Positive Effects of (+)-Epicatechin on Traumatic Spinal Cord Injury Recovery
Abstract
1. Introduction
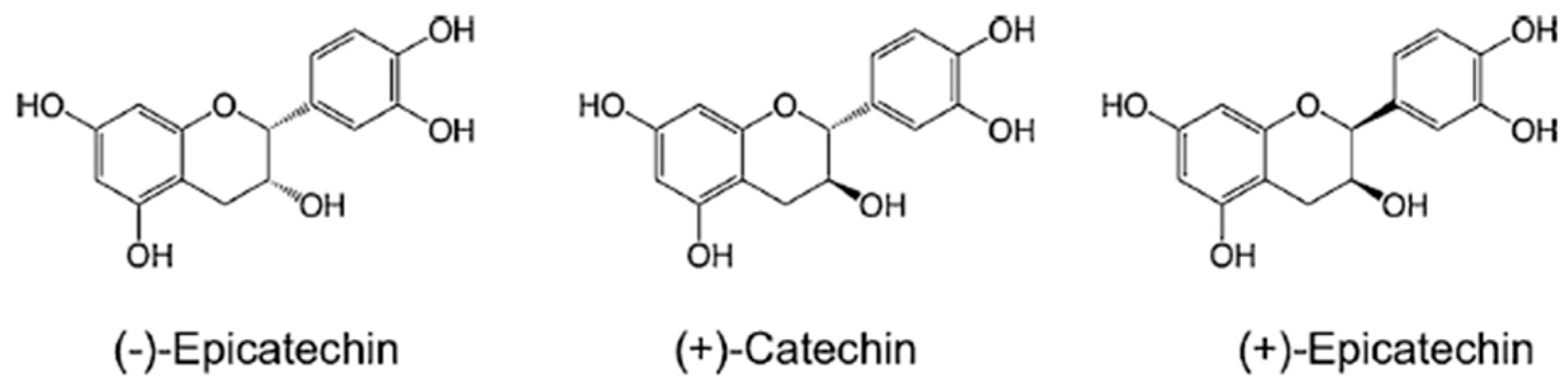
2. Materials and Methods
2.1. Ethical Approval
2.2. Surgical Procedure
2.3. Post-Surgical Care
2.4. Study Design
- (1)
- Sham group: Group with laminectomy without moderate contusion (n = 8);
- (2)
- Control group: Laminectomy + moderate contusion + administration of water as a vehicle [1 mL/kg of body weight/day] by oral gavage (n = 12 rats);
- (3)
- Treated group: Laminectomy + moderate contusion + administration of (+)-epicatechin in water [1 mg/mL/kg of body weight/day] by oral gavage (n = 12 rats).
2.5. Functional Recovery Analysis
2.6. Tissue Collection
2.7. Western Blot
2.8. Antibodies
2.9. Statistical Analysis
3. Results
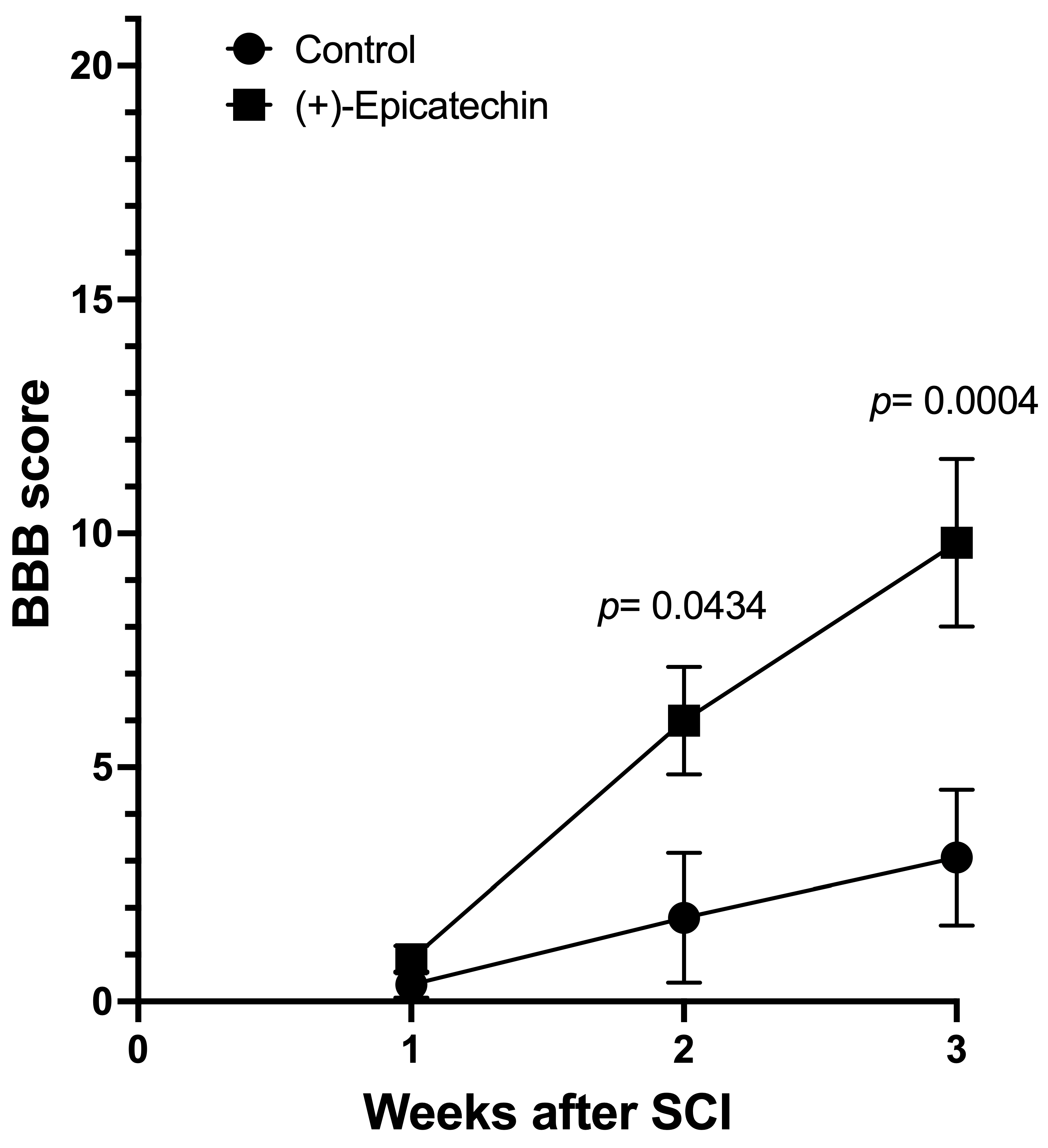
3.1. BBB Score

3.2. Protein Marker Changes



4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tallqvist, S.; Kauppila, A.M.; Vainionpää, A.; Koskinen, E.; Bergman, P.; Anttila, H.; Hiekkala, S. Prevalence of comorbidities and secondary health conditions among the Finnish population with spinal cord injury. Spinal Cord 2022, 60, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Moncada, S.; Carreon-Rodriguez, A.; Parra-Cid, M.C.; Ibarra-Ponce de Leon, C.; Velasquillo-Martinez, C.; Vacanti, C.A.; Belkind-Gerson, J. Lesión de médula espinal y medicina regenerativa. Salud Publica Mex. 2007, 49, 437–444. [Google Scholar] [CrossRef] [PubMed]
- GBD Spinal Cord Injuries Collaborators. Global, regional, and national burden of spinal cord injury,1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2023, 22, 1026–1047. [Google Scholar] [CrossRef]
- Yari, D.; Saberi, A.; Salmasi, S.; Alireza Ghoreishi, S.; Etemad, L.; Movaffagh, J.; Ganjeifar, B. Recent Advances in the Treatment of Spinal Cord Injury. Arch. Bone Jt. Surg. 2024, 12, 380–399. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Zhao, H. Thymoquinone reduces spinal cord injury by inhibiting inflammatory response, oxidative stress and apoptosis via PPAR-γ and PI3K/Akt pathways. Exp. Ther. Med. 2018, 15, 4987–4994. [Google Scholar] [CrossRef]
- Pallottie, A.; Ratnayake, A.; Ni, L.; Acioglu, C.; Li, L.; Mirabelli, E.; Elkabes, S. A toll-like receptor nine antagonist restores below-level glial glutamate transporter expression in the dorsal horn following spinal cord injury. Sci. Rep. 2018, 8, 8723. [Google Scholar] [CrossRef]
- Cheung, V.; Hoshide, R.; Bansal, V.; Kasper, E.; Chen, C.C. Methylprednisolone in managing spinal cord injuries: Lessons from randomized, controlled trials. Surg. Neurol. Int. 2015, 6, 142. [Google Scholar] [PubMed]
- Jones, L.L.; Oudega, M.; Bunge, M.B.; Tuszynski, M.H. Neurotrophic factors, cellular bridges, and gene therapy for spinal cord injury. J. Physiol. 2001, 533 Pt 1, 83–89. [Google Scholar] [CrossRef]
- Weissmiller, A.M.; Wu, C. Current advances in using neurotrophic factors to treat neurodegenerative disorders. Transl. Neurodegener. 2012, 1, 14. [Google Scholar] [CrossRef]
- Squair, J.W.; Ruiz, I.; Phillips, A.A.; Zheng, M.M.Z.; Sarafis, Z.K.; Sachdeva, R.; Krassioukov, A.V. Minocycline Reduces the Severity of Autonomic Dysreflexia after Experimental Spinal Cord Injury. J. Neurotrauma 2018, 35, 2861–2871. [Google Scholar] [CrossRef]
- Zhang, H.; Xiang, Z.; Duan, X.; Jiang, J.L.; Xing, Y.M.; Zhu, C.; Yu, Q.R. Antitumor and anti-inflammatory effects of oligosaccharides from Cistanche deserticola extract on spinal cord injury. Int. J. Biol. Macromol. 2019, 124, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Taub, P.R.; Ramirez-Sanchez, I.; Ciaraldi, T.P.; Gonzalez-Basurto, S.; Coral-Vazquez, R.; Perkins, G.; Hogan, M.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. Perturbations in skeletal muscle sarcomere structure in patients with heart failure and Type 2 diabetes: Restorative effects of (-)-epicatechin rich cocoa. Clin. Sci. 2013, 125, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.; Ramirez-Sanchez, I.; Perkins, G.A.; Murphy, A.; Taub, P.R.; Ceballos, G.; Villarreal, F.J.; Hogan, M.C.; Malek, M.H. (-)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J. Physiol. 2011, 589, 4615–4631. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ulloa, A.; Miranda-Cervantes, A.; Licea-Navarro, A.; Mansour, C.; Beltrán-Partida, E.; Donis-Maturano, L.; de la Herrán, H.C.D.; Villarreal, F.; Álvarez-Delgado, C. (-)-Epicatechin Stimulates Mitochondrial Biogenesis and Cell Growth in C2C12 Myotubes via the G-Protein Coupled Estrogen Receptor. Eur. J. Pharmacol. 2018, 822, 95. [Google Scholar] [CrossRef]
- Portilla-Martínez, A.; Ortiz-Flores, M.A.; Meaney, E.; Villarreal, F.; Nájera, N.; Ceballos, G. (-)-Epicatechin Is a Biased Ligand of Apelin Receptor. Int. J. Mol. Sci. 2022, 23, 8962. [Google Scholar] [CrossRef]
- Moreno-Ulloa, A.; Najera-Garcia, N.; Hernandez, M.; Ramirez-Sanchez, I.; Taub, P.R.; Beltran-Partida, E.; Ceballos, S.; Dugar, S.; Schreiner, G.; Best, B.M.; et al. A pilot study on clinical pharmacokinetics and preclinical pharmacodynamics of (+)-epicatechin on cardiometabolic end-points. Food Funct. 2018, 9, 307–319. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, T.; Ciaraldi, R.; Robert, R.H.; Villarreal, F. Evaluation and comparison of epicatechin epimer effects on skeletal muscle structure, function, and regulators of metabolism. Diabetes 2014, 63, A478. [Google Scholar]
- Dugar, S.; Villarreal, F.; Frank, H.; Mahajae, D.; Ramirez-Sanchez, I.; Moreno-Ulloa, A.; Ceballos, G.; Schreiner, G. 11-β-hydroxysterols as possible endogenous stimulators of mitochondrial biogenesis as inferred from epicatechin molecular mimicry. Pharmacol. Res. 2020, 151, 104540. [Google Scholar] [CrossRef]
- Gonzalez-Ruiz, C.; Cordero-Anguiano, P.; Morales-Guadarrama, A.; Mondragón-Lozano, R.; Sánchez-Torres, S.; Salgado-Ceballos, H.; Villarreal, F.; Meaney, E.; Ceballos, G.; Nájera, N. (-)-Epicatechin reduces muscle waste after complete spinal cord transection in a murine model: Role of ubiquitin-proteasome system. Mol. Biol. Rep. 2020, 47, 8975–8985. [Google Scholar] [CrossRef]
- Yin, J.; Yin, Z.; Wang, B.; Zhu, C.; Sun, C.; Liu, X.; Gong, G. Angiopoietin-1 Protects Spinal Cord Ischemia and Reperfusion Injury by Inhibiting Autophagy in Rats. Neurochem. Res. 2019, 44, 2746–2754. [Google Scholar] [CrossRef]
- Yan, Z.; Chu, L.; Jia, X.; Lin, L.; Cheng, S. Myelin basic protein enhances axonal regeneration from neural progenitor cells. Cell Biosci. 2021, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Darlot, F.; Vinit, S.; Matarazzo, V.; Kastner, A. Sustained cell body reactivity and loss of NeuN in a subset of axotomized bulbospinal neurons after a chronic high cervical spinal cord injury. Eur. J. Neurosci. 2017, 46, 2729–2745. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Yokobori, S.; Nakae, R.; Yokota, H. Neurofilaments light chain/Neurofilaments heavy chain. In Biomarkers for Traumatic Brain Injury; Chapter 13; Academic Press: New York, NY, USA, 2020; pp. 193–203. [Google Scholar]
- Datto, J.; Bastidas, J.; Miller, N.; Shah, A.; Arheart, K.; Marcillo, A.; Dietrich, D.; Pearse, D. Female Rats Demonstrate Improved Locomotor Recovery and Greater Preservation of White and Gray Matter after Traumatic Spinal Cord Injury Compared to Males. J. Neurotrauma 2015, 32, 1146–1157. [Google Scholar] [CrossRef]
- Verma, R.; Virdi, J.K.; Singh, N.; Jaggi, A.S. Animals models of spinal cord contusion injury. Korean J. Pain 2019, 32, 12–21. [Google Scholar] [CrossRef]
- Ramsey, J.B.G.; Ramer, L.M.; Inskip, J.A.; Alan, N.; Ramer, M.S.; Krassioukov, A.V. Care of rats with complete high-thoracic spinal cord injury. J. Neurotrauma 2010, 27, 1709–1722. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Yañez, V.; Garate-Carrillo, A.; Ayala, M.; Rodriguez-Castañeda, A.; Mendoza-Lorenzo, P.; Ceballos, G.; Ramirez-Sanchez, I. Stimulatory effects of (−)-epicatechin and its enantiomer (+)-epicatechin on mouse frontal cortex neurogenesis markers and short-term memory: Proof of concept. Food Funct. 2021, 12, 3504. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; Navarrete-Yanez, V.; Espinosa-Raya, J.; Leonor, G.; Rubio-Gayosso, I.; Palma-Flores, C.; Villarreal, F. Restorative effects of (+)-epicatechin in a rodent model of aging-induced sarcopenia: Underlying mechanisms. Food Funct. 2024, 15, 3669–3679. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Koopmans, G.C.; Deumens, R.; Honig, W.M.M.; Hamers, F.P.T.; Steinbusch, H.W.M.; Joosten, E.A.J. The assessment of locomotor function in spinal cord injured rats: The importance of objective analysis of coordination. J. Neurotrauma 2005, 22, 214–225. [Google Scholar] [CrossRef]
- Zhang, Y.; Al Mamun, A.; Yuan, Y.; Lu, Q.; Xiong, J.; Yang, S.; Wang, J. Acute spinal cord injury: Pathophysiology and pharmacological intervention (Review). Mol. Med. Rep. 2021, 23, 417. [Google Scholar] [CrossRef] [PubMed]
- Ritz, M.F.; Graumann, U.; Gutierrez, B.; Hausmann, O. Traumatic spinal cord injury alters angiogenic factors and TGF-beta1 that may affect vascular recovery. Curr. Neurovasc. Res. 2010, 7, 301–310. [Google Scholar] [CrossRef]
- Kumar, H.; Choi, H.; Jo, M.J.; Joshi, H.P.; Muttigi, M.; Bonanomi, D.; Han, I. Neutrophil elastase inhibition effectively rescued angiopoietin-1 decrease and inhibits glial scar after spinal cord injury. Acta Neuropathol. Commun. 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.J.; Sundberg, L.M.; Zentilin, L.; Giacca, M.; Narayana, P.A. Sustained expression of vascular endothelial growth factor and angiopoietin-1 improves blood-spinal cord barrier integrity and functional recovery after spinal cord injury. J. Neurotrauma 2010, 27, 2067–2076. [Google Scholar] [CrossRef]
- Kanno, H.; Ozawa, H.; Sekiguchi, A.; Itoi, E. Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death. Neurobiol. Dis. 2009, 33, 143–148. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Tumani, H. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.K.; Stammers, A.M.; Belanger, L.M.; Bernardo, A.; Chan, D.; Bishop, C.M.; Slobogean, G.P.; Zhang, H.; Umedaly, H.; Giffin, M.; et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J. Neurotrauma 2010, 27, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Holmström, U.; Tsitsopoulos, P.P.; Holtz, A.; Marklund, N. Cerebrospinal fluid levels of GFAP and pNF-H are elevated in patients with chronic spinal cord injury and neurological deterioration. Acta Neurochir. 2020, 162, 2075–2086. [Google Scholar] [CrossRef]
- Marinelli, S.; Vacca, V.; De Angelis, F.; Pieroni, L.; Orsini, T.; Parisi, C.; Pavone, F. Innovative mouse model mimicking human-like features of spinal cord injury: Efficacy of Docosahexaenoic acid on acute and chronic phases. Sci. Rep. 2019, 9, 8883. [Google Scholar] [CrossRef]
- Brenner, M. Role of GFAP in CNS injuries. Neurosci. Lett. 2014, 565, 7–13. [Google Scholar] [CrossRef]
- Albayar, A.A.; Roche, A.; Swiatkowski, P.; Antar, S.; Ouda, N.; Emara, E.; Awad, B.I. Biomarkers in Spinal Cord Injury: Prognostic Insights and Future Potentials. Front. Neurol. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; van der Heijden, R.; Heeringa, P.; Kaijzel, E.; Verschuren, L.; Blomhoff, R.; Kooistra, T.; Kleemann, R. Epicatechin attenuates atherosclerosis and exerts anti-inflammatory effects on diet-induced human-CRP and NFkB in vivo. Atherosclerosis 2014, 233, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, M.; Lu, G.; Wang, R.; Wei, Y.; Guo, Y.; Yu, Y.; Jiang, C. Anti-inflammation of epicatechin mediated by TMEM35A and TMPO in bovine mammary epithelial cell line cells and mouse mammary gland. J. Dairy Sci. 2021, 104, 12925–12938. [Google Scholar] [CrossRef]
- Quiñonez-Bastidas, G.N.; Pineda-Farias, J.B.; Flores-Murrieta, F.J.; Rodríguez-Silverio, J.; Reyes-García, J.G.; Godínez-Chaparro, B.; Granados-Soto, V.; Rocha-González, H.I. Antinociceptive effect of (-)-epicatechin in inflammatory and neuropathic pain in rats. Behav. Pharmacol. 2018, 29, 270–279. [Google Scholar] [CrossRef]
- Kang, J.; Wang, Z.; Oteiza, P.I. (−)-Epicatechin mitigates high fat diet-induced neuroinflammation and altered behavior in mice. Food Funct. 2020, 11, 5065–5076. [Google Scholar] [CrossRef]
- Shaki, F.; Shayeste, Y.; Karami, M.; Akbari, E.; Rezaei, M.; Ataee, R. The effect of epicatechin on oxidative stress and mitochondrial damage induced by homocycteine using isolated rat hippocampus mitochondria. Res. Pharm. Sci. 2017, 12, 119–127. [Google Scholar] [CrossRef]
- Tvrda, E.; Straka, P.; Galbavy, D.; Ivanic, P. Epicatechin Provides Antioxidant Protection to Bovine Spermatozoa Subjected to Induced Oxidative Stress. Molecules 2019, 24, 3226. [Google Scholar] [CrossRef]
- Keller, A.; Hull, S.E.; Elajaili, H.; Johnston, A.; Knaub, L.A.; Chun, J.H.; Reusch, J.E. (–)-Epicatechin Modulates Mitochondrial Redox in Vascular Cell Models of Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 6392629. [Google Scholar] [CrossRef]
- Martins de Almeida, F.; Adriani Marques, S.; Rodrigues dos Santos, A.; Andrade Prins, C.; Soares dos Santos Cardoso, F.; dos Santos Heringer, L.; Rocha Mendonça, H.; Blanco Martinez, A. Molecular approaches for spinal cord injury treatment. Neural Regen. Res. 2023, 18, 23–30. [Google Scholar]
- Huang, Z.; Liu, Q.; Guo, Q.; Gao, J.; Zhang, L.; Li, L. Effects and mechanisms of Apelin in treating central nervous system diseases. Neuroscience 2025, 566, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, S.; Wang, X.; Gu, C.; Guo, Q.; Li, X.; Zhang, C.; Zhang, N.; Zhang, L.; Huang, F. Apelin alleviated neuroinflammation and promoted endogenous neural stem cell proliferation and differentiation after spinal cord injury in rats. J. Neuroinflamm. 2022, 19, 160. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Ruiz, C.; Mondragón-Lozano, R.; Salgado-Ceballos, H.; Villarreal, F.; Martínez-Meza, Y.; Meaney, E.; Nájera, N.; Ceballos, G. Positive Effects of (+)-Epicatechin on Traumatic Spinal Cord Injury Recovery. Biomolecules 2025, 15, 869. https://doi.org/10.3390/biom15060869
Gonzalez-Ruiz C, Mondragón-Lozano R, Salgado-Ceballos H, Villarreal F, Martínez-Meza Y, Meaney E, Nájera N, Ceballos G. Positive Effects of (+)-Epicatechin on Traumatic Spinal Cord Injury Recovery. Biomolecules. 2025; 15(6):869. https://doi.org/10.3390/biom15060869
Chicago/Turabian StyleGonzalez-Ruiz, Cristian, Rodrigo Mondragón-Lozano, Hermelinda Salgado-Ceballos, Francisco Villarreal, Yuridia Martínez-Meza, Eduardo Meaney, Nayelli Nájera, and Guillermo Ceballos. 2025. "Positive Effects of (+)-Epicatechin on Traumatic Spinal Cord Injury Recovery" Biomolecules 15, no. 6: 869. https://doi.org/10.3390/biom15060869
APA StyleGonzalez-Ruiz, C., Mondragón-Lozano, R., Salgado-Ceballos, H., Villarreal, F., Martínez-Meza, Y., Meaney, E., Nájera, N., & Ceballos, G. (2025). Positive Effects of (+)-Epicatechin on Traumatic Spinal Cord Injury Recovery. Biomolecules, 15(6), 869. https://doi.org/10.3390/biom15060869





