Brianolide from Briareum stechei Attenuates Atopic Dermatitis-like Skin Lesions by Regulating the NFκB and MAPK Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Brianolide and Cell Line
2.2. Cell Culture
2.3. MTT Assay
2.4. Quantitative Polymerase Chain Reaction (qPCR)
2.5. Western Blot Assay Analysis
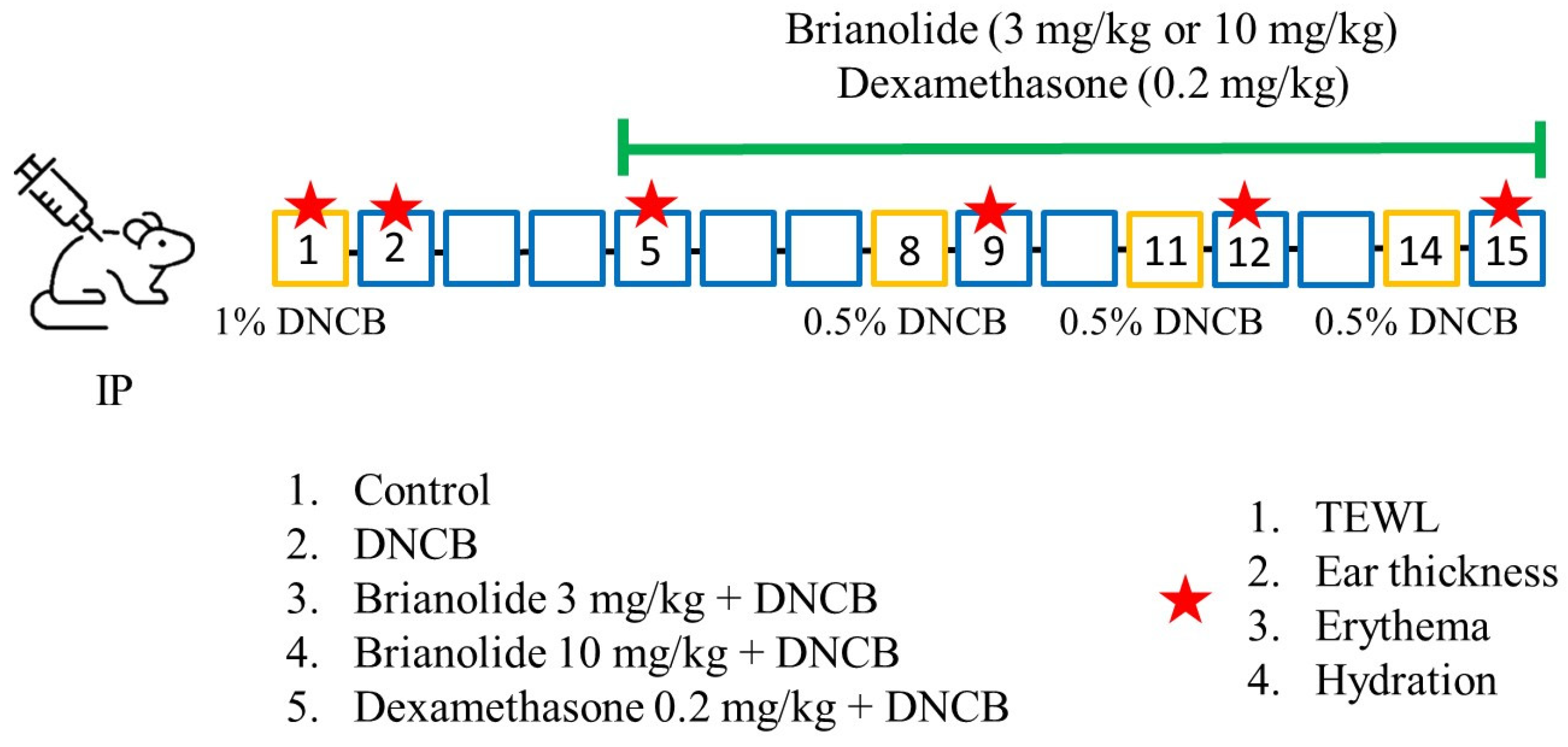
2.6. In Vivo Experiments
2.7. Data Analysis
3. Results
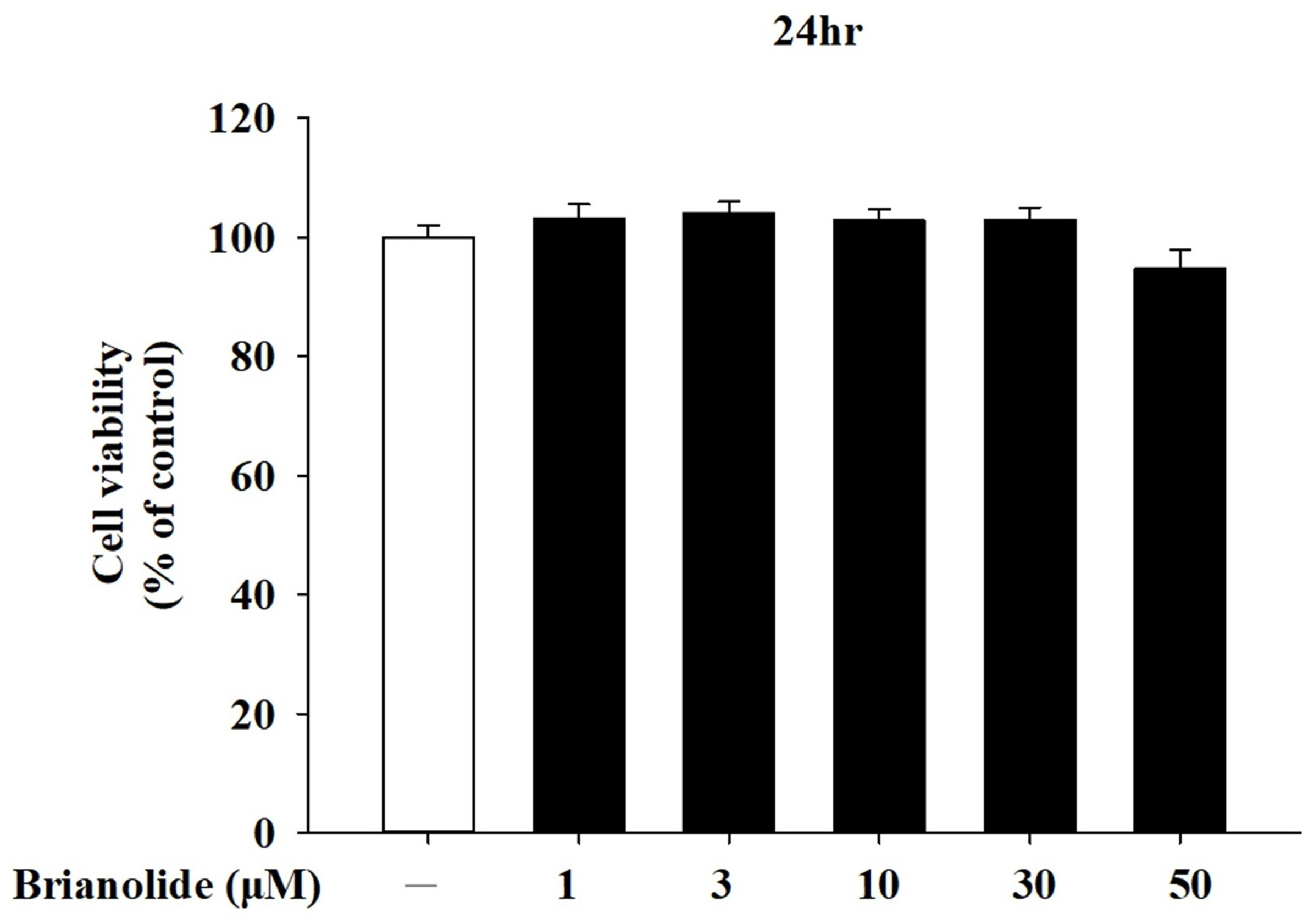
3.1. Analysis of Brianolide’s Effect on Keratinocyte Viability
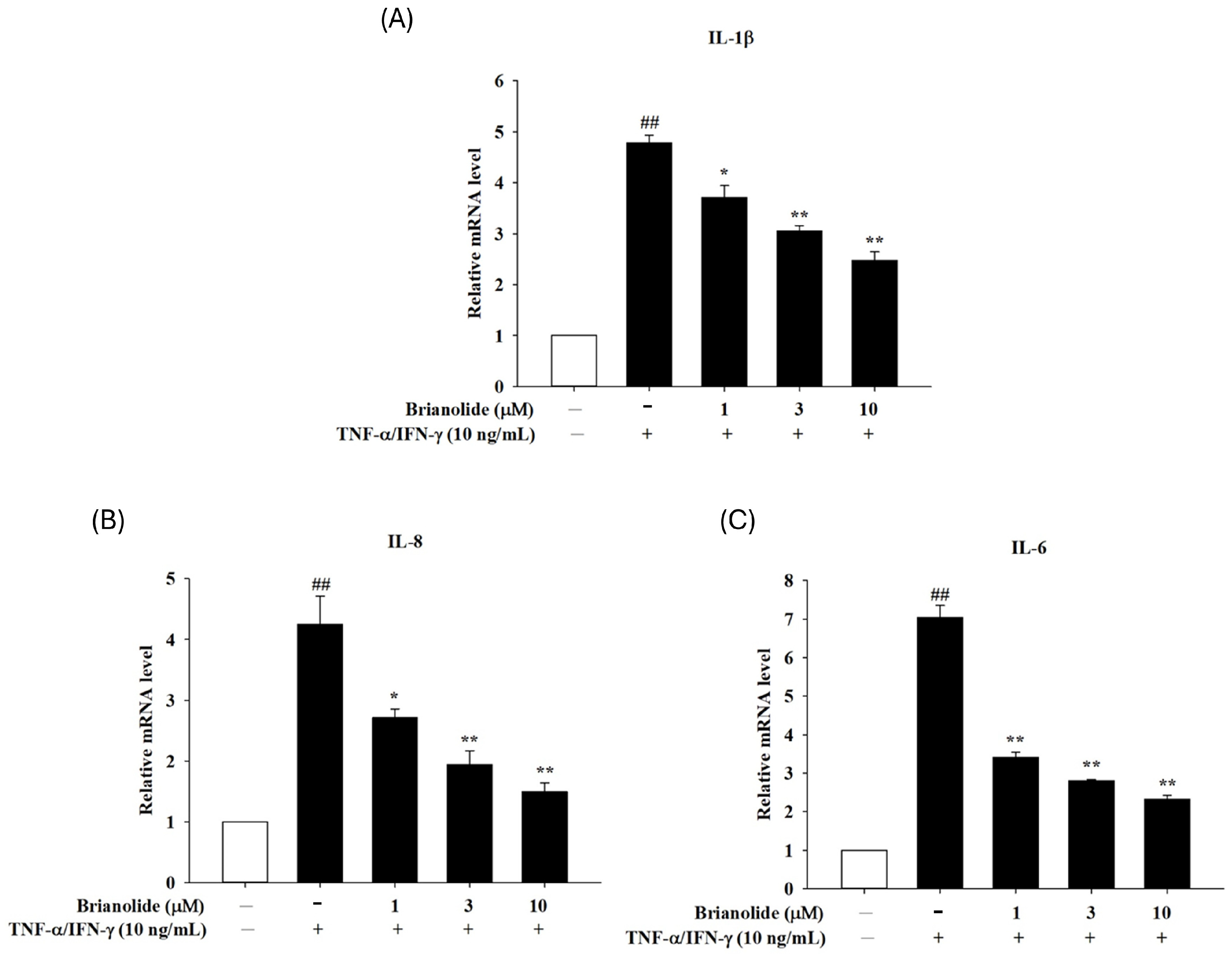
3.2. Downregulation of Pro-Inflammatory Cytokine mRNA Expression in HaCaT Following Treatment with Brianolide Under TNF-α/IFN-γ Stimulation
3.3. Brianolide Decreased Phosphorylation of Critical MAPK Proteins in TNF-α/IFN-γ-Stimulated HaCaT Cells
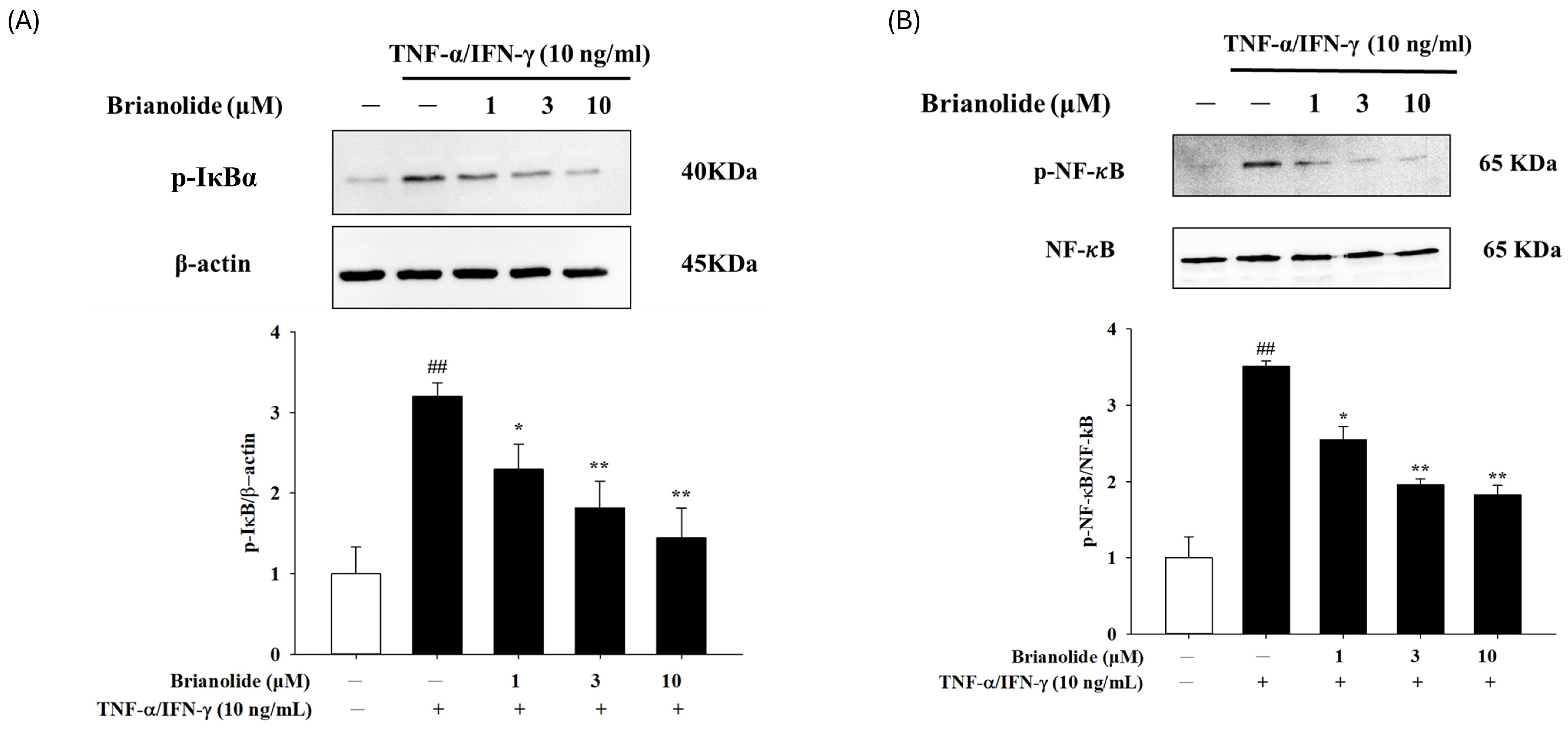
3.4. Brianolide Inhibited the Activation of IκB and NF-κB
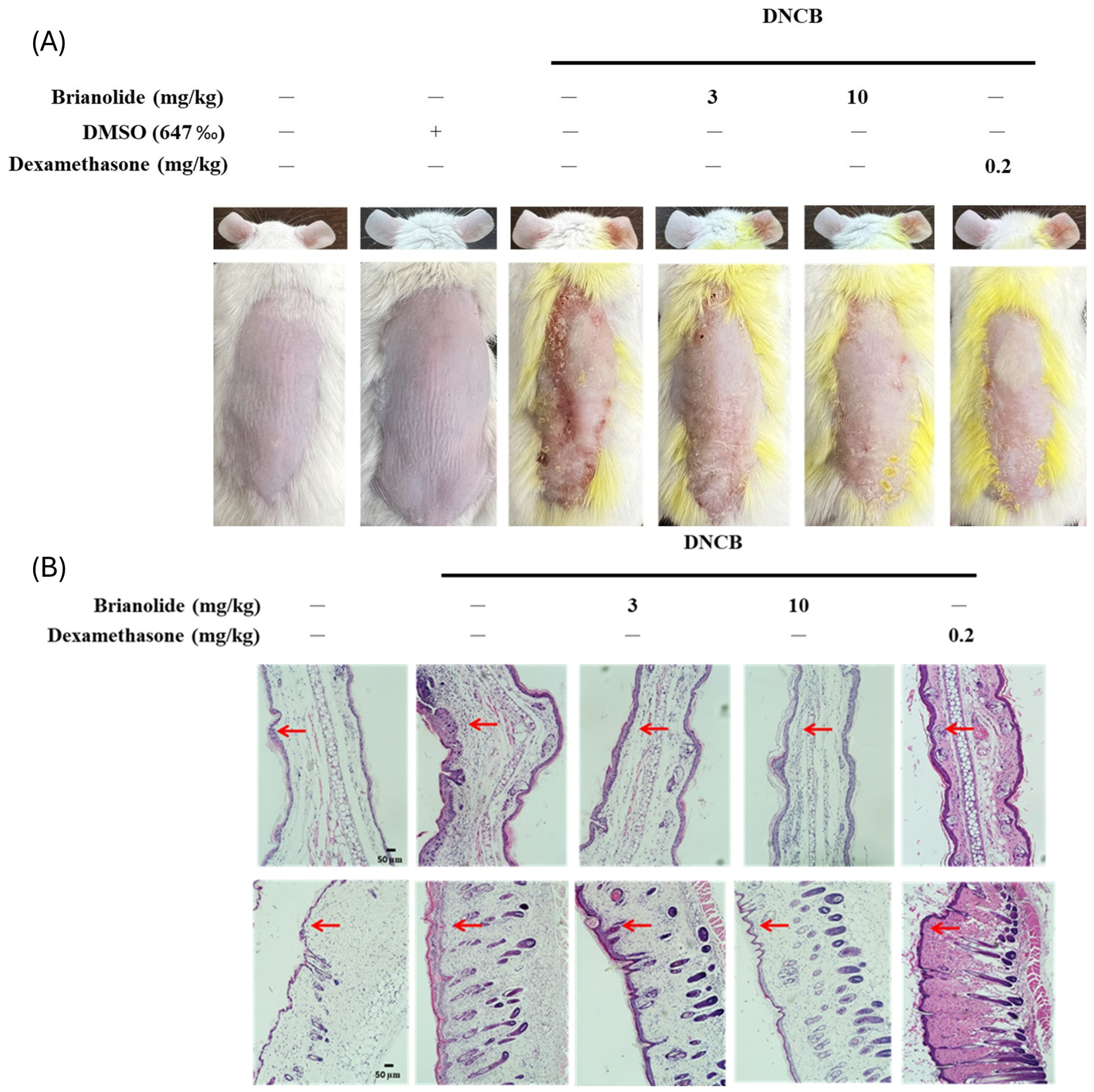
3.5. Brianolide Resulted in a Notable Decrease in Ear Thickness and Dorsal Skin Inflammation in DNCB-Induced Mice
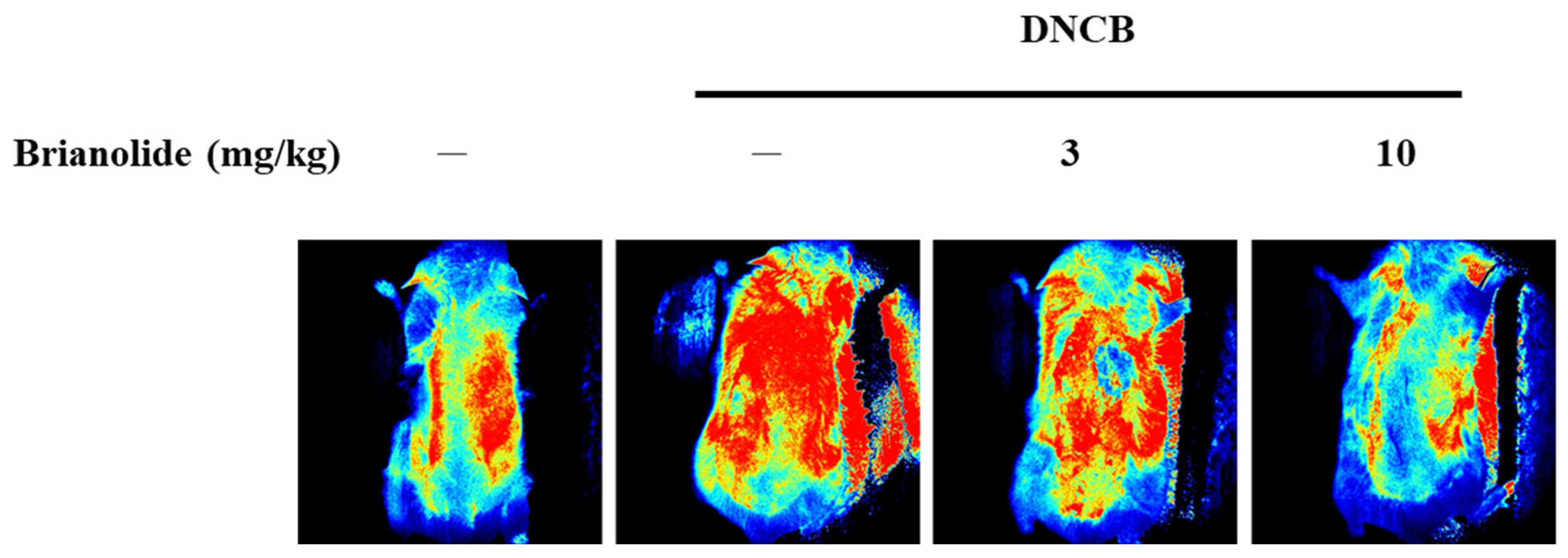
3.6. Brianolide’s Direct Effects on Blood Flow
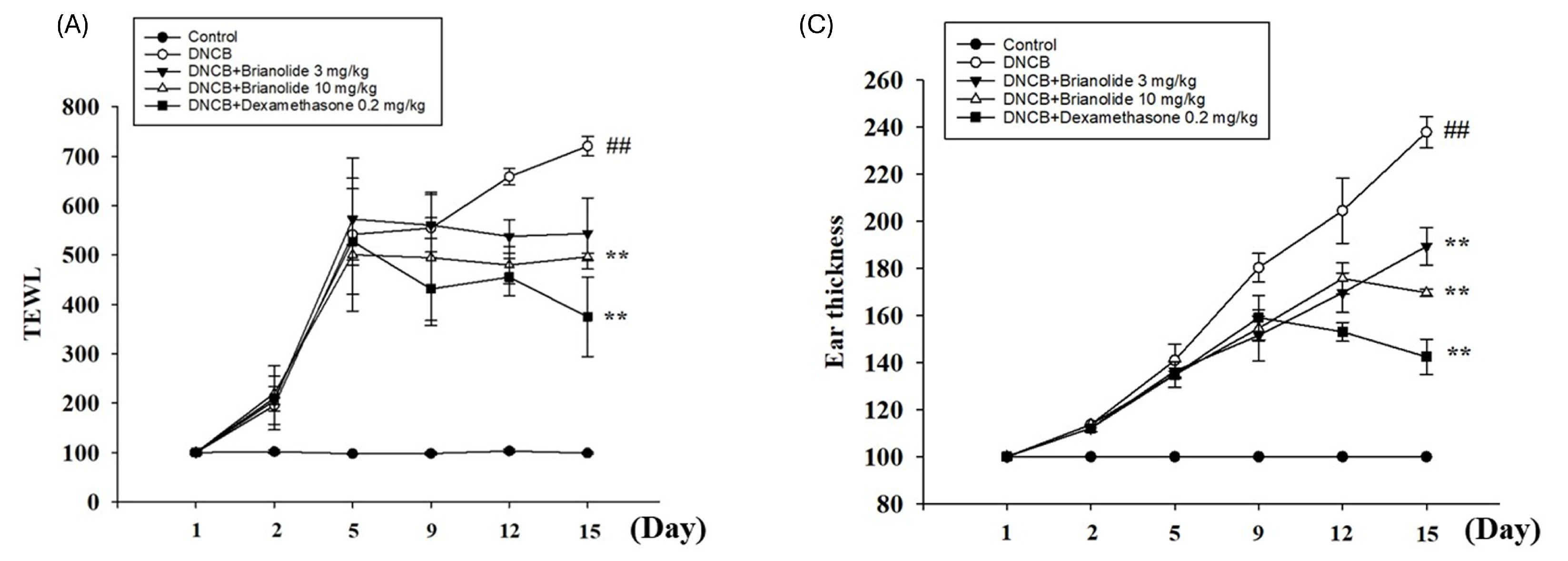
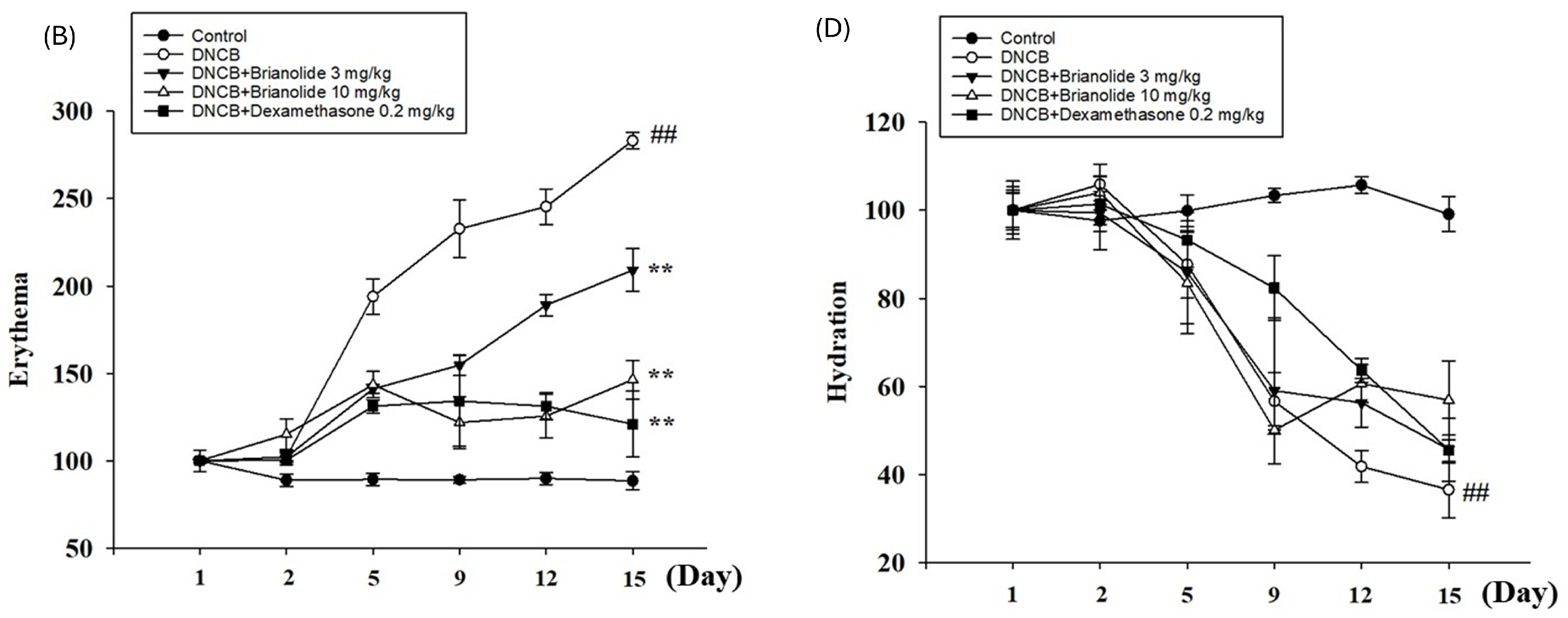
3.7. Brianolide Improves Physiological Parameters Such as TEWL, Hydration, and Erythema
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| HaCaT | Human keratinocyte cell line |
| TNF-α | Tumor necrosis factor-α |
| IFN-γ | Interferon-γ |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| DNCB | 2,4-Dinitrochlorobenzene |
| TEWL | Transepidermal water loss |
| IL-1β | Interleukin-1β |
| EXCB | Excavatolide B |
| DMSO | Dimethyl sulfoxide |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium |
| ELISA | Enzyme-linked immunosorbent assay |
| qPCR | Quantitative Polymerase Chain Reaction |
| LSCI | Laser speckle contrast imaging |
References
- Fitzmaurice, W.; Silverberg, N.B. Long-Term Impact of Atopic Dermatitis on Quality of Life. Dermatol. Clin. 2024, 42, 549–557. [Google Scholar] [CrossRef]
- Na, C.H.; Chung, J.; Simpson, E.L. Quality of Life and Disease Impact of Atopic Dermatitis and Psoriasis on Children and Their Families. Children 2019, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Kelbore, A.G.; Enbiale, W.; van Wyk, J.M.; Mosam, A. The Impact of Atopic Dermatitis on Caregivers’ Quality of Life in Ethiopia. Front. Med. 2025, 12, 1537089. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, D.; Yang, Y.; Huang, Y.; Wang, L.; Yao, X.; Lu, Q. Global Epidemiology of Atopic Dermatitis: A Comprehensive Systematic Analysis and Modelling Study. Br. J. Dermatol. 2023, 190, 55–61. [Google Scholar] [CrossRef]
- Baron, S.E.; Cohen, S.N.; Archer, C.B. British Association of Dermatologists and Royal College of General Practitioners Guidance on the Diagnosis and Clinical Management of Atopic Eczema. Clin. Exp. Dermatol. 2012, 37 (Suppl. S1), 7–12. [Google Scholar] [CrossRef]
- Avena-Woods, C. Overview of Atopic Dermatitis. Am. J. Manag. Care 2017, 23, S115–S123. [Google Scholar] [PubMed]
- Frazier, W.; Bhardwaj, N. Atopic Dermatitis: Diagnosis and Treatment. Am. Fam. Physician 2020, 101, 590–598. [Google Scholar] [PubMed]
- Lugović-Mihić, L.; Meštrović-Štefekov, J.; Potočnjak, I.; Cindrić, T.; Ilić, I.; Lovrić, I.; Skalicki, L.; Bešlić, I.; Pondeljak, N. Atopic Dermatitis: Disease Features, Therapeutic Options, and a Multidisciplinary Approach. Life 2023, 13, 1419. [Google Scholar] [CrossRef]
- Kasraie, S.; Werfel, T. Role of Macrophages in the Pathogenesis of Atopic Dermatitis. Mediat. Inflamm. 2013, 2013, 942375. [Google Scholar] [CrossRef]
- Lee, K.-S.; Chun, S.-Y.; Lee, M.-G.; Kim, S.; Jang, T.-J.; Nam, K.-S. The Prevention of TNF-α/IFN-γ Mixture-Induced Inflammation in Human Keratinocyte and Atopic Dermatitis-like Skin Lesions in Nc/Nga Mice by Mineral-Balanced Deep Sea Water. Biomed. Pharmacother. 2018, 97, 1331–1340. [Google Scholar] [CrossRef]
- Kolb, L.; Ferrer-Bruker, S.J. Atopic Dermatitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Pan, C.; Zhao, A.; Li, M. Atopic Dermatitis-like Genodermatosis: Disease Diagnosis and Management. Diagnostics 2022, 12, 2177. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, H.M. Atopic Dermatitis: A Comprehensive Updated Review of This Intriguing Disease with Futuristic Insights. Inflammopharmacology 2025, 33, 1161–1187. [Google Scholar] [CrossRef] [PubMed]
- Sadowsky, R.L.; Sulejmani, P.; Lio, P.A. Atopic Dermatitis: Beyond the Skin and Into the Gut. J. Clin. Med. 2023, 12, 5534. [Google Scholar] [CrossRef]
- Tier, H.L.; Balogh, E.A.; Bashyam, A.M.; Fleischer, A.B.; Spergel, J.M.; Masicampo, E.J.; Kammrath, L.K.; Strowd, L.C.; Feldman, S.R. Tolerability of and Adherence to Topical Treatments in Atopic Dermatitis: A Narrative Review. Dermatol. Ther. 2021, 11, 415–431. [Google Scholar] [CrossRef]
- Chu, D.K.; Schneider, L.; Asiniwasis, R.N.; Boguniewicz, M.; Benedetto, A.D.; Ellison, K.; Frazier, W.T.; Greenhawt, M.; Huynh, J.; Kim, E.; et al. Atopic Dermatitis (Eczema) Guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE- and Institute of Medicine-Based Recommendations. Ann. Allergy Asthma Immunol. 2024, 132, 274–312. [Google Scholar] [CrossRef]
- Ratchataswan, T.; Banzon, T.M.; Thyssen, J.P.; Weidinger, S.; Guttman-Yassky, E.; Phipatanakul, W. Biologics for Treatment of Atopic Dermatitis: Current Status and Future Prospect. J. Allergy Clin. Immunol. Pract. 2021, 9, 1053–1065. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-Derived Natural Products for Drug Discovery: Current Approaches and Prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Guo, C.; Huang, Q.; Wang, Y.; Yao, Y.; Li, J.; Chen, J.; Wu, M.; Zhang, Z.; Qi, H.; Ji, P.; et al. Therapeutic Application of Natural Products: NAD+ Metabolism as Potential Target. Phytomedicine 2023, 114, 154768. [Google Scholar] [CrossRef]
- Niseteo, T.; Hojsak, I.; Ožanić Bulić, S.; Pustišek, N. Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation on Clinical Outcome of Atopic Dermatitis in Children. Nutrients 2024, 16, 2829. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.-E.; Kim, K.H.; Kang, N.J. Beneficial Effects of Marine Algae-Derived Carbohydrates for Skin Health. Mar. Drugs 2018, 16, 459. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.-C.; Lin, S.-Y.; Chen, Y.-J.; Wen, C.-C.; Huang, C.-Y.; Palanisamy, A.; Yang, N.-S.; Sheu, J.-H. Topical Application of Marine Briarane-Type Diterpenes Effectively Inhibits 12-O-Tetradecanoylphorbol-13-Acetate-Induced Inflammation and Dermatitis in Murine Skin. J. Biomed. Sci. 2011, 18, 94. [Google Scholar] [CrossRef]
- Kim, A.-R.; Kim, M.-J.; Seo, J.; Moon, K.M.; Lee, B. The Beneficial Roles of Seaweed in Atopic Dermatitis. Mar. Drugs 2024, 22, 566. [Google Scholar] [CrossRef]
- Chen, H.-W.; Liu, F.-C.; Kuo, H.-M.; Tang, S.-H.; Niu, G.-H.; Zhang, M.M.; Tsou, L.K.; Sung, P.-J.; Wen, Z.-H. Immunomodulatory and Anti-Angiogenesis Effects of Excavatolide B and Its Derivatives in Alleviating Atopic Dermatitis. Biomed. Pharmacother. 2024, 172, 116279. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Lo, Y.-H.; Hsu, Y.-J.; Cheng, Y.-B.; Kung, C.-C.; Liang, C.-W.; Chang, D.-C.; Wang, K.-L.; Hung, C.-F. Anti-Atopic Dermatitis Activity of Epi-Oxyzoanthamine Isolated from Zoanthid. Mar. Drugs 2023, 21, 447. [Google Scholar] [CrossRef]
- Nguyen, N.B.A.; Chen, L.-Y.; El-Shazly, M.; Peng, B.-R.; Su, J.-H.; Wu, H.-C.; Lee, I.-T.; Lai, K.-H. Towards Sustainable Medicinal Resources through Marine Soft Coral Aquaculture: Insights into the Chemical Diversity and the Biological Potential. Mar. Drugs 2022, 20, 640. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Zhang, Y.-L.; Lee, G.-H.; Tsou, L.K.; Zhang, M.M.; Hsieh, H.-P.; Chen, J.-J.; Ko, C.-Y.; Wen, Z.-H.; Sung, P.-J. Briarenols W–Z: Chlorine-Containing Polyoxygenated Briaranes from Octocoral Briareum Stechei (Kükenthal, 1908). Mar. Drugs 2021, 19, 77. [Google Scholar] [CrossRef]
- Kwon, D.-J.; Bae, Y.-S.; Ju, S.M.; Goh, A.R.; Youn, G.S.; Choi, S.Y.; Park, J. Casuarinin Suppresses TARC/CCL17 and MDC/CCL22 Production via Blockade of NF-κB and STAT1 Activation in HaCaT Cells. Biochem. Biophys. Res. Commun. 2012, 417, 1254–1259. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Y.; Cui, L.; Shi, Y.; Guo, C. Advances in the Pathogenesis of Psoriasis: From Keratinocyte Perspective. Cell Death Dis. 2022, 13, 81. [Google Scholar] [CrossRef]
- Oh, J.-H.; Kim, S.-H.; Kwon, O.-K.; Kim, J.-H.; Oh, S.-R.; Han, S.-B.; Park, J.-W.; Ahn, K.-S. Purpurin Suppresses Atopic Dermatitis via TNF-α/IFN-γ-Induced Inflammation in HaCaT Cells. Int. J. Immunopathol. Pharmacol. 2022, 36, 1–12. [Google Scholar] [CrossRef]
- Hinz, M.; Scheidereit, C. The IκB Kinase Complex in NF-κB Regulation and Beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Valerio, M.R.; Arrivas Bajardi, E.; Arcara, C.C.; Borsellino, N.; Lo Mauro, M.; Cipolla, C.; Santarpia, M.; Firenze, A.; Motta, G.; Vigneri, P.; et al. Eribulin Mesylate for the Treatment of Metastatic Hormone-Refractory and Triple-Negative Breast Cancer: A Multi-Institutional Real-World Report on Efficacy and Safety. Am. J. Clin. Oncol. 2021, 44, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Kantarjian, H. Chronic Myeloid Leukemia: 2025 Update on Diagnosis, Therapy, and Monitoring. Am. J. Hematol. 2024, 99, 2191–2212. [Google Scholar] [CrossRef]
- Abramovits, W.; Rivas Bejarano, J.J.; Valdecantos, W.C. Role of Interleukin 1 in Atopic Dermatitis. Dermatol. Clin. 2013, 31, 437–444. [Google Scholar] [CrossRef]
- Boraschi, D. What Is IL-1 for? The Functions of Interleukin-1 Across Evolution. Front. Immunol. 2022, 13, 872155. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, Y.; Fang, R.; Zhao, J.; Huang, J. Exploring the Inhibition Mechanism of Interleukin-1-Beta in Gouty Arthritis by Polygonum Cuspidatum Using Network Pharmacology and Molecular Docking: A Review. Medicine 2023, 102, e34396. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 Chemokine Family and Its Receptors in Inflammatory Diseases. Expert Rev. Clin. Immunol. 2014, 10, 593–619. [Google Scholar] [CrossRef]
- Krause, K.; Metz, M.; Makris, M.; Zuberbier, T.; Maurer, M. The Role of Interleukin-1 in Allergy-Related Disorders. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 477–484. [Google Scholar] [CrossRef]
- Tanaka, A.; Muto, S.; Jung, K.; Itai, A.; Matsuda, H. Topical Application with a New NF-kappaB Inhibitor Improves Atopic Dermatitis in NC/NgaTnd Mice. J. Investig. Dermatol. 2007, 127, 855–863. [Google Scholar] [CrossRef]
- Shen, Y.; Boulton, A.P.R.; Yellon, R.L.; Cook, M.C. Skin Manifestations of Inborn Errors of NF-κB. Front. Pediatr. 2023, 10, 1098426. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, G.H.; Jin, S.W.; Kim, J.Y.; Hwang, Y.P.; Han, E.H.; Kim, Y.H.; Jeong, H.G. Impressic Acid Ameliorates Atopic Dermatitis-Like Skin Lesions by Inhibiting ERK1/2-Mediated Phosphorylation of NF-κB and STAT1. Int. J. Mol. Sci. 2021, 22, 2334. [Google Scholar] [CrossRef] [PubMed]
- Bystryn, J.C.; Hyman, C. Skin Blood Flow in Atopic Dermatitis. J. Investig. Dermatol. 1969, 52, 189–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steinhoff, M.; Steinhoff, A.; Homey, B.; Luger, T.A.; Schneider, S.W. Role of Vasculature in Atopic Dermatitis. J. Allergy Clin. Immunol. 2006, 118, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.W.; Ho, C.-C.; Chiu, H.-P.; Hsu, Y.-J.; Hung, C.-T.; Sung, C.-H.; Chang, D.-C.; Chang, H.-H.; Hung, C.-F. D-Limonene Inhibits Cytokines and Chemokines Expression by Regulating NF-kappaB and STAT in HaCat Cells and DNCB-Induced Atopic Dermatitis in BALB/c Mice. Int. Immunopharmacol. 2025, 148, 114082. [Google Scholar] [CrossRef]
- Hon, K.L.; Leung, A.K.C.; Barankin, B. Barrier Repair Therapy in Atopic Dermatitis: An Overview. Am. J. Clin. Dermatol. 2013, 14, 389–399. [Google Scholar] [CrossRef]


| Genes | Primers | Sequence(5′-3′) |
|---|---|---|
| IL-1β | Forward | CTC TCA CCT CTC CTA CTC ACT |
| Reverse | ATC AGA ATG TGG GAG CGA AT | |
| IL-6 | Forward | CGA GCC CAC CGG GAA CGA AA |
| Reverse | GGA CCG AAG GCG CTT GTG GAG | |
| IL-8 | Forward | ACT GAG AGT GAT TGA GAG TGG AC |
| Reverse | AAC CCT CTG CAC CCA GTT TTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-C.; Wang, K.-L.; Hsu, Y.-J.; Sung, C.-H.; Chen, M.-J.; Huang, M.-F.; Sung, P.-J.; Hung, C.-F. Brianolide from Briareum stechei Attenuates Atopic Dermatitis-like Skin Lesions by Regulating the NFκB and MAPK Pathways. Biomolecules 2025, 15, 871. https://doi.org/10.3390/biom15060871
Wang C-C, Wang K-L, Hsu Y-J, Sung C-H, Chen M-J, Huang M-F, Sung P-J, Hung C-F. Brianolide from Briareum stechei Attenuates Atopic Dermatitis-like Skin Lesions by Regulating the NFκB and MAPK Pathways. Biomolecules. 2025; 15(6):871. https://doi.org/10.3390/biom15060871
Chicago/Turabian StyleWang, Chia-Chen, Kang-Ling Wang, Yu-Jou Hsu, Chao-Hsien Sung, Mei-Jung Chen, Meng-Fang Huang, Ping-Jyun Sung, and Chi-Feng Hung. 2025. "Brianolide from Briareum stechei Attenuates Atopic Dermatitis-like Skin Lesions by Regulating the NFκB and MAPK Pathways" Biomolecules 15, no. 6: 871. https://doi.org/10.3390/biom15060871
APA StyleWang, C.-C., Wang, K.-L., Hsu, Y.-J., Sung, C.-H., Chen, M.-J., Huang, M.-F., Sung, P.-J., & Hung, C.-F. (2025). Brianolide from Briareum stechei Attenuates Atopic Dermatitis-like Skin Lesions by Regulating the NFκB and MAPK Pathways. Biomolecules, 15(6), 871. https://doi.org/10.3390/biom15060871









