Conditioned Generative Modeling of Molecular Glues: A Realistic AI Approach for Synthesizable Drug-like Molecules
Abstract
1. Introduction
2. Methods
2.1. Amyloid Beta 42 Structure
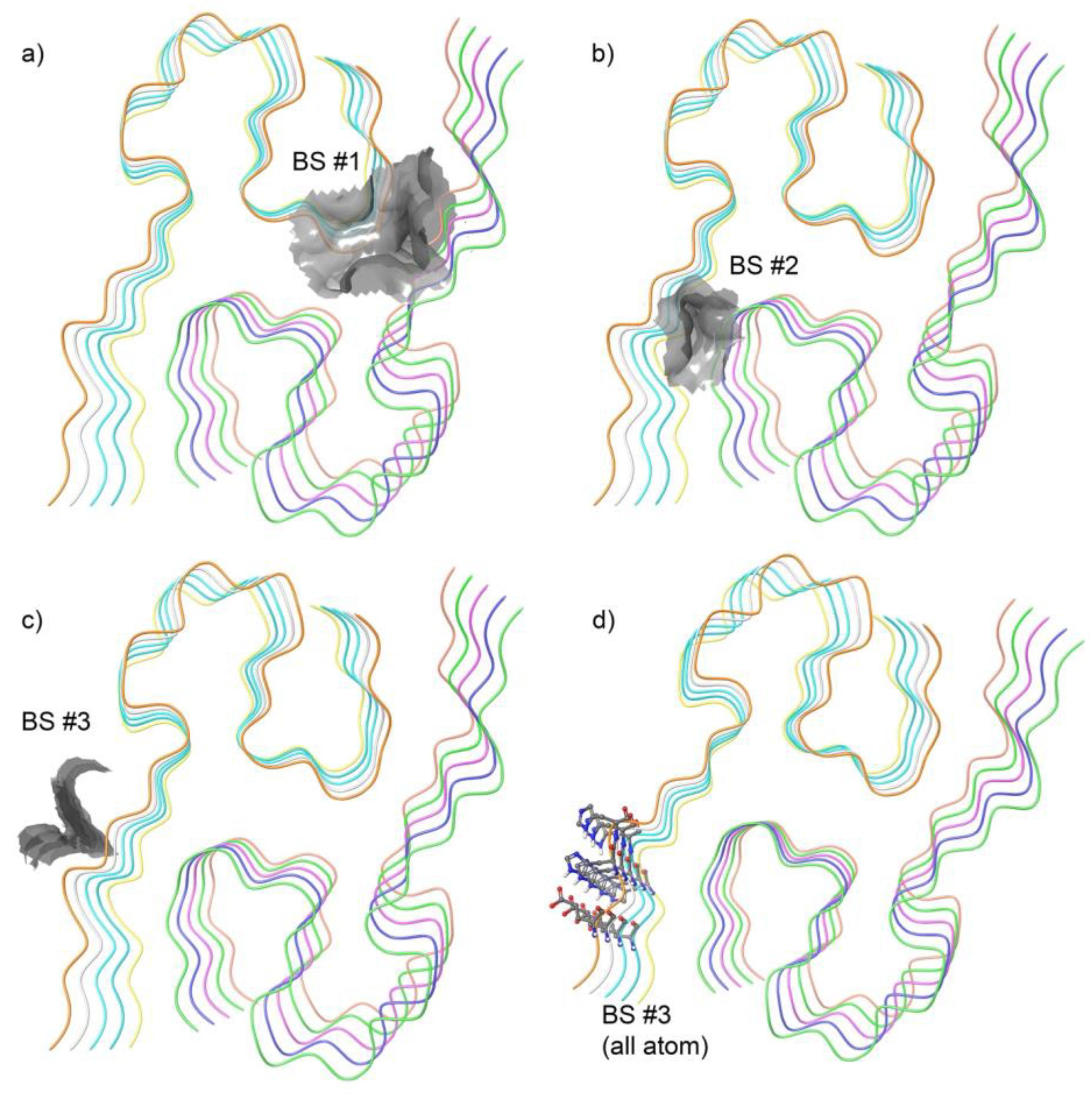
2.2. Binding Site Identified in Amyloid Beta42
2.3. E3 Ligase Structure Selection and Preparation
2.4. ADMET-Based Filtering and Docking
2.5. Dataset Construction and Feature Engineering for Ligase-Conditioned Molecular Glue Design
2.6. Development of Ligase-Conditioned JT-VAE Incorporating Torsional Features for Molecular Glue Design
2.7. Encoding E3 Ligase Binding Site Sequences
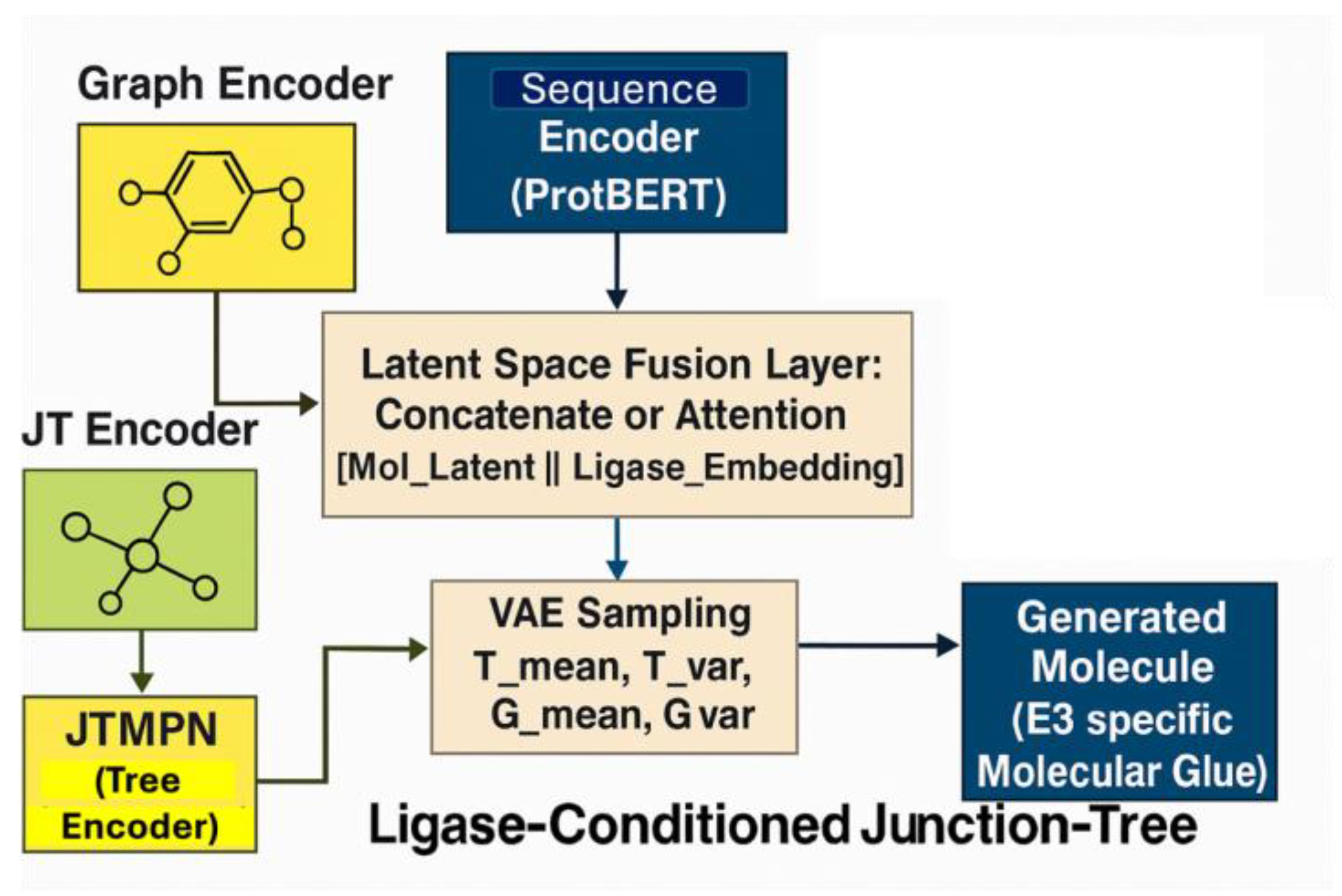
2.8. LC-JT-VAE Architecture and Latent Space Fusion
2.9. Detailed Architectural Breakdown and Conditioning Mechanism
- •
- Molecular Graph and Junction Tree Encoding: Molecules were processed as graphs and junction trees using MPN and JTMPN. Bond features were enhanced by incorporating torsional angles computed via RDKit, improving the model’s ability to capture conformational flexibility crucial for protein–ligand interactions.
- •
- Ligase Sequence Encoding: Binding site sequences were embedded using ProtBERT- based encoders and projected to match the molecular latent space dimensions.
- •
- Latent Space Fusion Strategies:
- o
- Concatenation and Linear Projection:z_fused = ReLU(W[z_mol; z_seq] + b)
- o
- Cross-Attention Mechanism:z_mol’ = MultiHeadAttention(z_mol, z_seq, z_seq)
- •
- Conditional Decoding:
- o
- Tree Decoder: Reconstructs the junction tree scaffold.
- o
- Graph Decoder: Rebuilds the full molecular graph conditioned on the fused latent vector.
2.10. Model Training, Optimization, and Implementation Details
3. Results
3.1. Identification of a Ligandable Interface on Amyloid-β42 Fibrils
3.2. ADMET-Based Filtering of Compound Libraries
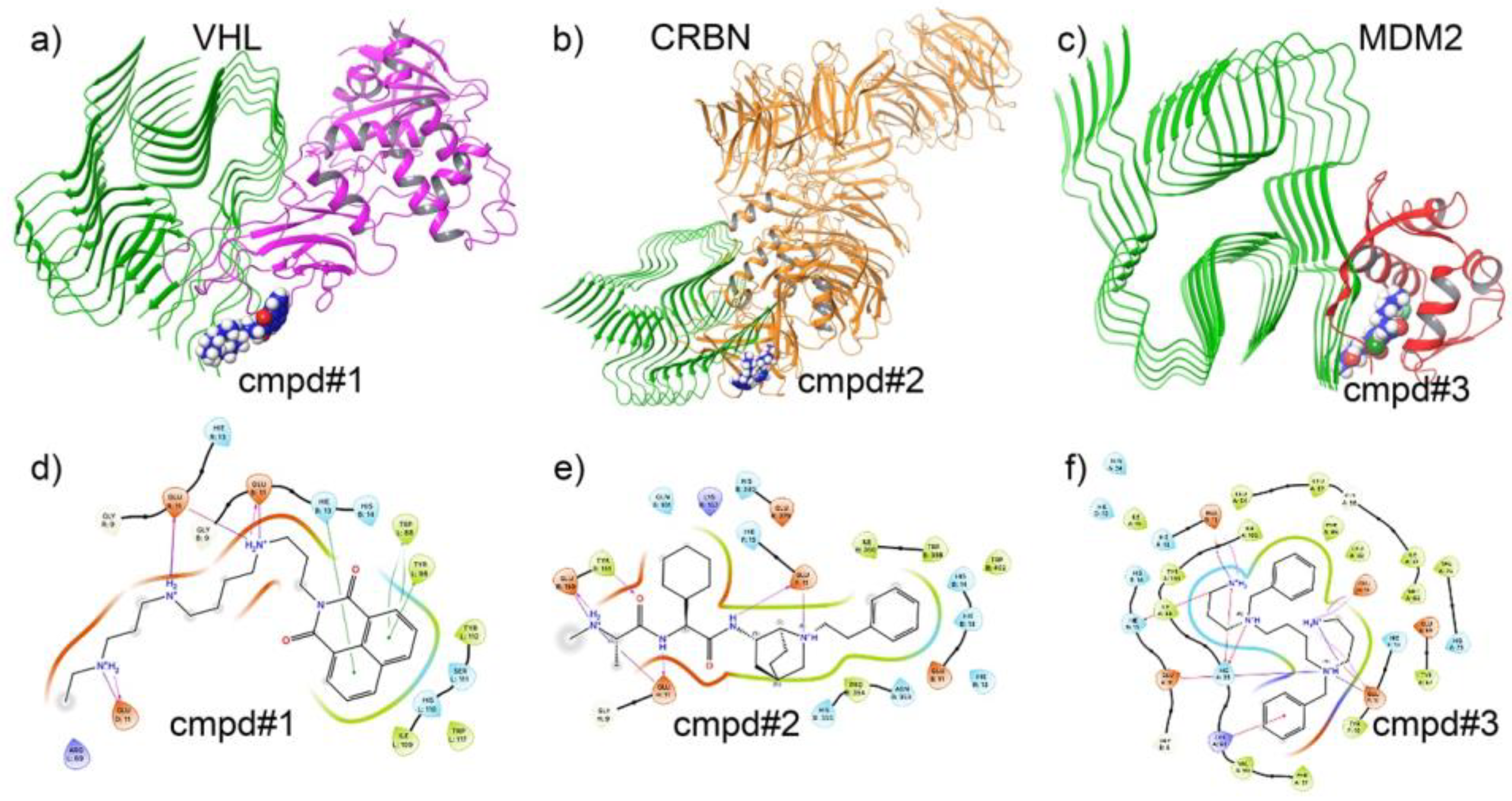
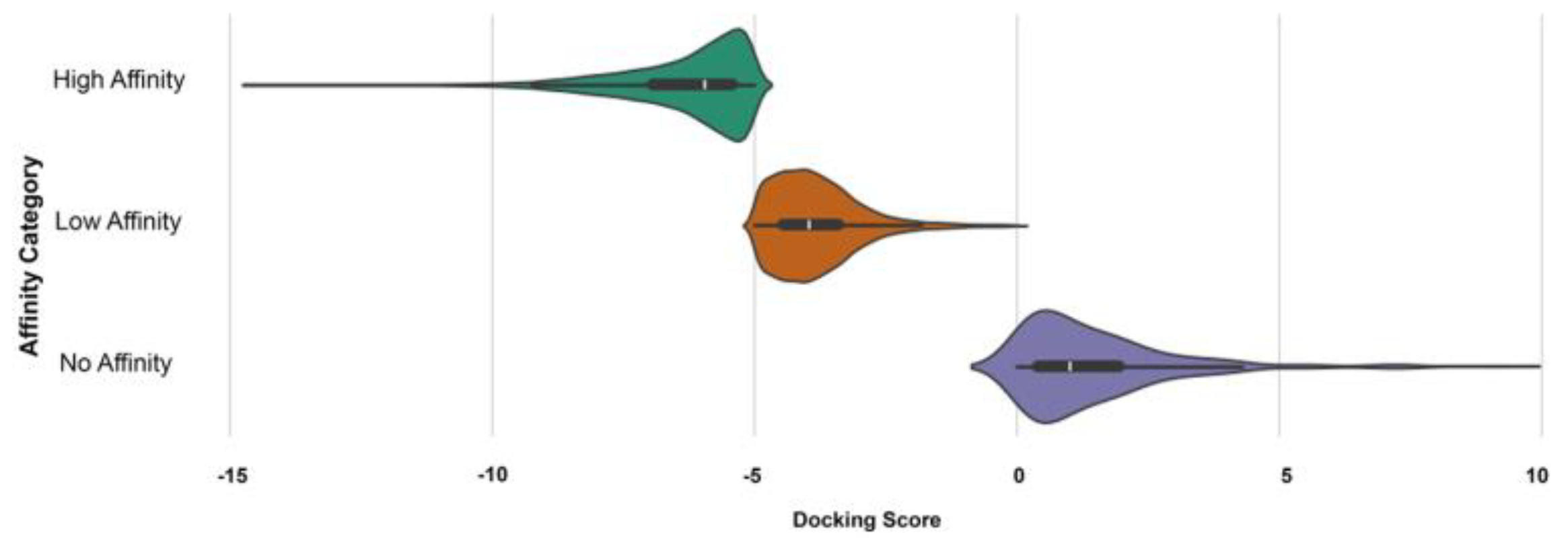
3.3. Docking Analysis of Ternary Complexes Formed by E3 Ligases and Amyloid Beta-42 via Molecular Glue Compounds
3.4. Training Data Compounds Characterization to Generate the Ligase-Conditioned Junction Tree Variational Autoencoder (LCJTVAE)
3.5. Graph and Junction Tree Representations of Molecules for Model Training
3.6. The Loss Function
3.7. AI-Generated Compounds and Model Performance
3.7.1. Molecular Diversity Visualization via t-SNE
3.7.2. Model Performance Across E3 Ligases
3.7.3. E3 Ligase Protein-Specific Structural Insights into AI-Generated Compounds
3.7.4. Three-Dimensional Conformational Plausibility of Ligase-Specific Compounds
3.8. Target-Specific Docking Validates the Precision of AI-Generated Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Hoe, H.S.; Lee, H.K.; Pak, D.T. The upside of APP at synapses. CNS Neurosci. Ther. 2012, 18, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Koch, L. Non-coding RNA: A protective role for TERRA at telomeres. Nat. Rev. Genet. 2017, 18, 453. [Google Scholar] [CrossRef]
- Li, Y.; Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Weiner, M.W.; Shaw, L.M.; Masters, C.L.; Fowler, C.J.; Trojanowski, J.Q.; et al. Validation of Plasma Amyloid-beta 42/40 for Detecting Alzheimer Disease Amyloid Plaques. Neurology 2022, 98, e688–e699. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Williamson, N.; Mishra, A.; Michel, C.H.; Kaminski, C.F.; Tunnacliffe, A.; Kaminski Schierle, G.S. Structural progression of amyloid-beta Arctic mutant aggregation in cells revealed by multiparametric imaging. J. Biol. Chem. 2019, 294, 1478–1487. [Google Scholar] [CrossRef]
- Lin, J.Y.; Wang, W.A.; Zhang, X.; Liu, H.Y.; Zhao, X.L.; Huang, F.D. Intraneuronal accumulation of Abeta42 induces age-dependent slowing of neuronal transmission in Drosophila. Neurosci. Bull. 2014, 30, 185–190. [Google Scholar] [CrossRef][Green Version]
- Zeidel, M.L.; Hammond, T.; Botelho, B.; Harris, H.W., Jr. Functional and structural characterization of endosomes from toad bladder epithelial cells. Am. J. Physiol. 1992, 263, F62–F76. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Imbimbo, B.P.; Panza, F.; Frisardi, V.; Solfrizzi, V.; D’Onofrio, G.; Logroscino, G.; Seripa, D.; Pilotto, A. Therapeutic intervention for Alzheimer’s disease with gamma-secretase inhibitors: Still a viable option? Expert. Opin. Investig. Drugs 2011, 20, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Sevigny, J.; Suhy, J.; Chiao, P.; Chen, T.; Klein, G.; Purcell, D.; Oh, J.; Verma, A.; Sampat, M.; Barakos, J. Amyloid PET Screening for Enrichment of Early-Stage Alzheimer Disease Clinical Trials: Experience in a Phase 1b Clinical Trial. Alzheimer Dis. Assoc. Disord. 2016, 30, 1–7. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Menzies, F.M.; Fleming, A.; Caricasole, A.; Bento, C.F.; Andrews, S.P.; Ashkenazi, A.; Fullgrabe, J.; Jackson, A.; Jimenez Sanchez, M.; Karabiyik, C.; et al. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron 2017, 93, 1015–1034. [Google Scholar] [CrossRef] [PubMed]
- Elsworthy, R.J.; Crowe, J.A.; King, M.C.; Dunleavy, C.; Fisher, E.; Ludlam, A.; Parri, H.R.; Hill, E.J.; Aldred, S. The effect of citalopram treatment on amyloid-beta precursor protein processing and oxidative stress in human hNSC-derived neurons. Transl. Psychiatry 2022, 12, 285. [Google Scholar] [CrossRef]
- Chung, C.W.; Stephens, A.D.; Konno, T.; Ward, E.; Avezov, E.; Kaminski, C.F.; Hassanali, A.A.; Kaminski Schierle, G.S. Intracellular Abeta42 Aggregation Leads to Cellular Thermogenesis. J. Am. Chem. Soc. 2022, 144, 10034–10041. [Google Scholar] [CrossRef] [PubMed]
- Ohyagi, Y.; Tsuruta, Y.; Motomura, K.; Miyoshi, K.; Kikuchi, H.; Iwaki, T.; Taniwaki, T.; Kira, J. Intraneuronal amyloid beta42 enhanced by heating but counteracted by formic acid. J. Neurosci. Methods 2007, 159, 134–138. [Google Scholar] [CrossRef]
- Kitajima, Y.; Yoshioka, K.; Suzuki, N. The ubiquitin–proteasome system in regulation of the skeletal muscle homeostasis and atrophy: From basic science to disorders. J. Physiol. Sci. 2020, 70, 40. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Wertz, I.E.; Wang, X. From Discovery to Bedside: Targeting the Ubiquitin System. Cell Chem. Biol. 2019, 26, 156–177. [Google Scholar] [CrossRef]
- Thibaudeau, T.A.; Anderson, R.T.; Smith, D.M. A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nat. Commun. 2018, 9, 1097. [Google Scholar] [CrossRef] [PubMed]
- King, E.A.; Cho, Y.; Hsu, N.S.; Dovala, D.; McKenna, J.M.; Tallarico, J.A.; Schirle, M.; Nomura, D.K. Chemoproteomics-enabled discovery of a covalent molecular glue degrader targeting NF-kappaB. Cell Chem. Biol. 2023, 30, 394–402.e9. [Google Scholar] [CrossRef] [PubMed]
- Hanzl, A.; Winter, G.E. Targeted protein degradation: Current and future challenges. Curr. Opin. Chem. Biol. 2020, 56, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Potjewyd, F.M.; Axtman, A.D. Exploration of Aberrant E3 Ligases Implicated in Alzheimer’s Disease and Development of Chemical Tools to Modulate Their Function. Front. Cell. Neurosci. 2021, 15, 768655. [Google Scholar] [CrossRef]
- Ivanenkov, Y.A.; Polykovskiy, D.; Bezrukov, D.; Zagribelnyy, B.; Aladinskiy, V.; Kamya, P.; Aliper, A.; Ren, F.; Zhavoronkov, A. Chemistry42: An AI-Driven Platform for Molecular Design and Optimization. J. Chem. Inf. Model. 2023, 63, 695–701. [Google Scholar] [CrossRef]
- McNair, D. Artificial Intelligence and Machine Learning for Lead-to-Candidate Decision-Making and Beyond. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 77–97. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, H.; Sharma, P.; Sahi, S. Advances in Artificial Intelligence (AI)-assisted approaches in drug screening. Artif. Intell. Chem. 2024, 2, 100039. [Google Scholar] [CrossRef]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial intelligence in drug discovery and development. Drug Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef]
- Perron, Q.; Mirguet, O.; Tajmouati, H.; Skiredj, A.; Rojas, A.; Gohier, A.; Ducrot, P.; Bourguignon, M.P.; Sansilvestri-Morel, P.; Do Huu, N.; et al. Deep generative models for ligand-based de novo design applied to multi-parametric optimization. J. Comput. Chem. 2022, 43, 692–703. [Google Scholar] [CrossRef]
- Luukkonen, S.; van den Maagdenberg, H.W.; Emmerich, M.T.M.; van Westen, G.J.P. Artificial intelligence in multi-objective drug design. Curr. Opin. Struct. Biol. 2023, 79, 102537. [Google Scholar] [CrossRef]
- Arus-Pous, J.; Johansson, S.V.; Prykhodko, O.; Bjerrum, E.J.; Tyrchan, C.; Reymond, J.L.; Chen, H.; Engkvist, O. Randomized SMILES strings improve the quality of molecular generative models. J. Cheminform. 2019, 11, 71. [Google Scholar] [CrossRef]
- Reiser, P.; Neubert, M.; Eberhard, A.; Torresi, L.; Zhou, C.; Shao, C.; Metni, H.; van Hoesel, C.; Schopmans, H.; Sommer, T.; et al. Graph neural networks for materials science and chemistry. Commun. Mater. 2022, 3, 93. [Google Scholar] [CrossRef]
- Meyers, J.; Fabian, B.; Brown, N. De novo molecular design and generative models. Drug Discov. Today 2021, 26, 2707–2715. [Google Scholar] [CrossRef]
- Ramirez-Mora, T.; Retana-Lobo, C.; Valle-Bourrouet, G. Biochemical characterization of extracellular polymeric substances from endodontic biofilms. PLoS ONE 2018, 13, e0204081. [Google Scholar] [CrossRef]
- Jin, W.; Barzilay, R.; Jaakkola, T. Junction Tree Variational Autoencoder for Molecular Graph Generation. arXiv 2019, arXiv:1802.04364. [Google Scholar]
- Truong, G.-B.; Pham, T.-A.; To, V.-T.; Le, H.-S.L.; Van Nguyen, P.-C.; Trinh, T.-C.; Phan, T.-L.; Truong, T.N. Discovery of Vascular Endothelial Growth Factor Receptor 2 Inhibitors Employing Junction Tree Variational Autoencoder with Bayesian Optimization and Gradient Ascent. ACS Omega 2024, 9, 47180–47193. [Google Scholar] [CrossRef]
- Kondratyev, V.; Dryzhakov, M.; Gimadiev, T.; Slutskiy, D. Generative model based on junction tree variational autoencoder for HOMO value prediction and molecular optimization. J. Cheminform. 2023, 15, 11. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Lee, M.; Yau, W.-M.; Louis, J.M.; Tycko, R. Structures of brain-derived 42-residue amyloid-β fibril polymorphs with unusual molecular conformations and intermolecular interactions. Proc. Natl. Acad. Sci. USA 2023, 120, e2218831120. [Google Scholar] [CrossRef]
- Dey, A.; Verma, A.; Bhaskar, U.; Sarkar, B.; Kallianpur, M.; Vishvakarma, V.; Das, A.K.; Garai, K.; Mukherjee, O.; Ishii, K.; et al. A Toxicogenic Interaction between Intracellular Amyloid-beta and Apolipoprotein-E. ACS Chem. Neurosci. 2024, 15, 1265–1275. [Google Scholar] [CrossRef]
- Bhachoo, J.; Beuming, T. Investigating Protein-Peptide Interactions Using the Schrodinger Computational Suite. Methods Mol. Biol. 2017, 1561, 235–254. [Google Scholar] [CrossRef]
- Hsu, P.W.; Lin, L.Z.; Hsu, S.D.; Hsu, J.B.; Huang, H.D. ViTa: Prediction of host microRNAs targets on viruses. Nucleic Acids Res. 2007, 35, D381–D385. [Google Scholar] [CrossRef]
- Zdrazil, B.; Felix, E.; Hunter, F.; Manners, E.J.; Blackshaw, J.; Corbett, S.; de Veij, M.; Ioannidis, H.; Lopez, D.M.; Mosquera, J.F.; et al. The ChEMBL Database in 2023: A drug discovery platform spanning multiple bioactivity data types and time periods. Nucleic Acids Res. 2024, 52, D1180–D1192. [Google Scholar] [CrossRef]
- Shaikh, F.; Siu, S.W. Identification of novel natural compound inhibitors for human complement component 5a receptor by homology modeling and virtual screening. Med. Chem. Res. 2016, 25, 1564–1573. [Google Scholar] [CrossRef]
- Jin, W.; Barzilay, R.; Jaakkola, T.S. Junction Tree Variational Autoencoder for Molecular Graph Generation. CoRR 2018, abs/1802.04364. [Google Scholar]
- Jin, W.; Barzilay, R.; Jaakkola, T. Junction Tree Variational Autoencoder for Molecular Graph Generation. In Proceedings of the 35th International Conference on Machine Learning, Stockholm, Sweden, 10–15 July 2018; pp. 2323–2332. [Google Scholar]
- Karauda, T.; Kornicki, K.; Jarri, A.; Antczak, A.; Milkowska-Dymanowska, J.; Piotrowski, W.J.; Majewski, S.; Gorski, P.; Bialas, A.J. Eosinopenia and neutrophil-to-lymphocyte count ratio as prognostic factors in exacerbation of COPD. Sci. Rep. 2021, 11, 4804. [Google Scholar] [CrossRef]
- Thireou, T.; Reczko, M. Bidirectional Long Short-Term Memory Networks for predicting the subcellular localization of eukaryotic proteins. IEEE/ACM Trans. Comput. Biol. Bioinform. 2007, 4, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Kingma, D.P.; Welling, M. Auto-Encoding Variational Bayes. arXiv 2022, arXiv:1312.6114. [Google Scholar]
- Csiszar, I. I-Divergence Geometry of Probability Distributions and Minimization Problems. Ann. Probab. 1975, 3, 146–158. [Google Scholar] [CrossRef]
- Colaboratory, G. Jupyter Notebook. Available online: https://colab.research.google.com (accessed on 10 November 2020).

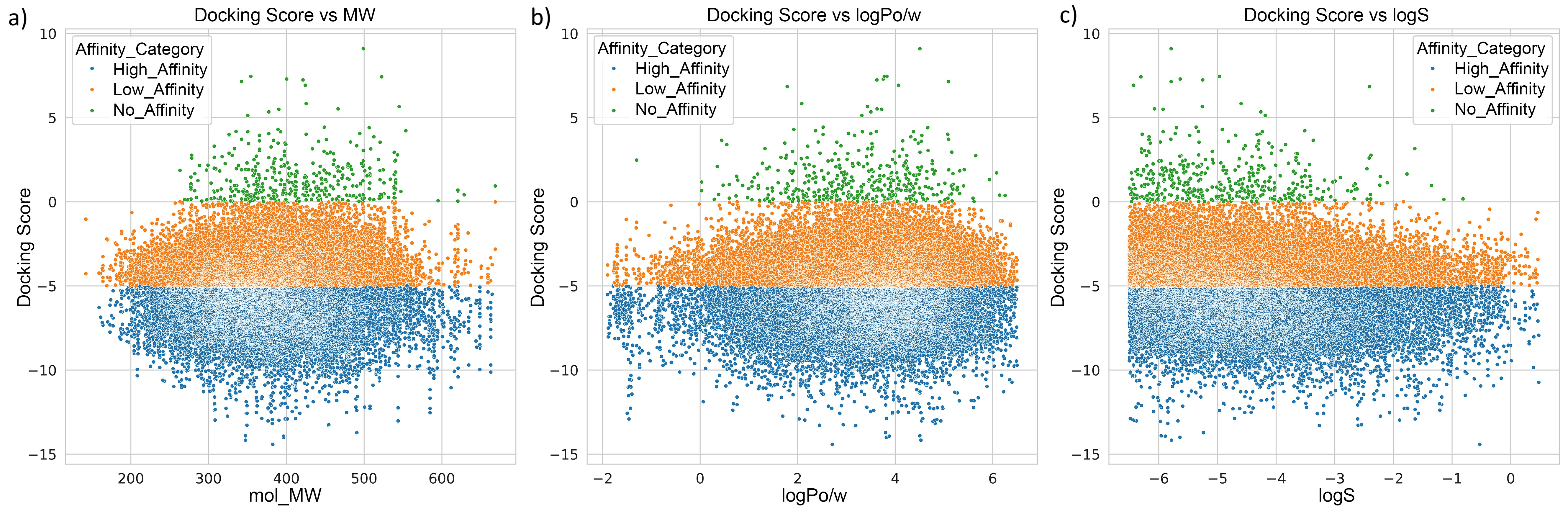

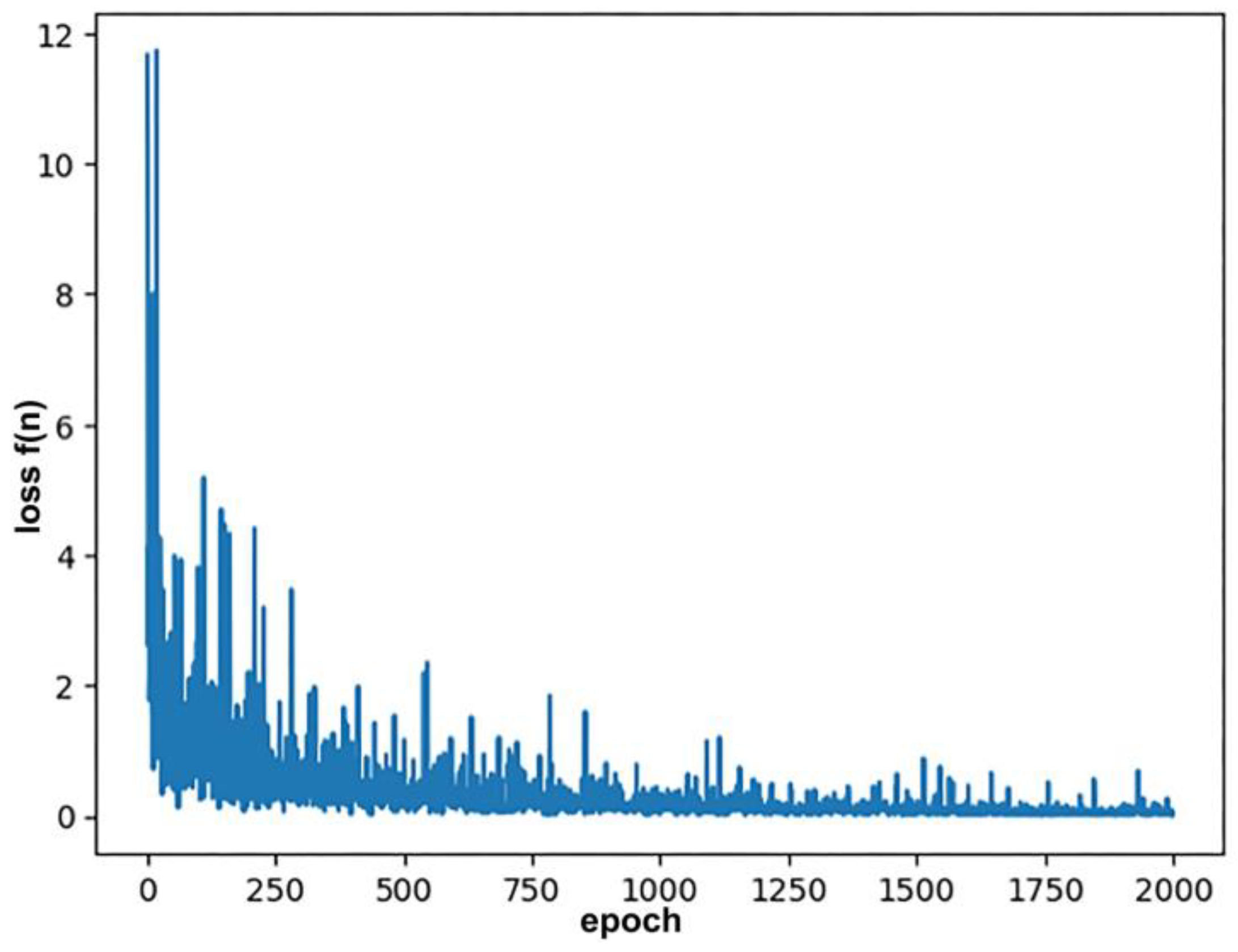
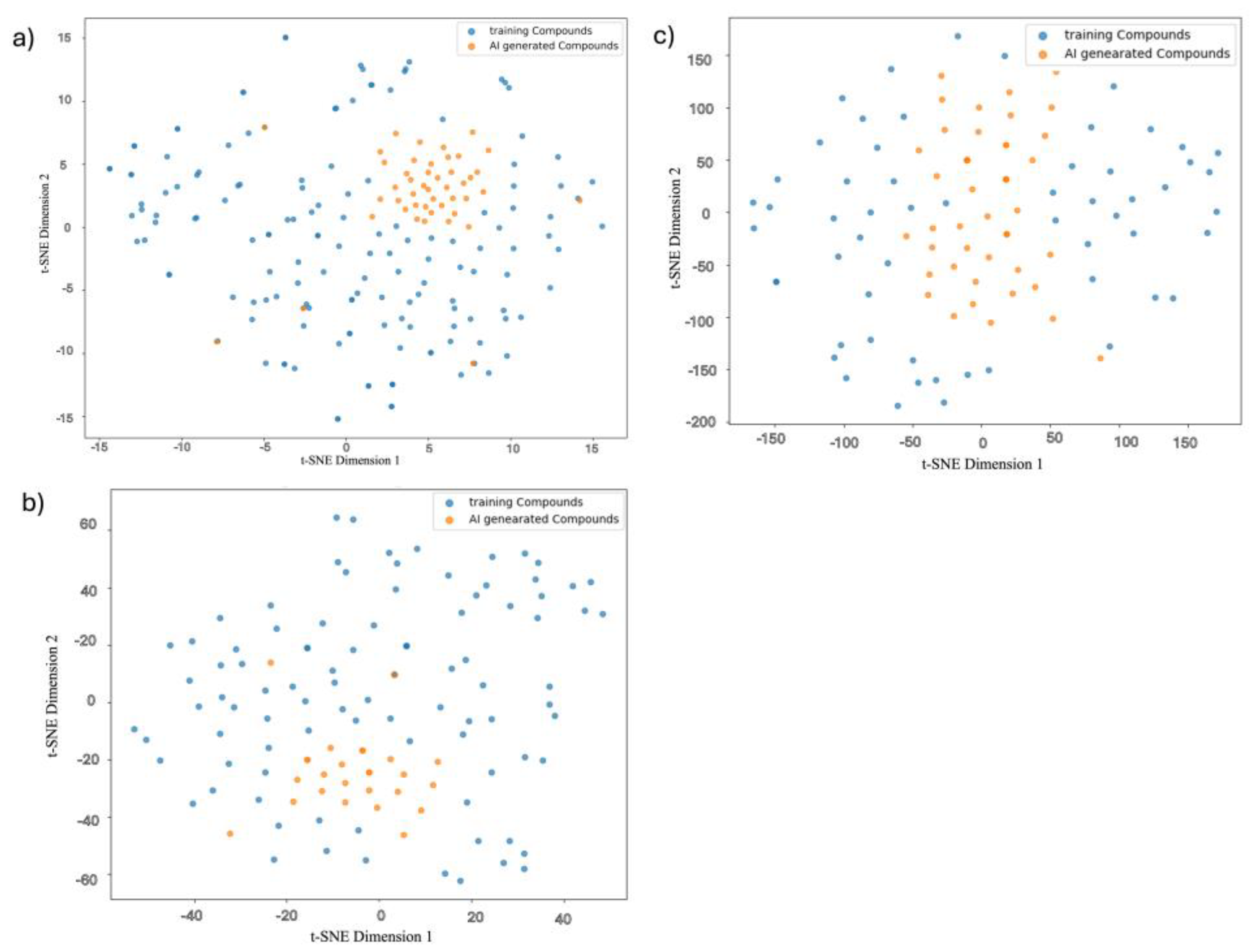
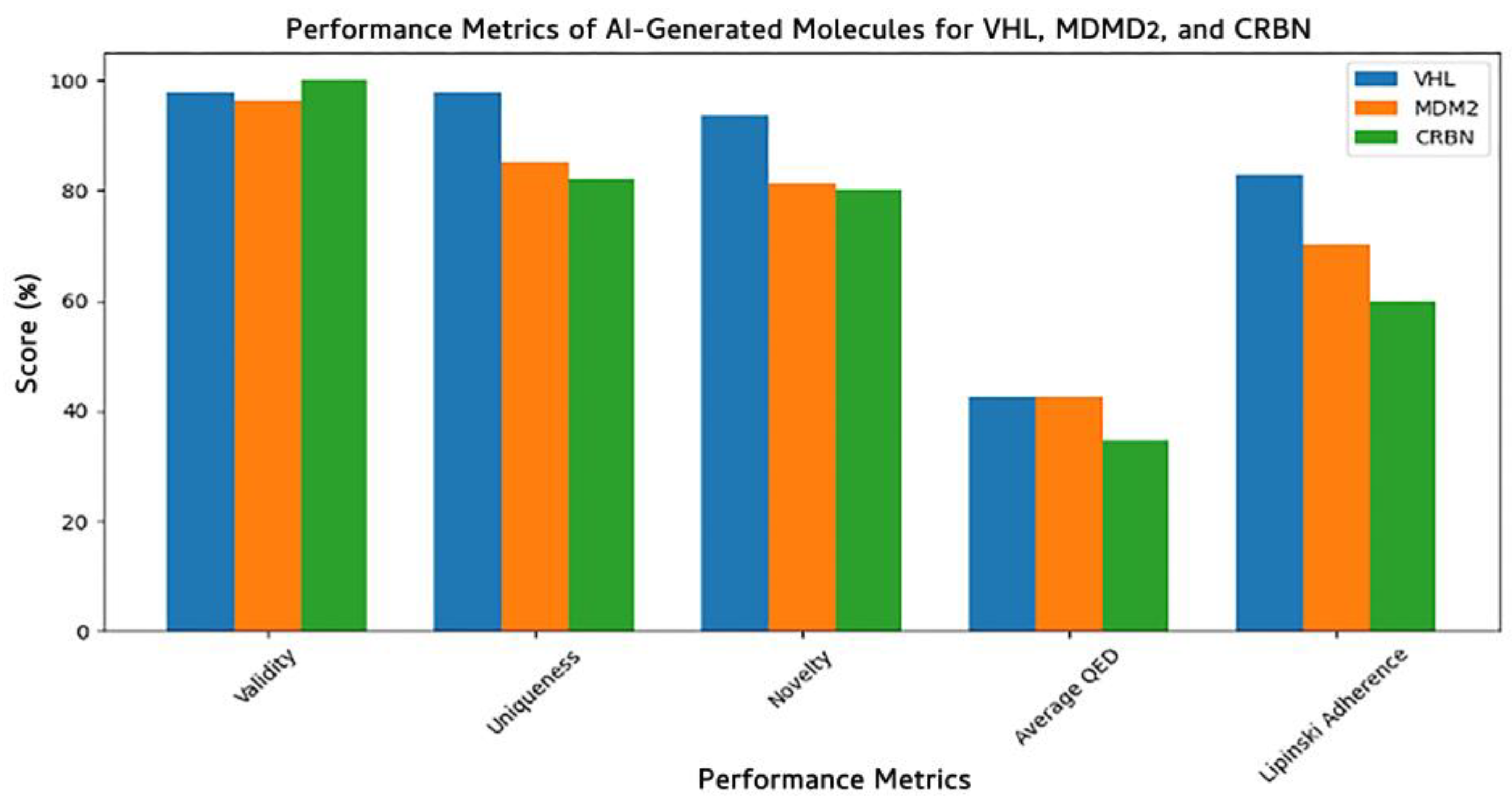
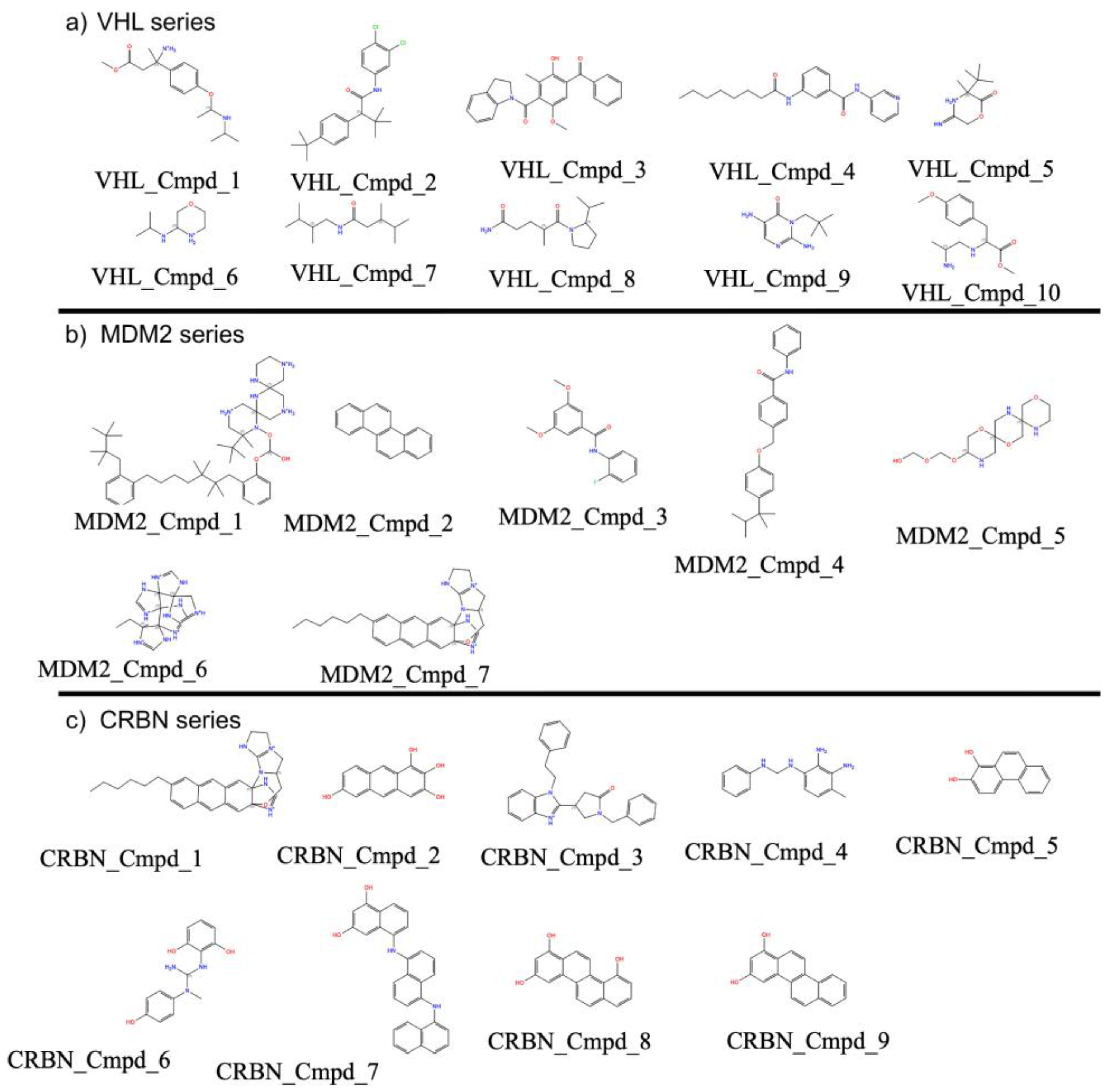
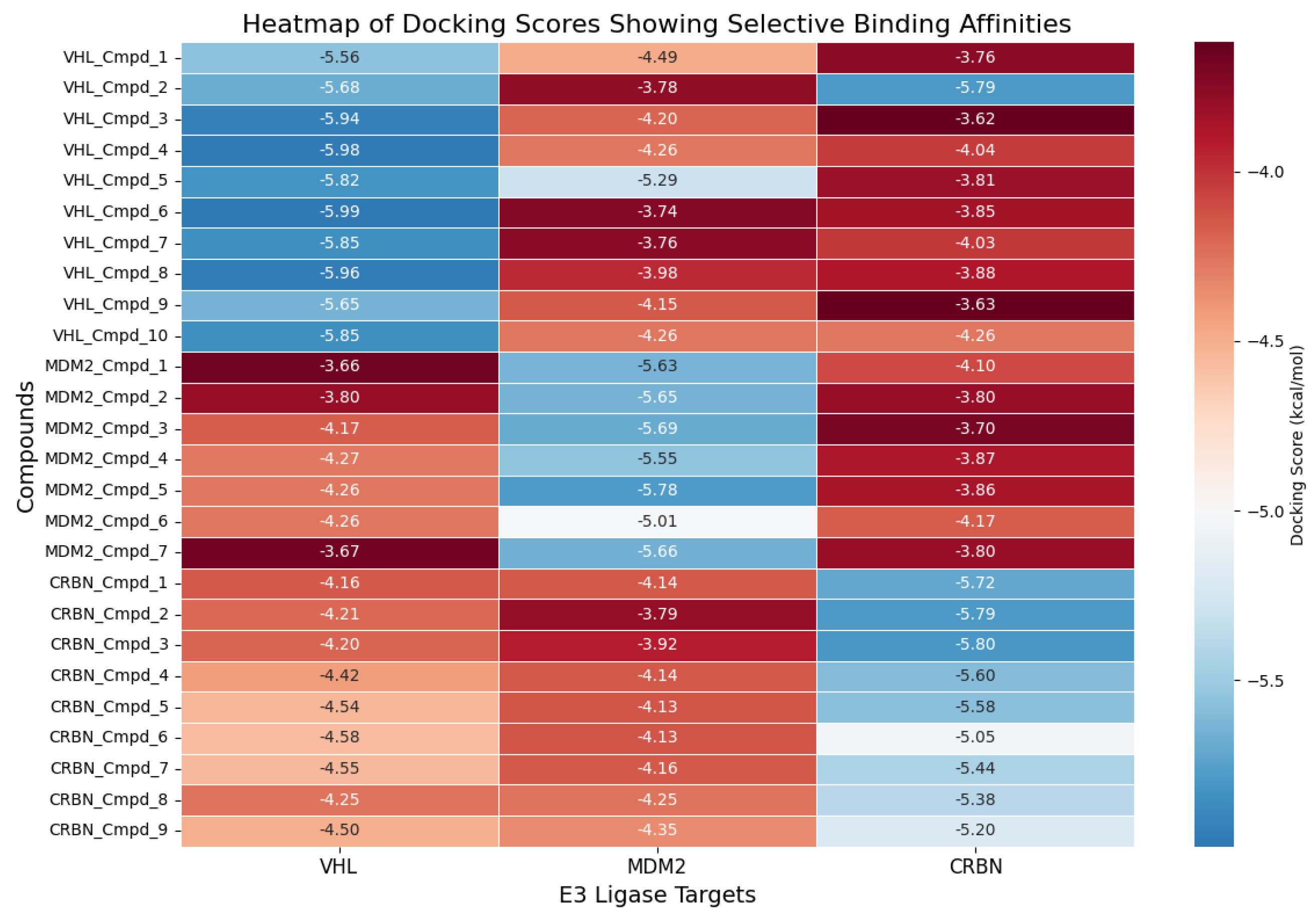
| Property | Count | Mean | Std Dev | Min | 25% | 50% | 75% | Max |
|---|---|---|---|---|---|---|---|---|
| MW | 65,998 | 366.68 | 70.31 | 142.24 | 316.42 | 361.35 | 411.45 | 668.72 |
| logPo/w | 65,998 | 3.39 | 1.29 | −1.89 | 2.60 | 3.54 | 4.31 | 6.49 |
| logS | 65,998 | −4.58 | 1.29 | −6.5 | −5.59 | −4.77 | −3.83 | 0.47 |
| #metab | 65,998 | 3.64 | 1.76 | 1 | 2 | 3 | 5 | 8 |
| Rule-Of-Five | 65,998 | 0.14 | 0.35 | 0 | 0 | 0 | 0 | 1 |
| Ligase | Library | High_Affinity | Low_Affinity | No_Affinity | Total |
|---|---|---|---|---|---|
| CRBN | ChEMBL | 4834 | 7448 | 36 | 12,318 |
| Vitas | 1748 | 11,949 | 73 | 13,770 | |
| MDM2 | ChEMBL | 131 | 51 | 0 | 182 |
| Vitas | 2302 | 11,361 | 86 | 13,749 | |
| VHL | ChEMBL | 8792 | 3414 | 80 | 12,286 |
| Vitas | 6607 | 6949 | 137 | 13,693 | |
| Total | 65,998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, N.N.; Caulfield, T.R. Conditioned Generative Modeling of Molecular Glues: A Realistic AI Approach for Synthesizable Drug-like Molecules. Biomolecules 2025, 15, 849. https://doi.org/10.3390/biom15060849
Islam NN, Caulfield TR. Conditioned Generative Modeling of Molecular Glues: A Realistic AI Approach for Synthesizable Drug-like Molecules. Biomolecules. 2025; 15(6):849. https://doi.org/10.3390/biom15060849
Chicago/Turabian StyleIslam, Naeyma N., and Thomas R. Caulfield. 2025. "Conditioned Generative Modeling of Molecular Glues: A Realistic AI Approach for Synthesizable Drug-like Molecules" Biomolecules 15, no. 6: 849. https://doi.org/10.3390/biom15060849
APA StyleIslam, N. N., & Caulfield, T. R. (2025). Conditioned Generative Modeling of Molecular Glues: A Realistic AI Approach for Synthesizable Drug-like Molecules. Biomolecules, 15(6), 849. https://doi.org/10.3390/biom15060849








