 5, 55–59. [Google Scholar] [CrossRef]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.-J.M.; Wells, J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, G851–G859. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, M.; Song, W.; Jiang, R.; Li, Y.Q. Effects of probiotics on chemotherapy in patients with lung cancer. Oncol. Lett. 2019, 17, 2836–2848. [Google Scholar] [CrossRef]
- Jonkers, D.; Penders, J.; Masclee, A.; Pierik, M. Probiotics in the management of inflammatory bowel disease: A systematic review of intervention studies in adult patients. Drugs 2012, 72, 803–823. [Google Scholar] [CrossRef]
- Yasueda, A.; Mizushima, T.; Nezu, R.; Sumi, R.; Tanaka, M.; Nishimura, J.; Kai, Y.; Hirota, M.; Osawa, H.; Nakajima, K.; et al. The effect of Clostridium butyricum MIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis. Surg. Today 2016, 46, 939–949. [Google Scholar] [CrossRef]
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A four-probiotics regimen reduces postoperative complications after colorectal surgery: A randomized, double-blind, placebo-controlled study. World J. Surg. 2015, 39, 2776–2783. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Heydari, Z.; Rahaie, M.; Alizadeh, A.M. Different anti-inflammatory effects of Lactobacillus acidophilus and Bifidobactrum bifidioum in hepatocellular carcinoma cancer mouse through impact on microRNAs and their target genes. J. Nutr. Intermed. Metab. 2019, 16, 100096. [Google Scholar] [CrossRef]
- Toubert, C.; Guiu, B.; Al Taweel, B.; Assenat, E.; Panaro, F.; Souche, F.-R.; Ursic-Bedoya, J.; Navarro, F.; Herrero, A. Prolonged survival after recurrence in HCC resected patients using repeated curative therapies: Never give up! Cancers 2022, 15, 232. [Google Scholar] [CrossRef]
- Wan, M.L.; El-Nezami, H. Targeting gut microbiota in hepatocellular carcinoma: Probiotics as a novel therapy. Hepatobiliary Surg. Nutr. 2018, 7, 11. [Google Scholar] [CrossRef]
- Kirpich, I.A.; McClain, C.J. Probiotics in the treatment of the liver diseases. J. Am. Coll. Nutr. 2012, 31, 14–23. [Google Scholar] [CrossRef]
- Nitin, J.; Mithun, S.; Rao, P.; Nageshwar Reddy, D. Liver diseases: The role of gut microbiota and probiotics. J. Prob. Health 2016, 4, 2. [Google Scholar] [CrossRef]
- Sharma, V.; Garg, S.; Aggarwal, S. Probiotics and liver disease. Perm. J. 2013, 17, 62. [Google Scholar] [CrossRef]
- Li, J.; Sung, C.Y.J.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef]
- Gratz, S.; Wu, Q.; El-Nezami, H.; Juvonen, R.; Mykkänen, H.; Turner, P. Lactobacillus rhamnosus strain GG reduces aflatoxin B1 transport, metabolism, and toxicity in Caco-2 cells. Appl. Environ. Microbiol. 2007, 73, 3958–3964. [Google Scholar] [CrossRef]
- Kratzer, T.B.; Bandi, P.; Freedman, N.D.; Smith, R.A.; Travis, W.D.; Jemal, A.; Siegel, R.L. Lung cancer statistics, 2023. Cancer 2024, 130, 1330–1348. [Google Scholar] [CrossRef]
- Wei, H.; Yue, Z.; Han, J.; Chen, P.; Xie, K.; Sun, Y.; Zhu, J. Oral compound probiotic supplements can improve the quality of life for patients with lung cancer during chemotherapy: A randomized placebo-controlled study. Thorac. Cancer 2024, 15, 182–191. [Google Scholar] [CrossRef]
- Sharma, A.; Viswanath, B.; Park, Y.-S. Role of probiotics in the management of lung cancer and related diseases: An update. J. Funct. Foods 2018, 40, 625–633. [Google Scholar] [CrossRef]
- Morita, A.; Ichihara, E.; Inoue, K.; Fujiwara, K.; Yokoyama, T.; Harada, D.; Ando, C.; Kano, H.; Oda, N.; Tamura, T.; et al. Impacts of probiotics on the efficacies of immune checkpoint inhibitors with or without chemotherapy for patients with advanced non-small-cell lung cancer. Int. J. Cancer 2024, 154, 1607–1615. [Google Scholar] [CrossRef]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Xu, L.; Yang, C.S.; Liu, Y.; Zhang, X. Effective regulation of gut microbiota with probiotics and prebiotics may prevent or alleviate COVID-19 through the gut-lung axis. Front. Pharmacol. 2022, 13, 895193. [Google Scholar] [CrossRef]
- Bingula, R.; Filaire, M.; Radosevic-Robin, N.; Bey, M.; Berthon, J.-Y.; Bernalier-Donadille, A.; Vasson, M.-P.; Filaire, E. Desired turbulence? Gut-lung axis, immunity, and lung cancer. J. Oncol. 2017, 2017, 5035371. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Immune boosting functional foods and their mechanisms: A critical evaluation of probiotics and prebiotics. Biomed. Pharmacother. 2020, 130, 110625. [Google Scholar] [CrossRef]
- Avasare, A.A. A Review on Immune-Boosting Activity of Functional Foods-Prebiotics and Probiotics. Bombay Technol. 2021, 68. [Google Scholar] [CrossRef]
- Gui, Q.; Lu, H.; Zhang, C.; Xu, Z.; Yang, Y. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet. Mol. Res. 2015, 14, 5642–5651. [Google Scholar] [CrossRef]
- Tanasienko, O.; Cheremshenko, N.; Titova, G.; Potebnya, M.; Gavrilenko, M.; Nagorna, S.; Kovalenko, N. Elevation of the efficacy of antitumor vaccine prepared on the base of lectines from B. subtilis B-7025 upon its combined application with probiotics in vivo. Exp. Oncol. 2005, 27, 336–338. [Google Scholar]
- MIu, S.; Urtenova, M.; Tkachenko, E.; Avalueva, E.; Orlov, S.; Ivanov, S.; Orishak, E.; Skazyvaeva, E. On the possibilities of correction of changes of the gastrointestinal tract microbiota in patients with lung cancer treated receiving chemotherapy. Eksperimental’naia I Klin. Gastroenterol. = Exp. Clin. Gastroenterol. 2013, 11, 15–20. [Google Scholar]
- Ranjbar, S.; Seyednejad, S.A.; Azimi, H.; Rezaeizadeh, H.; Rahimi, R. Emerging roles of probiotics in prevention and treatment of breast cancer: A comprehensive review of their therapeutic potential. Nutr. Cancer 2019, 71, 1–12. [Google Scholar] [CrossRef]
- Mendoza, L. Potential effect of probiotics in the treatment of breast cancer. Oncol. Rev. 2019, 13, 422. [Google Scholar] [CrossRef]
- Thu, M.S.; Ondee, T.; Nopsopon, T.; Farzana, I.A.; Fothergill, J.L.; Hirankarn, N.; Campbell, B.J.; Pongpirul, K.J.B. Effect of probiotics in breast cancer: A systematic review and meta-analysis. Biology 2023, 12, 280. [Google Scholar] [CrossRef]
- Summer, M.; Ali, S.; Fiaz, U.; Tahir, H.M.; Ijaz, M.; Mumtaz, S.; Mushtaq, R.; Khan, R.; Shahzad, H.; Fiaz, H. Therapeutic and immunomodulatory role of probiotics in breast cancer: A mechanistic review. Arch. Microbiol. 2023, 205, 296. [Google Scholar] [CrossRef]
- Juan, Z.; Chen, J.; Ding, B.; Yongping, L.; Liu, K.; Wang, L.; Le, Y.; Liao, Q.; Shi, J.; Huang, J.; et al. Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: A randomised, double-blind, and placebo-controlled trial. Eur. J. Cancer 2022, 161, 10–22. [Google Scholar] [CrossRef]
- Juan, Z.; Chen, J.; Ding, B.; Yongping, L.; Cai, H.; Chen, H.; Wang, L.; Le, Y.; Shi, J.; Wu, Y.; et al. Probiotics prevent pegylated liposomal doxorubicin-associated hand-foot syndrome and oral mucositis of breast cancer patients following surgery and chemotherapy: A randomized placebo-controlled trial. Int. J. Surg. 2025, 111, 2018–2030. [Google Scholar] [CrossRef]
- Juan, Z.; Qing, Z.; Yongping, L.; Qian, L.; Wu, W.; Wen, Y.; Tong, J.; Ding, B. Probiotics for the treatment of docetaxel-related weight gain of breast cancer patients—A single-center, randomized, double-blind, and placebo-controlled trial. Front. Nutr. 2021, 8, 762929. [Google Scholar] [CrossRef]
- Porter, C.M.; Shrestha, E.; Peiffer, L.B.; Sfanos, K.S. The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 345–354. [Google Scholar] [CrossRef]
- Pezeshki, B.; Abdulabbas, H.T.; Alturki, A.D.; Mansouri, P.; Zarenezhad, E.; Nasiri-Ghiri, M.; Ghasemian, A. Synergistic Interactions Between Probiotics and Anticancer Drugs: Mechanisms, Benefits, and Challenges. Robiotics Antimicrob. Proteins 2025, 1–14. [Google Scholar] [CrossRef]
- Rosa, L.S.; Santos, M.L.; Abreu, J.P.; Balthazar, C.F.; Rocha, R.S.; Silva, H.L.; Esmerino, E.A.; Duarte, M.C.K.; Pimentel, T.C.; Freitas, M.Q.; et al. Antiproliferative and apoptotic effects of probiotic whey dairy beverages in human prostate cell lines. Food Res. Int. 2020, 137, 109450. [Google Scholar] [CrossRef] [PubMed]
- Bitla, S.S.; Munirathinam, G. Anti-cancer effect of a probiotic bacteria-derived compound, 1, 4-Dihydroxy-2-naphthoic acid in prostate cancer. Cancer Res. 2023, 83, 4975. [Google Scholar] [CrossRef]
- Yadav, A.; Kaushik, M.; Tiwari, P.; Dada, R. From microbes to medicine: Harnessing the gut microbiota to combat prostate cancer. Microb. Cell 2024, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Galla, R.; Mulè, S.; Uberti, F. Analysis of the Beneficial Effects of Probiotics on the Gut–Prostate Axis Using Prostatic Co-Culture Model. Foods 2024, 13, 3647. [Google Scholar] [CrossRef]
- Singhal, B.; Mukherjee, A.; Srivastav, S. Role of probiotics in pancreatic cancer prevention: The prospects and challenges. Adv. Biosci. Biotechnol. 2016, 7, 468–500. [Google Scholar] [CrossRef]
- Chen, S.-M.; Chieng, W.-W.; Huang, S.-W.; Hsu, L.-J.; Jan, M.-S. The synergistic tumor growth-inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Sci. Rep. 2020, 10, 20319. [Google Scholar] [CrossRef]
- Lee, T.S. Are Probiotics Beneficial or Harmful for Pancreatic Cancer Outcomes? Probiotics Antimicrob Proteins 2024, 1–8. [Google Scholar] [CrossRef]
- Maher, S.; Elmeligy, H.A.; Aboushousha, T.; Helal, N.S.; Ossama, Y.; Rady, M.; Hassan, A.M.A.; Kamel, M. Synergistic immunomodulatory effect of synbiotics pre-and postoperative resection of pancreatic ductal adenocarcinoma: A randomized controlled study. Cancer Immunol. Immunother. 2024, 73, 109. [Google Scholar] [CrossRef]
- Chaib, M.; Hafeez, B.B.; Mandil, H.; Daria, D.; Pingili, A.K.; Kumari, S.; Sikander, M.; Kashyap, V.K.; Chen, G.-Y.; Anning, E.; et al. Reprogramming of pancreatic adenocarcinoma immunosurveillance by a microbial probiotic siderophore. Commun. Biol. 2022, 5, 1181. [Google Scholar] [CrossRef]
- Han, Z.-Y.; Fu, Z.-J.; Wang, Y.-Z.; Zhang, C.; Chen, Q.-W.; An, J.-X.; Zhang, X.-Z. Probiotics functionalized with a gallium-polyphenol network modulate the intratumor microbiota and promote anti-tumor immune responses in pancreatic cancer. Nat. Commun. 2024, 15, 7096. [Google Scholar] [CrossRef]
- Moreira, M.M.; Carriço, M.; Capelas, M.L.; Pimenta, N.; Santos, T.; Ganhão-Arranhado, S.; Mäkitie, A.; Ravasco, P. The impact of pre-, pro-and synbiotics supplementation in colorectal cancer treatment: A systematic review. Front. Oncol. 2024, 14, 1395966. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, S.; Ying, L.; Zhang, W.; Chen, X.; Liang, Y.; Chen, R.; Yao, K.; Li, C.; Yu, C.; et al. The effect of probiotics supplementation on cancer-treatment complications: A critical umbrella review of interventional meta-analyses. Crit. Rev. Food Sci. Nutr. 2024, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Minelli, E.B.; Benini, A. Relationship between number of bacteria and their probiotic effects. Microb. Ecol. Health Dis. 2008, 20, 180–183. [Google Scholar]
- Taylor, B.L.; Woodfall, G.E.; Sheedy, K.E.; O’Riley, M.L.; Rainbow, K.A.; Bramwell, E.L.; Kellow, N. Effect of probiotics on metabolic outcomes in pregnant women with gestational diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2017, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, M.; Musazadeh, V.; Faghfouri, A.H.; Sarmadi, B.; Jamilian, P.; Jamilian, P.; Tutunchi, H.; Dehghan, P. Probiotic therapy, a novel and efficient adjuvant approach to improve glycemic status: An umbrella meta-analysis. Pharmacol. Res. 2022, 183, 106397. [Google Scholar] [CrossRef]
- Musazadeh, V.; Zarezadeh, M.; Faghfouri, A.H.; Keramati, M.; Jamilian, P.; Jamilian, P.; Mohagheghi, A.; Farnam, A. Probiotics as an effective therapeutic approach in alleviating depression symptoms: An umbrella meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 8292–8300. [Google Scholar] [CrossRef]
- Cazzaniga, M.; Cardinali, M.; Di Pierro, F.; Zonzini, G.B.; Palazzi, C.M.; Gregoretti, A.; Zerbinati, N.; Guasti, L.; Bertuccioli, A. The potential role of probiotics, especially butyrate producers, in the Management of Gastrointestinal Mucositis Induced by oncologic chemo-radiotherapy. Int. J. Mol. Sci. 2024, 25, 2306. [Google Scholar] [CrossRef]
- Lu, S.; Wang, C.; Ma, J.; Wang, Y. Metabolic mediators: Microbial-derived metabolites as key regulators of anti-tumor immunity, immunotherapy, and chemotherapy. Front. Immunol 2024, 15, 1456030. [Google Scholar] [CrossRef]
- d’Ettorre, G.; Rossi, G.; Scagnolari, C.; Andreotti, M.; Giustini, N.; Serafino, S.; Schietroma, I.; Scheri, G.C.; Fard, S.N.; Trinchieri, V.; et al. Probiotic supplementation promotes a reduction in T-cell activation, an increase in Th17 frequencies, and a recovery of intestinal epithelium integrity and mitochondrial morphology in ART-treated HIV-1-positive patients. Immun Inflamm Dis. 2017, 5, 244–260. [Google Scholar] [CrossRef]
- Nicolini, A.; Ferrari, P. Involvement of tumor immune microenvironment metabolic reprogramming in colorectal cancer progression, immune escape, and response to immunotherapy. Front. Immunol 2024, 15, 1353787. [Google Scholar] [CrossRef]
- Pashenkov, M.V.; Murugina, N.E.; Budikhina, A.S.; Pinegin, B.V. Synergistic interactions between NOD receptors and TLRs: Mechanisms and clinical implications. J. Leukoc. Biol. 2019, 105, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Ali, S.A.; Function. Probiotics and gut microbiota: Mechanistic insights into gut immune homeostasis through TLR pathway regulation. Food Funct. 2022, 13, 7423–7447. [Google Scholar] [CrossRef]
- Giambra, V.; Pagliari, D.; Rio, P.; Totti, B.; Di Nunzio, C.; Bosi, A.; Giaroni, C.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Gut microbiota, inflammatory bowel disease, and cancer: The role of guardians of innate immunity. Cells 2023, 12, 2654. [Google Scholar] [CrossRef]
- De Kivit, S.; Tobin, M.C.; Forsyth, C.B.; Keshavarzian, A.; Landay, A.L. Regulation of intestinal immune responses through TLR activation: Implications for pro-and prebiotics. Front. Immunol. 2014, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Bamola, V.D.; Dubey, D.; Samanta, P.; Kedia, S.; Ahuja, V.; Madempudi, R.S.; Neelamraju, J.; Chaudhry, R. Role of a probiotic strain in the modulation of gut microbiota and cytokines in inflammatory bowel disease. Anaerobe 2022, 78, 102652. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, R.; Kakkar, P. Modulation of Bax/Bcl-2 and caspases by probiotics during acetaminophen induced apoptosis in primary hepatocytes. Food Chem. Toxicol. 2011, 49, 770–779. [Google Scholar] [CrossRef]
- Altonsy, M.O.; Andrews, S.C.; Tuohy, K.M. Differential induction of apoptosis in human colonic carcinoma cells (Caco-2) by Atopobium, and commensal, probiotic and enteropathogenic bacteria: Mediation by the mitochondrial pathway. Int. J. Food Microbiol. 2010, 137, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Malczewski, A.B.; Navarro, S.; Coward, J.I.; Ketheesan, N. Microbiome-derived metabolome as a potential predictor of response to cancer immunotherapy. J. Immunother. Cancer 2020, 8, e001383. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Shen, S.; Zhang, T.; Zhang, J.; Huang, S.; Sun, Z.; Zhang, H. Lacticaseibacillus rhamnosus Probio-M9 enhanced the antitumor response to anti-PD-1 therapy by modulating intestinal metabolites. EBioMedicine 2023, 91, 104533. [Google Scholar] [CrossRef]
- Danino, T.; Prindle, A.; Kwong, G.A.; Skalak, M.; Li, H.; Allen, K.; Hasty, J.; Bhatia, S.N. Programmable probiotics for detection of cancer in urine. Sci. Transl. Med. 2015, 7, ra284–ra289. [Google Scholar] [CrossRef]
- Ravnik, Z.; Muthiah, I.; Dhanaraj, P. Computational studies on bacterial secondary metabolites against breast cancer. J. Biomol. Struct. Dyn. 2021, 39, 7056–7064. [Google Scholar] [CrossRef] [PubMed]
- Zwezerijnen-Jiwa, F.H.; Sivov, H.; Paizs, P.; Zafeiropoulou, K.; Kinross, J. A systematic review of microbiome-derived biomarkers for early colorectal cancer detection. Neoplasia 2023, 36, 100868. [Google Scholar] [CrossRef]
- Gou, H.; Zeng, R.; Lau, H.C.H.; Yu, J. Gut microbial metabolites: Shaping future diagnosis and treatment against gastrointestinal cancer. Pharmacol. Res. 2024, 208, 107373. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, B.; Zheng, Q.; Li, H.; Meng, X.; Zhou, F.; Zhang, L. A review of gut microbiota-derived metabolites in tumor progression and cancer therapy. Adv. Sci. 2023, 10, 2207366. [Google Scholar] [CrossRef]
- León-Letelier, R.A.; Dou, R.; Vykoukal, J.; Yip-Schneider, M.T.; Maitra, A.; Irajizad, E.; Wu, R.; Dennison, J.B.; Do, K.-A.; Zhang, J.; et al. Contributions of the microbiome-derived metabolome for risk assessment and prognostication of pancreatic cancer. Clin. Chem. 2024, 70, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-Y.; Mei, J.-X.; Yu, G.; Lei, L.; Zhang, W.-H.; Liu, K.; Chen, X.-L.; Kołat, D.; Yang, K.; Hu, J.-K.; et al. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef]
- Cinque, B.; La Torre, C.; Lombardi, F.; Palumbo, P.; Van der Rest, M.; Cifone, M.G. Production conditions affect the in vitro anti-tumoral effects of a high concentration multi-strain probiotic preparation. PLoS ONE 2016, 11, e0163216. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Singh, R.P.; Shadan, A.; Ma, Y. Biotechnological applications of probiotics: A multifarious weapon to disease and metabolic abnormality. Probiotics Antimicrob. Proteins 2022, 14, 1184–1210. [Google Scholar] [CrossRef]
- Yazbeck, V.; Alesi, E.; Myers, J.; Hackney, M.H.; Cuttino, L.; Gewirtz, D.A. An overview of chemotoxicity and radiation toxicity in cancer therapy. Adv. Cancer Res. 2022, 155, 1–27. [Google Scholar]
- Waziri, A.; Bharti, C.; Aslam, M.; Jamil, P.; Mirza, M.; Javed, M.N.; Pottoo, U.; Ahmadi, A.; Alam, M.S. Probiotics for the chemoprotective role against the toxic effect of cancer chemotherapy. Anti-Cancer Agents Med. Chem. 2022, 22, 654–667. [Google Scholar] [CrossRef]
- Maria-Aggeliki, K.S.; Nikolaos, K.L.; Kyrias, G.M.; Vassilis, K.E. The potential clinical impact of probiotic treatment for the prevention and/or anti-inflammatory therapeutic effect against radiation induced intestinal mucositis. A review. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Holec, V.; Drgona, L.; Hainova, K.; Ciernikova, S.; Zajac, V. Probiotic bacteria in cancer patients undergoing chemotherapy and radiation therapy. Complement. Ther. Med. 2013, 21, 712–723. [Google Scholar] [CrossRef]
- Singh, N.K.; Beckett, J.M.; Kalpurath, K.; Ishaq, M.; Ahmad, T.; Eri, R.D. Synbiotics as supplemental therapy for the alleviation of chemotherapy-associated symptoms in patients with solid tumours. Nutrients 2023, 15, 1759. [Google Scholar] [CrossRef]
- López-Gómez, L.; Alcorta, A.; Abalo, R. Probiotics and probiotic-like agents against Chemotherapy-Induced Intestinal mucositis: A narrative review. J. Pers. Med. 2023, 13, 1487. [Google Scholar] [CrossRef]
- Guo, S.; Gillingham, T.; Guo, Y.; Meng, D.; Zhu, W.; Walker, W.A.; Ganguli, K. Secretions of Bifidobacterium infantis and Lactobacillus acidophilus protect intestinal epithelial barrier function. J. Pediatr. Gastroenterol Nutr. 2017, 64, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Q.; Niu, Y.; Zhang, X.; Lu, R. Surface-layer protein from Lactobacillus acidophilus NCFM attenuates tumor necrosis factor-α-induced intestinal barrier dysfunction and inflammation. Int. J. Biol. Macromol. 2019, 136, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Banna, G.L.; Torino, F.; Marletta, F.; Santagati, M.; Salemi, R.; Cannarozzo, E.; Falzone, L.; Ferraù, F.; Libra, M. Lactobacillus rhamnosus GG: An overview to explore the rationale of its use in cancer. Front. Pharmacol. 2017, 8, 603. [Google Scholar] [CrossRef]
- Garczyk, A.; Kaliciak, I.; Drogowski, K.; Horwat, P.; Kopeć, S.; Staręga, Z.; Bogdański, P.; Stelmach-Mardas, M.; Mardas, M. Influence of probiotics in prevention and treatment of patients who undergo chemotherapy or/and radiotherapy and suffer from mucositis, diarrhoea, constipation, nausea and vomiting. J. Clin. Med. 2022, 11, 3412. [Google Scholar] [CrossRef]
- Nenu, I.; Baldea, I.; Coadă, C.A.; Crăciun, R.C.; Moldovan, R.; Tudor, D.; Petrushev, B.; Toma, V.A.; Ştefanescu, H.; Procopeţ, B.; et al. Lactobacillus rhamnosus probiotic treatment modulates gut and liver inflammatory pathways in a hepatocellular carcinoma murine model. A preliminary study. Food Chem. Toxicol. 2024, 183, 114314. [Google Scholar] [CrossRef]
- Demers, M.; Dagnault, A.; Desjardins, J. A randomized double-blind controlled trial: Impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin. Nutr. 2014, 33, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Consoli, M.L.D.; da Silva, R.S.; Nicoli, J.R.; Bruña-Romero, O.; da Silva, R.G.; de Vasconcelos Generoso, S.; Correia, M.I.T. Randomized clinical trial: Impact of oral administration of Saccharomyces boulardii on gene expression of intestinal cytokines in patients undergoing colon resection. J. Parenter. Enter. Nutr. 2016, 40, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Redman, M.; Ward, E.; Phillips, R. The efficacy and safety of probiotics in people with cancer: A systematic review. Ann. Oncol. 2014, 25, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Patel, S.; Kim, S.-K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef]
- Mego, M.; Ebringer, L.; Drgoňa, L.; Mardiak, J.; Trupl, J.; Greksak, R.; Nemova, I.; Oravcova, E.; Zajac, V.; Koza, I. Prevention of febrile neutropenia in cancer patients by probiotic strain. Neoplasma 2005, 52, 159. [Google Scholar]
- Wada, M.; Nagata, S.; Saito, M.; Shimizu, T.; Yamashiro, Y.; Matsuki, T.; Asahara, T.; Nomoto, K. Effects of the enteral administration of Bifidobacterium breve on patients undergoing chemotherapy for pediatric malignancies. Support. Care Cancer 2010, 18, 751–759. [Google Scholar] [CrossRef]
- Wei, D.; Heus, P.; van de Wetering, F.T.; van Tienhoven, G.; Verleye, L.; Scholten, R.J. Probiotics for the prevention or treatment of chemotherapy-or radiotherapy-related diarrhoea in people with cancer. Cochrane Database Syst. Rev. 2018, 8, CD008831. [Google Scholar] [CrossRef]
- Saarela, M.; Matto, J.; Mattila-Sandholm, T. Safety aspects of Lactobacillus and Bifidobacterium species originating from human oro-gastrointestinal tract or from probiotic products. Microb. Ecol. Health Dis. 2002, 14, 234–241. [Google Scholar] [CrossRef]
- Liang, D.; Wu, F.; Zhou, D.; Tan, B.; Chen, T. Commercial probiotic products in public health: Current status and potential limitations. Crit. Rev. Food Sci. Nutr. 2024, 64, 6455–6476. [Google Scholar] [CrossRef]
- Arnold, R.; Gabrail, N.; Raut, M.; Kim, R.; Sung, J.; Zhou, Y. Clinical implications of chemotherapy-induced diarrhea in patients with cancer. J. Support. Oncol. 2005, 3, 227–232. [Google Scholar]
- Land, M.H.; Rouster-Stevens, K.; Woods, C.R.; Cannon, M.L.; Cnota, J.; Shetty, A.K. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005, 115, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Xie, J.; Du, H.; Xiao, H.; McClements, D.J.; Yao, M.; Li, L. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Yus, C.; Gracia, R.; Larrea, A.; Andreu, V.; Irusta, S.; Sebastian, V.; Mendoza, G.; Arruebo, M. Targeted release of probiotics from enteric microparticulated formulations. Polymers 2019, 11, 1668. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Production and evaluation of dry alginate-chitosan microcapsules as an enteric delivery vehicle for probiotic bacteria. Biomacromolecules 2011, 12, 2834–2840. [Google Scholar] [CrossRef]
- Ta, L.P.; Bujna, E.; Antal, O.; Ladányi, M.; Juhász, R.; Szécsi, A.; Kun, S.; Sudheer, S.; Gupta, V.K.; Nguyen, Q.D. Effects of various polysaccharides (alginate, carrageenan, gums, chitosan) and their combination with prebiotic saccharides (resistant starch, lactosucrose, lactulose) on the encapsulation of probiotic bacteria Lactobacillus casei 01 strain. Int. J. Biol. Macromol. 2021, 183, 1136–1144. [Google Scholar] [CrossRef]
- Fülöpová, N.; Brückner, K.; Muselík, J.; Pavloková, S.; Franc, A. Development and evaluation of innovative enteric-coated capsules for colon-specific delivery of hydrophilic biomaterials. Int. J. Pharm. 2025, 668, 124991. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; Babalghith, A.O.; Faidah, H.; Ahmed, F.; Khanam, A.; Mozaffar, B.; Kambal, N.; et al. Probiotics and Cancer: Mechanistic Insights and Organ-Specific Impact. Biomolecules 2025, 15, 879. https://doi.org/10.3390/biom15060879
Ahmad MF, Ahmad FA, Alsayegh AA, Zeyaullah M, Babalghith AO, Faidah H, Ahmed F, Khanam A, Mozaffar B, Kambal N, et al. Probiotics and Cancer: Mechanistic Insights and Organ-Specific Impact. Biomolecules. 2025; 15(6):879. https://doi.org/10.3390/biom15060879
Chicago/Turabian StyleAhmad, Md Faruque, Fakhruddin Ali Ahmad, Abdulrahman A. Alsayegh, Md. Zeyaullah, Ahmad O. Babalghith, Hani Faidah, Faiyaz Ahmed, Anjum Khanam, Boshra Mozaffar, Nahla Kambal, and et al. 2025. "Probiotics and Cancer: Mechanistic Insights and Organ-Specific Impact" Biomolecules 15, no. 6: 879. https://doi.org/10.3390/biom15060879
APA StyleAhmad, M. F., Ahmad, F. A., Alsayegh, A. A., Zeyaullah, M., Babalghith, A. O., Faidah, H., Ahmed, F., Khanam, A., Mozaffar, B., Kambal, N., & Bantun, F. (2025). Probiotics and Cancer: Mechanistic Insights and Organ-Specific Impact. Biomolecules, 15(6), 879. https://doi.org/10.3390/biom15060879





